Abstract
Background and Purpose
The spot sign score is a potent predictor of hematoma expansion in patients with primary intracerebral hemorrhage (ICH). We aim to determine the accuracy of this scoring system for the prediction of in-hospital mortality and poor outcome among survivors in patients with primary ICH.
Methods
Three neuroradiologists retrospectively reviewed CT angiograms (CTAs) performed in 573 consecutive patients who presented to our Emergency Department with primary ICH over a 9-year period to determine the presence and scoring of spot signs according to strict criteria. Baseline ICH and intraventricular hemorrhage volumes were independently determined by computer-assisted volumetric analysis. Medical records were independently reviewed for baseline clinical characteristics and modified Rankin Scale (mRS) at hospital discharge and 3-month follow-up. Poor outcome among survivors was defined as a mRS ≥4 at 3-month follow-up.
Results
We identified spot signs in 133 of 573 CTAs (23.2%), 11 of which were delayed spot signs (8.3%). The presence of any spot sign increased the risk of in-hospital mortality (55.6%, OR4.0, 95%CI 2.6–5.9, p<0.0001) and poor outcome among survivors at 3-month follow-up (50.8%, OR2.5, 95%CI 1.4–4.3, p 0.0014). The spot sign score successfully predicted an escalating risk of both outcome measures. In multivariate analysis, the spot sign score was an independent predictor of in-hospital mortality (OR1.5, 95%CI 1.2–1.9, p 0.0002) and poor outcome among survivors at 3-month follow-up (OR1.6, 95%CI 1.1–2.1, p 0.0065).
Conclusion
The spot sign score is an independent predictor of in-hospital mortality and poor outcome among survivors in primary ICH.
Keywords: CTA spot sign, Intracerebral hemorrhage, Emergency medicine, Outcome
INTRODUCTION
Non-traumatic intracerebral hemorrhage (ICH) accounts for 10–15% of cases of acute stroke in the United States1 and has a worse prognosis than ischemic stroke, with up to 50% 30-day mortality.2 The presence of active contrast extravasation into the hematoma at the time of multi-detector CT angiography (MDCTA), the spot sign, is an indicator of active hemorrhage and has been associated with an increased risk of hematoma expansion and mortality in patients with ICH in prior studies.3–10 Moreover, systematic characterization of the spot sign according to strict radiological criteria has made possible the development of a spot sign scoring system that identifies those ICH patients who are at highest risk of hematoma expansion.9 Hematoma expansion, in turn, has been shown to be an independent predictor of increased mortality and poor outcome in ICH.11 Identification of an accurate and reliable predictor of hematoma expansion, mortality and poor outcome in patients with ICH is important,12–14 as it may serve to select patients for early hemostatic therapies such as recombinant activated factor VII or intensive blood pressure reduction.11, 15–18
This study aims to assess whether the spot sign score can be utilized to identify primary ICH patients who are at highest risk of in-hospital mortality and poor outcome among survivors at 3-month follow-up.
METHODS
Patient Selection
Our study was approved by the hospital’s institutional review board. We conducted a retrospective review of all consecutive patients who presented to our Emergency Department from January 1st, 2000 until December 31st, 2008, with (1) evidence of non-traumatic ICH on a non-contrast CT examination (NCCT) of the head and (2) evaluation with a CT angiogram (CTA) of the intracranial circulation within 24 hours of presentation. Patient exclusion criteria were the presence of (1) associated subarachnoid hemorrhage (SAH) in the basal cisterns and/or a vascular lesion as the ICH etiology identified in the CTA, (2) loss of gray-white matter differentiation in a vascular territory suggesting a pre-established acute ischemic stroke, or (3) enrollment in a clinical trial of recombinant factor VIIa. A separate analysis of a subgroup of these patients with a different outcome measure has been previously published.9
Image Acquisition
NCCT and MDCTA acquisitions were performed according to standard departmental protocols on 16 or 64-section General Electric helical CT scanners (General Electric Medical Systems, Milwaukee, WI). NCCT examinations were performed using axial technique with 120–140kVp, 170mA, and 5mm slice thickness reconstruction. MDCTA was subsequently performed by scanning from the base of the C1 vertebral body to the vertex using axial technique, 0.5pitch, 1.25mm collimation, 350 maximal mA, 120kVp, 22cm field of view, and 65–85mL of iodinated contrast material administered by power injector at 4–5mL per second into an antecubital vein with either a fixed 25-second delay between the onset of contrast injection and the start of scanning, or Smart-Prep, an semi-automatic contrast bolus triggering technique. The decision to perform MDCTA and obtain delayed CTA acquisitions was at the discretion of the clinical providers. In general, delayed CTA images were acquired to (1) exclude dural venous sinus thrombosis as the ICH etiology, (2) assess for the presence of delayed spot signs, or (3) aid in the differentiation between spots signs and aneurysms or arteriovenous malformations in challenging cases.
