Summary
The dorsal raphe nucleus (DRN) contains the largest group of serotonin-producing neurons in the brain and projects to regions controlling reward. Although pharmacological studies suggest that serotonin inhibits reward-seeking, electrical stimulation of the DRN strongly reinforces instrumental behavior. Here, we provide a targeted assessment of the behavioral, anatomical, and electrophysiological contributions of serotonergic and non-serotonergic DRN neurons to reward processes. To explore DRN heterogeneity, we used a simultaneous two-vector knockout/optogenetic stimulation strategy, as well as cre-induced and cre-silenced vectors in several cre-expressing transgenic mouse lines. We found that the DRN is capable of reinforcing behavior primarily via non-serotonergic neurons, whose main projection target is the ventral tegmental area (VTA). Furthermore, these non-serotonergic projections provide glutamatergic excitation of VTA dopamine neurons and account for a large majority of the DRN-VTA pathway. These findings help to resolve apparent discrepancies between the roles of serotonin versus the DRN in behavioral reinforcement.
Introduction
Dopaminergic neurons of the ventral tegmental area (VTA) play a central role in reward learning (Wise, 2004). Whole-brain mapping studies have found the greatest density of VTA-projecting neurons to reside in the dorsal raphe nucleus (DRN) (Phillipson, 1979; Watabe-Uchida et al., 2012). The DRN contains the largest group of serotonin neurons in the brain, and supplies the vast majority of ascending serotonergic projections (Jacobs and Azmitia, 1992). The role of the DRN in reinforcement learning is unclear, with literature suggesting both excitatory and inhibitory functions. For example, electrical stimulation of the DRN is sufficient to vigorously reinforce instrumental behavior in rats (Corbett and Wise, 1979; Margules, 1969; Rompre and Miliaressis, 1985; Simon et al., 1976; Van Der Kooy et al., 1978). In contrast, drugs that selectively elevate levels of serotonin, the major neurotransmitter output of the DRN, possess very low abuse liability in humans and are not self-administered in laboratory animals (Gotestam and Andersson, 1975; Griffiths et al., 1976; Zawertailo et al., 1995).
A recent study provided evidence that optogenetic stimulation of serotonergic DRN cell bodies is capable of reinforcing instrumental behavior (Liu et al., 2014). However, a majority of the rewarding effects of electrical DRN stimulation act through fibers with refractory periods that are too rapid to be of serotonergic origin (Rompre and Miliaressis, 1987). These studies suggest that the DRN contains a population of non-serotonergic fibers capable of reinforcing behavior to a greater degree than serotonin-producing neurons. However, it is not known whether these fibers originate from neurons within the DRN, or instead represent axons of distal cell bodies projecting to or through the DRN.
Given recent demonstrations that DRN projection neurons are heterogeneous and include serotonergic, dopaminergic, GABAergic, and non-serotonergic glutamate populations (reviewed in Vasudeva et al., 2011), we explored the participation of these populations in reward circuitry and reinforcement learning. By testing self-stimulation behavior, anterograde/retrograde tracing, and electrophysiology, we found that the DRN reinforces behavior preferentially through non-serotonergic neurons, which make up the majority of the DRN-VTA pathway and produce strong glutamatergic excitation of VTA dopamine neurons.
Results
Stimulation of dopamine, but not serotonin, reinforces instrumental behavior
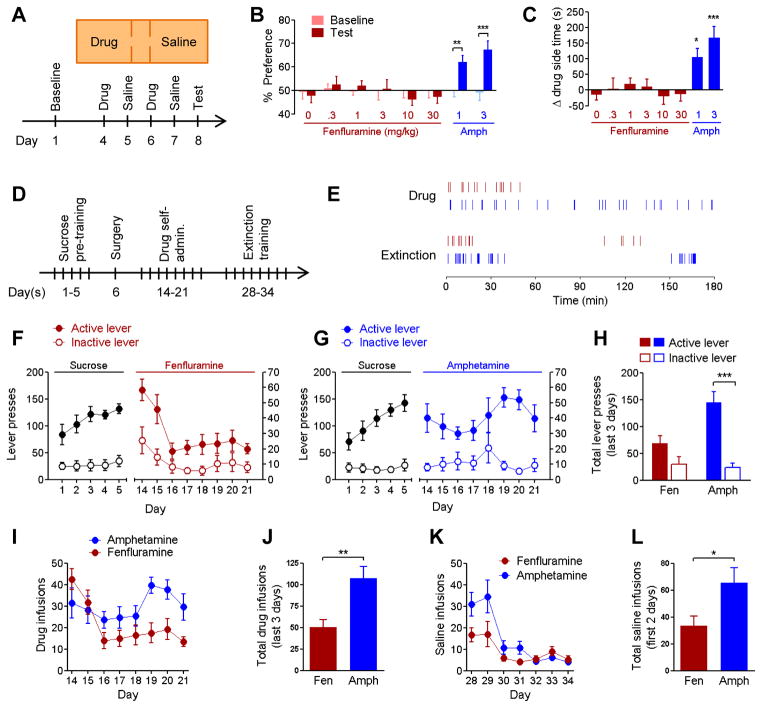
To test whether release of serotonin produces reward-related behavior, we examined the ability of the serotonin-releasing agent fenfluramine to elicit a conditioned place preference and to support self-administration in mice (Figure 1). Amphetamine, which is mechanistically similar to fenfluramine but acts preferentially upon dopaminergic reuptake sites (Rothman and Baumann, 2009), was used as a positive control. In the conditioned place preference paradigm, none of the five doses of fenfluramine we tested (0.3–30 mg/kg, i.p.) produced a significant preference or aversion (Figure 1A–C). In contrast, amphetamine (1 or 3 mg/kg, i.p.) elicited a strong preference. Similarly, mice that had self-administration access to amphetamine significantly lever-pressed more for drug infusions than mice with access to fenfluramine (Figure 1D–J). After one week of drug abstinence, each group of mice was also tested for extinction responding, in which lever presses resulted in saline infusions. Mice with previous access to amphetamine exhibited greater measures of drug-seeking (Figure 1K,L).
Figure 1. Pharmacological stimulation of dopamine but not serotonin release reinforces behavior.
A, Mice (n=9–18/group) were conditioned with injections of the serotonin-releasing agent fenfluramine (0–30 mg/kg, i.p.) or the dopamine-releasing agent amphetamine (1–3 mg/kg, i.p.). B, Percent of time spent in drug-paired chamber on baseline and test days. Repeated measures ANOVA (drug x day) interaction F(7,96)=4.166, p<0.001; ** p<0.01, *** p<0.001 post-hoc. C, Change in time spent on drug-paired chamber between test and baseline days. One-way ANOVA F(7,96)=5.318, p<0.0001; * p<0.05, *** p<0.001 Dunnett’s post-hoc vs. saline. D, Self-administration experiment in a separate cohort of mice. After pre-training for sucrose, mice were implanted with intravenous catheters and allowed to self-administer fenfluramine (0.03 mg/kg/infusion) or amphetamine (0.05 mg/kg/infusion), n=8/group. E, Sample data from individual self-administration sessions demonstrating timing of infusions for mice with access to fenfluramine (red) and amphetamine (blue), and during the first day of extinction training. F,G, daily lever-press counts during sucrose pre-training (left Y axis) and drug self-administration (right Y axis). These experiment phases are plotted on different scales because the number of maximally-allowed rewards differed. H, total number of lever presses during last three days of drug access. Two-way ANOVA (drug x lever) interaction F(1,28)=8.095, p<0.01; *** p<0.001 post-hoc. I, Daily drug infusions during drug self-administration phase. J, total number of drug infusions during last three days drug access, ** p<0.01. K, Daily counts of infusions of saline during extinction training. L, total number of saline infusions during the first two days of extinction, * p<0.05. Group data are presented here and in subsequent figures as mean ± SEM.
