Abstract
Background
Glycated albumin (GA) has been increasingly used as a reliable index for short-term glycemic monitoring, and is inversely associated with β-cell function. Because the pathophysiologic nature of type 2 diabetes (T2D) is characterized by progressive decline in insulin secretion, the aim was to determine whether GA levels were affected by diabetes duration in subjects with T2D.
Methods
To minimize the effect of glucose variability on GA, subjects with stably maintained HbA1c levels of <0.5% fluctuation across 6 months of measurements were included. Patients with newly diagnosed T2D (n = 1059) and with duration>1 year (n = 781) were recruited and categorized as New-T2D and Old-T2D, respectively. Biochemical, glycemic, and C-peptide parameters were measured.
Results
GA levels were significantly elevated in HbA1c-matched Old-T2D subjects compared to New-T2D subjects. Duration of diabetes was positively correlated with GA, whereas a negative relationship was found with C-peptide increment (ΔC-peptide). Among insulin secretory indices, dynamic parameters such as ΔC-peptide were inversely related to GA (r = −0.42, p<0.001). Multiple linear regression analyses showed that duration of diabetes was associated with GA (standardized β coefficient [STDβ] = 0.05, p<0.001), but not with HbA1c (STDβ = 0.04, p<0.095). This association disappeared after additional adjustment with ΔC-peptide (STDβ = 0.02, p = 0.372), suggesting that β-cell function might be a linking factor of close relationship between duration of diabetes and GA values.
Conclusions
The present study showed that GA levels were significantly increased in subjects with longer duration T2D and with decreased insulin secretory function. Additional caution should be taken when interpreting GA values to assess glycemic control status in these individuals.
Introduction
The United Kingdom Prospective Diabetes Study (UKPDS) [1] and Kumamoto Study [2] demonstrated that intensive glycemic control was essential for individuals with type 2 diabetes (T2D) to reduce risks of morbidity and mortality from diabetes-related complications and cardiovascular diseases. In line with these results, the purpose of glucose monitoring with glycemic indices, in conjunction with lifestyle modification, and pharmacological anti-diabetic drugs in diabetic subjects, is to achieve and maintain optimal blood glucose levels [3]. Clinical data of HbA1c levels have been shown to predict average glycemic status over several months, and to predict diabetic complications [1], [4]; therefore the American Diabetes Association recommended routine HbA1c testing in all diabetic patients as an initial assessment, and then as a part of continuing care [3].
By overcoming the unmet need of well-established or gold standard glycemic index of HbA1c [3], glycated albumin (GA) measurements have been increasingly used as another reliable index for 3-week intermediate glycation monitoring, and GA measurements are further preferred for use in several pathologic conditions, including anemia and chronic kidney disease (CKD) [5]–[7]. In addition, GA is closely associated with insulin secretory function of β-cells [8], [9]. The pathophysiologic nature of T2D is characterized by a progressive decline in pancreatic β-cell function, with deterioration and insulin resistance, resulting in the failure of insulin secretion from islet cells. As duration of diabetes increases, β-cell functions are well known to gradually decline, which may affect the levels of GA. Considering the greater association of GA with decreasing pancreatic β-cell functions than with HbA1c levels in subjects with T2D, the relationship between GA levels and diabetic duration may differ from what has been previously accepted. However, to date there is no report on the relationship among GA, β-cell function and duration of diabetes in subjects with T2D. On these bases, the aim of our study was to investigate whether GA levels are elevated in subjects with long duration of diabetes in close connection with their insulin secretory capacity.
Methods
Subjects and data collection
In this cross-sectional study, patients with T2D who visited the Diabetes Center at Severance Hospital, Seoul, South Korea between January 2009 and December 2012 were enrolled in the Diabetes Registry of Severance Hospital, and most of the subjects have been described in our previous studies [9]–[11]. The study subjects were classified into two groups according to the duration of diabetes. New-onset type 2 diabetes was defined as patients whose duration of diabetes was ≤1 year (New-T2D), and old T2D included patients with duration of diabetes >1 year (Old-T2D). Based on electronic medical records, a total of 1840 subjects satisfied the following inclusion criteria: age ≥18 years of age with stably maintained HbA1c levels of <0.5% fluctuation across 6 months of measurements and available laboratory data for HbA1c and GA prior to a standardized liquid meal test [Ensure, Meiji Dairies Corporation, Tokyo, Japan; 500 kcal, 17.5 g fat (31.5%), 68.5 g carbohydrate (54.5%), and 17.5 g protein (14.0%)] after an overnight fast. Patients were excluded for the following reasons: any medical conditions that could alter HbA1c or GA levels, including liver cirrhosis or kidney diseases (nephrotic syndrome or serum creatinine level ≥176.8 µM), pregnancy, steroid therapy, and hematologic disorders. The protocol of this study was approved by the Institutional Review Board at Severance Hospital (IRB No. 4-2009-0656, 4-2012-0398, 4-2013-0103, 4-2014-0507) and written informed consent for this study was not required by the Institutional Review Board because researchers only accessed the database for analysis purposes, and personal information was not used.
