Abstract
Approximately 39 million people are blind worldwide, with an estimated 285 million visually impaired. The developing world shoulders 90% of the world’s blindness, with 80% of causative diseases being preventable or treatable. Blindness has a major detrimental impact on the patient, community, and healthcare spending. Corneal diseases are significant causes of blindness, affecting at least 4 million people worldwide. The prevalence of corneal disease varies among parts of the world. Trachoma, for instance, is the second leading cause of blindness in Africa, after cataracts, but is rarely found today in developed nations.
When preventive strategies have failed, corneal transplantation is the most effective treatment for advanced corneal disease. The major surgical techniques for corneal transplantation include penetrating keratoplasty (PK), anterior lamellar keratoplasty (ALK), and endothelial keratoplasty (EK). Indications for corneal transplantation vary among countries, with Fuchs’ dystrophy being the leading indication in the U.S. and keratoconus in Australia. With the exception of the US, where EK will soon overtake PK as the most common surgical procedure, PK is the overwhelming procedure of choice.
Success using corneal grafts in developing nations, such as Nepal, demonstrates the feasibility of corneal transplantation on a global scale. The number of suitable corneas from deceased human donors that becomes available will never be sufficient, and so research into various alternatives, e.g., stem cells, amniotic membrane transplantation, synthetic and biosynthetic corneas, and xenotransplantation, is progressing. While each of these has potential, we suggest that xenotransplantation holds the greatest potential for a corneal replacement. With the increasing availability of genetically-engineered pigs, pig corneas may alleviate the global shortage of corneas in the near future.
Keywords: Corneal transplantation, Developing world, Donor shortage, corneas, Keratoplasty, Pig, Xenotransplantation
Introduction
“I wonder if people understand the loneliness of being blind. In a crowded room I could feel lonely. I’ve been to the things where not a soul has spoken to me. They speak to others, but not to me, because they see the white stick. They know I can’t see them.” [1].
Although there have been relatively recent reviews on the topic of corneal allotransplantation [2] and xenotransplantation [3–5], none has comprehensively reviewed the conditions for which corneal transplantation might be indicated, the global extent of the problem, the different surgical procedures that might be carried out, or the alternatives to corneal allotransplantation (which include xenotransplantation). Here, we attempt to cover these topics, and suggest why corneal xenotransplantation might provide a solution to the critical shortage of deceased human corneas for clinical transplantation.
Global prevalence of blindness
The most recent estimates place the prevalence of blindness worldwide at around 39 million, with 285 million people visually impaired [6,7]. The major causes of blindness have been listed by Pascolini 2012 [6] (Table 1). Cataracts account for approximately half of the cases, with corneal opacities from various causes representing approximately 10%. Based on data from the World Health Organization (WHO) and Pascolini et al., we can estimate that at least 4 million people worldwide suffer from corneal blindness [6,8]. Others estimate the figure to be between 4.9 and 10 million [9,10].
Table 1.
Major causes of blindness worldwide
| Cause (approximate % of total) |
|---|
| Cataract (50%) |
| Corneal blindness (10%) |
| Infectious keratitis - bacterial, fungal, trachomal, viral (herpes simplex) |
| Dystrophies - granular, macular, lattice, Fuchs |
| Degenerations - ICE, keratoconus, pterygium |
| Glaucoma (8%) |
| Age-related macular degeneration (5%) |
| Diabetes (1%) |
| Other (>20%) |
It is estimated that of the 39 million blind, 90% are in developing countries, and 80% of the causative diseases are either preventable or treatable by applying basic public health measures, available surgical technology, and/or drug therapy [7,8].
Blindness and visual impairment are important economically, both to the patient and to the community. Those who are visually impaired are more likely to be poor and unemployed [11], but blindness also impacts national budgets through medical care expenditures [12]. A US study estimated an annual economic impact of $5.5 billion spent on medical care and support of the blind [13]. Furthermore, the socio-emotional impact of sight loss has been associated with changed perceptions of self, decreased levels of social functioning and general well-being, and impaired social relationships [1]. The prevalence of depression is estimated to be twice as high in the visually impaired than in the general population [1]. Studies have indicated that vision loss is the most feared disability, and blindness and cancer are the two most feared health conditions [14].
The structure and function of the cornea
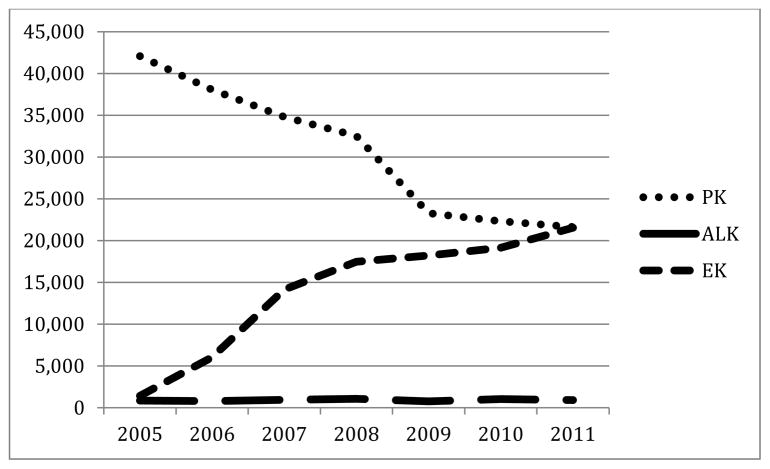
The cornea is approximately 500μm thick and consists of 5 major layers (Table 2 and Figure 1), and accounts for two-thirds of the refractive power of the eye [15]. The regular arrangement of collagen fibrils in the cornea and the relationship between collagen layers in the stroma are considered to be important for the maintenance of corneal transparency. Disordering of these fibrils through injury or disease can lead to corneal opacities and blindness [16]. Maintenance of corneal transparency depends primarily on the corneal endothelial monolayer of cells. A loss in endothelial cell density below a certain threshold compromises the barrier and ionic “pump” functions of the endothelium, which results in loss of visual acuity [17,18].
Table 2.
Major layers of the cornea (see also Figure 1)
| Corneal Layer | Structure/Function |
|---|---|
| Epithelium | Composed of about 6 layers of cells. Fast growing. Constantly replenished. |
| Bowman’s layer | Condensed collagenous layer under epithelial basement membrane.. |
| Stroma | Constitutes 90% of corneal thickness. Consists of collagen fibers with scarce stromal cells. |
| Descemet’s membrane | Thin acellular collagenous layer. Basement membrane of corneal endothelium. |
| Endothelium | Simple monolayer of mitochondria-rich cells. Responsible for regulating fluid balance in cornea. Cells do not regenerate. |
Figure 1.
The five layers of the cornea [84] and the types of corneal transplants
Feinberg has drawn attention to the unique properties of the cornea that differentiate it from other tissues in the body: (i) it is avascular, (ii) the stroma is primarily collagen type 1, (iii) the corneal endothelium is a monolayer of terminally-differentiated post-mitotic cells, and (iv) the stroma and endothelium are considered immunoprivileged [19].
Major causes of corneal blindness
Diseases affecting the cornea are significant causes of blindness worldwide (Table 1). The prevalence of corneal disease varies among countries and populations, and depends on many factors [20]. Some corneal conditions are prominent in certain parts of the world as a result of infectious disease associated with a poor public health infrastructure or genetic factors [21–23]. Trachoma is an infection associated with the bacterium Chlamydia trachomatis, which is endemic to many parts of the developing world, and causes a severe conjunctivitis that results in conjunctival scarring, lid distortion and inward turning of eyelashes, and corneal abrasions and scarring [24]. According to the International Trachoma Initiative this infection accounts for 1.3 million cases of blindness worldwide [25], making it the most common infectious cause of blindness in the world [26] and the second leading cause of blindness in Africa, second only to cataracts [27]. The majority of regions with a trachoma prevalence of >30% in children under age 10 are found in Africa [28], though it is also seen in the Middle East, Asia, and Latin America [28,29].
Other significant infections that can result in corneal blindness include leprosy and Lyme disease [30]. Fungal keratitis and pterygia can cause corneal damage in tropical developing countries [31–33]. Fungal keratitis has a high corneal perforation rate. Bhartiya et al demonstrate an incidence of perforations of 26% among patients with fungal keratitis, with approximately 80% of these subjects requiring full-thickness corneal transplantation (penetrating keratoplasty) [34]. Herpes simplex virus remains a leading cause of corneal blindness in the US and all developing countries [35], and, together with other neonatal eye infections, are responsible for much of childhood corneal blindness.
Vernal keratoconjunctivitis, an allergic reaction, and xerophthalmia (350,000 cases annually), stemming from vitamin A deficiency, also contribute to sight loss in children [20,36].
Keratoconus, which has a genetic contribution, appears to have a greater prevalence in people of Indian or Middle Eastern origin, but is seen in many countries [37–41]. Iridocorneal endothelial syndrome (ICE) and granular and lattice dystrophy seem to have a worldwide distribution. There appears to be evidence for a relatively high prevalence of macular dystrophy in Iceland and a low prevalence of Fuchs’ dystrophy in Japan and some other countries [42]. Other corneal pathologies may be manifestations of metabolic diseases, e.g., Hurler’s syndrome, dystrophies, or degeneration. Ocular trauma and corneal ulceration account for an estimated further 1.5–2.0 million new cases of monocular blindness every year [20].
Prevention and treatment of corneal disease
Nearly 80% of all corneal blindness is avoidable. Prevention is clearly more cost-effective than surgical intervention, as demonstrated by success in reducing corneal blindness from trachoma, vitamin A deficiency, and onchocerciasis [24,43,44]. Prevention strategies that erased trachoma from school children in the US (predominantly Native Americans), which in the early 1900s affected as many as 50–90% of children in certain settings [21], are necessary in Africa and the rest of the developing world.
Topical anti-fungal and anti-viral agents, antibiotics, and saline ointments can treat many conditions in the early stages. In its milder form, keratoconus can be corrected with glasses or contact lenses. Pterygia can be surgically excised, but recurrence rates can vary depending on surgical technique.
When preventive strategies have not been available, or have been unsuccessful, corneal transplantation is the only option, and is the most effective treatment for advanced corneal disease [36]. It is also the optimal option for the dystrophies and degenerations that are not currently preventable, such as Fuchs’ dystrophy, ICE, and keratoconus [45]. Figure 2 shows corneal diseases that may be indications for corneal transplantation.
