A strategy of programmed application of transforming growth factor β3 (TGFβ3) and Rac1 inhibitor NSC23766 to commit the hyaline cartilage differentiation of adipose-derived stem cells (ADSCs) for joint cartilage repair was developed. ADSC efficacy with TGFβ3 and NSC23766 for cartilage repair was studied in a rat osteochondral defect model. The combination of ADSCs, TGFβ3, and NSC23766 promoted quality osteochondral defect repair in rats with much less chondrocyte hypertrophy and significantly greater International Cartilage Repair Society macroscopic and microscopic scores.
Keywords: Adipose-derive stem cells, Rac1, Osteochondral defect, Hypertrophy
Abstract
Hyaline cartilage differentiation is always the challenge with application of stem cells for joint repair. Transforming growth factors (TGFs) and bone morphogenetic proteins can initiate cartilage differentiation but often lead to hypertrophy and calcification, related to abnormal Rac1 activity. In this study, we developed a strategy of programmed application of TGFβ3 and Rac1 inhibitor NSC23766 to commit the hyaline cartilage differentiation of adipose-derived stem cells (ADSCs) for joint cartilage repair. ADSCs were isolated and cultured in a micromass and pellet culture model to evaluate chondrogenic and hypertrophic differentiation. The function of Rac1 was investigated with constitutively active Rac1 mutant and dominant negative Rac1 mutant. The efficacy of ADSCs with programmed application of TGFβ3 and Rac1 inhibitor for cartilage repair was studied in a rat model of osteochondral defects. The results showed that TGFβ3 promoted ADSCs chondro-lineage differentiation and that NSC23766 prevented ADSC-derived chondrocytes from hypertrophy in vitro. The combination of ADSCs, TGFβ3, and NSC23766 promoted quality osteochondral defect repair in rats with much less chondrocytes hypertrophy and significantly higher International Cartilage Repair Society macroscopic and microscopic scores. The findings have illustrated that programmed application of TGFβ3 and Rac1 inhibitor NSC23766 can commit ADSCs to chondro-lineage differentiation and improve the efficacy of ADSCs for cartilage defect repair. These findings suggest a promising stem cell-based strategy for articular cartilage repair.
Introduction
Cartilage defect repair is a great clinical challenge owing to its avascularity and low cellular mitotic activity [1]. Conventional treatments have not elicited satisfactory outcomes and usually generate fibrous, rather than hyaline, cartilage [2, 3]. Emerging advances in cell-based articular cartilage tissue engineering provide promising strategies to this unsolved problem. Mesenchymal stem cells (MSCs) isolated from several different tissues, including adipose, bone marrow, and others, offer an attractive cell source for tissue engineering because of their great proliferation and differentiation capabilities [4]. Among them, adipose-derived stem cells (ADSCs) have drawn much attention recently because of their acquisition advantage and useful characteristics comparable to bone marrow stem cells (BMSCs) [5–7]. ADSCs have been intensively studied for the treatment of cartilage lesions since the first demonstration of their chondrogenic potential [8–10]. However, subsequent investigations revealed that ADSCs display a lower chondrogenic potential than BMSCs [11–13], although greater doses of growth factors such as transforming growth factor β (TGFβ) and insulin-like growth factor could overcome this disadvantage [14, 15]. Despite their inferior chondrogenic ability, ADSCs still deserve much more in depth investigation because of their huge advantage regarding acquisition.
Numerous in vitro [16–18] and in vivo [18] studies have shown that MSCs undergo chondrogenesis when supplemented with TGF family members. TGFβ3 supplementation can initiate chondro-lineage differentiation. However, TGFβ3 is unable to commit stem cells to stable chondrogenic differentiation. It has often been observed that stem cells treated with TGFβ3 will undergo hypertrophy and calcification [19], because TGFβ3 is also the active signal pathway during endochondral ossification in development [20, 21]. Also, clinical follow-up studies have shown that the newly formed cartilage was associated with osseous overgrowth in 25%–49% of cases [22–24]. Such bony overgrowths, not only have no function, but also impede the integrity of the newly formed tissue with adjacent healthy cartilage.
Rac1 belongs to small GTPases and can interchange between the GTP active form and GDP inactive form. Recent studies from different groups revealed that Rac1 functions as a positive regulator in governing chondrocyte hypertrophy, maturation, and calcification [25–27]. Studies from our laboratory and Long et al. [28] all showed that Rac1 was activated in osteoarthritic (OA) cartilage. We further demonstrated that activated Rac1 resulted in upregulation of hypertrophy related genes such as COLX, MMP13, and ADTAMTS-5 and chondrocyte calcification and that inhibition of Rac1 activity in vivo effectively ameliorated osteoarthritis progression in mice [29]. These findings suggest that inhibition of Rac1 could be an effective method to prevent chondrocyte hypertrophy and calcification.
The principal aim of the present study was to develop a strategy of programmed application of TGFβ3 and Rac1 inhibitor NSC23766 to commit the hyaline cartilage differentiation of ADSCs for joint cartilage repair. Our hypothesis was that TGFβ3 would promote ADSCs chondro-lineage differentiation and that local delivery of NSC23766 would prevent hypertrophy of the chondrocytes. The local delivery system was chitosan microspheres, because they are an inexpensive, biocompatible, biodegradable, and nontoxic natural polymer that enables the controlled release of many drugs [10, 30, 31].
In this study, we evaluated the chondro-lineage differentiation ability of ADSCs in the chondrogenic medium and the inhibitory effect of the Rac1 inhibitor NSC23766 on the hypertrophy of chondrocytes in vitro. From this, in vivo, we evaluated the cartilage reparative potential of ADSCs encapsulated in a biodegradable hydrogel (fibrin) containing TGFβ3. The NSC23766 control released by chitosan microspheres was then intra-articularly administered weekly, and the efficiency of the present strategy for cartilage regeneration was analyzed and evaluated.
Materials and Methods
ADSC Isolation and Characterization
Isolation of Rat ADSCs
Rat ADSCs were isolated from the inguinal area of 6-week-old male Sprague-Dawley rats weighing 160 ± 5 g, as described previously [32]. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, http://www.invitrogen.com). The fourth to sixth passage cells were used for the experiments.
Fluorescence-Activated Cell Sorting Analysis
The cells (1 × 106) were incubated with 1 μg of fluorescein isothiocyanate (FITC)-conjugated mouse anti-rat monoclonal antibodies specific to rat CD29, CD90, CD44, CD45. or FITC-conjugated isotype-matched immunoglobulin G (all from BD, Franklin Lakes, NJ, http://www.bd.com) for 1 hour at 4°C. After washing, the samples were analyzed using an Epics XL flow cytometer (Beckman Coulter, Fullerton, CA, http://www.beckmancoulter.com).
