A major advancement in obesity research over the past decade is the framing of obesity as a chronic inflammatory state. The nature of this inflammation is unique compared with classical inflammatory responses employed in host defense and pathogen recognition and has dramatic impacts on the metabolic control of nutrient flow. This “meta-inflammation” is low grade, long lasting, and enhanced in many metabolic tissues important in regulation of dietary nutrients, such as liver, muscle, pancreas, and adipose tissue.1 The cellular components of meta-inflammation include mediators of both innate and adaptive immunity and grow more diverse by the day. A study by Fabbrini et al2 in this issue of Gastroenterology advances our understanding of how interleukin (IL)-17–producing CD4+ lymphocytes may contribute to obesity-associated disease and provides a new perspective on the pathways that fan the flames of inflammation associated with metabolic disease.
Obesity generates a range of cellular stress in hypertrophic adipose tissue as well as in the fatty liver. Tissue leukocytes seem to respond directly to this stress, likely as a homeostatic mechanism to return the system to a normal baseline. However, as in many immunologic responses, dysregulation or chronic activation of inflammatory pathways can become pathologic if unchecked or the inciting stimulus is not removed. The epidemic of obesity and metabolic disease has drawn attention to these inflammatory responses with the hope that anti-inflammatory strategies that have been effective in mouse models may provide therapeutic benefits in clinical metabolic disease.3 Current challenges in the field are to better understand how leukocytes interact with each other in obesity and to unify data generated in animal models of obesity with what is observed in human obesity.
Current models suggest that leukocytes and inflammatory factors in adipose tissue contribute to dysfunctional adipocytes. In this setting, inflammation disrupts the functions of adipocytes in lipid storage, nutrient partitioning away from other organs (eg, liver), insulin responsivity, and the secretion of beneficial adipokines such as adiponectin. The mass of adipose tissue leukocytes in obese patients is substantial and it is likely that adipose tissue contributes to the majority of circulating cytokines in the serum that can impact other peripheral tissues. The article in this issue provides evidence to suggest that cytokines such as IL-17 and IL-22 may contribute to the systemic proinflammatory state in obese humans.
Although macrophages were among the leukocytes first identified in hypertrophied adipose tissue, the evidence for an important role for B and T lymphocytes in fat is growing. Both CD4+ and CD8+ T cells are found in adipose tissue and are expanded with obesity. In peripheral tissues, naïve CD4+ T cells can integrate local signals to differentiate down distinct pathways with discrete effector functions and cytokine production (eg, T helper [Th]1, Th2, Th9). Several reports have supported a role for interferon (IFN)-γ–expressing Th1 polarized T cells in promoting adipose tissue inflammation.4 Increased IFN-γ activity has been reported in adipose tissue in mice and humans5–8 as well as in circulating T cells from patients with type 2 diabetes.9 Furthermore, IFN-γ was shown to negatively impact glucose and lipid homeostasis in human adipocytes in vitro.10 Consistent with a pathogenic role of IFN-γ, whole body Ifng knockout mice were shown to be protected from insulin resistance with high fat diet feeding.8,11
However, several published reports have also suggested that Th17 polarized T cells were induced with obesity in mouse models.10 Th17 T cells are identified based on their preferential expression of cytokines that include IL-17 and IL-22 and play a critical role in the regulation of gut barrier function.11 As with many other Th subsets, dysregulation of Th17 activity can contribute to chronic inflammation in tissues like the gut and lung. Results of follow-up studies on IL-17 in obesity have been somewhat mixed. IL-17 was shown to be increased in obese women, although serum IL-17 did not correlate with central adiposity or insulin resistance.12 Two studies demonstrated a negative correlation between serum IL-17A and the metabolic syndrome or central adiposity.13,14 This is somewhat at odds with the observation that T cells from patients with type 2 diabetes are more susceptible to skewing toward a Th17 phenotype9 and that adipose tissue dendritic cells can induce a strong Th17 bias in naïve T cells.15 IL-17A was shown to be capable of inhibiting adipogenesis and triggering inflammation in vitro,16 suggesting that local effects of Th17 cells in fat promote adipocyte stress and block the formation of new adipocytes in the face of high caloric load.
