Dermal papilla cells (DPCs) as a dermal component in a permanent composite skin with human hair follicle stem cells (HFSCs) was evaluated. A porcine acellular dermal matrix was seeded with HFSCs alone and with human DPCs or dermal fibroblasts. The findings suggest that DPCs in the composite substitute favor early neovascularization after grafting and contribute to neovascular network maturation, which might reduce the inflammation process, resulting in better healing, with less scarring and wound contraction. Furthermore, only in grafted constructs containing DPC, embryonic hair bud-like structures were observed inside the remodeling dermal matrix.
Keywords: Adult stem cells, Skin grafts, Epidermis, Multipotential differentiation, Tissue regeneration
Abstract
Tissue-engineered skin represents a useful strategy for the treatment of deep skin injuries and might contribute to the understanding of skin regeneration. The use of dermal papilla cells (DPCs) as a dermal component in a permanent composite skin with human hair follicle stem cells (HFSCs) was evaluated by studying the tissue-engineered skin architecture, stem cell persistence, hair regeneration, and graft-take in nude mice. A porcine acellular dermal matrix was seeded with HFSCs alone and with HFSCs plus human DPCs or dermal fibroblasts (DFs). In vitro, the presence of DPCs induced a more regular and multilayered stratified epidermis with more basal p63-positive cells and invaginations. The DPC-containing constructs more accurately mimicked the skin architecture by properly stratifying the differentiating HFSCs and developing a well-ordered epithelia that contributed to more closely recapitulate an artificial human skin. This acellular dermal matrix previously repopulated in vitro with HFSCs and DFs or DPCs as the dermal component was grafted in nude mice. The presence of DPCs in the composite substitute not only favored early neovascularization, good assimilation and remodeling after grafting but also contributed to the neovascular network maturation, which might reduce the inflammation process, resulting in a better healing process, with less scarring and wound contraction. Interestingly, only DPC-containing constructs showed embryonic hair bud-like structures with cells of human origin, presence of precursor epithelial cells, and expression of a hair differentiation marker. Although preliminary, these findings have demonstrated the importance of the presence of DPCs for proper skin repair.
Introduction
Deep skin injuries, such as occur in severe burns and chronic wounds, produce complete destruction of the skin’s regenerative elements. These wounds heal by contraction, with epithelization only from the edges and extensive scarring, resulting in reduced joint movements and cosmetic defects [1]. Moreover, if this type of lesion is too extensive, the healing process will be unsuccessful, and the lesion becomes life-threatening for the patient.
Currently, the clinical reference standard treatment of full-thickness injuries is split-thickness autologous skin grafting [2]. Nevertheless, when the affected body surface area is greater than 50% or when smaller wounds cannot heal because of underlying pathologic factors, it is necessary to consider other therapeutic alternatives. To avoid the loss of heat and fluid from the wound, the generation of coverage becomes necessary. Autologous cultured epidermal substitutes can be another alternative [3], but when dermal bed has been completely destroyed, this approach drives to unsatisfactory results owing to the fragility of the graft and wound contraction and scar formation [4, 5].
Successful wound healing requires the presence of a dermis layer in the skin substitute [6, 7], and clinical trials have also indicated the importance of dermal pregraftment in the graft-take of the autologous epidermal substitute [8, 9] and in the reduction of graft contraction and scarring [10, 11]. Acellular dermal substitutes based on allogeneic, xenogeneic, or synthetic materials [12] are commercial alternatives. Nevertheless, it has been reported that contraction and scar formation cannot be completely prevented by an acellular dermal matrix unless the dermal and epidermal cells have been added [13]. In order to avoid those problems, many composite skin substitutes generated by tissue engineering were developed [12].
This type of constructs tries to mimic the normal histological structure of skin and is composed of a matrix component (tridimensional scaffold) and two cellular components (epidermal and dermal cells). The matrix component provides elasticity and strength to the epidermal layer and plays a very important role as a template for cellular anchorage and growth factor supply that guides survival, proliferation, migration, and differentiation in in vitro constructs and subsequent cellular host infiltration and remodeling after being grafted [14, 15]. A number of different types of scaffolds have already been designed and tested in many animal models and patients [12].
Tissue-engineered construct success depends on the selected epidermal cells, which must ensure the persistence and function of the grafted constructs throughout the patient’s lifetime [16]. Several studies have shown that progenitor epidermal cells are better than differentiated ones in the generation of successful tissue-engineered skin [17, 18].
Hair follicle stem cells (HFSCs) residing in the bulge contribute to hair follicle regeneration and wound repair [19] and can differentiate to epidermis, sebaceous glands, and eight different types of hair follicle epithelial cells [20–23]. The epithelial-mesenchymal interactions with the underlying dermal papilla play a pivotal role in embryonic hair genesis [24], the regulation of the postnatal hair follicle cyclical activity, and the repair of wounded skin [25–28]. Dermal papilla cells (DPCs) have shown inductive properties in the generation of bioengineered skin substitutes from basal epidermal stem cells [29].
The hair-differentiation potentiality of epidermal stem cells can be activated by inductive dermal cells [30]. The follicular dermal papilla cells can induce hair formation in rodents once they are located near the epithelium [31, 32] or generate hair follicle neogenesis in vivo when combined with hair follicle stem cells [33]. The DPCs are also able to induce the differentiation of bulge stem cells into hair-lineage cell types in vitro [34, 35].
The use of DPCs as a dermal component in a permanent composite skin with human HFSCs was evaluated in this work by studying its effect on tissue-engineered skin architecture, stem cell persistence, contraction of the healing skin, hair regeneration, and graft-take in nude mice.
Materials and Methods
Cell Cultures
Full-thickness skin samples were obtained, under written consent, from the occipital human scalp of 18 individuals undergoing corrective surgery for the treatment of androgenetic alopecia. The local institutional review board (Comité de Ética del Instituto de Ciencia y Tecnología “César Milstein”) approved the studies.
DPC primary cultures were established, as previously described [36]. In brief, anagen follicles were dissected, and the isolated hair bulbs were incubated in collagenase I (Sigma-Aldrich, Munich, Germany, http://www.sigmaaldrich.com) for 2 hours at 37°C. The papilla explants were grown in culture medium, Dulbecco’s Modified Eagle’s Medium (Gibco, Invitrogen, Life Technologies, Carlsbad, CA, http://www.lifetechnologies.com) containing penicillin (100 U/ml), streptomycin (100 mg/ml), and 10% fetal bovine serum (FBS) (Gibco, Invitrogen, Life Technologies).
The HF-enriched primary cultures (HFSCs) were obtained as previously described [34]. In brief, skin samples were incubated in 1 mg/ml collagenase/dispase (Sigma-Aldrich) overnight at 4°C, and telogen follicles were pulled out and incubated in 0.05% trypsin-0.23 mM EDTA (Gibco, Invitrogen, Life Technologies). The isolated cells were resuspended in cFAD medium [35] and enriched in stem cells by attachment to collagen IV (Sigma-Aldrich)-coated plates for 30 minutes at 37°C and then cultured on mitomycin C-inactivated 3T3-Swiss cells as a feeder layer, using cFAD medium. After 3 days, the cultures were supplemented with epidermal growth factor (10 ng/ml) (Sigma-Aldrich).
The immortalized human bulge stem cell line Tel-E6E7 [37] was kindly provided by Dr. Lyle (University of Massachusetts Medical School) and cultured in the same conditions.
