Abstract
Background
Pruritus has been anecdotally described in association with targeted cancer therapies. The risk of pruritus has not been systematically ascertained.
Objective
A systematic review and meta-analysis of the literature was conducted for axitinib, cetuximab, dasatinib, erlotinib, everolimus, gefitinib, imatinib, ipilimumab, lapatinib, nilotinib, panitumumab, pazopanib, rituximab, sorafenib, temsirolimus, tositumomab, vandetanib, and vemurafenib.
Methods
Databases from PubMed, Web of Science (01/1998–07/2012), and American Society of Clinical Oncology abstracts (2004–2012) were searched. Incidence and risk (RR) of pruritus were calculated using random or fixed effects model.
Results
The incidences of all-grade and high-grade pruritus were 17.4% (95% confidence interval (CI): 16.0%−19.0%) and 1.4% (95% CI: 1.2%−1.6%), respectively. There was an increased risk of all-grade pruritus (RR=2.90 (95% CI: 1.76–4.77, p<0.001)); and variation among different drugs (P<0.001).
Limitations
The reporting of pruritus may vary, resulting from concomitant medications, comorbidities, and underlying malignancies. We found a higher incidence of pruritus in patients with solid tumors, concordant with those targeted therapies with the highest pruritus incidences.
Conclusion
There is a significant risk of developing pruritus in patients receiving targeted therapies. In order to prevent suboptimal dosing and decreased quality of life, patients should be counseled and treated against this untoward symptom.
Keywords: Cancer, Pruritus, Itch, Inhibitor, Bcr-Abl, CD20, EGFR, VEGFR, mTOR, Raf
Introduction
Novel agents targeting specific cancer pathways or proteins have been shown to significantly increase the survival of patients with various malignancies1. The increased lifespan alongside the expanded use has led to a variety of therapy-associated adverse events (AE). These novel agents are associated with lower systemic toxicity than conventional chemotherapy, yet dermatologic events may affect the majority of treated patients2.
Dermatologic toxicities to targeted therapies manifest in cosmetically sensitive areas, are associated with symptoms, and can interfere with activities of daily living3. This results in a negative impact on quality of life, which may lead the physician to lower the dose4. Pruritus is a common but infrequently discussed AE; a survey of 379 cancer survivors reported 36% experienced pruritus during treatment, with 44% indicating a negative impact on quality of life (QoL)5. An analysis of anticancer therapies reported rash and pruritus to have the greatest negative impact on QoL among dermatologic AE including alopecia, nail changes, hand-foot syndrome, mucosal changes, and fissures6. Knowledge of these effects and which agents have a higher incidence of pruritus is important for patient counseling and directing supportive care efforts.
Whereas the acneiform (papulopustular) rash to EGFR inhibitors (EGFRIs) and hand-foot syndrome provoked by multikinase inhibitors have been extensively described, the overall risk of developing pruritus for patients receiving targeted therapies has not been systematically ascertained. We conducted a systematic review and meta-analysis of the literature to identify published clinical trials of targeted therapies to determine the incidence and risk of pruritus.
Methods
Data Source
The PubMed database was searched from January 1998 to July 2012 using the keywords of the name of the targeted agent (e.g. ‘axitinib’) and ‘clinical trials,’ and was limited to the English language and human studies. In addition, we reviewed abstracts and virtual meeting presentations that contained ‘axitinib’ presented at the American Society of Clinical Oncology (ASCO) annual meetings from 2004 through 2012. An independent search using the Web of Science database (a product developed by the Institute for Scientific Information) was also conducted to ensure that there were no additional studies. Only full publications from the Web of Science were added to the study selection. We reviewed each publication and used only complete or the most recent data reports when duplicate publications of the trial were identified. Information regarding patient characteristics, study design, treatment regimen, study results, and safety and tolerability were extracted from the publications. This systematic search was performed for axitinib, cetuximab, dasatinib, erlotinib, everolimus, gefitinib, imatinib, ipilimumab, lapatinib, nilotinib, panitumumab, pazopanib, rituximab, sorafenib, temsirolimus, tositumomab, vandetanib, and vemurafenib.
Study Selection
Each targeted therapy has been approved for treatment of malignancies in patients at a specific dose. It is therefore clinically significant to determine the incidence of pruritus at this dosing level. We excluded trials that treated at unapproved doses, including phase I studies. Since chemotherapy and radiation may cause pruritus, we excluded trials that combined targeted agents with chemotherapeutic agents and/or radiotherapy. Trials that met the following criteria were included for further analysis: (1) prospective phase II and phase III clinical trials in cancer patients; (2) assignment of participants to the treatment with and (3) clear data available for the incidence of pruritus.
Clinical End Points
The clinical end point of pruritus was extracted from the safety profile in each trial. Pruritus was recorded according to the National Cancer Institute Common Toxicity Criteria version 2 or Common Terminology Criteria for Adverse Events (CTCAE) version 3. We included the incidence of all patients with pruritus grade 1 and above. The grading of pruritus in version 2.0 is described with three grades, as follows: grade 1, mild or localized, relieved spontaneously or by local measures; grade 2, intense or widespread, relieved spontaneously or by systemic measures; grade 3, intense or widespread and poorly controlled despite treatment. In version 3.0, the descriptions of these three grades are updated to: grade 1, mild or localized; grade 2, intense or widespread; grade 3, intense or widespread and interfering with activities of daily living (ADL). Version 4.0 expands further upon the descriptions in version 3.0; however, none of the studies reviewed used version 4.0.
Statistical Analysis
All statistical analysis was performed using version 2 of the Comprehensive MetaAnalysis program (Biostat, Englewood, New Jersey, USA). The number of patients with all-grade and high-grade pruritus were extracted from the clinical trial data. For each study, the proportion of patients with pruritus was calculated and the 95% exact confidence interval (CI) was derived. For studies with a placebo-only control arm, the relative risk of rash among patients was also calculated.
For meta-analysis, both the fixed-effects model (weighted with inverse variance) and the random-effects model were considered 7. For each meta-analysis, the Cochran’s Q statistic was first calculated to assess the heterogeneity of the included trials. For p-value of Cochran’s Q statistic less than 0.1, the assumption of homogeneity was deemed invalid 8, and random-effects model was reported after exploring the causes of heterogeneity. Otherwise, both the fixed-effects model and the random-effects model results were reported. A two-tailed p-value of less than 0.05 was judged as statistically significant.
Results
Search Results
Our search yielded a total of 5065 potential articles on targeted therapies in the literature (see Figure 1 for the overall selection process). A total of 144 clinical trials were included for this analysis, including 116 phase II and 28 phase III trials9–148.
Figure 1.
Selection process for studies included in the meta-analysis.
Patients
A total of 20,532 (treated: 17,375; controls: 3157) patients from 144 clinical trials were included for analysis (see Table I for number of patients receiving each targeted therapy). Of these studies, 114 were solid organ malignancies and 30 were hematologic.
Table I.
