Abstract
Chronic myeloid leukemia (CML) is a hematologic neoplasm with a progressive, ultimately terminal, disease course. In most cases, CML arises owing to the aberrant formation of a chimeric gene for a constitutively active tyrosine kinase. Inhibition of the signaling activity of this kinase has proved to be a highly successful treatment target transforming the prognosis of patients with CML. New tyrosine kinase inhibitors (TKIs) continue to improve the management of CML, offering alternative options for those resistant to or intolerant of standard TKIs. Here we review the pathobiology of CML and explore emerging strategies to optimize the management of chronic-phase CML, particularly first-line treatment.
Keywords: tyrosine kinase inhibition, chronic myeloid leukemia, molecular response, suboptimal response
INTRODUCTION
Chronic myeloid leukemia (CML) is a hematologic neoplasm characterized by unregulated proliferation of myeloid cells in the bone marrow. CML is observed in all age groups, although it occurs most commonly in the middle aged and elderly, with a median age of onset of 67 years [201]. The estimated annual incidence of CML is 1–2 per 100,000 population, and the disease accounts for approximately 15% of all cases of leukemia in adults in western populations [201,202].
The disease begins with a chronic phase, which if untreated will progress to an accelerated phase within 3–5 years in the majority of patients, followed by the terminal blast crisis phase. However, improved understanding of the pathobiology of CML and the advent of the tyrosine kinase inhibitor (TKI) imatinib have expanded treatment options beyond interferon-α (IFN-α) or stem cell transplantation and transformed the prognosis for patients with CML. The 5-year survival rate is now >90% with current treatment options [1]. Two further tyrosine kinase agents, dasatinib and nilotinib, have been approved as first-line therapy for patients presenting with early chronic-phase CML (CP-CML). TKIs have become established as the standard of care for patients diagnosed with CP-CML [2, 201].
The utility of imatinib can, however, be limited by toxicity, lack of adherence to the prescribed regimen, and resistance to this agent, which may be pre-existing (innate) or emerge during treatment (acquired). Although dasatinib and nilotinib may be useful treatments for patients intolerant of imatinib, they are ineffective in patients with a common imatinib resistance-conferring mutation (T315I). Consequently, important clinical questions remain to be addressed, including how best to deploy the currently approved TKIs in first-line treatment strategies for individual patients.
Here we provide an overview of the pathophysiology of CML and the evolution of the management of the disease. We also focus on some ways in which current and emerging treatment options can be better deployed to improve outcomes for patients with CP-CML.
PATHOPHYSIOLOGY OF CML
CML arises due to the formation of a chimeric gene (BCR-ABL) following a reciprocal chromosomal translocation between chromosomes 9 and 22 that gives rise to the “Philadelphia” (Ph) chromosome [3]. The Ph chromosome is evident in 95% of patients with CML, although a small proportion of patients harbor molecular rearrangements not involving the breakpoint cluster region (BCR) and the ABL1 gene [4]. The resulting abnormal gene expresses a functional protein with constitutive tyrosine kinase activity: BCR-ABL. Aberrant activation of multiple intracellular signaling pathways has been demonstrated in response to the presence of BCR-ABL, resulting in accelerated cell cycle progression and inhibition of DNA repair, which lead to abnormal maturation and genomic instability of hematopoietic stem cells [5]. BCR-ABL expression is also associated with activation of anti-apoptotic pathways (and hence resistance to apoptosis) and with downregulation of expression of cell adhesion proteins, which leads to reduced adhesion to the bone marrow extracellular matrix and increased cell motility [6].
The phases of CML – chronic, accelerated, and blast crisis – are defined based on clinical characteristics and laboratory findings (Table 1) [7–9]. The chronic phase of CML is characterized by the proliferation of differentiated myeloid progenitors and mature cells; the more advanced stages are distinguished by the accumulation of undifferentiated immature myeloblast cells. The mechanisms underlying the transition between phases are unclear but the loss of differentiation is accompanied by increased BCR-ABL expression, genomic instability and the appearance of additional chromosomal abnormalities, most commonly double Ph chromosome, chromosome 8 and 19 trisomies, and isochromosome 17q [10]. Common molecular alterations include mutations in the p53 tumor suppressor gene [10].
Table 1.
MD Anderson and World Health Organization definitions of accelerated phase and blast crisis in chronic myeloid leukemia [7–9]
| Phase | MD Anderson criteria | World Health Organization criteria |
|---|---|---|
| Accelerated |
|
|
| Blast crisis |
|
The majority of patients diagnosed with CML initially present in the chronic phase. They are usually asymptomatic, and diagnosis occurs following a routine blood test or one conducted for unrelated reasons that reveals an elevated white blood cell count. Untreated, CML will inevitably progress from the indolent chronic phase to the accelerated phase in 3–5 years, and then to blast crisis within 1 year. Once patients exhibit blast crisis, their anticipated survival is less than 12 months. However, intervention during the chronic phase of the disease can prolong/prevent progression to the accelerated stage and the ultimate progression to the rapidly fatal blast crisis phase [11].
FIRST-LINE PHARMACOTHERAPY FOR CML
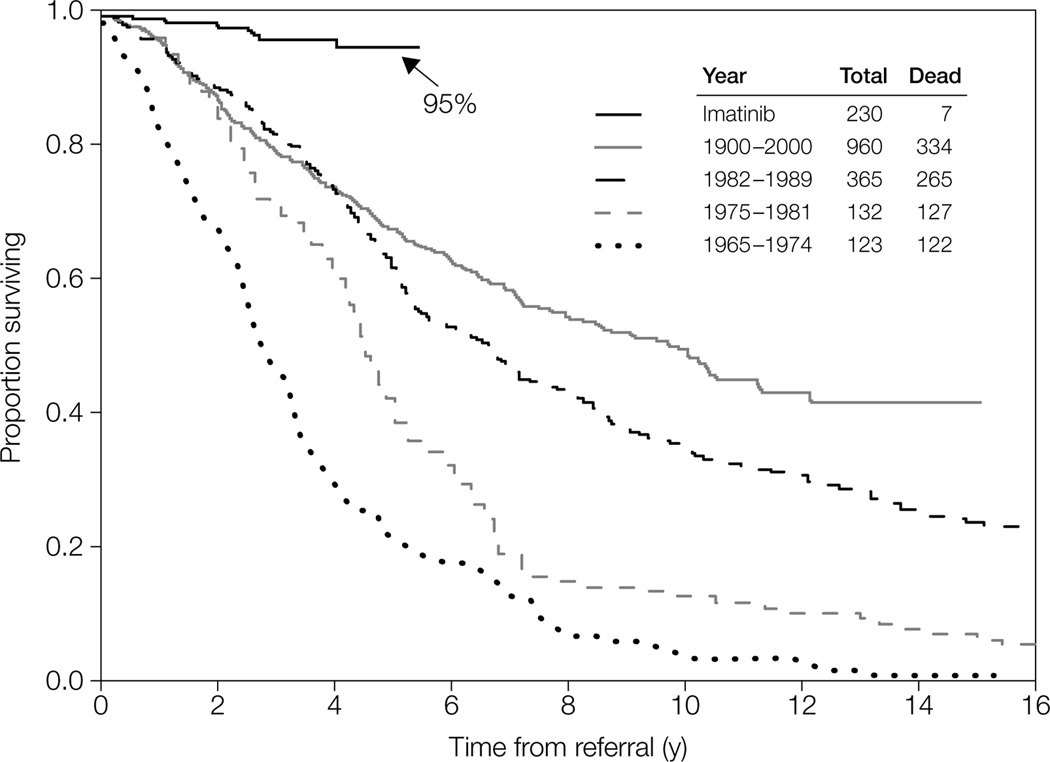
Before the advent of TKIs, treatment options included cytotoxic chemotherapy (cytarabine, busulfan, hydroxyurea) or IFN-α, and these treatments are still valuable and potentially curative for patients who do not respond to newer therapies. However, improved understanding of the pathophysiology of CML has opened the way for the development of agents specifically targeted toward the aberrant biologic process driving the disease. As a result of the introduction of the TKI imatinib, the treatment and natural history of CML have changed dramatically in recent years, with an improvement in the 5-year survival rate from little more than 20% to over 90% (Fig. 1) [12].
Figure 1.
Survival of patients with early chronic phase myeloid leukemia treated at the M. D. Anderson Cancer Center before and after the introduction of imatinib (reproduced from [12]).
Reprinted from Mayo Clinic Proceedings, 81(7), Quintas-Cardama A, Cortes JE. Chronic myeloid leukemia: diagnosis and treatment. 973–988 © (2006), with permission from Elsevier.
The goal of CML therapy is maintenance of remission and prevention of progression. Monitoring of response over the initial months of therapy is an integral component of the management of patients with CML, as it is essential to identify those with a suboptimal or lack of response to initial treatment. These patients will require an alternative treatment strategy if disease progression is to be halted. Response to treatment is measured in terms of hematologic, cytogenetic, and molecular parameters, as defined in Table 2 [2,13–15].
Table 2.
| Response type | Definition | Prognostic significance |
|---|---|---|
| Hematologic (HR) |
|
Failure to achieve an HR at 3 months predicts poor outcomes |
| Cytogenetic (CyR) |
|
Complete CyR (CCyR) is the best early predictor for good long-term outcomes Achievement of CyR at 3 and 6 months and of CCyR at 12 and 18 months are regarded as key targets |
| Molecular (MR) |
|
Although of prognostic value, the timing and grade of response remains controversial Loss of MR is also of prognostic significance |
Ph, Philadelphia chromosome.
Imatinib was first approved in the USA in 2001 for the treatment of the advanced phases of CML. Approval of this agent gave patients the potential to achieve a normal life span, although guidelines currently recommend therapy to be continued indefinitely [16,201]. Imatinib was established as the standard of care for patients with CP-CML based on the results of the pivotal International Randomized Study of Interferon and STI571 (IRIS) trial, which included 1,106 patients newly diagnosed with CML who were randomized to either imatinib or IFN plus cytarabine (Table 3) [13]. After a median follow-up of 19 months, the major cytogenetic response (MCyR) rate was statistically significantly higher with imatinib compared with the IFN–cytarabine combination (87.1% vs 34.7%, respectively; P < 0.001). The rate of freedom from progression to the accelerated phase at 18 months was also significantly higher in patients treated with imatinib than in those who received the IFN-α/cytarabine combination (96.7% vs 91.5%, respectively; P < 0.001). Six- and eight-year follow-up of patients who received imatinib in the IRIS trial demonstrated an overall survival (OS) rate of 88% and 85%, respectively [17,18]. An evaluation of data from the Imatinib Long-Term Side Effects trial has shown that for patients who achieve a stable cytogenetic response (CyR) with imatinib, OS is 95.2% at 8 years and is not statistically significantly different from that of the general population [19].
Table 3.