Anthropometric and laboratory measurements
Using electronic medical records, demographic and clinical data were retrospectively collected for age, gender, smoking history, and duration of diabetes. The duration of diabetes was defined by the date when patients were diagnosed as having diabetes by blood tests or by patient recall from interviews. Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters. During standardized liquid meal tests, blood samples were collected at 0 and 90 min (basal and stimulated levels, respectively) for glucose, insulin, and C-peptide analyses. Serum C-peptide levels were determined by an immunoradiometric assay method (Beckman Coulter, Fullerton, CA, USA). Pancreatic β-cell function and insulin sensitivity were assessed using the following indices: homeostasis model assessment of pancreatic β-cell function (HOMA-β) = basal insulin (pM)×0.48/[basal glucose (mM)−3.5], HOMA-IR = [basal insulin (pM)×glucose (mM)/156.3] [12] and C-peptide increment (ΔC-peptide = C-peptide, stimulated−C-peptide, basal) [13], respectively. Serum concentrations of fasting glucose, total cholesterol, and albumin were measured by standard methods. Serum creatinine levels were determined with a Hitachi 7600-110 automated chemistry analyzer (Hitachi Co., Tokyo, Japan) with CREA (Roche Diagnostics, Indianapolis, IN, USA). Serum GA was analyzed by an enzymatic method using an albumin-specific proteinase, ketoamine oxidase, and an albumin assay reagent (LUCICA GA-L; Asahi Kasei Pharma Co., Tokyo, Japan), and a Hitachi 7699 P module autoanalyzer (Hitachi Instruments Service, Tokyo, Japan) [14]. GA values (%) were the calculation of the ratio of GA to total serum albumin. The coefficient of variation was 1.43%. HbA1c was measured by high-performance liquid chromatography using Variant II Turbo (Bio-Rad Laboratories, Hercules, CA, USA). The reference ranges of HbA1c and GA were between 4.0% and 6.0%, and between 11.0% and 16.0%, respectively.
Statistical Analyses
All continuous variables were expressed as mean ± standard deviation (SD) or median (ranges) values, as appropriate. HOMA-β, HOMA-IR, and ΔC-peptide were log transformed for the analyses because the value distributions were skewed. Student's t test and Pearson's χ2 test were used to compare variables between two groups, as appropriate. The differences in GA and GA/HbA1c ratios, between two groups, were evaluated using Student's t test with Bonferroni correction after stratification of HbA1c levels, because GA/HbA1c ratios are affected by HbA1c levels [10]. One-way analysis of variance (ANOVA) was used to examine the differences of GA/HbA1c ratios, GA, and ΔC-peptide levels according to the duration of diabetes or tertiles of ΔC-peptide. We analyzed the relationship between GA/HbA1c ratios and ΔC-peptide, using Pearson's correlation coefficients with scatter plots. A spline curve was plotted for the relationship between GA and ΔC-peptide levels. A multivariable linear regression model was applied to assess various clinical and laboratory parameters associated with HbA1c or GA. HOMA-IR was removed from the final linear model because it had multiple collinearity with basal insulin. Results were expressed as values of standardized β coefficient and p. To minimize the possible effect of age on GA, propensity-score matching was used to match ages among subjects. A two-sided p value <0.05 was considered significant. Statistical analyses were carried out with SPSS version 20.0 for Windows (IBM Corp., Armonk, NY, USA) and SAS version 9.2 (SAS Institute).
Results
Study population characteristics
New-T2D of 1059 subjects, defined as ≤1 year of diabetes duration, and 781 subjects with Old-T2D defined as >1 year of diabetes duration, were included in the present study. The patient characteristics of the cohort are shown in Table 1. Median durations of diabetes were 0.5 year and 4.4 years in New-T2D and Old-T2D, respectively. Individuals in the New-T2D group were younger and had lower levels of glucose at 90 min, while they had significantly higher values of stimulated C-peptide, ΔC-peptide, and total cholesterol. While HbA1c levels were similar in the two groups (7.8±1.9 vs. 7.9±1.6, p = 0.159, respectively), both GA (19.6±7.8 vs. 20.9±7.4, p<0.001, respectively) and GA/HbA1c ratios (2.47±0.50 vs. 2.61±0.53, p<0.001, respectively) were significantly increased in Old-T2D subjects, when compared to the New-T2D subjects.