Incidences of all-grade and high-grade pruritus associated with targeted therapies
| Targeted therapy | Number of Studies* |
Number of Patients |
All-grade (95% CI) |
High-grade (95% CI) |
|---|---|---|---|---|
| mTOR inhibitors | 9 | 672 | 23.8% (15.0–35.7) | 1.2% (0.5–2.9) |
| Everolimus | 5 | 486 | 14.3% (11.5–17.7) | 1.3% (0.5–3.7) |
| Temsirolimus | 4 | 186 | 37.7% (20.9–58.0) | 1.0% (0.2–4.8) |
| Bcr-Abl inhibitors | 26 | 5036 | 12.8% (10.4–15.7) | 0.9% (0.6–1.3) |
| Dasatinib | 2 | 196 | 9.7% (6.3–14.7) | 0.8% (0.2–4.0) |
| Imatinib | 14 | 2351 | 10.2% (7.4–13.9) | 0.8% (0.5–1.3) |
| Nilotinib | 10 | 2489 | 17.1% (13.2–21.8) | 1.0% (0.7–1.6) |
| Raf kinase inhibitors | 19 | 1944 | 18.3% (12.9–25.2) | 1.3% (0.8–2.1) |
| Sorafenib | 16 | 1448 | 18.2% (12.3–26.1) | 1.0% (0.5–1.9) |
| Vemurafenib | 3 | 496 | 18.5% (6.3–43.4) | 1.7% (0.9–3.5) |
| VEGFR inhibitors | 4 | 158 | 3.0% (1.1–7.8) | 1.5% (0.4–5.7) |
| Axitinib Pazopanib | 1 | 12 | 8.3% (1.2–41.3) | 3.9% (0.2–40.3) |
| Pazopanib | 3 | 146 | 2.2% (0.7–6.6) | 1.1% (0.2–5.1) |
| EGFR inhibitors | 57 | 6809 | 22.7% (17.8–28.6) | 1.8% (1.5–2.3) |
| Cetuximab | 6 | 217 | 18.2% (10.8–28.8) | 2.1% (0.8–5.3) |
| Erlotinib | 24 | 2742 | 20.8% (14.3–29.3) | 2.3% (1.5–3.4) |
| Gefitinib | 22 | 3002 | 21.0% (15.3–28.3) | 1.0% (0.6–1.5) |
| Panitumumab | 5 | 848 | 54.9% (46.9–62.7) | 2.6% (1.7–4.0) |
| EGFR-HER2 inhibitors | 9 | 501 | 14.6% (9.9–21.0) | 1.0% (0.4–2.6) |
| Lapatinib | ||||
| EGFR-VEGFR inhibitor | 3 | 1261 | 9.1% (5.0–16.2) | 0.5% (0.2–1.5) |
| Vandetanib | ||||
| Monoclonal antibodies to CD20 | 13 | 658 | 11.3% (8.8–14.3) | 1.2% (0.5–2.7) |
| Rituximab | 10 | 519 | 10.2% (7.3–14.1) | 1.2% (0.5–2.9) |
| Tositumomab | 3 | 139 | 13.7% (8.9–20.5) | 0.8% (0.05–11.8) |
| Monoclonal antibody to CTLA4 | 4 | 336 | 30.7% (15.9–51.0) | 1.0% (0.3–3.9) |
| Ipilimumab | ||||
| Overall | 144 | 17,375 | 17.4% (16.0–19.0) | 1.4% (1.2–1.6) |
References: everolimus9–13, temsirolimus14–17, dasatinib46,47, imantinib55,58–70, nilotinib48–57, sorafenib18–33, vemurafenib37–39, axitinib93, pazopanib34–36, cetuximab40–45, erlotinib72,111–133, gefitinib71–92, panitumumab134–138, lapatinib36,139–146, vandetanib115,147,148, rituximab94–103, tositumomab108–110, ipilimumab104–107
Incidence of all-grade pruritus
Data for all-grade pruritus was available for analysis from a total of 17,368 patients treated with targeted therapies as a single agent from 141 clinical trials. Among these studies, the incidence of all-grade pruritus ranged between 3.0% (95% CI: 1.1%−7.8%) and 30.7% (95% CI: 15.9%−51.0%), with the lowest incidence in patients treated with VEGFR inhibitors (axitinib and pazopanib) and the highest in patients treated with CTLA4 inhibitor ipilimumab. The incidence of pruritus was determined to be 19.2% (95% CI: 16.2%−22.6%) in solid organ malignancies and 13.0% (95% CI: 10.7%−15.7%) in hematologic malignancies (p=0.003). Meta-analysis (heterogeneity test: Q=45.308, I2=80.136, P<0.001) revealed that the overall summary incidence of all-grade pruritus was 17.4% (95% CI: 16.0%−19.0%), according to a random-effects model (Table I).
Incidence of high-grade pruritus
High-grade (grade 3) pruritus is considered severe and can lead to dose reduction or treatment interruption. Data for high-grade pruritus was available for analysis from a total of 15,927 patients treated with targeted therapies as a single agent from 132 clinical trials. Among these studies, the incidence of high-grade pruritus ranged between 0.5% (95% CI: 0.2%−1.5%) and 1.8% (95% CI: 1.5%−2.3%), with the lowest incidence in patients treated with EGFR-VEGFR inhibitor, vandetanib, and the highest in patients treated with EGFRIs (gefitinib, cetuximab, panitumumab, and erlotinib). The overall incidence of high-grade pruritus in patients treated with CTLA4 inhibitor, ipilimumab, was 1.0% (95% CI: 0.3%−3.9%). The overall incidence of high-grade pruritus for all patients was 1.4% (95% CI: 1.2%−1.6%) (Table I).
Incidence of pruritus in patients with different EGFRIs
We investigated whether the specific EGFRI used as therapy has an impact on the incidence of pruritus. The incidences of all-grade pruritus were determined among cetuximab (n=217), erlotinib (n=2717), gefitinib (n=3002), and panitumumab (n=848), and ranged from 18.2% (95% CI: 10.8%−28.8%) to 54.9% (95% CI: 46.9%−62.7%), with the lowest incidence in cetuximab and the highest in panitumumab. The overall incidence of high-grade pruritus was determined among cetuximab (n=217), erlotinib (n=2263), gefitinib (n=3002), and panitumumab (n=842). There was a significant variation among these EGFRIs (P<0.001). The incidences of high-grade pruritus ranged from 1.0% (95% CI: 0.6%−1.5%) and 2.6% (95%CI: 1.7%−4.0%), with the lowest incidence in patients treated with gefitinib and the highest in patients treated with panitumumab (Table I).
Relative risk (RR) of developing pruritus
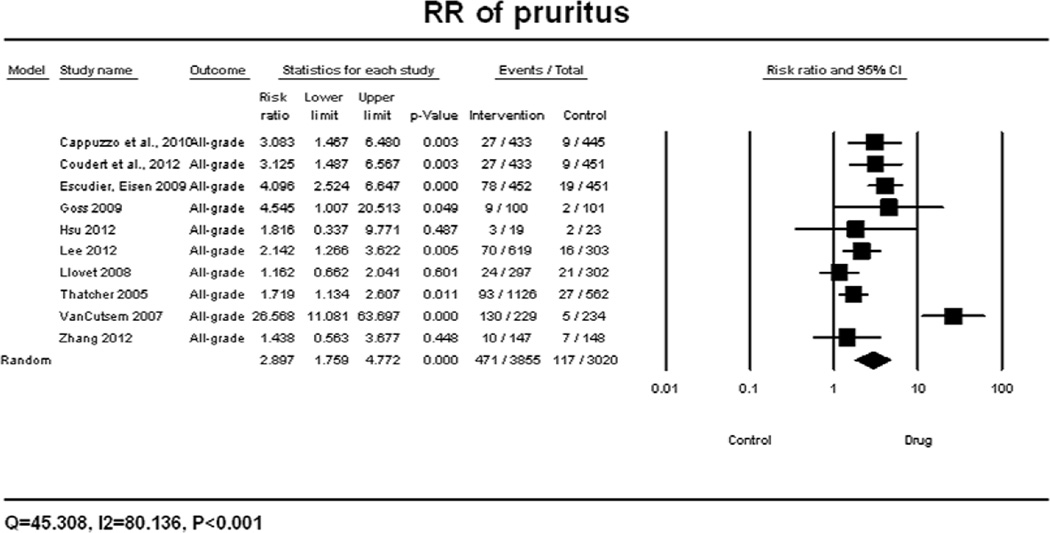
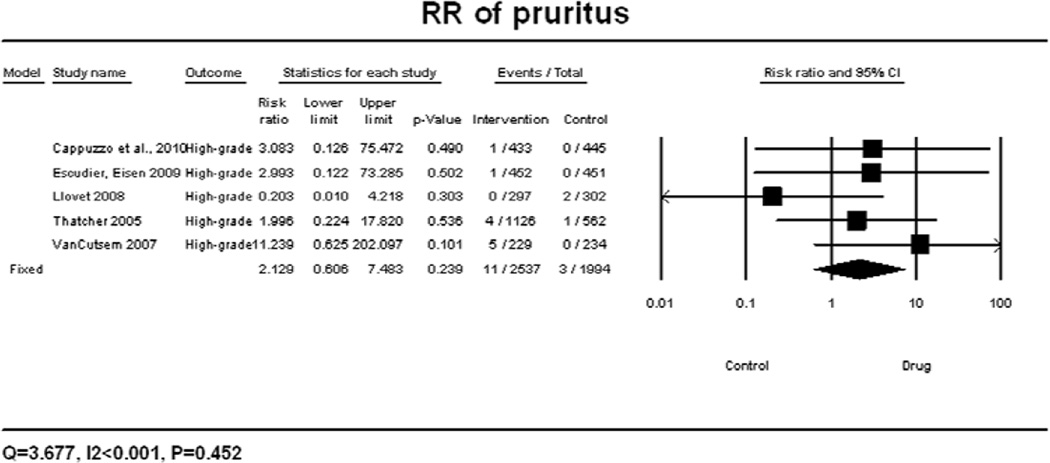
A meta-analysis of RR for all-grade pruritus associated with targeted agents versus controls was performed on eleven randomized control trials, in which the incidence of pruritus was reported for 2261 patients receiving best supportive care (BSC) alone. According to the random-effects model, the overall RR for all-grade pruritus was calculated to be 2.90 (95% CI: 1.76–4.77, p<0.001) (Figure 2A). There was significant variation among different classes of targeted therapies (P<0.001) and different EGFRIs (P<0.001). The RR for all-grade pruritus associated with specific EGFRIs was found to be 1.77 (95% CI: 1.23–2.56, p<0.001) for gefitinib and 26.57 (95% CI: 11.08–63.70, p<0.001) for panitumumab. The summary RR for high-grade pruritus associated with targeted agents versus controls was performed and found to be 2.13 (95% CI: 0.61–7.48, p=0.452), according to the fixed-effects model (Figure 2B).
Figure 2.
Relative risk of all-grade (A) and high-grade (B) pruritus associated with targeted therapies versus controls. RR was calculated using a random-effects model. The relative risk for each study is displayed numerically on the left and graphically on the right. Under study name, the first author’s name and publication year were used to represent each trial. The size of the squares is directly proportional to the amount of information available. For individual trials: filled-in square, incidence; lines, 95% confidence interval; diamond plot, overall results of the included trials.