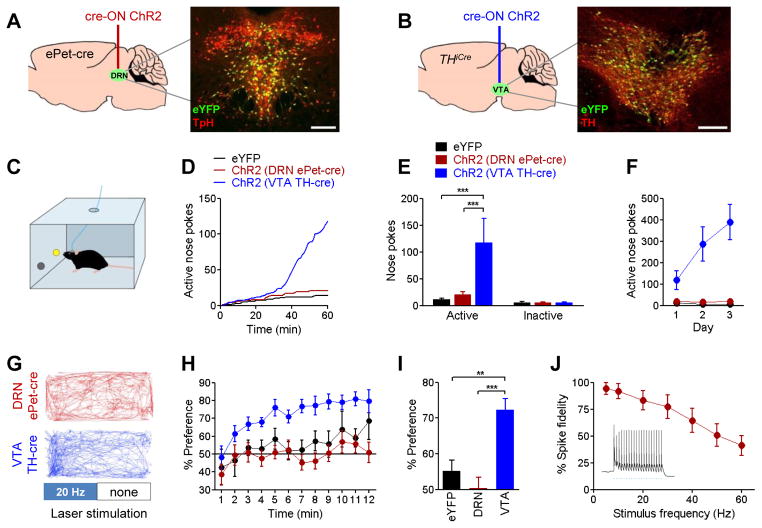
Although serotonin release was not sufficient to strongly elicit reward-related behavior, it is possible that such behavioral effects require precisely-timed neuronal activity within DRN serotonin neurons and/or co-release of other transmitters. To explore these possibilities, we used optogenetic stimulation with the light-activated ion channel channelrhodopsin-2 (ChR2) to activate neurons in vivo (Adamantidis et al., 2007) and test whether stimulation of serotonergic DRN cell bodies was sufficient to reinforce behavior (Figure 2A). Compared with classical electrical stimulation, this approach offers the advantages of restricting stimulation to genetically-defined populations of cells without affecting fibers of passage or terminals (Tye and Deisseroth, 2012). As a positive control, we also tested stimulation of dopaminergic VTA cell bodies (Figure 2B). Selective targeting of channelrhodopsin-2 (ChR2) to these cells was achieved by stereotaxically injecting cre-inducible (“cre-ON”) vectors expressing ChR2-eYFP or eYFP alone into the DRN of ePet-cre mice or the VTA of THiCre mice. These transgenic lines selectively express cre recombinase in serotonergic and catecholaminergic neurons, respectively. Nearly all (91.4%) of 1827 total eYFP-positive DRN neurons counted from 8 ePet-cre animals also tested positive for tryptophan hydroxylase, an enzyme essential for serotonin synthesis. Similarly, 88.9% of 903 eYFP-positive neurons counted from 4 THiCre animals also tested positive for tyrosine hydroxylase, an enzyme necessary for dopamine synthesis. Behavior from DRN and VTA-injected eYFP control mice did not differ, and they were combined into a single group. Mice were trained in an operant task (Figure 2C), in which nose pokes into an active port resulted in 3-second trains of 20 Hz laser stimulation. While stimulation of VTA dopamine neurons strongly reinforced behavior in this task, stimulation of DRN serotonin neurons had no effect (Figure 2D–F). We found the same pattern of results in a real-time place preference test (Figure 2G), in which presence of the mouse in one half of a chamber resulted in continuous 20 Hz stimulation. Stimulation of VTA dopamine neurons reinforced behavior in this task, whereas stimulation of DRN serotonin neurons did not (Figure 2H,I). Parametric variations of laser intensity, pulse frequency, and repeated days of training all failed to elicit statistically significant measures of reward or aversion in DRN serotonin stimulated mice (Figure S1). DRN serotonin neurons were capable of following 20 Hz optical stimulation with action potentials (Figure 2J, Figure S2), consistent with previous demonstrations that electrical stimulation produces increasing levels of serotonin release at increasing frequencies up to and including 20 Hz (Sharp et al., 1989). Together with the fenfluramine experiments, these data demonstrate that behavioral measures of reinforcement that are easily elicited by stimulation of dopamine release are much less sensitive to serotonin.
Figure 2. Optogenetic stimulation of VTA dopamine but not DRN serotonin cell bodies reinforces behavior.
Selective targeting of gene expression was achieved by injecting cre-induced (“cre-ON”) vectors expressing ChR2-eYFP or eYFP alone into the (A) DRN of ePet-cre mice or (B) VTA of THiCre mice. Insets depict expression of eYFP (green), double-labeled in red with tryptophan hydroxylase (TpH) for DRN tissue or tyrosine hydroxylase (TH) for VTA tissue. Scale bars = 200 μm. C, Mice were trained to nose-poke into an active port to receive 3-second trains of 20 Hz laser stimulation; nose-pokes into an inactive port were not reinforced. D,E, Representative cumulative-activity graph and group mean nose-pokes made in first behavioral session for VTA-dopamine (n=11), DRN-serotonin (n=18), and a combined control group expressing eYFP in DRN or VTA (n=17). Two-way ANOVA (group x port) interaction F(2,86)=8.317, p<0.001; *** p<0.001 post-hoc. F, Active nose poke responding on three consecutive days of testing. G, Mice underwent a real-time place preference task in which presence in one half of a chamber triggered continuous 20 Hz laser stimulation. Example tracks for a DRN serotonin stimulated mouse (top; red) and a VTA dopamine stimulated mouse (bottom; blue). H, Minute-by-minute percent of time spent in the laser-paired half of the chamber. I, Overall preference for laser-paired side during 12-minute session. One-way ANOVA F(2,38)=12.05, p<0.0001; ** p<0.01, *** p<0.001 post-hoc. J, Percent of laser pulses in a 20 pulse train resulting in action potentials in ChR2+ DRN serotonin cell bodies, recorded ex vivo in whole-cell current clamp. Inset, sample trace with 20Hz stimulation. See also Figures S1 and S2.
Stimulation of non-serotonergic DRN neurons reinforces instrumental behavior
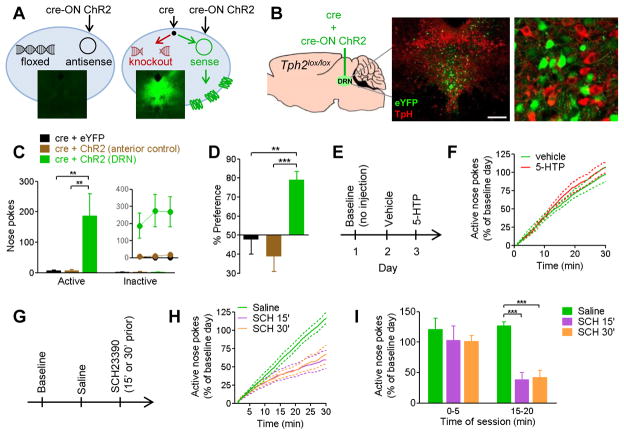
We next performed an experiment collectively stimulating all non-serotonergic DRN output, to test for non-serotonergic reward mechanisms. To do this we performed a combined knockout / stimulation experiment in mice homozygous for a floxed allele of Tph2, the gene encoding the rate-limiting enzyme for serotonin synthesis. Tph2lox/lox mice were co-injected with a virus mixture, containing a 1:10 ratio of a vector nonspecifically expressing cre recombinase and a second cre-ON vector expressing either ChR2-eYFP or eYFP alone (Figure 3A,B). Because cre-ON vector expression is dependent upon cre recombinase, which is not endogenously expressed in these mice, only cells transduced by both viral vectors will express functional ChR2 protein (Figure 3A). Although all DRN neuron types (serotonin, glutamate, GABA, dopamine) will be capable of laser-activated stimulation, cre recombinase will excise the Tph2 gene, preventing synthesis of serotonin without affecting other transmitters. Indeed, only 1.3% of 1276 total eYFP-positive DRN neurons counted from 3 animals also labeled for tryptophan hydroxylase. Optical stimulation of the DRN in these mice strongly reinforced behavior in nose-poke and real-time place preference tasks (Figure 3C,D). To test whether restoration of serotonin would alter the rewarding properties of DRN stimulation, we administered 5-hydroxytryptophan, the missing intermediate in the serotonin synthesis pathway of ChR2-expressing neurons. To ensure an ability to detect increases or decreases in rewarding properties of stimulation, laser pulse widths were adjusted for each mouse to deliver pulses that were empirically determined to produce 50% of maximal behavioral responding (Figure S3). Nose-poke behavior was not affected by administration of 5-hydroxytryptophan (40 mg/kg, i.p.) 30 minutes prior to testing (Figure 3F), a dose and time point that restores serotonin function in Tph2 knockout mice (Liu et al., 2011). Lastly, to ensure that behavioral effects were anatomically specific to the DRN, we included a control group in which ChR2 expression was targeted anterior to the DRN. The site of injection for anatomical control ChR2 mice ranged from 2.9 to 3.8mm posterior to bregma; injection sites for DRN-targeted ChR2 mice ranged from 3.9 to 4.4mm posterior to bregma (mean difference of 0.7mm). Optical stimulation of neurons anterior to the DRN did not reinforce behavior (Fig 3C,D). These results indicate that a population of DRN cell bodies is capable of driving reward-related behavior in a serotonin-independent fashion.
Figure 3. Optogenetic stimulation of DRN cell bodies reinforces behavior in a dopamine-dependent, serotonin-independent manner.
