Using induced pluripotent stem cell technology to alter the epigenetic status of differentiated cells, a recent study revealed that some types of cancer can develop through disruption of the epigenetic status triggered by dedifferentiation. This review addresses the epigenetic mechanism associated with maintenance or conversion of cell identity and introduces the system responsible for in vivo reprogramming to demonstrate the involvement of dedifferentiation-driven epigenetic disruption in cancer development.
Keywords: Induced pluripotent stem cells, Dedifferentiation, In vivo reprogramming, Epigenetic cancer, Wilms’ tumor
Abstract
The technology for generation of induced pluripotent stem cells (iPSCs) has made significant contributions to various scientific fields, and the field of cancer biology is no exception. Although cancer is generally believed to develop through accumulation of multiple genetic mutations, there is increasing evidence that cancer cells also acquire epigenetic abnormalities during development, maintenance, and progression. Because the epigenetic status of somatic cells changes dynamically through reprogramming, iPSC technology can be utilized to actively and globally alter the epigenetic status of differentiated cells. Using this technology, a recent study has revealed that some types of cancer can develop mainly through disruption of the epigenetic status triggered by dedifferentiation. In this paper, we outline the reprograming process and the epigenetic mechanism associated with the maintenance or conversion of cell identity. We then describe several observations suggesting that dedifferentiation can play an important role in cancer development. Finally, we introduce the system responsible for in vivo reprogramming to demonstrate the involvement of dedifferentiation-driven epigenetic disruption in cancer development, and propose that particular types of cancer can develop predominantly through epigenetic alterations.
Introduction
For a long time it has been believed that once a cell differentiates into a particular cell type that has a distinctive function in the human body, it permanently loses the potential for diverse functions and stably maintains its identity. This unidirectional developmental process is frequently compared with a ball rolling down a hill into a landscape with peaks and valleys. The pluripotent stem cell is located at the top of the hill, and each differentiated cell state lies at the bottom of each distinct valley. Once development proceeds, cells are unable to climb back to the top of the hill and become stem cells again, and they cannot cross the ridges separating the individual valleys and transform into other differentiated cell types (Fig. 1). We now know that this dogma is not necessarily valid and that cells can be altered by artificial reprogramming technology. The pioneering studies that demonstrated this involved the nuclear transfer technique, which showed that a somatic nucleus can be reprogrammed to have pluripotency [1, 2]. It is also known that expression of a transcription factor, MyoD, induces myogenic differentiation of most cell types [3]. The decisive breakthrough came when Takahashi and Yamanaka succeeded in generating induced pluripotent stem cells (iPSCs), which are able to differentiate into any of the body’s cell lineages [4, 5]. In the context of previous studies, they hypothesized that the factors playing important roles in the maintenance of embryonic stem cell (ESC) identity also play a critical role in the induction of pluripotency in somatic cells. As was expected, forced induction of four transcriptional factors—Oct3/4, Sox2, Klf4, and c-Myc, all of which are highly expressed and contribute to maintenance of ESC identity—resulted in generation of iPSCs. This iPSC-generation technique also opened the door to the direct reprogramming of specialized cells into other specialized types of cells [6–8]. As is the case in MyoD-induced transdifferentiation, direct reprogramming is basically achieved by inducing the transcription factors that govern the transcriptional network of the target cell lineage. Collectively, it is suggested that cell identity is controlled, at least in part, by a stable transcriptional network unique to the individual cell type and that, therefore, forced expression of transcription factors that activate the transcriptional network of the resulting cell type induce cell fate conversion (Fig. 1). A study demonstrating that expression of master transcription factors involving maintenance of cell identity interferes with reprogramming toward pluripotent stem cells supports this idea [9]. It is also important to note that the transcriptional network is closely linked with epigenetic modifications, suggesting that a stable transcriptional network in a particular cell type is maintained by epigenetic regulation [10].