Multipotent Differentiation
The multi-differentiation potential of the ADSCs toward osteogenesis, adipogenesis, and chondrogenesis was examined, as described previously [33]. In brief, to promote osteogenic differentiation, the cells were induced at a density of 5 × 103 cells per centimeter squared by 10 mm β-glycerol phosphate, 0.1 μM dexamethasone, and 50 μg/ml ascorbic acid (all Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) for 2 weeks. Positive induction was detected by alizarin red staining. To promote adipogenesis differentiation, the cells were cultured in DMEM supplemented with adipogenic stimulatory supplements (StemCell Technologies, Vancouver, BC, Canada, http://www.stemcell.com) for 2 weeks. Positive induction was detected by oil red staining for lipid vacuoles. To promote chondrogenic differentiation, the cells (103 cells per centimeter squared) were induced in pellet by culturing in DMEM supplemented with 50 μg/ml ascorbic acid (Sigma-Aldrich), 10 ng/ml TGF-β3 (Millipore, Billerica, MA, http://www.millipore.com), 10 mm of β-glycerol phosphate (Sigma-Aldrich), and 1 μM of dexamethasone (Sigma-Aldrich) for 3 weeks. Positive induction was detected by Safranin O staining after 3 weeks.
Micromass Cultures of ADSCs
For micromass cultures, the protocol described by De Bari et al. [34] with human periosteum-derived cells was used, with some modifications. In brief, confluent monolayer cultures of ADSC lines were released by trypsin-EDTA and resuspended in growth medium at a density of 2.5 × 107 viable cells per milliliter. Micromasses were obtained by pipetting 20 μl of cell suspension into individual wells of 24-well plates. After a 3-hour attachment period without medium, the growth medium was gently added, and the cultures were left to rest for an additional 24 hours. The medium was then changed to serum-free and phenol red-free medium (Gibco-BRL, Gaithersburg, MD, http://www.invitrogen.com) for 24 hours. Differentiation was promoted by 10 ng/ml TGFβ3 supplementation. On day 3 of the culture, fresh differentiation medium was added, and the treatments were performed as described in Results. After 14 days, some micromasses were harvested for Alcian blue (AB) or Safranin O staining. Others were harvested for quantitative reverse transcriptase-polymerase chain reaction (PCR) gene expression analysis of the marker genes for chondrocytes (COLII, COLX, SOX9, MMP13, ADAMTS-5), as described in Real-Time PCR. Positive induction was detected by AB and Safranin O staining.
Pellet Cultures of ADSCs
Differentiation of ADSCs was induced in pellet culture by exposure to 10 ng/ml TGF-β3 (Millipore) and 200 mM of ascorbic acid (Sigma-Aldrich) in DMEM-high glucose medium for 4 weeks. The treatments were performed as described in Results. Adherent cell colonies were trypsinized and counted. Aliquots of 2 × 105 cells in 0.5 ml of medium were then centrifuged at 500g in 15-ml polypropylene conical tubes. Within 24 hours after incubation, the cells had formed an aggregate that did not adhere to the tube walls. The medium was changed every 2 or 3 days, and cell aggregates were obtained at intervals of as long as 21 days. The pellets were harvested for Safranin O staining and immunostaining. Hyaline cartilage marker type II collagen expression was recognized by anti-COLII antibodies (ab116242, Abcam, Cambridge, U.K., http://www.abcam.com). The expression of the hypertrophy markers MMP13, ADAMTS-5, and COLX was recognized by anti-MMP13 antibodies (C0265, Anbobio, San Francisco, CA, http://www.anbobio.com/index.asp), anti-ADAMTS-5 antibodies (ab41037, Abcam), and anti-COLX antibodies (ab58632, Abcam). Data were confirmed in 3 of 4 independent experiments.
Pull-Down Assay for Rac1 Activity
Rac1 activation assays were performed using a commercially available Rac1 activation assay kit (16118, Thermo Pierce, Rockford, IL, http://www.piercenet.com/), in accordance with the manufacturer's protocol.
Western Blot Analysis
Cellular protein was extracted using RIPA lysis buffer, and the protein concentration was determined using a bicinchoninic acid assay kit (Thermo Pierce #23227). The extracted cellular protein was loaded on SDS-polyacrylamide gel electrophoresis denaturing gels. After electrophoresis, the proteins were transferred to a polyvinylidene difluoride membrane and blocked in 5% bovine serum albumin for 1 hour at room temperature. The membrane was incubated overnight at 4°C with anti-Rac1 (Thermo Pierce #16118) or anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Beyotime Institute of Biotechnology, Shanghai, People’s Republic of China) antibody. After washing in Tris-buffered saline with Tween (TBST), horseradish peroxidase secondary antibody was diluted 1:1000 in 5% bovine serum albumin solution and incubated with the membrane for 1 hour at room temperature. Excess secondary antibody was rinsed off the membrane with TBST, and a chemiluminescent signal was generated using Western blot detection reagents (BeyoECL; Beyotime Institute of Biotechnology) according to the manufacturer's protocol.
Lentivirus Methods
The ADSCs were transfected with lentivirus when the cells were 30%–50% confluent at a multiplicity of infection of 200. At 12 hours after infection, more than 95% of the cells were still viable, and the culture medium was then changed. Three days later, all transfected cells were passaged for use in additional experiments.
Real-Time PCR
The mRNA expression levels of genes associated with hypertrophy (MMP13, ADAMTS-5, COLX) or chondrogenesis (COLII and SOX-9) were assessed using real-time PCR. Total cellular RNA was isolated by lysis in TRIzol (Invitrogen, Carlsbad, CA, http://www.invitrogen.com). PCR was performed using Brilliant SYBR Green QPCR Master Mix (TakaRa Bio, Shiga, Japan, http://www.takara-bio.com) with a Light Cycler apparatus (ABI 7900HT). The amplification efficiencies of primer pairs were validated to enable quantitative comparison of gene expression. All primer sequences (Invitrogen) were designed using primer premier 5.0 software. Each qPCR was performed on at least three different experimental samples, and representative results are presented as the target gene expression normalized to the reference gene GAPDH, actin, β2-microglobulin, or hypoxanthine ribosyltransferase. We performed a negative control (without cDNA) for each of the genes we detected, and the negative control did not amplify (cycle threshold >32). Error bars represent 1 ± SD from the mean of the technical replicates. The following primer sequences were used: MMP13: sense 5′-ATGCAGTCTTTCTTCGGCTTAG-3′, antisense 5′-ATGCCATCGTGAAGTCTGGT-3′; ADAMTS-5: sense 5′-ATCACCCAATGCCAAGG-3′, antisense 5′-AGCAGAGTAGGAGACAAC-3′; COLX: sense 5′-GTGTTTTACGCTGAACGATACCAA-3′, antisense 5′-ACCTGGTTTCCCTACAGCTGATG-3′; COLII: sense 5′- GAGAACCTGGTACCCCTGGA, antisense 5′-CCTTATGACTCCCATCTG-3′; and SOX-9: sense 5′-GGAGCTCGAAACTGACTGGAA-3′, antisense 5′-GAGGCGAATTGGAGGAGGA-3′.
