More than 1,000 cultivated limbal epithelial transplantation (CLET) procedures have been reported from around the world, with varying degrees of success, for the treatment of limbal stem cell deficiency. The authors compare the methods of cultivation and the outcomes of CLET, introduce the new technique of simple limbal epithelial transplantation and discuss some problem areas, use of other cells as substitutes for limbal epithelium, and various carrier materials used in transplantation
Keywords: Ocular surface reconstruction, Corneal epithelial damage, Limbal stem cells, Kaplan-Meier analysis of outcomes, Oral mucosal cells, Simple limbal epithelial transplantation
Abstract
The cornea is a vital component of the eye because it provides approximately 70% of the refraction and focusing of incoming light. Being the outermost surface of the eye, it faces continuous stress from dryness, photodamage, infection, and injury; however, like the skin, the cornea regularly refreshes itself by shedding its epithelial cells, which are readily replaced, keeping the ocular surface stable and functional. This regular turnover of the corneal epithelial cells occurs through the stem cells in the limbus, an annular ring of a tissue surrounding the cornea, separating it from the sclera and the conjunctival membrane. The loss of this reserve of stem cells leads to a condition called limbal stem cell deficiency. Treatment for this disorder has evolved from transplanting whole limbal tissues to the affected eye to transplanting laboratory cultured limbal cells. This procedure is called cultivated limbal epithelial transplantation (CLET). Since its start in 1997, more than 1,000 CLET procedures have been reported from around the world, with varying degrees of success. In this paper, we compare the methods of cultivation and the outcomes and discuss some problem areas, use of other cells as substitutes for limbal epithelium, and various carrier materials used in transplantation. Our analysis suggests that CLET as a treatment for corneal surface damage has come of age. We also highlight a simpler procedure (simple limbal epithelial transplantation) that involves cultivation of limbal tissue in situ on the surface of the cornea in vivo and that has outcomes comparable to CLET.
Introduction
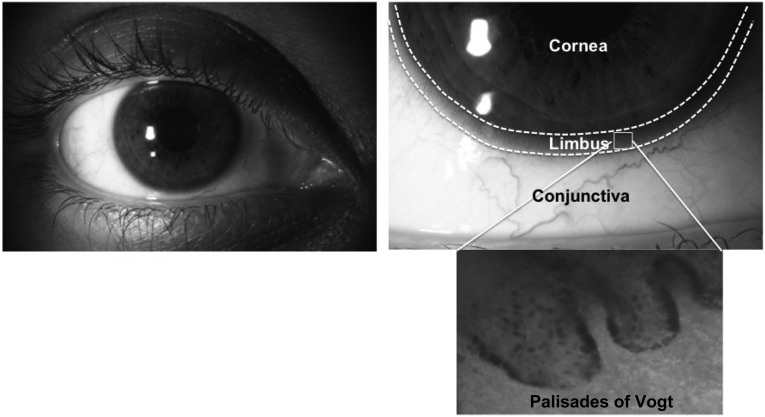
All regenerating tissues or organs in the body (e.g., gut epithelium, skin epithelium, blood) have reserves of tissue-specific stem cells that help maintain homeostasis and, more important, permit rapid healing in case of injury. This is also true of the ocular surface, which is composed of two main tissues: the cornea and the conjunctiva. Regeneration of the corneal surface is a multistep process involving cell division, migration, maturation, and finally death of the superficial squamous cells, which are removed through desquamation [1]. For constant repopulation and healing of epithelial defects, a reserve of stem cells is located in the limbal region, specifically, in the basal region of papillary structures called the palisades of Vogt (Fig. 1) [2–4].
Figure 1.
Anatomical location of limbal stem cells. This figure shows the region of the limbus (marked with dashes lines) located between the avascular, transparent cornea and the vascularized, nontransparent conjunctiva. In this limbal region, the corneal stem cells are located within finger-like projections called the palisades of Vogt, as can be seen in the phase-contrast image.
The limbus functions as a niche, a specialized microenvironment that supports the corneal epithelial stem cells and protects them from several intrinsic and environmental assaults. This complex structure is composed of several different populations of cells and a dense vasculature that, together, function to regulate the proliferation and differentiation of the limbal stem cells (LSCs). Any condition that affects the structural integrity of the limbus, thereby altering its support for the stem cells, will result in the loss of this cell population, leading to loss of corneal transparency and resulting in vision loss.
Several causative factors precipitate the loss of LSCs, resulting in either partial or total deficiency. The condition, clinically referred to as limbal stem cell deficiency (LSCD), can occur because of thermal or chemical injuries, surgeries involving the limbus, ocular cicatricial pemphigoid (an immune disorder involving the mucous membranes), microbial infection, chronic allergies (e.g., vernal keratoconjunctivitis), and/or when there is structural insufficiency of the limbal niche caused by developmental disorders (e.g., aniridia, or the absence of iris). These insults to the ocular surface lead to chronic inflammation, making it difficult for the tissue to heal itself and resulting in scarring of the cornea, the neighboring conjunctiva, and, in severe cases, the eyelids. In a recent review, Nakamura and Kinoshita [5] summarized the strategies for the treatment of severe ocular surface diseases. Our present concise review discusses the various clinical and research advances that are being made in treating LSCD and some persistent challenges and the approaches being used to address them.
Treatment for LSCD
Treatment for LSCD has evolved rapidly and effectively in the past few decades. The treatment strategy is based on whether the disease presentation is unilateral or bilateral and whether there is partial or total involvement of the limbus.
Management of partial LSCD, in which the deficiency involves only a few sectors of the cornea, typically involves either grafting of human amniotic membrane (hAM) or mechanical debridement of the encroaching conjunctiva, especially when the central cornea is spared and the defect is unilateral. Good clinical outcome (i.e., restoration of a stable ocular surface) is achieved, by and large, in this manner. The use of hAM has also been quite beneficial during the acute phase of injury or burns and in treatment of partial LSCD [6], owing to its anti-inflammatory property that allows natural restoration of a stable ocular surface. In a few cases of partial LSCD, ipsilateral translocation or transplantation of a small segment of tissue to the deficient region has also been reported [7]. In most cases of mild to moderate LSCD, engraftment of hAM or debridement of the encroaching conjunctiva, coupled with close follow-up of the patient, has been the preferred choice of treatment.
In the case of total LSCD, whether unilateral or bilateral, the treatment is surgical. In the early 1980s, transplantation of limbal tissue itself was initiated for the treatment of LSCD [8]. The outcome of this study confirmed that the limbus harbored corneal stem cells and, more important, that the ocular surface can be regenerated by transplanting healthy pieces of the limbus. Since then, several variations in allogeneic and autologous limbal transplantation with good clinical success have been reported, most notable of these advances being the culture of epithelial cells in the laboratory for transplantation. The culture technique was established primarily to offset the following drawbacks noted while transplanting the whole limbal tissue (also known as keratolimbal or conjunctival limbal transplantation): the need for a large section of the limbal tissue (approximately 3–6 clock hours) for restoration of the ocular surface and induced stem cell deficiency in the donor eye. The latter has been a major detriment to keratolimbal transplantation. A concise review of limbal stem cell deficiency, dysfunction, and distress has been provided by Ahmad [9].