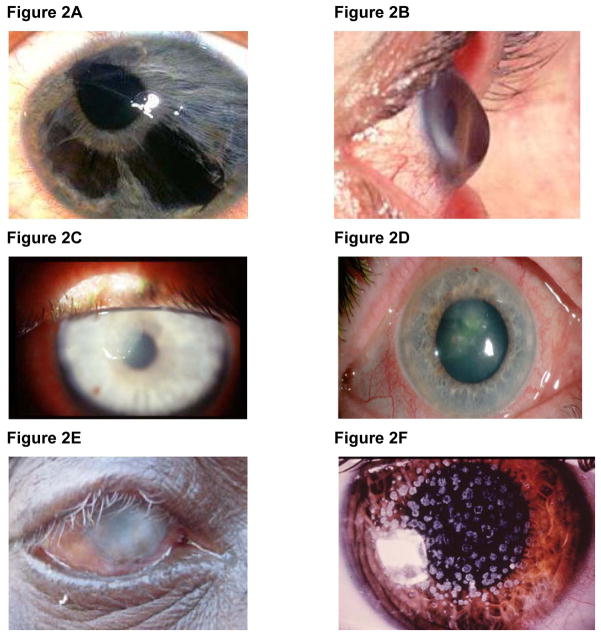
Figure 2. Corneal pathologies that may be indications for keratoplasty.
(A) Iridocorneal endothelial syndrome (ICE). There is iris atrophy with displacement of the pupil and polycoria (more than one pupil opening). The endothelium shows changes that resemble the small outgrowths of Descemet’s membrane seen in Fuchs’ [181].
(B) Ectasia of cornea characteristic of keratoconus [182].
(C) Bacterial keratitis. Grayish-white abscesses, formed in response to inflammatory response, are visible in the stroma [183].
(D) Fungal keratitis from Fusarium Solani [184].
(E) Trachoma. Blinding corneal opacification with entropion and trichiasis [26].
(F) Granular dystrophy - white granular stromal opacities [185].
(G) Type 1 lattice dystrophy - network of sub-epithelial thick linear opacities [186].
(H) Macular dystrophy showing opacities in the central stroma [187].
(I) Endothelial guttata of Fuchs’ dystrophy [188].
Corneal transplantation (keratoplasty)
Corneal allotransplantation is the most common and successful form of transplantation in humans [46,47]. Surgical procedures consist of three major types (Figure 1): (i) penetrating keratoplasty (PK, full-thickness), (ii) anterior lamellar keratoplasty (ALK, partial-thickness), and (iii) endothelial keratoplasty (EK) [37] (Tables 3 and 4). Indications for corneal transplantation differ among countries (Table 5).
Table 3.
Differences between the three main types of keratoplasty (PK, ALK, EK)
| Type of Keratoplasty | |||
|---|---|---|---|
| Penetrating (PK) | Anterior Lamellar (ALK) | Endothelial (EK) | |
| Layers transplanted/ techniques | Full-thickness host corneal tissue replaced with donor corneal tissue. | Limited to anterior portions of cornea. | Endothelium, Descemet’s membrane +/− some stroma. Two primary types:
|
| Advantages (ALK and EK each compared to PK) | No lamellar interface. Visual acuity better after PK than with lamellar grafts [132]. Compared to EK, lower risk of graft dislocation. Can treat conditions affecting various corneal layers. Familiar procedure for most corneal surgeons. |
Longer graft survival, less need for aftercare [58]. Less endothelial cell loss [133,134]. For keratoconus, corneas that have more resistance to deformation [135]. |
Safer and provides faster visual recovery [60,61]. Less astigmatism, better stability of graft, fewer suture related problems [60]. Ability to restore normal corneal topography of recipient [136]. Less rejection. |
| Disadvantages | Prolonged visual rehabilitation. High astigmatism. Suture-related complications. Higher likelihood of graft rejection [137]. | Average visual acuity lower [133]. Interface haze** |
Increased risk of graft dislocation [60]. |
DSEK includes endothelium, some stromal layer, and Descemet’s membrane. DMEK includes endothelium and Descemet’s membrane.
A slight obscuration may form in the interface between host and donor corneas.
Table 4.
Keratoplasty in the management/treatment of corneal diseases
| PK | ALK | EK | Comment | |
|---|---|---|---|---|
|
Infectious Keratitis Bacterial |
++ | + | − | PK has a lower rate of reinfection of graft than ALK [138]. |
| Fungal | + | + | − | Control of fungal process demonstrated in patients who underwent PK [139–141]. ALK is a viable option [32,142]. |
| Trachoma | ++ | + | − | Good prognosis only in carefully selected cases [143]. |
| Herpes simplex (HSV) | + | ++ | − | Despite higher preoperative risk, graft outcomes may be similar in patients with herpetic and non-herpetic keratitis [144]. High risk for poor outcomes post PK [145]. DALK shows comparable visual outcome and better graft survival rate than PK [146–148]. Though there is a high percentage of complications post DALK [149]. |
|
Dystrophies Granular |
+ | ++ | − | ≈ 85% of grafts after PK remain clear at 1 year [150]. DALK also a viable option [151–154]. |
| Macular | + | ++ | − | PK usually required earlier for macular dystrophy than for other stromal dystrophies [150]. PK entails favorable outcomes [155,156]. DALK [157] shows poorer visual acuity, but more durable stability of ocular surface and possibly fewer complications [158]. |
| Lattice | + | ++ | − | PK has good prognosis, although patients show delayed epithelial healing [159,160]. DALK is a safe alternative [157]. |
| Fuchs’ | + | − | ++ | More than 80% of corneas remain clear for 2 years or more after PK [161]. EK viable option with several advantages over PK in seemingly most [162–165], but not all [166] studies. |
|
Degenerations Iridocorneal Endothelial Syndrome (ICE) |
+ | − | + | PK, DSEK followed by favorable outcomes [167,168]. |
| Keratoconus | + | ++ | − | PK success rates >90% [169–172]. ALK is a safer technique with fewer complications [133,173–180]. |
Table 5.
Most common indications for corneal transplantation in selected countries for which data are available
| Indications for Corneal Transplantation | |||||
|---|---|---|---|---|---|
| US | Australia | Iran* | Israel* | India* | North China* |
|
|
|
|
|
|
PK is still the method of choice in many countries, with ALK and EK being relatively new techniques. We assume that indications for PK in Iran, Israel, India, and North China represent corneal transplantation as a whole in these countries.
Macular dystrophy was most common, followed by Fuchs’ dystrophy.
In the Australian Corneal Graft Registry 2012 Report, there appears to be an inconsistency in regard to data for re-transplantation.
Penetrating keratoplasty (PK)
PK has been significantly refined since the first PK procedure in 1905 and has been the gold standard of keratoplasty for the past 60 years [48,49]. Over the past decade, there has been a move towards ALK and EK, with the aim of replacing only the diseased portion of the cornea, leaving any healthy cornea untouched [49] (Table 3) (Figure 1). Nevertheless, PK is an effective and safe treatment and still accounts for >90% of grafts in the UK and Australia, and 47% in the US [37,38,50]. The long-term outcome of corneal grafts after PK is good, with 5 and 10-year survival rates of approximately 88% and 80%, respectively [51]. Other studies show 5-year survival rates for PK ranging from 69% to 90% and 10-year rates from 63% to 82% [52]. The success rate for PK can be affected by the primary corneal pathology that necessitates transplantation. For example, the 5 and 10-year survival rates for keratoconus treated by PK are particularly good at 97% and 92%, respectively [51].
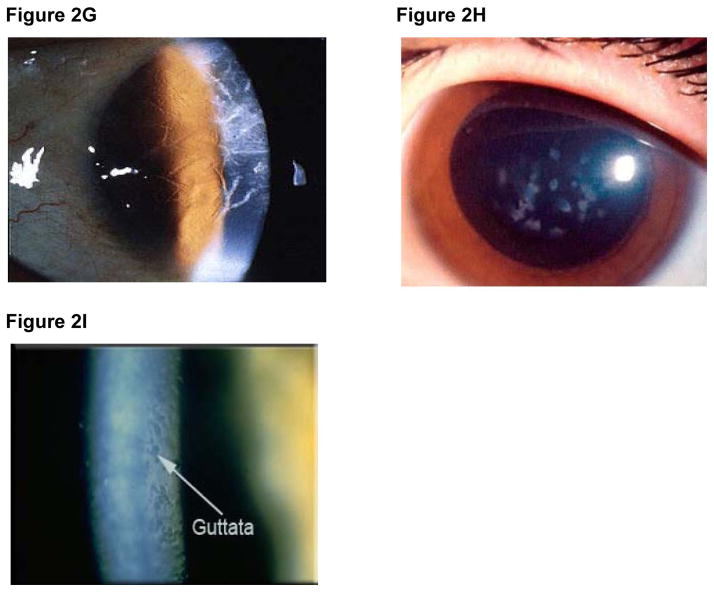
Indications for PK vary throughout the world (Table 5). For example, keratoconus is the most common indication in the US, Australia, Iran, and several developed countries [37–40], though the incidence of PK in the US is steadily declining (Figure 3). Infectious diseases and corneal scarring are the most common indications in many developing countries [53,54]. Bullous keratopathy, or corneal edema after damage to the endothelium during intraocular surgery, is a leading indication for PK and corneal transplantation in general [55]. Re-transplantation, resulting from graft failure, is one of the top three indications for PK in several countries, including the US. In the US, endothelial keratoplasty (EK), rather than PK, is the treatment of choice for bullous keratopathy and failure of an endothelial cell graft.
Figure 3. Keratoplasty procedures (PK, ALK, EK) performed in the US (2005 – 2011).
The success of PK depends to a large extent on the availability of high-quality donor corneas [37,38,39,53]. The age and mode of death of the donor, and the time interval between death and adequate storage can affect the quality of the donor cornea [56]. The popularity of Laser-Assisted in situ Keratomileusis (LASIK), which compromises structural and optical properties and renders corneas unsuitable for full-thickness corneal transplantation, threatens to reduce the donor cornea pool [57].
Anterior lamellar keratoplasty (ALK)
ALK is employed when the pathology is confined to the anterior layers of the cornea, such as epithelium and/or stroma (Figure 1). Advantages over PK include longer graft survival because of reduced immunogenicity, and the retention of the host corneal endothelial cells [58]. A variant of ALK is deep anterior lamellar keratoplasty (DALK), a procedure that removes the corneal stroma down to Descemet’s membrane (Figure 1). For corneal diseases not involving the endothelium, DALK is a good approach for younger patients due to a greater long-term survival than following PK [52].
ALK is a relatively new procedure and accounts for only approximately 3% of indications for corneal transplantation in the US and 8% in Australia [37,38]. In the US, the number of ALK procedures has remained relatively constant since 2005 (Figure 3). Keratoconus is the most common indication for ALK in the US and Australia [37,38].
Endothelial keratoplasty (EK)
Since the pump function of the cornea depends on a minimum endothelial cell density, significant endothelial cell loss results in corneal edema and necessitates keratoplasty [59]. EK, most commonly via Descemet stripping endothelial keratoplasty (DSEK), simply replaces the pathologic corneal endothelium with healthy donor endothelium and a thin layer of posterior stroma, and is appropriate for patients with endothelial dysfunction, e.g., Fuchs, pseudophakic bullous keratopathy, ICE (Table 3). Fuchs’ dystrophy is the most common indication for EK in the US and Australia, accounting for nearly half of EK procedures in both countries [37,38]. Re-transplantation is the third most common indication for EK in Australia [38].