A, Left panel, schematic view of a cell transduced with cre-induced (“cre-ON”) viral vector. In the absence of cre recombinase, viral plasmid DNA remains in antisense orientation and does not express functional protein. Inset, lack of eYFP signal in mouse injected with cre-ON ChR2-eYFP. Right panel, co-injection of cre-ON and cre-expressing viral vectors results in knockout of floxed genomic DNA and rearrangement of viral plasmid DNA into sense orientation, resulting in expression of ChR2-eYFP. Inset, robust eYFP expression in mouse co-injected with cre-expressing and cre-ON viral vectors. B, Tph2lox/lox mice were co-injected with viral vectors expressing cre and cre-ON ChR2-eYFP or eYFP into DRN. Insets, whole DRN (scale bar = 200 μm) and detail of non-overlapping expression of eYFP (green) and tryptophan hydroxylase (TpH, red). Thus, cells with ChR2 lack the enzyme necessary for serotonin synthesis. An additional anatomical control group was co-injected with cre and cre-ON ChR2 0.7mm anterior to the DRN. C, Nose pokes during first day of self-stimulation testing for non-serotonergic DRN stimulation (n=10), anterior controls (n=8), and eYFP controls (n=7). Two-way ANOVA (group x nose port) interaction F(2,44)=4.482, p<0.05; ** p<0.01 post-hoc. Inset, active nose pokes on 3 consecutive days of testing. D, Percent of time spent on laser side in a real-time place preference task. One-way ANOVA F(2,22)=11.24, p<0.001; ** p<0.01, *** p<0.001 post- hocs. E, Non-serotonin DRN stimulated mice (n=6) were tested for nose-poke optical self-stimulation 30 minutes after injection of vehicle or 5-hydroxytryptophan (5-HTP; 40 mg/kg i.p.), the intermediate in the serotonin synthesis pathway. 5-HTP is the product of the enzyme tryptophan hydroxylase, which is knocked out in ChR2-positive cells of these mice. F, Cumulative-activity graph of nose pokes in test sessions after injection of vehicle or 5-HTP. Individual data points were normalized to percent of nose pokes achieved during a 30-minute baseline session on day 1. G, Non-serotonin DRN stimulated mice (n=6) were tested after injection of the dopamine D1 receptor antagonist SCH23390 (SCH; 30 μg/kg, i.p.) at either 15 or 30 minutes before testing. H, Cumulative-activity graph of active nose pokes in 30-minute sessions following injection of saline or SCH. I, Active nose pokes during 5-minute bins at the beginning or in the middle of test depicted in panel H. Individual data points were normalized to percent of responses during baseline day. Repeated-measures ANOVA (drug x epoch) interaction F(2,15)=4.560, p<0.05; *** p<0.001 Dunnett’s post-hoc vs saline. See also Figures S3 and S4.
To examine whether these effects were dependent upon dopamine signaling, we tested effects of the D1 antagonist SCH23390 (SCH) on optical self-stimulation behavior (Figure 3G). On consecutive days, mice were injected 15 or 30 minutes prior to testing with either saline or a dose of SCH that did not prevent locomotion (30 μg/kg; Figure S4). To reduce inter-subject variability, data from each mouse were normalized to performance on the last day of baseline training. Regardless of injection timing, D1 receptor blockade significantly reduced nose-poke responses during the later period in the session, but not during the first 5 minutes (Figure 3H,I). This pattern of responding suggests a reduction in the rewarding effects of laser stimulation, rather than an impaired ability to perform the nose-poke behavior (Fouriezos and Wise, 1976).
Stimulation of dopaminergic or GABAergic DRN cell bodies do not reinforce instrumental behavior
The anterior DRN contains dopaminergic neurons that are involved in attention and arousal (Lu et al., 2006) that could underlie dopamine-dependent, serotonin-independent DRN reward. We tested this by injecting cre-ON vectors expressing ChR2-eYFP or eYFP alone into the DRN of THiCre mice (Fig 4A). In DRN tissue from 5 mice, 116 out of 240 eYFP-positive cells double-labeled for tyrosine hydroxylase (67.4%). While this specificity was lower than that obtained in the VTA, similar numbers have been reported for the specificity of GFP expression within the DRN of TH-GFP transgenic mice (Dougalis et al., 2012), suggesting that the tyrosine hydroxylase promoter may be active in cells expressing protein at levels below immunohistochemical detection. Nevertheless, stimulation of these cells failed to reinforce behavior in nose-poke or real-time place preference tasks (Figure 4B–D), which indicates that activity of DRN dopamine neurons cannot account for the reward-related behaviors seen previously.
Figure 4. Optogenetic stimulation of dopaminergic or GABAergic DRN cell bodies fails to reinforce nose-poke self-stimulation.
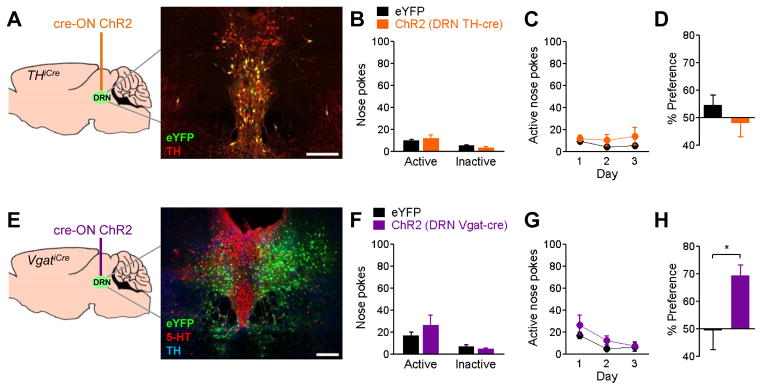
A, DRN dopamine neurons were targeted by injecting cre-induced (“cre-ON”) vectors expressing ChR2-eYFP (n=10) or eYFP (n=14) into the DRN of THiCre mice. Inset shows eYFP (green) double-labeled with tyrosine hydroxylase (TH, red). B, Nose pokes in the first day of testing. C, Active nose pokes on three consecutive days of testing. D, Percent of time spent on laser side in real-time place preference task. E, DRN GABA neurons were targeted by injecting cre-ON ChR2-eYFP (n=8) or eYFP (n=4) into the DRN of VgatiCre mice. Inset shows eYFP (green) cell bodies in the lateral DRN, which do not co-label for serotonin (5-HT, red) or tyrosine hydroxylase (TH, blue). Laser stimulation did not reinforce nose poke self-stimulation (F,G) but did induce a real-time place preference (H), p<0.05. Scale bars = 200 μm.
Thus far, we have observed that a population of DRN neurons is capable of reinforcing behavior, but this effect is not seen with selective stimulation of serotonergic or dopaminergic cell types. This suggests that the rewarding effects of DRN stimulation are driven by GABAergic and/or glutamatergic neurons, the two remaining major DRN cell types. The DRN contains GABAergic projection neurons (Bang and Commons, 2012), which could promote reward-related behavior by disinhibiting mesolimbic circuitry. To test the ability of DRN GABA neurons to reinforce behavior, we injected cre-ON vectors expressing ChR2-eYFP or eYFP alone into the DRN of VgatiCre mice (Figure 4E). Out of 1059 eYFP-positive neurons counted in DRN tissue from 3 mice, less than 1% of cells also labeled for tyrosine hydroxylase or serotonin. Stimulation of these neurons did not result in significant nose-poke behavior (Figure 4F,G), but it did induce a statistically significant real-time place preference (Figure 4H). To the extent that optical stimulation of DRN GABA neurons activates local inhibitory interneurons (Challis et al., 2013), this result is consistent with studies showing that local inhibition of the DRN can produce reward-related behavior in rats (Fletcher et al., 1993; Liu and Ikemoto, 2007). However, the lack of nose-poke behavior indicates that the rewarding effects of these neurons are minimal in the mouse, and they are not capable of driving the vigorous self-stimulation seen previously (Figure 3C).