Discussion
Our study has demonstrated that patients treated with targeted therapies have a significantly increased risk of developing pruritus. The overall incidence of all-grade pruritus is 17.4% (95% CI: 16.0%−19.0%) with a RR of 2.90 (95% CI: 1.76–4.77, p<0.001). Therefore, it is important for physicians and patients to recognize the risk in order to monitor and treat the toxicity adequately.
The pathophysiology of pruritus remains unclear. Our meta-analysis determined the incidence of all-grade pruritus from EGFRIs to be 22.7% (95% CI: 17.8%−28.6%). These targeted agents inhibit the EGFR of basal keratinocytes, perturbing normal epidermal physiology149, 163. During the first month of treatment with EGFRIs—cetuximab, erlotinib, or panitumumab—xerosis appears in 20% to 50% of patients150–153. Amongst individual EGFRIs and individual targeted agents included in this study, the highest overall incidence of pruritus of 54.9% (95% CI: 46.9%−62.7%) was seen with panitumumab, when compared to patients treated with cetuximab, erlotinib, or gefitinib (incidences were 18.2% (95% CI: 10.8%−28.8%), 20.8% (95% CI: 14.3%−29.3%), and 21.0% (95% CI: 15.3%−28.3%), respectively). These summary incidences are lower than in panitumumab, but are higher than the incidences in patients treated with dual inhibitors, such as 14.6% (95% CI: 9.9%−21.0%) in EGFR-HER2 inhibitor lapatinib and 9.1% (95% CI: 5.0%−16.2%) in EGFR-VEGFR inhibitor vandetanib.
With ipilimumab, pruritus appears to be a direct result of CTLA4 inhibition and subsequent enhanced immune system activation154. The incidence of all-grade pruritus in patients treated with ipilimumab was 30.7% (95% CI: 25.9%−51.0%). The skin is an immunologic organ, and dermatologic disorders may be caused by either exacerbation or reduction of cutaneous immune activity155. Ipilimumab abrogates CTLA4-induced inhibition of T cells, and results in increased activated T-cell function and thus enhances the immune response106. Cutaneous immune-related adverse events such as pruritus may be directly caused by this increased activation of the immune system. The incidence of pruritus with other monoclonal antibodies included in this study, rituximab and tositumomab, was found to be much lower than with ipilimumab (11.3%), likely due to their targeting of CD20 bearing cells.
Of patients treated with VEGFR inhibitors, axitinib and pazopanib had the lowest incidence of all-grade pruritus (3.0%), when compared to sorafenib. The incidences of pruritus among mTOR inhibitors (everolimus and temsirolimus), inhibitors of Bcr-Abl (dasatinib, imatinib, and nilotinib), and inhibitors of Raf (sorafenib and vemurafenib) were 23.8%, 12.8% and 18.3%, respectively.
Possible pathogenesis of pruritus may involve unmyelinated C fibers and neurotransmitters or receptor activation, such as serotonin, neurokinin 1 receptor, opioid receptors, and gamma-aminobutyric acid156, 157. In some cases, pruritus may be indirectly caused by targeted therapies. Indeed, xerosis is cited as the most frequent cause of pruritus in oncology, and pruritus also accompanies papulopustular rash156. Papulopustular (acneiform) rash is a common skin toxicity in patients treated with targeted therapies, and is the most common dermatologic AE that occurs in patients treated with EGFRIs156, 158. Recent research has proposed that patients with EGFRI-induced rash and pruritus may be associated with an increased number of dermal mast cells surrounding adnexal structures. A continued increase in mediators released from these cells may activate sensory nerves, ultimately resulting in itch, both of which have been associated with the acneiform rash in 62% of cases159, 160. Classically, mast cell mediators such as histamine are associated with non-allergic urticaria161.
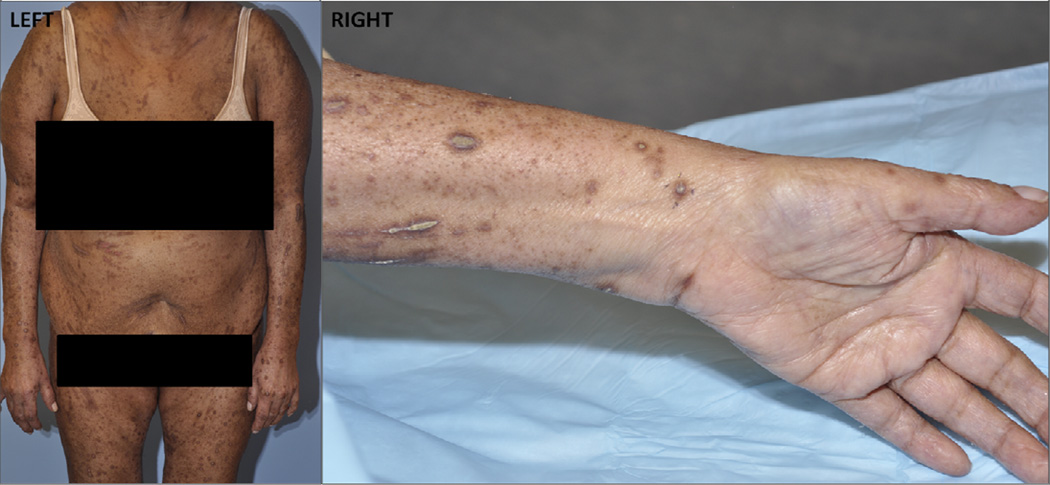
Currently, management options for pruritus in cancer patients require a tailored approach, which includes patient education, topical and systemic treatments. Patient education is key, as severe itching leads to scratching, causing secondary skin changes such as excoriations and infections (Fig. 3). Patients should be informed of how to break the “itch-scratch” cycle, for example by keeping fingernails short, wearing light clothing, using a humidifier, restricting bath and shower time and using lukewarm water, and avoiding cleansers with a high pH or containing alcohol162. Regular moisturizing and use of emollients are central to the management of pruritus, especially when associated with xerosis. Treatments for mild to moderate pruritus include topical corticosteroids, anesthetics (ie. lidocaine, prilocaine), capsaicin, salicylic acid, and menthol and for severe pruritus, oral agents such as antihistamines, anticonvulsants, antidepressants, mu antagonists, aprepitant, and phototherapy. These therapies have shown benefit in uncontrolled studies162, 163.
Figure 3.
Pruritus-induced excoriations associated with use of targeted therapies. Left panel: Excoriations induced by pruritus affecting anterior chest, abdomen, and upper legs; right panel: ventral arm and wrist, showing excoriations induced from pruritus.
Pruritus is common but often overlooked in cancer patients, as evidenced by it being reported in 2.8% of analyzed trials, likely due to seemingly more life-threatening side effects often taking precedence. Pruritus can impact on quality of life, often negatively affecting sleep, attention, and sexual function162. Preference-based quality of life measures have demonstrated that patients afflicted with pruritus would be willing to reduce their life expectancy by 13% to not have pruritus164. Although it was only reported to occur in 3 of the analyzed trials, patients may withdraw from treatment due to intolerable pruritus165–167.
There are several limitations to our meta-analysis. First, there is a large amount of heterogeneity amongst institutions in the assessment and reporting of pruritus. During our selection process, numerous studies either did not specify amongst dermatologic adverse events or would list pruritus in combination with rash and xerosis. Additionally, we came across a study that specified pruritus to be localized to the scalp of a patient with metastatic lymphoma18. Another study described that the only side effect seen in a patient treated with rituximab was “persistent itching”168. This wide variability and inconsistency in reporting pruritus may affect our ability to accurately assess the safety profiles of targeted agents, estimate the risk of pruritus development, and assess patient response to therapies. The development of an improved system for classification of dermatologic adverse events, with greater reflection upon their complexity and variability, may improve upon these areas. Another limitation of this study involves the possibility of sampling bias, as only 144 of 5065 studies initially found were included in our analysis, based on our selection criteria (see Study Selection in Methods for details). Due to the aforementioned reasons, it is possible that the clinical studies used in this meta-analysis over- or underestimated the incidence of pruritus associated with the use of targeted therapies. Furthermore, the results of our analysis may not apply to patients in the real-world setting, where patient and clinician AE reporting may differ from that in clinical trials.
Further studies are needed to investigate other factors that contribute to pruritus in this patient population. Concomitant medications and comorbid conditions, such as hepatic and renal impairment, and cancer itself, are common in these patients and can result in pruritus162. The type of cancer is a potential variable that may contribute to pruritus; hematologic malignancies per se, have been associated with paraneoplastic pruritus. However, our analysis found a higher incidence of pruritus in solid organ malignancies (19.2%) than hematologic tumors (13.0%) (p=0.003). This is consistent with the association of pruritus with drug rather than tumor type. Whereas the development of acneiform rash to EGFR inhibitors has been correlated with response, this observation has not been examined with pruritus, however the findings described herein warrant additional analyses to define whether pruritus correlates with clinical outcome. Additionally, analysis of other factors that could give clinical significance of pruritus such as allergic reactions, infections, and environmental factors (hot or cold weather, low humidity, bathing too frequently) should be considered.
Conclusion
Our results demonstrate that targeted cancer therapies are associated with a significant risk of developing pruritus. In order to prevent suboptimal dosing and reduction in patients’ quality of life, further research is needed to improve upon the current understanding of the pathogenesis of pruritus, risk factors, and management strategies. Prophylactic treatment, early detection and intervention, and close monitoring of this untoward event are critical to ensure patient adherence and maximize clinical benefit from optimal dosing.