Cultured Limbal Epithelial Transplantation: Techniques and Outcomes
A new chapter in the treatment of LSCD was started with the laboratory expansion of limbal epithelial cells (LECs) and transplantation in two patients, leading to complete regeneration of a stable ocular surface [10]. Since this first report on cultured limbal epithelial transplantation (CLET), there have been several modifications to the culture procedure that can be classified broadly as the “explant” and “suspension” culture techniques. The common factor of these techniques is that the limbal cells are expanded in culture from a small limbal biopsy of approximately 2 × 2 mm (less than 1 clock hour). The following are the main differences. (a) In the suspension technique, as the name suggests, the cells are separated by enzyme digestion from the limbal niche for culture. With the explant culture, limbal cells, along with the entire limbal niche, are placed in culture. (b) Because the cells are removed from the limbal niche in suspension culture, additional support in the form of feeder cells needs to be provided in order to maintain the stem cell population. This is not necessary for the explant culture since all the support cells, especially the limbal stromal cells, are retained within the original niche. It has been shown that the mesenchymal markers and basement membrane components between the limbal and bone marrow mesenchymal cells are comparable [11]. Thus, endogenous stromal cells in the biopsied limbus function as intrinsic feeders to support the culture of LSCs without the need for external feeder cells [12]. (c) The feeder cells that are used for providing support are at present the U.S. Food and Drug Administration-approved mouse NIH3T3-J2 fibroblasts. Although mitotically inhibited and quite safe, there is still a chance for microchimerism, prion disease, and undetected viral transmission to the recipient from the use of animal-derived cells. This is not a concern with explant culture. Further improvements to the culture techniques, such as the use of autologous serum, human recombinant growth factors, and hAM as carrier substrate for transplanting the cells, have made the technique completely xeno-free [13].
Regardless of the culture technique, the ability to obtain enough cells for transplantation from a very small amount of starting material has made the treatment of LSCD far simpler, more convenient, and safer. It has also allowed repeat CLET and other relevant procedures to be performed in cases in which the first CLET failed, without adversely affecting the health of the donor eye [14], giving this technique an edge over keratolimbal transplantation. It has been reported that by doing a repeat CLET 3–12 months after the first CLET, a stable ocular surface could be generated in more than 66% of the failed cases [14]. Likewise, in cases in which corneal transplantation (penetrating keratoplasty) was needed after CLET to restore vision, a two-step process appears to have enabled survival in 80% ± 6% of patients (median survival: 4 years), with vision recovery of 20/40 or better in 71.4% of the eyes [15] when compared with performance of the procedure simultaneously with CLET.
Outcomes of CLET
There are several reports on the success of CLET in treating LSCD, and the rate varies from 45% to 100%. A detailed summary of the outcomes of CLET, updated in 2011, was published by Baylis et al. [16], and a summary of the controversies and challenges of this therapy, also updated in 2011, was published by O’Callaghan and Daniels [17]. The primary measure of success in treatment for LSCD is the clinical presence of a stable ocular surface with no superficial corneal vascularization, conjunctivalization, or repeat epithelial breakdown. Vision improvement is typically the secondary measure.
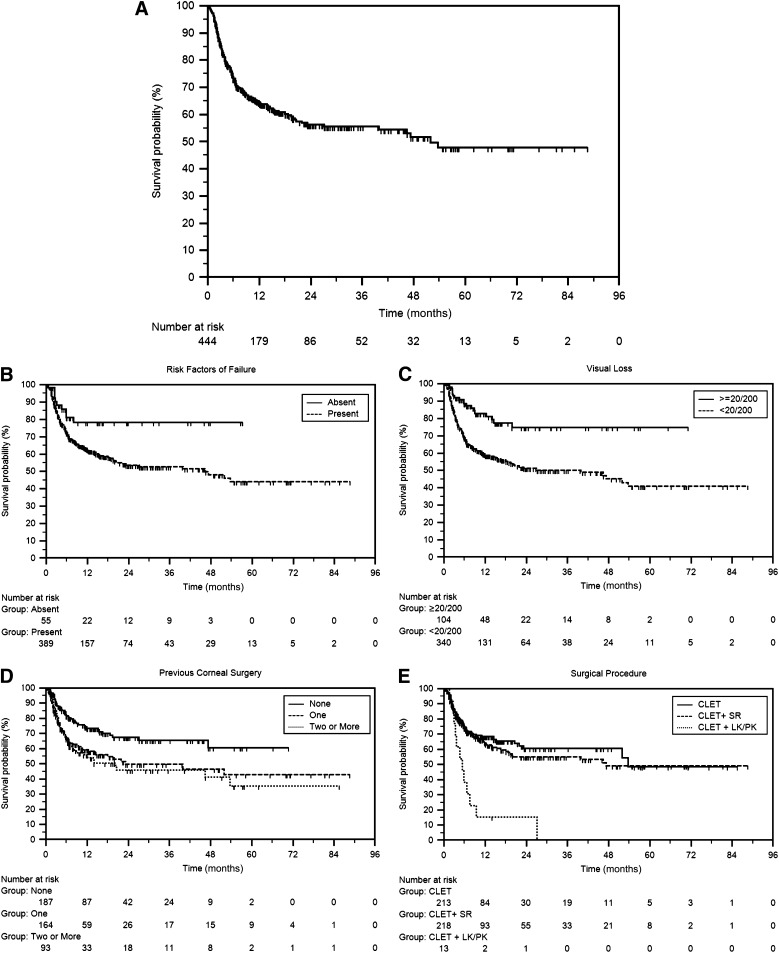
Our group has been performing CLET for more than a decade and probably has one of the largest patient databases, with more than 1,000 patients. Of these, we analyzed the outcomes of 444 patients that underwent autologous CLET for burn injuries. The median follow-up was 1.2 years, with a maximum follow-up of 7.2 years (Fig. 2A). CLET was successful in 279 patients (64.5%) with median survival of 4.3 years, and the majority of failures occurred within 8 months after surgery. When risk factors such as previous surgical interventions and combined procedures were removed from the equation, the outcome improved substantially to 80% (Fig. 2B). This is comparable to two previous reports, one from our group [18] and the other by Rama et al. [19], on selected cohorts that exhibited the lowest risk factors for failure. Looking at the individual impact of some of the risk factors on outcome success, it was found that risk of failure increased with an increase in the number of prior interventions in the form of corneal surgeries (Fig. 2E). The effect of combined surgeries (CLET plus keratoplasty) had by far the worst impact on outcome, with survival of 10% at the end of 1 year (Fig. 2E). Although this particular group has a very small sample size and the least follow-up, the findings are comparable to other previous reports. In contrast, symblepharon release (SR) did not have an adverse effect on the surgical outcome (CLET plus SR) (Fig. 2E). Interestingly, but not surprisingly, patients with vision of <20/200 before CLET had poor survival when compared with those with better vision (Fig. 2C). It could be argued that the disease condition is more aggressive in those with lower vision, resulting in poor surgical outcome. What has become evident from subgroup analysis and previous comparisons is that the surgery is likely to be 70% successful if the condition is unilateral, and thus permits autologous transplantation; if the cause of LSCD is burn; and if CLET is the only procedure required to restore vision.