Distribution of serotonergic and non-serotonergic DRN projections
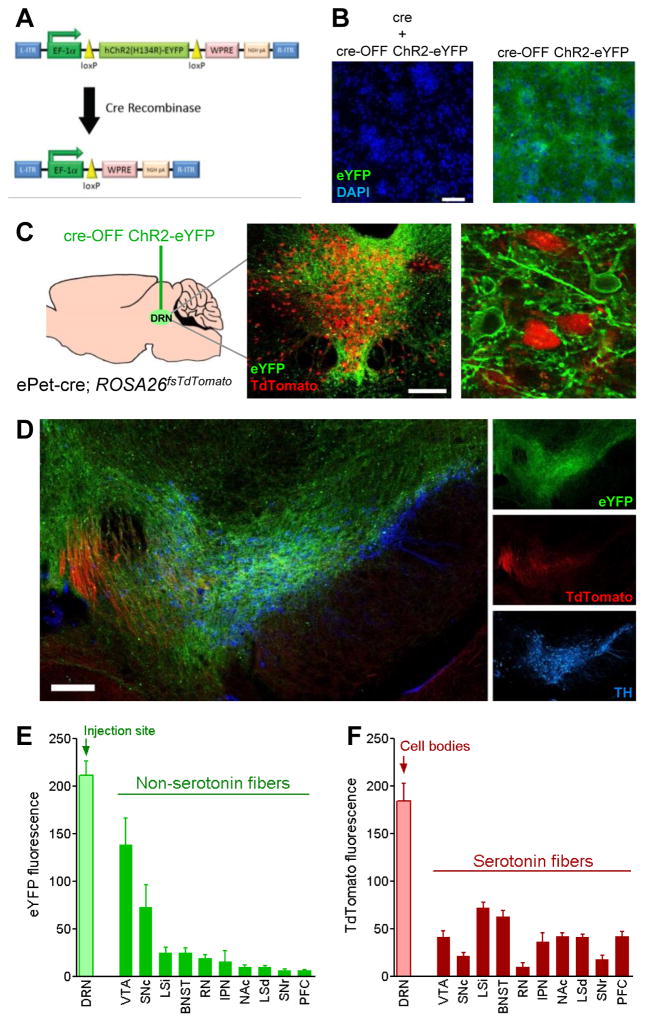
To explore potential interactions of the DRN with mesolimbic reward circuitry, we examined the ascending projections of both serotonergic and non-serotonergic DRN neurons. We constructed a cre-OFF viral vector (Figure 5A) containing a loxP-flanked ChR2-eYFP coding region, thereby expressing eYFP in the absence, but not in the presence, of cre recombinase (Figure 5B). To visualize serotonergic and non-serotonergic projections within the same mice, we injected cre-OFF ChR2-eYFP vector into the DRN of mice containing the serotonergic ePet-cre transgene and the cre-induced TdTomato fluorophore (ePet-cre; ROSA26fsTdTomato, Figure 4C). In these double transgenic mice, serotonergic cre-expressing neurons should express TdTomato but not eYFP. All other transduced neurons within the DRN should express only eYFP. Tissue was collected beginning at 12 weeks post-surgery to allow sufficient time for protein to fill distal processes. Red and green fluorescence within the DRN segregated into separate populations of cre-positive (serotonergic) and cre-negative (non-serotonergic) DRN cells (Figure 5C). Non-serotonergic (eYFP) projections were seen emanating rosto-ventrally from the DRN; this tract split bilaterally and entered the VTA, where it overlapped strongly with tyrosine hydroxylase-positive cell bodies of the lateral VTA (Figure 5D). This was the strongest site of eYFP expression outside of the DRN (Figure 5E). Faint eYFP expression was seen in a limited number of structures in the anterior brain, including the dorosolateral bed nucleus of the stria terminalis, lateral septum, and nucleus accumbens. In contrast, serotonergic (TdTomato) projections did not exhibit the same dramatic degree of variability (Figure 5F). At the level of the VTA, serotonergic projections appeared predominantly as a dense bundle of ascending fibers, located medially to the dopaminergic cells. These findings indicate that non-serotonergic neurons of the DRN project robustly to the dopamine-rich region of the lateral VTA.
Figure 5. Unlike serotonergic neurons, non-serotonergic DRN neurons preferentially project to the VTA.
A, Schematic of cre-silenced (“cre-OFF”) DNA construct containing loxP-flanked ChR2-eYFP coding region. B, Transduction of primary cultured rat neurons with cre-OFF ChR2-eYFP viral vector produces eYFP fluorescence (left) that is abolished in cells co-transduced with a vector expressing cre recombinase (right); DAPI nuclear staining (blue) is unaffected. Scale bar = 100 μm. C, Transgenic mice co-expressing cre and TdTomato in serotonergic neurons (ePet-cre; ROSA26fsTdTomato, n=4) were injected with cre-OFF ChR2-eYFP into the DRN. Inset depicts whole DRN tissue (scale bar = 200 μm) and detail demonstrating segregation of TdTomato and eYFP fluorescence into separate populations of cells. D, Serotonergic (red) and non-serotonergic (green) axons are visible in the VTA, identifiable by tyrosine hydroxylase immunoreactivity (TH, blue). Scale bar = 200 μm. E,F Quantitation of (E) eYFP and (F) TdTomato fluorescence intensity in brain regions with conspicuous eYFP expression. Abbreviations: ventral tegmental area (VTA), substantia nigra pars compacta (SNc), intermediate portion of the lateral septum (LSi), bed nucleus of the stria terminalis (BNST), red nucleus (RN), interpeduncular nucleus (IPN), nucleus accumbens (NAc), dorsal portion of the lateral septum (LSd), substantia nigra reticulata (SNr), prefrontal cortex (PFC).
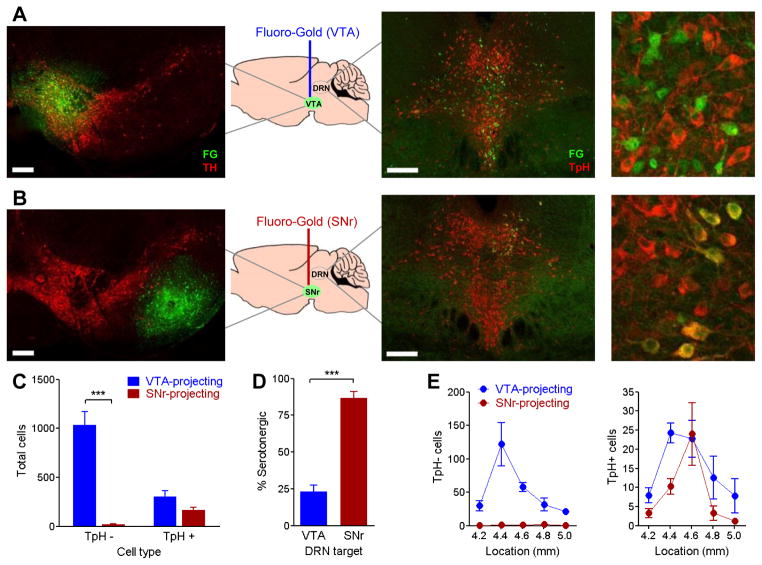
To determine whether non-serotonergic, VTA-projecting neurons reside within the traditional boundaries of the DRN, we iontophoretically infused the retrograde tracer Fluoro-Gold into the VTA or, for comparison, into the substantia nigra reticulata. In VTA-infused mice, strong retrograde labeling was observed medially within the DRN, concentrated at mid-rostral levels (Figure 6A,E). Very little was seen in the median raphe nucleus, similar to previous reports (Watabe-Uchida et al., 2012). Mice infused with tracer into the substantia nigra reticulata showed retrograde labeling that was tightly restricted to a small group of neurons in the dorsal DRN (Figure 6B). While these neighboring pathways both originated within the boundaries of the DRN, the number of non-serotonergic DRN neurons projecting to the VTA vastly outnumbered those projecting to the substantia nigra reticulata (Figure 6C,D). Thus, retrograde and anterograde measures indicate that the DRN-VTA circuit is a robust pathway comprised of primarily non-serotonergic neurons, whose major output is the VTA.
Figure 6. The majority of DRN cell bodies that project to VTA are non-serotonergic.
The retrograde tracer Fluoro-Gold was iontophoretically infused into the (A) VTA or (B) substantia nigra reticulata (n=4/group). Left panels, Fluoro-Gold (green) at infusion site, double-labeled with tyrosine hydroxylase (TH) to label dopamine neurons (red). Right panels, retrograde-labeled cells in DRN, double-labeled with tryptophan hydroxylase (TpH) to label serotonin neurons (red). Scale bars = 200 μm. C, Number of Fluoro-Gold-labeled cells in DRN tissue from mice injected with Fluoro-Gold in VTA or substantia nigra reticulata. Fluoro-Gold cells were grouped by presence or absence of tryptophan hydroxylase double-label (TpH+, TpH−). Two-way ANOVA (region x TpH label interaction) F(1,12)=34.11, p<0.0001; *** p<0.001 post-hoc. D, Percent of Fluoro-Gold labeled cells double-labeling for tryptophan hydroxylase. *** p<0.0001. E, Number of TpH− (left) and TpH+ (right) Fluoro-Gold labeled cells across the rostrocaudal axis of the DRN. X-axis indicates location of DRN tissue, in millimeters posterior to bregma.