Finally, dermal fibroblasts (DFs) were also obtained from scalp skin biopsies. For that purpose, explants of small pieces of skin dermis were placed in 35-mm plastic Petri dishes. Culture medium DMEM (Gibco, Invitrogen, Life Technologies) containing penicillin (100 U/ml) and streptomycin (100 mg/ml) and supplemented with 10% FBS was used (Gibco, Invitrogen, Life Technologies). Once explant outgrowth reached 70% of confluence, the cells were replated in culture flasks and maintained in the same conditions already described for the DPCs.
Human Composite Skin Substitute Generation
Porcine acellular dermal matrices (ADMs), 1 mm thick, were seeded with 5 × 105 cm−2 DFs or DPCs as the dermal cellular component and cultivated in DMEM supplemented with 10% FBS (Gibco, Invitrogen, Life Technologies). These matrices were cultured at 37°C for 7 days. Thereafter, the matrices were inverted and seeded on the opposite side with 5 × 105 cm−2 HFSCs or Tel-E6E7 as the epidermal cellular component in cFAD medium and cultured at 37°C for another 7 days. Some scaffolds were exclusively seeded with HFSCs. Half of these constructs were cultured 21 more days in submerged conditions and then formaldehyde-fixed and paraffin-embedded for subsequent histological studies (liquid-phase constructs). The other half were transferred to a Transwell device (Falcon Cell Culture Inserts, 1-µm pore size membrane; BD, Franklin Lakes, NJ, http://www.bd.com) to expose the dermal face to the culture medium and the epidermal face to air (air-liquid interphase) and cultured for 14 more days at 37°C. All these constructs were also formaldehyde-fixed and paraffin-embedded for subsequent histological studies (air-liquid interphase constructs). All constructs types were submitted to histological and immunohistochemical analysis. In all cases, three independent in vitro experiments were performed. In each, five constructions of each type were performed. Figure 1 shows the procedure scheme to generate the skin constructs using ADM as the scaffold.
Figure 1.

Procedure scheme to generate skin constructs using a porcine ADM as scaffold. (A): ADM was seeded with DFs or DPCs. (B): Matrix-side inversion. (C): The ADM was seeded with HFSCs. (D): Some matrices were transferred to Transwell plates and maintained in air-liquid interphase. Abbreviations: ADM, acellular dermal matrix; DF, dermal fibroblast; DPC, dermal papilla cell; HFSC, hair follicle stem cell; PET, polyethylene terephthalate.
Grafting of Skin Constructs in Nude Mice
A single full-thickness skin wound (1.5 cm in diameter) was created with surgical scissors in the back of each BALB/C nude mice aged 8 weeks (n = 6 per group) after being anesthetized with ketamine/xylazine (110 mg/10 mg per kilogram body weight). No shaving in the surgical area was necessary because nude mice were used. The air-liquid interphase skin constructs were grafted, locating the graft borders between the hypodermis and muscle-fascia without any type of surgical suture. The grafts were only coated by an auto-adhesive polyurethane transparent bandage (Tegaderm, 3M, St. Paul, MN, http://www.3m.com) that allows gas exchange and avoids fluid loss. A control group was grafted with ADM alone. In all cases, three independent grafting assays were performed. In each, six animals per group for each time point were grafted with the different types of constructs. The mice whose grafts were lost or had moved from their original place were discarded for statistical analysis (one in ADM control group at the 14-day point; two each in the HFSC-DF and HFSC-DPC groups at the 70-day point).
At the indicated time points (14, 30, and 70 days), the grafts were photographed, and the mice were sacrificed. The grafted skin constructs, including the rodent skin border, were dissected and fixed in formaldehyde and paraffin-embedded for histological and immunohistochemical analysis.
The skin contraction ratio (SCR) of the injured and grafted area was calculated as follows: SCR = 1 − (RLA/OLA), with RLA indicating the remaining lesion area 70 days after grafting and OLA, the original lesion area.
Histological and Immunohistochemical Analysis
Paraffin-embedded histological slides from in vitro and in vivo assays were stained with hematoxylin and eosin for tissue architecture analysis. The number of epidermal layers was evaluated in 10 fields of ×400 magnification, and epidermal invaginations were evaluated in six ×100 fields for each construct.
Immunohistochemical assays were performed with anti-p63 antibody (mouse monoclonal antibody IgG2a, Santa Cruz Biotechnology Inc., Dallas, TX, http://www.scbt.com) for epidermal stem cell detection, anti-murine CD34 antibody (mouse monoclonal antibody IgG2a, Abcam, Cambridge, U.K., http://www.abcam.com) for neovessel detection in the grafting experiments, anti-human leukocyte antigen type I (HLA I) ABC (mouse monoclonal antibody IgG2a, Abcam) for detection of human cells in the grafted mice, and anti-k6hf (polyclonal guinea pig antibody, Progen Biotechnik GmbH, Heidelberg, Germany, http://www.progen.de) for detection of hair committed cells in mouse skin injured areas grafted with the constructs containing DPCs and HFSCs. Primary antibodies were developed using Universal LSAB kit (Dako, Glostrup, Denmark, http://www.dako.com) according to the manufacturer’s recommendations. Positive epidermal cells for p63 in the in vitro and in vivo constructs were evaluated in ten ×1,000 and five ×400 fields, respectively, and were normalized to the total number of epidermal cells in each field. Neovessels in grafted constructs were evaluated by CD34 immunostaining in five ×400 fields and normalized to the dermis surface (number of blood vessels per 0.01 mm2) in each field.
All the results shown in bar graphs represent the mean values ± SD from three independent experiments.
Statistical Analysis
One-way analysis of variance to evaluate statistical significance was performed. For that purpose, we used the software Statistica 8 (StatSoft, Tulsa, OK, http://www.statsoft.com). Differences with p < .05 were considered statistically significant.
Results
Presence of DPCs in Air-Liquid Interphase Skin Constructs Leads to a Better Stratified Epidermis With the Highest Number of Epidermal Layers
In vitro skin constructs were generated using a porcine ADM as the scaffold, as described in “Materials and Methods” section and shown in Figure 1. The constructs were generated using both HF-enriched primary cultures and the immortalized human bulge stem cell line Tel-E6E7. Given that no differences were found regarding the histological structure among the pool of stem cells in both the in vitro and the in vivo assays and in the graft-take in the nude mice, we have referred to the epidermal component collectively as HFSCs.
One type of construct was seeded with HFSCs and DPCs (HFSC-DPC), a second type was seeded with HFSCs and DFs (HFSC-DF), and a third type was seeded exclusively with HFSCs without the dermal component.
When these in vitro skin constructs were generated in liquid phase, a low number of epidermal layers was observed that never exceeded four and did not display the proper order for stratified epithelium; nor were significant differences found among them (supplemental online Fig. 1).