Overview of the pivotal Phase III clinical trial data for imatinib, dasatinib, and nilotinib in patients with newly diagnosed early chronic phase CML
| Trial | Treatment | No. of patients | Primary endpoint | Follow-up data | ||
|---|---|---|---|---|---|---|
| MMR | PFS | Overall survival | ||||
|
IRIS [13,17,18] |
Imatinib 400 mg qd vs IFN + cytarabine |
Imatinib: 553 IFN: 553 |
PFS at 18 months: Imatinib: 96.7% IFN + cytarabine: 91.5% (P < 0.001) |
6 years / 8 years Imatiniba: 93% / 92% |
6 years / 8 years Imatinib: 88% / 85% |
|
|
ENESTnd [22–25] |
Nilotinib 300 mg bid or Nilotinib 400 mg bid vs Imatinib 400 mg qd |
Nilotinib 300 mg bid: 282 Nilotinib 400 mg bid: 281 Imatinib: 283 |
MMR at 12 months: Nilotinib 300 mg bid: 44% Nilotinib 400 mg bid: 43% Imatinib: 22% (P < 0.001 for both comparisons) |
2 years / 3 years / 4 years Nilotinib 300 mg bid: 71% / 73% / 76% Nilotinib 400 mg bid: 67% / 70% / 73% Imatinib: 44% (P < 0.0001 vs both nilotinib doses) / 53% (P <0.0001 for both comparisons) / 56% (P < 0.0001 vs both nilotinib doses) |
2 years / 3 years / 4 years Nilotinib 300 mg bid: 98.0% / 96.9% / 96.1% Nilotinib 400 mg bid: 97.7% (P<0.05 vs imatinib) / 98.3% (P<0.05 vs imatinib) / 98.3% Imatinib: 95.2% / 94.7% / 94.7% |
2 yearsb / 3 yearsb 4 yearsb Nilotinib 300 mg bid: 97.4% / 95.1% / 94.3% Nilotinib 400 mg bid: 97.8% / 97.0% 96.7% Imatinib: 96.3% / 94.0% / 93.3% |
|
DASISION [26–28] |
Dasatinib 100 mg qd vs Imatinib 400 mg qd |
Dasatinib: 259 Imatinib: 260 |
cCCyR at 12 months: Dasatinib: 77% Imatinib: 66% (P = 0.007) Secondary endpoint: MMR at 12 months: Dasatinib: 46% Imatinib: 28% (P < 0.0001) |
2 years / 3 years Dasatinib: 64% / 68% Imatinib: 46% / 55% |
1 year / 2 years / 3 years Dasatinib: 96% / 93.7% / 93.7% Imatinib:97% / 92.1% / 93.2% |
1 year / 2 years / 3 years Dasatinib: 97% / 95.3% / 91.0% Imatinib: 99% / 95.2% / 90.9% |
Free from progression to accelerated phase or blast crisis
(c)CCyR, (confirmed) complete cytogenetic response; CML, chronic myeloid leukemia; DASISION, Dasatinib Versus Imatinib Study in Treatment-Naïve CML Patients; ENESTnd, Evaluating Nilotinib Efficacy and Safety in Clinical Trials – Newly Diagnosed Patients; IFN, interferon; IRIS, International Randomized Study of Interferon and STI571; MMR, major molecular response; PFS, progression-free survival; qd, once daily.
Unfortunately, a significant proportion of patients respond suboptimally or have no response to imatinib and they require an alternative treatment strategy to prevent progression to the accelerated phase. In IRIS for example, at the 8-year data cut-off 16% of patients had discontinued because of an unsatisfactory therapeutic response to imatinib treatment [18].
Two “second-generation” TKIs have been approved for the first-line treatment of CML (Table 3). Dasatinib was initially approved in 2007 for the treatment of patients who are either resistant to or intolerant of imatinib; nilotinib was subsequently approved for the same indication. Both dasatinib and nilotinib were approved as first-line treatment options in 2010 following demonstration of high CyR and molecular response (MR) rates. Among 50 patients with early CP-CML treated with dasatinib as initial therapy, 49 (98%) achieved a complete CyR (CCyR) with 41 (82%) achieving a major MR (MMR) after at least 3 months of follow-up [20]. Similarly encouraging response rates were reported for nilotinib, with CyR rates >96% and MR rates >76% in several independent cohorts of patients with CML [21].
In the pivotal Phase III trials for the TKIs, high progression-free survival (PFS) and OS rates were achieved in treatment-naïve patients (Table 3) [22–28]. The Phase III Evaluating Nilotinib Efficacy and Safety in Clinical Trials – Newly Diagnosed Patients (ENESTnd) study was designed to compare nilotinib 300 mg and 400 mg twice daily (bid) with imatinib 400 mg once daily in patients with newly diagnosed CP-CML [22]. Both nilotinib doses proved significantly superior to the standard imatinib dose, with almost twice as many patients in each of the nilotinib arms achieving MMRs at 12 months (imatinib 22%, nilotinib 300 mg bid 44%, nilotinib 400 mg bid 43%; P < 0.001 for both comparisons vs imatinib). Follow-up data showed that the higher rates of MMR were maintained after 2 years of treatment (Table 3) [23]. Three- and four-year follow-up data confirmed the superiority of nilotinib [24,25]. At 4 years the probability of progression to advanced- or blast-phase CML was significantly lower than with imatinib treatment (P < 0.05) and nilotinib was associated with significantly higher rates of MMR, MR4 and MR4.5 (ie 4 or 4.5 log reduction in the transcript level, respectively, according to the international scale [IS]) [25].
The Phase III DASISION trial compared standard-dose imatinib with dasatinib 100 mg in patients newly diagnosed with CP-CML. Higher rates of CCyR and MMR were seen after 12 months of treatment in patients randomized to dasatinib versus imatinib [26]. Significantly higher rates of confirmed CCyR (CCyR on two consecutive assessments; primary endpoint) and MMR were observed with dasatinib compared with imatinib at 12 months. Follow-up data showed that patients randomized to dasatinib continue to exhibit higher rates of both CCyR and MMR (Table 3) [27,28]. At 3 years, patients receiving dasatinib and imatinib achieved cumulative MMRs of 68% and 55% and MR4.5 (IS) rates of 36% and 22%, respectively [28].
Longer follow-up of these cohorts is now required to determine whether these earlier, deeper, sustained responses translate into long-term survival benefits.
Another second-generation TKI – bosutinib – has also been compared with imatinib in a Phase III study in patients with newly diagnosed CP-CML. After 1 year, although the MMR rate was significantly higher with bosutinib than imatinib (P < 0.001) and the times to achieve CCyR and MMR were significantly faster with bosutinib (both P < 0.01 vs imatinib), the study did not achieve the primary endpoint in that the CCyR rate was not significantly different for bosutinib compared with imatinib [29]. At 2 years, CCyR rates and Kaplan-Meier OS estimates were similar for the two TKIs [30]. Long-term outcomes are awaited.
The latest European Society for Medical Oncology guidelines and National Comprehensive Cancer Network Guidelines In Oncology (NCCN Guidelines®) (Table 4) recommend imatinib, nilotinib, or dasatinib as first-line therapy for patients newly diagnosed with CP-CML [2,201]. The European Leukemia Net (ELN) guidelines have also recently been updated to include recommendation of nilotinib or dasatinib, as well as imatinib, in this indication [16]. There is, however, little guidance on choosing between the three agents for individual patients, with only a reference in the NCCN Guidelines® to preliminary data from clinical studies that suggest that ‘patients with an intermediate or high risk score may preferentially benefit from dasatinib or nilotinib’ [201].
Table 4.
Current NCCN Guideline recommendations for the assessment and treatment of CML [201]
| Diagnosis of Ph+ or BCR-ABL+ chronic phase CML | |
|---|---|
| Discussion of treatment options: TKI, role of HSCT, clinical trial TKIs: imatinib 400 mg OR nilotinib 600 mg OR dasatinib 100 mg daily | |
| 3-month follow-upb | |
|
Partial cytogenetic response or BCR-ABL/ABL transcript level ≤10%(IS) |
|
|
Less than partial cytogenetic response or BCR-ABL transcript level >10% by qPCR (IS) or relapse |
|
| 12-month follow-upb | |
| Complete cytogenetic response |
|
| Partial cytogenetic response | |
| Minor or no cytogenetic response | |
| Cytogenetic relapse | |
| 18-month follow-upb | |
| Complete cytogenetic response |
|
|
Partial cytogenetic response or cytogenetic relapse |
|
qPCR should be conducted every 3 months in patients responding to treatment; after CCyR has been achieved it should be conducted every 3 months for 3 years and every 3–6 months thereafter.
In cases of inadequate response: evaluate patient compliance and drug-drug interactions, and (unless otherwise stated) perform mutational analysis.
Patients failing to respond to first-line imatinib should be treated with nilotinib, dasatinib, bosutinib or ponatinib in second-line setting; patients failing to respond to first-line nilotinib or dasatinib could be treated with another TKI (not imatinib) in the second-line setting.
Consider IFN/PEG-IFN, allogeneic HSCT, omacetaxine, or clinical trial for rare patients unable to tolerate TKI therapy.
Omacetaxine is treatment option for patients with resistance and/or intolerance to two or more TKIs.
Adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Chronic Myelogenous Leukemia V.4.2013. © 2013 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, NCCN GUIDELINES®, and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc.
HSCT, Hematopoietic stem cell transplantation; IFN, interferon; IS, international scale; PEG, pegylated; Ph, Philadelphia chromosome; qPCR, quantitative real time polymerase chain reaction; TKI, tyrosine kinase inhibitor.
Outcomes in Clinical Practice
The advent of imatinib and TKI-based therapy has undoubtedly transformed the treatment and life expectations of patients with CML. For now, these agents should usually be a physician’s first choice when initiating treatment for a patient presenting with this disease. Now that we have an effective treatment strategy that controls the disease and extends patients’ life expectancy in many cases, we can turn our attention to ensuring this outcome for all patients presenting with CML. Initial treatment interventions should be planned that not only optimize treatment outcomes but also minimize the need for future interventions. As such, it is necessary to first consider the long-term implications of the initial treatment choice and ask a number of questions: should initial management be early and aggressive treatment? Are there differences between current TKIs in terms of the need for or time to subsequent therapy?
Real-world studies are beginning to provide the answers to such questions and suggest that despite the efficacy of imatinib in gaining control of the disease, a significant proportion of patients newly diagnosed with CML and initiated on this agent will require alternative therapies [31–33]. In 2008, Lucas and co-workers reported the results of a population-based study in the northwest of England in which they found that the efficacy of imatinib was considerably lower in the real-world setting than previously observed in clinical trials [32]. Among 84 patients newly diagnosed with CP-CML and initiated on imatinib 400 mg, 17 withdrew from treatment during the first 2 years, at which point only 28 patients (33%) had achieved and maintained a CCyR [32]. Also in 2008, de Lavallade and co-workers reported the 5-year follow-up of a cohort of 204 adult patients newly diagnosed with CP-CML who were initiated on imatinib as first-line treatment [31]. As expected, the majority of patients (82.7%) achieved a CCyR and half achieved an MMR, with OS and PFS rates of 83.2% and 82.7%, respectively. At the 5-year follow-up, the probability of maintaining a CCyR while still receiving imatinib was 62.7% and one-quarter of all patients had discontinued imatinib treatment due to either an unsatisfactory response or toxicity issues [31]. A Europe-wide observational study, the Unmet Need in CML study, is underway. In 2010, Michallet and co-workers reported on the cohort of 654 French patients in this study [33]. The majority of patients (95.9%) were initiated on imatinib; however, 44% required dose modifications and 23% discontinued treatment during follow-up. Although some of the below-expected responses may result from poor patient adherence or non-optimized dosing, the results of these observational studies suggest that there is still considerable room for improvement in long-term outcomes for a significant proportion of patients presenting with CP-CML.
Should We Be More Aggressive From the Start of Therapy?
The timing and grade of the various levels of response to TKI therapy are regarded as key milestones in the ongoing management of patients with CML and offer valuable prognostic information (Table 2) [34–37]. Data from the IRIS trial in patients newly diagnosed with CP-CML and initiated on imatinib 400 mg underlie the rationale for the current milestones [1,18,38]. While failure to achieve a hematologic response after 3 months of treatment is a predictor for poor long-term outcome, CyR is regarded as the more relevant target and prognostic indicator. After 3 months of treatment, patients should have achieved at least a partial CyR and ideally a CCyR. The 5-year follow-up of the IRIS trial cohort found that patients with CP-CML initiated on imatinib 400 mg who failed to achieve a CCyR after 12 months of continuous treatment were at a significantly greater risk for disease progression than those with a CCyR (P < 0.001) [1]. A more recent 8-year follow-up of this cohort [18] supports a 3-month target of at least a CyR for such patients. The investigators found that those patients with at least a minor-partial CyR at 3 months were more likely to achieve a stable CCyR during follow-up than to experience a disease-related event including death, progression, or an increase in white blood cell count to >20 × 109/L [18]. Consequently, failure to achieve these targets should prompt re-evaluation of the patient and a change in therapy. This may be an increased dose of the initial therapy or a switch to an alternative therapy.