Figure 2.
Kaplan-Meier survival curves of eyes that underwent autologous CLET. (A): Analysis of 444 eyes that underwent CLET for ocular burn-induced limbal stem cell deficiency. A successful outcome was noted in 279 (62.8%) of the 444 eyes. The survival probability of autologous CLET was 95.7% ± 0.01% at 42 days and 64.5% ± 0.02% at 1 year with median survival of 4.3 years. (B): Survival was significantly longer and more stable in eyes without risk factors for failure (p = .012). (C): Survival was greater in eyes with best corrected visual acuity of 20/200 or better (p = .003). (D): Survival was shorter in eyes with one corneal surgery or more prior to CLET (p = .0009). (E): Survival was shorter in eyes with simultaneous keratoplasty performed along with CLET (p = .0012). Abbreviations: CLET, cultivated limbal epithelial transplantation; LK/PK, lamellar keratoplasty or penetrating keratoplasty; SR, symblepharon release.
Another noteworthy finding from our analysis is that success of CLET in children younger than 15 years was much lower (45% of 107 subjects) than in adults (68% of 200 subjects) with similar injuries at 2 years after surgery [18, 20]. This appears to indicate that the protocols of treating adults may not apply to children. An important consideration is the vulnerability of this age group to developing deprivation amblyopia (lazy eye) and strabismus (misalignment of the two eyes) when the condition is long standing. One of the conundrums for the clinician when treating a juvenile patient with LSCD is when to commence treatment. Intervening too early (less than 4 months after injury) in the disease phase could increase the chance of failure, but late intervention could mean development of amblyopia and strabismus, resulting in poor visual prognosis despite a stable ocular surface. It is not clear what causes the poor outcome, but there is a clear need for more studies that focus specifically on this age group to determine an optimal treatment regimen and to understand the reason for the failures.
Simple Limbal Epithelial Transplantation
Although CLET has been of help to many suffering from corneal surface damage, the high cost involved in setting up a clinical-grade culture facility for the culture of these cells has effectively made this treatment expensive not only for patients but also for hospitals. Expenses incurred in CLET include clean-room facilities, nutrient medium, and trained personnel for the culture of the cells. Simple limbal epithelial transplantation (SLET) is a surgical technique that has been introduced to reduce the cost of treatment for LSCD without compromising the visual outcomes [21].
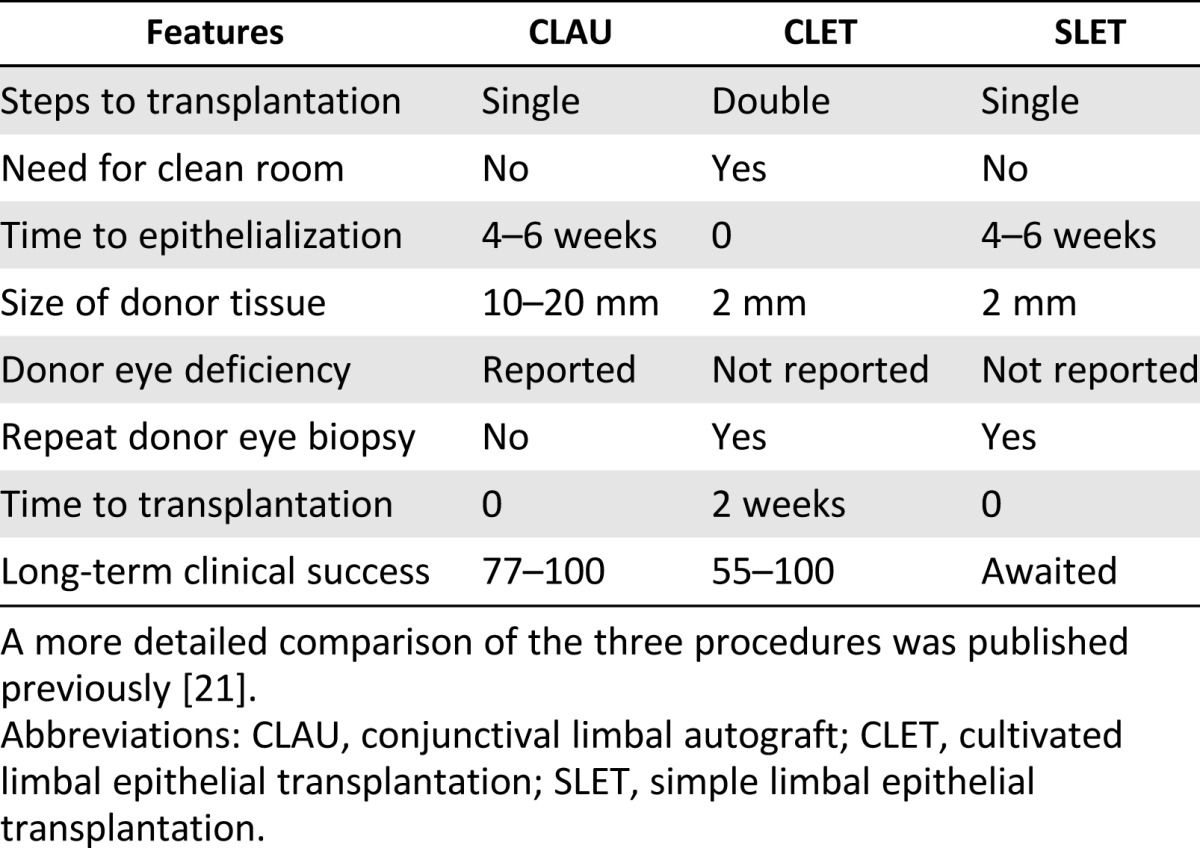
Both conjunctival-limbal autograft (CLAU) and CLET provide good surgical and visual outcomes; however, these techniques have certain inherent drawbacks. In CLAU, the main drawback is the possibility of inducing LSCD in the donor eye because at least 3 clock hours of tissue is taken for transplantation in the affected eye. Furthermore, delayed corneal epithelialization, prolonged ocular surface inflammation, and significantly greater scarring were reported with CLAU when compared with CLET. In CLET, the advantage is that the cells are expanded in the laboratory, using far less than 1 clock hour of tissue from the donor eye. The main drawback with this technique has been the high cost involved in culturing the cells and the patient wait time, which has restricted limbal stem cell transplantation to specialized centers across the globe. SLET was conceptualized as minimizing the drawbacks while maximizing the benefits of both CLET and CLAU (Table 1).