Image Analysis
The NCCT examinations were reviewed by three experienced neuroradiologists to determine the ICH location (lobar, deep gray matter or infratentorial), presence of associated intraventricular hemorrhage (IVH), and presence of calcifications within or adjacent to the ICH. Subsequently, the 1.25mm axial CTA source images were independently reviewed in “Spot Windows” (width 200, level 110) by the same three neuroradiologists to determine the presence and scoring of spot signs according to previously-described strict radiological criteria (Table 1) and scoring system (Table 2).9
Table 1.
Spot Sign Criteria
| ≥1 focus of contrast pooling within the ICH |
| Attenuation ≥120 Hounsfield units |
| Discontinuous from normal or abnormal vasculature adjacent to the ICH |
| Any size and morphology |
ICH: intracerebral hemorrhage.
Table 2.
Calculation of the Spot Sign Score
| Spot Sign Characteristic* | Points |
|---|---|
| Number of spot signs | |
| 1 – 2: | 1 |
| ≥3: | 2 |
| Maximum axial dimension | |
| 1–4 mm: | 0 |
| ≥5 mm: | 1 |
| Maximum attenuation | |
| 120–179 HU: | 0 |
| ≥180 HU: | 1 |
The spot sign characterization is performed in the first CTA acquisition in which a spot sign is identified. For CTAs with more than 1 spot sign, the maximum dimension in a single axial CTA source image and maximum attenuation of the largest spot sign is determined. The spot sign score is obtained by adding the total number of points for the CTA.
HU: Hounsfield unit; CTA: CT angiogram.
If a delayed CTA acquisition was obtained, it was reviewed by the same three neuroradiologists, blinded to the first-pass CTA, to determine the presence and scoring of spot signs according to the same criteria. The spot sign score obtained in the first CTA acquisition in which a spot sign was identified was utilized for the purpose of this study. Differences in reader interpretation for the presence and/or scoring of spot signs were adjudicated by consensus.
Determination of the initial ICH and IVH volumes was performed independently and blinded to the CTA categorization, with the Analyze 9.0 software (Mayo Clinic, Rochester, MN) by thresholding with manual hematoma outline adjustment in the baseline NCCT examinations.
Medical Record Review
Medical records were independently reviewed for time of ictus, patient age, gender, admission mean arterial blood pressure (MABP), International Normalized Ratio (INR), Glasgow Coma Scale (GCS) and blood glucose level, history of hypertension, anti-platelet therapy, surgical intervention, length of hospital stay and modified Rankin Scale (mRS) at hospital discharge and 3-month follow-up. A known time of ictus was only recorded if it was witnessed or self-reported by the patient within a 15-minute margin of error as documented in the Neurology Emergency Department consultation note.
Three-month follow-up was available in 477 patients (83.2%). In the remaining 96 patients (16.8%), we carried forward the last clinical observation, mRS at hospital discharge, for the purpose of this study. Poor outcome among survivors at 3-month follow-up was defined as (1) the inability to walk or attend to own bodily needs without assistance (mRS 4), (2) being bedridden, incontinent and requiring constant nursing care and attention (mRS 5), or (3) death after hospital discharge (mRS 6).
Statistical Analysis
Statistical analysis was performed utilizing the SAS 9.1 software package (SAS Institute Inc., Cary, NC). We constructed a multivariate logistic regression model to determine the correlation of the NCCT and clinical variables with the presence of a spot sign, in-hospital mortality and poor outcome among survivors at 3-month follow-up. Subsequently, the multivariate logistic regression analysis was repeated for the prediction of in-hospital mortality and poor outcome among survivors at 3-month follow-up including as an additional variable first (1) the presence of any spot sign, and then (2) the spot sign score. We utilized receiver operating characteristic analysis to determine the area under the curve for the spot sign score in the prediction of in-hospital mortality and poor outcome among survivors at 3-month follow-up. Inter-observer agreement for the identification and scoring of spot signs was determined with the kappa statistic. A p-value ≤0.05 was considered statistically significant.
RESULTS
From January 1st, 2000 until December 31st, 2008, a total of 818 patients presented to our Emergency Department with non-traumatic ICH on a NCCT examination and were evaluated with MDCTA of the intracranial circulation within 24 hours of admission. Two-hundred and forty-five patients were excluded from the study (30.0%): 152 due to the presence of associated SAH within the basal cisterns and/or a vascular lesion as the ICH etiology, 75 due to incomplete admission or discharge clinical data, 15 due to loss of gray-white matter differentiation in a vascular territory, 2 due to enrollment in a clinical trial of recombinant factor VIIa, and 1 due to administration of gadolinium contrast material for the CTA.