Recent data suggest that the relevant response targets for the second-generation TKIs may differ from those established for imatinib because high CCyR rates are achievable much earlier with the newer TKIs (see below) [39]. In a study of 167 patients newly diagnosed with CP-CML treated with second-generation TKIs in Phase II studies, 93% achieved a CCyR and 87% achieved an MMR. An analysis of outcomes found that attainment of a CCyR at 3 months was the critical response target in these patients, regardless of the level of MR achieved. At this timepoint, patients with less than a CCyR had a poor outcome. Thus for second-generation TKIs, the pivotal evaluation may be at 3 months when anything less than a CCyR indicates a need for further frequent monitoring or a therapy change.
Should We Strive for an Early Molecular Response?
Beyond CyR, the more stringent criteria of an MR may also offer prognostic information. Recently, much attention has focused on the potential for an early MR to be indicative of favorable long-term outcomes, including survival, and for guiding treatment decisions.
The potential significance of MMR has, of course, been investigated. Some studies noted that achievement of MMR at 12 or 18 months was not associated with any benefit in long-term OS, although other benefits were observed [40–42]. For example, in an analysis of 7-year follow-up data from the IRIS study, event-free survival (EFS) and progression to advanced/blast-phase CML could be predicted at 12 and 18 months by achievement of an MMR (BCR-ABL ≤0.1% [IS]) compared with no MMR. In contrast, OS could be distinguished at 6, 12, or 18 months only by an MR threshold of 10% (IS), not by MMR [41]. In the German CML Study IV of imatinib with or without IFN-α in newly diagnosed CP-CML, achieving an MMR by 12 months was associated with a significant increase in 3-year OS compared with not achieving an MMR (99% vs 95%; P = 0.016) [43]. However, the investigators noted that as a prognostic indicator, MMR at 12 months was no better than CCyR at 12 months. This highlights the fact that an MR needs to be considered in the context of the cytogenetic response. When simply comparing outcomes for patients with versus without an MMR, it must be borne in mind that those without an MMR comprise a heterogeneous group that includes patients with varying degrees of cytogenetic and hematologic response. Several studies have investigated the prognostic implications of achieving an MMR specifically in subsets of patients with a CCyR and found that although achieving a CCyR in response to imatinib was associated with a significant beneficial effect on survival, in those patients who had a CCyR, achieving an MMR did not confer significantly greater OS advantages [31,37,39–45]. However some association has been reported with improved PFS, EFS, time to transformation to accelerated/blast phase, and maintenance of CCyR or MMR [40–42,44,45].
Hanfstein and co-workers [46] further investigated the potential correlations between molecular and cytogenetic responses and survival in the German CML Study IV. They found that the persistence of BCR-ABLIS transcript levels >10% at 3 months after treatment initiation identified a group of patients who were at increased risk for death and disease progression. Patients with BCR-ABLIS >10% at 3 months had a 5-year survival rate of 87.0%, compared with 95.2% in patients with BCR-ABLIS ≤10% (P < 0.001) and 93.9% in patients with BCR-ABLIS >1%–10% (P = 0.012). At the 6-month landmark analysis, significant differences in 5-year survival were seen between patients achieving BCR-ABLIS ≤1% and those with >1%–10% (96.9% vs 89.6% survival; P = 0.002). The investigators suggest that failure to achieve BCR-ABLIS transcript levels of <10% at 3 months or ≤1% at 6 months when treated with imatinib should prompt treatment change.
In an exploratory analysis of data from the DASISION trial, Saglio and co-workers [47] reported that among patients newly diagnosed with CP-CML and initiated on TKI therapy (imatinib 400 mg or dasatinib 100 mg), those who achieved a reduction in BCR-ABLIS transcripts to ≤10% at 3 months had significantly improved 3-year survival outcomes compared with patients with BCR-ABL transcript levels >10%. Three-year OS for patients receiving imatinib was 96.0% versus 88.0% (P = 0.0036) in those with versus without BCR-ABLIS ≤10%, respectively; in patients receiving dasatinib, it was 95.9% versus 85.9% (P = 0.0348). The risk of transformation within 3 months was also decreased in patients with BCR-ABL ≤10% (vs >10%) and ≤1% (vs >1%) at 3 months. The advantage of an early MR to dasatinib treatment was also demonstrated in the Phase III SPIRIT 2 trial in patients newly diagnosed with CP-CML [48]. Patients with a BCR-ABL1:ABL1 ratio of >10% at 3 months had significantly poorer 2-year cytogenetic and molecular responses than patients achieving a ratio of ≤10%.
Similar results have been demonstrated for nilotinib. In the analysis of 3-year follow-up data from the Phase III ENESTnd study, treatment with either nilotinib or imatinib was associated with a higher OS rate in patients with a 3-month BCR-ABLIS transcript level ≤10% compared with those with a level >10% [49].
However, in another study it was noted that patients receiving imatinib plus pegylated (PEG)-IFN were at very low risk of disease progression even if they did not achieve a BCR-ABLIS transcript level of ≤10% at 3 months or 6 months [50].
The NCCN Guidelines currently recommend that if the BCR-ABL:ABL ratio is >10% (IS; by quantitative polymerase chain reaction [PCR]) at 3 months then the patient should be evaluated for treatment compliance and drug-drug interactions, and mutational analysis conducted, with the possibility of changing treatment (Table 4) [201].
Marin and co-workers have recently suggested that more precise predictive 3-month MR thresholds could be developed specific to the individual TKIs [48,51]. In 282 patients newly diagnosed with CP-CML and initiated on imatinib 400 mg (followed by dasatinib or nilotinib if imatinib failed), the authors identified BCR-ABL transcript thresholds for low and high risk for each clinical outcome investigated at the 8-year follow-up [51]. For OS, the BCR-ABL1:ABL1IS transcript threshold was identified to be 9.84% at 3 months, 1.67% at 6 months and 0.53% at 12 months. Attainment of a BCR-ABL transcript level below this threshold at 3 months was associated with a significantly increased 8-year OS rate (93.3% for patients with BCR-ABL levels below this threshold vs 56.9% for those above; P < 0.001). For PFS, EFS, CCyR, MMR and complete MR (CMR) the 3-month predictive thresholds were defined as BCR-ABL1:ABL1IS levels of 9.54%, 9.84%, 8.58%, 2.81%, and 0.61%, respectively. The authors noted that the 6- and 12-month assessments did not contribute further to the identification of patients at high risk of progression [51,52]. In the subset of patients who achieved a CCyR at 12 months, MMR at 12 and 18 months had no prognostic power, but a transcript level of 0.53% at 6 or 12 months was identified as having prognostic significance in this patient group [51].
In a similar analysis of a study in patients treated with dasatinib as a first-line treatment, BCR-ABL1:ABL1IS transcript thresholds of 2.2%, 0.92%, and 0.57% were identified as being optimally predictive for the 2-year cumulative incidence of CCyR, MMR, and MR4.5, respectively [48]. Marin and co-workers have suggested that a single measurement of BCR-ABL1 transcript levels 3 months after initiating TKI therapy is sufficient to identify patients at increased risk for adverse outcomes and to prompt treatment optimization [51].
Other investigators have reported that a 3-month MR (BCR-ABL transcripts ≤1%, 1–10%, and ≥10%) was not predictive of 3-year OS in patients treated with first-line TKIs (imatinib, nilotinib, or dasatinib), although the 3-month cytogenetic responses (≤0%, 1–35%, and >35% Ph+) significantly discriminated 3-year OS [53]. Notably, the outcome of analyses of OS, EFS, and transformation-free survival by molecular and cytogenetic responses was the same regardless of whether the analyses were based on 3-month or 6-month responses, with the exception of a 6-month MR predicting improved 3-year OS [53]. Given that the differences may be minimal between 3 and 6 months and that the long-term outcome of early switching is still unclear, for patients who have a suboptimal MR (but who retain a CCyR) it may be more beneficial to continue to monitor response until a trend becomes evident rather than implement a treatment switch at 3 months.
In 2009, we reported the results of a study designed to examine the clinical significance of minimal residual disease, ie the presence of detectable BCR-ABL transcript levels, in patients with CP-CML who had achieved a durable CCyR (>18 months) with imatinib treatment [45]. We showed that the majority of patients who achieve a stable CCyR and experience an increase in BCR-ABL transcript levels will remain in CCyR; however, a subset of these patients will lose an MMR or will never achieve an MMR, and it is these patients who are most at risk for subsequent CML progression. In terms of clinical practice, these results suggest that, in general, cytogenetic and molecular monitoring every 6 months is sufficient for patients with an MMR. More frequent monitoring (every 3 months) and possibly treatment escalation might be considered for those who achieve a CCyR but not an MMR and who exhibit a ≥1 log increase in BCR-ABL transcript levels, and for those who lose an MMR. In clinical practice, modest increases in BCR-ABL transcript levels revealed by molecular monitoring in patients with a CCyR should not automatically prompt a change in treatment – not least because of assay variability. Such an intervention could result in an unnecessary increase in toxicity or switch from a still-effective treatment. The NCCN Guidelines note that changes of therapy solely on the basis of rising BCR-ABL levels should only be considered within clinical trials [201].
The study results discussed above have implications for both when to monitor response and when to change treatment. Clearly, as treatment evolves and more long-term analyses become available, there is an ongoing need to identify the most relevant prognostic indicators and adapt the milestones accordingly.
Stepping Up First-Line Therapy
Increasing the dose
Currently, three agents are approved for the first-line treatment of CP-CML – imatinib, dasatinib, and nilotinib. The majority of patients with CP-CML will start their TKI therapy with a standard imatinib dose of 400 mg daily. However, as noted above, this regimen is less than optimal for a considerable proportion of patients. These patients will not achieve the depth of response that is associated with the best long-term outcomes. Should we then consider an alternative or more aggressive initial regimen as the first-line treatment of choice for patients newly diagnosed with CP-CML?
A number of studies have examined the benefit of higher initial doses of imatinib for newly diagnosed patients (Table 5) [43,54–59]. In 2008, Hughes and co-workers reported the results of a trial of imatinib 600 mg daily with escalation to 800 mg for suboptimal response in patients with newly diagnosed CP-CML [54]. This study showed that superior responses were achieved in patients who tolerated the higher imatinib doses. For example, the MMR rate was 55% and 77% at 12 and 24 months, respectively, after treatment initiation in patients able to maintain an average daily dose of 600 mg compared with 32% and 53%, respectively, in those with an average daily dose below 600 mg (P = 0.037 and P= 0.016, respectively). However, only 60 of the initial 103 patients were able to maintain an average daily dose of 600 mg during the first 6 months (reasons for not maintaining this dose included toxicity, withdrawal from treatment, and imatinib resistance). Somewhat better results were reported by Kantarjian and co-workers in a cohort of newly diagnosed patients initiated on imatinib 400 mg bid [55]. Overall, 90% of patients achieved a CCyR, a rate that was significantly higher than in a historical cohort of patients treated with the standard imatinib dose (400 mg daily; P = 0.0005). In this study, 82% of patients continued to receive imatinib ≥600 mg daily [55]. A small cohort of patients with newly diagnosed CP-CML, the majority of whom (70%) were at low risk for disease progression, achieved an MMR with imatinib 400 mg bid more rapidly than historical cohorts treated with the standard imatinib dose [51]. Similarly, a Phase II study of high-dose imatinib in patients with previously untreated CP-CML at intermediate risk for disease progression achieved high rates of both CCyR and MMR more rapidly than a historical cohort (IRIS) and only three patients progressed to accelerated-phase disease [57]. Studies have also been conducted to directly compare standard- and high-dose regimens [58,59].