Table 1.
Summary comparison of various features of CLET, CLAU, and SLET
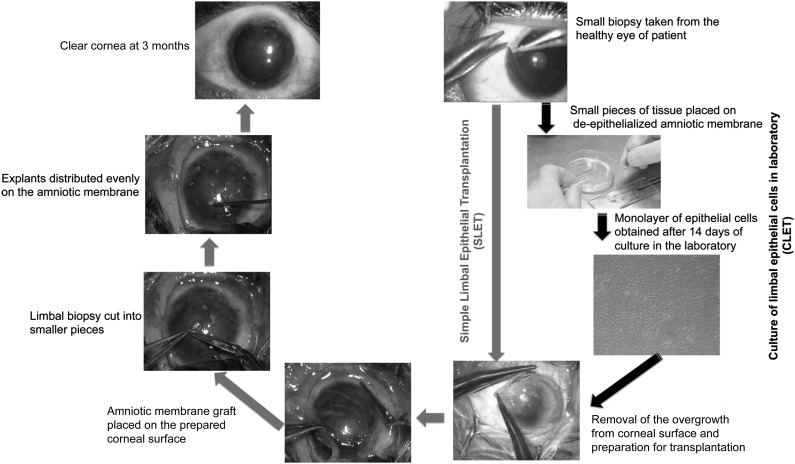
As shown in Figure 3, SLET is a one-step surgical procedure in which a limbal biopsy of 2 × 2 mm is obtained from the healthy eye, chopped into small pieces, stuck to the hAM using fibrin glue, and then placed on the prepared recipient cornea [21]. The growth of cells occurs in situ instead of in a laboratory. It is similar to CLET in that only a small biopsy is taken, but it differs in that the cost of the procedure and the patient wait time are dramatically reduced. In a clinical trial conducted on six patients, a stable, epithelialized, and avascular ocular surface was observed within 6 weeks after surgery. A 11-month follow-up of the patients showed restoration of a stable epithelial surface plus considerable improvement in the visual acuity of all patients and no complications [21]. This work was replicated by another group in four patients, and similar outcomes were found in terms of visual improvement and stable ocular surface regeneration [22]. The long-term success of this procedure in restoring ocular surface stability, once established, would simplify the treatment of LSCD and be easily accessible to surgeons and patients worldwide.
Figure 3.
Comparison of the surgical technique of CLET and SLET. Both techniques start with the same amount of limbal biopsy (top right) but differ in that SLET bypasses the need for the laboratory culture of limbal epithelial cells prior to transplantation, as indicated by the gray vertical arrow, whereas CLET goes through the clockwise steps of laboratory cultivation; both procedures lead to the same clear cornea (top left). Unlike CLET, cells are cultured in situ on the human amniotic membrane in SLET, without the need for special nutrients or incubators or a special cultivation laboratory. Abbreviations: CLET, cultivated limbal epithelial transplantation; SLET, simple limbal epithelial transplantation.
Problem Areas
The success with CLET has been encouraging and probably exemplary in regenerative medicine. However, a few basic questions must be addressed to further improve the success of this procedure. A pressing question is, what happens to the transplanted cells? Do they repopulate the limbal region to form a reserve, or do they remain dispersed on the corneal surface and differentiate within a period of time? The second major challenge is to develop an objective means of grading the severity of the disease and quantifying the minimum number of stem cells required for successful long-term restoration of the ocular surface. This would not only help the clinician predict the outcome for a given patient but also would allow us to compare data across studies and maybe devise a better strategy for improving success in cases with poor prognosis. Described below are some areas of LSCD treatment that continue to be challenging and the approaches being taken to address these challenges.
LSCD With Extensive Conjunctival Involvement: Coculturing Limbal and Conjunctival Epithelial Cells Together
In many cases of LSCD, there is additional involvement of conjunctiva that can be extensive enough to affect the long-term survival of the transplanted LECs. This occurs because the goblet cells of the conjunctiva secrete the mucin component of tears, essential for maintaining a smooth optical interface, and their loss in LSCD results in poor wettability of the ocular surface (i.e., dry eye). In such cases, although the patients exhibited all the clinical signs of LSCD, goblet cells were not detected on the conjunctivalized corneal surface, as confirmed with impression cytology [23]. Although the presence of goblet cells on the cornea is confirmatory of LSCD, its absence does not rule out the disease condition. In cases with such severe involvement of the ocular surface, it is common to first reconstruct the conjunctival surface by transplanting conjunctival grafts, similar to keratolimbal transplantation. This step is seen not only to reduce inflammation but also to improve the quality of tears for better survival of the transplanted limbal cells. When the loss is bilateral, autologous sources such as oral mucosa or nasal mucosa offer a reasonably good alternative for conjunctival reconstruction. These alternatives are comparable to the conjunctiva in their basic structure and function. Importantly, both alternatives secrete mucin and possess stem cells within their structures for continued maintenance of the transplanted cells. However, the major drawback is limited availability, which can be circumvented by transplanting cultured conjunctival, nasal, or oral mucosal cells, similar to CLET.
This two-step procedure for reconstructing the conjunctiva before LEC transplantation is cumbersome and twice as expensive for patients and surgeons. It has been possible to change this two-step procedure into a single step by simultaneously culturing both the limbal and conjunctival epithelial cells on the same hAM for transplantation [24, 25]; an artificial ring barrier is placed on the membrane, culturing the conjunctival cells outside and the LECs inside the ring. The effectiveness of the barrier in separating the two cell types was confirmed by marker-assisted characterization of the limbal and conjunctival cultures. When this cocultured material was transplanted on the affected ocular surface, the outcome was successful in 60% of patients at 1 year; however, this dropped to 45% beyond 4 years from intervention. One of the major risk factors for failure was any form of intervention during the acute phase of injury. More than 40% of the patients did not have recurrence of symblepharon. This novel procedure appears to reconstruct surfaces (lids, conjunctiva, and cornea) in a single step, reducing the cost of the procedure and the number of visits to the clinic by the patient.