Providing a safer and faster visual recovery time compared to PK [60, 61], EK has overtaken PK as the procedure of choice for endothelial dysfunction in the US, accounting for 47% of corneal grafts in 2011 (Figure 3). However, EK currently accounts for only 6% of keratoplasties in Australia and an even lower percentage in the UK. During the next few years, as pathology of the corneal endothelium plays an increasing role in developed countries, EK is expected to overtake PK as the most common keratoplasty procedure in these countries.
Corneal transplantation in the developing world
The US accounts for approximately half of all corneal transplants performed worldwide, with 46,196 in 2011 [37]. In the developing world, which carries most of the burden for corneal blindness, it is estimated that corneal transplantation could be of value in the treatment of 80–90% of patients with corneal blindness [62,63]. However, some or all of the critical components required for successful keratoplasty, such as availability of high-quality corneal tissue, skilled surgeons, and anti-rejection medications, are limited.
Can corneal transplantation ease this burden in regions with developing public health infrastructures and facilities? While many developing nations lack eye bank facilities and trained surgeons, the establishment of eye banks and developing infrastructures for keratoplasty, e.g., in Ethiopia and Nigeria, demonstrate the feasibility of corneal transplantation on a global scale [64,65]. The growing performance of keratoplasty in Nepal is a case in point, where the establishment of the Nepal Eye Bank in 1996, public campaigns, and the media resulted in the acquisition of trained surgeons, staff, and modern facilities, enabling keratoplasty to become a viable treatment option in that country [66].
Vieira Silva et al reported unfavorable results after keratoplasty in a poor society [67], but others have demonstrated more successful outcomes [62,66,68,69]. In patients undergoing PK, 60–70% graft clarity is attainable, even among those with severe corneal disease [66]. Furthermore, costly surgical equipment is not required for Descemet membrane endothelial keratoplasty (DMEK) - although it is still required for Descemet stripping endothelial keratoplasty (DSEK) - and thus holds the promise of increasing its usage [70].
Shortage of corneal grafts: an international crisis
Although at least 4 million people worldwide suffer from corneal blindness [6,8], only 100,000 corneal transplants are performed each year, primarily as a result of lack of access to suitable donor tissue. Even in the US, corneas considered acceptable for transplantation are limited; in 2011, 29,407 corneas were deemed unsuitable for transplantation for reasons that included (i) positive serology, (ii) structural defects, and (iii) adverse findings in the medical records [37]. In India, with a population of >1 billion, approximately 20,500 donor corneas are obtained annually, but <10,000 can be utilized for transplantation [36]. Here, the requirement for donor corneas is estimated to be at least 20 times the current number transplanted. In South Africa, the number of corneal transplants performed annually has fallen by 50% in recent years because of the high incidence of HIV-positivity in the general population, limiting the number of acceptable donors [4]. The number of people aged 50 years and older has significantly increased worldwide over the past several decades and, as >80% of all blind people are in this age group, this continuing trend will likely result in an increasing shortage of donor corneas [63,71].
Clearly, there is an enormous gap between supply and demand in countries where corneal disease is prevalent [36,4]. This lack of access has been described as an international crisis in public health [10]. Research in various fields hopes to address this shortage.
Research to address the shortage of corneal grafts
The worldwide inadequacy of the number and quality of deceased human corneas necessitates a search for a form of corneal replacement that is readily available and convenient for clinical application [72,73]. Several approaches are being explored (Table 6). For those interested in developing the field of corneal xenotransplantation, it will be essential to demonstrate superiority over the other approaches.
Table 6.
Approaches that may address the shortage of deceased human corneal grafts
| Research | Hypothesis | Current State | Advantages | Disadvantages & areas to address | Conclusions |
|---|---|---|---|---|---|
| Stem cells (stromal & hESCs) | Can differentiate into different layers of the cornea. | hESCs can form epithelial-like cells. Human stromal corneal stem cells show favorable results in animal models. | Can theoretically provide unlimited corneal grafts. | Differentiation of hESCs into complete epithelium layer and other layers has not been achieved. Applicability of stromal stem cells in clinical models. | Not currently clinically applicable. |
| Stem cells (limbal) | Can reverse corneal epithelial damage. | Clinical trial has shown good results. | Autologous stem cells may avoid immunogenicity of xenografts. | Applicability to wide range of patients. Differentiation of stems cells into corneal layers other than epithelium. |
Presently, limited to only a small subset of patients. |
| Human amniotic membrane transplantation | Can provide tissue suitable for a corneal patch and/or partial thickness graft | Can be used in patients with corneal ulcers, stromal thinning, and other indications. | No risk of allograft rejection. | Time needed to produce enough transplantable material. Cannot provide tectonic support Limited in scope of indications. |
Limited to a small subset of indications for keratoplasty. |
| Synthetic corneas | Can serve as a corneal replacement. | Have been used to treat patients with severe corneal pathology. | Can be mass produced. Less expensive than human donor tissue. Non-immunogenic. | Applicability to wide range of corneal pathologies. Visual acuity. Stromal melting. | Not suitable for all corneal pathologies. Long-term retention is an issue. |
| Biosynthetic corneas | Cornea will regenerate around a biopolymer matrix. | Clinical study has had favorable results with epithelial transplantation. | Can be mass produced. | Not currently applicable to corneal stroma. Nerve regeneration slow. Issues with regenerating endothelium. |
Not currently clinically applicable |
| Xeno-transplantation | Corneal grafts from animals can be successfully transplanted into human recipients. | Decellularization, cultivated CECs, and genetic engineering may bring xeno-transplantation to keratoplasty. | Potential for unlimited corneal xenografts that are comparable or better than stored human corneas. | Immunogenicity of corneal xenograft. Biomechanical issues. |
Greatest potential for a corneal replacement. |
Human stem cells
Some groups have studied the ability of human stem cells to correct stromal opacities in animals models [74,75]. Du et al showed restoration of corneal transparency after direct injection of stem cells isolated from adult human corneal stroma into murine corneal stroma [74]. While these results are promising, the mouse cornea differs significantly from the human cornea. Human corneas are thicker, have more extensively cross-linked collagen, and are potentially less responsive to stem cell therapy. This type of treatment based on animal models has yet to reach pre-clinical or clinical trials.
The limbus, the narrow junction of cornea and sclera, provides a reservoir of stem cells to maintain transparency of the corneal epithelium [76]. In limbal stem cell deficiency, the corneal epithelium cannot be maintained, and corneal epithelial defects appear [77]. Limbal stem cell transplantation, using donor tissue containing limbal stem cells, aims to restore the corneal epithelium. This donor tissue can be from the patient’s own healthy fellow eye, or from a living-related of cadaveric donor, or from sheets of ex vivo expanded bioengineered epithelial cells [78]. Using autologous limbal stem cells to treat 112 patients with burn-related corneal damage, Rama et al reported permanent restoration of a transparent corneal epithelium in 77% of treated eyes [79]. However, normal vision was restored in only those patients with undamaged corneal stroma. At present, this form of treatment is applicable to only a very small cohort of patients with corneal epithelial dysfunction.
Human embryonic stem cells (hESCs) are pluripotent, having the potential to differentiate into all cell types. While experiments had been carried out in animal models [80–82], the first report of hESCs growing on damaged human corneal tissue was in 2012. When established on human Bowman’s membrane, hESCs were expanded and differentiated into corneal epithelial-like cells [83]. However, the epithelium represents only the outermost layer of the cornea.
While stem cells could, theoretically, provide unlimited corneal tissue, including corneal endothelial cells, this area of research is in its infancy. Currently, no known stem cells are able to be expanded and differentiated into corneal endothelial cells [19].
Synthetic corneas
Keratoprostheses are completely synthetic and designed to restore visual acuity with a biologically-inert material, and are already in use in patients with severely damaged or diseased corneas in which tissue grafts would not likely succeed, e.g., in certain autoimmune diseases, such as Stevens-Johnson syndrome [84]. Newer designs that allow for a more firm anchorage and are composed of various materials to enhance strength have been reported [85]. However, keratoprostheses in clinical use have been reported to be associated with stromal “melting” (a disorder in which the corneal stroma breaks down), glaucoma, retinal detachment, and other complications. While artificial corneas can address severe pathologies, they are not currently suitable for long-term use in the majority of patients requiring corneal transplantation [86], and it is questionable whether they will solve the donor shortage.
Clinical indications for a keratoprosthesis differ from those for the biosynthetic cornea [86]. They are used in severe cases of disease or trauma where human donor tissue has failed repeatedly or where a graft cannot be supported in the presence of the specific eye pathology. Keratoprostheses address an important range of severe pathologies that human tissue cannot, and will continue to be an option in corneal replacement therapy. However, they are not suitable for the majority of indications for corneal transplantation, and therefore do not supplement the severely limited pool of donor corneas.
Biosynthetic corneas
Unlike fully artificial corneas, biosynthetic corneas are designed to stimulate endogenous corneal regeneration by seeding and proliferating autologous or allogeneic cells within a biopolymer matrix [86–89]. In 1999, Griffith et al constructed human corneal equivalents that contained the three main corneal layers (epithelium, stroma, and endothelium). These corneal equivalents mimicked human corneas in key functions, including transparency, gross morphology, gene expression, and endothelium pump transport [90]. In animal models, collagen-based corneal substitutes have been successfully transplanted as lamellar or full-thickness grafts [88].
In a phase 1 clinical trial, using collagen-based biosynthetic corneal substitutes to replace anterior portions of the cornea, Fagerholm et al demonstrated corneal epithelialization and in-growth of keratocytes and superficial nerves [82]. This was one of the first studies that demonstrated nerve regeneration in corneal substitutes in humans [91,92]. Innervation of the cornea is required for normal function, including pain sensation and tissue repair [93]. Nevertheless, further optimization is needed, and issues with deeper stromal nerve regeneration remain challenges.
In addition, biosynthetic corneas have not yet addressed conditions affecting the corneal endothelium, which comprise the majority of cases requiring PK or EK [86]. Engineering the corneal endothelium, which is non-regenerating, is a major problem [19]. Feinberg and his colleagues have been attempting to bioengineer the corneal endothelium by manipulating the cell microenvironment to better maintain cell phenotype in vitro [94]. Engineering the pores necessary to allow the endothelium to pump fluid is another challenge. While this research is promising, a bioengineered corneal endothelium is not currently clinically applicable.
Other studies have demonstrated favorable results with cell sheet engineering to reconstruct the corneal epithelium [95–97], but this technique cannot be applied to corneal stroma [87].