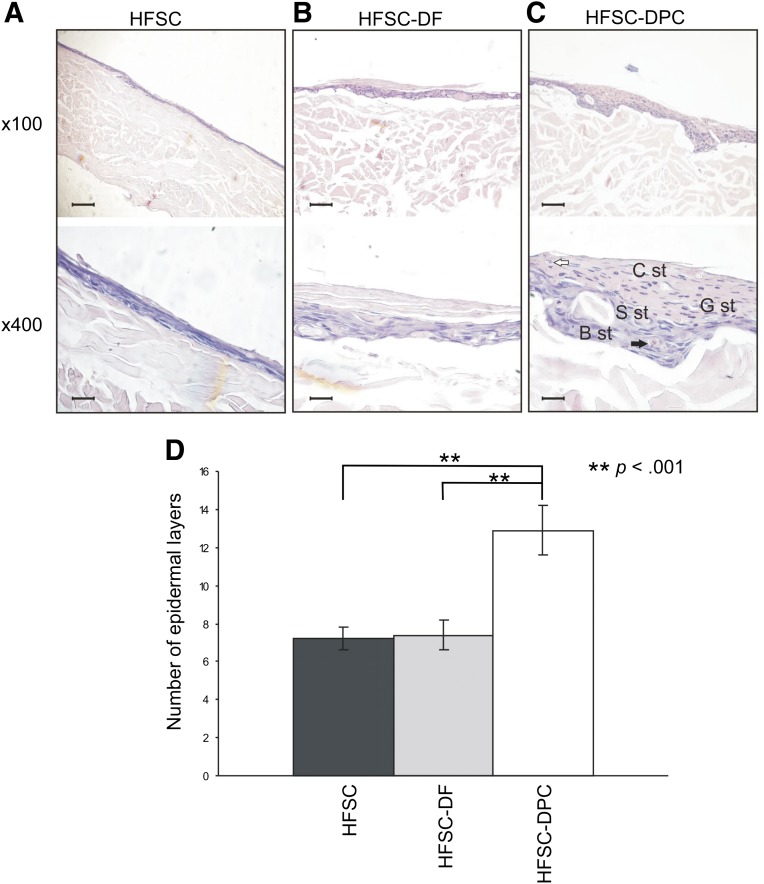
Notable differences were observed when the same skin constructs were generated in air-liquid interphase. Skin constructs with HFSCs alone (Fig. 2A) or with DFs (HFSC-DF) (Fig. 2B) showed an epidermis with a proliferative basal layer, an incipient and irregular stratum spinosum, frequent dyskeratosis, and a cornified layer. In the HFSC-DPC skin constructs (Fig. 2C), we observed the most regular epidermis, consisting of a proliferative basal layer and stratum spinosum with less dyskeratosis. A granulosum stratum, parakeratosis areas, and cornified layer were also observed. This construction showed a significantly higher number of epidermal layers typical of human epidermis (Fig. 2D).
Figure 2.
Histology of skin constructs generated in air-liquid interphase. (A): HFSC. (B): HFSC-DF. (C): HFSC-DPC. Black arrow indicates dyskeratosis; white arrow, parakeratosis. Scale bars = 200 µm (×100 fields) and 50 µm (×400 fields). (D): Bar graph shows the number of epidermal layers from each construct. ∗∗, p < .001. Abbreviations: B st, basal stratum; C st, cornified stratum; DF, dermal fibroblast; DPC, dermal papilla cell; G st, granulosum stratum; HFSC, hair follicle stem cell; S st, spinosum stratum.
In Vitro Skin Constructs With DPCs Showed a Higher Number of p63-Positive Epidermal Basal Cells and Epidermal Invaginations
The expression of p63 is considered a stemness marker of epithelial cells in tissues such as the epidermis and corneal limbus [38].
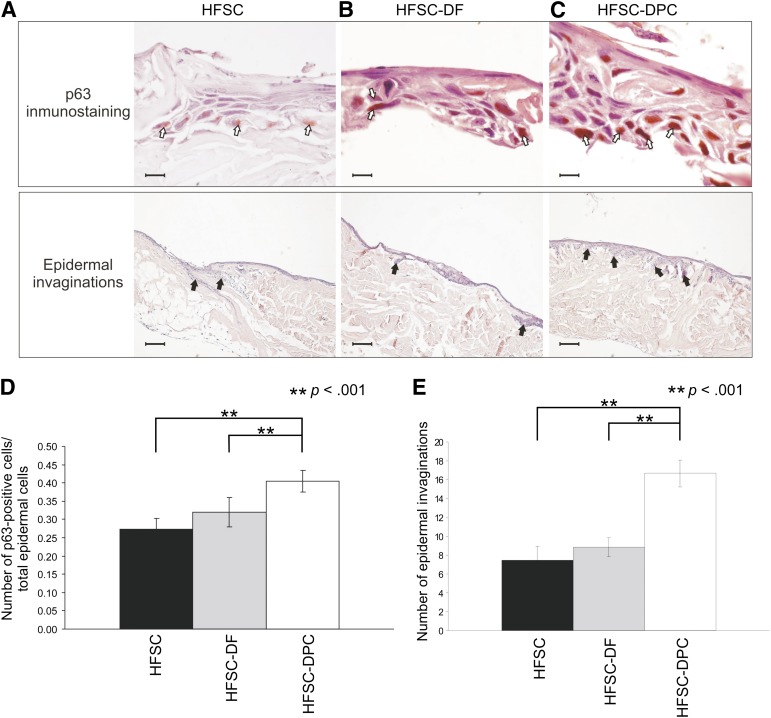
Skin constructs generated with HFSCs and DPCs (Fig. 3C) showed a significantly higher number of p63-positive cells (Fig. 3D) than did the constructs with HFSCs alone (Fig. 3A) or with HFSCs and DFs (Fig. 3B). The p63-positive cells were mainly located in the basal region, suggesting that the presence of DPCs favored the survival of a greater population of precursor cells. The HFSC-DPC skin constructs (Fig. 3C) showed, in vitro, a significantly higher number of epidermal invaginations (Fig. 3E) than did the HFSC and HFSC-DF constructs (Fig. 3A, 3B). The presence of DPC generated a papillary architecture, reminiscent of normal human skin.
Figure 3.
p63 immunohistochemistry and epidermal invaginations of skin constructs generated in air-liquid interphase. (A): HFSC. (B): HFSC-DF. (C): HFSC-DPC. Scale bars = 20 µm (p63 immunostaining) and 200 µm (epidermal invaginations). (D): Bar graph of p63-positive per total epidermal cells in ×1,000 fields. (E): Bar graph of epidermal invaginations in ×100 fields. White arrows show some p63-positive nucleus and black arrows, some epidermal invaginations. ∗∗, p < .001. Abbreviations: ADM, acellular dermal matrix; DF, dermal fibroblast; DPC, dermal papilla cell; HFSC, hair follicle stem cell.
Presence of DPCs Favored the Graft-Take of Composite Skin and Improved the Wound Healing Process
The in vitro skin constructs generated in air-liquid interphase were grafted into the deep skin lesions generated in the nude mice, and the graft-take was evaluated.