Table 5.
Overview of the clinical trial data to support high-dose imatinib or TKI combination therapy as an alternative first-line option for patients newly diagnosed with chronic phase CML
| Study | Treatments | No. of patients | Efficacy | Tolerability/acceptability |
|---|---|---|---|---|
| High-dose imatinib | ||||
| Hughes et al. 2008 [54] | Imatinib 600 mg with dose escalation to 800 mg for suboptimal response |
103 | Cumulative CCyR
Cumulative MMR
|
Only 60 patients were able to maintain an average daily dose of 600 mg |
| Kantarjian et al. 2004 [55] |
Imatinib 400 mg twice daily |
114 | CCyR: 90% Estimated 2-year survival: 94% |
More frequent myelosuppression vs standard-dose imatinib 82% of patients were able to continue at ≥600 mg daily |
| Cortes et al. 2009 [56] RIGHT study |
Imatinib 400 mg twice daily | 115 (Sokal low risk) |
MMR
|
Most frequent AEs were myelosuppression, rash, fatigue, and musculoskeletal symptoms |
| Castagnetti et al. 2009 [57] |
Imatinib 400 mg twice daily |
78 (Sokal intermediate risk) |
Cumulative CCyR
Three patients progressed during a median follow-up of 24 months |
Incidence of AEs was slightly higher vs standard-dose imatinib in historical cohorts |
| Baccarani et al. 2009 [58] |
Imatinib 400 mg or 800 mg daily |
216 (Sokal high risk) |
CCyR at 12 months
No difference in MR rate at any timepoint |
Only 25 patients were able to tolerate the 800 mg daily dose |
| Cortes et al. 2010 [59] | Imatinib 400 mg or 800 mg daily |
476 | MMR at 12 months
MR was achieved faster in the 800 mg arm with higher rates at 3 and 6 months |
Higher AE burden associated with the 800 mg dose including grade 3 and 4 hematologic toxicities |
| Hehlmann et al. 2011 [43] |
Imatinib 400 mg daily, 800 mg daily, or 400 mg daily + IFN-α |
1014 | MMR at 12 months
|
Similar frequency of grade 3 and 4 AEs in all three arms |
| Combination TKI regimens | ||||
| Cortes et al. 2011 [64] | Imatinib 800 mg daily alone or in combination with PEG-IFN-α-2b |
94 | CCyR rates
MMR rates
|
PEG-IFN-α-2b was discontinued in all patients due to tolerability issues |
| Simonsson et al. 2011 [65] |
Imatinib 400 mg daily alone or in combination with PEG-IFN-α-2 |
112 (Sokal low or intermediate risk) |
MMR at 12 months
|
61% of patients in the combination arm discontinued PEG-IFN-α-2b due to tolerability issues |
| Preudhomme et al. 2010 [66] | Imatinib 400 mg daily alone or in combination with PEG-IFN-α-2a or cyt; or imatinib 600 mg daily |
636 | MMR at 12 months
SMR at 12 months
|
PEG-IFN-α-2a associated with higher rates of grade 3/4 rash, depression, asthenia and edema vs other treatments; higher rates of grade 3/4 neutropenia and thrombocytopenia vs imatinib 400 mg |
AE, adverse event; CCyR, complete cytogenetic response; CML, chronic myeloid leukemia; cyt, cytarabine; IFN, interferon; MR, molecular response; PEG, polyethylene glycol; RIGHT, Rationale and Insight for Gleevec High-Dose Therapy; SMR, superior molecular response (≥4 log10 reduction from standardized baseline level); TKI, tyrosine kinase inhibitor
Cortes and co-workers [59] reported the results of a Phase III study that directly compared the standard imatinib starting dose (400 mg) with a high-dose regimen of 800 mg daily in 476 patients newly diagnosed with CP-CML. They demonstrated that CCyR and MMR rates were comparable after 1 year of treatment, but that both endpoints were achieved earlier with the 800 mg regimen. The overall adverse-event burden was higher with the 800 mg dose, including rates of grade 3 and 4 hematologic toxicity. Baccarani and co-workers [58] compared the standard- and high-dose imatinib regimens in patients at high risk for disease progression. No differences were found in the rate of CCyR or MMR and the authors concluded that the results did not support the routine use of an 800 mg starting dose even for patients at high risk for disease progression. Most recently, Hehlmann and co-workers [43] reported the results of a study comparing imatinib 800 mg, imatinib 400 mg, and imatinib 400 mg plus IFN-α as initial therapy for patients with CP-CML. The protocol encouraged dose adaptation to avoid higher-grade toxicity, and patients were followed for 3 years. Early high-dose imatinib with subsequent dose adaptation (median dose 628 mg/d) to manage high-grade toxicities was associated with a higher rate of MMR at 12 months than either of the two standard regimens (59% vs 44% for the imatinib 400 mg regimen [P < 0.001] and 46% for the imatinib 400 mg plus IFN-α regimen [P = 0.002]). The patients treated with dose-optimized imatinib also achieved CMR4.5 in a shorter time than those receiving the standard dose [60].
Changing the treatment
An alternative to high-dose imatinib might be to initiate patients on dasatinib or nilotinib. Indeed, recent data suggest that the newer TKIs may offer deeper and more durable responses than imatinib [61]. Both agents have been compared directly with standard-dose imatinib in Phase III studies and were found to lead to earlier and sustained CCyRs and a more rapid reduction in BCR-ABL transcript levels (Table 3). However, a considerable challenge remains in understanding the differences between the three approved TKIs because of the non-uniform definitions of PFS (or EFS) used in their pivotal trials [34]. An analysis of outcomes for 435 patients with early CP-CML treated with imatinib, dasatinib, or nilotinib revealed that PFS ranged from 81% to 96% depending on the definition used [34].
Combination treatment
Combining a TKI with another drug may also be a feasible first-line treatment choice. However, while mathematical and theoretical modeling of the combination of two TKIs suggest that such an approach should be viable [62,63], clinical trial data are currently lacking. Data are, however, available for the combination of a TKI with agents from a different drug class such as PEG-IFNs (Table 5) [64–66]. The addition of PEG-IFN-α-2b to standard-dose imatinib significantly improved the MMR rate after 12 months of treatment (from 54% to 82%; P = 0.002) in a cohort of patients with CP-CML at low or intermediate risk for progression [65]. Cortes and co-workers [64] evaluated the ability of combining high-dose imatinib with PEG-IFN-α-2b to improve CMR rates in 94 patients with CP-CML. However, this study failed to demonstrate any improvement versus high-dose imatinib alone, mainly owing to toxicity issues; for this reason PEG-IFN-α-2b was eventually discontinued in all patients.
PEG IFN-α-2a has also demonstrated efficacy as monotherapy in patients with CP-CML, achieving higher 12-month CyR rates than unpegylated IFN-α-2a [67]. A Phase III trial conducted by Preudhomme and co-workers [66] showed that treatment with PEG-IFN-α-2a in combination with imatinib 400 mg was associated with a significantly higher 12-month MMR rate compared with imatinib alone (57% vs 38%; P < 0.001) in patients with untreated CP-CML. MR rates were also higher with the imatinib plus PEG-IFN-α-2a combination treatment than with imatinib 600 mg alone or imatinib 400 mg plus cytarabine. Rates of grade 3 or 4 neutropenia and thrombocytopenia were significantly higher with the IFN combination than with imatinib 400 mg alone (P < 0.001 and P = 0.002, respectively). Early data from a Phase II study also suggest a good response to the combination of PEG-IFN-α-2a and nilotinib, although transient grade 3 or 4 hematologic toxicities were common in the first few months of treatment [68].
Improving tolerability
Tolerability remains a significant concern when considering strategies to improve early response rates in patients newly diagnosed with CP-CML. Emergence of adverse events can lead to clinically necessary withdrawal of treatment and can also result in poor patient persistence with therapy [69]. For high-dose imatinib, improved response rates appear to be achieved at the expense of an increased adverse-event burden and an impact on short-term quality of life, even with protocols designed to minimize the occurrence of high-grade toxicities. Similarly, adverse-event issues may limit the suitability of combining even standard-dose imatinib with PEG-IFN-α. The long-term safety of TKI treatment must also be considered but long-term data are not yet available for the TKIs and current recommendations are to maintain TKI treatment indefinitely. Toxicities may emerge with continuing treatment, for example there have been reports of hepatic failure with imatinib [70,71], pulmonary arterial hypertension with dasatinib [72,73], and peripheral arterial occlusive disease with nilotinib [74].
PHARMACOLOGIC OPTIONS FOR PATIENTS WITH A SUBOPTIMAL OR NO RESPONSE TO THEIR FIRST-LINE THERAPY
Patients who respond to their initial treatment but do not reach the response targets as defined in current treatment guidelines for CML [16,201] can be described as having a suboptimal response. Reasons for suboptimal or lack of response may include drug resistance [75,76], tolerability issues, and poor patient adherence to treatment in clinical practice [77–80]. In cases of inadequate response or loss of response, guidelines recommend evaluation of patient compliance and possible drug-drug interactions, and mutational analysis [16,201]. There are many possible reasons for resistance to TKIs, but mutations in the BCL-ABL kinase domain are believed to be a key cause. A full review of the topic is beyond the scope of this article, however, for a few specific BCR-ABL mutations associated with resistance to TKIs there are recommendations for appropriate treatment options based on in vitro and clinical evidence [201]. BCR-ABL-independent mechanisms also contribute to resistance and another cause of TKI failure is thought to be the activation of alternative intracellular signaling pathways. Most notably, the SRC family kinases (SFKs) – in particular LYN and HCK – have been implicated in imatinib resistance [81–85] and SFK activation has been demonstrated in more than 50% of cases of TKI resistance in patients with CML [86,87].
Given the relationship between the depth of early responses and long-term outcomes discussed above, it is important to optimize treatment early. The most appropriate treatment strategy in the case of suboptimal response to imatinib is still under evaluation [88]. However, potential strategies are similar to those discussed above as alternative first-line approaches to standard-dose imatinib, and may include increasing the dose [89], introducing an additional agent such as another TKI or an agent from another drug class, for example IFN, or switching to an alternative TKI.
A switch to a second- or third-generation TKI has been explored as a potential option for patients responding suboptimally to an initial course of imatinib [90–92]. Preliminary data in 30 patients reported by Hyun-Gyung and co-workers [90] found that switching to nilotinib offered a significantly higher MMR rate at 12 months compared with an increase from a standard to a high dose of imatinib (59% vs 27%, respectively; P = 0.047). Similarly, the Therapeutic Intensification in De Novo Leukemia (TIDEL)-II study investigators reported data from two sequential cohorts of 105 patients, both of which were initiated on imatinib 600 mg daily but which employed different strategies if a response was not obtained. The patients who switched to nilotinib demonstrated higher rates of MMR at 12 months than those who had their imatinib dose increased to 800 mg (69% vs 47%, respectively) [92]. However, among the patients who failed to attain BCR-ABL levels ≤10% at 3 months, only 16% achieved MMR (IS) and none achieved MR4.5 despite switching at as early as 3 months [93].