Table 1. Baseline characteristics of the study population.
| New T2D | Old T2D | P | |
| diabetes duration ≤1 y (N = 1059) | diabetes duration >1 y (N = 781) | ||
| Age (years) | 56.8±12.3 | 60.7±11.3 | <0.001 |
| Sex (M/F, %Female) | 625/434 (41) | 435/346 (44) | 0.154 |
| Duration of diabetes (years) | 0.5 (0–0.99) | 4.4 (1.00–43.44) | <0.001 |
| BMI (kg/m2) | 25.3±3.6 | 25.1±3.7 | 0.486 |
| Smoking (never/past/current) | 645/224/190 | 502/139/140 | 0.184 |
| Glycemic profiles | |||
| Glucose, basal (mM) | 7.6±2.7 | 7.5±2.5 | 0.768 |
| Glucose, stimulated (mM) | 12.2±4.9 | 12.8±4.4 | 0.005 |
| HbA1c (%) | 7.8±1.9 | 7.9±1.6 | 0.159 |
| HbA1c (mM/M) | 61.6±20.3 | 62.8±17.0 | 0.159 |
| Glycated albumin (%) | 19.6±7.8 | 20.9±7.4 | <0.001 |
| GA/HbA1c ratio | 2.47±0.50 | 2.61±0.53 | <0.001 |
| C-peptide, basal (nM) | 0.82±0.45 | .79±0.43 | 0.188 |
| C-peptide, stimulated (nM) | 2.10±1.03 | 1.90±0.94 | <0.001 |
| ΔC-peptide (nM)* | 1.29±0.87 | 1.11±0.76 | <0.001 |
| Insulin, basal (pM) | 82.8±91.1 | 83.5±97.2 | 0.903 |
| Insulin, stimulated (pM) | 400.1±355.2 | 348.6±280.6 | 0.003 |
| HOMA-IR* | 3.5±4.4 | 3.9±5.1 | 0.097 |
| HOMA-β* | 85.4±188.2 | 82.6±160.7 | 0.776 |
| Biochemistry profiles | |||
| Total cholesterol (mM) | 4.7±1.2 | 4.3±1.0 | <0.001 |
| HDL cholesterol (mM) | 1.2±0.3 | 1.2±0.3 | 0.660 |
| LDL cholesterol (mM) | 2.8±1.0 | 2.4±0.8 | <0.001 |
| Albumin (g/L) | 45.0±3.7 | 45.1±3.4 | 0.763 |
| Creatinine (µM) | 81.8±20.5 | 86.0±23.1 | <0.001 |
*log transformed.
Variables were described as mean ± SD or median (ranges).
BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-β, homeostasis model assessment of pancreatic β-cell function.
Glycated albumin levels were elevated in subjects with long duration of diabetes
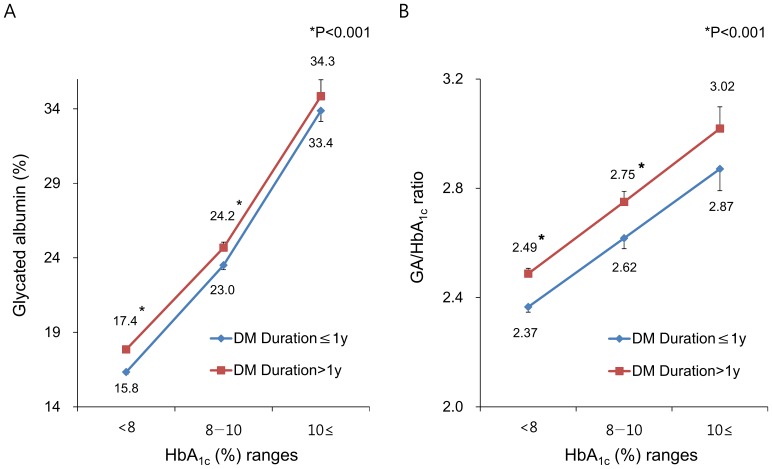
Based on recent studies, the levels of GA and GA/HbA1c ratios were higher in subjects with poorly controlled diabetes than in subjects with well-controlled diabetes, whereas HbA1c levels were not different [10]. Likewise, patients with longer diabetes duration tend to have higher HbA1c levels [15]. Therefore, GA and GA/HbA1c ratios in this study were compared, according to HbA1c strata (Fig. 1). In the ranges of HbA1c levels <8%, and 8 to 10%, both GA and GA/HbA1c ratios in Old-T2D were significantly higher than in New-T2D. Average GA values in Old-T2D subjects were 0.9 to 1.6% higher than in New-T2D subjects.
Figure 1. Differences of glycated albumin (GA) and GA/HbA1c ratios according to the duration of diabetes by HbA1c ranges.