A more severe form of mucosal involvement is Stevens-Johnson syndrome, which has a systemic component underlying the ocular manifestation of LSCD. Decreased tear secretion, squamous metaplasia of conjunctival epithelium, and lid margin keratinization adversely affect the outcome of limbal epithelial transplantation [26]. In these patients, the chances of performing autograft transplantation is minimal when compared with unilateral LSCD caused by chemical or thermal burns because the disease affects the quality of cells in both eyes. Even when transplanted, the chances of cells surviving in the hostile environment appear to be minimal. A recent analysis by Shortt et al. [27] of the 3-year outcome of allogeneic ex vivo CLET in patients with bilateral total LSCD secondary to aniridia and Stevens-Johnson syndrome, using the Clinical Outcome Assessment in Surgical Trials (COASTL) tool, showed that although LSCD decreased and visual acuity increased up to 12 months after treatment, progressive deterioration occurred thereafter. Whether a staggered approach for constructing a stable ocular surface before limbal stem cell transplantation will help in this disease remains to be seen.
Bilateral LSCD
Bilateral LSCD can be more visually devastating than unilateral disease and offers a bigger challenge for treatment. This is because in most cases there is very little or no healthy limbus left following injury with which to perform CLET or SLET. In cases with some healthy tissue available in at least one eye, CLET can be used to expand the patient’s own cells for transplantation [24]. Alternatively, a small biopsy from a living relative could be used for culturing the cells [28]. The reported outcomes for this procedure have been quite successful, with 60% of patients maintaining a stable ocular surface beyond 3 years of surgery. However, the risk of rejection remains high in these patients, and long-term immunosuppression is a major drawback (both topical and systemic immunosuppressants were used in this study). Alternative sources for deriving autologous cells have been explored, and oral mucosa is one of the most widely studied options.
Oral Mucosa
Oral mucosa exhibits structural similarity to other stratified epithelia in that it consists of several layers of cells that become progressively differentiated, marked by the expression of specific cytokeratins and loss of organelles, as they reach the tissue surface. What makes these cells suitable for use in ocular surface reconstruction is that they are less differentiated than epidermal keratinocytes, need less time to grow in culture, and do not undergo keratinization when maintained in culture; in addition, scarring of the biopsy location is inconspicuous [29, 30], and, most important, they are devoid of secondary structures such as hair follicles and sweat glands. Transplantation of sheets of oral epithelium onto the abraded corneas of rabbits was shown to generate a clear ocular surface [31]. Subsequently, several groups have transplanted cultured oral mucosa cells to the ocular surface in humans [32–35]. In a recent paper, the authors showed good visual outcome in 48% of 15 patients who underwent transplantation of cultured oral mucosa cells for bilateral LSCD with a median follow-up of 24 months [36]. A substantial number of these patients either had Stevens-Johnson syndrome or ocular cicatricial pemphigoid, conditions that afflict mucosal linings of the body, suggesting that this could be an alternate autologous option for treating LSCD, provided the oral mucosa remains unaffected.
Although the short-term effectiveness of oral mucosa epithelial cells in replenishing the ocular surface and relieving symptoms has been promising, long-term success has been poorer due to persistent epithelial defects and the tendency to develop varying degrees of corneal vascularization with time. This is in contrast to transplanting LECs that result in the regression of corneal blood vessels developed during the wound-healing phase. Studies to understand the molecular basis for developing corneal vascularization have shown that specific antiangiogenic factors produced by the corneal epithelium are lacking in both the conjunctiva and oral mucosal cells [37]. These factors (soluble FLT1, TIMP3, and TSP1) are expressed in the normal cornea but not in the normal oral mucosa or conjunctiva. More pertinent is the finding that the expression of these factors, in addition to Pax6 and keratin 12, is absent in the transplanted oral mucosal cells, suggesting that, at best, these cells undergo a partial transformation to the ocular phenotype [38].
Studies performed both in vitro and in vivo have shown that the intercellular barrier formed by oral mucosal cells is leaky when compared with corneal epithelial cells and is probably attributable to a fundamental difference in the distribution of junction proteins between the two tissue types. In addition, the stratification of oral mucosal epithelial cells is nonuniform, forming 4–5 layers in some regions and 10 layers in other regions, resulting in an uneven corneal surface that may contribute to poor visual outcomes [39]. The most pertinent question regards the long-term survival of the oral mucosal epithelial stem cell population required for sustaining the regenerated ocular surface. Based on marker expression, their slow-cycling nature, and clonogenic potential, the epithelial stem cells of oral mucosa are thought to be located in the basal layer of the mucosal tissue. Unlike the limbus, there does not appear to be a specific niche structure within which the stem cells are located; instead, they are distributed throughout the tissue. Studies have demonstrated the persistence of oral mucosal cells even after 2 years of transplantation in some patients, suggesting that the cells are capable of adapting to the ocular environment and replenishing the ocular surface [40]. It is unclear at this point whether these cells can eventually home to the limbal niche or whether they can survive for much longer on the ocular surface, given the differences of the ocular and oral environments.
Other Cells as Substitutes
Besides oral mucosal cells, some other sources of cells are being tested for their potential to regenerate the ocular surface. Some of these are human embryonic stem cells, skin epidermal stem cells, hair follicle stem cells, bone marrow-derived mesenchymal stem cells, immature dental pulp stem cells, and induced pluripotent stem (iPS) cells [41–45]. Ahmad et al. [41] have shown that human embryonic stem cells can be induced to take up a corneal epithelial-like phenotype using limbal fibroblast-conditioned medium and have suggested in vivo animal studies to check their suitability and efficiency. Others have attempted to derive corneal epithelial-like cells from rhesus monkey skin epidermal stem cells, which, when cocultured along with human limbal stroma and corneal epithelial cells, express cytokeratins K3, K12, and K15 and β1-integrin typical of the latter cells [46]. Similarly, coculturing hair follicle stem cells with limbal stromal fibroblasts resulted in the generation of corneal epithelium-like cells [42]. Human bone marrow mesenchymal stem cells have been able to transdifferentiate with some success into corneal epithelial lineage using limbal medium. Cells derived this way were cultured on hAM and transplanted in nude rats with corneas that were alkali injured. Corneal healing was observed after 8 weeks [45]. Similarly, a Brazilian group has used immature dental pulp stem cells and cultured them on a temperature-responsive cell culture dish using a slightly modified Dulbecco's modified Eagle’s medium: Nutrient Mixture F-12 to generate a cell sheet. This was then transplanted in rabbits with corneas that were alkali burned. The treated rabbits were found to have a well-defined stromal layer and stratified epithelium [44]. Hayashi et al. [43] demonstrated that iPS cell lines derived from the corneal limbal epithelium and dermal fibroblasts can be induced to differentiate into the corneal epithelial cells. An interesting finding of this study was the higher propensity for the corneal epithelium-derived iPS cells to give rise to differentiated corneal epithelium compared with the dermal fibroblast-derived iPS cells. It would be interesting to see whether any component of epigenomic memory of the original cells is retained, even after reprogramming. In addition, it would be valuable to do animal experiments and then attempt human trials in cases with total bilateral LSCD.
