Proteins require correct folding to be functional and this is achieved primarily with the aid of chaperones. Since protein folding is an extremely intricate and error-prone process, it is highly susceptible to failure in the presence of stress. Misfolded proteins are not only non-functional but they also tend to form a large mass called a pre-amyloid oligomer (PAO). Formation of PAO is believed to be detrimental to cells because it interferes with the function of other nearby proteins through sticky interactions, which in turn induces ER stress and oxidative stress, culminating in global cellular and organ dysfunction, generally referred to as proteotoxicity and proteinopathy, respectively. Increasing lines of evidence suggest that protein misfolding contributes to the pathogenesis of many forms of cardiac disease and heart failure and, in this regard, they can be classified as proteinopathies. In order to control protein turnover (which can be as high as approximately 2% of total protein per day) and prevent accumulation of misfolded proteins, cardiomyocytes employ vigorous mechanisms for protein quality control (PQC) 1, including molecular chaperones, the unfolded protein response (UPR) in the ER and mitochondria, and the degradation mechanisms, namely the ubiquitin proteasome system (UPS) and autophagy (Figure). In the UPS, the 26S proteasome degrades damaged and/or misfolded proteins following tagging with ubiquitin to identify them for degradation 2, 3. Autophagy is a bulk degradation system in which damaged proteins and organelles are degraded by lysosomal proteases 4, 5. Despite the importance of PQC for the maintenance of proper function in the heart, the detailed molecular mechanisms by which the heart develops amyloid oligomers remain poorly understood and it is not clear whether modulation of such mechanisms could be a method of treatment in patients with heart disease..
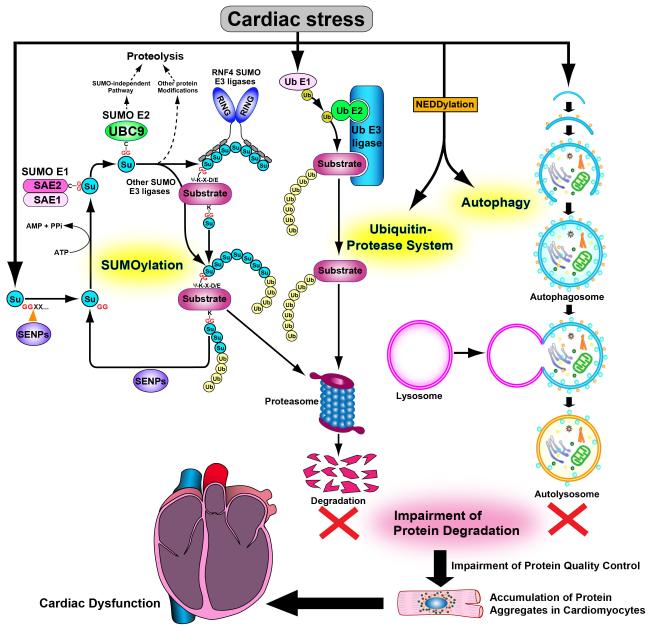
Figure. A diagram depicting the hypothesis that impairment of three targeted proteolytic mechanisms leads to cardiac dysfunction.
Damaged and/or misfolded proteins generated in increased amounts in response to various cardiac stresses are eliminated by a SUMOylation-mediated proteolytic mechanism, as well as by the ubiquitin-proteasome system and/or autophagy. Once these proteolytic mechanisms are impaired, damaged and/or misfolded proteins are accumulated as protein aggregates in cardiomyocytes, thereby damaging the cell and leading to cardiac dysfunction. Su: SUMO (small ubiquitin-related modifier), Ub: Ubiquitin, SAE1/2:SUMO-activating enzyme E1/2, UBC9: Ubiquitin-conjugating enzyme 9, RNF4: Ring finger protein 4, SENPs: Sentrin-specific proteases, G: Glycine, C: Cysteine, Ψ: hydrophobic amino acid, E: Glutamic acid, D: Aspartic acid, X: any amino acid.