Amniotic membrane transplantation
The human amniotic membrane is thin, avascular, and composes the innermost layer of fetal membranes. Preserved amniotic membrane is composed of epithelium, a basement membrane, and stroma. It may be used as a graft and/or patch to correct epithelial and stromal defects. As a graft, amniotic membrane provides a stroma and basement membrane on which normal corneal epithelium grows. It can also heal corneal ulcers with stromal thinning [98]. The ability of amniotic membrane to inhibit a lymphocyte reaction contributes to the low immunogenicity of the tissue [99–102]. Amniotic membrane transplantation is, therefore, a relatively simple procedure with little risk of allograft rejection. It has been suggested as an alternative to PK in countries with a shortage of corneas [103]. However, it cannot be used to replace a full-thickness cornea. There is a lack of evidence that amniotic membrane transplantation is better than existing treatments for various corneal conditions [104]. At present, its use is too limited to make a significant contribution in overcoming the shortage of corneas.
Xenotransplantation
It is not our intention to review this field of research comprehensively as this has been done by us [3,4] and others [5] relatively recently. We shall confine ourselves to a brief overview of certain aspects of the topic.
The first recorded attempt at clinical corneal transplantation was in 1838 by Richard Kissam, who transplanted the cornea from a 6-month old pig into a blind patient. Though this xenograft ultimately failed, it was the earliest precursor to the immense success of corneal allotransplantation [4,47]. The worldwide corneal donor shortage has stimulated renewed interest in corneal xenotransplantation. Modern xenotransplantation, using pigs as the source animal, offers the potential of an unlimited number of corneas for PK, ALK, and EK procedures [4].
Biomechanical parameters (strength, stress, plasticity) suggest that porcine corneas can provide an alternative to human corneas [4]. The proliferative capacity of corneal endothelial cells from young genetically-modified pigs has been demonstrated to be greater than that of the average human corneal endothelial cells, which increasingly are from older donors. As the proliferative capacity varies between young and old donors [105–107], the quality of young pig corneas, which have greater corneal endothelial cell density, may be better that that of the average human donor [108].
The immune-privileged environment of the cornea provides a corneal xenograft with some degree of protection [4,47]. Nevertheless, the humoral and cellular immune responses remain challenges.
The results of pig corneal transplantation in nonhuman primates have been encouraging [4,109]. Because the cornea is an avascular tissue, hyperacute rejection, which results from the sudden occlusion of blood vessels and is common in vascularized solid organ xenografts, has not been observed in corneal xenografts in animal models [4,109–113]. ALK xenografts avoid the stronger immune response stimulated by the presence of the corneal endothelium. Survival after pig ALK in rhesus monkeys (which survived for >3 months) was longer than that of full-thickness xenografts, which were rejected within about 2 weeks [109]. However, with corticosteriod therapy, PK grafts from wild-type pigs have survived in monkeys for >4 months [109]. Testing of cultured corneal endothelial cells in an in vivo EK model has been carried out [114]. Following the transplantation of sheets constructed from monkey corneal endothelial cells, the corneas remained clear for >6 months.
The greatest hurdle for corneal xenografts is the immune response generated against corneal endothelial cells. However, corneal endothelial cells from genetically-modified pigs lacking the α1,3-galactosyltransferase gene [115,116], particularly if also expressing a human complement-regulatory protein, CD46 [105], stimulate significantly weaker human humoral and cellular responses compared with those from wild-type pigs. Genetic modification of pig corneal endothelial cells does not seem to affect cell density, size, or morphology, when compared to those from wild-type pigs [108]. In our opinion, genetic engineering of the source pig will be essential if PK, ALK, and EK xenotransplantation are to prove fully successful. Achieving this goal will require multiple genetic modifications to overcome the human immune response [4].
Decellularization techniques, which reduce antigenicity, have been explored in preclinical models of pig corneal xenotransplantation [72,117–120]. Dehydrating porcine anterior lamellar xenografts removes stromal cells, which play important roles in the maintenance and metabolism of the cornea [72], and relies on repopulation of the stroma with recipient cells. Corneal xenografts treated in this way have maintained transparency for up to 6 months after transplantation into monkeys [89,121]. (In a clinical trial, Li J et al showed that deep ALK using human decellularized donor corneal allografts had a significantly higher graft survival rate compared to untreated corneal tissue [118.) Furthermore, decellularized corneas can be stored indefinitely and easily distributed [89], an important issue when considering corneal transplantation in developing countries where the need is greatest.
Formation of the ethical and regulatory guidelines necessary to conduct clinical trials using corneal xenografts is underway. A Korean group has recently published recommendations on source pigs, considering the risk/benefit ratio for trial participants, and differentiating between potential regulations for cellular and acellular (decellularized) pig corneas [122,123]. In regard to key ethical requirements [124,125], source pigs [126,127] pre-clinical efficacy to justify a clinical trial, and the informed consent process [128], some of the guidelines borrow from existing criteria from the Declaration of Helsinki [129], the International Xenotransplantation Association [130], and the US Secretary’s Advisory Committee on Xenotransplantation [128].
Because the cornea is considered a relatively immune-privileged site and thus less immunosuppressive therapy may be required, there may be advantages in initiating clinical trials with corneal xenotransplantation rather than with porcine islet or solid organ transplantation. The risk-versus-benefit ratio would be assessed differently between patients with corneal blindness and those, for example, with diabetes. Although corneal blindness is not life-threatening, the quality of life of the blind is in many ways more challenging than those of other disabled persons.
The Korean group proposed strategies to prevent or reduce the transfer of porcine endogenous retroviruses (by decellularization) [122]. If there is conclusive evidence that decellularization of the cornea abrogates the risk of transfer of porcine endogenous retroviruses with the graft, then this may have a significant impact on how the various national regulatory authorities view corneal transplantation, as a case could then be made to consider the cornea as a ‘medical device’, for which the regulatory pathway is simpler and more rapid. However, decellularization is only of relevance to ALK, the indications for which are limited.
The Korean guidelines will likely serve as the basis for similar proposals in other countries.
Conclusions
In summary, corneal blindness is a major global health problem, particularly in the developing world. When prevention or basic treatment fails, corneal transplantation can provide curative therapy. Limitations with regard to availability of deceased human donor corneas necessitate exploration of alternative approaches. We are optimistic that, with the increasing generation of genetically-engineered pigs, with or without advances in decellularization and long-term preservation techniques, pig corneas may begin to alleviate the shortage of corneas in the near future. Clinical trials of corneal xenotransplantation may be imminent.
Acknowledgments
Hidetaka Hara MD, PhD is supported in part by NIH grant # 1RO3A1096296-01. Research on xenotransplantation at the University of Pittsburgh is funded in part by NIH Grants # IU19A1090959-01, #U01A1066331, and #5P01 HL107152-02, by an Ocular Tissue Engineering and Regenerative Ophthalmology (OTERO) Postdoctoral Fellowship from the University of Pittsburgh, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor Inc., Blacksburg, VA.
ABBREVIATIONS
- ALK
anterior lamellar keratoplasty
- EK
endothelial keratoplasty
- GTKO
α1,3-galactosyltransferase gene-knockout
- hESC
human embryonic stem cells
- ICE
iridocorneal endothelial syndrome
- PK
penetrating keratoplasty
Footnotes
Disclosure of conflict of interest
No author reports a conflict of interest.
References
- 1.THURSTON M, THURSTON A, MCLEOD J. Socio-emotional effects of the transition from sight to blindness. Br J Vis Impair. 2010;28:90–112. [Google Scholar]
- 2.TAN DT, DART JK, HOLLAND EJ, et al. Corneal transplantation. Lancet. 2012;379:1749–1761. doi: 10.1016/S0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]
- 3.HARA H, COOPER DKC. The immunology of corneal xenotransplantation: a review of the literature. Xenotransplantation. 2010;17:338–349. doi: 10.1111/j.1399-3089.2010.00608.x. [DOI] [PubMed] [Google Scholar]
- 4.HARA H, COOPER DKC. Xenotransplantation--the future of corneal transplantation? Cornea. 2011;30:371–378. doi: 10.1097/ICO.0b013e3181f237ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.KIM MK, WE WR, PARK CG, et al. Xenocorneal transplantation. Curr Opin Organ Transplant. 2011;16:231–236. doi: 10.1097/MOT.0b013e328344870c. [DOI] [PubMed] [Google Scholar]
- 6.PASCOLINI D, MARIOTTI SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 7.WHO. [accessed December 19, 2012];Visual impairment and blindness. http://www.who.int/mediacentre/factsheets/fs282/en/
- 8.WHO. Action plan 2006–2011. Geneva: WHO Press; 2007. Vision 2020 Global initiative for the elimination of avoidable blindness. [Google Scholar]
- 9.GULATI M, OLIVA M, SCHOTTMAN T. Turning the tide of corneal blindness. Indian J Ophthalmol. 2012;60:423–427. doi: 10.4103/0301-4738.100540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CORNEA RESEARCH FOUNDATION OF AMERICA. [accessed December 17, 2012]; http://www.cornea.org/index.php/research/corneal_transplant/artificial_cornea/
- 11.ULLDEMOLINS AR, LANSINGH VC, VALENCIA LG, et al. Social inequalities in blindness and visual impairment: a review of social determinants. Indian J Ophthalmol. 2012;60:368–375. doi: 10.4103/0301-4738.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CHIANG YP, BASSI LJ, JAVITT JC. Federal budgetary costs of blindness. Milbank Q. 1992;70:319–340. [PubMed] [Google Scholar]
- 13.FRICK KD, GOWER EW, KEMPEN JH, et al. Economic impact of visual impairment and blindness in the United States. Arch Ophthalmol. 2007;125:544–550. doi: 10.1001/archopht.125.4.544. [DOI] [PubMed] [Google Scholar]
- 14.TAYLOR HR, PEZZULLO ML, KEEFFE JE. The economic impact and cost of visual impairment in Australia. Br J Ophthalmol. 2006;90:272–275. doi: 10.1136/bjo.2005.080986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.PIATIGORSKY J. Enigma of the abundant water-soluble cytoplasmic proteins of the cornea: the “refracton” hypothesis. Cornea. 2001;20:853–858. doi: 10.1097/00003226-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 16.KOMAI Y, USHIKI T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci. 1991;32:2244–2258. [PubMed] [Google Scholar]
- 17.PIPPARELLI A, ARSENIJEVIC Y, THURET G, et al. ROCK inhibitor enhances adhesion and wound healing of human corneal endothelial cells. PLoS One. 2013;8:e62095. doi: 10.1371/journal.pone.0062095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.JOYCE NC. Proliferative capacity of corneal endothelial cells. Exp Eye Res. 2012;95:16–23. doi: 10.1016/j.exer.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FEINBERG AW. Engineered tissue grafts: opportunities and challenges in regenerative medicine. WIREs Syst Biol Med. 2012;4:207–220. doi: 10.1002/wsbm.164. [DOI] [PubMed] [Google Scholar]
- 20.WHITCHER JP, SRINIVASAN M, UPADHYAY MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 21.ALLEN SK, SEMBA RD. The trachoma menace in the United States, 1897–1960. Surv Opthalmol. 2002;47:500–509. doi: 10.1016/s0039-6257(02)00340-5. [DOI] [PubMed] [Google Scholar]
- 22.THOMAS PA, GERALDINE P. Infectious keratitis. Curr Opin Infect Dis. 2007;20:129–141. doi: 10.1097/QCO.0b013e328017f878. [DOI] [PubMed] [Google Scholar]
- 23.KLINTWORTH GK, JESTER JV. Genetic basis of corneal diseases and the role of keratocytes in corneal transparency – a review. Clin Experiment Ophthalmol. 2010;38:23–33. [Google Scholar]
- 24.WRIGHT HR, TURNER A, TAYLOR HR. Trachoma. Lancet. 2008;371:1945–1954. doi: 10.1016/S0140-6736(08)60836-3. [DOI] [PubMed] [Google Scholar]
- 25.INTERNATIONAL TRACHOMA INITIATIVE. [accessed March 26, 2013];Mapping the magnitude of blinding trachoma. http://trachoma.org/news-releases/2011/mapping-magnitude-blinding-trachoma.