Non-serotonergic DRN-VTA pathway mediates behavioral reinforcement and excites VTA dopamine neurons
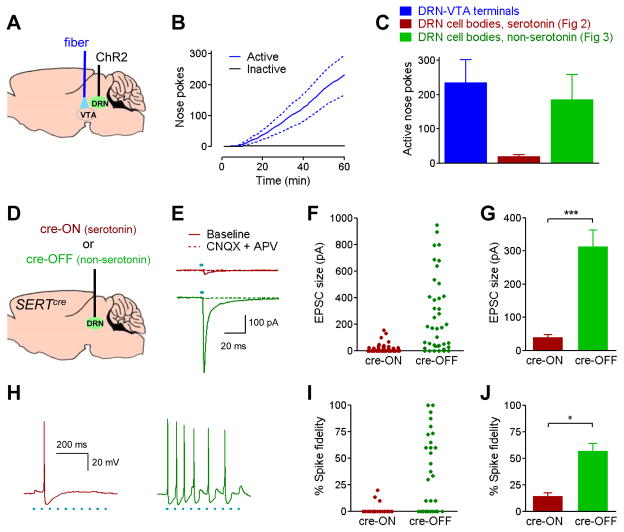
To determine if selective stimulation of DRN-VTA projections was sufficient to reinforce behavior, we targeted ChR2 expression to the DRN of wild-type mice and implanted optical fibers in the VTA (Figure 7A). Mice vigorously nose-poked for stimulation of the DRN-VTA pathway (Figure 7B), indicating that DRN-VTA projections are sufficient to drive reward-related behavior. The lack of rewarding effects from stimulation of DRN serotonin, dopamine, and GABA cell bodies strongly suggests that this rewarding DRN-VTA pathway contains a glutamatergic component. To compare glutamatergic contributions of serotonergic and non-serotonergic DRN-VTA projections, we used cre-ON and cre-OFF vectors to express ChR2-eYFP in SERTcre mice (Figure 7D). SERTcre and ePet-cre mouse lines demonstrate similar selectively for expression in serotonergic neurons, as 90.3% of 651 total eYFP-positive cells from five SERTcre mice injected with cre-ON eYFP virus were found to double label with tryptophan hydroxylase. However, preliminary results in TdTomato reporter mice suggested greater penetrance of expression in serotonergic populations with the SERTcre line (data not shown), therefore we used these mice in electrophysiology experiments to ensure that cre-OFF viral expression was limited to non-serotonergic neurons. We obtained whole-cell patch clamp recordings in horizontal slices containing VTA tissue beginning 12 weeks after surgery. Stimulation of the non-serotonin pathway produced excitatory post-synaptic currents (EPSCs) in 34 out of 43 cells tested (79.1%, Figure 6F), with average latency of 1.84 ± 0.15 ms. Stimulation of the serotonin pathway produced EPSCs in 21 out of 47 cells tested (44.7%), with an average latency of 2.51 ± 0.11 ms. The rapid latency and low jitter of current response times suggest monosynaptic connections. The magnitude of EPSCs produced by stimulation of the non-serotonin pathway were significantly larger than those produced by the serotonin pathway (Figure 7G). Bath application of selective AMPA and NMDA antagonists completely abolished evoked currents (Figure 7E). When light-responsive cells were tested for action potentials in current-clamp (Figure 7H), stimulation of the non-serotonin pathway produced action potentials in 19 / 28 cells tested (67.9%, Figure 7I). The serotonin pathway only produced spikes in 3 / 17 cells (17.6%). In addition, spike fidelity was greater in response to optical stimulation of the non-serotonin pathway (Figure 7J). These data demonstrate that non-serotonergic, glutamatergic DRN neurons directly target VTA dopamine neurons and are highly efficacious in enhancing their rate of firing.
Figure 7. DRN-VTA projections reinforce behavior and provide synaptic glutamatergic excitation of VTA dopamine neurons primarily via non-serotonergic projections.
A, Mice (n=11) were injected with non-specific ChR2 viral vector in the DRN, and implanted with fiber optic cables in the VTA. B, Cumulative activity-graph of nose pokes into active and inactive ports on the first day of training. Total number of responses was greater into the active port (p<0.01). C, Total number of active nose pokes on day 1 from DRN-VTA mice. For comparison, data is reconstituted from previous experiments stimulating serotonergic and non-serotonergic DRN cell bodies. D, Serotonergic and non-serotonergic DRN projections were targeted by injecting cre-induced (“cre-ON”) (n=6) or cre-silenced (“cre-OFF”) (n=4) vectors expressing ChR2-eYFP in SERTcre mice. E, Representative voltage-clamp traces of VTA dopamine neurons showing optically-evoked glutamatergic excitatory post-synaptic current (EPSC) resulting from stimulation of terminals of the serotonergic (top trace) or non-serotonergic (bottom trace) DRN-VTA pathway. F, EPSC amplitudes in response to optical stimulation. Graph includes cells that did not respond to light (plotted as 0 pA). G, Average amplitude of light-responsive EPSCs, ***, p<0.0001. H, Representative current-clamp traces of a VTA dopamine neuron spiking in response to 20 Hz laser stimulation of DRN-VTA serotonin (left) or non-serotonin (right) pathways. I, Individual spike fidelity measurements; represented as percent of laser pulses during a 0.5 second, 20 Hz train that resulted in action potentials. J, Average spike fidelity in cells that responded to light with at least one action potential, p<0.05.
Discussion
In the present study we found that stimulation of DRN cell bodies was capable of strongly reinforcing instrumental behavior, with a level of vigor comparable to direct stimulation of VTA dopamine neurons. Although serotonergic neurons are the largest population of projection neurons in the DRN, selective stimulation of these cells did not reinforce behavior. Rather, self-stimulation was preferentially elicited by targeting non-serotonergic DRN neurons, which we showed to comprise the majority of DRN-VTA projections. Furthermore, stimulation of the DRN-VTA pathway was sufficient to fully reinforce instrumental learning. Because self-stimulation was not supported by DRN dopaminergic or GABAergic cell bodies, our observations suggest that the rewarding effects of DRN stimulation are mediated by non-serotonergic glutamate neurons. Accordingly, in vitro stimulation of the non-serotonergic DRN-VTA pathway increased VTA dopamine neuron firing rates and produced monosynaptic glutamatergic currents that were substantially larger than those elicited by stimulating the serotonergic pathway.
The DRN contains the largest group of serotonergic neurons in the brain, a subset of which encode information about magnitude of reward received (Inaba et al., 2013; Liu et al., 2014; Nakamura et al., 2008). We employed a variety of optogenetic and pharmacological methodologies to test the possibility that such activity drives reinforcement learning. In all cases, the results of our experiments did not support this conclusion. This is consistent with reports that rats and primates do not self-administer serotonergic drugs (Gotestam and Andersson, 1975; Griffiths et al., 1976), which we now extend to mice. In both fenfluramine and optogenetic place preference experiments, maximal trends towards rewarding effects were seen at low doses/frequencies, suggesting that serotonin may exert pro-reward effects in an inverted-U shaped manner. A recent study demonstrated that optogenetic stimulation of serotonergic DRN neurons in mice can reinforce a variety of instrumental tasks (Liu et al., 2014). There were several methodological differences between this study and our present work that likely afforded Liu et al greater sensitivity in detecting behavioral effects of laser stimulation which, in our experiments, produced non-significant trends toward the same direction of effect. Nevertheless, using identical behavioral and optical parameters across experiments, we found that reinforcement learning was preferentially supported in this region by a population of non-serotonergic neurons.
Mice in our study vigorously nose-poked for optogenetic stimulation of non-serotonergic DRN neurons. This finding is consistent with reports that rats will respond for electrical DRN stimulation in a serotonin-independent manner (Margules, 1969; Rompre and Miliaressis, 1987; Simon et al., 1976) – but see (Van Der Kooy et al., 1978). Although electrical self-stimulation literature provides the foundation for our understanding of brain reward circuitry, interpretation of this work is inherently limited by the fact that electrical stimulation of brain tissue excites both cell bodies and axonal fibers. In fact, action potentials are preferentially induced in axons, due to a far greater surface density of sodium channels (Nowak and Bullier, 1998). This issue is of particular importance because the DRN is bordered by dense fiber tracts; furthermore, it receives strong projections from several brain regions which are each individually sufficient to support reward learning, including the lateral hypothalamus, laterodorsal tegmental nucleus, and medial prefrontal cortex (Britt et al., 2012a; Kempadoo et al., 2013; Lammel et al., 2012; Lee et al., 2003). By using optogenetic methodology, we are able to negate the influence of stimulating fibers and localize the reward-relevant neuronal cell bodies.