Osteochondral Defect Model
In the present study, fifteen 12-week-old Sprague-Dawley rats were used and all were treated according to the standard guidelines approved by the Zhejiang University ethics committee (no. ZJU2007105002). Under intramuscular droperidol (0.25 mg/kg), intravenous pentobarbital (20 mg/kg) and general 2% isoflurane (vol/vol) anesthesia, the knee joint was opened using a medial parapatellar approach. The trochlear groove was exposed by lateral dislocation of the patella. A full-thickness cylindrical cartilage defect 2 mm in diameter and 2 mm deep was created in the trochlear groove of the femur using a stainless-steel punch. The defects were managed using one of the five methods listed in Table 1.
Table 1.
Description of methods used to manage the defects
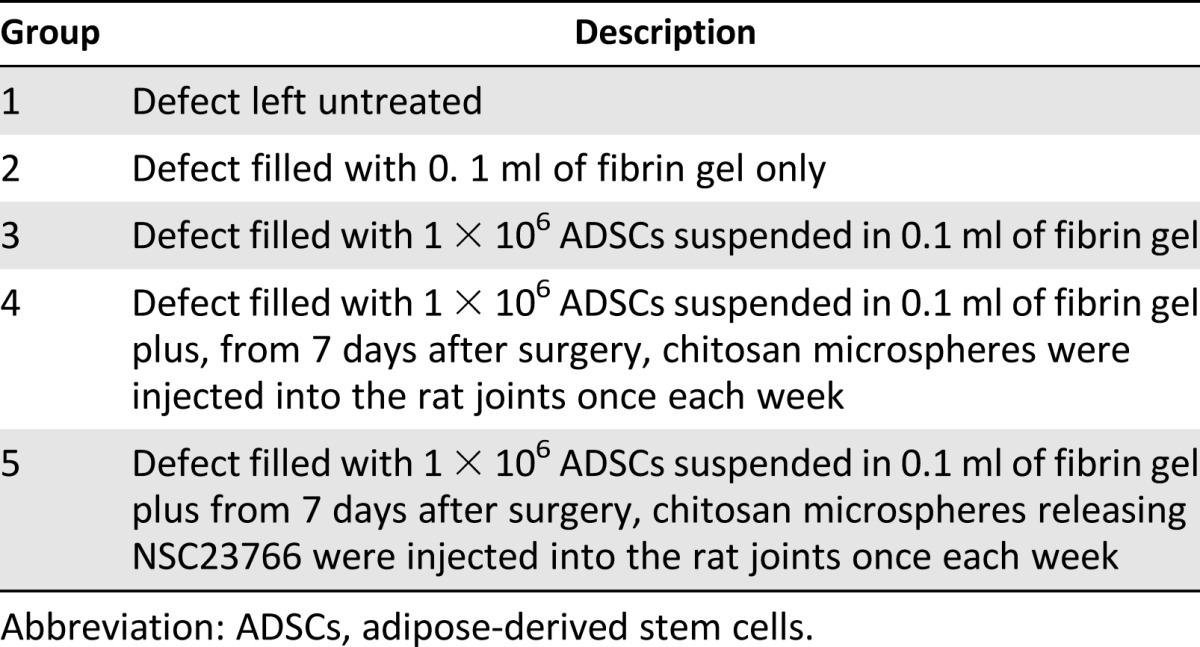
Each group consisted of 3 rats. Immediately after surgery, the rats were returned to their individual cages without joint immobilization. A postoperative antibiotic (gentamicin) was administered intramuscularly at 6 mg/kg daily for 3 days. After 6 weeks, the rats were sacrificed.
The specimens were investigated by Safranin O staining and immunostaining. The hyaline cartilage marker COLII expression was recognized by anti-COLII antibodies. The hypertrophy marker (MMP13, ADAMTS-5, and COLX) expression was recognized by the antibodies reported in the Pellet Cultures of ADSCs. The regenerated cartilage was grossly examined using the International Cartilage Repair Society (ICRS) macroscopic score [35], which evaluates the degree of defect repair, integration to the border zone, and the macroscopic appearance. The ICRS Visual Histological Assessment Scale [36] was used to evaluate the quality of the repaired tissue.
Preparation of Chitosan Microspheres
Chitosan microspheres were prepared through the water-in-oil emulsion solvent diffusion method. The chitosan solution (2% wt/vol) was prepared by dissolving chitosan (Shanghai Bio Science and Technology, Shanghai, http://www.sh-bio.com/eweb/index.asp) in 2.5% (vol/vol) acetic acid aqueous solution (Sinopharm Chemical Reagent) at room temperature. The chitosan solution was mixed with inhibitor by stirring overnight with a magnetic stirrer to produce a homogeneous mixture. Then, 5 ml of the mixture was aspirated into a syringe pump and then added drop by drop into the oil phase (24.72 ml) consisting of 14 ml of liquid paraffin (Sinopharm Chemical Reagent), 10 ml of petroleum ether, and 0.72 ml of Span 80 (Sangon Biotech) at a flow rate of 4 ml/hour with continuous stirring at 1500 rpm. A syringe needle with a 0.2-mm internal diameter was used for this process. After the solvent diffusion procedure, the suspension was cross-linked using 25% (vol/vol) glutaraldehyde solution as a cross-linking agent. The addition of the cross-linker was performed three times at intervals of 15 minutes, with the following volumes of glutaraldehyde: 0.64, 0.64, and 0.32 ml. Subsequently, the suspension was stirred at room temperature for cross-linking and then centrifuged at 3000 rpm for 5 minutes, followed by discarding the supernatant fluid. The microspheres were then washed three times with petroleum ether, two times with methanol, one time with acetone, one time with isopropyl alcohol (all from Sinopharm Chemical Reagent), one time with ethanol, and three times with distilled water. After washing, the microspheres were collected by lyophilizing with a freeze dryer to remove residual water. For the control group, pure chitosan microspheres were also prepared by directly dropping chitosan solution into the oil phase under the same conditions.
Statistical Analysis
All quantitative data are presented as the mean ± SD. Nonparametric statistical tests were performed to assess statistically significant differences in the data between groups. Values of p < .05 were considered statistically significant. The significance level is presented as either p < .05 or p < .01.
Results
Immunophenotypic Characterization and Multilineage Differentiation Ability of ADSCs
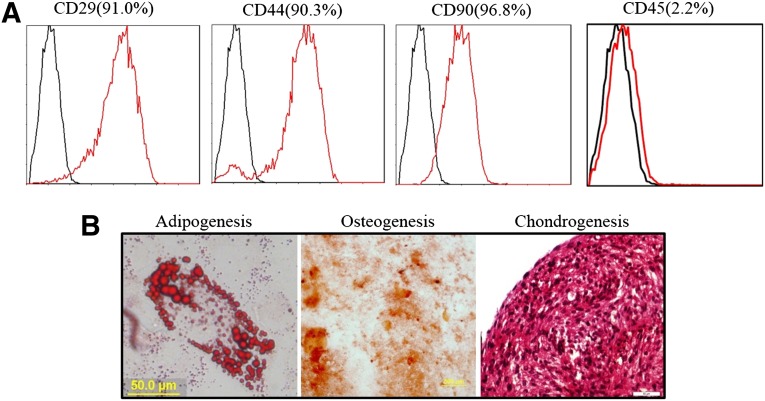
To verify that the stem cell phenotypes were maintained in the subcultures of ADSCs, the mesenchymal characteristics were evaluated using the positive MSC markers, CD90, CD29, and CD44, and the negative MSC marker CD45. The MSC markers CD90, CD29, and CD44 were observed in more than 90% of the cells (Fig. 1A), and the MSC negative marker CD45 was observed in less than 10% of the cells (Fig. 1B). An osteogenic differentiation assay confirmed that most of the cells had mineralized calcium deposits after 2 weeks of induction, as shown by alizarin red staining (Fig. 1C). Oil red O and Safranin O staining demonstrated that the cells after induction were able to differentiate into adipogenic and chondrogenic lineages, respectively (Fig. 1C). Taken together, our results suggest that we successfully isolated and maintained ADSCs.