A total of 573 patients met our study’s inclusion criteria, with a mean age of 66.7 years (median 69 years, range 8–94 years). Median time from Emergency Department admission to MDCTA evaluation was 1.33 hours (mean 2.5 hours, range 0.25–24 hours), and median length of hospital stay was 6 days (mean 9.5 days, range 1–88 days). A total of 314 patients had a known time of ictus (54.8%, median time of ictus 7.25 hours prior to MDCTA evaluation, mean 7.4 hours, range 0.5–80 hours). Median initial ICH volume was 21.5mL (mean 33.8mL, range 0.2–169mL), and median initial IVH volume was 4.9mL (mean 14.9mL, range 0.1–365mL). Delayed CTA images were acquired in 116 patients (20.2%, median delay time 116 seconds after the first-pass CTA, mean 173 seconds, range 17–689 seconds).
A total of 180 patients expired during the hospitalization (31.4%). Among the 393 survivors, 129 had poor outcome at 3-month follow-up (32.8%), including 2 patients who expired after hospital discharge.
Predictors of a Spot Sign and Clinical Outcome in Primary ICH
Table 3 provides a summary of the predictors of a spot sign, in-hospital mortality and poor outcome among survivors in primary ICH. We identified at least 1 spot sign in 122 of the 573 first-pass CTAs (21.3%) and in 45 of the 116 delayed CTA acquisitions (38.8%). In 34 cases spot signs were present in both the first-pass and delayed CTA acquisitions, in 11 cases spot signs were present in the delayed CTA acquisition only, and in 1 case a spot sign was present in the first-pass CTA but not in the delayed acquisition. Overall, we identified at least 1 spot sign in 133 CTAs (23.2%). The mean number of spot signs per hematoma was 3 (median 2, range 1–30), the mean maximum spot sign axial size was 4.9mm (median 4mm, range 1–16mm) and the mean maximum spot sign attenuation was 214 Hounsfield units (HU, median 198 HU, range 120–456 HU). The mean spot sign score for patients with spot signs was 2.3 (median 2, range 1–4). Inter-observer agreement for both the identification and scoring of spot signs was almost perfect, with kappa statistics ranging from 0.86 to 0.92 between the 3 readers (95% confidence interval [CI] 0.82–0.96).
Table 3.
Clinical and NCCT Predictors of a Spot Sign, In-Hospital Mortality and Poor Outcome among Survivors in Primary ICH
| Spot Sign Frequency n (%) |
p | In-Hospital Mortality n (%) |
p | Poor Outcome* n (%) |
p | |
|---|---|---|---|---|---|---|
| All patients, n=573 | 133 (23) | n/a | 180 (31) | n/a | 129 (23) | n/a |
| Gender | 0.19 | 0.86 | 0.023 | |||
| Males, n=312 | 79 (25) | 97 (31) | 60 (28) | |||
| Females, n=261 | 54 (21) | 83 (32) | 69 (39) | |||
| Age, years | 0.03 | <0.001† | <0.0001† | |||
| 8–45, n=55 | 5 (9) | 8 (15) | 5 (11) | |||
| 46–70, n=248 | 59 (24) | 59 (24) | 47 (25) | |||
| 71–94, n=270 | 69 (26) | 113 (42) | 77 (49) | |||
| Time from ictus to CTA, h | <0.0001† | <0.0001 | 0.66 | |||
| ≤3, n=71 | 47 (66) | 30 (42) | 15 (37) | |||
| >3 – ≤6, n=110 | 27 (25) | 52 (47) | 20 (34) | |||
| >6, n=133 | 18 (14) | 23 (17) | 31 (28) | |||
| Unknown, n=259 | 41 (16) | 75 (29) | 63 (34) | |||
| Admission MABP, mmHg | <0.0001† | 0.76 | 0.009† | |||
| ≤100, n=234 | 33 (14) | 70 (30) | 45 (27) | |||
| 101 – 120, n=193 | 42 (22) | 61 (32) | 40 (30) | |||
| >120, n=146 | 58 (40) | 49 (34) | 44 (45) | |||
| Admission INR | <0.0001† | 0.1 | 0.10 | |||
| <1.5, n=475 | 94 (20) | 142 (30) | 108 (32) | |||
| 1.5 – 2.5, n=59 | 14 (24) | 20 (34) | 10 (26) | |||
| >2.5, n=39 | 25 (64) | 18 (46) | 11 (52) | |||
| Admission GCS | <0.0001 | <0.0001† | <0.0001† | |||
| ≤8, n=177 | 58 (33) | 115 (65) | 32 (52) | |||
| 9–12, n=83 | 27 (33) | 36 (43) | 22 (47) | |||
| ≥13, n=313 | 48 (15) | 29 (9) | 75 (26) | |||
| Blood glucose ≥170 mg/dL | 0.007 | <0.0001 | 0.013 | |||
| Yes, n=147 | 46 (31) | 74 (50) | 96 (30) | |||
| No, n=426 | 87 (20) | 106 (25) | 33 (45) | |||