In this issue of Circulation Research, Gupta et al demonstrate that SUMOylation, a form of posttranslational modification involving attachment of small ubiquitin-related modifier (SUMO) proteins, plays an important role in mediating PQC in cardiomyocytes by stimulating degradation through the UPS 6. SUMOylation is a process in which SUMO proteins are covalently attached to specific lysine residues in target proteins, thereby regulating various aspects of protein function, including transcription, subcellular localization, DNA repair and cell cycle 7. Unlike the UPS, however, it does not directly degrade its target proteins. To date, four SUMO isoforms (SUMO1 to SUMO4) have been isolated in mammalian cells. Like ubiquitination, SUMOylation occurs through a series of enzymatic reactions. In the first step, SUMO precursors are converted to the active form of SUMO protein, exposing a glycine (Gly)-Gly motif through cleavage of the carboxyl-terminal tails of the SUMO precursors via the hydrolase activity of sentrin-specific proteases (SENPs). The Gly-Gly motif of the mature SUMO protein is covalently conjugated with a conserved catalytic cysteine in the heterodimeric SUMO-activating enzyme E1 (SAE1/SAE2) via a thioester bond in an ATP-dependent reaction. UBC9 (ubiquitin-conjugating enzyme 9), the only SUMO-conjugating E2 enzyme identified in mammalian cells, then attaches the SUMO protein directly to a lysine located within the consensus sequence, Ψ-K-X-E/D (Ψ: hydrophobic amino acid, X: any amino acid), in the substrate. SUMO E3 ligases, such as the protein inhibitor of activated STAT (PIAS) family of proteins (PIAS1, PIAS3, PIASx and PIASy), ring finger protein 4 (RNF4), Ran binding protein 2 (RANBP2), and the polycomb protein 2 (Pc2), stimulate protein SUMOylation by associating with both UBC9 and substrates, thereby promoting poly-SUMO chain formation. Finally, SUMO proteins are removed from substrates by a family of isopeptidases called the SUMO-specific proteases, such as SENPs (Figure). Although SUMOylation is controlled primarily at the levels of E3 and SUMO proteases, the fact that UBC9 is the only E2 and that UBC9 is subjected to posttranslational modifications, such as oxidation, nitrosylation and SUMOylation, suggest the possibility that the function of UBC9 is regulated by stress and, in turn, it globally affects SUMOylation in cardiomyocytes in the heart.
Gupta et al found that expression of UBC9 is upregulated in response to the accumulation of misfolded proteins in cardiomyocytes and transgenic mouse hearts with overexpression of an αB-crystallin (CryAB) mutant, well-established models of proteotoxicity. UBC9 was also upregulated in the heart in response to pressure overload. Using gain- and loss-of-function experiments, Gupta et al have shown that UBC9 has a strong ability to eliminate accumulation of PAO by stimulating UPS-mediated degradation. The study suggests that upregulation of UBC9 is a compensatory mechanism to eliminate PAO in the desmin-related cardiomyopathic heart .
The molecular mechanisms by which UBC9 mediates degradation of PAO remain to be elucidated. Since elimination of PAO by UBC9 was attenuated in the presence of an inhibitor of the UPS, the protective effect of UBC9 is most likely mediated through stimulation of the UPS. Perhaps the most straightforward hypothesis is that SUMOylation of the constituents of PAO, such as CryAB(R120G), induces degradation through recruitment of RNF4, an E3 ubiquitin ligase. However, in theory, the effect of UBC9 could be mediated through SUMOylation of any molecule involved in PQC, which, in turn, affects the activity of the UPS and the accumulation of PAO. For example, autophagy is a master regulator of PQC and eliminates misfolded proteins and damaged organelles. Growing lines of evidence point to an important role for SUMO in regulating autophagy. Beclin1 forms a complex with Vps34, thereby promoting both autophagosome formation and autophagosome-lysosome fusion. Formation of the Beclin1-Vps34 complex is tightly regulated by various posttranslational modifications, such as phosphorylation 5, 8. The Beclin1-Vps34 complex physically interacts with acetylated Hsp70, which in turn associates with SUMO E3 ligase KAP1. Vps34 is then SUMOylated at its Lys840, thereby stabilizing the interaction of Vps34 with Beclin1 9.