Figure 1.
Immunophenotypic characterization and multilineage differentiation ability of adipose-derived stem cells (ADSCs). (A): Positive markers, CD29, CD44, and CD90, were observed in more than 90% of the cells. The negative marker, CD45, was observed in less than 10% of the cells. (B): Identification of multilineage differentiation ability of ADSCs. Scale bars = 50 μm.
Inhibition of Rac1 Activity in ADSCs Reduced Chondrocyte Hypertrophy-Like Changes Without Affecting Chondrogenesis In Vitro
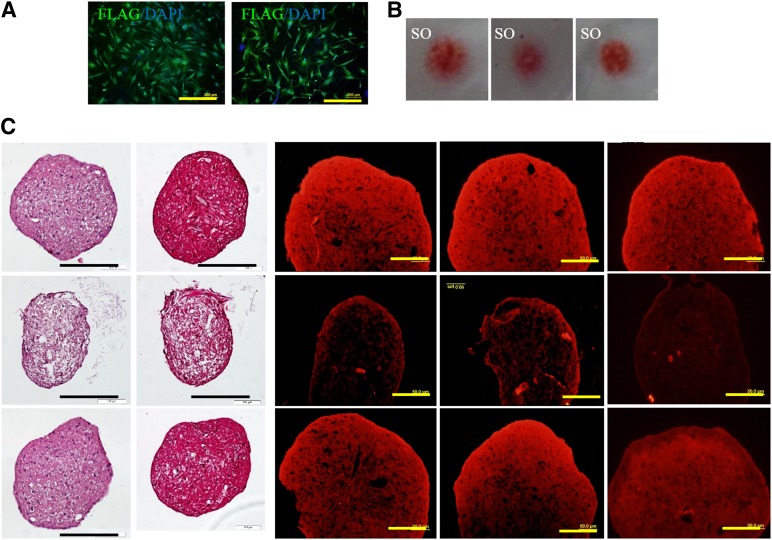
We have previously reported that activation of Rac1 in chondrocytes promotes chondrocyte hypertrophy and calcification. In this study, we checked the role of Rac1 in ADSC chondrogenic differentiation. ADSCs were infected with constitutively active Rac1 mutant (Ca-Rac1) and dominant negative Rac1 (DN-Rac1) virus (Fig. 2A). In a micromass culture model, CA-Rac1 or DN-Rac1 expression did not affect the chondrogenic capability of the ADSCs, demonstrated by Safranin O staining (Fig. 2B). In another chondrogenic model of pellet culture, TGFβ3 unavoidably induced expression of chondrocyte hypotrophy, and calcification-related genes, including ADAMTS-5, MMP13, and COLX (Fig. 2C), could, to a great extent, be reduced by DN-Rac1. These results indicate that TGFβ3 promotes chondrogenic differentiation of ADSCs, and inhibition of Rac1 activity prevents ADSCs from undergoing hypertrophy.
Figure 2.
Inhibition of Rac1 activity in adipose-derived stem cells (ADSCs) reduces chondrocyte hypertrophy-like changes without affecting chondrogenesis in vitro. (A): ADSCs were successfully transfected with ectopic Rac1 detected by immunostaining of a FLAG tag (green). Scale bars = 400 μm. (B): Safranin O staining demonstrated that constitutively active Rac1 mutant or dominant negative Rac1 expression will not affect chondrogenesis of ADSCs induced by transforming growth factor β3 (TGFβ3). (C): Immunostaining results for hypertrophic marker expression. Scale bars = 200 or 100 μm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; SO, Safranin O.
TGFβ3 Promoted ADSC Chondrogenic Differentiation and Rac1 Inhibitor NSC23766 Inhibited Hypertrophy In Vitro
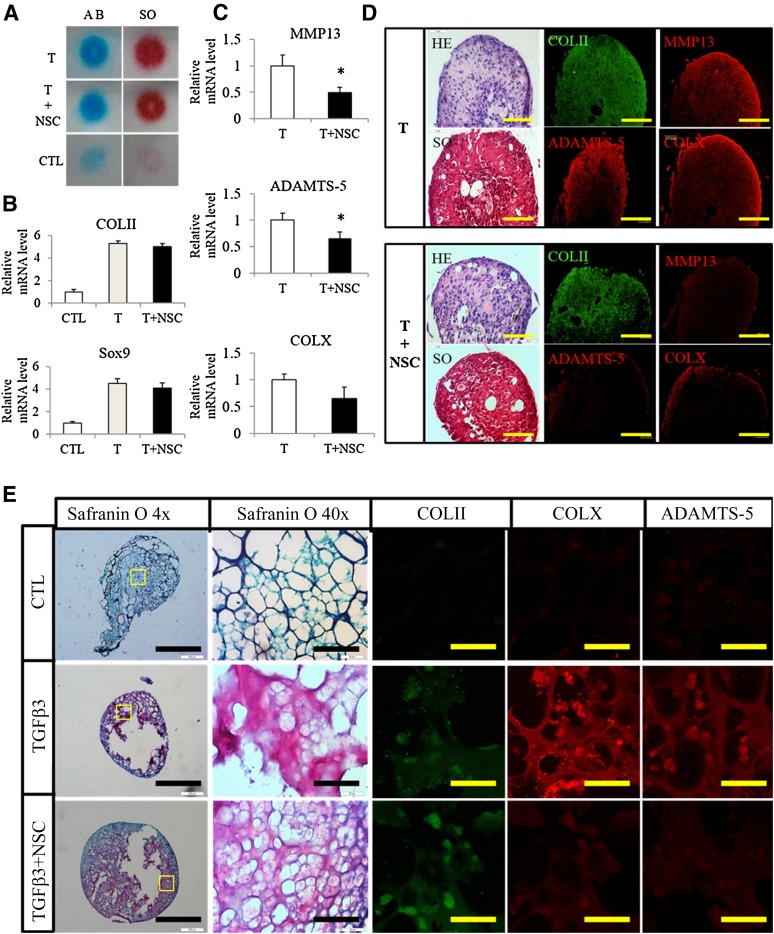
We also studied the collaborative roles of TGFβ3 and Rac1 inhibitor NSC23766 on ADSC chondrogenic differentiation. ADSCs were cultured in high density using a micromass culture model. Real-time quantitative polymerase chain reaction (qPCR) was performed for selected chondrogenic (type II collagen and Sox9) and hypertrophic (MMP13, COLX, and ADAMTS-5) markers. Supplementation with TGFβ3 greatly promoted chondrogenesis, and the addition of the Rac1 inhibitor NSC23766 did not affect the chondrogenic differentiation of ADSCs, demonstrated by both Alcian blue and Safranin O staining (Fig. 3A) and the qPCR results for COLII and Sox9 (Fig. 3B). Supplementation with NSC23766 significantly suppressed TGFβ3-induced hypertrophy gene upregulation (Fig. 3C). Testing of several other reference genes demonstrated that the qPCR is robust (supplemental online Fig. 1). We also tested the effect of NSC23766 in another chondrogenic model of pellet culture. Histological staining suggested NSC23766 had no effect on chondrogenic differentiation of ADSCs; nonetheless, inhibition of Rac1, to a great extent, decreased expression of the hypertrophic markers such as MMP13, ADAMTS-5, and COLX (Fig. 3D).
