This study tested the adaptive alloimmunity response of nonactivated human T cells to human pluripotent stem cell (hPSC)-derived pericytes compared with that of pericytes from brain and full-term placenta in cocultures and following implantation into immunodeficient mice. It was found that under steady-state conditions, hPSC pericytes, like their native tissue-derived counterparts, maintained poor immunogenicity and immunomodulation capabilities, favoring allostimulation of regulatory T cells over T-cell activation.
Keywords: Human pluripotent stem cells, Pericytes, Immunomodulation, Regulatory T cells
Abstract
Isolated microvessel-residing pericytes and pericytes from human pluripotent stem cells (hPSCs) exhibit mesenchymal stem cell-like characteristics and therapeutic properties. Despite growing interest in pericyte-based stem cell therapy, their immunogenicity and immunomodulatory effects on nonactivated T cells are still poorly defined, in particular those of vasculogenic hPSC pericytes. We found that tissue-embedded and unstimulated cultured hPSC- or tissue-derived pericytes constitutively expressed major histocompatibility complex (MHC) class I and the inhibitory programmed cell death-ligand 1/2 (PD-L1/2) molecules but not MHC class II or CD80/CD86 costimulatory molecules. Pretreatment with inflammatory mediators failed to induce an antigen-presenting cell-like phenotype in stimulated pericytes. CD146+ pericytes from hPSCs did not induce activation and proliferation of allogeneic resting T cells independent of interferon (IFN)-γ prestimulation, similarly to pericytes from human brain or placenta. Instead, pericytes mediated a significant increase in the frequency of allogeneic CD25highFoxP3+ regulatory T cells when cocultured with nonactivated peripheral blood T cells. Furthermore, when peripheral blood CD25high regulatory T cells (Tregs) were depleted from isolated CD3+ T cells, pericytes preferentially induced de novo formation of CD4+CD25highFoxP3+CD127−, suppressive regulatory T cells. Constitutive expression of PD-L1/2 and secretion of transforming growth factor-β by hPSC pericytes directly regulated generation of pericyte-induced Tregs. Pericytes cotransplanted into immunodeficient mice with allogeneic CD25− T cells maintained a nonimmunogenic phenotype and mediated the development of functional regulatory T cells. Together, these findings reveal a novel feature of pericyte-mediated immunomodulation distinguished from immunosuppression, shared by native tissue pericytes and hPSC pericytes, and support the notion that pericytes can be applied for allogeneic cell therapy.
Introduction
Small blood vessels such as arterioles, capillaries, and venules are composed of two cell types: an inner layer of blood-facing endothelial cells (ECs) and an outer layer of perivascular pericytes, which are embedded in and partially surround the endothelial layer [1, 2]. Microvessels play a crucial role in the early stages of cell-mediated immune responses, wherein vascular cellular components and immobilized secreted factors coordinate to regulate activation of circulating leukocytes and extravasation into peripheral tissues, mostly through postcapillary venules [3–5]. The role of ECs in the recruitment and activation of circulating T cells is well defined, yet little is known about how T cells interact with pericytes either integrated within the vascular niche or retrospectively isolated and expanded.
Pericytes can be isolated from multiple human tissues and have been shown to exhibit multipotent mesenchymal stem cell (MSC) features in vitro, including osteogenic, adipogenic, chondrogenic, and myogenic differentiation capabilities. Pericytes coexpress characteristic MSCs and a pericytic set of markers, as well as having clonogenic and vasculogenic properties [6]. However, not all tissue-derived MSCs are of perivascular origin: for example, expression of CD146 differentiates between perivascular and endosteal CD271+ MSCs in human bone marrow [7]. In accordance, we have recently demonstrated that human pluripotent stem cells (hPSCs), either embryonic or induced, can be used as an alternative source for the generation of α-smooth muscle actin-negative CD146+ pericytic derivatives with properties compatible with those of their human native tissue-derived counterparts [8].