| History of hypertension | 0.012 | 0.16 | 0.11 | |||
| Yes, n=361 | 96 (27) | 121 (34) | 86 (36) | |||
| No, n=212 | 37 (17) | 59 (28) | 43 (28) | |||
| Anti-platelet therapy | 0.013 | 0.031 | 0.12 | |||
| Yes, n=178 | 53 (30) | 67 (38) | 43 (39) | |||
| No, n=395 | 80 (20) | 113 (29) | 86 (30) | |||
| IC and anti-platelet therapy | 0.0003 | 0.14 | 0.58 | |||
| Yes, n=27 | 14 (52) | 12 (44) | 5 (33) | |||
| No, n=546 | 119 (22) | 168 (31) | 124 (33) | |||
| ICH site | 0.8 | 0.37 | 0.44 | |||
| Lobar, n=325 | 74 (23) | 95 (29) | 71 (31) | |||
| Deep gray, n=186 | 46 (25) | 62 (33) | 42 (34) | |||
| Infra, n=62 | 13 (21) | 23 (37) | 16 (41) | |||
| Initial ICH volume, mL | <0.001† | <0.00001† | <0.0001† | |||
| 0.2–29.9, n=345 | 51 (15) | 53 (15) | 84 (29) | |||
| 30–59.9, n=106 | 26 (25) | 37 (35) | 21 (30) | |||
| ≥60, n=122 | 56 (46) | 90 (74) | 24 (75) | |||
| Initial IVH volume, mL | <0.0001† | <0.0001† | 0.001† | |||
| 0, n=306 | 46 (16) | 48 (16) | 68 (26) | |||
| 0.1–4.9, n=136 | 34 (25) | 44 (32) | 38 (41) | |||
| 5–14.9, n=63 | 21 (33) | 35 (56) | 15 (54) | |||
| ≥15, n=68 | 32 (47) | 53 (78) | 8 (53) | |||
| Surgical evacuation | 0.14 | 0.25 | 0.006 | |||
| Yes, n=81 | 24 (30) | 21 (26) | 29 (48) | |||
| No, n=492 | 109 (22) | 159 (32) | 100 (30) |
The numbers in the table represent number of patients and the numbers in parenthesis represent percentages.
Defined as a modified Rankin Scale of ≥4 at 3-month follow-up among the 393 survivors.
Independent predictor in multivariate logistic regression analysis (p-value ≤0.05). The multivariate logistic regression analyses for in-hospital mortality and poor outcome among survivors at 3-month follow-up exclude the presence of a spot sign.
NCCT: non-contrast CT; p: p-value using Pearson’s chi square test; n: number of patients; n/a: not applicable; h: hours; CTA: CT angiogram; MABP: mean arterial blood pressure; INR: international normalized ratio; IC: impaired coagulation (defined as INR ≥1.5); ICH: intracerebral hemorrhage; Infra: infratentorial; IVH: intraventricular hemorrhage.
There was a significantly higher frequency of spot signs in the 116 delayed CTA acquisitions (38.8%) compared to the 573 first-pass CTAs (21.3%, p<0.0001, Pearson’s chi square test). Indeed, 11 spot signs were seen in the delayed CTA acquisition only (8.3%). Nonetheless, these delayed spot signs were equally predictive of in-hospital mortality (positive predictive value [PPV] 63.6%) and poor outcome among survivors at 3-month follow-up (PPV 50%).
In the 92 patients with spot signs and a known time of ictus, there was a significant difference between the median spot sign score for patients imaged ≤3 hours from ictus (median score 3, mean score 2.7, range 1–4), >3 to ≤6 hours from ictus (median score 2, mean 2.1, range 1–4), and >6 hours from ictus (median 1, mean score 1.4, range 1–2, p<0.0001, Kruskal-Wallis test). The median score for the 41 patients with spot signs and an unknown time of ictus was 2 (mean 2.4, range 1–4).
The highest spot sign scores in our population were observed in patients with initial ICH volumes of 30–59.9mL (mean score 2.8, median 3, range 1–4), MDCTA evaluation within 3 hours of ictus (mean score 2.7, median 3, range 1–4), admission GCS 9–12 (mean score 2.7, median 3, range 1–4), admission MABP>120mmHg (mean score 2.6, median 3, range 1–4), and deep gray matter ICH (mean score 2.6, median 3, range 1–4). Patients with spot sign scores of 1–4 are illustrated in the on-line figure.
Accuracy of the Spot Sign and Spot Sign Score for the Prediction of Clinical Outcome in Primary ICH
The presence of any spot sign at MDCTA markedly increased the risk of in-hospital mortality (PPV 55.6%, odds ratio [OR] 4.0 [95% CI 2.6–5.9], p<0.0001) and poor outcome among survivors at 3-month follow-up (PPV 50.8%, OR 2.5 [95% CI 1.4–4.3], p 0.0014, Table 4).