The newer TKIs bosutinib [94–96] and ponatinib [97] potentially expand the TKI treatment options; both were recently approved by the US Food and Drug Administration (FDA) for the treatment of CML in patients with resistance or intolerance to prior TKI therapy. In an open-label phase I/II study, bosutinib was investigated as second-, third-, or fourth-line treatment, following other TKIs, in CP-CML. In patients who had previously only been treated with imatinib, the 2-year estimates of OS and PFS in patients treated with bosutinib were 92% and 79%, respectively [95]. After a minimum follow-up of 36 months, a CCyR was achieved or maintained in 48% of imatinib-resistant patients and 51% of imatinib-intolerant patients [96]. In patients who had been treated with imatinib followed by dasatinib and/or nilotinib, the 2-year estimates of OS and PFS with bosutinib treatment were 83% and 73%, respectively. At a median follow-up of 28.5 months, CCyR was achieved by 24% of patients [94]. Ponatinib – a “third-generation” TKI – has been investigated in a Phase II open-label study in patients with CML who were resistant to or intolerant of dasatinib or nilotinib or who were positive for the T315I mutation. After a minimum of 12 months’ follow-up, 56% of patients with CP-CML achieved an MCyR and 46% achieved a CCyR [97]. Of great interest is the finding that 70% of patients with the T315I mutation achieved an MCyR.
Recent evidence suggests that the level of response to initial TKI therapy may have prognostic significance with regard to the outcomes that can be expected from a second-line TKI regimen. For example, a recent study of patients with CML treated with second-generation TKIs after imatinib failure demonstrated that patients without a previous CyR to imatinib are unlikely to respond to further TKI therapy [91]. Alternative non-TKI-based treatment strategies may thus be required for these patients. Emerging options in this case include the cytotoxic agent omacetaxine mepesuccinate (“omacetaxine”) [98–104].
Omacetaxine represents an alternative treatment strategy to current TKI-based approaches, as this agent induces apoptosis by inhibition of protein synthesis. In a Phase II/III study in patients with resistance and/or intolerance to at least two TKIs, omacetaxine achieved a complete hematologic response in 80% of patients with CP-CML, MCyR in 20% and MMR in 10% [100]. A second Phase II study in patients who had failed to respond to imatinib therapy and who carried the T315I mutation, demonstrated that approximately 23% of patients with CP-CML achieved an MCyR, including 16% with a CCyR, and 77% achieved or maintained a hematologic response [102]. Long-term follow-up of patients with CP-CML from these studies demonstrated sustained cytogenetic responses [103]. There is also some evidence that omacetaxine may be active against leukemic stem cells (LSCs) [98,104]. Omacetaxine has recently been approved by the FDA for the treatment of adult patients with chronic- or accelerated-phase CML with resistance and/or intolerance to two or more TKIs.
OPTIONS FOR PATIENTS WHO RESPOND WELL TO FIRST-LINE THERAPY
A number of studies suggest that, with careful molecular monitoring, treatment can be stopped in some patients who respond well to their first-line therapy and achieve and maintain a CMR (also termed undetectable minimal residual disease [UMRD] or molecularly undetectable leukemia). This may be a preferred option for some patients for a variety of reasons, such as the wish to conceive in female patients; long-term safety concerns; drug resistance; and cost. Data from the Stop Imatinib (STIM) study in 100 patients with CML who achieved and maintained a CMR for at least 2 years suggest that treatment can be safely discontinued or at least suspended [105,106]. The authors reported that molecular relapse occurred in 61 of the 100 patients (with 58 occurring during the first 7 months), but 56 of these patients regained a CMR on imatinib rechallenge. Similar results have emerged from an ongoing Australasian study (CML8) in which approximately 40% of patients who sustained UMRD for ≥2 years with imatinib retained UMRD on cessation of imatinib [107]. The investigators estimated that approximately 12% of all patients with CML who receive imatinib as first-line treatment may eventually achieve treatment-free remission. A retrospective analysis of clinical practice data for Japanese patients has also reported that 56% of those who discontinued imatinib treatment after achieving CMR maintained their response without molecular recurrence [108]. One small study (n=16) has investigated the possibility of discontinuing imatinib for a second time in patients who achieved a second sustained CMR after imatinib cessation [109]. A quarter of patients retained a drug-free MMR with a median follow-up of 32 months, with 12.5% remaining in CMR.
Case reports are also emerging of sustained stable remissions among patients treated with second-generation TKIs [110] and interim results of a study in 34 patients found that discontinuation of dasatinib or nilotinib treatment was feasible in patients with a sufficient response [111].
The use of IFN-α as maintenance therapy following imatinib cessation has also been explored. In one study, IFN-α was administered daily to Japanese patients who discontinued imatinib after achieving a CMR and having had an MR for at least 2 years [112]. After a median 23 months’ follow-up, 9 of 12 patients maintained a CMR and the three patients who relapsed regained an MMR on resumption of imatinib treatment. Similarly, Burchert and co-workers [113] reported sustained remission in patients treated with IFN-2α after discontinuation of treatment with imatinib and IFN-2α.
Several studies have reported possible risk factors for relapse following imatinib discontinuation. In the STIM study, risk factors for molecular relapse were high Sokal score at diagnosis and duration of imatinib treatment <60 months [106]. In the CML8 trial, high Sokal score was identified as a risk factor for relapse and, in patients who had received IFN in combination with imatinib, the risk was lower in those patients who received IFN for longer (>12 months vs ≤12 months) [107]. Other factors identified in CML8 as being predictive of achieving the discontinuation criterion while receiving imatinib (undetectable BCR-ABL1 at a PCR sensitivity of 4.5 log) include time taken to achieve MMR, sex and the 3-month BCR-ABL1 level [114]. Higher imatinib dose intensity and prior IFN-α administration were associated with sustained MR after imatinib discontinuation in a study in Japanese patients [108]. Others have reported longer duration of CMR to be associated with prolonged drug-free survival [115]. The immunologic activation status of natural killer cells and CD8+ T cells may also predict the risk of relapse [116,117]. This accruing evidence raises the future possibility of developing an algorithm by which to select patients who may safely discontinue TKI treatment.
Of course, patient preference also needs to be taken into account when considering stopping treatment, with some patients likely to be anxious about potential relapse. A recent survey found that patients did not feel confident in stopping their TKI treatment when informed that the current evidence suggested a relapse rate of 60% [118].
The possibility of safely discontinuing treatment may only apply to a small proportion of patients; however, the viability of this approach, particularly with regard to any impact on long-term survival and overall cost, warrants further detailed investigation. The NCCN Guidelines recommend that in patients with a satisfactory response, the same dose of TKI should be continued indefinitely, with discontinuation only considered in the setting of a clinical trial [201].
The high rate of relapse following imatinib discontinuation corroborates other evidence for the persistence of BCR-ABL-positive stem/progenitor cells in the bone marrow [119]. These primitive LSCs lack sensitivity to currently available TKIs. This is probably due to a variety of causes, including cellular quiescence and oncogene independence [120–122]. Using a PCR technique that is more sensitive than those usually used to define MR, Ross and co-workers demonstrated that even patients with sustained CMR after discontinuation of imatinib harbored BCR-ABL DNA [123]. In patients who relapsed shortly after discontinuation, the BCR-ABL DNA levels increased, while the DNA levels remained stable in those who maintained a CMR. The results suggest that complete eradication of the residual leukemic cells with imatinib may not be necessary, and that in some patients a process of “leukemic suppression” is occurring [123]. However, abolition of LSCs that give rise to disease persistence would be beneficial in the majority of patients and will most likely require an alternative, non-TKI approach to treatment. A number of intracellular signaling pathways and molecules have been identified that are potentially involved in LSC survival and these are being investigated as potential therapeutic targets [124,125]. The role of the bone marrow microenvironment in protecting LSCs is also under scrutiny [126,127].
EXPERT COMMENTARY
CML is a life-shortening hematologic malignancy. However, in recent years the advent of TKI therapy has significantly changed the management of the disease and has presented the possibility of long-term remission. The introduction of imatinib significantly improved survival in patients with CML and the more recent availability of a range of TKI options means that there is now the potential for selecting a more potent TKI or one better suited to an individual patient. Taking all the above evidence together, second-generation TKIs would appear to offer a more appropriate alternative to high-dose imatinib at present. In addition, there is renewed interest in alternative therapies such as omacetaxine and IFN-α (as PEG-IFN-α) and clinical trials of these agents are establishing their potential value in the era of TKI therapy.
Despite advances in our understanding of molecular responses, CCyR remains the crucial goal of treatment. Caution is urged in making treatment decisions based on molecular responses, particularly given assay variability. The possibility of stopping therapy in patients who respond well is also attracting considerable attention. However, at present the data supporting safe discontinuation of treatment are immature and stopping cannot be recommended in clinical practice.
Clinical challenges now include evolving strategies to meet the needs of patients who respond suboptimally, are intolerant of, or who acquire resistance to current treatment options. Increasing the dose, switching, and combining therapies are all possibilities.
FIVE-YEAR VIEW
We now have access to several effective TKIs but at present it is unclear which presents the optimal first-line option. The early outcomes are of course encouraging but long-term outcomes are unknown. Long-term survival data are keenly awaited. Vigilance is also required in the coming years with regard to emerging adverse events as we do not yet know the long-term side effects of these treatments.
Although physicians agree that early responses to treatment are important, there is still much to be learned about optimal treatment for those patients who do not achieve an early response. Although switching is widely advocated, we are currently lacking evidence on the best approach to switching therapy: when should we switch and what should we switch to? Data from well-conducted clinical trials of switching will be crucial. Cost may also become an important issue in treatment decisions as the US patent for imatinib expires in 2015, after which generic imatinib will become available.
The question of whether we can stop TKI therapy in patients who respond well also needs to be addressed. Stopping would be beneficial for some patients for a variety of reasons, including avoiding treatment-related adverse events, and on economic grounds. Long-term data are needed to determine the possibility of terminating ongoing TKI therapy without exposing patients to the risk of relapse, and to determine the most appropriate early strategies to achieve remission and disease eradication. In the future, based on more extensive clinical evidence, it may be feasible to develop a strategy by which we can confidently stop treatment in patients meeting certain criteria.
KEY ISSUES.
TKIs have transformed the management of CML, however a significant proportion of patients respond suboptimally or fail to respond to first-line TKIs.
The optimal first-line treatment is unclear; long-term survival data for the different TKIs are lacking.
Achieving CCyR is the key goal of treatment; in patients with a CCyR, changing treatment in response to modest increases in BCR-ABL transcript levels may not be the optimal approach.
Clinical evidence is currently lacking for the outcomes of switching TKI treatment. For patients who fail to achieve an early response we need more information to guide decisions on when to switch and what to switch to.
Physicians must be vigilant for treatment-related adverse effects that may emerge with long-term therapy.
Non-TKI therapies, such as omacetaxine and PEG-IFN-α also have a role in the management of CML.
Although there is some evidence that TKI therapy can be safely stopped in some patients who respond well, further investigation is required and at present stopping is not recommended in clinical practice.
Acknowledgments
Medical writing support was provided by Dr Tracey Lonergan and Dr Julie Ponting of Anthemis Consulting Ltd and was funded by Teva Pharmaceutical Industries. Teva provided a single medical accuracy review of the final draft. The authors were not compensated and retained full editorial control over the content of the paper.