Figure 3.
Transforming growth factor β3 (TGFβ3) promotes adipose-derived stem cell (ADSC) chondrogenic differentiation and NSC23766 inhibits hypertrophy in vitro. (A, B): Micromass culture model demonstrated TGFβ3-induced chondrogenesis. The addition of the Rac1 inhibitor did not impede chondrogenesis (A) or COLII and Sox9 expression (B). (C): Addition of Rac1 inhibitor NSC23766 decreased TGFβ3-induced MMP13, ADAMTS-5, and COLX expression. (D): Pellet culture model also confirmed that supplementation with NSC23766 prevented hypertrophy without affecting the chondrogenesis of ADSCs, demonstrated by immunostaining of COLII, COLX, MMP13, and ADAMTS-5. Scale bars = 100 μm. (E): Safranin O staining of ADSCs treated with TGFβ3 or TGFβ3+NSC23766 and the detection of COLII, COLX, and ADAMTS-5 by immunostaining. Scale bars = 1 mm or 100 μm. Abbreviations: AB, Alcian blue staining; ADSCs, adipose-derived stem cells; CLT, control; HE, hematoxylin and eosin stain; NSC, NSC23766; SO, Safranin O; T, transforming growth factor β3.
In the next step, we tested the feasibility of promoting cartilage repair using these two reagents. We prepared fibrin gel encapsulated ADSCs cultured in medium supplemented with TGFβ3 or TGFβ3 plus NSC23766 for 21 days. COLII expression, a characteristic of differentiated chondrocytes, could be detected in the cells with TGFβ3 but not in the control group. Consistently, the Safranin O-positive proteoglycan showed remarkable increases in the TGFβ3 treated group compared with the control group (Fig. 3E). Also, inhibition of Rac1 decreased the expression of hypertrophic markers such as ADAMTS-5 and COLX (Fig. 3E). These results encouraged us to further apply this strategy to cartilage repair and regeneration.
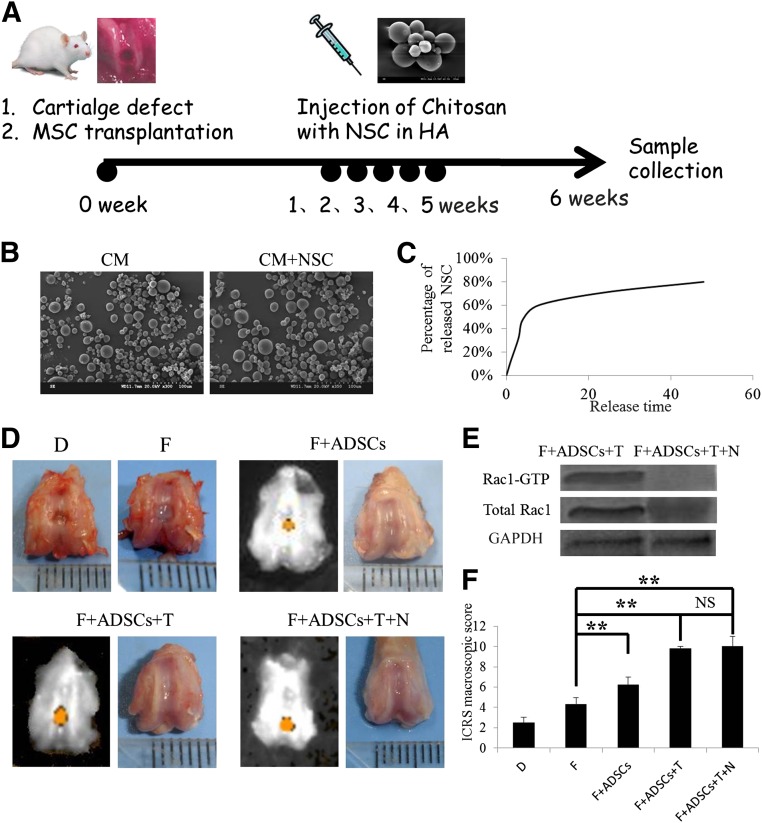
Programmed Addition of TGFβ3 and NSC23766 Promoted ADSCs for Cartilage Repair In Vivo
Osteochondral defects were created in the patellar groove of rat distal femurs, and ADSCs encapsulated in fibrin gel supplemented with or without TGFβ3 were implanted in the defects. From 7 days after surgery, chitosan microspheres containing NSC23766 were injected into the rat joints once a week; the whole joints were collected 6 weeks after surgery (Fig. 4A). The chitosan microspheres were spherical, with a mean size of around 100 μm and had a smooth surface without cracks or wrinkles, demonstrated by scanning electron micrographs (Fig. 4B). The control release ability of chitosan microspheres was determined by measuring the optical density of the extracts at 360 nm (which is specific for NSC23766). NSC23766 was released from the microspheres in a biphasic fashion, characterized by a fast release phase on the initial day 1, followed by slower release on the remaining days (Fig. 4C). In three groups (F+ADSCs [10% fetal bovine serum plus ADSCs], F+ADSCs+T [10% fetal bovine serum plus ADSCs plus TGFβ2], and F+ADSCs+T+NSC [10% fetal bovine serum plus ADSCs plus TGFβ2 plus NSC23766]), the defect had become firm and smooth after 6 weeks. In contrast, a soft and friable tissue was present in groups D (Defect) and F (Fibrin), demonstrated by macroscopic assessment (Fig. 4D). A Rac1 pull-down assay demonstrated that Rac1 activity had been significantly inhibited by NSC23766 (Fig. 4E). The ICRS macroscopic score was highest in groups F+ADSCs+T+NSC (10.0) and F+ADSCs+T (9.8), followed by group F+ADSCs (6.2) and group F (4.3). These results suggest that ADSCs encapsulated in fibrin gel supplemented with TGFβ3 can greatly promote the repair of osteochondral defects.
Figure 4.