(A) glycated albumin; (B) GA/HbA1c ratio. Data are shown as mean with SD (bars). *P<0.001 compared to those from subjects with New-T2D.
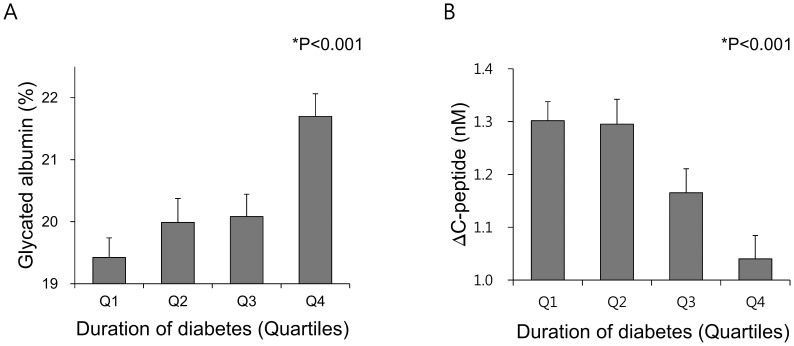
To assess the association between GA-related indices and diabetes duration, the duration of diabetes was categorized into quartiles. Consistent with the results shown in Fig. 1, GA (Fig. 2A) and GA/HbA1c ratios (Fig. S1) were significantly and sequentially increased across the quartiles of diabetes duration. However, as diabetes duration increased, the level of ΔC-peptide, an insulin secretory index of β-cells, was statistically decreased (Fig. 2B), indicating the gradual deterioration of reserve β-cell function over time.
Figure 2. Association between duration of diabetes and glycated albumin or C-peptide increment (ΔC-peptide).
(A) Difference of glycated albumin levelsaccording to the duration of diabetes; (B) Difference of ΔC-peptide levels according to the duration of diabetes. Data are shown as mean with SD (bars).
Glycated albumin was negatively correlated with insulin secretory indices
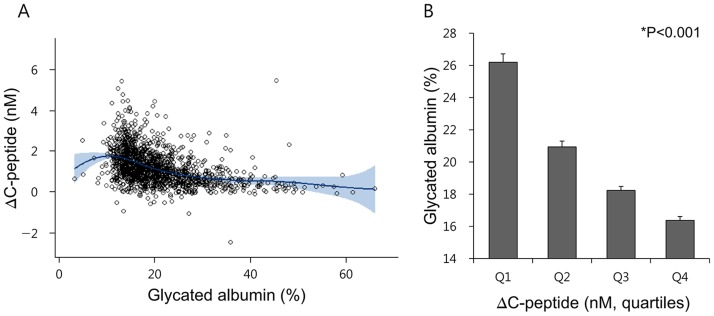
Considering the detrimental effects of diabetes duration on GA/HbA1c ratio and ΔC-peptide levels, correlation analysis was performed to assess the link between GA-related indices and parameters of β-cell functions (Table 2 and Fig. 3). Simple and partial correlation analyses both showed that GA levels were negatively related to insulin secretory indices such as HOMA-β, Δinsulin, and basal C-peptide, whereas basal insulin levels had no significant correlation with GA (Table 2 and Fig. S2). Duration of diabetes was also positively related to GA levels (r = 0.12, p<0.001), regardless of age. Strong inverse correlation was found between ΔC-peptide levels and GA (r = −0.41, p<0.001 in Table 2, or Fig. 3A) or GA/HbA1c ratios (r = −0.35, p<0.001, Fig. S3), regardless of age and gender. Values of both GA (Fig. 3B) and GA/HbA1c ratios (Fig. S3) were gradually decreased as quartiles of ΔC-peptide levels increased.
Table 2. Correlation analyses to determine the association between glycated albumin and other variables including insulin secretory indices and the duration of diabetes.
| Glycated albumin | ||||
| Simple correlation | Partial correlation* | |||
| r | P | r | P | |
| Age (years) | −0.010 | 0.680 | – | – |
| BMI (kg/m2) | −0.185 | <0.001 | −0.200 | <0.001 |
| Glucose, basal (mM) | 0.614 | <0.001 | 0.670 | <0.001 |
| Glucose, stimulated (mM) | 0.676 | <0.001 | 0.704 | <0.001 |
| Total cholesterol (mM) | 0.033 | 0.181 | 0.005 | 0.878 |
| HOMA-IR | 0.186 | <0.001 | 0.201 | <0.001 |
| HOMA-β | −0.072 | 0.006 | −0.120 | <0.001 |
| Insulin, basal (pM) | 0.026 | 0.314 | 0.029 | 0.355 |
| ΔInsulin (pM) | −0.295 | <0.001 | −0.318 | <0.001 |
| C-peptide, basal (nM) | −0.148 | <0.001 | −0.128 | <0.001 |
| ΔC-peptide (nM) | −0.412 | <0.001 | −0.442 | <0.001 |
| Albumin (g/L) | −0.091 | <0.001 | −0.108 | <0.001 |
| Creatinine (µM) | 0.026 | 0.306 | −0.005 | 0.882 |
| Duration of diabetes (years) | 0.123 | <0.001 | 0.120 | <0.001 |
Partial correlation* was conducted with adjustment for age and sex.