Footnotes
Financial and competing interests disclosure
Dr Jabbour has received honoraria from Bristol-Myers Squibb, Novartis, Pfizer, Ariad, and Teva; Dr Cortes is a consultant for Ariad, Teva, and Pfizer and has received grant support from Bristol-Myers Squibb, Novartis, Ariad, Teva and Pfizer; Dr Kantarjian has received research grants from Novartis, Bristol-Myers Squibb, Pfizer, Ariad, and ChemGenex, and is an unpaid consultant to Novartis.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
REFERENCES
- 1.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 2006;355(23):2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 2.Baccarani M, Pileri S, Steegmann J-L, et al. ESMO Guidelines Working Group. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012;23(Suppl. 7):vii72–vii77. doi: 10.1093/annonc/mds228. [DOI] [PubMed] [Google Scholar]
- 3.Bartram CR, de Klein A, Hagemeijer A, et al. Translocation of c-ab 1 oncogene correlates with the presence of a Philadelphia chromosome in chronic myelogenous leukemia. Nature. 1983;306(5940):277–280. doi: 10.1038/306277a0. [DOI] [PubMed] [Google Scholar]
- 4.Shtalrid M, Talpaz M, Blick M, et al. Philadelphia-negative chronic myelogenous leukemia with breakpoint cluster region rearrangement: molecular analysis, clinical characteristics, and response to therapy. J. Clin. Oncol. 1988;6(10):1569–1575. doi: 10.1200/JCO.1988.6.10.1569. [DOI] [PubMed] [Google Scholar]
- 5.Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat. Rev. Cancer. 2005;5(3):172–183. doi: 10.1038/nrc1567. [DOI] [PubMed] [Google Scholar]
- 6.Thielen N, Ossenkoppele GJ, Schuurhuis G-J, Janssen JJWM. New insights into the pathogenesis of chronic myeloid leukemia: towards a path to cure. Neth. J. Med. 2011;69(10):430–440. [PubMed] [Google Scholar]
- 7.Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. WHO Classification of Tumours, Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer; 2001. [Google Scholar]
- 8.Kantarjian HM, Deisseroth A, Kurzrock R, Estrov Z, Talpaz M. Chronic myelogenous leukemia: a concise update. Blood. 1993;82(3):691–703. [PubMed] [Google Scholar]
- 9.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 10.Calabretta B, Perrotti D. The biology of CML blast crisis. Blood. 2004;103(11):4010–4022. doi: 10.1182/blood-2003-12-4111. [DOI] [PubMed] [Google Scholar]
- 11.Sokal JE, Baccarani M, Russo D, Tura S. Staging and prognosis in chronic myelogenous leukemia. Semin. Hematol. 1988;25(1):49–61. [PubMed] [Google Scholar]
- 12.Quintas-Cardama A, Cortes JE. Chronic myeloid leukemia: diagnosis and treatment. Mayo Clin. Proc. 2006;81(7):973–988. doi: 10.4065/81.7.973. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien S, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia N. Engl. J. Med 200334811994–1004.First full report of results from the pivotal IRIS trial that demonstrated the efficacy of imatinib in first-line treatment of CML and led to a dramatic change in CML treatment [DOI] [PubMed] [Google Scholar]
- 14.Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N. Engl. J. Med. 2003;349(15):1423–1432. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 15.Faderl S, Kurzrock R, Estrov Z. Chronic myelogenous leukemia: biology and therapy. Ann. Intern. Med. 1999;131(3):207–219. doi: 10.7326/0003-4819-131-3-199908030-00008. [DOI] [PubMed] [Google Scholar]
- 16.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hochhaus A, O’Brien SG, Guilhot F, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia Leukemia 20092361054–1061.Report of follow-up demonstrating durable responses to first-line imatinib [DOI] [PubMed] [Google Scholar]
- 18.Deininger M, O’Brien SG, Guilhot F, et al. International randomized study of interferon vs ST1571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood; New Orleans, LA, USA. Presented at: American Society of Hematology 51st Annual Meeting; 2009. Dec 5–8, Abstract 1126 (2009) [Google Scholar]
- 19.Gambacorti-Passerini C, Antolini L, Mahon FX, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J. Natl. Cancer Inst. 2011;103(7):553–561. doi: 10.1093/jnci/djr060. [DOI] [PubMed] [Google Scholar]
- 20.Cortes JE, Jones D, O’Brien S, Jabbour E. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J. Clin. Oncol. 2010;28(3):398–404. doi: 10.1200/JCO.2009.25.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fullmer A, Kantarjian H, Cortes J, et al. Nilotinib for the treatment of Philadelphia-chromosome-positive chronic myeloid leukemia. Expert Opin. Pharmacother. 2010;11(18):3065–3072. doi: 10.1517/14656566.2010.535655. [DOI] [PubMed] [Google Scholar]
- 22.Saglio G, Kim DW, Issaragrisil SM, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia N. Engl. J. Med 2010362242251–2259.Report of pivotal study demonstrating superior efficacy of nilotinib in first-line treatment [DOI] [PubMed] [Google Scholar]
- 23.Kantarjian HM, Hochhaus A, Saglio G, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12(9):841–851. doi: 10.1016/S1470-2045(11)70201-7. [DOI] [PubMed] [Google Scholar]
- 24.Larson RA, Hochhaus A, Hughes TP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26(10):2197–2203. doi: 10.1038/leu.2012.134. [DOI] [PubMed] [Google Scholar]
- 25.Hochhaus A, Saglio G, Larson R, et al. Nilotinib shows sustained benefit compared with imatinib in patients (Pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): ENESTnd 4-year follow-up (F/U). Haematologica; Stockholm, Sweden. Presented at: 18th Congress of the European Hematology Association; 2013. Jun 13–16, Abstract P712 (2013) [Google Scholar]
- 26.Kantarjian HM, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia N. Engl. J. Med 2010362242260–2270.Report of study demonstrating significantly higher and faster responses with dasatinib than imatinib as first-line therapy [DOI] [PubMed] [Google Scholar]
- 27.Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119(5):1123–1129. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hochhaus A, Shah NP, Cortes JE, et al. Dasatinib versus imatinib (IM) in newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): DASISION 3-year follow-up. J. Clin. Oncol; Chicago, IL, USA. Presented at: American Society of Clinical Oncology Annual Meeting; 2012. Jun 1–5, Abstract 6504 (2012) [Google Scholar]
- 29.Cortes JE, Kim DW, Kantarjian HM, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J. Clin. Oncol. 2012;30(28):3486–3492. doi: 10.1200/JCO.2011.38.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gambacorti-Passerini C, Lipton JH, Tee GY, et al. BELA trial update: Bosutinib (BOS) versus imatinib (IM) in patients (pts) with newly diagnosed chronic phase chronic myeloid leukemia (CP CML) after 30 months of follow-up. J. Clin. Oncol; Chicago, IL, USA. Presented at: American Society of Clinical Oncology Annual Meeting; 2012. Jun 1–5, Abstract 6512 (2012) [Google Scholar]
- 31.de Lavallade H, Apperley JF, Khirashad JS, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J. Clin. Oncol. 2008;26(20):3358–3363. doi: 10.1200/JCO.2007.15.8154. [DOI] [PubMed] [Google Scholar]
- 32.Lucas CM, Wang L, Austin GM, et al. A population study of imatinib in chronic myeloid leukaemia demonstrates lower efficacy than in clinical trials. Leukemia. 2008;22(10):1963–1966. doi: 10.1038/leu.2008.225. [DOI] [PubMed] [Google Scholar]
- 33.Michallet M, Tulliez M, Corm S, et al. Management of chronic myeloid leukaemia in clinical practice in France: results of the French subset of patients from the UNIC study. Curr. Med. Res. Opin. 2010;26(2):307–317. doi: 10.1185/03007990903479299. [DOI] [PubMed] [Google Scholar]
- 34.Kantarjian HM, O’Brien S, Jabbour E, et al. Impact of treatment end point definitions on perceived differences in long-term outcome with tyrosine kinase inhibitor therapy in chronic myeloid leukemia. J. Clin. Oncol. 2011;29(23):3173–3178. doi: 10.1200/JCO.2010.33.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fava C, Kantarjian HM, Jabbour E, et al. Failure to achieve a complete hematologic response at the time of a major cytogenetic response with second-generation tyrosine kinase inhibitors is associated with a poor prognosis among patients with chronic myeloid leukemia in accelerated or blast phase. Blood. 2009;113(21):5058–5063. doi: 10.1182/blood-2008-10-184960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jabbour E, Cortes JE, Kantarjian HM. Molecular monitoring in chronic myeloid leukemia: response to tyrosine kinase inhibitors and prognostic implications. Cancer. 2008;112(10):2112–2118. doi: 10.1002/cncr.23427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jabbour E, Kantarjian H, O’Brien S, et al. The achievement of an early complete cytogenetic response is a major determinant of outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors. Blood. 2011;118(17):4541–4546. doi: 10.1182/blood-2011-04-348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guilhot F, Druker B, Larson RA, et al. High rates of durable response are achieved with imatinib after treatment with interferon alpha plus cytarabine: results from the International Randomized Study of Interferon and STI571 (IRIS) trial. Haematologica. 2009;94(12):1669–1675. doi: 10.3324/haematol.2009.010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jabbour E, Kantarjian HM, O’Brien S, et al. Front-line therapy with second-generation tyrosine kinase inhibitors in patients with early chronic phase chronic myeloid leukemia: what is the optimal response? J. Clin. Oncol. 2011;29(32):4260–4265. doi: 10.1200/JCO.2011.36.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marin D, Milojkovic D, Olavarria E, et al. European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood. 2008;112(12):4437–4444. doi: 10.1182/blood-2008-06-162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes T, Hochhaus A, Branford S, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS) Blood. 2010;116(19):3758–3765. doi: 10.1182/blood-2010-03-273979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kantarjian H, O’Brien S, Shan J, et al. Cytogenetic and molecular responses and outcome in chronic myelogenous leukemia. Need for new response definitions? Cancer. 2008;112(4):837–845. doi: 10.1002/cncr.23238. [DOI] [PubMed] [Google Scholar]
- 43.Hehlmann R, Lauseker M, Jung-Munkwitz S, et al. Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon-α in newly diagnosed chronic myeloid leukemia. J. Clin. Oncol. 2011;29(12):1634–1642. doi: 10.1200/JCO.2010.32.0598. [DOI] [PubMed] [Google Scholar]
- 44.Falchi L, Kantarjian HM, Wang X, et al. Significance of deeper molecular responses in patients with chronic myeloid leukemia in early chronic phase treated with tyrosine kinase inhibitors. Am. J Hematol. 2013 doi: 10.1002/ajh.23560. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kantarjian HM, Shan J, Jones D, et al. Significance of increasing levels of minimal residual disease in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in complete cytogenetic response. J. Clin. Oncol. 2009;27(22):3659–3663. doi: 10.1200/JCO.2008.18.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanfstein B, Muller MC, Hehlmann R, et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML) Leukemia. 2012;26(9):2096–2102. doi: 10.1038/leu.2012.85. [DOI] [PubMed] [Google Scholar]
- 47.Saglio G, Kantarjian HM, Shah N, et al. Early response (molecular and cytogenetic) and long-term outcomes in newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): exploratory analysis of DASISION 3-year data. Blood; Atlanta, GA, USA. Presented at: American Society of Hematology 54th Annual Meeting; 2012. Dec 8–11, Abstract 1675 (2012) [Google Scholar]
- 48.Marin D, Hedgley C, Clark RE, et al. Predictive value of early molecular response in patients with chronic myeloid leukemia treated with first-line dasatinib. Blood. 2012;120(2):291–294. doi: 10.1182/blood-2012-01-407486. [DOI] [PubMed] [Google Scholar]