Capsule.
Pruritus is frequent in patients receiving targeted anticancer therapies, but its incidence is unknown. Use of these novel agents may be hindered due to widespread dermatologic adverse events, such as pruritus.
This manuscript assesses the incidence and relative risk of developing pruritus among patients treated with targeted anticancer therapies.
Patients with pruritus should be counseled and symptomatically treated in order to prevent suboptimal dosing and significant reduction in quality of life.
Acknowledgments
Funding sources:
Memorial Sloan-Kettering; Dermatology Foundation Career and Development Award
Abbreviations and Acronyms List
- CI
confidence interval
- CD20
B-lymphocyte antigen CD20
- CTLA4
T-lymphocyte antigen 4
- CTCAE
Common Terminology Criteria for Adverse Events
- EGFR
epidermal growth factor receptor
- EGFRIs
epidermal growth factor receptor inhibitors
- HER2
human epidermal growth factor receptor 2
- Mtor
mammalian target of rapamycin
- RR
relative risk
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests:
Dr. Lacouture has a consultant role with AstraZeneca, Roche, Bayer, Exelixis, and Advancell. He is also receiving research funding from Berg, Roche. Dr. Wu has received honoraria from Onyx Pharmaceuticals, Pfizer, Jansen, and Novartis, and is a speaker for Onyx, Pfizer, Jansen, and Novartis.
References
- 1.Ricciardi S, Tomao S, de Marinis F. Toxicity of targeted therapy in non-small-cell lung cancer management. Clinical lung cancer. 2009;10:28–35. doi: 10.3816/CLC.2009.n.004. [DOI] [PubMed] [Google Scholar]
- 2.Balagula Y, Lacouture ME, Cotliar JA. Dermatologic toxicities of targeted anticancer therapies. The journal of supportive oncology. 2010;8:149–161. [PubMed] [Google Scholar]
- 3.Wagner LI, Berg SR, Gandhi M, Hlubocky FJ, Webster K, Aneja M, et al. The development of a Functional Assessment of Cancer Therapy (FACT) questionnaire to assess dermatologic symptoms associated with epidermal growth factor receptor inhibitors (FACT-EGFRI-18) Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2012 doi: 10.1007/s00520-012-1623-4. [DOI] [PubMed] [Google Scholar]
- 4.Boone SL, Rademaker A, Liu D, Pfeiffer C, Mauro DJ, Lacouture ME. Impact and management of skin toxicity associated with anti-epidermal growth factor receptor therapy: survey results. Oncology. 2007;72:152–159. doi: 10.1159/000112795. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi M, Oishi K, Zubal B, Lacouture ME. Unanticipated toxicities from anticancer therapies: survivors’ perspectives. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2010;18:1461–1468. doi: 10.1007/s00520-009-0769-1. [DOI] [PubMed] [Google Scholar]
- 6.Rosen ACE, Dusza S, Gordon J, West D, Lacouture ME. Impact of anticancer therapies on quality of life: is the skin the target? 2011 [Google Scholar]
- 7.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 8.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 9.Renner C, Zinzani PL, Gressin R, Klingbiel D, Dietrich PY, Hitz F, et al. A multicenter phase II trial (SAKK 36/06) of single-agent everolimus (RAD001) in patients with relapsed or refractory mantle cell lymphoma. Haematologica. 2012;97:1085–1091. doi: 10.3324/haematol.2011.053173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doi T, Muro K, Boku N, Yamada Y, Nishina T, Takiuchi H, et al. Multicenter phase II study of everolimus in patients with previously treated metastatic gastric cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1904–1910. doi: 10.1200/JCO.2009.26.2923. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma : final results and analysis of prognostic factors. Cancer. 2010;116:4256–4265. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 12.Yao JC, Lombard-Bohas C, Baudin E, Kvols LK, Rougier P, Ruszniewski P, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:69–76. doi: 10.1200/JCO.2009.24.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varadarajan PKA, Martin D, Gutkind JS, Gibson MK, Argiris A. Phase II trial of everolimus in patients with previously treated recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30 (suppl; abstr 5541) [Google Scholar]
- 14.Oza AM, Elit L, Tsao MS, Kamel-Reid S, Biagi J, Provencher DM, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3278–3285. doi: 10.1200/JCO.2010.34.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess G, Herbrecht R, Romaguera J, Verhoef G, Crump M, Gisselbrecht C, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 16.Duran I, Kortmansky J, Singh D, Hirte H, Kocha W, Goss G, et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. British journal of cancer. 2006;95:1148–1154. doi: 10.1038/sj.bjc.6603419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atkins MB, Hidalgo M, Stadler WM, Logan TF, Dutcher JP, Hudes GR, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 18.Guidetti A, Carlo-Stella C, Locatelli SL, Malorni W, Pierdominici M, Barbati C, et al. Phase II study of sorafenib in patients with relapsed or refractory lymphoma. British journal of haematology. 2012;158:108–119. doi: 10.1111/j.1365-2141.2012.09139.x. [DOI] [PubMed] [Google Scholar]
- 19.Grignani G, Palmerini E, Dileo P, Asaftei SD, D’Ambrosio L, Pignochino Y, et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:508–516. doi: 10.1093/annonc/mdr151. [DOI] [PubMed] [Google Scholar]
- 20.Naito S, Tsukamoto T, Murai M, Fukino K, Akaza H. Overall survival and good tolerability of long-term use of sorafenib after cytokine treatment: final results of a phase II trial of sorafenib in Japanese patients with metastatic renal cell carcinoma. BJU international. 2011;108:1813–1819. doi: 10.1111/j.1464-410X.2011.10281.x. [DOI] [PubMed] [Google Scholar]
- 21.Procopio G, Verzoni E, Bracarda S, Ricci S, Sacco C, Ridolfi L, et al. Sorafenib with interleukin-2 vs sorafenib alone in metastatic renal cell carcinoma: the ROSORC trial. British journal of cancer. 2011;104:1256–1261. doi: 10.1038/bjc.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam ET, Ringel MD, Kloos RT, Prior TW, Knopp MV, Liang J, et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:2323–2330. doi: 10.1200/JCO.2009.25.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Safarinejad MR. Safety and efficacy of sorafenib in patients with castrate resistant prostate cancer: a Phase II study. Urologic oncology. 2010;28:21–27. doi: 10.1016/j.urolonc.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Bianchi G, Loibl S, Zamagni C, Salvagni S, Raab G, Siena S, et al. Phase II multicenter, uncontrolled trial of sorafenib in patients with metastatic breast cancer. Anti-cancer drugs. 2009;20:616–624. [PubMed] [Google Scholar]
- 25.Blumenschein GR, Jr., Gatzemeier U, Fossella F, Stewart DJ, Cupit L, Cihon F, et al. Phase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:4274–4280. doi: 10.1200/JCO.2009.22.0541. [DOI] [PubMed] [Google Scholar]
- 26.Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:1280–1289. doi: 10.1200/JCO.2008.19.3342. [DOI] [PubMed] [Google Scholar]
- 27.Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, et al. Phase II trial of sorafenib in metastatic thyroid cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:1675–1684. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chi KN, Ellard SL, Hotte SJ, Czaykowski P, Moore M, Ruether JD, et al. A phase II study of sorafenib in patients with chemo-naive castration-resistant prostate cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2008;19:746–751. doi: 10.1093/annonc/mdm554. [DOI] [PubMed] [Google Scholar]
- 29.Dahut WL, Scripture C, Posadas E, Jain L, Gulley JL, Arlen PM, et al. A phase II clinical trial of sorafenib in androgen-independent prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:209–214. doi: 10.1158/1078-0432.CCR-07-1355. [DOI] [PubMed] [Google Scholar]
- 30.Gupta-Abramson V, Troxel AB, Nellore A, Puttaswamy K, Redlinger M, Ransone K, et al. Phase II trial of sorafenib in advanced thyroid cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:4714–4719. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. The New England journal of medicine. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 32.Flaherty KTRM, Schuchter LM, Lathia CD, Weber BL, O’Dwyer PJ. Phase I/II, pharmacokinetic and pharmacodynamic trial of BAY 43–9006 alone in patients with metastatic melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23 (suppl; abstr 3037) [Google Scholar]
- 33.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 34.Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. The lancet oncology. 2010;11:962–972. doi: 10.1016/S1470-2045(10)70203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwamoto FM, Lamborn KR, Robins HI, Mehta MP, Chang SM, Butowski NA, et al. Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06-02) Neuro-oncology. 2010;12:855–861. doi: 10.1093/neuonc/noq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monk BJ, Mas Lopez L, Zarba JJ, Oaknin A, Tarpin C, Termrungruanglert W, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:3562–3569. doi: 10.1200/JCO.2009.26.9571. [DOI] [PubMed] [Google Scholar]