Electrical mapping studies indicate that rewarding sites in the brain are not restricted to the DRN, but extend rostrally in a continuous band before bifurcating laterally and merging with the VTA (Rompre and Miliaressis, 1985). We found that stimulation of cell bodies rostral to the DRN did not produce behavioral measures of reward, although we did observe efferent fibers of DRN neurons in an identical pattern to the rewarding region described. Thus, the rewarding effects of electrical stimulation in this region are likely mediated by activation of axonal fibers originating from non-serotonergic cell bodies in the DRN. Furthermore, two-electrode collision experiments within this region suggest that the reward-relevant axons are highly branched between VTA and DRN (Boye and Rompre, 1996), suggestive of the dense network of non-serotonergic fibers that we observed in the VTA. The rewarding properties of DRN stimulation were dependent upon dopamine receptor activation. Although the DRN contains dopaminergic cell bodies (Dougalis et al., 2012; Lu et al., 2006), stimulation of these cells did not evoke reward-related behavior, suggesting action on mesolimbic dopamine circuitry. Accordingly, we found that non-serotonergic DRN neurons primarily project to the VTA, with comparatively sparse projections to the nucleus accumbens and other forebrain structures. Furthermore, stimulation of the DRN-VTA pathway was sufficient to fully reproduce the rewarding effects of DRN cell body stimulation. Although other projection targets may contribute, these findings suggest that the DRN is capable of driving reinforcement learning primarily through its projection to the VTA.
Because individual stimulation of serotonergic, GABAergic, and dopaminergic DRN cell bodies failed to reinforce behavior, we infer that the rewarding effects seen in our non-serotonergic stimulation experiment were mediated through a distinct population of cell bodies. The largest remaining population of cells, accounting for approximately 10% of DRN neuronal cell bodies, are non-serotonergic neurons expressing vesicular glutamate transporter 3 (Commons, 2009; Hioki et al., 2010). Indeed, we observed that stimulation of the non-serotonergic DRN-VTA pathway produced strong monosynaptic glutamatergic currents and drove spiking activity in VTA dopamine neurons. Comparatively weak currents were observed following stimulation of the serotonergic DRN-VTA pathway. Because direct excitation of VTA dopamine neurons is sufficient to powerfully reinforce instrumental learning (Witten et al., 2011), it seems reasonable to propose that the rewarding effects of non-serotonergic DRN stimulation were driven, at least in part, by a glutamatergic DRN-VTA mechanism. However, the DRN is also noted to contain several peptidergic cell types, including corticotropin-releasing factor and substance P (Valentino and Commons, 2005). Although these peptides are aversive when administered intracerebroventricularly (Cador et al., 1992; Elliott, 1988), we cannot rule out the possibility that these or other DRN cell types contribute to reinforcement learning. With these caveats in mind, the most parsimonious interpretation of the data presented is that this population of non-serotonergic glutamate neurons is highly efficacious in driving reward-related behavior.
It has been shown that the DRN sends projections to mesolimbic circuitry, with the VTA receiving notably stronger innervation than nucleus accumbens (Vertes, 1991). Retrograde studies mapping whole-brain inputs to the VTA have noted the DRN as a major input (Geisler et al., 2007; Phillipson, 1979; Watabe-Uchida et al., 2012). None of these reports, however, examined the serotonergic composition of this projection. Early studies of DRN anatomy led to the view that nearly all of its projection neurons are serotonergic (reviewed in Jacobs and Azmitia, 1992). We have quantitatively compared the composition of the DRN-VTA projections using anterograde, retrograde, and electrophysiological techniques. All three approaches supported the same conclusion: the majority of this pathway consists of non-serotonergic projections. Our study raises important questions and open new avenues of investigation with respect to the role of this circuit in normal function and disease states.
Experimental procedures
Animals
Adult (8+ weeks) male and female mice were housed with food and water available ad libitum. Mice were housed on a 12/12 hour light cycle with lights on at 7:00 AM. All experiments except intravenous self-administration were carried out during the animals’ light cycle. Wild-type C57Bl6/J mice were ordered from Jackson Laboratories (Bar Harbor, ME); transgenic mice were bred in-house. Transgenic expression of cre recombinase was achieved in serotonin neurons using ePet-cre (Scott et al., 2005) or Sl6a4cre/+ mice, referred to herein as SERTcre (Zhuang et al., 2005). ePet-cre mice were considered advantageous for behavioral experiments (Figure 2) because SERTcre mice are heterozygous knockouts for the serotonin transporter, a manipulation that alters basal extracellular serotonin levels (Mathews et al., 2004) and could possibly confound behavioral data. Additionally, ePet-cre mice do not demonstrate ectopic cre expression during early development like the SERTcre line (Scott et al., 2005; Zhuang et al., 2005), and were therefore used to selectively induce recombination in serotonergic neurons for genetic fluorescent labeling (Figure 5). Dopaminergic and GABAergic neurons were targeted using THiCre/+ (Lindeberg et al., 2004) and VgatiCre/+ (Vong et al., 2011) mice. Deletion of serotonin synthesis was carried out in Tph2lox/lox mice (Wu et al., 2012). Cre-mediated fluorescence was produced using ROSA26fsTdTomato/+ mice, which carry a floxed stop cassette preceding a coding region for the TdTomato gene (Madisen et al., 2010). All lines were backcrossed onto a C57Bl6/J background. Mice were surgically injected with viral vectors (Table S1) and implanted with fiber optic cables (Britt et al., 2012b), the details of which are described in Supplemental Experimental Procedures. All animal procedures were approved by the National Institute on Drug Abuse’s animal care and use committee and carried out in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Optogenetic real-time place preference
Mice were placed in a 24 × 36 cm plastic chamber with walls of opposite halves identified by horizontal or vertical stripes, and a small 0.5 cm barrier on the floor at the division site. A cohort of naïve wild-type mice tested in these chambers did not display a preference for either side (51.8 ±2.6% preference for horizontally-striped half; n=6). Mouse movement was tracked with Ethovision computer software (Noldus; Wageningen, Netherlands) and presence in the randomly-assigned laser-paired half resulted in 15 mW 473nm laser stimulation at 20 Hz, with 5 ms pulses. Mice remained in the chamber for 12 minutes. Electrical self-stimulation studies indicate that the reward substrate within the DRN is increasingly responsive to higher stimulation frequencies, with reward thresholds occurring within the range of 13–40 Hz, depending on electrode placement (Rompre and Miliaressis, 1985). ChR2 protein is capable of inducing action potentials in a variety of neuron types up to 20 Hz, above which spike fidelity is less reliable (Tye and Deisseroth, 2012). Therefore in the present study, 20 Hz optogenetic stimulation was used to drive action potentials in serotonergic and non-serotonergic DRN cells (Figure S2). Although serotonergic DRN neurons typically have baseline firing rates below 5 Hz, they were recently shown to briefly fire at 20–30 Hz during a reward task (Liu et al., 2014). DRN serotonin neurons are capable of following extrinsic 20 Hz stimulation without entering depolarization block, as assessed with whole-cell patch clamp (Figure S2) and in vivo microdialysis (Sharp et al., 1989).
Optogenetic nose-poke self-stimulation
One week after the place preference task, mice were allowed to self-stimulate by performing a nose-poke instrumental response (Stuber et al., 2011). On a habituation day, mice were placed in operant chambers (Med Associates; St Albans, VT) for 45 minutes with ports closed off to prevent access. Mice were then given access to ports for 3 days of testing. Mice were placed in the chamber for 1 hour. A nose poke into the active port resulted in a 3-second train of laser pulses (30mW for midline DRN stimulation, 2 × 15mW for bilateral VTA stimulation) at 20 Hz with 5 ms pulses, accompanied by dimming of the house light and an auditory cue. Nose pokes during the 3 second stimulation period had no consequence and were not counted towards the active nose poke total.
Electrophysiology
Details of procedures and recipes can be found in supplemental methods. Briefly, cells within 250 μm slices of tissue containing DRN or VTA were recorded in whole-cell patch clamp using a potassium gluconate-based internal solution. VTA dopamine cells were identified by morphology, tonic spike rate, and presence of a hyperpolarization-induced Ih current, which can be a reasonable predictor of dopaminergic identity in mice (Margolis et al., 2006; Wanat et al., 2008; Zhang et al., 2010). Cells were optically stimulated with 473 nm laser light, directed at tissue through a fiber optic cable submerged in the bath and aimed at the region of interest.
Supplementary Material
Table S1, related to Figures 2,3,4,5,7. List of viral vectors used in experiments. Adeno-associated virus (AAV) vectors are listed in the order they are introduced in the manuscript. All ChR2 constructs contain the H134R mutation to increase amplitude of currents (Nagel et al., 2005). Cre-ON vectors contain a double-inverted open reading frame (Atasoy et al., 2008). Cre-OFF vectors contain a loxP-flanked open reading frame, use a similar strategy to other recently characterized vectors (Saunders et al., 2012). AAV-1 serotypes were packaged in-house by the National Institute on Drug Abuse Optogenetics and Transgenic Technology Core. AAV-5 vector was packaged at the University of North Carolina Vector Core.