Figure 3. Association between glycated albumin and C-peptide increment (ΔC-peptide).
(A) A plot of spline curve for association between glycated albumin and ΔC-peptide levels. Data plotted by a spline curve (dark blue) and 95% confidence interval (light blue); (B) Difference of glycated albumin levels according to the ΔC-peptide levels.
The association between glycated albumin and C-peptide increment (ΔC-peptide) accounted for increased levels of glycated albumin in subjects with longer duration of diabetes
Multiple linear regression models were applied to determine the clinical and laboratory parameters associated with HbA1c or GA (Table 3). In model 1, which included clinically and statistically important variables except ΔC-peptide levels, duration of diabetes had a positive correlation with both GA (standardized [STD] β = 0.05, p = 0.02) and GA/HbA1c ratio (STD β = 0.05, p = 0.03, Table S1), but not with HbA1c levels (STD β = 0.04, p = 0.10). Contrary to glucose profiles, both age and serum albumin levels were negatively correlated with HbA1c levels, while BMI, total cholesterol, and albumin levels were negatively correlated with GA (model 1). After additional adjustment for ΔC-peptide levels in the existing model (model 2), the significant correlation of duration of diabetes with GA (STD β = 0.02, p = 0.37) or with GA/HbA1c ratio (STD β = 0.02, p = 0.43, Table S1) disappeared. However, ΔC-peptide level was strongly associated with both GA and GA/HbA1c ratio (STD β = −0.22 and −0.21, respectively; both p<0.001), which led to increments of R2 values (from 0.36 in Model 1 to 0.40 in Model 2). These findings remained consistent in further analysis with age-matched study population (Table S2).
Table 3. Multiple linear regression analyses to determine the variables associated with HbA1c or glycated albumin.
| HbA1c | Glycated albumin | |||||||
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| STD β | P | STD β | P | STD β | P | STD β | P | |
| Age (years) | −0.087 | <0.001 | −0.044 | 0.075 | −0.010 | 0.654 | 0.046 | 0.040 |
| Sex (F = 0, M = 1) | −0.030 | 0.329 | −0.010 | 0.751 | −0.022 | 0.428 | 0.006 | 0.825 |
| BMI (kg/m2) | 0.005 | 0.828 | 0.024 | 0.317 | −0.113 | <0.001 | −0.073 | 0.001 |
| Smoking (never = 0, ever = 1) | 0.013 | 0.633 | 0.012 | 0.661 | −0.010 | 0.679 | −0.013 | 0.579 |
| Glucose, basal (mM) | 0.300 | <0.001 | 0.236 | <0.001 | 0.351 | <0.001 | 0.305 | <0.001 |
| Glucose, stimulated (mM) | 0.428 | <0.001 | 0.414 | <0.001 | 0.419 | <0.001 | 0.392 | <0.001 |
| Total cholesterol (mM) | −0.001 | 0.978 | 0.012 | 0.599 | −0.050 | 0.018 | −0.038 | 0.069 |
| Insulin, basal (pM) | 0.049 | 0.035 | 0.028 | 0.240 | 0.070 | 0.001 | 0.038 | 0.079 |
| C-peptide, basal (nM) | −0.051 | 0.042 | 0.009 | 0.735 | −0.130 | <0.001 | −0.075 | 0.002 |
| Albumin (g/L) | −0.113 | <0.001 | −0.091 | <0.001 | −0.038 | 0.064 | −0.006 | 0.760 |
| Creatinine (µM) | −0.030 | 0.250 | −0.056 | 0.042 | 0.049 | 0.046 | 0.013 | 0.605 |
| Duration of diabetes (years) | 0.037 | 0.095 | 0.022 | 0.330 | 0.047 | 0.023 | 0.018 | 0.372 |
| ΔC-peptide (nM) * | – | – | −0.196 | <0.001 | – | – | −0.223 | <0.001 |
*log transformed.
STD β, standardized β coefficient; BMI, body mass index.