Substrates Used for Limbal Stem Cell Transplantation
Two important considerations are relevant for regenerative medicine to be successful. The first is the technique used for generating the tissue or organ and the second is the substrate material used for transplantation. The latter is an important consideration, especially for stem cells, because the interaction between the cells and the substrate can define the differentiation characteristics of the stem cells. In the treatment of LSCD, the use of hAM, fibrin, and postmitotic feeder cells is widely accepted because these substrates support the expansion and maintenance of the LSCs in culture. When compared with the fibrin and postmitotic feeder cells, the advantages with hAM are its anti-inflammatory and antimicrobial properties in addition to low immunogenicity. hAM helps reduce inflammation by suppressing the expression of inflammatory cytokines such as interleukin-1α (IL-1α), IL-2, IL-8, interferon-γ, tumor necrosis factor-β, basic fibroblast growth factor, and platelet-derived growth factor, thereby pacifying the inflamed ocular surface and preparing it to receive the transplanted cells [47, 48].
A few drawbacks have been cited with the use of hAM, the most important being the high cost involved in the testing for pathogens, preparation of the tissue, storage, and the need for a clean room facility. This is true even for the use of feeder cells, for which there is an additional risk of transmitting unknown pathogens from the use of animal cells. Although no reported evidence shows the transmission of pathogens from either of these sources, it is a concern that has warranted more research.
Several alternatives to replace hAM and feeder cells in the culture of limbal cells have been tested and compared with the gold standard of hAM. Some of these include thermosensitive substrates, recombinant collagen III scaffolds, lens capsule, surface-treated contact lenses, Mebio-1 gel, and synthetic polymer scaffolds [49–56]. The challenge is to develop a product that is cost-effective and easily available and that should not only support the stem cell population sufficiently but also allow cells to be transferred to the ocular surface, not produce any toxic breakdown products, lend to bulk production, and, most important, be safe for use in humans. Most of these alternatives seem to provide good support for the cells to grow, specifically, the stem cell population. A few of them have been tested in animals, and when applied to the ocular surface, the carrier materials allow for the cultured cells to be transferred without eliciting any inflammatory or tissue toxic reactions. Oie and Nishida [57] described a cell transportation device, using a temperature-responsive set of culture dishes, that allows the transport of cultured epithelial cell sheets to multiple clinics. A comprehensive review by Feng et al. [53] describes the various materials that have been studied so far and compares their advantages and disadvantages.
More recently, synthetic polymer scaffolds made of polylactic and glycolic acids (PLGA), components of dissolvable sutures [55], and plasma polymer-coated contact lenses [56] are being developed as potential transfer materials for LECs. These materials are biocompatible, provide sufficient support for the LSCs, and allow the transfer of the cultured cells to the ocular surface (albeit to a lesser extent from the contact lenses). The PLGA scaffolds, unlike collagen or contact lenses, are translucent but break down within 4–6 weeks, leaving behind a clear ocular surface. Each of these materials has advantages and a few disadvantages when compared with hAM; therefore, hAM remains the substrate of choice for now.
With our knowledge of the niche for housing the LSCs, the scaffolds being developed for the limbal cells are also taking on a more complicated appearance [58]. These three-dimensional structures are created to mimic the limbal niche so as to provide the cells with an environment that is closer to home, thereby providing long-term support for the stem cell population. Although we are concentrating on replicating the structure of the limbal niche, it becomes more important to understand the functional significance of its various cellular components. As we acquire more knowledge about the structural and functional composition of the limbus, we might even be able to create a whole new limbus in the laboratory for transplantation.
Conclusion
It is clear that in the 17 years since making its debut, CLET has turned out to be an increasingly practiced and successful example of stem cell therapy and is becoming regarded as the standard of care in several places for repair of corneal damage, just as the use of bone marrow-derived hematopoietic stem cells is for hematological disorders. Besides the above-cited examples, reports of corneal epithelial cell culture and CLET have come from various countries across the globe, including Brazil [59], Iran [60], India [61], Thailand [62], and Malaysia [45] (just to name those published in the public domain). The ease with which CLET can be successfully simplified and used as SLET makes this form of stem cell therapy available to qualified corneal surgeons in centers and countries without the availability of or, indeed, the need for laboratory culture facilities. However, it is also clear that the success rate needs to improve from the present 55%–70%, and ways to do that present the challenges that need to be addressed.
Acknowledgments
We thank Drs. Vivek Singh and Sachin Shukla for helpful comments. This work was supported by competitive research grants from the Department of Biotechnology, India (BT/CoE/06/02/10), Wellcome Trust, U.K. (Grant 091128), and the Champalimaud Foundation, Portugal (C-TRACER-1, 2008-2013).