The combination of ADSCs, transforming growth factor β3, and NSC23766 promoted cartilage repair in vivo. (A): Experimental design for in vivo treatment of osteochondral defects. (B): Scanning electron micrographs of the chitosan microspheres with or without NSC23766. (C): Average cumulative NSC23766 release from the microspheres. (D): Gross morphology of joint samples. (E): Rac1 pull-down assay of Rac1-GTP level. (F): International Cartilage Repair Society macroscopic score. n = 3, ∗∗, p < .01. Abbreviations: ADSCs, adipose-derived stem cells; CM, chitosan microsphere; D, defect; F, fibrin, HA, hyaluronic acid; ICRS, International Cartilage Repair Society; MSC, mesenchymal stem cell; N, NSC23766; NS, not significant; NSC, NSC23766; T, transforming growth factor β3.
Histological Assessment of Repair
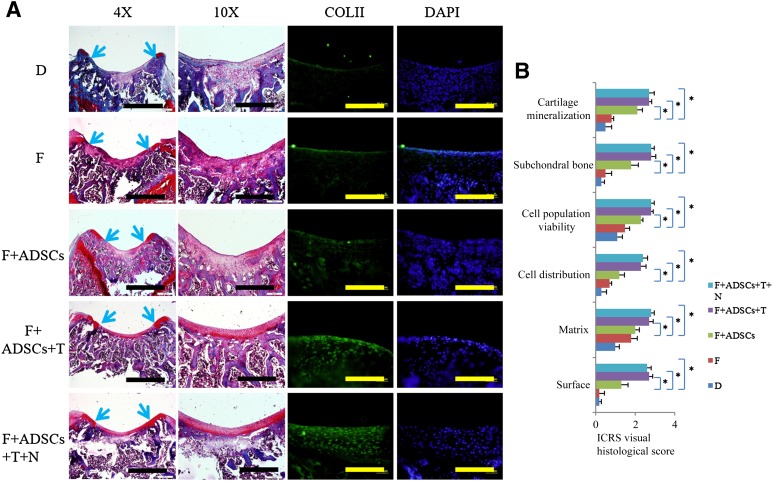
Histological analysis at 6 weeks after surgery demonstrated a smooth articular surface with restoration of hyaline cartilage-like tissue stained with Safranin O in groups F+ADSCs+T+NSC and F+ADSCs+T. The defects in group F+ADSCs had incomplete healing with hyaline cartilage-like tissue limited to a few area of the defects, with most of the defect area filled with fibrous tissue (Fig. 5A). In group F, the defects were almost completely filled with fibrous tissue with little Safranin O staining (Fig. 5A). Group D displayed disruption of subchondral bone and a thin layer of fibrous tissue (Fig. 5A). Also, the immunohistochemical results demonstrated that TGFβ3 stimulated hyaline cartilage marker type II collagen expression, and the addition of NSC23766 did not interfere with this effect (Fig. 5A). The ICRS Visual Histological Assessment Scale showed that groups F+ADSCs+T+NSC and F+ADSCs+T had much better results than groups F+ADSCs and F (Fig. 5B). These results suggest that supplementation with TGFβ3 could to a large extent promote ADSC chondrogenic differentiation and lead to better articular cartilage repair.
Figure 5.
Histological assessment of repair. (A): Safranin O staining of cartilage sections (blue arrows denote the margin of the defects and immunofluorescence with antibody to type II collagen). Scale bars = 1 mm, 400 μm, or 100 μm. (B): ICRS visual histological score. n = 3; ∗, p < .05. Abbreviations: ADSCs, adipose-derived stem cells; D, defect; DAPI, 4′,6-diamidino-2-phenylindole; F, fibrin; ICRS, International Cartilage Repair Society; N, NSC23766; T, transforming growth factor β3.
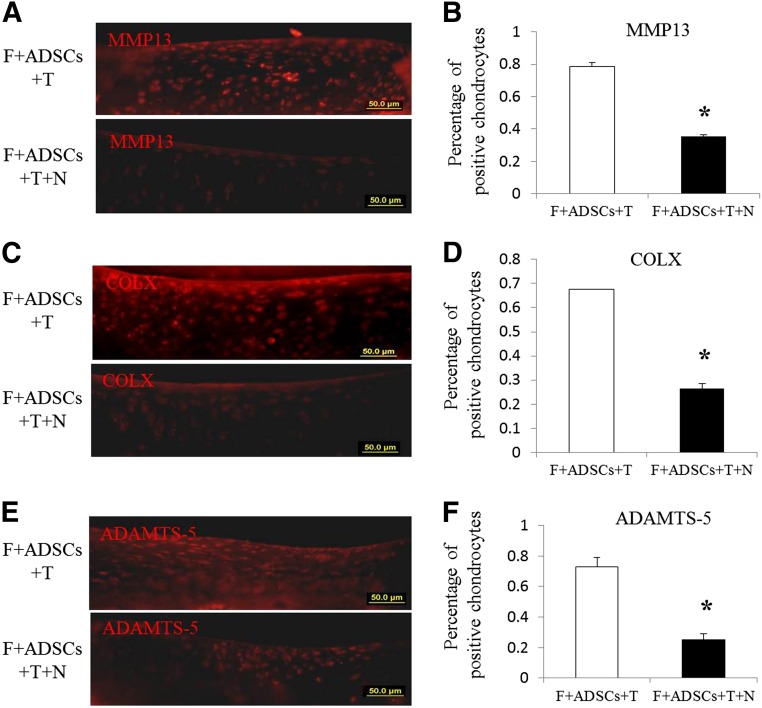
Effect of Rac1 Inhibitor NSC23766 on TGFβ3 Induced Chondrocyte Hypertrophy In Vivo
Because TGFβ3 supplementation promotes chondrogenesis and causes undesired events such as hypertrophy and calcification, we checked whether NSC23766 could ameliorate these side effects using an immunohistochemical assay for hypertrophy marker (MMP13, ADAMTS-5, and COLX) expression. We found many fewer chondrocytes in group F+ADSCs+T+NSC cartilage expressing MMP13, ADAMTS-5, and COLX than in group F+ADSCs+T (Fig. 6A, 6C, 6E). In addition, MMP13- and COLX-positive chondrocytes were limited to a deep area in group F+ADSCs+T+NSC, and in group F+ADSCs+T, the MMP13- and COLX-positive chondrocytes were distributed over the whole thickness of the cartilage (Fig. 6A, 6C). The quantitative results showed that controlled release of NSC23766 in rat joints significantly decreased the percentage of MMP13-, COLX-, and ADAMTS-5-positive chondrocytes (Fig. 6B, 6D, 6F). These results demonstrated that controlled release of NSC23766 by chitosan microspheres greatly ameliorated the TGFβ3-induced chondrocyte hypertrophy.
Figure 6.
Effect of Rac1 inhibitor NSC23766 on transforming growth factor β3-induced chondrocyte hypertrophy. Immunocytochemistry of MMP13, ADAMTS-5, and COLX was performed (A, C, E) and quantified (B, D, F). Scale bars = 50 μm. ∗, p < .05. Abbreviations: ADSCs, adipose-derived stem cells; F, fibrin; N, NSC23766; T, transforming growth factor β3.