- 26.HU VH, HARDING-ESCH EM, BURTON MJ, et al. Epidemiology and control of trachoma: systematic review. Trop Med Int Health. 2010;15:673–691. doi: 10.1111/j.1365-3156.2010.02521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LEWALLEN S, COURTRIGHT P. Blindness in Africa: present situation and future needs. Br J Ophthalmol. 2001;85:897–903. doi: 10.1136/bjo.85.8.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.POLACK S, BROOKER S, KUPER H, et al. Mapping the global distribution of trachoma. Bull World Health Organ. 2005;83:913–919. [PMC free article] [PubMed] [Google Scholar]
- 29.WHO. [accessed October 16, 2012];Global Health Atlas. http://apps.who.int/globalatlas/default.asp.
- 30.BERBOS ZJ, KRACHMER JH. Infectious disease: corneal manifestations. In: Krachmer JJ, Mannis MJ, Holland EJ, editors. Cornea. St. Louis: Mosby-Year Book Inc; 2005. pp. 763–775. [Google Scholar]
- 31.GAUJOUX T, BORSALI E, GOLDSCHMIDT P, et al. Fungal keratitis in France. Acta Ophthalmol. 2011;89:e215–216. doi: 10.1111/j.1755-3768.2010.01946.x. [DOI] [PubMed] [Google Scholar]
- 32.XIE L, HU J, SHI W. Treatment failure after lamellar keratoplasty for fungal keratitis. Ophthalmology. 2008;115:33–36. doi: 10.1016/j.ophtha.2007.03.072. [DOI] [PubMed] [Google Scholar]
- 33.FURTADO JM, LANSINGH VC, CARTER MJ, et al. Causes of blindness and visual impairment in Latin America. Surv Ophthalmol. 2012;57:149–177. doi: 10.1016/j.survophthal.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 34.BHARTIYA P, DANIELL M, CONSTANTINOU M, et al. Fungal keratitis in Melbourne. Clin Experiment Opthamol. 2007;35:124–130. doi: 10.1111/j.1442-9071.2006.01405.x. [DOI] [PubMed] [Google Scholar]
- 35.KAYE S, CHOUDHARY A. Herpes simplex keratitis. Prog Retin Eye Res. 2006;25:355–380. doi: 10.1016/j.preteyeres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 36.GARG P, KRISHNA PV, STRATIS AK, et al. The value of corneal transplantation in reducing blindness. Eye. 2005;19:1106–1114. doi: 10.1038/sj.eye.6701968. [DOI] [PubMed] [Google Scholar]
- 37.EYE BANK ASSOCIATION OF AMERICA. 2011 Eye Banking Statistical Report. Washington DC: EBBA; 2011. [Google Scholar]
- 38.ACGR. The Australian Corneal Graft Registry 2012 Report. Registry; Bedford Park, Australia: 2012. [Google Scholar]
- 39.YAHALOM C, MECHOULAM H, SOLOMON A, et al. Forty years of changing indications in penetrating keratoplasty in Israel. Cornea. 2005;24:256–258. doi: 10.1097/01.ico.0000148310.63755.74. [DOI] [PubMed] [Google Scholar]
- 40.KANAVI MR, JAVADI MA, SANAGOO M. Indications for penetrating keratoplasty in Iran. Cornea. 2007;26:561–563. doi: 10.1097/ICO.0b013e318041f05c. [DOI] [PubMed] [Google Scholar]
- 41.KOK YO, TAN GF, LOON SC. Review: keratoconus in Asia. Cornea. 2012;31:581–593. doi: 10.1097/ICO.0b013e31820cd61d. [DOI] [PubMed] [Google Scholar]
- 42.MUSCH DC, NIZIOL LM, STEIN JD, et al. Prevalence of corneal dystrophies in the United States: estimates from claims data. Invest Ophthalmol Vis Sci. 2011;52:6959–6963. doi: 10.1167/iovs.11-7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WHO. WHO Global Database on Vitamin A Deficiency. Geneva: WHO Press; 2009. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. [Google Scholar]
- 44.WHO. [accessed February 25, 2013];Onchocerciasis Control Programme in West Africa (OCP) http://www.who.int/apoc/onchocerciasis/ocp/en/index.html.
- 45.LEIBOWITZ HM, MOORE TE. Keratoplasty. In: Leibowitz HM, Waring GO III, editors. Corneal Disorders: Clinical Diagnosis and Management. Philadelphia: W.B. Saunders; 1998. pp. 842–869. [Google Scholar]
- 46.QIAN Y, DANA MR. Molecular mechanisms of immunity in corneal allotransplantation and xenotransplantation. Expert Rev Mol Med. 2001;3:1–21. doi: 10.1017/S1462399401003246. [DOI] [PubMed] [Google Scholar]
- 47.NIEDERKORN JY. Immune privilege and immune regulation in the eye. Adv Immunol. 1990;48:191–226. doi: 10.1016/s0065-2776(08)60755-5. [DOI] [PubMed] [Google Scholar]
- 48.MOFFATT SL, CARTWRIGHT VA, STUMPF TH. Centennial review of corneal transplantation. Clin Experiment Ophthalmol. 2005;33:642–657. doi: 10.1111/j.1442-9071.2005.01134.x. [DOI] [PubMed] [Google Scholar]
- 49.VERDIER DD. Penetrating keratoplasty. In: Krachmer JJ, Mannis MJ, Holland EJ, editors. Cornea. St. Louis: Mosby-Year Book Inc; 2005. pp. 1335–1348. [Google Scholar]
- 50.NATIONAL HEALTH SERVICE. Transplant activity in the UK. Bristol, UK: NHS Blood and Transplant; 2010. Nov, [Google Scholar]
- 51.THOMPSON RW, PRICE MO, BOWERS PJ, et al. Long-term graft survival after penetrating keratoplasty. Ophthalmology. 2003;110:1396–1402. doi: 10.1016/S0161-6420(03)00463-9. [DOI] [PubMed] [Google Scholar]
- 52.BORDERIE VM, BOËLLE PY, TOUZEAU O, et al. Predicted long-term outcome of corneal transplantation. Ophthalmology. 2009;116:2354–2360. doi: 10.1016/j.ophtha.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 53.DANDONA L, RAGU K, JANARTHANAN M, et al. Indications for penetrating keratoplasty in India. Indian J Ophthalmol. 1997;45:163–168. [PubMed] [Google Scholar]
- 54.XIE L, SONG Z, ZHAO J, et al. Indications for penetrating keratoplasty in north China. Cornea. 2007;26:1070–1073. doi: 10.1097/ICO.0b013e318093de07. [DOI] [PubMed] [Google Scholar]
- 55.MIAN SI, SUGAR A. Corneal complications of intraocular surgery. In: Krachmer JJ, Mannis MJ, Holland EJ, editors. Cornea. St. Louis: Mosby-Year Book Inc; 2005. pp. 115–1168. [Google Scholar]
- 56.MOORE TE, ARONSON SB. The role of surgical factors in corneal graft failure. In: Porter R, Knight J, editors. Corneal Graft Failure; Ciba Foundation Symposium; Chichester, UK: John Wiley & Sons, Ltd; 2008. pp. 209–220. [Google Scholar]
- 57.MOOTHA VV, DAWSON D, KUMAR A, et al. Slitlamp, specular, and light microscopic findings of human donor corneas after laser-assisted in situ keratomileusis. Arch Ophthalmol. 2004;122:686–692. doi: 10.1001/archopht.122.5.686. [DOI] [PubMed] [Google Scholar]
- 58.BORDERIE VM, GUILBERT E, TOUZEAU O, et al. Graft rejection and graft failure after anterior lamellar versus penetrating keratoplasty. Am J Ophthalmol. 2011;151:1024–1029. doi: 10.1016/j.ajo.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 59.ENGELMANN K, BEDNARZ J, VALTINK M. Prospects for endothelial transplantation. Exp Eye Res. 2004;78:573–578. doi: 10.1016/s0014-4835(03)00209-4. [DOI] [PubMed] [Google Scholar]
- 60.VAN DEN BIGGELAAR FJ, CHENG YY, NUIJTS RM, et al. Economic evaluation of endothelial keratoplasty techniques and penetrating keratoplasty in the Netherlands. Am J Ophthalmol. 2012;154:272–281. doi: 10.1016/j.ajo.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 61.TERRY MA, OUSLEY PJ. Rapid visual rehabilitation after endothelial transplants with deep lamellar endothelial keratoplasty (DLEK) Cornea. 2004;23:143–153. doi: 10.1097/00003226-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 62.FEILMEIER MR, TABIN GC, WILLIAMS L, et al. The use of glycerol-preserved corneas in the developing world. Middle East Afr J Ophthalmol. 2010;17:38–43. doi: 10.4103/0974-9233.61215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.RESNIKOFF S, PASCOLINI D, ETYA’ALE D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 64.RAO GN, GOPINATHAN U. Eye banking: an introduction. Community Eye Health. 2009;22:46–47. [PMC free article] [PubMed] [Google Scholar]
- 65.NBF News. [accessed March 3, 2013];Eye Donation: The dead can offer something to the living ophthalmologist. http://www.thenigerianvoice.com/nvnews/43067/1/eye-donation-the-dead-can-offer-something-to-the-l.html.