Recent evidence suggests that NEDDylation, another posttranslational modification with ubiquitin-like protein NEDD8, also plays a critical role in PQC in cardiomyocytes by regulating both UPS and the autophagic pathway. Similar to SUMOylation, NEDDylation regulates a variety of biological processes, including DNA repair, cell cycle, signaling, nuclear transport and transcription. NEDD8 is conjugated with the heterodimeric NEDD-activating enzyme E1 (APP-BP1/Uba3) via a thioester bond in an ATP-dependent reaction. Subsequently, UBC12 (ubiquitin-conjugating enzyme 12), the NEDD-conjugating E2 enzyme, attaches directly to the NEDD8. NEDD8 then interacts with cullin to form a complex with cullin-based RING ligases (CRLs), a group of E3 ubiquitin ligases. The appropriate functioning of CRLs also requires deNEDDylation of cullin, catalyzed by the COP9 signalosome (CSN), a multiprotein complex consisting of 8 unique subunits (CSN1 through CSN8). Recently, the critical roles of CSN8/CSN in the cardiac PQC system have been revealed 10. Genetic ablation of the CSN8 gene resulted in impairment of both proteasomal function and autophagosome formation, which, in turn, caused cardiomyocyte necrosis and severe dilated cardiomyopathy. The results of the loss-of-function experiments suggest that SUMOylation and NEDDylation are not redundant. Although it is reasonable that important cellular functions, including UPS and autophagy, would be subjected to multiple layers of control, it would be worthwhile to investigate further the specific role of each mode of posttranslational modification in controlling proteotoxicity in cardiomyocytes.
Dysregulation of SUMOylation contributes to a number of congenital and acquired heart diseases 11. SUMOylation-deficient mutations in lamin A, a protein responsible for maintaining nuclear structure and function, are associated with familial dilated cardiomyopathy and abnormalities in the cardiac conduction system 12. Cardiac transcription factors essential for development, including GATA4, TBXs and Nkx2.5, are regulated by SUMOylation, and transduction of a non-SUMOylation mutant of Nkx2.5 in Nkx2.5+/− mice leads to congenital heart defects 13. SUMO1 is downregulated in failing hearts in humans and animals14. Both SUMO1+/− and SUMO1−/− mice exhibit congenital heart defects with high mortality, whereas the phenotype of these mice is rescued by transduction of the SUMO1 gene 15. SUMOylation of SERCA2a on two lysine residues, Lys480 and Lys585, is required for stabilization and enhancement of the activity of SERCA2a, thereby positively regulating cardiac contractility 14, 16, 17. The level of SUMO-specific protease 1 (SENP1) was increased after ischemia/reperfusion (I/R) and the infarct size after I/R was greater in SENP1+/− than in wild-type mice 18. SUMOylation also modulates cardiac ion channel activity and mitochondrial dynamics. The activities of both Kv2.1 and Kv1.5, the voltage-gated potassium channels, are suppressed by SUMOylation 19. Mitochondrial fission is regulated by SENP5-mediated SUMOylation of Drp1, a GTPase that promotes scission of the mitochondrial outer membrane 20. A cautionary note here is that, since SUMOylation has such diverse functions in the heart in addition to its direct effects upon PQC, modulation of SUMOylation may also affect PAO through many indirect mechanisms in vivo.