- 37.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. The New England journal of medicine. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldinger SMRJ, Belloni B, Eggmann N, Dummer R. Cutaneous side effects (cAE) of vemurafenib (V): Single-center cohort study of 28 metastatic melanoma (mM) patients (pts) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30 (suppl; abstr e19017) [Google Scholar]
- 40.Chan JA, Blaszkowsky LS, Enzinger PC, Ryan DP, Abrams TA, Zhu AX, et al. A multicenter phase II trial of single-agent cetuximab in advanced esophageal and gastric adenocarcinoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2011;22:1367–1373. doi: 10.1093/annonc/mdq604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maubec E, Petrow P, Scheer-Senyarich I, Duvillard P, Lacroix L, Gelly J, et al. Phase II study of cetuximab as first-line single-drug therapy in patients with unresectable squamous cell carcinoma of the skin. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3419–3426. doi: 10.1200/JCO.2010.34.1735. [DOI] [PubMed] [Google Scholar]
- 42.Ramalingam SS, Lee JW, Belani CP, Aisner SC, Kolesar J, Howe C, et al. Cetuximab for the treatment of advanced bronchioloalveolar carcinoma (BAC): an Eastern Cooperative Oncology Group phase II study (ECOG 1504) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:1709–1714. doi: 10.1200/JCO.2010.33.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neal JW, Heist RS, Fidias P, Temel JS, Huberman M, Marcoux JP, et al. Cetuximab monotherapy in patients with advanced non-small cell lung cancer after prior epidermal growth factor receptor tyrosine kinase inhibitor therapy. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2010;5:1855–1858. doi: 10.1097/JTO.0b013e3181f0bee0. [DOI] [PubMed] [Google Scholar]
- 44.Locati LD, Bossi P, Perrone F, Potepan P, Crippa F, Mariani L, et al. Cetuximab in recurrent and/or metastatic salivary gland carcinomas: A phase II study. Oral oncology. 2009;45:574–578. doi: 10.1016/j.oraloncology.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Zhu AX, Stuart K, Blaszkowsky LS, Muzikansky A, Reitberg DP, Clark JW, et al. Phase 2 study of cetuximab in patients with advanced hepatocellular carcinoma. Cancer. 2007;110:581–589. doi: 10.1002/cncr.22829. [DOI] [PubMed] [Google Scholar]
- 46.Cortes JE, Jones D, O’Brien S, Jabbour E, Ravandi F, Koller C, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:398–404. doi: 10.1200/JCO.2009.25.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah NP, Kim DW, Kantarjian H, Rousselot P, Llacer PE, Enrico A, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica. 2010;95:232–240. doi: 10.3324/haematol.2009.011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cauchi C, Somaiah N, Engstrom PF, Litwin S, Lopez M, Lee J, et al. Evaluation of nilotinib in advanced GIST previously treated with imatinib and sunitinib. Cancer chemotherapy and pharmacology. 2012;69:977–982. doi: 10.1007/s00280-011-1785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giles FJ, le Coutre PD, Pinilla-Ibarz J, Larson RA, Gattermann N, Ottmann OG, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2012 doi: 10.1038/leu.2012.181. [DOI] [PubMed] [Google Scholar]
- 50.Nicolini FE, Turkina A, Shen ZX, Gallagher N, Jootar S, Powell BL, et al. Expanding Nilotinib Access in Clinical Trials (ENACT): an open-label, multicenter study of oral nilotinib in adult patients with imatinib-resistant or imatinib-intolerant Philadelphia chromosome-positive chronic myeloid leukemia in the chronic phase. Cancer. 2012;118:118–126. doi: 10.1002/cncr.26249. [DOI] [PubMed] [Google Scholar]
- 51.Reichardt P, Blay JY, Gelderblom H, Schlemmer M, Demetri GD, Bui-Nguyen B, et al. Phase III study of nilotinib versus best supportive care with or without a TKI in patients with gastrointestinal stromal tumors resistant to or intolerant of imatinib and sunitinib. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:1680–1687. doi: 10.1093/annonc/mdr598. [DOI] [PubMed] [Google Scholar]
- 52.Usuki K, Tojo A, Maeda Y, Kobayashi Y, Matsuda A, Ohyashiki K, et al. Efficacy and safety of nilotinib in Japanese patients with imatinib-resistant or -intolerant Ph+ CML or relapsed/refractory Ph+ ALL: a 36-month analysis of a phase I and II study. International journal of hematology. 2012;95:409–419. doi: 10.1007/s12185-012-1026-9. [DOI] [PubMed] [Google Scholar]
- 53.Cho JH, Kim KM, Kwon M, Kim JH, Lee J. Nilotinib in patients with metastatic melanoma harboring KIT gene aberration. Investigational new drugs. 2012;30:2008–2014. doi: 10.1007/s10637-011-9763-9. [DOI] [PubMed] [Google Scholar]
- 54.Giles FJ, Abruzzese E, Rosti G, Kim DW, Bhatia R, Bosly A, et al. Nilotinib is active in chronic and accelerated phase chronic myeloid leukemia following failure of imatinib and dasatinib therapy. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2010;24:1299–1301. doi: 10.1038/leu.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. The New England journal of medicine. 2010;362:2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 56.Rosti G, Palandri F, Castagnetti F, Breccia M, Levato L, Gugliotta G, et al. Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood. 2009;114:4933–4938. doi: 10.1182/blood-2009-07-232595. [DOI] [PubMed] [Google Scholar]
- 57.le Coutre P, Ottmann OG, Giles F, Kim DW, Cortes J, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is active in patients with imatinib-resistant or -intolerant accelerated-phase chronic myelogenous leukemia. Blood. 2008;111:1834–1839. doi: 10.1182/blood-2007-04-083196. [DOI] [PubMed] [Google Scholar]
- 58.Schlemmer M, Bauer S, Schutte R, Hartmann JT, Bokemeyer C, Hosius C, et al. Activity and side effects of imatinib in patients with gastrointestinal stromal tumors: data from a German multicenter trial. European journal of medical research. 2011;16:206–212. doi: 10.1186/2047-783X-16-5-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kerob D, Porcher R, Verola O, Dalle S, Maubec E, Aubin F, et al. Imatinib mesylate as a preoperative therapy in dermatofibrosarcoma: results of a multicenter phase II study on 25 patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:3288–3295. doi: 10.1158/1078-0432.CCR-09-3401. [DOI] [PubMed] [Google Scholar]
- 60.Lin AY, Fisher GA, So S, Tang C, Levitt L. Phase II study of imatinib in unresectable hepatocellular carcinoma. American journal of clinical oncology. 2008;31:84–88. doi: 10.1097/COC.0b013e3181131db9. [DOI] [PubMed] [Google Scholar]
- 61.Nishida T, Shirao K, Sawaki A, Koseki M, Okamura T, Ohtsu A, et al. Efficacy and safety profile of imatinib mesylate (ST1571) in Japanese patients with advanced gastrointestinal stromal tumors: a phase II study (STI571B1202) International journal of clinical oncology / Japan Society of Clinical Oncology. 2008;13:244–251. doi: 10.1007/s10147-007-0746-y. [DOI] [PubMed] [Google Scholar]
- 62.Bajaj GK, Zhang Z, Garrett-Mayer E, Drew R, Sinibaldi V, Pili R, et al. Phase II study of imatinib mesylate in patients with prostate cancer with evidence of biochemical relapse after definitive radical retropubic prostatectomy or radiotherapy. Urology. 2007;69:526–531. doi: 10.1016/j.urology.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 63.Wen PY, Yung WK, Lamborn KR, Dahia PL, Wang Y, Peng B, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:4899–4907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 64.Hess G, Bunjes D, Siegert W, Schwerdtfeger R, Ledderose G, Wassmann B, et al. Sustained complete molecular remissions after treatment with imatinib-mesylate in patients with failure after allogeneic stem cell transplantation for chronic myelogenous leukemia: results of a prospective phase II open-label multicenter study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:7583–7593. doi: 10.1200/JCO.2005.01.3110. [DOI] [PubMed] [Google Scholar]
- 65.Rao K, Goodin S, Levitt MJ, Dave N, Shih WJ, Lin Y, et al. A phase II trial of imatinib mesylate in patients with prostate specific antigen progression after local therapy for prostate cancer. The Prostate. 2005;62:115–122. doi: 10.1002/pros.20130. [DOI] [PubMed] [Google Scholar]
- 66.Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–1134. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 67.O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. The New England journal of medicine. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 68.Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. The New England journal of medicine. 2002;346:645–652. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 69.Talpaz M, Silver RT, Druker BJ, Goldman JM, Gambacorti-Passerini C, Guilhot F, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood. 2002;99:1928–1937. doi: 10.1182/blood.v99.6.1928. [DOI] [PubMed] [Google Scholar]
- 70.Kang BLJ, Ryu M, Im S, Park S, Kang W, et al. A phase II study of imatinib mesylate as adjuvant treatment for curatively resected high-risk localized gastrointestinal stromal tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27 (suppl; abstr e21515) [Google Scholar]