- 66.TABIN GC, GURUNG R, PAUDYAL G, et al. Penetrating keratoplasty in Nepal. Cornea. 2004;23:589–596. doi: 10.1097/01.ico.0000121712.36593.0d. [DOI] [PubMed] [Google Scholar]
- 67.VIEIRA SILVA J, JÚLIO DE FARIA E, SOUSA S, MAFALDA FERRANTE A. Corneal transplantation in a developing country: problems associated with technology transfer from rich to poor societies. Acta Ophthalmol Scand. 2006;84:396–400. doi: 10.1111/j.1600-0420.2006.00663.x. [DOI] [PubMed] [Google Scholar]
- 68.PAN Q, LI X, GU Y. Indications and outcomes of penetrating keratoplasty in a tertiary hospital in the developing world. Clin Experiment Ophthalmol. 2012;40:232–238. doi: 10.1111/j.1442-9071.2011.02598.x. [DOI] [PubMed] [Google Scholar]
- 69.TILAHUN Y, SHIMELASH D. The outcome of corneal transplantation versus indications in a tertiary eye care center in Ethiopia. Ethiop Med J. 2010;48:35–39. [PubMed] [Google Scholar]
- 70.FERNANDEZ MM, AFSHARI NA. Endothelial keratoplasty: from DLEK to DMEK. Middle East Afr J Ophthalmol. 2010;17:5–8. doi: 10.4103/0974-9233.61210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.PEH GS, BEUERMAN RW, COLMAN A, et al. Human corneal endothelial cell expansion for corneal endothelium transplantation: an overview. Transplantation. 2011;91:811–819. doi: 10.1097/TP.0b013e3182111f01. [DOI] [PubMed] [Google Scholar]
- 72.OH JY, KIM MK, LEE HJ, et al. Processing porcine cornea for biomedical applications. Tissue Eng Part C Methods. 2009;15:635–645. doi: 10.1089/ten.TEC.2009.0022. [DOI] [PubMed] [Google Scholar]
- 73.EKSER B, EZZELARAB M, HARA H, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379:672–683. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 74.DU Y, CARLSON EC, FUNDERBURGH ML, et al. Stem cell therapy restores transparency to defective murine corneas. Stem Cells. 2009;27:1635–1642. doi: 10.1002/stem.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.ARNALICH-MONTIEL F, PASTOR S, BLAZQUEZ-MARTINEZ A, et al. Adipose derived stem cells are a source for cell therapy of the corneal stroma. Stem Cells. 2008;26:570–579. doi: 10.1634/stemcells.2007-0653. [DOI] [PubMed] [Google Scholar]
- 76.DANIELS JT, DART JKG, TUFT SJ, et al. Corneal stem cells in review. Wound Repair Regen. 2001;9:483–494. doi: 10.1046/j.1524-475x.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- 77.AHMAD S. Concise review: limbal stem cell deficiency, dysfunction, and distress. Stem Cells Transl Med. 2012;1:110–115. doi: 10.5966/sctm.2011-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.GICQUEL JJ. Limbal stem cell transplantation techniques. Acta Ophthalmol. 2011;89:0. [Google Scholar]
- 79.RAMA P, MATUSKA S, PAGANONI G, et al. Limbal stem-cell therapy and long-term corneal regeneration. New Engl J Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 80.HOMMA R, YOSHIKAWA H, TAKENO M, et al. Induction of epithelial progenitors in in vitro from mouse embryonic stem cells and application for reconstruction of damaged cornea in mice. Invest Ophthalmol Vis Sci. 2004;45:4320–4326. doi: 10.1167/iovs.04-0044. [DOI] [PubMed] [Google Scholar]
- 81.KUMAGAI Y, KUROKAWA MS, UENO H, et al. Induction of corneal epithelium-like cells from cynomolgus monkey embryonic stem cells and their experimental transplantation to damaged cornea. Cornea. 2010;29:432–438. doi: 10.1097/ICO.0b013e3181b9ffcc. [DOI] [PubMed] [Google Scholar]
- 82.LIN HF, LAI YC, TAI CF, et al. Effects of cultured human adipose-derived stem cells transplantation on rabbit cornea regeneration after alkaline chemical burn. Kaohsiung J Med Sci. 2013;29:14–18. doi: 10.1016/j.kjms.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.HANSON C, HARDARSON T, ELLERSTRÖM C, et al. Transplantation of human embryonic stem cells onto a partially wounded human cornea in vitro. Acta Ophthalmol. 2013;91:127–130. doi: 10.1111/j.1755-3768.2011.02358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.SAYEGH RR, ANG LP, FOSTER CS, et al. The Boston keratoprosthesis in Stevens-Johnson syndrome. Am J Ophthalmol. 2008;145:438–444. doi: 10.1016/j.ajo.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 85.GRIFFITH M, JACKSON WB, LAGALI N, et al. Artificial corneas: a regenerative medicine approach. Eye. 2009;23:1985–1989. doi: 10.1038/eye.2008.409. [DOI] [PubMed] [Google Scholar]
- 86.LAGALI N, FAGERHOLM P, GRIFFITH M. Biosynthetic corneas: prospects for supplementing the human donor cornea supply. Expert Rev Med Devices. 2011;8:127–130. doi: 10.1586/erd.10.89. [DOI] [PubMed] [Google Scholar]
- 87.HASHIMOTO Y, FUNAMOTO S, SASAKI S, et al. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials. 2010;31:3941–3948. doi: 10.1016/j.biomaterials.2010.01.122. [DOI] [PubMed] [Google Scholar]
- 88.MCLAUGHLIN CR, TSAI RJ, LATORRE MA, et al. Bioengineered corneas for transplantation and in vitro toxicology. Front Biosci. 2009;14:3326–3337. doi: 10.2741/3455. [DOI] [PubMed] [Google Scholar]
- 89.LI A, PAN Z, JIE Y, et al. Comparison of immunogenicity and porcine-to-rhesus lamellar corneal xenografts survival between fresh preserved and dehydrated porcine corneas. Xenotransplantation. 2011;18:46–55. doi: 10.1111/j.1399-3089.2011.00626.x. [DOI] [PubMed] [Google Scholar]
- 90.GRIFFITH M, OSBORNE R, MUNGER R, et al. Functional human corneal equivalents constructed from cell lines. Science. 1999;286:2169–2172. doi: 10.1126/science.286.5447.2169. [DOI] [PubMed] [Google Scholar]
- 91.FAGERHOLM P, LAGALI NS, MERRETT K, et al. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci Transl Med. 2010;2:46ra61. doi: 10.1126/scitranslmed.3001022. [DOI] [PubMed] [Google Scholar]
- 92.FAGERHOLM P, LAGALI NS, CARLSSON DJ, et al. Corneal regeneration following implantation of a biomimetic tissue-engineered substitute. Clinc Transl Sci. 2009;2:162–164. doi: 10.1111/j.1752-8062.2008.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.NISHIDA T, SAIKA S. Fundamentals of cornea and external disease. In: Krachmer JJ, Mannis MJ, Holland EJ, editors. Cornea. St. Louis: Mosby-Year Book Inc; 2005. pp. 3–24. [Google Scholar]
- 94.FEINBERG AW. Corneal endothelium: the Holy Grail of bioengineered tissue. Presentation to the Pediatric Ophthalmology Conferenence: The World through a Child’s Eye; November 8, 2012; Pittsburgh, PA. [Google Scholar]
- 95.NISHIDA K, YAMATO M, HAYASHIDA Y, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 96.KINOSHITA S, KOIZUMI N, NAKAMURA T. Transplantable cultivated mucosal epithelial sheet for ocular surface reconstruction. Exp Eye Res. 2004;78:483–491. doi: 10.1016/j.exer.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 97.TSUBOTA K, SATAKE Y, KAIDO M, et al. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med. 1999;340:1697–1703. doi: 10.1056/NEJM199906033402201. [DOI] [PubMed] [Google Scholar]
- 98.GÜELL JL, GRIS O, MANERO F, et al. Indications for and uses of amniotic membrane. In: Krachmer JJ, Mannis MJ, Holland EJ, editors. Cornea. St. Louis: Mosby-Year Book Inc; 2005. pp. 1647–1654. [Google Scholar]
- 99.NAKAMURA T, YOSHITANI M, RIGBY H, et al. Sterilized, freeze-dried amniotic membrane: A useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci. 2004;45:93–99. doi: 10.1167/iovs.03-0752. [DOI] [PubMed] [Google Scholar]
- 100.ADINOLFI M, AKLE CA, MCCOLL I, et al. Expression of HLA antigens, beta 2-microglobulin and enzymes by human amniotic epithelial cells. Nature. 1982;295:325–327. doi: 10.1038/295325a0. [DOI] [PubMed] [Google Scholar]
- 101.AKLE CA, ADINOLFI M, WELSH KI, et al. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2:1003–1005. doi: 10.1016/s0140-6736(81)91212-5. [DOI] [PubMed] [Google Scholar]
- 102.KUBO M, SONODA Y, MURAMATSU R, et al. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci. 2001;42:1539–1546. [PubMed] [Google Scholar]
- 103.PRABHASAWAT P, TESAVIBUL N, KOMOLSURADEJ W. Single and multilayer amniotic membrane transplantation for persistent corneal epithelial defect with and without stromal thinning and perforation. Br J Ophthalmol. 2001;85:1455–1463. doi: 10.1136/bjo.85.12.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.SANGWAN VS, BURMAN S, TEJWANI S, et al. Amniotic membrane transplantation: A review of current indications in the management of ophthalmic disorders. Indian J Ophthalmol. 2007;55:251–260. doi: 10.4103/0301-4738.33036. [DOI] [PubMed] [Google Scholar]
- 105.HARA H, KOIKE N, LONG C, et al. Initial in vitro investigation of the human immune response to corneal cells from genetically engineered pigs. Invest Ophthalmol Vis Sci. 2011;52:5278–5286. doi: 10.1167/iovs.10-6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.FUJITA M, MEHRA R, LEE SE, et al. Comparison of proliferative capacity of genetically-engineered pig and human corneal endothelial cells. Ophthalmic Res. 2012;49:127–318. doi: 10.1159/000342978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.SENOO T, JOYCE NC. Cell cycle kinetics in corneal endothelium from old and young donors. Invest Ophthalmol Vis Sci. 2000;41:660–667. [PubMed] [Google Scholar]