Table 4.
Accuracy of the Spot Sign for the Prediction of In-Hospital Mortality and Poor Outcome among Survivors in Primary ICH
| Accuracy Parameter | In-Hospital Mortality (95% CI) |
Poor Outcome* (95% CI) |
|---|---|---|
| Sensitivity | 41 (34 – 49) | 23 (17 – 32) |
| Specificity | 85 (81 – 88) | 89 (85 – 92) |
| PPV | 56 (47 – 64) | 51 (38 – 64) |
| NPV | 76 (72 – 80) | 70 (65 – 75) |
| Positive LR | 2.7 (2.0 – 3.7) | 2.2 (1.4 – 3.4) |
| Negative LR | 0.69 (0.61 – 0.78) | 0.86 (0.78 – 0.95) |
| Accuracy | 71 | 67 |
| Prevalence | 31 | 33 |
For sensitivity, specificity, PPV, NPV, accuracy and prevalence, the numbers in the table represent percentages. The numbers in parenthesis represent the 95% CI.
Defined as a modified Rankin Scale of ≥4 at 3-month follow-up among the 393 survivors.
ICH: intracerebral hemorrhage; CI: confidence interval; PPV: positive predictive value; NPV: negative predictive value; LR: likelihood ratio.
Overall, there was no significant difference in length of hospital stay for patients with spot signs (median 6 days, mean 9.4 days, range 1–51 days) compared to those without spot signs (median 6 days, mean 9.6 days, range 1–88 days, p 0.3, Mann-Whitney test). However, among the 393 survivors, patients with spot signs had a significantly longer hospital stay (median 11 days, mean 15.2 days, range 2–51 days) compared to those without spot signs (median 6 days, mean 10.1 days, range 1–70 days, p<0.0001, Mann-Whitney test). Likewise, among the 180 patients who expired during the hospitalization, patients with spot signs had a significantly shorter hospital stay (median 3 days, mean 4.9 days, range 1–24 days) compared to those without spot signs (median 4 days, mean 8 days, range 1–88 days, p 0.033, Mann-Whitney test).
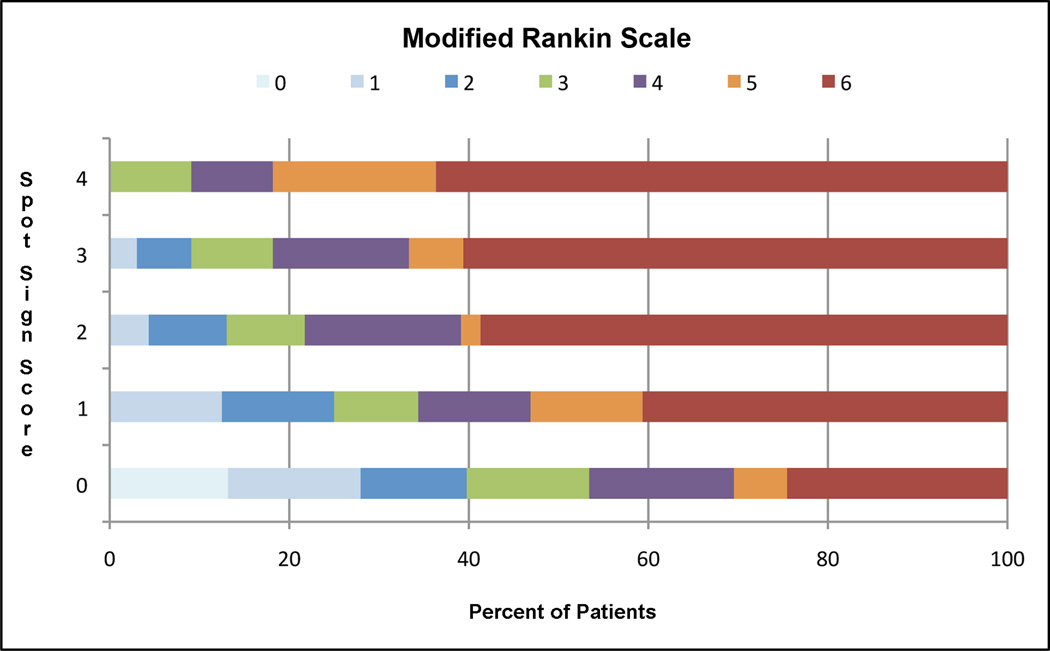
The spot sign score successfully predicted longer hospital stays among survivors and overall increasing 3-month mRS (Figure 1), as well as an increasing risk in-hospital mortality and poor outcome among survivors at 3-month follow-up (Table 5).
FIGURE 1.
Modified Rankin Scale at 3-Month Follow-up by Spot Sign Score
Table 5.