Figure S1, related to Figure 2. Parametric variations of laser stimulus do not result in statistically significant preference or avoidance of laser-paired chamber in mice with optogenetic stimulation of DRN serotonin neurons. A, Mice were tested on four consecutive days for real-time place preference of optogenetic stimulation on one half of a chamber. Stimulation was delivered at 15 mW strength, in 5 ms pulses at 20 Hz. Serotonin-stimulated ePet-cre mice received DRN injections of cre-ON ChR2-eYFP (n=18). Also included were positive control mice with stimulation of VTA dopamine neurons via cre-ON ChR2-eYFP in TH-cre mice (n=10) and negative control mice receiving cre-ON eYFP alone, injected into DRN or VTA (n=10). B, One cohort of DRN serotonin-stimulated mice (n=10) was tested in “context B,” a chamber with different markings from the one used in the repeated-days experiment in panel A. Testing was conducted one week after nose-poke self-administration experiment (Figure 2F). Laser intensity was increased in four consecutive daily tests, with stimulation delivered in 5 ms pulses at 20 Hz. C, A second cohort of DRN serotonin-stimulated mice (n=8) were tested in context B, as described above. Laser stimulus was applied at 15 mW in 5 ms pulses, with frequency varying in daily tests.
Figure S2, related to Figure 2. Serotonergic DRN neurons do not enter depolarization block with 20 Hz optogenetic stimulation. A, DRN neurons from mice injected with nonspecific ChR2 virus were recorded in current clamp, as in Figure 2J. When stimulated with 20 Hz 5-msec pulses of blue light, neurons followed with action potentials throughout the 6-second stimulation period. B, Spike fidelity in the first and last second of the stimulation period did not differ between putative serotonergic (n=4 cells; 4 mice) and non-serotonergic (n=6 cells; 5 mice) neurons, identified by presence or absence of hyperpolarization in response to bath application of 100 μM serotonin at the end of the experiment, a response mediated by 5-HT1A receptors that are a preferential marker of serotonergic identity within the DRN (Day et al., 2004; Kirby et al., 2003). C, In contrast to pulsed light, DRN neurons respond to constant illumination by initial spiking, followed by depolarization without action potentials. D, In all cells examined, spikes were present in the first but not in the last second of a 6-second period of illumination.
Figure S3, related to Figure 3. Optical self-stimulation for VTA dopamine cell bodies and non-serotonergic DRN cell bodies demonstrate similar input-output curves. Well-trained mice were given access to self-stimulation for VTA dopamine cell bodies (n=7) or DRN non-serotonin cell bodies (n=5). Mice were placed in operant chambers and allowed to nose-poke for 3-second trains of 20 Hz 5ms pulses of light for a baseline period of 10 minutes. Afterwards, mice were given 5 minutes of access to nose-poking for trains with descending pulse widths, from 20 to 0.2 ms.
Figure S4, related to Figure 3. SCH23390 dose-dependently reduces novelty-induced locomotion. Naïve wild-type mice were injected with saline or the dopamine D1 antagonist SCH23390 at 30, 100, or 300 μg/kg, i.p. (n=4/group) and immediately placed in a novel environment, consisting of a plastic 33 × 40 cm tub containing fresh bedding. Novelty-induced locomotion was monitored for 90 minutes A, Distance traveled during 5-minute bins. Panels B and C depict drug effects during time periods used in nose-poke experiment in Figure 3. B, Distance traveled during minutes 15–45 after injection. One-way ANOVA F(3,12)=3.966, p<0.05; * p<0.05 Dunnett’s post-hoc vs saline. C, Distance traveled during minutes 30–60 after injection. One-way ANOVA F(3,12)=6.080, p<0.01; * p<0.05, **p<0.01 Dunnett’s post-hoc vs saline.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse. We are grateful to Dr. Karl Deisseroth for providing cre-ON ChR2 plasmid DNA, Dr. Zheng-Xiong Xi for assistance with mouse self-administration, Dr. Francois Vautier and Joni McKenzie for assistance with transgenic mouse colonies, Zachary Fusfeld, Christina Hatch, India J. Kawata, and Martha Zemen for assistance with surgery and behavioral testing, Doug Howard and Lowella Fortuno for assistance with cre-OFF vector preparation, and the NIDA Optogenetic and Transgenic Technology Core for production of other vectors.
Footnotes
The authors declare no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang SJ, Commons KG. Forebrain GABAergic projections from the dorsal raphe nucleus identified by using GAD67-GFP knock-in mice. J Comp Neurol. 2012;520:4157–4167. doi: 10.1002/cne.23146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye SM, Rompre PP. Mesencephalic substrate of reward: axonal connections. J Neurosci. 1996;16:3511–3520. doi: 10.1523/JNEUROSCI.16-10-03511.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012a;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, McDevitt RA, Bonci A. Use of channelrhodopsin for activation of CNS neurons. In: Crawley Jacqueline N, et al., editors. Current protocols in neuroscience. Unit2. Chapter 2. 2012b. p. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cador M, Ahmed SH, Koob GF, Le Moal M, Stinus L. Corticotropin-releasing factor induces a place aversion independent of its neuroendocrine role. Brain Res. 1992;597:304–309. doi: 10.1016/0006-8993(92)91487-y. [DOI] [PubMed] [Google Scholar]
- Challis C, Boulden J, Veerakumar A, Espallergues J, Vassoler FM, Pierce RC, Beck SG, Berton O. Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. J Neurosci. 2013;33:13978–13988. 13988a. doi: 10.1523/JNEUROSCI.2383-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG. Locally collateralizing glutamate neurons in the dorsal raphe nucleus responsive to substance P contain vesicular glutamate transporter 3 (VGLUT3) J Chem Neuroanat. 2009;38:273–281. doi: 10.1016/j.jchemneu.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett D, Wise RA. Intracranial self-stimulation in relation to the ascending noradrenergic fiber systems of the pontine tegmentum and caudal midbrain: a moveable electrode mapping study. Brain Res. 1979;177:423–436. doi: 10.1016/0006-8993(79)90461-x. [DOI] [PubMed] [Google Scholar]
- Dougalis AG, Matthews GA, Bishop MW, Brischoux F, Kobayashi K, Ungless MA. Functional properties of dopamine neurons and co-expression of vasoactive intestinal polypeptide in the dorsal raphe nucleus and ventro-lateral periaqueductal grey. Eur J Neurosci. 2012;36:3322–3332. doi: 10.1111/j.1460-9568.2012.08255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott PJ. Place aversion induced by the substance P analogue, dimethyl-C7, is not state dependent: implication of substance P in aversion. Experimental brain research. 1988;73:354–356. doi: 10.1007/BF00248227. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Ming ZH, Higgins GA. Conditioned place preference induced by microinjection of 8-OH-DPAT into the dorsal or median raphe nucleus. Psychopharmacology. 1993;113:31–36. doi: 10.1007/BF02244330. [DOI] [PubMed] [Google Scholar]
- Fouriezos G, Wise RA. Pimozide-induced extinction of intracranial self-stimulation: response patterns rule out motor or performance deficits. Brain Res. 1976;103:377–380. doi: 10.1016/0006-8993(76)90809-x. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotestam KG, Andersson BE. Self-administration of amphetamine analogues in rats. Pharmacology, biochemistry, and behavior. 1975;3:229–233. doi: 10.1016/0091-3057(75)90152-5. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Winger G, Brady JV, Snell JD. Comparison of behavior maintained by infusions of eight phenylethylamines in baboons. Psychopharmacology. 1976;50:251–258. doi: 10.1007/BF00426841. [DOI] [PubMed] [Google Scholar]
- Hioki H, Nakamura H, Ma YF, Konno M, Hayakawa T, Nakamura KC, Fujiyama F, Kaneko T. Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J Comp Neurol. 2010;518:668–686. doi: 10.1002/cne.22237. [DOI] [PubMed] [Google Scholar]
- Inaba K, Mizuhiki T, Setogawa T, Toda K, Richmond BJ, Shidara M. Neurons in monkey dorsal raphe nucleus code beginning and progress of step-by-step schedule, reward expectation, and amount of reward outcome in the reward schedule task. J Neurosci. 2013;33:3477–3491. doi: 10.1523/JNEUROSCI.4388-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Kempadoo KA, Tourino C, Cho SL, Magnani F, Leinninger GM, Stuber GD, Zhang F, Myers MG, Deisseroth K, de Lecea L, Bonci A. Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J Neurosci. 2013;33:7618–7626. doi: 10.1523/JNEUROSCI.2588-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Kim MA, Valentino RJ, Waterhouse BD. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res. 2003;963:57–71. doi: 10.1016/s0006-8993(02)03841-6. [DOI] [PubMed] [Google Scholar]
- Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Soderstrom S, Ebendal T. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis. 2004;40:67–73. doi: 10.1002/gene.20065. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jiang Y, Si Y, Kim JY, Chen ZF, Rao Y. Molecular regulation of sexual preference revealed by genetic studies of 5-HT in the brains of male mice. Nature. 2011;472:95–99. doi: 10.1038/nature09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhou J, Li Y, Hu F, Lu Y, Ma M, Feng Q, Zhang JE, Wang D, Zeng J, et al. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron. 2014;81:1360–1374. doi: 10.1016/j.neuron.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZH, Ikemoto S. The midbrain raphe nuclei mediate primary reinforcement via GABA(A) receptors. Eur J Neurosci. 2007;25:735–743. doi: 10.1111/j.1460-9568.2007.05319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Jhou TC, Saper CB. Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci. 2006;26:193–202. doi: 10.1523/JNEUROSCI.2244-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margules DL. Noradrenergic rather than serotonergic basis of reward in the dorsal tegmentum. Journal of comparative and physiological psychology. 1969;67:32–35. doi: 10.1037/h0026652. [DOI] [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumoto M, Hikosaka O. Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. J Neurosci. 2008;28:5331–5343. doi: 10.1523/JNEUROSCI.0021-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak LG, Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. I. Evidence from chronaxie measurements. Experimental brain research. 1998;118:477–488. doi: 10.1007/s002210050304. [DOI] [PubMed] [Google Scholar]
- Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol. 1979;187:117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- Rompre PP, Miliaressis E. Pontine and mesencephalic substrates of self-stimulation. Brain Res. 1985;359:246–259. doi: 10.1016/0006-8993(85)91435-0. [DOI] [PubMed] [Google Scholar]
- Rompre PP, Miliaressis E. Behavioral determination of refractory periods of the brainstem substrates of self-stimulation. Behavioural brain research. 1987;23:205–219. doi: 10.1016/0166-4328(87)90021-0. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Appetite suppressants, cardiac valve disease and combination pharmacotherapy. American journal of therapeutics. 2009;16:354–364. doi: 10.1097/MJT.0b013e31817fde95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci U S A. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp T, Bramwell SR, Clark D, Grahame-Smith DG. In vivo measurement of extracellular 5-hydroxytryptamine in hippocampus of the anaesthetized rat using microdialysis: changes in relation to 5-hydroxytryptaminergic neuronal activity. J Neurochem. 1989;53:234–240. doi: 10.1111/j.1471-4159.1989.tb07319.x. [DOI] [PubMed] [Google Scholar]
- Simon H, Le Moal M, Cardo B. Intracranial self-stimulation from the dorsal raphe nucleus of the rat: effects of the injection of para-chlorophenylalanine and of alpha-methylparatyrosine. Behavioral biology. 1976;16:353–364. doi: 10.1016/s0091-6773(76)91486-3. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13:251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Commons KG. Peptides that fine-tune the serotonin system. Neuropeptides. 2005;39:1–8. doi: 10.1016/j.npep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Van Der Kooy D, Fibiger HC, Phillips AG. An analysis of dorsal and median raphe self-stimulation: effects of parachlorophenylalanine. Pharmacology, biochemistry, and behavior. 1978;8:441–445. doi: 10.1016/0091-3057(78)90083-7. [DOI] [PubMed] [Google Scholar]
- Vasudeva RK, Lin RC, Simpson KL, Waterhouse BD. Functional organization of the dorsal raphe efferent system with special consideration of nitrergic cell groups. J Chem Neuroanat. 2011;41:281–293. doi: 10.1016/j.jchemneu.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483:594–597. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawertailo LA, Busto U, Kaplan HL, Sellers EM. Comparative abuse liability of sertraline, alprazolam, and dextroamphetamine in humans. Journal of clinical psychopharmacology. 1995;15:117–124. doi: 10.1097/00004714-199504000-00007. [DOI] [PubMed] [Google Scholar]
- Zhang TA, Placzek AN, Dani JA. In vitro identification and electrophysiological characterization of dopamine neurons in the ventral tegmental area. Neuropharmacology. 2010;59:431–436. doi: 10.1016/j.neuropharm.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R. Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J Neurosci Methods. 2005;143:27–32. doi: 10.1016/j.jneumeth.2004.09.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1, related to Figures 2,3,4,5,7. List of viral vectors used in experiments. Adeno-associated virus (AAV) vectors are listed in the order they are introduced in the manuscript. All ChR2 constructs contain the H134R mutation to increase amplitude of currents (Nagel et al., 2005). Cre-ON vectors contain a double-inverted open reading frame (Atasoy et al., 2008). Cre-OFF vectors contain a loxP-flanked open reading frame, use a similar strategy to other recently characterized vectors (Saunders et al., 2012). AAV-1 serotypes were packaged in-house by the National Institute on Drug Abuse Optogenetics and Transgenic Technology Core. AAV-5 vector was packaged at the University of North Carolina Vector Core.
Figure S1, related to Figure 2. Parametric variations of laser stimulus do not result in statistically significant preference or avoidance of laser-paired chamber in mice with optogenetic stimulation of DRN serotonin neurons. A, Mice were tested on four consecutive days for real-time place preference of optogenetic stimulation on one half of a chamber. Stimulation was delivered at 15 mW strength, in 5 ms pulses at 20 Hz. Serotonin-stimulated ePet-cre mice received DRN injections of cre-ON ChR2-eYFP (n=18). Also included were positive control mice with stimulation of VTA dopamine neurons via cre-ON ChR2-eYFP in TH-cre mice (n=10) and negative control mice receiving cre-ON eYFP alone, injected into DRN or VTA (n=10). B, One cohort of DRN serotonin-stimulated mice (n=10) was tested in “context B,” a chamber with different markings from the one used in the repeated-days experiment in panel A. Testing was conducted one week after nose-poke self-administration experiment (Figure 2F). Laser intensity was increased in four consecutive daily tests, with stimulation delivered in 5 ms pulses at 20 Hz. C, A second cohort of DRN serotonin-stimulated mice (n=8) were tested in context B, as described above. Laser stimulus was applied at 15 mW in 5 ms pulses, with frequency varying in daily tests.
Figure S2, related to Figure 2. Serotonergic DRN neurons do not enter depolarization block with 20 Hz optogenetic stimulation. A, DRN neurons from mice injected with nonspecific ChR2 virus were recorded in current clamp, as in Figure 2J. When stimulated with 20 Hz 5-msec pulses of blue light, neurons followed with action potentials throughout the 6-second stimulation period. B, Spike fidelity in the first and last second of the stimulation period did not differ between putative serotonergic (n=4 cells; 4 mice) and non-serotonergic (n=6 cells; 5 mice) neurons, identified by presence or absence of hyperpolarization in response to bath application of 100 μM serotonin at the end of the experiment, a response mediated by 5-HT1A receptors that are a preferential marker of serotonergic identity within the DRN (Day et al., 2004; Kirby et al., 2003). C, In contrast to pulsed light, DRN neurons respond to constant illumination by initial spiking, followed by depolarization without action potentials. D, In all cells examined, spikes were present in the first but not in the last second of a 6-second period of illumination.
Figure S3, related to Figure 3. Optical self-stimulation for VTA dopamine cell bodies and non-serotonergic DRN cell bodies demonstrate similar input-output curves. Well-trained mice were given access to self-stimulation for VTA dopamine cell bodies (n=7) or DRN non-serotonin cell bodies (n=5). Mice were placed in operant chambers and allowed to nose-poke for 3-second trains of 20 Hz 5ms pulses of light for a baseline period of 10 minutes. Afterwards, mice were given 5 minutes of access to nose-poking for trains with descending pulse widths, from 20 to 0.2 ms.
Figure S4, related to Figure 3. SCH23390 dose-dependently reduces novelty-induced locomotion. Naïve wild-type mice were injected with saline or the dopamine D1 antagonist SCH23390 at 30, 100, or 300 μg/kg, i.p. (n=4/group) and immediately placed in a novel environment, consisting of a plastic 33 × 40 cm tub containing fresh bedding. Novelty-induced locomotion was monitored for 90 minutes A, Distance traveled during 5-minute bins. Panels B and C depict drug effects during time periods used in nose-poke experiment in Figure 3. B, Distance traveled during minutes 15–45 after injection. One-way ANOVA F(3,12)=3.966, p<0.05; * p<0.05 Dunnett’s post-hoc vs saline. C, Distance traveled during minutes 30–60 after injection. One-way ANOVA F(3,12)=6.080, p<0.01; * p<0.05, **p<0.01 Dunnett’s post-hoc vs saline.