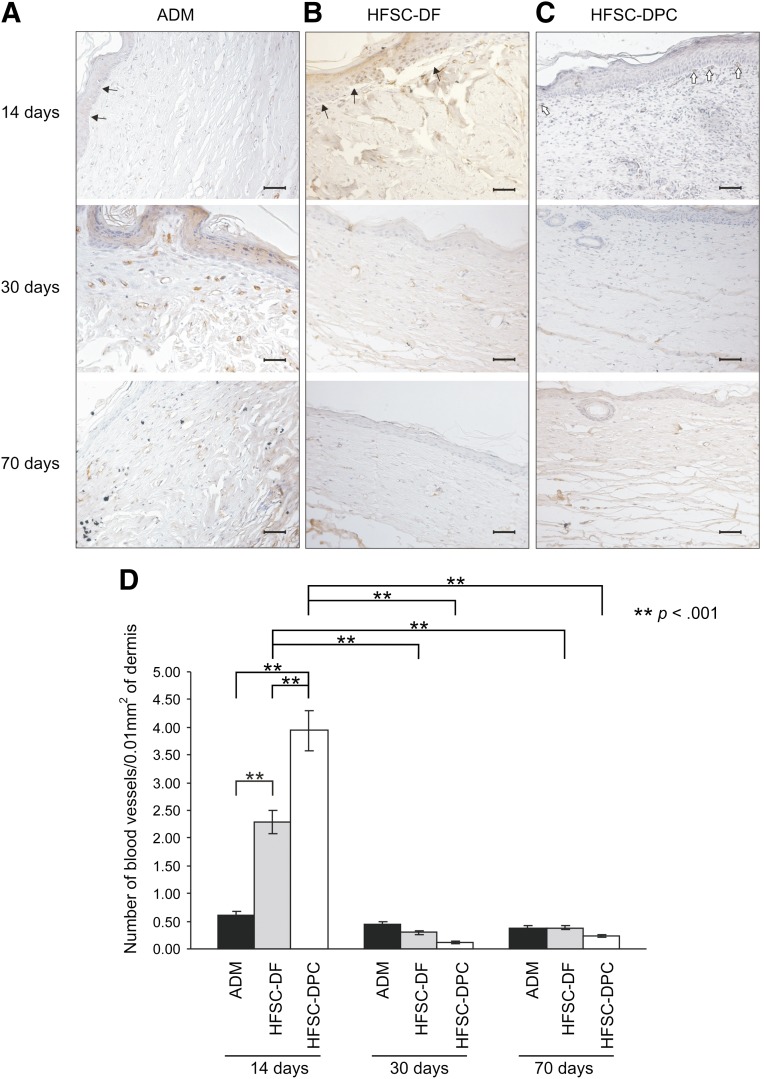
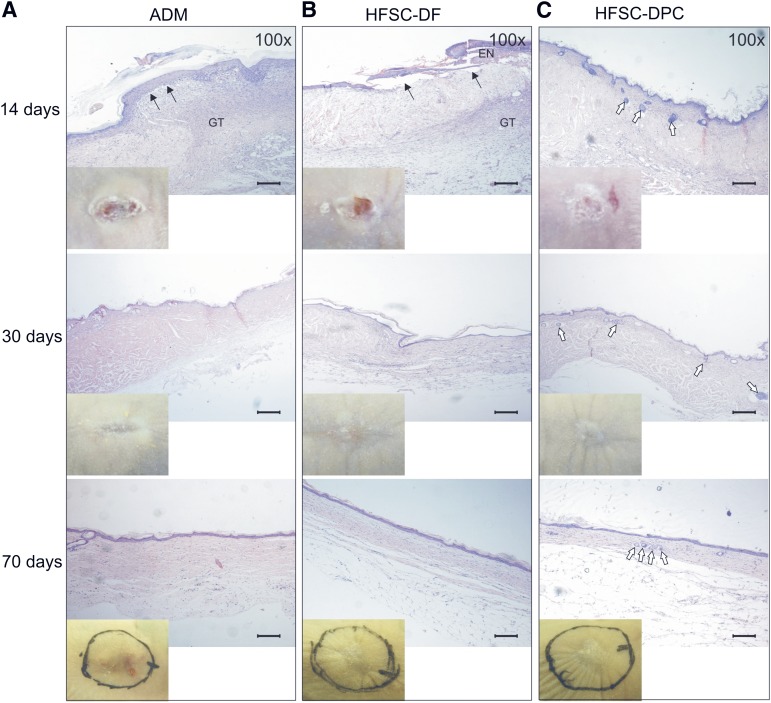
When neovascularization of the grafts was evaluated by immunohistochemistry for CD34 at day 14, the HFSC-DPC constructs showed the highest number of neovessels (Fig. 4D), mainly located immediately below the epidermis and probably supporting its survival (Fig. 4C). Even if the HFSC-DF constructs showed a higher number of neovessels than the ADM constructs without a human cellular component, in both cases, a process of dermal-epidermal detachment (Figs. 4, 5A, 5B) was observed. However, the HFSC-DF constructs also showed epidermis focal necrosis (Fig. 5B). In grafting experiments using ADM alone, a re-epithelization process at the edges was observed, but this new epidermis generated by the rodent was detached, at least partly, at 14 days after grafting (Figs. 4, 5A). In accordance with the neovascularization pattern described, at 30 days after grafting, the vessel number in both composite constructs had dramatically decreased to the ADM level (Fig. 4D). The same features were seen 70 days after grafting.
Figure 4.
Immunoassay for CD34 neovessel marker in skin constructs grafted in nude mice. The constructs were generated with ADM alone (A), HFSC-DF (B), and HFSC-DPC (C). Scale bars = 50 µm. (D): Bar graph of vessels per dermis surface unit in ×400 fields from each construct. White arrows indicate neovessels below graft epidermis; black arrows, dermal-epidermal detachment. ∗∗, p < .001. Abbreviations: ADM, acellular dermal matrix; DF, dermal fibroblast; DPC, dermal papilla cell; HFSC, hair follicle stem cell.
Figure 5.
Histology of skin constructs grafted in nude mice. Constructs were generated with ADM alone (A), HFSC-DF (B), and HFSC-DPC (C). Insets: Macroscopic views of the grafts. Black arrows indicate dermal-epidermal detachment; white arrows, epithelial cyst-like structures reminiscent of embryonic hair buds. Scale bars = 200 µm. Abbreviations: ADM, acellular dermal matrix; DF, dermal fibroblast; DPC, dermal papilla cell; EN, epidermis necrosis; GT, granulation tissue; HFSC, hair follicle stem cell.
Surprisingly, at 14 days after grafting, the HFSC-DPC constructs showed the highest number of blood neovessels, concordant with an early beginning of the matrix remodeling process. In contrast, ADM alone or the HFSC-DF constructs showed a more intense inflammatory response and formation of granulation tissue (Fig. 5A–5C). At day 30, both HFSC-DPC (Fig. 5C) and HFSC-DF (Fig. 5B) constructs showed a dermis with a loose aspect, a lower cellular content, and fewer blood vessels (Fig. 4D).
When the secretion of vascular epidermal growth factor (VEGF) by the DPC and DF cultures used in the constructs was quantified by enzyme-linked immunosorbent assay, the amount of VEGF secreted by the DPC cultures was sixfold higher than the amount in the DF cultures (927 ± 87 pg/ml vs. 147 ± 54 pg/ml, p < .0001) after 3 days in culture. This result is in accordance with the highest number of neovessels observed at 14 days in HFSC-DPC grafts.
Interestingly, a stable population of p63-positive cells was maintained in DPC-containing substitutes after grafting onto nude mice, contrary to what was observed in the DF-containing ones, which showed a significant reduction of these cells (supplemental online Fig. 2). These results indicate that the presence of DPCs contributes to the maintenance of a precursor cell population.