Predictive Value of the Spot Sign Score for Length of Hospital Stay, 3-Month Modified Rankin Scale, In-Hospital Mortality and Poor Outcome among Survivors in Primary ICH
| Spot Sign Score* |
Mean/Median LOS, days† |
Mean mRS in All Patients‡ |
Mean mRS in Survivors‡ |
In-Hospital Mortality, % |
Poor Outcome,§ % |
|---|---|---|---|---|---|
| 0 | |||||
| n = 440 | 10.1 / 6 | 3.2 | 2.3 | 24 | 29 |
| ns = 334 | |||||
| 1 | |||||
| n = 32 | 10.4 / 8 | 4.2 | 3.0 | 41 | 42 |
| ns = 19 | |||||
| 2 | |||||
| n = 46 | 15.3 / 13 | 4.8 | 3.1 | 59 | 47 |
| ns = 19 | |||||
| 3 | |||||
| n = 33 | 19.1 / 19 | 5.0 | 3.4 | 61 | 54 |
| ns = 13 | |||||
| 4 | |||||
| n = 22 | 19.8 / 17 | 5.4 | 4.3 | 64 | 75 |
| ns = 8 | |||||
| AUC | n/a | n/a | n/a | 0.64 | 0.56 |
| (95% CI) | (0.60–0.68) | (0.51–0.61) | |||
| p-value | n/a | n/a | n/a | <0.0001 | 0.04 |
A score of zero indicates that no spot sign is identified in the CT angiogram.
Length of hospital stay among the 393 survivors.
Modified Rankin Scale at 3-month follow-up.
Defined as a modified Rankin Scale of ≥4 at 3-month follow-up among the 393 survivors.
ICH: intracerebral hemorrhage; LOS: length of hospital stay; mRS: modified Rankin Scale; n: number of patients within spot sign score group in the entire cohort; ns: number of patients within spot sign score group among survivors; AUC: area under the curve after receiver operating characteristic analysis; CI: confidence interval; n/a: not applicable.
Effect of the Spot Sign and Spot Sign Score on the Statistical Model for the Prediction of Clinical Outcome in Primary ICH
When a patient’s spot sign status was entered into the multivariate logistic regression model either as a binary variable or as the spot sign score (in addition to the variables provided in Table 2), both the presence of any spot sign (OR 2.5, 95% CI 1.3–4.7, p 0.0052) and the spot sign score (OR 1.5, 95% CI 1.2–1.9, p 0.0002) were independent predictors of in-hospital mortality (in addition to patient age, admission GCS, and initial ICH and IVH volumes). Similarly, both the presence of any spot sign (OR 2.4, 95% CI 1.1–4.9, p 0.02) and the spot sign score (OR 1.6, 95% CI 1.1–2.1, p 0.0065) were independent predictors of poor outcome among survivors at 3-month follow-up (in addition to patient age, admission GCS and MABP, as well as initial ICH and IVH volumes).
DISCUSSION
In this study, we demonstrated that (1) strict radiological criteria for the identification and scoring of the MDCTA spot sign can be applied reliably in patients with primary ICH, and (2) the spot sign has good accuracy for the prediction of in-hospital mortality (71%) and poor outcome among survivors at 3-month follow-up (67%) in this patient population. For the prediction of in-hospital mortality, our accuracy results are similar to those reported by Becker et al (74%)3 and Kim et al (73%),5 and higher than reported by Wada et al (64%)4 as well as the previously reported subset of our patient population that used a less strict spot sign definition (56%).6
Spot sign criteria similar to ours have been proposed by Thompson et al,10 with the salient differences being that (1) we use an absolute attenuation cut-off for the definition of a spot sign (≥120 HU), compared to a relative attenuation cut-off of at least twice the mean background hematoma attenuation; and (2) we do not impose a minimum size cut-off for a spot sign, compared to a minimum axial size of >1.5mm. We find that determining absolute spot sign attenuation is more practical and less prone to operator error than determining relative attenuation as it relies on a single attenuation measurement rather than two. Furthermore, in our patient cohort, we identified 38 spot signs in the 120–150 HU attenuation range (28.6%) and 7 spot signs measuring 1mm in maximum axial dimension (5.3%), which may have been excluded using Thompson et al’s criteria. Adhering to a uniform set of strict radiological criteria while evaluating CTAs for the presence and scoring of spot signs is important, as this determination will be the cornerstone of future clinical trials that will rely on the MDCTA “spot sign status” for the selection of patients to receive early hemostatic therapy or surgery.
Although delayed images were not routinely acquired at our institution at the time of this study, we found that the frequency of spot signs in the delayed CTA acquisitions was significantly higher than in the first-pass CTAs, which is similar to the results reported by Ederies et al.8 This is a rather important finding because acquiring delayed images through the ICH approximately 2 to 3 minutes after contrast injection, either routinely or if a spot sign is not identified in the first-pass CTA, is likely to identify additional delayed spot signs that would increase this finding’s sensitivity for the prediction of hematoma expansion, in-hospital mortality and poor outcome among survivors.