- 108.LEE SE, MEHRA R, FUJITA M, et al. Characterization of porcine corneal endothelium for xenotransplantation. Semin Ophthalmol. 2013 doi: 10.3109/08820538.2013.787104. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 109.PAN Z, SUN C, JIE Y, et al. WZS-pig is a potential donor alternative in corneal xenotransplantation. Xenotransplantation. 2007;14:603–611. doi: 10.1111/j.1399-3089.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- 110.ROSS JR, HOWELL DN, SANFILIPPO FP. Characteristics of corneal xenograft rejection in a discordant species combination. Invest Ophthalmol Vis Sci. 1993;34:2469–2476. [PubMed] [Google Scholar]
- 111.LARKIN DF, TAKANO T, STANDFIELD SD, et al. Experimental orthotopic corneal xenotransplantation in the rat. Mechanisms of graft rejection. Transplantation. 1995;60:491–497. doi: 10.1097/00007890-199509000-00015. [DOI] [PubMed] [Google Scholar]
- 112.TANAKA, YAMADA J, STREILEIN JW. Xenoreactive CD4+ T cells and acute rejection of orthotopic guinea pig corneas in mice. Invest Ophthalmol Vis Sci. 2000;41:1827–1832. [PubMed] [Google Scholar]
- 113.OH JY, KIM MK, KO JH, et al. Histological differences in full-thickness vs. lamellar corneal pig-to-rabbit xenotransplantation. Vet Ophthalmol. 2009;12:78–82. doi: 10.1111/j.1463-5224.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- 114.KOIZUMI N, SAKAMOTO Y, OKUMURA N, et al. Cultivated corneal endothelial cell sheet transplantation in a primate model. Invest Ophthalmol Vis Sci. 2007;48:4519–4526. doi: 10.1167/iovs.07-0567. [DOI] [PubMed] [Google Scholar]
- 115.KIM YG, OH JY, GIL GC, et al. Identification of alpha-Gal and non-Gal epitopes in pig corneal endothelial cells and keratocytes by using mass spectrometry. Curr Eye Res. 2009;34:877–895. doi: 10.3109/02713680903184243. [DOI] [PubMed] [Google Scholar]
- 116.CHOI HJ, KIM MK, LEE HJ, et al. Effect of αGal on corneal xenotransplantation in a mouse model. Xenotransplantation. 2011;18:176–182. doi: 10.1111/j.1399-3089.2011.00641.x. [DOI] [PubMed] [Google Scholar]
- 117.LIN XC, HUI YN, WANG YS, et al. Lamellar keratoplasty with a graft of lyophilized acellular porcine corneal stroma in the rabbit. Vet Ophthalmol. 2008;11:61–66. doi: 10.1111/j.1463-5224.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- 118.LI J, YU L, DENG Z, et al. Deep anterior lamellar keratoplasty using acellular corneal tissue for prevention of allograft rejection in high-risk corneas. Am J Ophthalmol. 2011;152:762–770. doi: 10.1016/j.ajo.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 119.DU L, WU X. Development and characterization of a full-thickness acellular porcine cornea matrix for tissue engineering. Artif Organs. 2011;35:691–705. doi: 10.1111/j.1525-1594.2010.01174.x. [DOI] [PubMed] [Google Scholar]
- 120.YOERUEK E, BAYYOUD T, MAURUS C, et al. Decellularization of porcine corneas and repopulation with human corneal cells for tissue-engineered xenografts. Acta Ophthalmol. 2012;90:e-125–131. doi: 10.1111/j.1755-3768.2011.02261.x. [DOI] [PubMed] [Google Scholar]
- 121.CHOI HJ, KIM MK, LEE HJ, et al. Efficacy of pig-to-rhesus lamellar corneal xenotransplantation. Invest Ophthalmol Vis Sci. 2011;52:6643–6650. doi: 10.1167/iovs.11-7273. [DOI] [PubMed] [Google Scholar]
- 122.KIM MK, LEE JJ, CHOI HJ, et al. Ethical and regulatory guidelines in clinical trials of xenocorneal transplantation in Korea; the Korean xenocorneal transplantation consensus statement. Xenotransplantation. 2013;20:209–218. doi: 10.1111/xen.12036. [DOI] [PubMed] [Google Scholar]
- 123.Iceland Regulation on Scientific Research in the Health Sector. [accessed October 13, 2013];Regulation on Medical Devices, No. 934/2010. 2010 Novemeber; Available at: http://eng.velferdarraduneyti.is/media/Reglugerdir-enska/Medical-Devices-934_2010.pdf.
- 124.US Department of Health and Human Services Secretary’s Advisory Committee on Xenotransplantation. [accessed October 13, 2013];Informed Consent in Clinical Research Involving Xenotransplantation. 2004 Available at: http://wwwtransplantationsocorg/downloads/SACX-informedconsentpdf.
- 125.World Health Organization. First WHO Global Consultation on Regulatory Requirements for Xenotransplantation Clinical Trials. [accessed October 13, 2013];Changsha: The Changsha Communique. 2008 Available at: http://wwwwhoint/transplantation/xeno/ChangshaCommuniquepdf.
- 126.Centers for Disease Control and Prevention. U.S public health service guideline on infectious disease issues in xenotransplantation. [accessed October 13, 2013];MMWR. 2001 50:1–46. Available at: http://www.cdc.gov/mmwr/PDF/rr/rr5015.pdf. [PubMed] [Google Scholar]
- 127.U.S. Food and Drug Administration. [accessed October 13, 2013];Guidance for Industry: Source Animal, Product, Preclinical, and Clinical Issues Concerning the Use of Xenotransplantation Products in Humans. 2003 Available at: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Xenotransplantation/ucm074354.htm.
- 128.Xenotransplantation U.S. Department of Health and Human Services Secretary’s Advisory Committee on Xenotransplantation. [accessed October 13, 2013];Informed Consent in Clinical Research Involving Xenotransplantation. 2004 Available at: http://wwwtransplantation-socorg/downloads/SACXinformedconsentpdf.
- 129.World Medical Association. Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. finally amended by the 59th WMA General Assembly; Seoul. October 2008. [Google Scholar]
- 130.HERING BJ, COOPER DKC, COZZI E, et al. The International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes – Executive summary. Xenotransplantation. 2009;16:196–202. doi: 10.1111/j.1399-3089.2009.00547.x. [DOI] [PubMed] [Google Scholar]
- 131.DAPENA I, HAM L, MELLES GR. Endothelial keratoplasty: DSEK/DSAEK or DMEK--the thinner the better? Curr Opin Ophthalmol. 2009;20:299–307. doi: 10.1097/ICU.0b013e32832b8d18. [DOI] [PubMed] [Google Scholar]
- 132.REINHART WJ, MUSCH DC, JACOBS DS, et al. Deep anterior lamellar keratoplasty as an alternative to penetrating keratoplasty: a report by the American Academy of Ophthalmology. Ophthalmology. 2011;118:209–218. doi: 10.1016/j.ophtha.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 133.BORDERIE VM, SANDALI O, BULLET J, et al. Long-term results of deep anterior lamellar versus penetrating keratoplasty. Ophthalmology. 2012;119:249–255. doi: 10.1016/j.ophtha.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 134.KIM MH, CHUNG TY, CHUNG ES. A retrospective contralateral study comparing deep anterior lamellar keratoplasty with penetrating keratoplasty. Cornea. 2013;32:385–389. doi: 10.1097/ICO.0b013e318254be4e. [DOI] [PubMed] [Google Scholar]
- 135.ACAR BT, AKDEMIR MO, ACAR S. Corneal biomechanical properties in eyes with no previous surgery, with previous penetrating keratoplasty and with deep anterior lamellar keratoplasty. Jpn J Ophthalmol. 2013;57:85–89. doi: 10.1007/s10384-012-0197-5. [DOI] [PubMed] [Google Scholar]
- 136.TERRY MA, WALL JM, HOAR KL, et al. A prospective study of endothelial cell loss during the 2 years after deep lamellar endothelial keratoplasty. Ophthalmology. 2007;114:631–639. doi: 10.1016/j.ophtha.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 137.BAHAR I, KAISERMAN I, MCALLUM P, et al. Comparison of posterior lamellar keratoplasty techniques to penetrating keratoplasty. Ophthalmology. 2008;115:1525–1533. doi: 10.1016/j.ophtha.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 138.MALIK SRK, SINGH G. Therapeutic keratoplasty in Pseuodomonas pyocyanea corneal ulcers. Br J Ophthalmol. 1971;55:326–330. doi: 10.1136/bjo.55.5.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.DONNENFELD ED, SOLOMON R, PERRY A. Therapeutic keratoplasty. In: Krachmer JJ, Mannis MJ, Holland EJ, editors. Cornea. St. Louis: Mosby-Year Book Inc; 2005. pp. 1593–1603. [Google Scholar]
- 140.XIE L, DONG X, SHI W. Treatment of fungal keratitis by penetrating keratoplasty. Br J Ophthalmol. 2001;85:1070–1074. doi: 10.1136/bjo.85.9.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.XIE L, ZHAI H, SHI W. Penetrating keratoplasty for corneal perforations in fungal keratitis. Cornea. 2007;26:158–162. doi: 10.1097/01.ico.0000248381.24519.0d. [DOI] [PubMed] [Google Scholar]
- 142.XIE L, SHI W, LIU Z, et al. Lamellar keratoplasty for the treatment of fungal keratitis. Cornea. 2002;21:33–37. doi: 10.1097/00003226-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 143.AL-FAWAZ A, WAGONER MD. Penetrating keratoplasty for trachomatous corneal scarring. Cornea. 2008;27:129–132. doi: 10.1097/ICO.0b013e318158b49e. [DOI] [PubMed] [Google Scholar]
- 144.HALBERSTADT M, MACHENS M, GAHLENBEK KA, et al. The outcome of corneal grafting in patients with stromal keratitis of herpetic and non-herpetic origin. Br J Ophthalmol. 2002;86:646–652. doi: 10.1136/bjo.86.6.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.SHTEIN RM, ELNER VM. Herpes simplex virus keratitis: histopathology and corneal allograft outcomes. Expert Rev Ophthalmol. 2010;5:129–134. doi: 10.1586/eop.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.AWAN MA, ROBERTS F, HEGARTY B, et al. The outcome of deep anterior lamellar keratoplasty in herpes simplex virus-related corneal scarring, complications and graft survival. Br J Ophthalmol. 2010;94:1300–1303. doi: 10.1136/bjo.2009.169300. [DOI] [PubMed] [Google Scholar]
- 147.WU SQ, ZHOU P, ZHANG B, et al. Long-term comparison of full-bed deep lamellar keratoplasty with penetrating keratoplasty in treating corneal leucoma caused by herpes simplex keratitis. Am J Ophthalmol. 2012;153:291–299. e2. doi: 10.1016/j.ajo.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 148.SARNICOLA V, TORO P. Deep anterior lamellar keratoplasty in herpes simplex corneal opacities. Cornea. 2010;29:60–64. doi: 10.1097/ICO.0b013e3181a317d3. [DOI] [PubMed] [Google Scholar]