A significant negative outcome of skin wound healing after deep injuries is the skin contraction, which compromises the appearance of the healing skin and can cause scar contractures that limit movement and function. In this way, the appearance of the healing skin was evaluated at macroscopic level. In the mice grafted with composite skin constructs (HFSC-DF or HFSC-DPC in insets of Fig. 5B, 5C), less skin contraction was observed than in the mice grafted with ADM without the cellular components (Fig. 5A, insets). In fact, the SCR, calculated as indicated in the “Materials and Methods” section, was significantly lower in the mice grafted with composite skin constructs (HFSC-DPC or HFSC-DF) than in mice grafted with ADM alone after 70 days (supplemental online Fig. 3).
Presence of DPCs in Skin Constructs Induced Epidermal Hair Buds
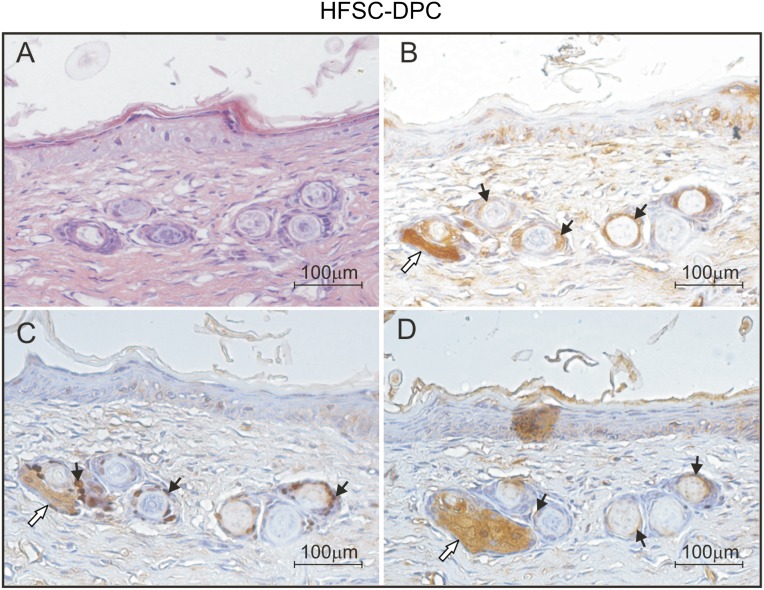
Only the HFSC-DPC constructs grafted in the nude mice showed notable epithelial cyst-like inclusions in the remodeling dermis. These histological structures, very similar to those observed during hair follicle embryonic development (supplemental online Fig. 4) were found at times as short as 14 days after grafting (Fig. 5C). The presence of “hair buds” was detected in 60% of the mice at 14 days, in 80% of the mice at 30 days, and in 100% of the mice sacrificed 70 days after grafting with the constructs containing DPCs. These structures, reminiscent of embryonic development of the hair follicle (Fig. 6A; supplemental online Fig. 4), might represent hair follicle neogenesis in the HFSC-DPC constructs. The cells forming these structures were recognized by an antibody specific for HLA I ABC (Fig. 6B), demonstrating their human origin. Some cells from these buds were also positive for a p63-specific antibody, indicating the persistence of a precursor cell population in these structures (Fig. 6C).
Figure 6.
Evaluation of embryonic hair bud-like structures in grafted skin constructs containing HFSC-DPC after 30 days. (A): Hematoxylin and eosin staining. (B): Immunoassay for human leukocyte antigen I. (C): Immunoassay for p63 antigen. (D): Immunoassay for k6hf antigen. Black arrows indicate positive cells for the corresponding antigen; white arrows, the sebaceous glands (unspecifically stained). Abbreviations: DPC, dermal papilla cell; HFSC, hair follicle stem cell.
Finally, we observed expression of k6hf in these hair bud-like structures inside the remodeling matrix (Fig. 6D). This keratin is expressed by the companion layer of anagen hair follicles, indicating a differentiation commitment to hair lineage.
The histological slides used to evaluate HLA I ABC, p63, and k6hf and the hematoxylin and eosin staining were performed in sequential histological slides, representing the same tissue area and showing that the structures observed contained cells positive for all the markers tested (Fig. 6).
Discussion
The tissue-engineering technology exploits the ability of cells to form a tissue under appropriate culture conditions that include the use of a scaffold. The acellular porcine dermis used in deep skin injuries without any cellular component at the Hospital de Quemados de la Ciudad de Buenos Aires has proved to be able to integrate and vascularized well, without rejection, allowing patient self-epithelization or the posterior use of a split autologous skin graft or other epidermal substitute [39].
In the present study, this acellular dermal matrix previously repopulated in vitro with HFSCs and DFs or DPCs as the dermal component, has been shown to support the generation of a stratified epidermis in vitro, with a reservoir of precursor basal cells and a good assimilation and remodeling process in vivo.
Previous work suggests that the success of tissue engineering depends on the choice of suitable seeded cells in culture [16]. The long-term function of the graft is presumably limited by the maintenance of the epidermal stem cell compartment during in vitro culture, and, to achieve this goal, the use of a starting pure population of stem cells is very important [40]. In our work, the presence of DPCs in the skin constructs in vitro showed a more regular stratification pattern and a higher number of p63-positive basal epidermal cells compared with those carrying fibroblasts, indicating an improved precursor cell reservoir. Accordingly, grafted DPC-containing substitutes maintained over time a constant number of p63-positive cells, indicating that the presence of DPCs contributes to the maintenance of a precursor cell population in the new epidermis. These results indicate that this type of skin substitute could represent a true permanent device because of the continue presence of an epidermal stem cell population probably involved in epidermis tissue turnover.
Many growth factors have been reported as derived from DPCs [25, 41–43]. Some of these factors might contribute to the higher number of epidermis layers and the stem cell population present in the DPC composite skin constructs in vitro. Thus, the presence of DPCs more accurately mimics the skin architecture by properly stratifying the differentiating HFSCs and developing a well-ordered epithelia in vitro that contributes to more closely recapitulate human skin. An in vitro model might provide, not only answers to physiological questions that cannot be answered using monolayer tissue cultures, but might also represent a time- and cost-effective alternative to the use of laboratory animals in cosmetic testing, photoaging and cancer models, and pharmacological analyses.
When the tissue-engineered constructs are grafted, vascularization of the dermal support remains a major challenge, limiting their survival and take rates, even when autologous cellular components are used [44]. In the present report, we observed that the grafted DPC-containing composite skin retained the living epidermis generated in vitro, and the DF-containing ones showed a dermal-epidermal detachment associated with a focal necrosis process. This phenomenon correlated with a higher number of neovessels observed in presence of DPCs 14 days after grafting in nude mice. Surprisingly, this higher number of neovessels was not associated with an intense inflammatory process, but rather with an early process of tissue remodeling. Actually, we cannot explain properly which are the factors and mechanisms that DPCs could drive, but we can speculate that DPCs help to avoid an excessive inflammatory response, favoring a regenerative environment, rather than a cicatricial one, and somehow resembling that seen in wound healing during embryonic development [45].
Neovascularization of dermal substitutes is slow and begins around 10–15 days in patients with acute burns [46]. It is known that several soluble factors are involved in the neovascularization process. Among them, VEGF [47, 48], fibroblast growth factor [47], platelet-derived growth factor, hepatocyte growth factor, keratinocyte growth factor, and angiopoietin, have been shown to have potential tissue neovascularization inductivity [47, 49]. DPCs play a key role in hair cycle induction that includes the secretion of angiogenic factors, such as VEGF [50] and angiogenin, which have been proved to promote skin angiogenesis and the anagen phase in the hair cycle when injected into mice [51]. The DPC cultures used in our study secreted both angiogenin (observed by protein microarrays; data not shown) and VEGF. These potent angiogenic factors might be responsible for the rapid neovascularization process observed in the DPC-containing constructs, although further studies are necessary to elucidate which are the growth factors and cytokines involved.