Importantly, patient populations with the highest mean spot sign scores - those with medium initial ICH volumes, moderate depression of consciousness at admission, high admission MABP, deep gray matter ICH and short time interval from ictus to MDCTA evaluation, - may benefit the most from early hemostatic therapies such as recombinant activated factor VII or intensive blood pressure reduction. Future studies are needed to examine these treatment possibilities and determine which populations would derive the most benefit.
This study’s limitations are its retrospective design, as well as the lack of delayed CTA acquisitions and 3-month follow-up in all patients. The lack of delayed CTA acquisitions in all patients has likely led to an underestimation of (1) the frequency of spot signs in our study group, as well as (2) the spot sign’s sensitivity for the prediction in-hospital mortality and poor outcome among survivors at 3-month follow-up. The lack of 3-month follow-up in all patients may have led to an overestimation of the spot sign’s predictive value for poor outcome among survivors, as some patients may have regained some neurological function during rehabilitation following hospital discharge.
CONCLUSION
The spot sign score is a reliable independent predictor of mortality and poor outcome among survivors in primary ICH.
Supplementary Material
A: 67 year-old female presents with a right thalamic ICH with intraventricular extension. Axial CTA source image in “spot windows” demonstrates a focus of contrast pooling within the ICH with a maximum axial dimension of 2 mm and a maximum attenuation of 157 HU (spot sign score 1). The patient was discharged 9 days after admission but had an mRS at 3-month follow-up of 5. B: 77 year-old female presents with a left fronto-temporo-parietal ICH with intraventricular extension. Axial CTA source image in “spot windows” demonstrates 2 foci of contrast pooling within the ICH, the largest of which has a maximum axial dimension of 5 mm and a maximum attenuation of 176 HU (spot sign score 2). The patient expired 3 days after admission. C: 61 year-old male presents with a right temporal ICH. Axial CTA source image in “spot windows” demonstrates a focus of contrast pooling within the ICH with a maximum axial dimension of 14 mm and a maximum attenuation of 302 HU (spot sign score 3). The patient was discharged 6 days after admission but had an mRS at 3-month follow-up of 4. D: 90 year-old female presents with a left parietal ICH. Axial CTA source image in “spot windows” demonstrates 3 foci of contrast pooling within the ICH, the largest of which has a maximum axial dimension of 12 mm and a maximum attenuation of 331 HU (spot sign score 4). The patient expired 6 days after admission.
ACKNOWLEDGEMENTS
FUNDING
The authors would like to thank Elkan Halpern, Ph.D., for his contribution in the statistical analysis, and Eleni K. Balasalle, B.A., for her contribution in the artwork for this manuscript.
Dr. Jonathan Rosand is the recipient of the American Heart Association Grant-in-Aid #0755984T. Dr. Joshua N. Goldstein is the recipient of the National Institute of Neurological Disorders and Stroke grant #K23NS059774. Dr. Michael H. Lev is part of the medical advisory board and has received lecture honoraria from General Electric Healthcare.
Footnotes
CONFLICTS OF INTEREST
All other authors do not know of any potential conflicts of interests pertaining to this research project with regards to relationships with pharmaceutical companies, device manufacturers or other corporations whose services or products are directly related to the subject matter of this article.
REFERENCES
- 1.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 2.Broderick JP, Brott T, Tomsick T, Huster G, Miller R. The risk of subarachnoid and intracerebral hemorrhages in blacks as compared with whites. N Engl J Med. 1992;326:733–736. doi: 10.1056/NEJM199203123261103. [DOI] [PubMed] [Google Scholar]
- 3.Becker KJ, Baxter AB, Bybee HM, Tirschwell DL, Abouelsaad T, Cohen WA. Extravasation of radiographic contrast is an independent predictor of death in primary intracerebral hemorrhage. Stroke. 1999;30:2025–2032. doi: 10.1161/01.str.30.10.2025. [DOI] [PubMed] [Google Scholar]
- 4.Wada R, Aviv RI, Fox AJ, Sahlas DJ, Gladstone DJ, Tomlinson G, Symons SP. CT angiographyti “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38:1257–1262. doi: 10.1161/01.STR.0000259633.59404.f3. [DOI] [PubMed] [Google Scholar]
- 5.Kim J, Smith A, Hemphill JC, III, Smith WS, Lu Y, Dillon WP, Wintermark M. Contrast extravasation on CT predicts mortality in primary intracerebral hemorrhage. Am J Neuroradiol. 2008;29:520–525. doi: 10.3174/ajnr.A0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein JN, Fazen LE, Snider R, Schwab K, Greenberg SM, Smith EE, Lev MH, Rosand J. Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology. 2007;68:889–894. doi: 10.1212/01.wnl.0000257087.22852.21. [DOI] [PubMed] [Google Scholar]