In summary, the study by Gutpa et al suggests that UBC9 is a promising therapeutic target for the proteinopathies through modulation of SUMOylation-mediated protein degradation, as speculated by Gupta et al in the article. However, additional investigations are needed to advance UBC9 as a target for PQC enhancement. First, whether the beneficial effect of UBC9 is mediated primarily through enhancement of SUMOylation remains to be demonstrated. UBC9 has SUMOylation-independent effects, including direct stimulation of the UPR, another mechanism of PQC. The authors discussed the possibility that UBC9 may directly reduce protein misfolding by acting on protein folding. Since cardiac stress downregulates the level of free SUMO proteins, augmentation of UBC9 alone may not be able to restore the level of SUMOylation in cardiomyocytes with proteotoxicity. It would be interesting to test the effect of another intervention to stimulate SUMOylation, including upregulation of E3 ligases and/or SUMOs. Second, if SUMOylation is in fact able to facilitate PQC, identifying the direct target of SUMOylation that is responsible for the improvement of PQC would dramatically advance our understanding as to how the formation of PAO is controlled. Identifying the specific E3 ligase and SUMO isoform responsible for the SUMOylation of the target may lead to development of more specific interventions. Finally, it remains unknown whether changes in SUMOylation are involved in the pathogenesis of proteotoxicity in more common forms of heart disease and heart failure. It would, therefore, be helpful to conduct in vivo experiments in conjunction with the generation of gain- and loss-of-function mouse models of SUMOylation. Although it is generally accepted that both aging and oxidative stress facilitate proteotoxicity, the signaling mechanisms in the heart through which the production of misfolded proteins is increased and/or PQC is impaired are poorly understood. Identifying these endogenous mechanisms would provide us with valuable information that will be useful in combating heart failure.
Acknowledgement
The authors thank Daniela Zablocki for critical reading of the manuscript.
Source of Funding
This work was supported in part by U.S. Public Health Service Grants HL67724, HL91469, HL102738, HL112330 and AG23039 (JS). This work was also supported by the Fondation Leducq Transatlantic Networks of Excellence (JS), JSPS KAKENHI Grant-in-Aid for Scientific Research (C) 26461126 (YM), and American Heart Association Scientist Development Grant 12SDG12070262 (YM).
Footnotes
Disclosures
None.
References
- 1.Hedhli N, Pelat M, Depre C. Protein turnover in cardiac cell growth and survival. Cardiovasc Res. 2005;68:186–196. doi: 10.1016/j.cardiores.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 2.Usui S, Maejima Y, Pain J, Hong C, Cho J, Park JY, Zablocki D, Tian B, Glass DJ, Sadoshima J. Endogenous muscle atrophy F-box mediates pressure overload-induced cardiac hypertrophy through regulation of nuclear factor-kappaB. Circ Res. 2011;109:161–171. doi: 10.1161/CIRCRESAHA.110.238717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maejima Y, Usui S, Zhai P, Takamura M, Kaneko S, Zablocki D, Yokota M, Isobe M, Sadoshima J. Muscle-specific RING finger 1 negatively regulates pathological cardiac hypertrophy through downregulation of calcineurin A. Circ Heart Fail. 2014;7:479–490. doi: 10.1161/CIRCHEARTFAILURE.113.000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhuiyan MS, Pattison JS, Osinska H, James J, Gulick J, McLendon PM, Hill JA, Sadoshima J, Robbins J. Enhanced autophagy ameliorates cardiac proteinopathy. J Clin Invest. 2013;123:5284–5297. doi: 10.1172/JCI70877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maejima Y, Kyoi S, Zhai P, Liu T, Li H, Ivessa A, Sciarretta S, Del Re DP, Zablocki DK, Hsu CP, Lim DS, Isobe M, Sadoshima J. Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2. Nat Med. 2013;19:1478–1488. doi: 10.1038/nm.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta MK, Gulick J, Liu R, Wang X, Molkentin JD, Robbins J. Sumo E2 Ligase UBC9 is Required for Efficient Protein Quality Control in Cardiomyocytes. Circ Res. 2014;115:xxx–xxx. doi: 10.1161/CIRCRESAHA.115.304760. in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flotho A, Melchior F. Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem. 2013;82:357–385. doi: 10.1146/annurev-biochem-061909-093311. [DOI] [PubMed] [Google Scholar]
- 8.Wei Y, Zou Z, Becker N, Anderson M, Sumpter R, Xiao G, Kinch L, Koduru P, Christudass CS, Veltri RW, Grishin NV, Peyton M, Minna J, Bhagat G, Levine B. EGFR-mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell. 2013;154:1269–1284. doi: 10.1016/j.cell.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Fiskus W, Yong B, Atadja P, Takahashi Y, Pandita TK, Wang HG, Bhalla KN. Acetylated hsp70 and KAP1-mediated Vps34 SUMOylation is required for autophagosome creation in autophagy. Proc Natl Acad Sci U S A. 2013;110:6841–6846. doi: 10.1073/pnas.1217692110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su H, Li J, Osinska H, Li F, Robbins J, Liu J, Wei N, Wang X. The COP9 signalosome is required for autophagy, proteasome-mediated proteolysis, and cardiomyocyte survival in adult mice. Circ Heart Fail. 2013;6:1049–1057. doi: 10.1161/CIRCHEARTFAILURE.113.000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Schwartz RJ. Sumoylation and regulation of cardiac gene expression. Circ Res. 2010;107:19–29. doi: 10.1161/CIRCRESAHA.110.220491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang YQ, Sarge KD. Sumoylation regulates lamin A function and is lost in lamin A mutants associated with familial cardiomyopathies. J Cell Biol. 2008;182:35–39. doi: 10.1083/jcb.200712124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim EY, Chen L, Ma Y, Yu W, Chang J, Moskowitz IP, Wang J. Expression of sumoylation deficient Nkx2.5 mutant in Nkx2.5 haploinsufficient mice leads to congenital heart defects. PLoS One. 2011;6:e20803. doi: 10.1371/journal.pone.0020803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E, Park WJ, Hajjar RJ. SUMO1-dependent modulation of SERCA2a in heart failure. Nature. 2011;477:601–605. doi: 10.1038/nature10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Chen L, Wen S, Zhu H, Yu W, Moskowitz IP, Shaw GM, Finnell RH, Schwartz RJ. Defective sumoylation pathway directs congenital heart disease. Birth Defects Res A Clin Mol Teratol. 2011;91:468–476. doi: 10.1002/bdra.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tilemann L, Lee A, Ishikawa K, Aguero J, Rapti K, Santos-Gallego C, Kohlbrenner E, Fish KM, Kho C, Hajjar RJ. SUMO-1 gene transfer improves cardiac function in a large-animal model of heart failure. Sci Transl Med. 2013;5:211ra159. doi: 10.1126/scitranslmed.3006487. [DOI] [PubMed] [Google Scholar]
- 17.Lee A, Jeong D, Mitsuyama S, Oh JG, Liang L, Ikeda Y, Sadoshima J, Hajjar R, Kho C. The Role of SUMO-1 in Cardiac Oxidative Stress and Hypertrophy. Antioxid Redox Signal. 2014 Jun 3; doi: 10.1089/ars.2014.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu J, Fan Y, Liu X, Zhou L, Cheng J, Cai R, Xue S. SENP1 protects against myocardial ischemia/reperfusion injury via a HIF1alpha-dependent pathway. Cardiovasc Res. 2014 Jul 31;pii:cvu177. doi: 10.1093/cvr/cvu177. [DOI] [PubMed] [Google Scholar]
- 19.Rougier JS, Albesa M, Abriel H. Ubiquitylation and SUMOylation of cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:22–28. doi: 10.1097/FJC.0b013e3181daaff9. [DOI] [PubMed] [Google Scholar]
- 20.Zunino R, Schauss A, Rippstein P, Andrade-Navarro M, McBride HM. The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J Cell Sci. 2007;120:1178–1188. doi: 10.1242/jcs.03418. [DOI] [PubMed] [Google Scholar]