During wound healing, sprouting and branching of neovessels results in an extensive immature and leaky neovascular network followed by a controlled phase of blood vessel maturation that results in a well-perfused vascular network [52, 53]. In our experimental results 14 days after grafting, DPC-containing constructs showed higher neovascularization compared with DF-containing constructs or ADM alone. Interestingly, this pattern was completely inverted 30 days after grafting. This result suggests that the presence of DPCs in the composite substitutes not only favors early neovascularization but also contributes to neovascular network maturation, which could reduce inflammation, resulting in a better healing process. Further studies should be performed to elucidate the mechanism by which DPCs contribute both to the initial process of neovascularization and to the subsequent maturation of the vascular network in the neodermis.
Both of our composite skin constructs showed notably less skin contraction compared with what was observed using the matrix alone. It has been reported that fibroblasts seeded in collagen sponge inhibit host fibroblast infiltration [54, 55] and secrete basic fibroblast growth factor that induces the apoptosis of myofibroblasts [56]. Both phenomena might contribute to the reduction of skin wound contraction. Therefore, in our study, the presence of DFs or DPCs in the composite skin constructs might play an important role in reducing skin wound contraction, either expressing such cytokines or inducing their expression by macrophages or other inflammatory cells recruited to the area of injury.
Given that basal cell carcinoma of in the skin is the most common cancer and earlier case reports have described an increased risk of basal cell carcinoma associated with wounds, skin malignant transformation is an issue that should be considered in skin tissue engineering.
In fact, recently [57, 58], a link between epidermal wounds and skin cancer risk was established. It was demonstrated that the tumor-promoting effect of the wound environment results from the recruitment of tumor-initiating cells originating from the neighboring hair follicles. Our histological observations did not reveal any basal cell carcinoma-like lesions at any of the measurement points studied. However, given the slow growing nature of these tumors, it would be interesting to analyze in future studies the presence of transformed stem cells that could promote the development of tumors associated with wound healing.
Many dermal-epidermal substitutes composed of dermal fibroblasts have been described for use in the clinic or to model skin development [12]; however, the inability of these skin constructs to regenerate skin appendages such as hair follicles has limited their use. The lack of skin appendages not only affects patients' psychological well-being but also endangers the inherent functions of the skin.
Even if total keratinocytes can be obtained from a small piece of skin and expanded sufficiently to cover patients with large burns, this coverage would have its aesthetic and functional properties reduced. Instead, HFSCs with their potentiality to generate skin appendages are promising for the treatment of deep skin injuries. One major challenge for its clinical application in patients with major skin loss would be to sufficiently expand them from such a small skin biopsy. Nevertheless, less extensive full-thickness skin wounds can benefit from the cellular types used in this work.
During embryogenesis, signals from mesenchymal cells into epithelium induce the formation of the hair follicle. In adults, the dermal papilla retains the ability to induce regeneration of HFs [27]. Although several hair reconstitution models have been proposed, most of them depended on the use of noncultured fetal or newborn inductive cells. This limits their use for regeneration of hair follicles in tissue-engineered skin.
Sriwiriyanont et al. [59] observed neofollicles in grafted engineered skin substitutes with human epidermal keratinocytes and murine dermal papilla cells but not when the dermal cells were of human origin. In other study [60], they grafted in nude mice, chimeric populations of cultured human keratinocytes from neonatal foreskins and cultured murine dermal papilla cells from adult green fluorescent protein transgenic mice. In their study, the neonatal murine-only skin substitutes formed external hairs and sebaceous glands, the chimeric skin substitutes formed pigmented hairs without sebaceous glands, and the human-only skin substitutes formed no follicles or glands.
Other work [61] has demonstrated that epithelial stem cells can be kept in vitro in a permissive tissue-engineered dermal environment without losing their potential to induce hair growth after grafting. However, the use of newborn mouse hair buds was necessary, and adult fibroblasts of human origin did not sustain the formation of normal hair follicles from these newborn mouse hair buds.
Recently, it was reported [62] that cultured human dermal papilla cells from the temporal scalp can induce complete pilosebaceous units in vivo, 8 weeks after the skin substitutes were grafted into nude mice. In that case, success using dermal papilla cells appears to require human neonatal foreskin keratinocytes and not adult cells.
In this work, we report, for the first time to our knowledge, the appearance of embryonic hair bud-like structures in vivo with cultured human adult cells. Notably, using a population of adult HFSCs and adult DPCs, we observed, as early as 14 days after grafting, structures reminiscent of embryonic hair buds inside the remodeling ADM. Furthermore, the cells constituting these structures are of human origin, with the presence of precursor epithelial cells and expression of a hair differentiation marker.
In fact, histological structures very similar to those present during hair follicle embryonic development were observed. The early stages in the development of the hair follicle can be recapitulated in histological sections, showing both the hair germ and peg and the concentric layer organization of the developing hair follicle and sebaceous glands normally expected in later stages (supplemental online Fig. 4). Even if the tissue-engineered dermal environment was permissive to induce hair follicle buds, the molecular and architectural environmental conditions of each embryonic stage of hair development are different from the conditions created in the experiment. The absence of such environmental factors and the remodeling dermal collagen could not favor maturational development. Further studies should improve the conditions necessary to the development of the mature hair follicle in a tissue-engineered dermal environment.
All these observations indicate that these structures could be hair follicle neogenesis attempts. When constructs containing HFSCs and dermal fibroblasts were grafted in the same conditions and environment, this phenomenon was not observed. We conclude that the type of dermal component in the skin constructs influences, not only the skin architecture and stem cell persistence, but also the differentiation fate of the epidermal stem cells and their potential to regenerate hair follicles. As we have already stated, the presence of hair follicles improves the functional and aesthetic aspect of repaired skin by providing the progenitor cells involved in skin wound healing and in the generation of other functional appendages such as sebaceous glands. Based on our results, the adult cells present in the hair follicle, such as HFSCs and DPCs, should allow the development of skin substitutes using autologous cells, avoiding tissue immune rejection, and on the other hand restoring the epithelial-mesenchymal interactions that recapitulate the embryonic events involved in hair follicle neogenesis.
Although further studies are necessary to improve the conditions to achieve fully developed skin appendages, the combination of human adult cells used in this study hold out promise to produce fully functional true skin equivalents.
Conclusion
In the present work, we have observed that the presence of DPCs in composite skin constructs generated in air-liquid interphase led to the formation of an epidermal-like structure with the most regular stratification, more invaginations that could indicate hair follicle neogenesis attempts, and maintenance of an epidermal stem cell pool. Moreover, the results obtained in grafting experiments suggest that the presence of DPCs in composite skin substitutes favors its graft-take and stimulates the wound healing process. This phenomenon is supported by a very early beginning of the graft angiogenesis process that helps graft-epidermal survival, ensuring rapid wound covering and a fast remodeling of the matrix with consequently lower skin contraction that ameliorates the functional and aesthetic aspect of the healing skin.
These results indicate that constructs generated from HFSCs and DPCs are able to provide permanent skin lesion coverage, supported by the maintenance of a p63-positive cell population. However, only the presence of DPCs in the grafted-composite skin was able to induce hair bud-like structures reminiscent of the hair follicle neogenesis process.