- 49.Hochhaus A, Hughes TP, Saglio G, et al. Outcome of patients with chronic myeloid leukemia in chronic phase (CML-CP) based on early molecular response and factors associated with early response: 4-year follow-up data from Enestnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials newly diagnosed patients). Blood; Atlanta, GA, USA. Presented at: American Society of Hematology 54th Annual Meeting; 2012. Dec 8–11, Abstract 167 (2012) [Google Scholar]
- 50.Rousselot P, Guilhot J, Preudhomme C, Mahon FX, Rea D, Nicolini FE, et al. Relationship between molecular responses and disease progression in patients (pts) treated first line with imatinib (Im) based regimens: impact of treatment arm within the French Spirit trial from the French CML group (FI LMC). Blood; Atlanta, GA, USA. Presented at: American Society of Hematology 54th Annual Meeting; 2012. Dec 8–11, Abstract 168 (2012) [Google Scholar]
- 51.Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J. Clin. Oncol. 2012;30(3):232–238. doi: 10.1200/JCO.2011.38.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neelakantan P, Gerrard G, Foroni L, et al. Can the combination of the measurement of BCR-ABL1 transcript levels at 3 and 6 months improve the prognostic value of the 3 month measurement?. Blood; Atlanta, GA, USA. Presented at: American Society of Hematology 54th Annual Meeting; 2012. Dec 8–11, Abstract 68 (2012) [Google Scholar]
- 53.Jain P, Kantarjian HM, Nazha A, et al. Early molecular and cytogenetic responses predicts for significantly longer event free survival (EFS) and overall survival (OS) in patients (pts) with newly diagnosed chronic myeloid leukemia (CML) in chronic phase (CP) – an analysis of 4 tyrosine kinase inhibitor (TKI) modalities (standard dose imatinib, high dose imatinib, dasatinib and nilotinib). Blood; Atlanta, GA, USA. Presented at: American Society of Hematology 54th Annual Meeting; 2012. Dec 8–11, Abstract 70 (2012) [Google Scholar]
- 54.Hughes T, Branford S, White DL, et al. Impact of early dose intensity on cytogenetic and molecular responses in chronic-phase CML patients receiving 600 mg/day of imatinib as initial therapy. Blood. 2008;112(10):3965–3973. doi: 10.1182/blood-2008-06-161737. [DOI] [PubMed] [Google Scholar]
- 55.Kantarjian HM, Talpaz M, O’Brien S, et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood. 2004;103(8):2873–2878. doi: 10.1182/blood-2003-11-3800. [DOI] [PubMed] [Google Scholar]
- 56.Cortes JE, Kantarjian HM, Goldberg SL, et al. High-dose imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: high rates of rapid cytogenetic and molecular responses. J. Clin. Oncol. 2009;27(28):4754–4759. doi: 10.1200/JCO.2008.20.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castagnetti F, Palandri F, Amabie M, et al. Results of high dose imatinib mesylate in intermediate Sokal risk chronic myeloid leukemia patients in early chronic phase: a phase 2 trial of the GIMEMA CML Working Party. Blood. 2009;113(15):3428–3434. doi: 10.1182/blood-2007-08-103499. [DOI] [PubMed] [Google Scholar]
- 58.Baccarani M, Rosti G, Castagnetti F, et al. Comparison of imatinib 400 mg and 800 mg daily in the front-line treatment of high-risk, Philadelphia-positive chronic myeloid leukemia: a European LeukemiaNet Study. Blood. 2009;113(19):4497–4504. doi: 10.1182/blood-2008-12-191254. [DOI] [PubMed] [Google Scholar]
- 59.Cortes JE, Baccarani M, Guilhot F, et al. Phase III, randomized, open-label study of daily imatinib mesylate 400 mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: tyrosine kinase inhibitor optimization and selectivity study. J. Clin. Oncol. 2010;28(3):424–430. doi: 10.1200/JCO.2009.25.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hehlmann R, Lauseker M, Hanfstein B, et al. Complete molecular remission (CMR4.5) of CML is induced faster by dose-optimized imatinib and predicts better survival – results from the randomized CML-Study IV. Blood; Atlanta, GA, USA. Presented at: American Society of Hematology 54th Annual Meeting; 2012. Dec 8–11, Abstract 67 (2012) [Google Scholar]
- 61.Breccia M, Alimena G. Second-generation tyrosine kinase inhibitors as first-line treatment strategy in newly diagnosed chronic phase chronic myeloid leukemia patients. Curr. Cancer Drug Targets. 2012;12(4):391–401. doi: 10.2174/156800912800190965. [DOI] [PubMed] [Google Scholar]
- 62.Katouli AA, Komarova NL. Optimizing combination therapies with existing and future CML drugs. PLoS. One. 2010;5:e12300. doi: 10.1371/journal.pone.0012300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Komarova NL, Katouli AA, Wodarz D. Combination of two but not three current targeted drugs can improve therapy of chronic myeloid leukemia. PLoS. One. 2009;4:e4423. doi: 10.1371/journal.pone.0004423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cortes JE, Quintas-Cardama A, Jones D, et al. Immune modulation of minimal residual disease in early chronic phase chronic myelogenous leukemia: a randomized trial of frontline high-dose imatinib mesylate with or without pegylated interferon alpha-2b and granulocyte-macrophage colony-stimulating factor. Cancer. 2011;117(3):572–580. doi: 10.1002/cncr.25438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simonsson B, Gedde-Dahl T, Markevarn B, et al. Combination of pegylated IFN-α2b with imatinib increases molecular response rates in patients with low- or intermediate-risk chronic myeloid leukemia. Blood. 2011;118(12):3228–3235. doi: 10.1182/blood-2011-02-336685. [DOI] [PubMed] [Google Scholar]
- 66.Preudhomme C, Guilhot J, Nicolini FE, et al. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N. Engl. J. Med. 2010;363(26):2511–2521. doi: 10.1056/NEJMoa1004095. [DOI] [PubMed] [Google Scholar]
- 67.Lipton JH, Khoroshko N, Golenkov A, et al. Phase II, randomized, multicenter, comparative study of peginterferon-alpha-2a (40 kD) (Pegasys) versus interferon alpha-2a (Roferon-A) in patients with treatment-naïve, chronic-phase chronic myelogenous leukemia. Leuk. Lymphoma. 2007;48(3):497–505. doi: 10.1080/10428190601175393. [DOI] [PubMed] [Google Scholar]
- 68.Nicolini FE, Etienne G, Dubruille V, et al. Pegylated interferon-α 2a in combination to nilotinib as first line therapy in newly diagnosed chronic phase chronic myelogenous leukemia provides high rates of MR4.5. Preliminary results of a Phase II study. Blood; Atlanta, GA, USA. Presented at: American Society of Hematology 54th Annual Meeting; 2012. Dec 8–11, Abstract 166 (2012) [Google Scholar]
- 69.Eliasson L, Clifford S, Barber N, Marin D. Exploring chronic myeloid leukemia patients’ reasons for not adhering to the oral anticancer drug imatinib as prescribed. Leuk. Res. 2011;35(5):626–630. doi: 10.1016/j.leukres.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 70.Cross TJ, Bagot C, Portmann B, et al. Imatinib mesylate as a cause of acute liver failure. Am. J. Hematol. 2006;81(3):189–192. doi: 10.1002/ajh.20486. [DOI] [PubMed] [Google Scholar]
- 71.Mindikoglu AL, Regev A, Bejarano PA, et al. Imatinib mesylate (Gleevec) hepatotoxicity. Dig. Dis. Sci. 2007;52(2):598–601. doi: 10.1007/s10620-006-9117-1. [DOI] [PubMed] [Google Scholar]
- 72.Montani D, Bergot E, Gunther S, et al. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation. 2012;125(17):2128–2137. doi: 10.1161/CIRCULATIONAHA.111.079921. [DOI] [PubMed] [Google Scholar]
- 73.Sano M, Saotome M, Urushida T, et al. Pulmonary arterial hypertension caused by treatment with dasatinib for chronic myeloid leukemia -critical alert- Intern. Med. 2012;51(17):2337–2340. doi: 10.2169/internalmedicine.51.7472. [DOI] [PubMed] [Google Scholar]
- 74.Le Coutre P, Rea D, Abruzzese E, et al. Severe peripheral arterial disease during nilotinib therapy. J. Natl. Cancer Inst. 2011;103(17):1347–1348. doi: 10.1093/jnci/djr292. [DOI] [PubMed] [Google Scholar]
- 75.Gambacorti-Passerini C, Zucchetti M, Russo D, et al. Alpha1 acid glycoprotein binds to imatinib (STI571) and substantially alters its pharmacokinetics in chronic myeloid leukemia patients. Clin. Cancer Res. 2003;9(2):625–632. [PubMed] [Google Scholar]
- 76.Soverini S, Martinelli G, Rosti G, et al. ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: a study by the GIMEMA Working Party on Chronic Myeloid Leukemia. J. Clin. Oncol. 2005;23(18):4100–4109. doi: 10.1200/JCO.2005.05.531. [DOI] [PubMed] [Google Scholar]
- 77.Wei G, Rafiyath S, Liu D. First-line treatment for chronic myeloid leukemia: dasatinib, nilotinib, or imatinib. J. Hematol. Oncol. 2010;3:47. doi: 10.1186/1756-8722-3-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J. Clin. Oncol. 2010;28(14):2381–2388. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Darkow T, Henk HJ, Thomas SK, et al. Treatment interruptions and non-adherence with imatinib and associated healthcare costs: a retrospective analysis among managed care patients with chronic myelogenous leukaemia. Pharmacoeconomics. 2007;25(6):481–496. doi: 10.2165/00019053-200725060-00004. [DOI] [PubMed] [Google Scholar]
- 80.Noens L, van Lierde MA, De De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113(22):5401–5411. doi: 10.1182/blood-2008-12-196543. [DOI] [PubMed] [Google Scholar]
- 81.Donato NJ, Wu JY, Stapley J, et al. BCR-ABL independence and LYN kinase overexpression in chronic Myelogenous leukemia cells selected for resistance to ST571. Blood. 2003;101(2):690–698. doi: 10.1182/blood.V101.2.690. [DOI] [PubMed] [Google Scholar]
- 82.Donato NJ, Wu JY, Stapley J, et al. Imatinib mesylate resistance through BCR-ABL independence in chronic myelogenous leukemia. Cancer Res. 2004;64(2):672–677. doi: 10.1158/0008-5472.can-03-1484. [DOI] [PubMed] [Google Scholar]
- 83.Wu J, Meng F, Lu H, et al. Lyn regulates BCR-ABL and Gab2 tyrosine phosphorylation and c-Chl protein stability in imatinib-resistant chronic myelogenous leukemia cells. Blood. 2008;111(7):3821–3829. doi: 10.1182/blood-2007-08-109330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu Y, Swerdlow S, Duffy TM, et al. Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice. Proc. Natl. Acad. Sci. U.S.A. 2006;103(45):16870–16875. doi: 10.1073/pnas.0606509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pene-Dumitrescu T, Peterson LF, Donato NJ, Smithgall TE. An inhibitor-resistant mutant of Hck protects CML cells against the antiproliferative and apoptotic effects of the broad-spectrum Src family kinase inhibitor A-419259. Oncogene. 2008;27(56):7055–7069. doi: 10.1038/onc.2008.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hayette S, Chabane K, Michallet M, et al. Longitudinal studies of SRC family kinases in imatinib- and dasatinib-resistant chronic myelogenous leukemia patients. Leuk. Res. 2011;35(1):38–43. doi: 10.1016/j.leukres.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 87.Wu J, Meng F, Kong LY, et al. Association between imatinib-resistant BCR-ABL mutation-negative leukemia and persistent activation of LYN kinase. J. Natl. Cancer Inst. 2008;100(13):926–939. doi: 10.1093/jnci/djn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jabbour E, Fullmer A, Cortes JE, Kantarjian H. Clinical algorithms for the treatment of patients with chronic myeloid leukemia: the 2010 perspective. Clin. Lymphoma Myeloma Leuk. 2010;10(Suppl 1):S6–S13. doi: 10.3816/CLML.2010.s.001. [DOI] [PubMed] [Google Scholar]
- 89.Kantarjian HM, Larson RA, Guilhot F, et al. Efficacy of imatinib dose escalation in patients with chronic myeloid leukemia in chronic phase. Cancer. 2009;115(3):551–560. doi: 10.1002/cncr.24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hyun-Gyung G, Jootar S, Kim HJ, et al. Efficacy of nilotinib versus high-dose imatinib in early chronic phase CML patients who have suboptimal molecular responses to standard dose imatinib (RE-NICE Multicenter Study). Blood; San Diego, CA, USA. Presented at: American Society of Hematology 53rd Annual Meeting; 2011. Dec 10–13, Abstract 2765 (2011) [Google Scholar]