- 71.Lewis CM, Glisson BS, Feng L, Wan F, Tang X, Wistuba II, et al. A phase II study of gefitinib for aggressive cutaneous squamous cell carcinoma of the head and neck. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:1435–1446. doi: 10.1158/1078-0432.CCR-11-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park SJ, Kim HT, Lee DH, Kim KP, Kim SW, Suh C, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012;77:556–560. doi: 10.1016/j.lungcan.2012.05.092. [DOI] [PubMed] [Google Scholar]
- 73.Zhang L, Ma S, Song X, Han B, Cheng Y, Huang C, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. The lancet oncology. 2012;13:466–475. doi: 10.1016/S1470-2045(12)70117-1. [DOI] [PubMed] [Google Scholar]
- 74.Asami K, Koizumi T, Hirai K, Ameshima S, Tsukadaira A, Morozumi N, et al. Gefitinib as first-line treatment in elderly epidermal growth factor receptor-mutated patients with advanced lung adenocarcinoma: results of a Nagano Lung Cancer Research Group study. Clinical lung cancer. 2011;12:387–392. doi: 10.1016/j.cllc.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 75.Chen X, Li W, Hu X, Geng Y, Wang R, Yin Y, et al. Effect of gefitinib challenge to initial treatment with non-small cell lung cancer. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2011;65:542–546. doi: 10.1016/j.biopha.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 76.Kim DW, Lee SH, Lee JS, Lee MA, Kang JH, Kim SY, et al. A multicenter phase II study to evaluate the efficacy and safety of gefitinib as first-line treatment for Korean patients with advanced pulmonary adenocarcinoma harboring EGFR mutations. Lung Cancer. 2011;71:65–69. doi: 10.1016/j.lungcan.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 77.Lee DH, Park K, Kim JH, Lee JS, Shin SW, Kang JH, et al. Randomized Phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:1307–1314. doi: 10.1158/1078-0432.CCR-09-1903. [DOI] [PubMed] [Google Scholar]
- 78.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. The New England journal of medicine. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 79.Goss G, Ferry D, Wierzbicki R, Laurie SA, Thompson J, Biesma B, et al. Randomized phase II study of gefitinib compared with placebo in chemotherapy-naive patients with advanced non-small-cell lung cancer and poor performance status. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:2253–2260. doi: 10.1200/JCO.2008.18.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lara-Guerra H, Waddell TK, Salvarrey MA, Joshua AM, Chung CT, Paul N, et al. Phase II study of preoperative gefitinib in clinical stage I non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:6229–6236. doi: 10.1200/JCO.2009.22.3370. [DOI] [PubMed] [Google Scholar]
- 81.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. The New England journal of medicine. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 82.Maruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, Nakagawa K, et al. Phase III study, V-15–32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:4244–4252. doi: 10.1200/JCO.2007.15.0185. [DOI] [PubMed] [Google Scholar]
- 83.Sunaga N, Tomizawa Y, Yanagitani N, Iijima H, Kaira K, Shimizu K, et al. Phase II prospective study of the efficacy of gefitinib for the treatment of stage III/IV non-small cell lung cancer with EGFR mutations, irrespective of previous chemotherapy. Lung Cancer. 2007;56:383–389. doi: 10.1016/j.lungcan.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 84.Asahina H, Yamazaki K, Kinoshita I, Sukoh N, Harada M, Yokouchi H, et al. A phase II trial of gefitinib as first-line therapy for advanced non-small cell lung cancer with epidermal growth factor receptor mutations. British journal of cancer. 2006;95:998–1004. doi: 10.1038/sj.bjc.6603393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cufer T, Vrdoljak E, Gaafar R, Erensoy I, Pemberton K. Phase II, open-label, randomized study (SIGN) of single-agent gefitinib (IRESSA) or docetaxel as second-line therapy in patients with advanced (stage IIIb or IV) non-small-cell lung cancer. Anti-cancer drugs. 2006;17:401–409. doi: 10.1097/01.cad.0000203381.99490.ab. [DOI] [PubMed] [Google Scholar]
- 86.Xu JM, Han Y, Li YM, Zhao CH, Wang Y, Paradiso A. Phase II trial of sequential gefitinib after minor response or partial response to chemotherapy in Chinese patients with advanced non-small-cell lung cancer. BMC cancer. 2006;6:288. doi: 10.1186/1471-2407-6-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee DH, Han JY, Lee HG, Lee JJ, Lee EK, Kim HY, et al. Gefitinib as a first-line therapy of advanced or metastatic adenocarcinoma of the lung in never-smokers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:3032–3037. doi: 10.1158/1078-0432.CCR-04-2149. [DOI] [PubMed] [Google Scholar]
- 88.Polychronis A, Sinnett HD, Hadjiminas D, Singhal H, Mansi JL, Shivapatham D, et al. Preoperative gefitinib versus gefitinib and anastrozole in postmenopausal patients with oestrogen-receptor positive and epidermal-growth-factor-receptor-positive primary breast cancer: a double-blind placebo-controlled phase II randomised trial. The lancet oncology. 2005;6:383–391. doi: 10.1016/S1470-2045(05)70176-5. [DOI] [PubMed] [Google Scholar]
- 89.Rothenberg ML, LaFleur B, Levy DE, Washington MK, Morgan-Meadows SL, Ramanathan RK, et al. Randomized phase II trial of the clinical and biological effects of two dose levels of gefitinib in patients with recurrent colorectal adenocarcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:9265–9274. doi: 10.1200/JCO.2005.03.0536. [DOI] [PubMed] [Google Scholar]
- 90.Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 91.Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 92.Defferrari CLM, Brianti A, Catania G, Grossi F, Pronzato P. Difference between skin toxicities in erlotinib (E) and gefitinib (G) in the treatment of advanced non-small cell lung cancer (NSCLC) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25 (suppl; abstr 18187) [Google Scholar]
- 93.Giles FJ, Bellamy WT, Estrov Z, O’Brien SM, Verstovsek S, Ravandi F, et al. The anti-angiogenesis agent, AG-013736, has minimal activity in elderly patients with poor prognosis acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) Leukemia research. 2006;30:801–811. doi: 10.1016/j.leukres.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 94.Tobinai K, Igarashi T, Itoh K, Kurosawa M, Nagai H, Hiraoka A, et al. Rituximab monotherapy with eight weekly infusions for relapsed or refractory patients with indolent B cell non-Hodgkin lymphoma mostly pretreated with rituximab: a multicenter phase II study. Cancer science. 2011;102:1698–1705. doi: 10.1111/j.1349-7006.2011.02001.x. [DOI] [PubMed] [Google Scholar]
- 95.Gertz MA, Rue M, Blood E, Kaminer LS, Vesole DH, Greipp PR. Multicenter phase 2 trial of rituximab for Waldenstrom macroglobulinemia (WM): an Eastern Cooperative Oncology Group Study (E3A98) Leukemia & lymphoma. 2004;45:2047–2055. doi: 10.1080/10428190410001714043. [DOI] [PubMed] [Google Scholar]
- 96.Tobinai K, Igarashi T, Itoh K, Kobayashi Y, Taniwaki M, Ogura M, et al. Japanese multicenter phase II and pharmacokinetic study of rituximab in relapsed or refractory patients with aggressive B-cell lymphoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2004;15:821–830. doi: 10.1093/annonc/mdh176. [DOI] [PubMed] [Google Scholar]
- 97.Rehwald U, Schulz H, Reiser M, Sieber M, Staak JO, Morschhauser F, et al. Treatment of relapsed CD20+ Hodgkin lymphoma with the monoclonal antibody rituximab is effective and well tolerated: results of a phase 2 trial of the German Hodgkin Lymphoma Study Group. Blood. 2003;101:420–424. doi: 10.1182/blood.V101.2.420. [DOI] [PubMed] [Google Scholar]
- 98.Igarashi T, Kobayashi Y, Ogura M, Kinoshita T, Ohtsu T, Sasaki Y, et al. Factors affecting toxicity, response and progression-free survival in relapsed patients with indolent B-cell lymphoma and mantle cell lymphoma treated with rituximab: a Japanese phase II study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2002;13:928–943. doi: 10.1093/annonc/mdf155. [DOI] [PubMed] [Google Scholar]
- 99.Davis TA, Grillo-Lopez AJ, White CA, McLaughlin P, Czuczman MS, Link BK, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: safety and efficacy of re-treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18:3135–3143. doi: 10.1200/JCO.2000.18.17.3135. [DOI] [PubMed] [Google Scholar]
- 100.Foran JM, Gupta RK, Cunningham D, Popescu RA, Goldstone AH, Sweetenham JW, et al. A UK multicentre phase II study of rituximab (chimaeric anti-CD20 monoclonal antibody) in patients with follicular lymphoma, with PCR monitoring of molecular response. British journal of haematology. 2000;109:81–88. doi: 10.1046/j.1365-2141.2000.01965.x. [DOI] [PubMed] [Google Scholar]
- 101.Piro LD, White CA, Grillo-Lopez AJ, Janakiraman N, Saven A, Beck TM, et al. Extended Rituximab (anti-CD20 monoclonal antibody) therapy for relapsed or refractory low-grade or follicular non-Hodgkin’s lymphoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 1999;10:655–661. doi: 10.1023/a:1008389119525. [DOI] [PubMed] [Google Scholar]