Supplementary Material
Acknowledgments
We thank Dr. Alicia Lorenti for her review of our report. This work was supported by the Agencia Nacional de Producción Científica y Tecnológica (Grant ANR BIO 0032/10). M.E.B. is a researcher at Consejo Nacional de Investigaciones Científicas y Técnicas.
Author Contributions
G.J.L.: conception and design, data analysis and interpretation, manuscript writing; A.G.K., M.L.C.: collection and/or assembly of data; H.D., S.B., and F.S.: provision of study material or patients; I.Y.S.: provision of study material or patients, data analysis and interpretation; M.E.B.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Papini R. Management of burn injuries of various depths. BMJ. 2004;329:158–160. doi: 10.1136/bmj.329.7458.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanton RA, Billmire DA. Skin resurfacing for the burned patient. Clin Plast Surg. 2002;29:29–51. doi: 10.1016/s0094-1298(03)00085-3. [DOI] [PubMed] [Google Scholar]
- 3.Gallico GG, III, O’Connor NE, Compton CC, et al. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984;311:448–451. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- 4.Carsin H, Ainaud P, Le Bever H, et al. Cultured epithelial autografts in extensive burn coverage of severely traumatized patients: A five year single-center experience with 30 patients. Burns. 2000;26:379–387. doi: 10.1016/s0305-4179(99)00143-6. [DOI] [PubMed] [Google Scholar]
- 5.Hafemann B, Ensslen S, Erdmann C, et al. Use of a collagen/elastin-membrane for the tissue engineering of dermis. Burns. 1999;25:373–384. doi: 10.1016/s0305-4179(98)00162-4. [DOI] [PubMed] [Google Scholar]
- 6.Dai NT, Williamson MR, Khammo N, et al. Composite cell support membranes based on collagen and polycaprolactone for tissue engineering of skin. Biomaterials. 2004;25:4263–4271. doi: 10.1016/j.biomaterials.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Ruszczak Z. Effect of collagen matrices on dermal wound healing. Adv Drug Deliv Rev. 2003;55:1595–1611. doi: 10.1016/j.addr.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Rennekampff HO, Kiessig V, Griffey S, et al. Acellular human dermis promotes cultured keratinocyte engraftment. J Burn Care Rehabil. 1997;18:535–544. doi: 10.1097/00004630-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Orgill DP, Butler C, Regan JF, et al. Vascularized collagen-glycosaminoglycan matrix provides a dermal substrate and improves take of cultured epithelial autografts. Plast Reconstr Surg. 1998;102:423–429. doi: 10.1097/00006534-199808000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Walden JL, Garcia H, Hawkins H, et al. Both dermal matrix and epidermis contribute to an inhibition of wound contraction. Ann Plast Surg. 2000;45:162–166. doi: 10.1097/00000637-200045020-00011. [DOI] [PubMed] [Google Scholar]
- 11.Jiong C, Jiake C, Chunmao H, et al. Clinical application and long-term follow-up study of porcine acellular dermal matrix combined with autoskin grafting. J Burn Care Res. 2010;31:280–285. doi: 10.1097/BCR.0b013e3181d0f42d. [DOI] [PubMed] [Google Scholar]
- 12.Shevchenko RV, James SL, James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface. 2010;7:229–258. doi: 10.1098/rsif.2009.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yannas IV. Similarities and differences between induced organ regeneration in adults and early foetal regeneration. J R Soc Interface. 2005;2:403–417. doi: 10.1098/rsif.2005.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutmacher DW, Cool S. Concepts of scaffold-based tissue engineering—The rationale to use solid free-form fabrication techniques. J Cell Mol Med. 2007;11:654–669. doi: 10.1111/j.1582-4934.2007.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansilla E, Drago H, Sturla F, et al. Matrix superhighways configurations: New concepts for complex organ regeneration. Transplant Proc. 2007;39:2431–2433. doi: 10.1016/j.transproceed.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 16.Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414:118–121. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 17.Dunnwald M, Tomanek-Chalkley A, Alexandrunas D, et al. Isolating a pure population of epidermal stem cells for use in tissue engineering. Exp Dermatol. 2001;10:45–54. doi: 10.1034/j.1600-0625.2001.100106.x. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrini G, Ranno R, Stracuzzi G, et al. The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin. Transplantation. 1999;68:868–879. doi: 10.1097/00007890-199909270-00021. [DOI] [PubMed] [Google Scholar]
- 19.Ito M, Liu Y, Yang Z, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 20.Alonso L, Fuchs E. The hair cycle. J Cell Sci. 2006;119:391–393. doi: 10.1242/jcs.02793. [DOI] [PubMed] [Google Scholar]
- 21.Blanpain C, Lowry WE, Geoghegan A, et al. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Tumbar T. Epithelial skin stem cells. Methods Enzymol. 2006;419:73–99. doi: 10.1016/S0076-6879(06)19004-7. [DOI] [PubMed] [Google Scholar]
- 23.Tausche AK, Skaria M, Böhlen L, et al. An autologous epidermal equivalent tissue-engineered from follicular outer root sheath keratinocytes is as effective as split-thickness skin autograft in recalcitrant vascular leg ulcers. Wound Repair Regen. 2003;11:248–252. doi: 10.1046/j.1524-475x.2003.11403.x. [DOI] [PubMed] [Google Scholar]
- 24.Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 25.Botchkarev VA, Kishimoto J. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J Investig Dermatol Symp Proc. 2003;8:46–55. doi: 10.1046/j.1523-1747.2003.12171.x. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs E, Merrill BJ, Jamora C, et al. At the roots of a never-ending cycle. Dev Cell. 2001;1:13–25. doi: 10.1016/s1534-5807(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 27.Gharzi A, Reynolds AJ, Jahoda CA. Plasticity of hair follicle dermal cells in wound healing and induction. Exp Dermatol. 2003;12:126–136. doi: 10.1034/j.1600-0625.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 28.Jahoda CA, Reynolds AJ. Hair follicle dermal sheath cells: Unsung participants in wound healing. Lancet. 2001;358:1445–1448. doi: 10.1016/S0140-6736(01)06532-1. [DOI] [PubMed] [Google Scholar]
- 29.Qi SH, Liu P, Xie JL, et al. Experimental study on repairing of nude mice skin defects with composite skin consisting of xenogeneic dermis and epidermal stem cells and hair follicle dermal papilla cells. Burns. 2008;34:385–392. doi: 10.1016/j.burns.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Ohyama M, Zheng Y, Paus R, et al. The mesenchymal component of hair follicle neogenesis: Background, methods and molecular characterization. Exp Dermatol. 2010;19:89–99. doi: 10.1111/j.1600-0625.2009.00935.x. [DOI] [PubMed] [Google Scholar]
- 31.Jahoda CA, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311:560–562. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- 32.Xing L, Kobayashi K. Ability of transplanted cultured epithelium to respond to dermal papillae. Tissue Eng. 2001;7:535–544. doi: 10.1089/107632701753213165. [DOI] [PubMed] [Google Scholar]