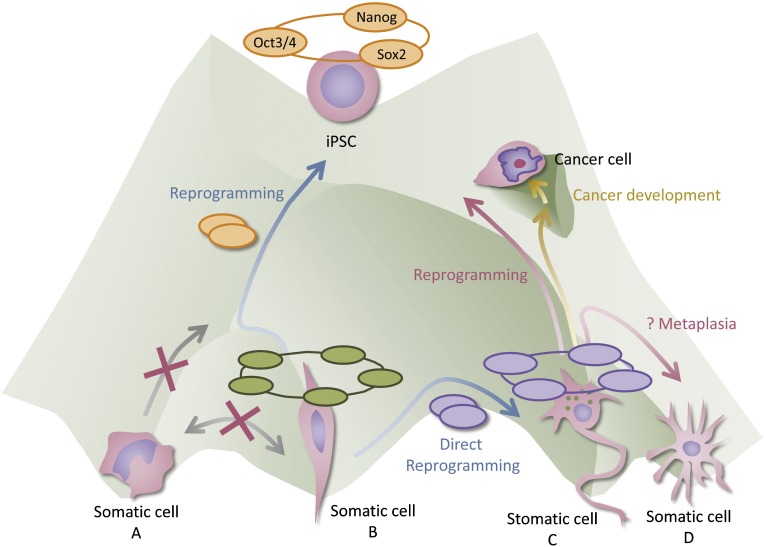
Figure 1.
Dedifferentiation-driven cancer development in an epigenetic landscape. As a general rule, the developmental process is unidirectional and irreversible, like a ball rolling downhill (gray arrows). It is suggested that maintenance of cell identity is governed by cell type-specific transcriptional networks (halos over each cell type), accompanied by epigenetic regulation. Forced expression of the transcriptional factors (orange and violet ellipsoids) that activate the network of the target cells can induce lineage conversion, namely, reprogramming or direct reprogramming (blue arrows). External stimuli (e.g., infection, inflammation) and/or intrinsic plasticity might lead to dedifferentiation of mature cells as a natural phenomenon (red arrows). On the way toward pluripotency (by transcription factors or as a natural phenomenon), there may be a “hollow” near the path, leading to cancer development. Some partially reprogrammed cells that may already possess some cancer-like properties can fall to the hollow and expand as cancer cells (gold arrows). Abbreviation: iPSC, induced pluripotent stem cell.
Epigenetic Regulation During Cell Identity Conversion
Epigenetics is defined as meiotically and mitotically inherited regulation of gene expression that is not accompanied by alteration of the DNA sequence. DNA methylation and histone modifications, both of which are major epigenetic modifications, also play critical roles in normal development. During the reprogramming process, dynamic alteration of epigenetic modifications can be observed, whereas the underlying DNA sequence remains unchanged. Consequently, epigenetic regulation during cell-fate conversion has been vigorously studied in the context of induced pluripotency. Although the road map has not been fully clarified, a number of detailed investigations have partly unraveled the processes operating during reprogramming [11–13]. Polo et al. have indicated that two epigenetic waves occur during reprogramming. In the first wave, which is driven by c-Myc or Klf4, an active mark, histone H3 lysine 4 (H3K4) trimethylation, becomes enriched at promoters for pluripotency-related genes. Gradual enrichment of H3K27 trimethylation then occurs at the same locus (bivalent domain, a characteristic mark of pluripotent cells) [12]. Finally, changes in DNA methylation take place after the second wave (Oct4-, Sox2-, or Klf4-driven wave), together with the altered histone modifications. Conversely, functional analyses have revealed that failure of epigenetic alteration can hamper the reprogramming process. It has been shown, for example, that failure to remove H3K9 methylation is one of the barriers preventing complete reprogramming into iPSCs [14].
As in the case of cell-fate conversion, evidence suggests that epigenetic regulation plays a key role in cancer initiation and progression. In the following section, we describe how the abnormality of epigenetic regulation occupies an important place in cancer development.
Epigenetic Abnormality During Cancer Initiation and Progression
Previous studies using the reverse genetics approach have revealed that cancer is caused by genetic mutations. Specifically, genetically modified rodents with mutations in oncogenes or tumor suppressor genes develop cancers, and this is a powerful tool for demonstrating that a mutation is functionally involved in cancer development in vivo. Mutation of the APC gene, for example, is known to be the first event in multistep carcinogenesis in the colon [15], and a causal relationship between APC gene mutation and colon cancer development has been established from the fact that Apc-mutant mice develop multiple intestinal tumors [16].