Author Contributions
C.R., S.B., and V.S.S.: conception and design, manuscript writing, final approval of manuscript; D.B.: data analysis, manuscript writing, financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24:1442–1443. [PubMed] [Google Scholar]
- 2.Pellegrini G, Golisano O, Paterna P, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145:769–782. doi: 10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanmuganathan VA, Foster T, Kulkarni BB, et al. Morphological characteristics of the limbal epithelial crypt. Br J Ophthalmol. 2007;91:514–519. doi: 10.1136/bjo.2006.102640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shortt AJ, Secker GA, Munro PM, et al. Characterization of the limbal epithelial stem cell niche: Novel imaging techniques permit in vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells. 2007;25:1402–1409. doi: 10.1634/stemcells.2006-0580. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura T, Kinoshita S. New hopes and strategies for the treatment of severe ocular surface disease. Curr Opin Ophthalmol. 2011;22:274–278. doi: 10.1097/ICU.0b013e3283477d4d. [DOI] [PubMed] [Google Scholar]
- 6.Sridhar MS, Bansal AK, Sangwan VS, et al. Amniotic membrane transplantation in acute chemical and thermal injury. Am J Ophthalmol. 2000;130:134–137. doi: 10.1016/s0002-9394(00)00500-6. [DOI] [PubMed] [Google Scholar]
- 7.Nishiwaki-Dantas MC, Dantas PE, Reggi JR. Ipsilateral limbal translocation for treatment of partial limbal deficiency secondary to ocular alkali burn. Br J Ophthalmol. 2001;85:1031–1033. doi: 10.1136/bjo.85.9.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thoft RA. Keratoepithelioplasty. Am J Ophthalmol. 1984;97:1–6. doi: 10.1016/0002-9394(84)90438-0. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad S. Concise review: Limbal stem cell deficiency, dysfunction, and distress. Stem Cells Translational Medicine. 2012;1:110–115. doi: 10.5966/sctm.2011-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 11.Polisetty N, Fatima A, Madhira SL, et al. Mesenchymal cells from limbal stroma of human eye. Mol Vis. 2008;14:431–442. [PMC free article] [PubMed] [Google Scholar]
- 12.González S, Deng SX. Presence of native limbal stromal cells increases the expansion efficiency of limbal stem/progenitor cells in culture. Exp Eye Res. 2013;116:169–176. doi: 10.1016/j.exer.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mariappan I, Maddileti S, Savy S, et al. In vitro culture and expansion of human limbal epithelial cells. Nat Protoc. 2010;5:1470–1479. doi: 10.1038/nprot.2010.115. [DOI] [PubMed] [Google Scholar]
- 14.Basu S, Ali H, Sangwan VS. Clinical outcomes of repeat autologous cultivated limbal epithelial transplantation for ocular surface burns. Am J Ophthalmol. 2012;153:643–650, 650.e1–650.e2. doi: 10.1016/j.ajo.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Basu S, Mohamed A, Chaurasia S, et al. Clinical outcomes of penetrating keratoplasty after autologous cultivated limbal epithelial transplantation for ocular surface burns. Am J Ophthalmol. 2011;152:917.e1–924.e1. doi: 10.1016/j.ajo.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Baylis O, Figueiredo F, Henein C, et al. 13 years of cultured limbal epithelial cell therapy: A review of the outcomes. J Cell Biochem. 2011;112:993–1002. doi: 10.1002/jcb.23028. [DOI] [PubMed] [Google Scholar]
- 17.O’Callaghan AR, Daniels JT. Concise review: Limbal epithelial stem cell therapy: Controversies and challenges. Stem Cells. 2011;29:1923–1932. doi: 10.1002/stem.756. [DOI] [PubMed] [Google Scholar]
- 18.Sangwan VS, Basu S, Vemuganti GK, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: A 10-year study. Br J Ophthalmol. 2011;95:1525–1529. doi: 10.1136/bjophthalmol-2011-300352. [DOI] [PubMed] [Google Scholar]
- 19.Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 20.Sejpal K, Ali MH, Maddileti S, et al. Cultivated limbal epithelial transplantation in children with ocular surface burns. JAMA Ophthalmol. 2013;131:731–736. doi: 10.1001/jamaophthalmol.2013.2308. [DOI] [PubMed] [Google Scholar]
- 21.Sangwan VS, Basu S, MacNeil S, et al. Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:931–934. doi: 10.1136/bjophthalmol-2011-301164. [DOI] [PubMed] [Google Scholar]
- 22.Amescua G, Atallah M, Nikpoor N, et al. Modified simple limbal epithelial transplantation using cryopreserved amniotic membrane for unilateral limbal stem cell deficiency. Am J Ophthalmol. 2014;158:469.e2–475.e2. doi: 10.1016/j.ajo.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Fatima A, Iftekhar G, Sangwan VS, et al. Ocular surface changes in limbal stem cell deficiency caused by chemical injury: A histologic study of excised pannus from recipients of cultured corneal epithelium. Eye (Lond) 2008;22:1161–1167. doi: 10.1038/sj.eye.6702895. [DOI] [PubMed] [Google Scholar]
- 24.Sangwan VS, Vemuganti GK, Iftekhar G, et al. Use of autologous cultured limbal and conjunctival epithelium in a patient with severe bilateral ocular surface disease induced by acid injury: A case report of unique application. Cornea. 2003;22:478–481. doi: 10.1097/00003226-200307000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Subramaniam SV, Sejpal K, Fatima A, et al. Coculture of autologous limbal and conjunctival epithelial cells to treat severe ocular surface disorders: Long-term survival analysis. Indian J Ophthalmol. 2013;61:202–207. doi: 10.4103/0301-4738.99840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimazaki J, Higa K, Morito F, et al. Factors influencing outcomes in cultivated limbal epithelial transplantation for chronic cicatricial ocular surface disorders. Am J Ophthalmol. 2007;143:945–953. doi: 10.1016/j.ajo.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Shortt AJ, Bunce C, Levis HJ, et al. Three-year outcomes of cultured limbal epithelial allografts in aniridia and Stevens-Johnson syndrome evaluated using the Clinical Outcome Assessment in Surgical Trials assessment tool. Stem Cells Translational Medicine. 2014;3:265–275. doi: 10.5966/sctm.2013-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basu S, Fernandez MM, Das S, et al. Clinical outcomes of xeno-free allogeneic cultivated limbal epithelial transplantation for bilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:1504–1509. doi: 10.1136/bjophthalmol-2012-301869. [DOI] [PubMed] [Google Scholar]
- 29.Hata K, Kagami H, Ueda M, et al. The characteristics of cultured mucosal cell sheet as a material for grafting; comparison with cultured epidermal cell sheet. Ann Plast Surg. 1995;34:530–538. doi: 10.1097/00000637-199505000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Juhl M, Reibel J, Stoltze K. Immunohistochemical distribution of keratin proteins in clinically healthy human gingival epithelia. Scand J Dent Res. 1989;97:159–170. doi: 10.1111/j.1600-0722.1989.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura T, Endo K, Cooper LJ, et al. The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci. 2003;44:106–116. doi: 10.1167/iovs.02-0195. [DOI] [PubMed] [Google Scholar]
- 32.Burillon C, Huot L, Justin V, et al. Cultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency. Invest Ophthalmol Vis Sci. 2012;53:1325–1331. doi: 10.1167/iovs.11-7744. [DOI] [PubMed] [Google Scholar]