- 7.Gazzola S, Aviv RI, Gladstone DJ, Mallia G, Li V, Fox AJ, Symons SP. Vascular and nonvascular mimics of the CT angiography “spot sign” in patients with secondary intracerebral hemorrhage. Stroke. 2008;39:1177–1183. doi: 10.1161/STROKEAHA.107.499442. [DOI] [PubMed] [Google Scholar]
- 8.Ederies A, Demchuk A, Chia T, Gladstone DJ, Dowlatshahi D, Bendavit G, Wong K, Symons SP, Aviv RI. Postcontrast CT extravasation is associated with hematoma expansion in CTA spot negative patients. Stroke. 2009;40:1672–1676. doi: 10.1161/STROKEAHA.108.541201. [DOI] [PubMed] [Google Scholar]
- 9.Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Goldstein JN, Rosand J, Oleinik A, Lev MH, Gonzalez RG, Romero JM. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion. The spot sign score. Stroke. 2009 Jul 2; doi: 10.1161/STROKEAHA.109.554667. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson AL, Kosior JC, Gladstone DJ, Hopyan JJ, Symons SP, Romero F, Dzialowski I, Roy J, Demchuk AM, Aviv RI. PREDICTS/Sunnybrook ICH CTA Study Group. Defining the CT angiography 'spot sign' in primary intracerebral hemorrhage. Can J Neurol Sci. 2009;36:456–461. doi: 10.1017/s0317167100007782. [DOI] [PubMed] [Google Scholar]
- 11.Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, Begtrup K, Steiner T Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 12.Becker KJ, Tirschwell DL. Intraparenchymal hemorrhage, bleeding, hemostasis, and the utility of CT angiography. Int J Stroke. 2008;3:11–13. doi: 10.1111/j.1747-4949.2008.00179.x. [DOI] [PubMed] [Google Scholar]
- 13.Aviv RI, Gladstone D, Goldstein J, Flaherty M, Broderick J, Demchuk A Spot Sign for Predicting and Treating ICH Growth and Spot Sign Selection of ICH to Guide Hemostatic Therapy Investigators. Contrast extravasation predicts hematoma growth: where to now? Am J Neuroradiol. 2008;29:520–525. doi: 10.3174/ajnr.A1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saposnik G. Looking for the 'spot sign': enlightening the management of intracranial hemorrhage. Can J Neurol Sci. 2009;36:407–408. doi: 10.1017/s0317167100007721. [DOI] [PubMed] [Google Scholar]
- 15.Mayer SA, Brun NC, Broderick J, Davis S, Diringer MN, Skilnock BE, Steiner T Europe/AustralAsia NovoSeven ICH Trial Investigators. Safety and feasibility of recombinant factor VIIa for acute intracerebral hemorrhage. Stroke. 2005;36:74–79. doi: 10.1161/01.STR.0000149628.80251.b8. [DOI] [PubMed] [Google Scholar]
- 16.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, Skolnick Be, Steiner T Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–785. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- 17.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, Skolnick BE, Steiner T Factor Seven for Acute Hemorrhagic Stroke (FAST) Trial Investigators. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–2137. [Google Scholar]
- 18.Anderson CS, Huang Y, Wang JG, Arima H, Neal B, Peng B, Heeley E, Skulina C, Parsons MW, Kim JS, Tao QL, Li YC, Jiang JD, Tai LW, Zhang JL, Xu E, Cheng Y, Heritier S, Morgenstern LB, Chalmers J INTERACT Investigators. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7:391–399. doi: 10.1016/S1474-4422(08)70069-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: 67 year-old female presents with a right thalamic ICH with intraventricular extension. Axial CTA source image in “spot windows” demonstrates a focus of contrast pooling within the ICH with a maximum axial dimension of 2 mm and a maximum attenuation of 157 HU (spot sign score 1). The patient was discharged 9 days after admission but had an mRS at 3-month follow-up of 5. B: 77 year-old female presents with a left fronto-temporo-parietal ICH with intraventricular extension. Axial CTA source image in “spot windows” demonstrates 2 foci of contrast pooling within the ICH, the largest of which has a maximum axial dimension of 5 mm and a maximum attenuation of 176 HU (spot sign score 2). The patient expired 3 days after admission. C: 61 year-old male presents with a right temporal ICH. Axial CTA source image in “spot windows” demonstrates a focus of contrast pooling within the ICH with a maximum axial dimension of 14 mm and a maximum attenuation of 302 HU (spot sign score 3). The patient was discharged 6 days after admission but had an mRS at 3-month follow-up of 4. D: 90 year-old female presents with a left parietal ICH. Axial CTA source image in “spot windows” demonstrates 3 foci of contrast pooling within the ICH, the largest of which has a maximum axial dimension of 12 mm and a maximum attenuation of 331 HU (spot sign score 4). The patient expired 6 days after admission.