In contrast, accumulated evidence suggests that most cancer cells harbor alterations in epigenetic modifications, in addition to genetic mutations. Alteration of the DNA methylation pattern is the type of abnormality that has been analyzed most extensively. In particular, site-specific DNA hypermethylation and global DNA hypomethylation have been observed in the vast majority of cancers [17]. Although it remains unclear how genetic mutations affect epigenetic abnormalities, the functional involvement of epigenetic abnormalities in both cancer initiation and progression has also been highlighted by previous in vivo studies. Particularly noteworthy are detailed studies using Apc-mutant mice that have revealed the role of abnormal DNA methylation in colon tumor development [18–21]. These studies suggest that forced reduction of DNA methylation causes chromosomal instability resulting in loss of Apc heterozygosity and promotes neoplastic transformation of colonic mucosa, whereas it suppresses the progression of early microadenomas into macroscopic tumors. In addition, de novo overexpression of DNA methyltransferase Dnmt3b accelerates the progression of colonic microadenoma to a macroscopic tumor, whereas deletion of Dnmt3b suppresses this progression.
Taken together, the available data suggest that cancer progresses through multistep processes involving both genetic mutations and epigenetic abnormalities; however, it still remains unclear how epigenetic abnormality occurs during cancer development. Previous studies have demonstrated that cancer-promoting inflammatory stimuli induce drastic changes in DNA methylation patterns [22]. These results suggest that external signals could be a cause of epigenetic abnormalities in cancer cells. In contrast, large-scale sequencing projects have identified a number of mutations of epigenetic regulator genes across a wide variety of cancer types [23]. These results clearly demonstrate that some of the epigenetic abnormalities observed in cancers are attributable to genetic mutations and highlight the primary role of genetic mutations, even against a background of epigenetic alterations.
Dedifferentiation in Cancer Initiation and Progression
Previous studies suggested that the concept of cancer stem cells is closely related to dedifferentiation of cancer cells. Because the role of dedifferentiation in cancer cell heterogeneity has been nicely described in other reviews [24, 25], we have not attempted comprehensive coverage of this topic in this review but rather focused on the possible role of dedifferentiation on cancer initiation and promotion.
Several studies suggested that cancer cells can arise from somatic stem cells [26, 27]. Baker et al. demonstrated that intestinal stem/progenitor cells are prone to transformation [26]. In contrast, other studies proposed that dedifferentiation of mature cells triggers cancer development [28–31]. Schwitalla et al. demonstrated that activated Wnt signaling together with elevated nuclear factor-κB (NF-kB) signaling can induce dedifferentiation of nonstem cells in the intestine, resulting in acquisition of tumor-initiating capacity with stem cell properties [28]. Using a conditional knockout system for PAX5, the master regulator of B-cell lineage, another group has shown that mature B cells can develop into aggressive lymphoma through dedifferentiation into the progenitor state [29]. Moreover, several studies that have explored the origin of tumor cells in glioma have suggested that this tumor can arise in differentiated lineages through dedifferentiation [30, 31].
In the field of surgical pathology, dedifferentiation (i.e.,morphological loss of lineage identity with tumor progression) is often recognized. Well-differentiated liposarcoma, for example, a common soft tissue sarcoma that shows characteristics of adipose tissue such as lipid droplets in cytoplasm, can progress to dedifferentiated liposarcoma, a high-grade malignancy lacking morphological features of fat tissue. Collectively, these findings suggest that dedifferentiation is involved in both initiation and promotion of cancer development; however, it is still unclear whether dedifferentiation is a driver of cancer development.
It is interesting to note that the reprogramming process, namely, dedifferentiation, has characteristics similar to those of cancer development. During reprogramming, somatic cells acquire unlimited proliferation properties and self-renewing activities, which are also well-accepted characteristics of cancer cells. It is also suggested that iPS cells and cancer cells share a similar metabolic status [32, 33]. Furthermore, poorly differentiated aggressive cancers have been shown to have an ESC-like transcriptional signature [34]. These similarities suggest that the processes of tumor development and reprogramming may be promoted by overlapping factors. Indeed, depletion of p53, a major tumor suppressor gene, significantly promotes the derivation of iPSCs, demonstrating that tumor suppression could be a roadblock on the path toward pluripotency [35, 36].