Discussion
In this study, we developed a new stem cell-based strategy for cartilage defect repair in which a chondro-lineage differentiation activator (TGFβ3) and chondrocyte hypertrophy inhibitor (Rac1 inhibitor NSC23766) were applied. Our study had several major findings. First, genetic modulation of Rac1 activity by DN-Rac1 suppressed cell hypertrophy without affecting the chondrogenic ability of the ADSCs. Second, programmed application of TGFβ3 and the Rac1 inhibitor NSC23766 committed the ADSCs to chondro-lineage differentiation without hypertrophy. Finally, the strategy of programmed application of TGFβ3 and NSC23766 improved the efficacy of ADSCs for cartilage healing in the rat knee joint. These data collectively suggest that programmed application of TGFβ3 and Rac1 inhibitor NSC23766 can commit ADSCs to chondro-lineage differentiation and improve the efficacy of ADSCs for cartilage defect repair.
The present study has shown that supplementation of TGFβ3 greatly promotes ADSC chondrogenic differentiation. ADSCs could be an alternative autologous cell source for cartilage regeneration that is easier to obtain, has lower donor-site morbidity, and is available in larger numbers than stem cells harvested using bone marrow aspiration [37–39]. Generally, in vitro chondrogenic differentiation of ADSCs is induced by culturing ADSCs in a pellet with supplementation of TGFβ3 [40]. However, many issues still need to be considered, such as whether during induction of chondrogenesis in vitro, cells adopt natural differentiation stages, and whether they can form ectopic stable cartilage that is resistant to hypertrophy and calcification in vivo [41].
Previously, we reported that Rac1 was aberrantly activated in OA chondrocytes and inhibition of Rac1 activity in vivo protected cartilage from deterioration by reducing chondrocyte hypertrophy [29]. In this study, we used the Rac1 inhibitor NSC23766 to facilitate the efficiency of TGFβ3-induced ADSC chondrogenesis by suppressing chondrocytes from undergoing hypertrophy and calcification.
For cartilage defects, many surgical treatments involving microfracture, joint debridement and drilling, autologous chondrocyte/bone marrow mesenchymal stem cell implantation have been successfully used in clinics [42]. However, these therapeutic methods mainly focused on the supply of cells and ignored the hypertrophy and calcification caused by the cytokines. In our study, we developed a strategy of programmed application of TGFβ3 and the Rac1 inhibitor NSC23766 to commit the hyaline cartilage differentiation of ADSCs for joint cartilage repair.
Characterized by biocompatibility, nontoxicity, a lack of allergenicity, and biodegradability, chitosan microspheres are an attractive biopolymer for drug delivery systems in a controlled and/or sustained manner and can be successfully targeted for site-specific drug delivery [32, 43]. Hyaluronic acid (HA) is a sterile, viscoelastic, nonpyogenic glycosaminoglycan that is indicated as a medical device for the treatment of pain in patients with knee injuries [44–46]. In this study, HA-containing chitosan microspheres encapsulating the Rac1 inhibitor NSC23766 were injected into rat joints for sustained release to lubricate the joints and remit the pain. However, the controlled release system used in this study could only guarantee sustained release for 3 days owing to the relative inhomogeneous and large size of the chitosan microspheres. Additional work will aim to develop a more efficient delivery system by controlling the size and homogeneity of the biomaterial spheres to maintain the drug concentration in the OA joint.
Conclusion
In this study, we have shown that programmed application of TGFβ3 and Rac1 inhibitor NSC23766 can commit ADSCs to chondro-lineage differentiation and improve the efficacy of ADSCs for cartilage defect repair by slowing the progression to hypertrophy. Our findings have also provided additional support for the importance of Rac1 as a therapeutic target for cartilage degeneration.
Supplementary Material
Acknowledgments
This work was supported by the National Program on Key Basic Research project (Grant 2012CB966604), the National Natural Science Fund (Grants 81125014, 81101356, 81201395, and 31000436), Zhejiang Province Public Welfare Fund (Grant 2012C3112), Zhejiang Provincial Natural Science Foundation of China (Grant LY13C100001), and Postdoctoral Foundation of China (Grant 2013M531465), and was sponsored by the Regenerative Medicine in Innovative Medical Subjects of Zhejiang Province and Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents. We also thank the care facilities of Zhejiang University School of Medicine for technical assistance.
Author Contributions
S.Z. and P.C.: in vitro cell culture and histological assessment; Y.W.: osteochondral defect surgery; S.X. chitosan microsphere preparation; H.S., Q.X., and L.S.: fluorescence-activated cell sorting analysis and Western blotting; H.L. and H.W.O. manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Hunziker EB. Articular cartilage repair: Basic science and clinical progress: A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 2.Camp CL, Stuart MJ, Krych AJ. Current concepts of articular cartilage restoration techniques in the knee. Sports Health. 2014;6:265–273. doi: 10.1177/1941738113508917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Merchan EC. Regeneration of articular cartilage of the knee. Rheumatol Int. 2013;33:837–845. doi: 10.1007/s00296-012-2601-3. [DOI] [PubMed] [Google Scholar]
- 4.Caplan AI. Review: Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 5.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez AM, Elabd C, Amri EZ, et al. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005;87:125–128. doi: 10.1016/j.biochi.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 8.Erickson GR, Gimble JM, Franklin DM, et al. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;290:763–769. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- 9.Masuoka K, Asazuma T, Hattori H, et al. Tissue engineering of articular cartilage with autologous cultured adipose tissue-derived stromal cells using atelocollagen honeycomb-shaped scaffold with a membrane sealing in rabbits. J Biomed Mater Res B Appl Biomater. 2006;79:25–34. doi: 10.1002/jbm.b.30507. [DOI] [PubMed] [Google Scholar]
- 10.Dragoo JL, Carlson G, McCormick F, et al. Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Eng. 2007;13:1615–1621. doi: 10.1089/ten.2006.0249. [DOI] [PubMed] [Google Scholar]
- 11.Sakaguchi Y, Sekiya I, Yagishita K, et al. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 12.Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage. 2005;13:845–853. doi: 10.1016/j.joca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Afizah H, Yang Z, Hui JH, et al. A comparison between the chondrogenic potential of human bone marrow stem cells (BMSCs) and adipose-derived stem cells (ADSCs) taken from the same donors. Tissue Eng. 2007;13:659–666. doi: 10.1089/ten.2006.0118. [DOI] [PubMed] [Google Scholar]