Discussion
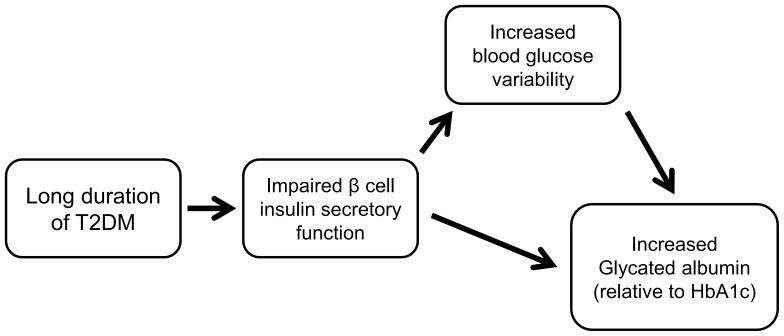
The present study observed that GA levels or GA/HbA1c ratios were elevated in Old-T2D subjects than in New-T2D subjects, regardless of their glycemic control status (HbA1c levels). With respect to insulin-related parameters, GA was negatively correlated with dynamic insulin secretory indices, but not with basal insulin levels. In addition, GA, but not HbA1c was significantly associated with duration of diabetes. This association did not remain after additional adjustment with ΔC-peptide in the regression model, indicating that insulin secretory function played a major role in this close relationship between GA and duration of diabetes. Although this is a cross-sectional study which cannot draw any causal relationships, we may suggest that GA values are indirectly associated with the long duration of T2D, because β-cell function gradually decreases as duration of diabetes increases (Fig. 4). Impaired insulin secretion from β-cells can increase blood glucose excursions, which is more sensitively reflected by GA compared to HbA1c.
Figure 4. A putative diagram of the relationship among glycated albumin, β-cell function and duration of diabetes.
HbA1c levels depend on glucose transport from plasma into erythrocytes and on intracellular glucose and protein metabolism, which indirectly reflect glycemic status. However, GA is not affected by extracellular–intracellular glucose dynamics [16], but directly produced by the glycation process in the plasma. In addition, GA level is not changed by serum concentration of albumin, because it is calculated by the glycated proportion of total serum albumin [17]. However, several factors can influence GA values. It is well known that obesity (BMI) is inversely associated with GA [18], which is consistent with our regression analyses. This result may be due to increased albumin catabolism induced by chronic micro-inflammatory conditions in obese subjects [19]. Likewise, GA levels are more inaccurate compared to HbA1c values in certain diseases affecting albumin metabolism [17]. During hyper-metabolic states of albumin, such as nephrotic syndrome, hyperthyroidism, and glucocorticoid treatment, GA increases in relation to blood glucose, while it decreases in subjects with diminished albumin catabolism, including liver cirrhosis and hypothyroidism [17]. To minimize the effects of erythrocytes or albumin turnover on HbA1c or GA values, we excluded subjects who had liver cirrhosis or CKD, pregnancy, steroid therapy, and hematologic disorders such as anemia.
In addition to the above listed pathologic conditions, the present study showed that GA or GA/HbA1c ratios were significantly increased in subjects with longer duration of T2D. Duration of diabetes was indirectly associated with GA and decreased β-cell function might be the linking factor between duration of diabetes and GA values. This was supported by our results from linear regression analyses showing that diabetic duration was associated with GA, of which association disappeared after adjustment with β-cell function index (ΔC-peptide). Among various insulin secretory indices, static parameters such as basal insulin or HOMA-β showed no or minimal relationships with GA, whereas dynamic parameters such as ΔC-peptide or Δinsulin had strong correlation with GA. To date, it is still not known why GA is closely correlated with endogenous insulin secretory function. GA is known to function as a pathogenic protein as well as an index for glycemic status, because it is an early precursor of advanced glycation end-products (AGE), which cause alterations in various cellular proteins and organelles, leading to apoptosis [5]. A recent report suggested that GA induced pancreatic β-cell dysfunction and death by disrupting cellular defense homeostasis [20]. Similarly, GA directly suppressed glucose-stimulated insulin secretion from rat pancreatic β-cells through impairment of intracellular glucose metabolism [21]. These may support the inverse relationship found between GA and β-cell function, because increased circulating levels of GA can lead to secretory dysfunction in β-cells, a pathophysiology known as glucolipotoxicity. In addition, considering the role of GA as a short-term (3-week) glycemic index, GA may reflect glucose fluctuation and postprandial glucose more sensitively than HbA1c [10], [22]–[24].