The fact that conversion of somatic cells to iPSCs is accompanied by dynamic changes in epigenetic status raises the possibility that epigenetic regulation of derivation of iPSCs might drive dedifferentiation-associated cancer development. Moreover, considering that iPSC derivation does not require any particular change in the underlying DNA sequence, it is possible that dedifferentiation-driven epigenetic disruption could be a primary driving force of a particular type of cancer development, independent of genetic mutations. Together, these findings provide a rationale for exploring the causal relationship between dedifferentiation and cancer development, using technology for iPSC derivation.
Dedifferentiation Can Induce Cancer Development: Indication of Epigenetics-Driven Cancer Development
We recently tried to elucidate the close association between dedifferentiation and cancer development using in vivo reprogrammable mice that have a transgenic system similar to that utilized in previous studies [37–40]. We used chimeric mice that were generated using ESCs in which reprogramming factors were inducible under the control of doxycycline. Continuous expression of reprogramming factors resulted in the development of multiple teratomas in various organs. It was noteworthy that in vitro culture of these teratoma cells resulted in derivation of iPSCs capable of chimeric contribution, confirming that somatic cells can be reprogrammed in vivo. Interestingly, when incomplete reprogramming was induced in these mice by withdrawal of doxycycline treatment before teratoma formation, the mice developed tumors consisting of undifferentiated dysplastic cells that were distinct from teratoma cells. These tumors showed invasion into the surrounding tissues, one of the hallmarks of cancer [41]. The doxycycline-withdrawn cancer cells arising in the kidney were considered to be intermediate between a differentiated (renal tubule cell) state and an iPSC state in terms of gene expression profile and DNA methylation pattern, consistent with the notion that these cancer cells are partially reprogrammed cells. Interestingly, these cancers resembled Wilms’ tumor, a common pediatric kidney cancer, in many respects including histology, gene expression profile, and an abnormal expression pattern of Ifg2, a common imprinting gene, suggesting that this mouse could be a model for human Wilms’ tumor. Importantly, no remarkable genetic mutations were observed in doxycycline-withdrawn cancer cells, and these cancer cells were readily reprogrammed into iPSCs with shorter latency and higher efficiency when compared with the process of reprogramming from normal kidney cells. Surprisingly, these kidney tumor-derived iPSCs contributed to chimeric mice and differentiated into apparently normal non-neoplastic kidney cells. Considering that iPSC derivation and the differentiation process are not accompanied by changes in genomic sequence, these findings indicate that the kidney cancer genome in this model supports normal kidney development, resulting in terminal differentiation into non-neoplastic kidney cells. The results provide proof of concept for epigenetic cancer and suggest that particular types of cancer, such as Wilms’ tumor, can arise mainly as a result of epigenetic disruption triggered by dedifferentiation (Fig. 2). The fact that human Wilms’ tumors sometimes lack detectable genetic mutations at well-known genes for Wilms’ tumor development supports this notion. Further analysis would be required to uncover molecular mechanisms for how somatic cells overcome the limited length of telomeres and the limited ability for cell proliferation with partial reprogramming [24, 41, 42], leading to the acquisition of neoplastic properties.
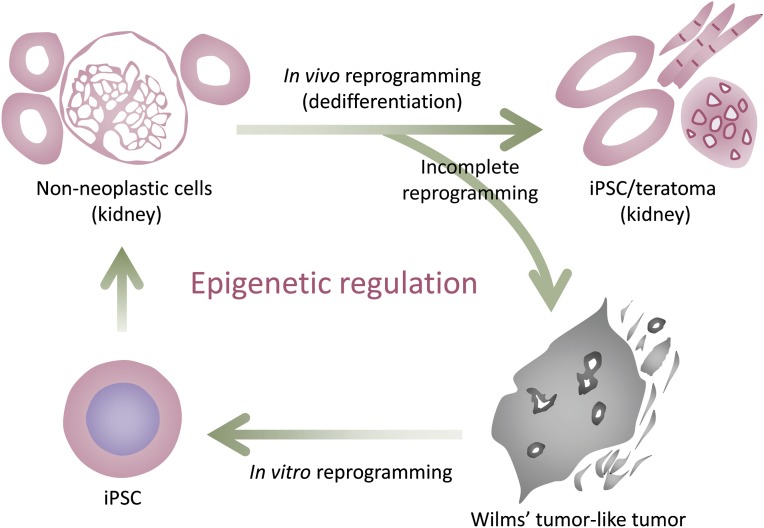
Figure 2.