- 14.Im GI, Lee JH. Repair of osteochondral defects with adipose stem cells and a dual growth factor-releasing scaffold in rabbits. J Biomed Mater Res B Appl Biomater. 2010;92:552–560. doi: 10.1002/jbm.b.31552. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Im GI. Chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells: Greater doses of growth factor are necessary. J Orthop Res. 2009;27:612–619. doi: 10.1002/jor.20766. [DOI] [PubMed] [Google Scholar]
- 16.Huang AH, Farrell MJ, Mauck RL. Mechanics and mechanobiology of mesenchymal stem cell-based engineered cartilage. J Biomech. 2010;43:128–136. doi: 10.1016/j.jbiomech.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim M, Erickson IE, Choudhury M, et al. Transient exposure to TGF-β3 improves the functional chondrogenesis of MSC-laden hyaluronic acid hydrogels. J Mech Behav Biomed Mater. 2012;11:92–101. doi: 10.1016/j.jmbbm.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ronzière MC, Perrier E, Mallein-Gerin F, et al. Chondrogenic potential of bone marrow- and adipose tissue-derived adult human mesenchymal stem cells. Biomed Mater Eng. 2010;20:145–158. doi: 10.3233/BME-2010-0626. [DOI] [PubMed] [Google Scholar]
- 19.Barry F, Boynton RE, Liu B, et al. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: Differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 20.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 21.Tamamura Y, Otani T, Kanatani N, et al. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;280:19185–19195. doi: 10.1074/jbc.M414275200. [DOI] [PubMed] [Google Scholar]
- 22.Mithoefer K, Williams RJ, III, Warren RF, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee: A prospective cohort study. J Bone Joint Surg Am. 2005;87:1911–1920. doi: 10.2106/JBJS.D.02846. [DOI] [PubMed] [Google Scholar]
- 23.Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14:1119–1125. doi: 10.1016/j.joca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Brown WE, Potter HG, Marx RG, et al. Magnetic resonance imaging appearance of cartilage repair in the knee. Clin Orthop Relat Res. 2004;(422):214–223. doi: 10.1097/01.blo.0000129162.36302.4f. [DOI] [PubMed] [Google Scholar]
- 25.Woods A, Wang G, Dupuis H, et al. Rac1 signaling stimulates N-cadherin expression, mesenchymal condensation, and chondrogenesis. J Biol Chem. 2007;282:23500–23508. doi: 10.1074/jbc.M700680200. [DOI] [PubMed] [Google Scholar]
- 26.Wang G, Beier F. Rac1/Cdc42 and RhoA GTPases antagonistically regulate chondrocyte proliferation, hypertrophy, and apoptosis. J Bone Miner Res. 2005;20:1022–1031. doi: 10.1359/JBMR.050113. [DOI] [PubMed] [Google Scholar]
- 27.Wang G, Woods A, Agoston H, et al. Genetic ablation of Rac1 in cartilage results in chondrodysplasia. Dev Biol. 2007;306:612–623. doi: 10.1016/j.ydbio.2007.03.520. [DOI] [PubMed] [Google Scholar]
- 28.Long DL, Willey JS, Loeser RF. Rac1 is required for matrix metalloproteinase-13 production by chondrocytes in response to fibronectin fragments. Arthritis Rheum. 2013;65:1561–1568. doi: 10.1002/art.37922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu S, Lu P, Liu H, et al. Inhibition of Rac1 activity by controlled release of NSC23766 from chitosan microspheres effectively ameliorates osteoarthritis development in vivo. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203901. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alpar HO, Somavarapu S, Atuah KN, et al. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv Drug Deliv Rev. 2005;57:411–430. doi: 10.1016/j.addr.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Illum L, Watts P, Fisher AN, et al. Intranasal delivery of morphine. J Pharmacol Exp Ther. 2002;301:391–400. doi: 10.1124/jpet.301.1.391. [DOI] [PubMed] [Google Scholar]
- 32.Jain SK, Chourasia MK, Jain AK, et al. Development and characterization of mucoadhesive microspheres bearing salbutamol for nasal delivery. Drug Deliv. 2004;11:113–122. doi: 10.1080/10717540490280750. [DOI] [PubMed] [Google Scholar]
- 33.Lee JM, Im GI. SOX trio-co-transduced adipose stem cells in fibrin gel to enhance cartilage repair and delay the progression of osteoarthritis in the rat. Biomaterials. 2012;33:2016–2024. doi: 10.1016/j.biomaterials.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, Song XH, Yin Z, et al. Stepwise differentiation of human embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. Stem Cells. 2009;27:1276–1287. doi: 10.1002/stem.61. [DOI] [PubMed] [Google Scholar]
- 35.De Bari C, Dell’Accio F, Luyten FP. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001;44:85–95. doi: 10.1002/1529-0131(200101)44:1<85::AID-ANR12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 36.van den Borne MP, Raijmakers NJ, Vanlauwe J, et al. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in autologous chondrocyte implantation (ACI) and microfracture. Osteoarthritis Cartilage. 2007;15:1397–1402. doi: 10.1016/j.joca.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Mainil-Varlet P, Aigner T, Brittberg M, et al. Histological assessment of cartilage repair: A report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS) J Bone Joint Surg Am. 2003;85-A(suppl 2):45–57. [PubMed] [Google Scholar]
- 38.Yoon HH, Bhang SH, Shin JY, et al. Enhanced cartilage formation via three-dimensional cell engineering of human adipose-derived stem cells. Tissue Eng Part A. 2012;18:1949–1956. doi: 10.1089/ten.tea.2011.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veronesi F, Maglio M, Tschon M, et al. Adipose-derived mesenchymal stem cells for cartilage tissue engineering: State-of-the-art in in vivo studies. J Biomed Mater Res A. 2014;102:2448–2466. doi: 10.1002/jbm.a.34896. [DOI] [PubMed] [Google Scholar]
- 40.Technau A, Froelich K, Hagen R, et al. Adipose tissue-derived stem cells show both immunogenic and immunosuppressive properties after chondrogenic differentiation. Cytotherapy. 2011;13:310–317. doi: 10.3109/14653249.2010.504769. [DOI] [PubMed] [Google Scholar]
- 41.Pelttari K, Winter A, Steck E, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed TA, Hincke MT. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev. 2010;16:305–329. doi: 10.1089/ten.TEB.2009.0590. [DOI] [PubMed] [Google Scholar]
- 43.Kang ML, Kang SG, Jiang HL, et al. In vivo induction of mucosal immune responses by intranasal administration of chitosan microspheres containing Bordetella bronchiseptica DNT. Eur J Pharm Biopharm. 006;63:215–220. doi: 10.1016/j.ejpb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Curran MP. Hyaluronic acid (Supartz®): A review of its use in osteoarthritis of the knee. Drugs Aging. 2010;27:925–941. doi: 10.2165/11205920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Munteanu SE, Zammit GV, Menz HB, et al. Effectiveness of intra-articular hyaluronan (Synvisc, hylan G-F 20) for the treatment of first metatarsophalangeal joint osteoarthritis: A randomised placebo-controlled trial. Ann Rheum Dis. 2011;70:1838–1841. doi: 10.1136/ard.2011.153049. [DOI] [PubMed] [Google Scholar]
- 46.Yatabe T, Mochizuki S, Takizawa M, et al. Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann Rheum Dis. 2009;68:1051–1058. doi: 10.1136/ard.2007.086884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.