- 149.LYALL DA, TARAFDAR S, GILHOOLY MJ, et al. Long term visual outcomes, graft survival and complications of deep anterior lamellar keratoplasty in patients with herpes simplex related corneal scarring. Br J Ophthalmol. 2012;96:1200–1203. doi: 10.1136/bjophthalmol-2012-301947. [DOI] [PubMed] [Google Scholar]
- 150.WARING GO, MBEKANI JN. Differential diagnosis of localized deposits (spots and dots) in the cornea. In: Leibowitz HM, Waring GO III, editors. Corneal Disorders: Clinical Diagnosis and Management. Philadelphia: W.B. Saunders; 1998. pp. 224–286. [Google Scholar]
- 151.KARIMIAN F, FEIZI S. Deep anterior lamellar keratoplasty: indications, surgical techniques and complications. Middle East Afr J Ophthalmol. 2010;17:28–37. doi: 10.4103/0974-9233.61214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.UNAL M, ARSLAN OS, ATALAY E, et al. Deep anterior lamellar keratoplasty for the treatment of stromal corneal dystrophies. Cornea. 2013;32:301–305. doi: 10.1097/ICO.0b013e31825718ca. [DOI] [PubMed] [Google Scholar]
- 153.COMBILLET F, TOUBOUL D, LEGER F, et al. Granular corneal dystrophy treated with deep anterior lamellar keratoplasty: comparing histological analysis and optical coherence tomography. J Fr Ophthalmol. 2012;35:e1–5. doi: 10.1016/j.jfo.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 154.SALOUTI R, HOSSEINI H, EGHTEDARI M, et al. Deep anterior lamellar keratoplasty with melles technique for granular corneal dystrophy. Cornea. 2009;28:140–143. doi: 10.1097/ICO.0b013e3181861cdd. [DOI] [PubMed] [Google Scholar]
- 155.KARIMIAN F, BARADARAN-RAFII AR, FEIZI S, et al. Outcomes of penetrating keratoplasty for macular corneal dystrophy. J Ophthalmic Vis Res. 2009;4:14–18. [PMC free article] [PubMed] [Google Scholar]
- 156.AL-SWAILEM SA, AL-RAJHI AA, WAGONER MD. Penetrating keratoplasty for macular corneal dystrophy. Opthalmology. 2005;112:220–224. doi: 10.1016/j.ophtha.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 157.KAWASHIMA M, KAWAKITA T, DEN S, et al. Comparison of deep lamellar keratoplasty and penetrating keratoplasty for lattice and macular corneal dystrophies. Am J Ophthalmol. 2006;142:304–309. doi: 10.1016/j.ajo.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 158.CHENG J, QI X, ZHAO J, et al. Comparison of penetrating keratoplasty and deep lamellar keratoplasty for macular corneal dystrophy and risk factors of recurrence. Ophthalmology. 2013;120:34–39. doi: 10.1016/j.ophtha.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 159.KAWAMOTO K, MORISHIGE N, YAMADA N, et al. Delayed corneal epithelial wound healing after penetrating keratoplasty in individuals with lattice corneal dystrophy. Am J Ophthalmol. 2006;142:173–174. doi: 10.1016/j.ajo.2006.01.077. [DOI] [PubMed] [Google Scholar]
- 160.FOERSTER CG, LANGENBUCHER A, CURSIEFEN C, et al. Delayed epithelial healing after keratoplasty for lattice corneal dystrophy. Cornea. 2007;26:1182–1183. doi: 10.1097/ICO.0b013e318151f8cc. [DOI] [PubMed] [Google Scholar]
- 161.PINEROS O, COHEN EJ, RAPUANO CJ, et al. Long-term results after penetrating keratoplasty for Fuchs’ endothelial dystrophy. Arch Ophthalmol. 1996;114:15–18. doi: 10.1001/archopht.1996.01100130013002. [DOI] [PubMed] [Google Scholar]
- 162.PRICE MO, PRICE FW. Indications for endothelial keratoplasty. In: Krachmer JJ, Mannis MJ, Holland EJ, editors. Cornea. St. Louis: Mosby-Year Book Inc; 2005. pp. 1531–1534. [Google Scholar]
- 163.HEIDEMANN DG, DUNN SP, CHOW CY. Comparison of deep lamellar endothelial keratoplasty and penetrating keratoplasty in patients with Fuchs endothelial dystrophy. Cornea. 2008;27:161–167. doi: 10.1097/ICO.0b013e31815b8304. [DOI] [PubMed] [Google Scholar]
- 164.HJORTDAL J, EHLERS N. Descemet’s stripping automated endothelial keratoplasty and penetrating keratoplasty for Fuchs’ endothelial dystrophy. Acta Ophthalmol. 2009;87:310–314. doi: 10.1111/j.1755-3768.2008.01492.x. [DOI] [PubMed] [Google Scholar]
- 165.HJORTDAL J, PEDERSEN IB, BAK-NIELSEN S, et al. Graft rejection and graft failure after penetrating keratoplasty or posterior lamellar keratoplasty for Fuchs endothelial dystrophy. Cornea. 2013;32:e60–63. doi: 10.1097/ICO.0b013e3182687ff3. [DOI] [PubMed] [Google Scholar]
- 166.NANAVATY MA, SHORTT AJ. Endothelial keratoplasty versus penetrating keratoplasty for Fuchs endothelial dystrophy. Cochrane Database Syst Rev. 2011;7:6959–6963. doi: 10.1002/14651858.CD008420.pub2. [DOI] [PubMed] [Google Scholar]
- 167.PRICE MO, PRICE FW. Descemet stripping with endothelial keratoplasty for treatment of iridocorneal endothelial syndrome. Cornea. 2007;26:493–497. doi: 10.1097/ICO.0b013e318030d274. [DOI] [PubMed] [Google Scholar]
- 168.HUANG T, WANG Y, JI J, et al. Deep lamellar endothelial keratoplasty for iridocorneal endothelial syndrome in phakic eyes. Arch Ophthalmol. 2009;127:33–36. doi: 10.1001/archophthalmol.2008.537. [DOI] [PubMed] [Google Scholar]
- 169.SHARIF KW, CASEY TA. Penetrating keratoplasty for keratoconus: complications and long-term success. Br J Ophthalmol. 1991;75:142–146. doi: 10.1136/bjo.75.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.PRICE FW, JR, WHITSON WE, MARKS RG. Graft survival in four common groups of patients undergoing penetrating keratoplasty. Ophthalmology. 1991;98:322–328. doi: 10.1016/s0161-6420(91)32292-9. [DOI] [PubMed] [Google Scholar]
- 171.LINDQUIST TD, MCGLOTHAN JS, ROTKIS WM. Indications for penetrating keratoplasty: 1980–1988. Cornea. 1991;10:210–216. doi: 10.1097/00003226-199105000-00005. [DOI] [PubMed] [Google Scholar]
- 172.KIRKNESS CM, FICKER LA, STEELE AD, et al. The success of penetrating keratoplasty for keratoconus. Eye (Lond) 1990;4:673–688. doi: 10.1038/eye.1990.95. [DOI] [PubMed] [Google Scholar]
- 173.AL-TORBAK AA, AL-MOTOWA S, AL-ASSIRI A, et al. Deep anterior lamellar keratoplasty for keratoconus. Cornea. 2006;25:408–412. doi: 10.1097/01.ico.0000220777.70981.46. [DOI] [PubMed] [Google Scholar]
- 174.PARENTE G, FONTANA L, TASSINARI G. Quality of vision following penetrating keratoplasty and deep anterior lamellar keratoplasty for keratoconus. Acta Ophthalmol. 2008;86:0. [Google Scholar]
- 175.KUBALOGLU A, SARI ES, UNAL M, et al. Long-term results of deep anterior lamellar keratoplasty for the treatment of keratoconus. Am J Ophthalmol. 2011;151:760–767. doi: 10.1016/j.ajo.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 176.AMAYEM AF, HAMDI IM, HAMDI MM. Refractive and visual outcomes of penetrating keratoplasty versus deep anterior lamellar keratoplasty with hydrodissection for treatment of keratoconus. Cornea. 2013;32:e2–5. doi: 10.1097/ICO.0b013e31825ca70b. [DOI] [PubMed] [Google Scholar]
- 177.SMADJA D, COLIN J, KRUEGER RR, et al. Outcomes of deep anterior lamellar keratoplasty for keratoconus: learning curve and advantages of the big bubble technique. Cornea. 2012;31:859–863. doi: 10.1097/ICO.0b013e318242fdae. [DOI] [PubMed] [Google Scholar]
- 178.MASHOR RS, ROOTMAN DB, BAHAR I, et al. Outcomes of deep anterior lamellar keratoplasty versus intralase enabled penetrating keratoplasty in keratoconus. Can J Ophthalmol. 2011;46:403–407. doi: 10.1016/j.jcjo.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 179.FONTANA L, PARENTE G, SINCICH A, et al. Influence of graft-host interface on the quality of vision after deep anterior lamellar keratoplasty in patients with keratoconus. Cornea. 2011;30:497–502. doi: 10.1097/ico.0b013e3181d25e4d. [DOI] [PubMed] [Google Scholar]
- 180.KUBALOGLU A, KOYTAK A, SARI ES, et al. Corneal endothelium after deep anterior lamellar keratoplasty and penetrating keratoplasty for keratoconus: a four-year comparative study. Indian J Ophthalmol. 2012;60:35–40. doi: 10.4103/0301-4738.90490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.MRCOphth. [accessed April 15, 2013];ICE. http://www.mrcophth.com/glaucoma/ice.jpg.
- 182.Seeability. [accessed April 15, 2013];Keratoconus Eye. http://www.seeability.org/images/Our_Services/Eye_care_hub/Factsheets/Keratoconus/keratoconuseye_01.jpg.
- 183.Cyber Rounds. [accessed April 15, 2013];Bacterial keratitis. http://www.cyberounds.com/assets/09/58/958/figure6.jpg.
- 184.Review of Optometry. [accessed April 15, 2013];Fungal keratitis. http://www.revoptom.com/CMSImagesContent/2007/1/2_13103_0.gif.
- 185.The University of Arizona. A database of hereditary ocular disease. [accessed April 15, 2013];Granular dystrophy. http://disorders.eyes.arizona.edu/sites/disorders.eyes.arizona.edu/files/disorder_images/slidescan_091.jpg.
- 186.Wikimedia. [accessed April 15, 2013];Type 1 lattice dystrophy. http://upload.wikimedia.org/wikipedia/commons/b/bc/Lattice_corneal_dystrophy_type_1.JPEG.
- 187.Review of Cornea and Contact Lenses. [accessed April 15, 2013];Macular dystrophy. http://www.reviewofcontactlenses.com/CMSImagesContent/2010/6/Fig-6.gif.
- 188.Stephen M, Johnson MD. [accessed April 15, 2013];Endothelial guttata of Fuchs’ dystrophy. http://www.smjohnsonmd.com/Images/guttata%20arrow%20.jpg.
- 189.EYE BANK ASSOCIATION OF AMERICA. 2009 Eye Banking Statistical Report. Washington DC: EBBA; 2009. [Google Scholar]