Proof of concept for epigenetic cancer. Non-neoplastic cells in the kidney can be reprogrammed into iPSCs in teratoma in vivo. However, incomplete reprogramming caused by transient expression of reprogramming factors results in the development of a cancer resembling Wilms’ tumor, a common pediatric kidney cancer. The tumor cells are easily fully reprogrammed into iPSCs by additional expression of reprogramming factors in vitro. The kidney tumor-derived iPSCs differentiate into non-neoplastic kidney cells in chimeric mice, proving that kidney cancer cells in this model have not undergone irreversible genetic transformation. This result suggests that particular types of cancer can develop mainly through disruption of epigenetic regulation. Abbreviation: iPSC, induced pluripotent stem cell.
Given that epigenetic regulation can be modulated by chemical compounds, the epigenetic abnormality that drives cancer development could be a promising therapeutic target. It would be exciting to explore cancer types that depend mainly on epigenetic regulation for their development and maintenance. Childhood tumors, which appear not to accumulate genetic mutations in terms of patient age, may be candidates for epigenetically driven cancer. In fact, recent studies have demonstrated that childhood cancers, such as medulloblastoma, neuroblastoma, and rhabdoid tumor, have very few recurrent mutated genes [43–45]. Our experimental model described above utilizes artificial expression of reprogramming factors, and the present study does not provide direct evidence that dedifferentiation is actually involved in the development of human Wilms’ tumor. Further detailed studies using human samples are needed to clarify the role of reprogramming in human cancer development. In addition, because it is unlikely that expression of Yamanaka factors is directly responsible for development of human cancers, it would be of great interest to identify a natural phenomenon that induces dedifferentiation.
The Possibility of Dedifferentiation as a Natural Phenomenon
Metaplasia is a reversible change in which one differentiated cell type is replaced by another mature cell type. It is a very common phenomenon and is regarded as an adaptive substitution of cells that are sensitive to stress by cell types better able to withstand the adverse environment. Importantly, metaplasia is of clinical significance because some metaplasias, such as Barrett esophagus and intestinal metaplasia of the stomach, are well known to have an increased risk of cancer development.
Nevertheless, the detailed mechanism of metaplasia still seems to be controversial [46, 47]. Although Wang et al. proposed that residual embryonic cells can be precursors of a Barrett-like metaplasia [46], Fujii et al. reported in a study of intestinal metaplasia of the stomach that chronic infection with Helicobacter pylori converts gastric epithelial cells to intestinal epithelial cells via tissue stem-like progenitor cells [48]: H. pylori infection induces aberrant expression of the intestine-specific caudal-related homeobox (CDX) transcription factors CDX1 and CDX2. Ectopic CDX1 activates the stemness-associated reprogrmming factors SALL4 and KLF5, resulting in the reprogramming of gastric epithelial cells into tissue-stem like progenitors and leading to transdifferentiation into intestinal epithelial cells. This study supports the idea that external stimuli (i.e., a natural phenomenon) such as infection by a pathogenic organism (e.g., H. pylori) and subsequent inflammation can induce dedifferentiation of somatic cells. As mentioned above, it is noteworthy that inflammation-inducible NF-kB signaling, one of the common cytokine signals, accelerates intestinal tumor formation initiated by dedifferentiation [28]. It is possible that the dedifferentiated cells, arising as a result of inflammation, may easily acquire cancer cell properties or already possess some aspects of them (Fig. 1). Given that accumulation of DNA methylation is observed at some loci depending on age [49], it is also possible that somatic cells in children may have more flexible plasticity than those of adults and that the characteristics of such cells may make them more prone to dedifferentiation, leading to tumor development (Fig. 1). The possibility of naturally occurring dedifferentiation is worth further exploration.