- 33.Gaddipati S, Muralidhar R, Sangwan VS, et al. Oral epithelial cells transplanted on to corneal surface tend to adapt to the ocular phenotype. Indian J Ophthalmol. 2014;62:644–648. doi: 10.4103/0301-4738.109517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura T, Takeda K, Inatomi T, et al. Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br J Ophthalmol. 2011;95:942–946. doi: 10.1136/bjo.2010.188714. [DOI] [PubMed] [Google Scholar]
- 35.Priya CG, Arpitha P, Vaishali S, et al. Adult human buccal epithelial stem cells: Identification, ex-vivo expansion, and transplantation for corneal surface reconstruction. Eye (Lond) 2011;25:1641–1649. doi: 10.1038/eye.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sotozono C, Inatomi T, Nakamura T, et al. Visual improvement after cultivated oral mucosal epithelial transplantation. Ophthalmology. 2013;120:193–200. doi: 10.1016/j.ophtha.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 37.Chen HC, Yeh LK, Tsai YJ, et al. Expression of angiogenesis-related factors in human corneas after cultivated oral mucosal epithelial transplantation. Invest Ophthalmol Vis Sci. 2012;53:5615–5623. doi: 10.1167/iovs.11-9293. [DOI] [PubMed] [Google Scholar]
- 38.Madhira SL, Vemuganti G, Bhaduri A, et al. Culture and characterization of oral mucosal epithelial cells on human amniotic membrane for ocular surface reconstruction. Mol Vis. 2008;14:189–196. [PMC free article] [PubMed] [Google Scholar]
- 39.Shimazaki J, Higa K, Kato N, et al. Barrier function of cultivated limbal and oral mucosal epithelial cell sheets. Invest Ophthalmol Vis Sci. 2009;50:5672–5680. doi: 10.1167/iovs.09-3820. [DOI] [PubMed] [Google Scholar]
- 40.Satake Y, Dogru M, Yamane GY, et al. Barrier function and cytologic features of the ocular surface epithelium after autologous cultivated oral mucosal epithelial transplantation. Arch Ophthalmol. 2008;126:23–28. doi: 10.1001/archopht.126.1.23. [DOI] [PubMed] [Google Scholar]
- 41.Ahmad S, Stewart R, Yung S, et al. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells. 2007;25:1145–1155. doi: 10.1634/stemcells.2006-0516. [DOI] [PubMed] [Google Scholar]
- 42.Blazejewska EA, Schlötzer-Schrehardt U, Zenkel M, et al. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells. 2009;27:642–652. doi: 10.1634/stemcells.2008-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayashi R, Ishikawa Y, Ito M, et al. Generation of corneal epithelial cells from induced pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium. PLoS One. 2012;7:e45435. doi: 10.1371/journal.pone.0045435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monteiro BG, Serafim RC, Melo GB, et al. Human immature dental pulp stem cells share key characteristic features with limbal stem cells. Cell Prolif. 2009;42:587–594. doi: 10.1111/j.1365-2184.2009.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rohaina CM, Then KY, Ng AM, et al. Reconstruction of limbal stem cell deficient corneal surface with induced human bone marrow mesenchymal stem cells on amniotic membrane. Transl Res. 2014;163:200–210. doi: 10.1016/j.trsl.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Gao N, Wang Z, Huang B, et al. Putative epidermal stem cell convert into corneal epithelium-like cell under corneal tissue in vitro. Sci China C Life Sci. 2007;50:101–110. doi: 10.1007/s11427-007-0006-4. [DOI] [PubMed] [Google Scholar]
- 47.Fernandes M, Sridhar MS, Sangwan VS, et al. Amniotic membrane transplantation for ocular surface reconstruction. Cornea. 2005;24:643–653. doi: 10.1097/01.ico.0000151501.80952.c5. [DOI] [PubMed] [Google Scholar]
- 48.Sangwan VS, Basu S. Antimicrobial properties of amniotic membrane. Br J Ophthalmol. 2011;95:1–2. doi: 10.1136/bjo.2010.184259. [DOI] [PubMed] [Google Scholar]
- 49.Nishida K, Yamato M, Hayashida Y, et al. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation. 2004;77:379–385. doi: 10.1097/01.TP.0000110320.45678.30. [DOI] [PubMed] [Google Scholar]
- 50.Nithya J, Kumar PR, Tilak P, et al. Intelligent thermoresponsive substrate from modified overhead projection sheet as a tool for construction and support of cell sheets in vitro. Tissue Eng Part C Methods. 2011;17:181–191. doi: 10.1089/ten.TEC.2009.0783. [DOI] [PubMed] [Google Scholar]
- 51.Albert R, Veréb Z, Csomós K, et al. Cultivation and characterization of cornea limbal epithelial stem cells on lens capsule in animal material-free medium. PLoS One. 2012;7:e47187. doi: 10.1371/journal.pone.0047187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dravida S, Gaddipati S, Griffith M, et al. A biomimetic scaffold for culturing limbal stem cells: A promising alternative for clinical transplantation. J Tissue Eng Regen Med. 2008;2:263–271. doi: 10.1002/term.91. [DOI] [PubMed] [Google Scholar]
- 53.Feng Y, Borrelli M, Reichl S, et al. Review of alternative carrier materials for ocular surface reconstruction. Curr Eye Res. 2014;39:541–552. doi: 10.3109/02713683.2013.853803. [DOI] [PubMed] [Google Scholar]
- 54.Deshpande P, Notara M, Bullett N, et al. Development of a surface-modified contact lens for the transfer of cultured limbal epithelial cells to the cornea for ocular surface diseases. Tissue Eng Part A. 2009;15:2889–2902. doi: 10.1089/ten.tea.2008.0528. [DOI] [PubMed] [Google Scholar]
- 55.Deshpande P, Ramachandran C, Sefat F, et al. Simplifying corneal surface regeneration using a biodegradable synthetic membrane and limbal tissue explants. Biomaterials. 2013;34:5088–5106. doi: 10.1016/j.biomaterials.2013.03.064. [DOI] [PubMed] [Google Scholar]
- 56.Brown KD, Low S, Mariappan I, et al. Plasma polymer-coated contact lenses for the culture and transfer of corneal epithelial cells in the treatment of limbal stem cell deficiency. Tissue Eng Part A. 2014;20:646–655. doi: 10.1089/ten.tea.2013.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oie Y, Nishida K. Regenerative medicine for the cornea. Biomed Res Int. 2013:428247. doi: 10.1155/2013/428247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ortega Í, Deshpande P, Gill AA, et al. Development of a microfabricated artificial limbus with micropockets for cell delivery to the cornea. Biofabrication. 2013;5:025008. doi: 10.1088/1758-5082/5/2/025008. [DOI] [PubMed] [Google Scholar]
- 59.Ricardo JR, Cristovam PC, Filho PA, et al. Transplantation of conjunctival epithelial cells cultivated ex vivo in patients with total limbal stem cell deficiency. Cornea. 2013;32:221–228. doi: 10.1097/ICO.0b013e31825034be. [DOI] [PubMed] [Google Scholar]
- 60.Baradaran-Rafii A, Ebrahimi M, Kanavi MR, et al. Midterm outcomes of autologous cultivated limbal stem cell transplantation with or without penetrating keratoplasty. Cornea. 2010;29:502–509. doi: 10.1097/ICO.0b013e3181bd9f60. [DOI] [PubMed] [Google Scholar]
- 61.Sharma S, Tandon R, Mohanty S, et al. Culture of corneal limbal epithelial stem cells: Experience from benchtop to bedside in a tertiary care hospital in India. Cornea. 2011;30:1223–1232. doi: 10.1097/ICO.0b013e3181dc81f1. [DOI] [PubMed] [Google Scholar]
- 62.Prabhasawat P, Ekpo P, Uiprasertkul M, et al. Efficacy of cultivated corneal epithelial stem cells for ocular surface reconstruction. Clin Ophthalmol. 2012;6:1483–1492. doi: 10.2147/OPTH.S33951. [DOI] [PMC free article] [PubMed] [Google Scholar]