- 91.Jabbour E, Kantarjian E, Shan J, et al. The achievement of a 3-month complete cytogenetic response (3-mo CCyR) to second generation (2nd) tyrosine kinase inhibitors (TKI) post imatinib failure is the only predictive factor for event-free (EFS) and overall survival (OS). Blood; Orlando, FL, USA, mDecember. Presented at: American Society of Hematology 52nd Annual Meeting; 2010. Dec 4–7, Abstract 2289 (2010) [Google Scholar]
- 92.Yeung DT, Osborn M, White DL, et al. Upfront imatinib therapy in CML patients with rapid switching to nilotinib for failure to achieve molecular targets or intolerance achieves high overall rates of molecular response and a low risk of progression – an update of the TIDEL-II trial. Blood; San Diego, CA, USA. Presented at: American Society of Hematology 53rd Annual Meeting; 2011. Dec 10–13, Abstract 451 (2011) [Google Scholar]
- 93.Yeung DT, Osborn MP, White DL, et al. Early switch to nilotinib does not overcome the adverse outcome for CML patients failing to achieve early molecular response on imatinib, depite excellent overall outcomes in the TIDEL II trial. Blood; Atlanta, GA, USA. Presented at: American Society of Hematology 54th Annual Meeting; 2012. Dec 8–11, Abstract 3771 (2012) [Google Scholar]
- 94.Khoury HJ, Cortes JE, Kantarjian HM, et al. Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. Blood. 2012;119(15):3403–3412. doi: 10.1182/blood-2011-11-390120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cortes JE, Kantarjian HM, Brummendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118(17):4567–4576. doi: 10.1182/blood-2011-05-355594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cortes JE, Kantarjian HM, Kim DW, et al. Bosutinib as therapy for chronic phase chronic myeloid leukemia following resistance or intolerance to imatinib: 36-month minimum follow-up update. Blood; Atlanta, GA, USA. American Society of Hematology 54th Annual Meeting; 2012. Dec 8–11, Abstract 3779 (2012a) [Google Scholar]
- 97.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. PACE: A pivotal phase 2 trial of ponatinib in patients with chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL) resistant or intolerant to dasatinib or nilotinib, or with the T315I mutation: 12-month follow-up of the PACE trial Blood 2012December8–1112021Atlanta, GA, USAPresented at: American Society of Hematology 54th Annual MeetingAbstract 163 (2012)Report of pivotal clinical trial describing the efficacy of a new TK that has activity in patients with the T315I mutation [Google Scholar]
- 98.Allan EK, Holyoake TL, Craig AR, Jørgensen HG. Omacetaxine may have a role in chronic myeloid leukaemia eradication through downregulation of Mcl-1 and induction of apoptosis in stem/progenitor cells. Leukemia. 2011;25(6):985–994. doi: 10.1038/leu.2011.55. [DOI] [PubMed] [Google Scholar]
- 99.Ayoubi M, Kantarjan HM, Wierda W, et al. A Phase 2 study of the combination of omacetaxine and imatinib in the treatment of patients with chronic myeloid leukemia (CML) in advanced stages or after failure to imatinib. Blood; New Orleans, LA, USA. Presented at: American Society of Hematology 51st Annual Meeting; 2009. Dec 5–8, Abstract 2193 (2009) [Google Scholar]
- 100.Cortes-Franco J, Raghunadharao D, Parikh P, et al. Safety and efficacy of subcutaneous-administered omacetaxine mepesuccinate in chronic myeloid leukemia (CML) patients who are resistant or intolerant to two or more tyrosine kinase inhibitors – results of a multicenter phase 2/3 study. Blood; New Orleans, LA, USA. Presented at: American Society of Hematology 51st Annual Meeting; 2009. Dec 5–8, Abstract 861 (2009) [Google Scholar]
- 101.Cortes JE, Nicolini FE, Wetzler M, et al. Subcutaneous omacetaxine in chronic or accelerated chronic myeloid leukemia resistant to two or more tyrosine-kinase inhibitors including imatinib. Blood; San Diego, CA, USA. Presented at: American Society of Hematology 53rd Annual Meeting; 2011. Dec 10–13, Abstract 3761 (2011) [Google Scholar]
- 102.Cortes J, Lipton JH, Rea D, et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation Blood 2012120132573–2580.Report of clinical trial demonstrating the efficacy of an agent in treating patients who have the T315I mutation and have failed TKI therapy [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kantarjian HM, Lipton JH, Baccarani M, et al. Long-term follow-up of ongoing patients in 2 studies of omacetaxine mepesuccinate for chronic myeloid leukemia. Blood; Atlanta, GA, USA. Presented at: American Society of Hematology 54th Annual Meeting; 2012. Dec 8–11, Abstract 2787 (2012) [Google Scholar]
- 104.Chen Y, Hu Y, Michaels S, et al. Inhibitory effects of omacetaxine on leukemic stem cells and BCR-ABL-induced chronic myeloid leukemia and acute lymphoblastic leukemia in mice. Leukemia. 2009;23(8):1446–1454. doi: 10.1038/leu.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mahon FX, Rea D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial Lancet Oncol 201011111029–1035.Report of clinical study designed to specifically investigate whether imatinib can be safety discontinued in specific patients [DOI] [PubMed] [Google Scholar]
- 106.Mahon FX, Rea D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukemia who have maintained complete molecular response: update results of the STIM study. Blood; San Diego, CA, USA. Presented at: American Society of Hematology 53rd Annual Meeting; 2011. Dec 10–13, Abstract 603 (2011) [Google Scholar]
- 107.Ross M, Branford S, Seymour JF, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122(4):515–522. doi: 10.1182/blood-2013-02-483750. [DOI] [PubMed] [Google Scholar]
- 108.Takahashi N, Kyo T, Maeda Y, et al. Discontinuation of imatinib in Japanese patients with chronic myeloid leukemia. Haematologica. 2012;97(6):903–906. doi: 10.3324/haematol.2011.056853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Legros L, Rousselot P, Giraudier S, et al. Second attempt to discontinue imatinib in CP-CML patients with a second sustained complete molecular response. Blood. 2012;120(9):1959–1960. doi: 10.1182/blood-2012-02-408229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ross DM, Bartley PA, Goyne J, et al. Durable complete molecular remission of chronic myeloid leukemia following dasatinib cessation, despite adverse disease features. Haematologica. 2011;96(11):1720–1722. doi: 10.3324/haematol.2011.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rea D, Rousselot P, Guilhot F, et al. Discontinuation of second generation (2G) tyrosine kinase inhibitors (TKI) in chronic phase (CP) chronic myeloid leukemia (CML) patients with stable undetectable BCR-ABL transcripts. Blood; Atlanta, GA, USA. Presented at: American Society of Hematology 54th Annual Meeting; 2012. Dec 8–11, Abstract 916 (2012) [Google Scholar]
- 112.Usuki k, Yusa N, Hangaishi A, et al. Sustained molecular response with maintenance dose of interferon alfa after imatinib discontinuation in patients with chronic myeloid leukemia. Blood; Atlanta, GA, USA. Presented at: American Society of Hematology 54th Annual Meeting; 2012. Dec 8–11, Abstract 1684 (2012) [Google Scholar]
- 113.Burchert A, Müller MC, Kostrewa P, et al. Sustained molecular response with interferon alfa maintenance after induction therapy with imatinib plus interferon alfa in patients with chronic myeloid leukemia. J. Clin. Oncol. 2010;28(8):1429–1435. doi: 10.1200/JCO.2009.25.5075. [DOI] [PubMed] [Google Scholar]
- 114.Branford S, Ross D, Prime J, et al. Early molecular response and female sex strongly predict achievement of stable undetectable BCR-ABL1, a criterion for imatinib discontinuation in patients with CML. Blood; Atlanta, GA, USA. Presented at: American Society of Hematology 54th Annual Meeting; 2012. Dec 8–11, Abstract 165 (2012) [DOI] [PubMed] [Google Scholar]
- 115.Matsuki E, Ono Y, Tonegawa K, et al. Detailed investigation on characteristics of Japanese patients with chronic phase CML who achieved a durable CMR after discontinuation of imatinib – an updated result of the Keio STIM study. Blood; Atlanta, GA, USA. Presented at: American Society of Hematology 54th Annual Meeting; 2012. Dec 8–11, Abstract 2788 (2012) [Google Scholar]
- 116.Ohyashiki K, Katagiri S, Tauchi T, et al. Increased natural killer cells and decreased CD3+CD8+CD62L+ T cells in CML patients who sustained complete molecular remission after discontinuation of imatinib. Br. J. Haematol. 2012;157(2):254–256. doi: 10.1111/j.1365-2141.2011.08939.x. [DOI] [PubMed] [Google Scholar]
- 117.Katagiri S, Mizoguchi I, Yoshimoto T, et al. Activation levels of natural killer cells and CD8+ T cells correlate highly with sustained complete molecular response after discontinuation of imatinib in chronic myeloid leukemia patients. Blood; Atlanta, GA, USA. Presented at: American Society of Hematology 54th Annual Meeting; 2012. Dec 8–11, Abstract 3745 (2012) [Google Scholar]
- 118.Kyle RMA, Lazo-Langner A, Xenocostas A, et al. Patient preferences for stopping tyrosine kinase inhibitors in chronic myeloid leukemia. Blood; Atlanta, GA, USA. Presented at: American Society of Hematology 54th Annual Meeting; 2012. Dec 8–11, Abstract 4274 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chu S, McDonald T, Lin A, et al. Persistence of leukemia stem cells in chronic myelogenous leukemia patients in prolonged remission with imatinib treatment. Blood. 2011;118(20):5565–5572. doi: 10.1182/blood-2010-12-327437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Graham S, Jorgenson H, Allan E, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to ST1571 in vitro. Blood. 2002;99(1):319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- 121.Corbin A, Agarwal A, Loriaux M, et al. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J. Clin. Invest. 2011;121(1):396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hamilton A, Helgason GV, Schemionek M, et al. Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival. Blood. 2011;119(6):1501–1510. doi: 10.1182/blood-2010-12-326843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ross DM, Branford S, Seymour JF, et al. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia. 2010;24(10):1719–1724. doi: 10.1038/leu.2010.185. [DOI] [PubMed] [Google Scholar]
- 124.Hamad A, Sahli Z, El Sabban M, Mouteirik M, Nasr R. Emerging therapeutic strategies for targeting chronic myeloid leukemia stem cells. Stem Cells Int. 2013:724360. doi: 10.1155/2013/724360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sinclair A, Latif AL, Holyoke TL. Targeting survival pathways in chronic myeloid leukemia stem cells. Br. J. Pharmacol. 2013;169(8):1693–1707. doi: 10.1111/bph.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Konopleva M, Jordan C. Leukemic stem cells and microenvironment: biology and therapeutic targeting. J. Clin. Oncol. 2011;29(5):591–599. doi: 10.1200/JCO.2010.31.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nwajei F, Konopleva M. The bone marrow microenvironment aos niche retreats for hemtopoietic and leukemic stem cells. Adv. Hematol. Article ID 953982 (2013). doi: 10.1155/2013/953982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 201. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Chronic Myelogenous Leukemia V.4.2013. © National Comprehensive Cancer Network, Inc 2013. Accessed September 2013. All rights reserved. To view the most recent and complete version of the guideline, go online to www.nccn.org. NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, NCCN GUIDELINES®, and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc. Available from: http://www.nccn.org/professionals/physician_gls/pdf/cml.pdf
- 202.Surveillance Epidemiology and End Results. SEER Stat Fact Sheets: Chronic myeloid leukemia. 2012 [cited 9 November, 2012] Available from: http://seer.cancer.gov/statfacts/html/cmyl.html.