Recently, much attention has been given to “super-enhancers” for maintenance of cell identity. Super-enhancers are large clusters of transcription enhancers that have much stronger transcriptional activity than classic promoters and enhancers. Each cell type has unique super-enhancers, and these are involved in the proper expression of genes that define the distinct characteristics of both normal cells and cancer cells. Moreover, single nucleotide polymorphisms in super-enhancers are significantly correlated with lineage-associated disease [50, 51]. These findings indicate that loss of cell identity (i.e., dedifferentiation) plays a role in the pathogenesis of diverse diseases, including cancer.
Conclusion
Although the rationale for somatic dedifferentiation as a driver for general cancer development remains unclear, previous studies have suggested the involvement of dedifferentiation in cancer development. A recent study using iPSC technology that allows global changes in epigenetic status without affecting the underlying DNA sequence has provided stronger evidence for a causative and primary role of dedifferentiation-associated epigenetic regulation in a particular type of cancer development. Further studies aimed at identifying external stimuli that induce loss of cellular identity are warranted to explore the possibility of dedifferentiation as a natural causative phenomenon in human cancer development. The data obtained would be applicable for devising novel strategies for both prevention and treatment of specific types of cancer.
Acknowledgments
We thank members of the Yamada laboratory for helpful discussions. The authors were supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT); by the Ministry of Health, Labor, and Welfare of Japan; by the Japan Science and Technology Agency; by the Takeda Science Foundation; and by the Naito Foundation. The Institute for Integrated Cell-Material Sciences is supported by the World Premier International Research Center Initiative, MEXT, Japan.
Author Contributions
Yosuke Yamada and H.H.: manuscript writing; Yasuhiro Yamada: manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morphol. 1962;10:622–640. [PubMed] [Google Scholar]
- 2.Wilmut I, Schnieke AE, McWhir J, et al. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 3.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 8.Vierbuchen T, Ostermeier A, Pang ZP, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hikichi T, Matoba R, Ikeda T, et al. Transcription factors interfering with dedifferentiation induce cell type-specific transcriptional profiles. Proc Natl Acad Sci USA. 2013;110:6412–6417. doi: 10.1073/pnas.1220200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gifford CA, Ziller MJ, Gu H, et al. Transcriptional and epigenetic dynamics during specification of human embryonic stem cells. Cell. 2013;153:1149–1163. doi: 10.1016/j.cell.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koche RP, Smith ZD, Adli M, et al. Reprogramming factor expression initiates widespread targeted chromatin remodeling. Cell Stem Cell. 2011;8:96–105. doi: 10.1016/j.stem.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polo JM, Anderssen E, Walsh RM, et al. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell. 2012;151:1617–1632. doi: 10.1016/j.cell.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onder TT, Kara N, Cherry A, et al. Chromatin-modifying enzymes as modulators of reprogramming. Nature. 2012;483:598–602. doi: 10.1038/nature10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Liu H, Liu J, et al. H3K9 methylation is a barrier during somatic cell reprogramming into iPSCs. Nat Genet. 2013;45:34–42. doi: 10.1038/ng.2491. [DOI] [PubMed] [Google Scholar]
- 15.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 16.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 17.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laird PW, Jackson-Grusby L, Fazeli A, et al. Suppression of intestinal neoplasia by DNA hypomethylation. Cell. 1995;81:197–205. doi: 10.1016/0092-8674(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 19.Yamada Y, Jackson-Grusby L, Linhart H, et al. Opposing effects of DNA hypomethylation on intestinal and liver carcinogenesis. Proc Natl Acad Sci USA. 2005;102:13580–13585. doi: 10.1073/pnas.0506612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin H, Yamada Y, Nguyen S, et al. Suppression of intestinal neoplasia by deletion of Dnmt3b. Mol Cell Biol. 2006;26:2976–2983. doi: 10.1128/MCB.26.8.2976-2983.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linhart HG, Lin H, Yamada Y, et al. Dnmt3b promotes tumorigenesis in vivo by gene-specific de novo methylation and transcriptional silencing. Genes Dev. 2007;21:3110–3122. doi: 10.1101/gad.1594007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niwa T, Tsukamoto T, Toyoda T, et al. Inflammatory processes triggered by Helicobacter pylori infection cause aberrant DNA methylation in gastric epithelial cells. Cancer Res. 2010;70:1430–1440. doi: 10.1158/0008-5472.CAN-09-2755. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence MS, Stojanov P, Mermel CH, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daley GQ. Common themes of dedifferentiation in somatic cell reprogramming and cancer. Cold Spring Harb Symp Quant Biol. 2008;73:171–174. doi: 10.1101/sqb.2008.73.041. [DOI] [PubMed] [Google Scholar]