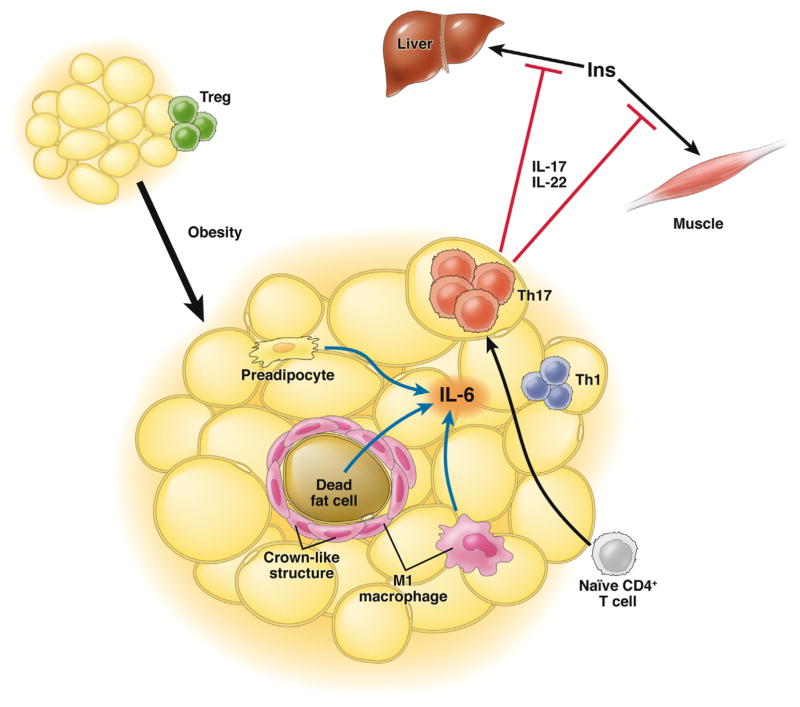
Fabbrini et al2 approached this problem from a non-biased perspective using subjects who underwent detailed metabolic phenotyping to identify the lymphocyte effector pathways that best correlate with metabolic dysfunction. A major strength of the study is the inclusion of samples from obese patients with normal metabolism based on hyperinsulinemic–euglycemic clamp data. This metabolically healthy obese population had intrahepatic triglyceride content and homeostatic model assessment of insulin resistance that did not differ from lean controls and permitted the identification of inflammatory signals associated with insulin resistance independent of body fat mass. They demonstrate a preferential skewing of adipose tissue T cells toward a Th17 phenotype in subcutaneous fat from obese metabolically abnormal subjects. This correlated with increased serum levels of the Th17-related cytokines IL-22 and IL-6. In contrast with other reports, IL-17 was at levels too low to accurately detect in serum. They went on to confirm the presence of IL-17 and IL-22 receptors in muscle and liver and demonstrate that activation of these receptors in vitro block insulin receptor signaling and impair normal glucose homeostasis. The model that extends from their results is summarized in Figure 1.
Figure 1.
T helper cell (Th)17 activation in obese adipose tissue. Adipose tissue in lean subjects is dominated by the presence of regulatory T cells (Treg). With obesity associated with metabolic dysfunction, naïve CD4+ T cells in subcutaneous adipose tissue are skewed toward at Th17 phenotype characterized by interleukin (IL)-17 and IL-22 production. Adipose tissue IL-6 may be an inducer of the Th17 phenotype and may originate from hypertrophic adipocytes, pre-adipocytes, and/or M1-like macrophages in adipose tissue. Th17 cytokines are produced in excess by adipose tissue and block insulin receptor signaling in liver and muscle and contribute to the metabolic dysfunction by impairing insulin action.
In conflict with some previous studies, Fabbrini et al2 did not identify a correlation between obesity and adipose tissue Th1 (IFN-γ+) or Th2 (IL-13+) balance. This may be related to differences in sampling site or in the protocols used to evaluate T cells in fat samples. Techniques to efficiently purify adipose tissue CD4+ cells in large numbers for definitive assays remain elusive in both human and mouse samples, thus the authors had to stimulate and expand the cells ex vivo before analysis, which potentially skewed the T-cell populations. In addition, there is significant plasticity in Th17 cells, which includes the induction of Th1 cell cytokines (Th17/Th1 cells) and direct generation of Th1 cells from Th17 precursors depending on the cytokines in the local environment.11
Another outstanding question is the source of IL-6 that may be a proximal trigger for Th17 T-cell generation. IL-6 has been long known to be induced in obese adipose tissue. Studies have suggested that preadipocytes, adipocytes, and macrophages may all be sources of IL-6 production in fat.17 The relative contribution of these cells to IL-6 production as well as the upstream signals that induce IL-6 remain unclear. It is possible that adipose tissue macrophages, in the context of their function as antigen-presenting cells,19 secrete IL-6 that participates in Th17 polarization. Regardless of its cellular source, IL-6 seems to be important in the generation of the Th17 lineage in the context murine obesity, as obese IL-6–null mice do not develop the Th17 bias observed in obese wild-type animals.10 The present data from Fabbrini et al2 suggest the possibility of a similar link between IL-6 and Th17 cells in human obesity.