Our study has some distinct strengths. This was a rather large population-based study consisting of both newly diagnosed and long duration of T2D subjects, which could detect mild association of diabetes duration with GA and enhance the statistical reliability of the results. Our findings that GA is significantly elevated relative to HbA1c in certain patients with T2D, may raise serious issues regarding GA as an adequate index for monitoring glycemic control, especially in subjects with longer duration of diabetes, or severely impaired endogenous insulin secretion, such as during type 1 diabetes. Consistent with our results, Koga et al. reported that GA/HbA1c ratios were elevated in T2D patients treated with insulin, as well as in autoimmune acute-onset type 1 diabetic patients [8], [25]. This was attributed to strong links between GA and β-cell function. Because GA is less consistent in predicting blood glucose levels among subjects who have newly-diagnosed or long-standing T2D compared to HbA1c, clinicians should be cautious when using GA as a substitute index for monitoring glycemic status, especially in terms of long-term follow-up. Although GA is a superior index to HbA1c for subjects with anemia or CKD, duration of diabetes as well as insulin secretory function should be taken into consideration when interpreting GA values.
The current study has several potential limitations, which should be addressed by future research. First, the cross-sectional approach did not allow us to draw complete conclusions regarding time-dependent changes of GA and GA/HbA1c ratios in subjects with T2D or with transition in β-cell function. Second, assessment of endogenous insulin secretory function was confined to C-peptide-related indices. Due to lack of data from oral glucose tolerance tests, various insulin secretory parameters were not analyzed in this study. Third, other factors such as anti-diabetic medication, which may affect the variation in GA levels, were not evaluated. Lastly, duration of diabetes was unable to be assessed precisely in each individual due to slow-progressive characteristics of T2D, but was clinically defined, similar to previous studies [26]. Therefore, its pathognomic meaning is feeble.
In conclusion, the present study clearly showed that GA/HbA1c ratios were significantly elevated in subjects with longer duration of T2D, largely due to inverse relationships between GA and β-cell secretory indices. Clinicians should be careful in interpreting GA values as assessment indices of glycemic control in these individuals. Future investigation is warranted to elucidate the mechanism of the negative association between GA and β-cell function, and to validate the role of GA as a reliable index for long-term glycemic monitoring in various clinical conditions.
Supporting Information
Difference of GA/HbA1c ratios according to the duration of diabetes.
(DOCX)
Association between glycated albumin and insulin or C-peptide-related parameters. Correlation analysis of glycated albumin with basal insulin levels (A); Δinsulin levels (stimulated insulin – basal insulin) (B); HOMA-β (C).
(DOCX)
Correlation analysis of GA/HbA1c ratios with ΔC-peptide levels (A); Difference of GA/HbA1c ratios according to the ΔC-peptide levels (B).
(DOCX)
Multiple linear regression analyses to determine the variables associated with GA/HbA1c ratio.
(DOCX)
Multiple linear regression analyses to determine the variables associated with HbA1c or glycated albumin in age-matched study cohort (N = 1562).
(DOCX)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Data are available upon request because of an ethical restriction governed by the Institutional Review Board from Severance Hospital. Data are from the Diabetes Registry of Severance Hospital whose authors may be contacted at yholee@yuhs.ac.
Funding Statement
The authors have no support or funding to report.
References
- 1. UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352: 837–853. [PubMed] [Google Scholar]
- 2. Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, et al. (1995) Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 28: 103–117. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes A (2014) Standards of medical care in diabetes–2014. Diabetes Care 37 Suppl 1: S14–80. [DOI] [PubMed] [Google Scholar]
- 4. Lee EJ, Kim YJ, Kim TN, Lee WK, Kim M, et al. (2013) A1c variability can predict coronary artery disease in patients with type 2 diabetes with mean a1c levels greater than 7. Endocrinol Metab 28: 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim KJ, Lee BW (2012) The roles of glycated albumin as intermediate glycation index and pathogenic protein. Diabetes Metab J 36: 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inaba M, Okuno S, Kumeda Y, Yamada S, Imanishi Y, et al. (2007) Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol 18: 896–903. [DOI] [PubMed] [Google Scholar]
- 7. Koga M, Hashimoto K, Murai J, Saito H, Mukai M, et al. (2011) Usefulness of glycated albumin as an indicator of glycemic control status in patients with hemolytic anemia. Clin Chim Acta 412: 253–257. [DOI] [PubMed] [Google Scholar]