- 25.Friedmann-Morvinski D, Verma IM. Dedifferentiation and reprogramming: Origins of cancer stem cells. EMBO Rep. 2014;15:244–253. doi: 10.1002/embr.201338254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 27.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 28.Schwitalla S, Fingerle AA, Cammareri P, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Cobaleda C, Jochum W, Busslinger M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature. 2007;449:473–477. doi: 10.1038/nature06159. [DOI] [PubMed] [Google Scholar]
- 30.Liu C, Sage JC, Miller MR, et al. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell. 2011;146:209–221. doi: 10.1016/j.cell.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedmann-Morvinski D, Bushong EA, Ke E, et al. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science. 2012;338:1080–1084. doi: 10.1126/science.1226929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folmes CD, Nelson TJ, Martinez-Fernandez A, et al. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011;14:264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panopoulos AD, Yanes O, Ruiz S, et al. The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res. 2012;22:168–177. doi: 10.1038/cr.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ben-Porath I, Thomson MW, Carey VJ, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong H, Takahashi K, Ichisaka T, et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Utikal J, Polo JM, Stadtfeld M, et al. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460:1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carey BW, Markoulaki S, Beard C, et al. Single-gene transgenic mouse strains for reprogramming adult somatic cells. Nat Methods. 2010;7:56–59. doi: 10.1038/nmeth.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stadtfeld M, Maherali N, Borkent M, et al. A reprogrammable mouse strain from gene-targeted embryonic stem cells. Nat Methods. 2010;7:53–55. doi: 10.1038/nmeth.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abad M, Mosteiro L, Pantoja C, et al. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature. 2013;502:340–345. doi: 10.1038/nature12586. [DOI] [PubMed] [Google Scholar]
- 40.Ohnishi K, Semi K, Yamamoto T, et al. Premature termination of reprogramming in vivo leads to cancer development through altered epigenetic regulation. Cell. 2014;156:663–677. doi: 10.1016/j.cell.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 43.Rausch T, Jones DT, Zapatka M, et al. Genome sequencing of pediatric medulloblastoma links catastrophic DNA rearrangements with TP53 mutations. Cell. 2012;148:59–71. doi: 10.1016/j.cell.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molenaar JJ, Koster J, Zwijnenburg DA, et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature. 2012;483:589–593. doi: 10.1038/nature10910. [DOI] [PubMed] [Google Scholar]
- 45.Lee RS, Stewart C, Carter SL, et al. A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J Clin Invest. 2012;122:2983–2988. doi: 10.1172/JCI64400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Ouyang H, Yamamoto Y, et al. Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell. 2011;145:1023–1035. doi: 10.1016/j.cell.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xian W, Ho KY, Crum CP, et al. Cellular origin of Barrett’s esophagus: Controversy and therapeutic implications. Gastroenterology. 2012;142:1424–1430. doi: 10.1053/j.gastro.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujii Y, Yoshihashi K, Suzuki H, et al. CDX1 confers intestinal phenotype on gastric epithelial cells via induction of stemness-associated reprogramming factors SALL4 and KLF5. Proc Natl Acad Sci U S A. 2012;109:20584–20589. doi: 10.1073/pnas.1208651109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Issa JP, Ottaviano YL, Celano P, et al. Methylation of the oestrogen receptor CpG island links ageing and neoplasia in human colon. Nat Genet. 1994;7:536–540. doi: 10.1038/ng0894-536. [DOI] [PubMed] [Google Scholar]
- 50.Whyte WA, Orlando DA, Hnisz D, et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hnisz D, Abraham BJ, Lee TI, et al. Super-enhancers in the control of cell identity and disease. Cell. 2013;155:934–947. doi: 10.1016/j.cell.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]