The important inclusion and stratification of metabolic healthy and abnormal obese patients in this study permits the distinction between T-cell activation associated with obesity from that associated with metabolic dysfunction. This obviously leads to the question of why Th17 signals are induced in some obese patients and not others. Conversely, why are some patients protected from the induction of Th17 cells in fat? Alternatively, it may be that the location of adipose tissue inflammation is important. Future studies examining visceral or deep subcutaneous adipose tissue samples for Th17 cells are an important next step. Finally, rather than a single culprit, it is likely that multiple leukocyte subsets act in concert to determine metabolic phenotype; a decreased peripheral regulatory T cells/Th17 ratio, for example, is associated with an increased risk of type II diabetes in humans.18 These data, along with those from Fabbrini et al,2 suggest that the balance of T-cell subpopulations dictates metabolic output in obesity.
Future challenges in this field are to better unify what is observed in mouse models of obesity with what is observed in patients. This study suggests that the spotlight should shift away from Th1 polarization as a primary mediator of metabolic dysfunction or at the very least the beam should be widened. The authors did not evaluate whether regulatory T cells contribute to the phenotype of metabolically abnormal obese patients, and this would be an interesting next step to take in their studies. The challenge of unifying observations in mouse models and human studies is not unique to the fields of immunology or metabolism, but careful studies such as this can certainly move the field forward.
Acknowledgments
Funding
This work is supported by National Institutes of Health Grants DK074397 (RWO), DK095050 (RWO), DK097449 (RWO), DK090262 (CNL), and DK092873 (CNL).
Footnotes
Conflicts of interest
The authors disclose no conflicts.
Contributor Information
ROBERT W. O’ROURKE, Department of Surgery, University of Michigan, Ann Arbor, Michigan
CAREY N. LUMENG, Department of Pediatrics and Communicable Disease, University of Michigan, Ann Arbor, Michigan
References
- 1.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 2.Fabbrini E, Cella M, McCartney SA, et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology. 2013;145:366–374. doi: 10.1053/j.gastro.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 4.Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy: CD4+ T cells control glucose homeostasis. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffaut C, Zakaroff-Girard A, Bourlier V, et al. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol. 2009;29:1608–1614. doi: 10.1161/ATVBAHA.109.192583. [DOI] [PubMed] [Google Scholar]
- 6.Kintscher U, Hartge M, Hess K, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–1310. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 7.O’Rourke RW, Metcalf MD, White AE, et al. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-gamma in inflammation in human adipose tissue. Int J Obes (Lond) 2009;33:978–990. doi: 10.1038/ijo.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocha VZ, Folco EJ, Sukhova G, et al. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jagannathan-Bogdan M, McDonnell ME, Shin H, et al. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol. 2011;186:1162–1172. doi: 10.4049/jimmunol.1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winer S, Paltser G, Chan Y, et al. Obesity predisposes to Th17 bias. Eur J Immunol. 2009;39:2629–2635. doi: 10.1002/eji.200838893. [DOI] [PubMed] [Google Scholar]
- 11.Weaver CT, Elson EO, Fouser LA, et al. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol Mech Dis. 2013;8:477–512. doi: 10.1146/annurev-pathol-011110-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumarac-Dumanovic M, Stevanovic D, Ljubic A, et al. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes (Lond) 2009;33:151–156. doi: 10.1038/ijo.2008.216. [DOI] [PubMed] [Google Scholar]
- 13.Surendar J, Aravindhan V, Rao MM, et al. Decreased serum interleukin-17 and increased transforming growth factor-beta levels in subjects with metabolic syndrome (Chennai Urban Rural Epidemiology Study-95) Metabolism. 2011;60:586–590. doi: 10.1016/j.metabol.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Zizza A, Guido M, Grima P. Interleukin-17 regulates visceral obesity in HIV-1-infected patients. HIV Med. 2012;13:574–577. doi: 10.1111/j.1468-1293.2012.01014.x. [DOI] [PubMed] [Google Scholar]
- 15.Bertola A, Ciucci T, Rousseau D, et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61:2238–2247. doi: 10.2337/db11-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin JH, Shin DW, Noh M. Interleukin-17A inhibits adipocyte differentiation in human mesenchymal stem cells and regulates pro-inflammatory responses in adipocytes. Biochem Pharmacol. 2009;77:1835–1844. doi: 10.1016/j.bcp.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Harkins JM, Moustaid-Moussa N, Chung YJ, et al. Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3-L1 cells and C57BL/6J and ob/ob mice. J Nutr. 2004;134:2673–2677. doi: 10.1093/jn/134.10.2673. [DOI] [PubMed] [Google Scholar]
- 18.Zeng C, Shi X, Zhang B, et al. The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: relationship with metabolic factors and complications. J Mol Med (Berl) 2012;90:175–186. doi: 10.1007/s00109-011-0816-5. [DOI] [PubMed] [Google Scholar]
- 19.Morris DL, Cho KW, Delproposto J, et al. Adipose tissue macrophages function as antigen presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes. 2013 doi: 10.2337/db12-1404. Epub ahead of press. http://dx.doi.org/10.2337/db12-1404. [DOI] [PMC free article] [PubMed]