- 102.Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998;92:1927–1932. [PubMed] [Google Scholar]
- 103.Cortes-Funes HdlSJ, Flores E, Perez Manga G, Lopez A, Palacios C, et al. Rituximab in Monotherapy in Patients with Relapsed Follicular or Low Grade B-Cell Non-Hodgkin’s Lymphoma: Results After a Six Month Follow-up Cut-off. Proc Am Soc Clin Oncol. 2000;19 [Google Scholar]
- 104.Hersh EM, O’Day SJ, Powderly J, Khan KD, Pavlick AC, Cranmer LD, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naive patients with advanced melanoma. Investigational new drugs. 2011;29:489–498. doi: 10.1007/s10637-009-9376-8. [DOI] [PubMed] [Google Scholar]
- 105.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. The lancet oncology. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 107.Simeone EGG, Romano A, Daponte A, Caraco C, Grimaldi AM, et al. Immunological and biological changes during ipilimumab (Ipi) treatment and their correlation with clinical response and survival. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30 [Google Scholar]
- 108.Kaminski MS, Radford JA, Gregory SA, Leonard JP, Knox SJ, Kroll S, et al. Re-treatment with I-131 tositumomab in patients with non-Hodgkin’s lymphoma who had previously responded to I-131 tositumomab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:7985–7993. doi: 10.1200/JCO.2005.01.0892. [DOI] [PubMed] [Google Scholar]
- 109.Kaminski MS, Zelenetz AD, Press OW, Saleh M, Leonard J, Fehrenbacher L, et al. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin’s lymphomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19:3918–3928. doi: 10.1200/JCO.2001.19.19.3918. [DOI] [PubMed] [Google Scholar]
- 110.Vose JM, Wahl RL, Saleh M, Rohatiner AZ, Knox SJ, Radford JA, et al. Multicenter phase II study of iodine-131 tositumomab for chemotherapy-relapsed/refractory low-grade and transformed low-grade B-cell non-Hodgkin’s lymphomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18:1316–1323. doi: 10.1200/JCO.2000.18.6.1316. [DOI] [PubMed] [Google Scholar]
- 111.Ciuleanu T, Stelmakh L, Cicenas S, Miliauskas S, Grigorescu AC, Hillenbach C, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. The lancet oncology. 2012;13:300–308. doi: 10.1016/S1470-2045(11)70385-0. [DOI] [PubMed] [Google Scholar]
- 112.Coudert B, Ciuleanu T, Park K, Wu YL, Giaccone G, Brugger W, et al. Survival benefit with erlotinib maintenance therapy in patients with advanced non-small-cell lung cancer (NSCLC) according to response to first-line chemotherapy. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:388–394. doi: 10.1093/annonc/mdr125. [DOI] [PubMed] [Google Scholar]
- 113.Choi DR, Lee DH, Choi CM, Kim SW, Suh C, Lee JS. Erlotinib in first-line therapy for non-small cell lung cancer: a prospective phase II study. Anticancer research. 2011;31:3457–3462. [PubMed] [Google Scholar]
- 114.Lee DH, Kim SW, Suh C, Han YH, Lee JS. Phase II study of erlotinib for chemotherapy-naive patients with advanced or metastatic non-small cell lung cancer who are ineligible for platinum doublets. Cancer chemotherapy and pharmacology. 2011;67:35–39. doi: 10.1007/s00280-010-1280-6. [DOI] [PubMed] [Google Scholar]
- 115.Natale RB, Thongprasert S, Greco FA, Thomas M, Tsai CM, Sunpaweravong P, et al. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:1059–1066. doi: 10.1200/JCO.2010.28.5981. [DOI] [PubMed] [Google Scholar]
- 116.Sequist LV, von Pawel J, Garmey EG, Akerley WL, Brugger W, Ferrari D, et al. Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3307–3315. doi: 10.1200/JCO.2010.34.0570. [DOI] [PubMed] [Google Scholar]
- 117.Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczesna A, Juhasz E, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. The lancet oncology. 2010;11:521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 118.Takahashi T, Yamamoto N, Nukiwa T, Mori K, Tsuboi M, Horai T, et al. Phase II study of erlotinib in Japanese patients with advanced non-small cell lung cancer. Anticancer research. 2010;30:557–563. [PubMed] [Google Scholar]
- 119.Dickler MN, Cobleigh MA, Miller KD, Klein PM, Winer EP. Efficacy and safety of erlotinib in patients with locally advanced or metastatic breast cancer. Breast cancer research and treatment. 2009;115:115–121. doi: 10.1007/s10549-008-0055-9. [DOI] [PubMed] [Google Scholar]
- 120.Kubota K, Nishiwaki Y, Tamura T, Nakagawa K, Matsui K, Watanabe K, et al. Efficacy and safety of erlotinib monotherapy for Japanese patients with advanced non-small cell lung cancer: a phase II study. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2008;3:1439–1445. doi: 10.1097/JTO.0b013e31818d6702. [DOI] [PubMed] [Google Scholar]
- 121.Oza AM, Eisenhauer EA, Elit L, Cutz JC, Sakurada A, Tsao MS, et al. Phase II study of erlotinib in recurrent or metastatic endometrial cancer: NCIC IND-148. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:4319–4325. doi: 10.1200/JCO.2007.15.8808. [DOI] [PubMed] [Google Scholar]
- 122.Garland LL, Rankin C, Gandara DR, Rivkin SE, Scott KM, Nagle RB, et al. Phase II study of erlotinib in patients with malignant pleural mesothelioma: a Southwest Oncology Group Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:2406–2413. doi: 10.1200/JCO.2006.09.7634. [DOI] [PubMed] [Google Scholar]
- 123.Thomas MB, Chadha R, Glover K, Wang X, Morris J, Brown T, et al. Phase 2 study of erlotinib in patients with unresectable hepatocellular carcinoma. Cancer. 2007;110:1059–1067. doi: 10.1002/cncr.22886. [DOI] [PubMed] [Google Scholar]
- 124.Dragovich T, McCoy S, Fenoglio-Preiser CM, Wang J, Benedetti JK, Baker AF, et al. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:4922–4927. doi: 10.1200/JCO.2006.07.1316. [DOI] [PubMed] [Google Scholar]
- 125.Gordon AN, Finkler N, Edwards RP, Garcia AA, Crozier M, Irwin DH, et al. Efficacy and safety of erlotinib HCl, an epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor, in patients with advanced ovarian carcinoma: results from a phase II multicenter study. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2005;15:785–792. doi: 10.1111/j.1525-1438.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- 126.Philip PA, Mahoney MR, Allmer C, Thomas J, Pitot HC, Kim G, et al. Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:6657–6663. doi: 10.1200/JCO.2005.14.696. [DOI] [PubMed] [Google Scholar]
- 127.Perez-Soler R. Phase II clinical trial data with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib (OSI-774) in non-small-cell lung cancer. Clinical lung cancer. 2004;6(Suppl 1):S20–S23. doi: 10.3816/clc.2004.s.010. [DOI] [PubMed] [Google Scholar]
- 128.Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:77–85. doi: 10.1200/JCO.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 129.Neal JWPN, Goodgame BW, Lanuti M, Heist RS, Shaw AT, et al. A multicenter phase II trial of adjuvant erlotinib in patients with resected non-small cell lung cancer (NSCLC) and mutations in the epidermal growth factor receptor (EGFR): Toxicity evaluation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010;28:suppl; abstr. 7078 [Google Scholar]
- 130.Jasas KVFA, Elit L, Hoskins PJ, Biagi J, Dubuc-Lissoir J, et al. Phase II study of erlotinib (OSI 774) in women with recurrent or metastatic endometrial cancer: NCIC CTG IND-148. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2004;22:suppl; abstr. 5019 doi: 10.1200/JCO.2007.15.8808. [DOI] [PubMed] [Google Scholar]
- 131.Horowitz NS, Olawaiye AB, Borger DR, Growdon WB, Krasner CN, Matulonis UA, et al. Phase II trial of erlotinib in women with squamous cell carcinoma of the vulva. Gynecologic oncology. 2012;127:141–146. doi: 10.1016/j.ygyno.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 132.Ramalingam SS, Blackhall F, Krzakowski M, Barrios CH, Park K, Bover I, et al. Randomized Phase II Study of Dacomitinib (PF-00299804), an Irreversible Pan-Human Epidermal Growth Factor Receptor Inhibitor, Versus Erlotinib in Patients With Advanced Non-Small-Cell Lung Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:3337–3344. doi: 10.1200/JCO.2011.40.9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schaake EE, Kappers I, Codrington HE, Valdes Olmos RA, Teertstra HJ, van Pel R, et al. Tumor response and toxicity of neoadjuvant erlotinib in patients with early-stage non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2731–2738. doi: 10.1200/JCO.2011.39.4882. [DOI] [PubMed] [Google Scholar]
- 134.Hecht JR, Mitchell E, Neubauer MA, Burris HA, 3rd, Swanson P, Lopez T, et al. Lack of correlation between epidermal growth factor receptor status and response to Panitumumab monotherapy in metastatic colorectal cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:2205–2213. doi: 10.1158/1078-0432.CCR-09-2017. [DOI] [PubMed] [Google Scholar]
- 135.Muro K, Yoshino T, Doi T, Shirao K, Takiuchi H, Hamamoto Y, et al. A phase 2 clinical trial of panitumumab monotherapy in Japanese patients with metastatic colorectal cancer. Japanese journal of clinical oncology. 2009;39:321–326. doi: 10.1093/jjco/hyp016. [DOI] [PubMed] [Google Scholar]