- 33.Lichti U, Weinberg WC, Goodman L, et al. In vivo regulation of murine hair growth: Insights from grafting defined cell populations onto nude mice. J Invest Dermatol. 1993;101(suppl):124S–129S. doi: 10.1111/1523-1747.ep12363165. [DOI] [PubMed] [Google Scholar]
- 34.Leirós GJ, Attorresi AI, Balañá ME. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br J Dermatol. 2012;166:1035–1042. doi: 10.1111/j.1365-2133.2012.10856.x. [DOI] [PubMed] [Google Scholar]
- 35.Roh C, Tao Q, Lyle S. Dermal papilla-induced hair differentiation of adult epithelial stem cells from human skin. Physiol Genomics. 2004;19:207–217. doi: 10.1152/physiolgenomics.00134.2004. [DOI] [PubMed] [Google Scholar]
- 36.Balañá ME, Alvarez Roger C, Dugour AV, et al. Antiandrogen oligonucleotides: Active principles in hair- and skin-derived culture cells. J Drugs Dermatol. 2004;3:287–294. [PubMed] [Google Scholar]
- 37.Roh C, Roche M, Guo Z, et al. Multi-potentiality of a new immortalized epithelial stem cell line derived from human hair follicles. In Vitro Cell Dev Biol Anim. 2008;44:236–244. doi: 10.1007/s11626-008-9084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mansilla E, Arrúa J, Salas E, et al. The Derma Project: Present and future possibilities of skin procurement for the treatment of large burns in Argentina, Tissue Engineering and the Cadaver Skin Bank. Transplant Proc. 2001;33:637–639. doi: 10.1016/s0041-1345(00)02179-5. [DOI] [PubMed] [Google Scholar]
- 40.Charruyer A, Ghadially R. Stem cells and tissue-engineered skin. Skin Pharmacol Physiol. 2009;22:55–62. doi: 10.1159/000178864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paus R, Foitzik K. In search of the “hair cycle clock”: A guided tour. Differentiation. 2004;72:489–511. doi: 10.1111/j.1432-0436.2004.07209004.x. [DOI] [PubMed] [Google Scholar]
- 42.Fujie T, Katoh S, Oura H, et al. The chemotactic effect of a dermal papilla cell-derived factor on outer root sheath cells. J Dermatol Sci. 2001;25:206–212. doi: 10.1016/s0923-1811(00)00130-4. [DOI] [PubMed] [Google Scholar]
- 43.Itami S, Kurata S, Sonoda T, et al. Interaction between dermal papilla cells and follicular epithelial cells in vitro: Effect of androgen. Br J Dermatol. 1995;132:527–532. [PubMed] [Google Scholar]
- 44.Wood FM, Kolybaba ML, Allen P. The use of cultured epithelial autograft in the treatment of major burn injuries: A critical review of the literature. Burns. 2006;32:395–401. doi: 10.1016/j.burns.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Bullard KM, Longaker MT, Lorenz HP. Fetal wound healing: Current biology. World J Surg. 2003;27:54–61. doi: 10.1007/s00268-002-6737-2. [DOI] [PubMed] [Google Scholar]
- 46.Stern R, McPherson M, Longaker MT. Histologic study of artificial skin used in the treatment of full-thickness thermal injury. J Burn Care Rehabil. 1990;11:7–13. doi: 10.1097/00004630-199001000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Tabata Y. Tissue regeneration based on growth factor release. Tissue Eng. 2003;9(suppl 1):S5–S15. doi: 10.1089/10763270360696941. [DOI] [PubMed] [Google Scholar]
- 48.Lugo LM, Lei P, Andreadis ST. Vascularization of the dermal support enhances wound re-epithelialization by in situ delivery of epidermal keratinocytes. Tissue Eng Part A. 2011;17:665–675. doi: 10.1089/ten.tea.2010.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nomi M, Atala A, Coppi PD, et al. Principals of neovascularization for tissue engineering. Mol Aspects Med. 2002;23:463–483. doi: 10.1016/s0098-2997(02)00008-0. [DOI] [PubMed] [Google Scholar]
- 50.Lachgar S, Moukadiri H, Jonca F, et al. Vascular endothelial growth factor is an autocrine growth factor for hair dermal papilla cells. J Invest Dermatol. 1996;106:17–23. doi: 10.1111/1523-1747.ep12326964. [DOI] [PubMed] [Google Scholar]
- 51.Zhou N, Fan W, Li M. Angiogenin is expressed in human dermal papilla cells and stimulates hair growth. Arch Dermatol Res. 2009;301:139–149. doi: 10.1007/s00403-008-0907-5. [DOI] [PubMed] [Google Scholar]
- 52.Egginton S, Gaffney E. Tissue capillary supply—It’s quality not quantity that counts! Exp Physiol. 2010;95:971–979. doi: 10.1113/expphysiol.2010.053421. [DOI] [PubMed] [Google Scholar]
- 53.Owen MR, Alarcón T, Maini PK, et al. Angiogenesis and vascular remodelling in normal and cancerous tissues. J Math Biol. 2009;58:689–721. doi: 10.1007/s00285-008-0213-z. [DOI] [PubMed] [Google Scholar]
- 54.Lamme EN, Van Leeuwen RT, Brandsma K, et al. Higher numbers of autologous fibroblasts in an artificial dermal substitute improve tissue regeneration and modulate scar tissue formation. J Pathol. 2000;190:595–603. doi: 10.1002/(SICI)1096-9896(200004)190:5<595::AID-PATH572>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 55.de Vries HJ, Middelkoop E, van Heemstra-Hoen M, et al. Stromal cells from subcutaneous adipose tissue seeded in a native collagen/elastin dermal substitute reduce wound contraction in full thickness skin defects. Lab Invest. 1995;73:532–540. [PubMed] [Google Scholar]
- 56.Akasaka Y, Ono I, Tominaga A, et al. Basic fibroblast growth factor in an artificial dermis promotes apoptosis and inhibits expression of alpha-smooth muscle actin, leading to reduction of wound contraction. Wound Repair Regen. 2007;15:378–389. doi: 10.1111/j.1524-475X.2007.00240.x. [DOI] [PubMed] [Google Scholar]
- 57.Kasper M, Jaks V, Are A, et al. Wounding enhances epidermal tumorigenesis by recruiting hair follicle keratinocytes. Proc Natl Acad Sci USA. 2011;108:4099–4104. doi: 10.1073/pnas.1014489108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong SY, Reiter JF. Wounding mobilizes hair follicle stem cells to form tumors. Proc Natl Acad Sci USA. 2011;108:4093–4098. doi: 10.1073/pnas.1013098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sriwiriyanont P, Lynch KA, Maier EA, et al. Morphogenesis of chimeric hair follicles in engineered skin substitutes with human keratinocytes and murine dermal papilla cells. Exp Dermatol. 2012;21:783–785. doi: 10.1111/exd.12003. [DOI] [PubMed] [Google Scholar]
- 60.Sriwiriyanont P, Lynch KA, McFarland KL, et al. Characterization of hair follicle development in engineered skin substitutes. PLoS One. 2013;8:e65664. doi: 10.1371/journal.pone.0065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Larouche D, Cuffley K, Paquet C, et al. Tissue-engineered skin preserving the potential of epithelial cells to differentiate into hair after grafting. Tissue Eng Part A. 2011;17:819–830. doi: 10.1089/ten.TEA.2010.0403. [DOI] [PubMed] [Google Scholar]
- 62.Thangapazham RL, Klover P, Wang JA, et al. Dissociated human dermal papilla cells induce hair follicle neogenesis in grafted dermal-epidermal composites. J Invest Dermatol. 2014;134:538–540. doi: 10.1038/jid.2013.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.