- 8. Koga M, Murai J, Saito H, Kasayama S (2010) Glycated albumin and glycated hemoglobin are influenced differently by endogenous insulin secretion in patients with type 2 diabetes. Diabetes Care 33: 270–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim D, Kim KJ, Huh JH, Lee BW, Kang ES, et al. (2012) The ratio of glycated albumin to glycated haemoglobin correlates with insulin secretory function. Clin Endocrinol (Oxf) 77: 679–683. [DOI] [PubMed] [Google Scholar]
- 10. Lee EY, Lee BW, Kim D, Lee YH, Kim KJ, et al. (2011) Glycated albumin is a useful glycation index for monitoring fluctuating and poorly controlled type 2 diabetic patients. Acta Diabetol 48: 167–172. [DOI] [PubMed] [Google Scholar]
- 11. Park KH, Kim KJ, Lee BW, Kang ES, Cha BS, et al. (2014) The effect of insulin resistance on postprandial triglycerides in Korean type 2 diabetic patients. Acta Diabetol 51: 15–22. [DOI] [PubMed] [Google Scholar]
- 12. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 13. Lee YH, Lee BW, Kwon HJ, Kang ES, Cha BS, et al. (2012) Higher morning to evening ratio in total dose of twice-daily biphasic insulin analog might be effective in achieving glucose control in patients with poorly controlled type 2 diabetes. Diabetes Technol Ther 14: 508–514. [DOI] [PubMed] [Google Scholar]
- 14. Kouzuma T, Usami T, Yamakoshi M, Takahashi M, Imamura S (2002) An enzymatic method for the measurement of glycated albumin in biological samples. Clin Chim Acta 324: 61–71. [DOI] [PubMed] [Google Scholar]
- 15. Ko S, Park S, Cho J, Shin K, Lee S, et al. (2012) Influence of the duration of diabetes on the outcome of a diabetes self-management education program. Diabetes Metab J 36: 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zafon C, Ciudin A, Valladares S, Mesa J, Simo R (2013) Variables involved in the discordance between HbA1c and fructosamine: the glycation gap revisited. PLoS One 8: e66696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koga M, Kasayama S (2010) Clinical impact of glycated albumin as another glycemic control marker. Endocr J 57: 751–762. [DOI] [PubMed] [Google Scholar]
- 18. Koga M, Otsuki M, Matsumoto S, Saito H, Mukai M, et al. (2007) Negative association of obesity and its related chronic inflammation with serum glycated albumin but not glycated hemoglobin levels. Clin Chim Acta 378: 48–52. [DOI] [PubMed] [Google Scholar]
- 19. Don BR, Kaysen G (2004) Serum albumin: relationship to inflammation and nutrition. Semin Dial 17: 432–437. [DOI] [PubMed] [Google Scholar]
- 20. Song YM, Song SO, You YH, Yoon KH, Kang ES, et al. (2013) Glycated albumin causes pancreatic beta-cells dysfunction through autophagy dysfunction. Endocrinology 154: 2626–2639. [DOI] [PubMed] [Google Scholar]
- 21. Shiraki T, Miura Y, Sawada T, Okada T, Sakuraoka Y, et al. (2011) Glycated albumin suppresses glucose-induced insulin secretion by impairing glucose metabolism in rat pancreatic beta-cells. Nutr Metab (Lond) 8: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koga M, Murai J, Morita S, Saito H, Kasayama S (2013) Comparison of annual variability in HbA1c and glycated albumin in patients with type 1 vs. type 2 diabetes mellitus. J Diabetes Complications 27: 211–213. [DOI] [PubMed] [Google Scholar]
- 23. Suwa T, Ohta A, Matsui T, Koganei R, Kato H, et al. (2010) Relationship between clinical markers of glycemia and glucose excursion evaluated by continuous glucose monitoring (CGM). Endocr J 57: 135–140. [DOI] [PubMed] [Google Scholar]
- 24. Ogawa A, Hayashi A, Kishihara E, Yoshino S, Takeuchi A, et al. (2012) New indices for predicting glycaemic variability. PLoS One 7: e46517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koga M, Murai J, Saito H, Aoki K, Kanehara H, et al. (2011) Glycated albumin levels are higher relative to HbA1c levels in people with autoimmune acute-onset type 1 diabetes mellitus than in people with type 2 diabetes mellitus at the time of diagnosis. Diabetes Res Clin Pract 94: e12–e14. [DOI] [PubMed] [Google Scholar]
- 26. Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA (1994) Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet 344: 283–287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Difference of GA/HbA1c ratios according to the duration of diabetes.
(DOCX)
Association between glycated albumin and insulin or C-peptide-related parameters. Correlation analysis of glycated albumin with basal insulin levels (A); Δinsulin levels (stimulated insulin – basal insulin) (B); HOMA-β (C).
(DOCX)
Correlation analysis of GA/HbA1c ratios with ΔC-peptide levels (A); Difference of GA/HbA1c ratios according to the ΔC-peptide levels (B).
(DOCX)
Multiple linear regression analyses to determine the variables associated with GA/HbA1c ratio.
(DOCX)
Multiple linear regression analyses to determine the variables associated with HbA1c or glycated albumin in age-matched study cohort (N = 1562).
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Data are available upon request because of an ethical restriction governed by the Institutional Review Board from Severance Hospital. Data are from the Diabetes Registry of Severance Hospital whose authors may be contacted at yholee@yuhs.ac.