- 136.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 137.Van Cutsem E, Siena S, Humblet Y, Canon JL, Maurel J, Bajetta E, et al. An open-label, single-arm study assessing safety and efficacy of panitumumab in patients with metastatic colorectal cancer refractory to standard chemotherapy. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2008;19:92–98. doi: 10.1093/annonc/mdm399. [DOI] [PubMed] [Google Scholar]
- 138.Yamada YTT, Shirao K, Doi T, Tahara M, Minashi K, et al. Safety and pharmacokinetics (PK) of panitumumab in Japanese patients (pts) with advanced solid malignancies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007 susuppl; abstr385. [Google Scholar]
- 139.Whang YE, Armstrong AJ, Rathmell WK, Godley PA, Kim WY, Pruthi RS, et al. A phase II study of lapatinib, a dual EGFR and HER-2 tyrosine kinase inhibitor, in patients with castration-resistant prostate cancer. Urologic oncology. 2011 doi: 10.1016/j.urolonc.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 140.Thiessen B, Stewart C, Tsao M, Kamel-Reid S, Schaiquevich P, Mason W, et al. A phase I/II trial of GW572016 (lapatinib) in recurrent glioblastoma multiforme: clinical outcomes, pharmacokinetics and molecular correlation. Cancer chemotherapy and pharmacology. 2010;65:353–361. doi: 10.1007/s00280-009-1041-6. [DOI] [PubMed] [Google Scholar]
- 141.Bekaii-Saab T, Markowitz J, Prescott N, Sadee W, Heerema N, Wei L, et al. A multi-institutional phase II study of the efficacy and tolerability of lapatinib in patients with advanced hepatocellular carcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:5895–5901. doi: 10.1158/1078-0432.CCR-09-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Blackwell KL, Pegram MD, Tan-Chiu E, Schwartzberg LS, Arbushites MC, Maltzman JD, et al. Single-agent lapatinib for HER2-overexpressing advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab-containing regimens. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2009;20:1026–1031. doi: 10.1093/annonc/mdn759. [DOI] [PubMed] [Google Scholar]
- 143.Toi M, Iwata H, Fujiwara Y, Ito Y, Nakamura S, Tokuda Y, et al. Lapatinib monotherapy in patients with relapsed, advanced, or metastatic breast cancer: efficacy, safety, and biomarker results from Japanese patients phase II studies. British journal of cancer. 2009;101:1676–1682. doi: 10.1038/sj.bjc.6605343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gomez HL, Doval DC, Chavez MA, Ang PC, Aziz Z, Nag S, et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:2999–3005. doi: 10.1200/JCO.2007.14.0590. [DOI] [PubMed] [Google Scholar]
- 145.Johnston S, Trudeau M, Kaufman B, Boussen H, Blackwell K, LoRusso P, et al. Phase II study of predictive biomarker profiles for response targeting human epidermal growth factor receptor 2 (HER-2) in advanced inflammatory breast cancer with lapatinib monotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:1066–1072. doi: 10.1200/JCO.2007.13.9949. [DOI] [PubMed] [Google Scholar]
- 146.Agulnik M, Cohen EW, Cohen RB, Chen EX, Vokes EE, Hotte SJ, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:3978–3984. doi: 10.1200/JCO.2007.11.8612. [DOI] [PubMed] [Google Scholar]
- 147.Hsu C, Yang TS, Huo TI, Hsieh RK, Yu CW, Hwang WS, et al. Vandetanib in patients with inoperable hepatocellular carcinoma: a phase II, randomized, double-blind, placebo-controlled study. Journal of hepatology. 2012;56:1097–1103. doi: 10.1016/j.jhep.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 148.Lee JS, Hirsh V, Park K, Qin S, Blajman CR, Perng RP, et al. Vandetanib Versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase III trial (ZEPHYR) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:1114–1121. doi: 10.1200/JCO.2011.36.1709. [DOI] [PubMed] [Google Scholar]
- 149.Peuvrel L, Bachmeyer C, Reguiai Z, Bachet JB, Andre T, Bensadoun RJ, et al. Semiology of skin toxicity associated with epidermal growth factor receptor (EGFR) inhibitors. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2012;20:909–921. doi: 10.1007/s00520-012-1404-0. [DOI] [PubMed] [Google Scholar]
- 150.Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2005;16:1425–1433. doi: 10.1093/annonc/mdi279. [DOI] [PubMed] [Google Scholar]
- 151.Galimont-Collen AF, Vos LE, Lavrijsen AP, Ouwerkerk J, Gelderblom H. Classification and management of skin, hair, nail and mucosal side-effects of epidermal growth factor receptor (EGFR) inhibitors. Eur J Cancer. 2007;43:845–851. doi: 10.1016/j.ejca.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 152.Roe E, Garcia Muret MP, Marcuello E, Capdevila J, Pallares C, Alomar A. Description and management of cutaneous side effects during cetuximab or erlotinib treatments: a prospective study of 30 patients. Journal of the American Academy of Dermatology. 2006;55:429–437. doi: 10.1016/j.jaad.2006.04.062. [DOI] [PubMed] [Google Scholar]
- 153.Karthaus MHR, Mineur L, Letocha H, Greil R, Thaler J, et al. Epidermal growth factor receptor (EGFR) inhibitor- related skin toxicity: review of interim data from a phase II study (2260314) of panitumumab with FOLFIRI in the first-line treat- ment of metastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:e20364. [Google Scholar]
- 154.Ledezma B. Ipilimumab for advanced melanoma: a nursing perspective. Oncology nursing forum. 2009;36:97–104. doi: 10.1188/09.ONF.97-104. [DOI] [PubMed] [Google Scholar]
- 155.Fallen RS, Terpstra CR, Lima HC. Immunotherapies in dermatologic disorders. The Medical clinics of North America. 2012;96:565–582. x–xi. doi: 10.1016/j.mcna.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 156.Balagula Y, Wu S, Su X, Lacouture ME. The effect of cytotoxic chemotherapy on the risk of high-grade acneiform rash to cetuximab in cancer patients: a meta-analysis. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2011;22:2366–2374. doi: 10.1093/annonc/mdr016. [DOI] [PubMed] [Google Scholar]
- 157.Yosipovitch G, Bernhard JDClinical practice. Chronic pruritus. The New England journal of medicine. 2013;368:1625–1634. doi: 10.1056/NEJMcp1208814. [DOI] [PubMed] [Google Scholar]
- 158.Su X, Lacouture ME, Jia Y, Wu S. Risk of high-grade skin rash in cancer patients treated with cetuximab--an antibody against epidermal growth factor receptor: systemic review and meta-analysis. Oncology. 2009;77:124–133. doi: 10.1159/000229752. [DOI] [PubMed] [Google Scholar]
- 159.Inami Y, Andoh T, Sasaki A, Kuraishi Y. Topical surfactant-induced pruritus: involvement of histamine released from epidermal keratinocytes. The Journal of pharmacology and experimental therapeutics. 2013;344:459–466. doi: 10.1124/jpet.112.200063. [DOI] [PubMed] [Google Scholar]
- 160.Gerber PA, Buhren BA, Cevikbas F, Bolke E, Steinhoff M, Homey B. Preliminary evidence for a role of mast cells in epidermal growth factor receptor inhibitor-induced pruritus. Journal of the American Academy of Dermatology. 2010;63:163–165. doi: 10.1016/j.jaad.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 161.Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nature reviews Neuroscience. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 162.Yosipovitch Pa. Dermatologic Principles & Practice in Oncology. Hoboken, NJ: Wiley; 2013. Pruritus. [Google Scholar]
- 163.Santini D, Vincenzi B, Guida FM, Imperatori M, Schiavon G, Venditti O, et al. Aprepitant for management of severe pruritus related to biological cancer treatments: a pilot study. The lancet oncology. 2012;13:1020–1024. doi: 10.1016/S1470-2045(12)70373-X. [DOI] [PubMed] [Google Scholar]
- 164.Chen SC. Pruritus. Dermatologic clinics. 2012;30:309–321. ix. doi: 10.1016/j.det.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 165.Penel N, Le Cesne A, Bui BN, Perol D, Brain EG, Ray-Coquard I, et al. Imatinib for progressive and recurrent aggressive fibromatosis (desmoid tumors): an FNCLCC/French Sarcoma Group phase II trial with a long-term follow-up. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2011;22:452–457. doi: 10.1093/annonc/mdq341. [DOI] [PubMed] [Google Scholar]
- 166.Graziadei IWFA, Stauber R, Trauner M, Mueller C, Vogel W. Imatinib treatment for patients with advanced stage hepatocellular carcinoma: A multicenter phase II trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28 suppl; abstr e14629. [Google Scholar]
- 167.Lin AM, Rini BI, Weinberg V, Fong K, Ryan CJ, Rosenberg JE, et al. A phase II trial of imatinib mesylate in patients with biochemical relapse of prostate cancer after definitive local therapy. BJU international. 2006;98:763–769. doi: 10.1111/j.1464-410X.2006.06396.x. [DOI] [PubMed] [Google Scholar]
- 168.Gellrich S, Muche JM, Wilks A, Jasch KC, Voit C, Fischer T, et al. Systemic eight-cycle anti-CD20 monoclonal antibody (rituximab) therapy in primary cutaneous B-cell lymphomas--an applicational observation. The British journal of dermatology. 2005;153:167–173. doi: 10.1111/j.1365-2133.2005.06659.x. [DOI] [PubMed] [Google Scholar]