Recently, accumulating evidence, based mostly on rodent experimental models, demonstrates that (like tissue-derived MSCs [9]) hPSC-MSCs [10], mesoangioblasts [11], and normal and tumor-derived pericytes display immunosuppressive properties: human placenta and brain pericytes induce T cell receptor (TCR)-dependent T-cell anergy upon stimulation with IFN-γ [12] and human brain or retinal pericytes repress activated T cells via cell-cell contact through PD-L1 or soluble mediators [13, 14]. However, very little is known about the immunogenic properties of pericytes in steady-state homeostasis or in the absence of polyclonal activation, with no information at all on the immunogenicity of hPSC pericytes. Considering the vast potential of multipotent pericytes for cellular therapy, it is important to establish human experimental models for studying the alloimmunogenicity of hPSC pericytes and native pericytes before pericyte-based therapy can be applied to the clinic. Furthermore, the mechanisms of MSC-induced immunomodulation is dependent on the species or tissue source [15, 16], and since pericytes are removed from their anatomical localization in the vasculature or from differentiating hPSC prior to transplantation, they are exposed to possible immunophenotypic modifications during long-term culture.
Recognition of alloantigens by T cells is a major cause for transplant rejection, either directly in the transplant site early after transplantation or indirectly in the lymph nodes at later phase of transplant rejection with multiple mechanisms determining the type and strength of the adaptive immune responses [17]. Specifically, activation of T cells is tightly regulated by three signals: (a) antigen presentation via cell surface major histocompatibility complex (MHC) class I or II stimulatory molecules (affinity- and avidity-dependent) by professional antigen presenting cells, (b) costimulation with costimulatory molecules (e.g., CD80, CD86), and (c) secretion of cytokines (e.g., tumor necrosis factor [TNF]-α and IFN-γ), interleukins (e.g., interleukin 1 [IL-1]–IL-17), and other soluble mediators. Alterations of the tightly coordinated stimulus shift T-cell alloresponses from activation and proliferation to anergy, apoptosis, or exhaustion [18]. In addition, the secretion profile of soluble immunomodulators by stromal cells determines directly or indirectly through monocyte and dendritic cells the type, the proportion and functionality of T-cell subsets [19]. CD4+ T cells can differentiate into effector Th1, Th2, Th9, Th17, or Tfh cells or other functional subsets, including central and effector memory T cells, which are generally characterized based on cell surface markers and/or the cytokines they produce [20]. Alternatively, regulatory T cells (Tregs) can be induced and require transforming growth factor (TGF)-β or IL-10 and the absence of IL-6 [21, 22] or cell-cell interactions via PD-L1 [23]. Importantly, mouse and human Tregs exhibit many differences in phenotype, development, and suppressive mechanism [24]. Normally, human MSCs are poorly immunogenic because of induction of immunosuppression [25], do not constitutively express MHC class II or CD80/CD86, and downregulate T-cell activation through secretion of indoleamine 2,3-dioxygenase (IDO) or the Fas/FasL pathway [26, 27].
To better understand the immunological characteristics of hPSC pericytes, as an essential determinant of their potential clinical application, we tested the adaptive alloimmunity response of nonactivated human T cells to hPSC pericytes in comparison with that of pericytes from brain and full-term placenta in cocultures and following implantation into immunodeficient mice. Our data reveal that native-tissue and hPSC pericytes are poorly immunogenic, expressing MHC class I and functional T-cell inhibitory ligands PD-L1/2 but not the costimulatory molecules CD80/CD86, and secrete substantial amounts of TGF-β. We demonstrate here that these characteristics of pericytes induced the formation of suppressive allogeneic CD4+CD25highFoxP3+CD127− Tregs in a TGF-β-dependent and PD-L1-dependent manner.
Materials and Methods
Cell Cultures
Human ESC lines H9.2 [28] and I6 [29] and human hair follicle keratinocyte-derived induced pluripotent stem cell (iPSC) line KTN [30] were used for generation of CD146+CD105+ pericytes (hPSC pericytes) as previously described [8]. Primary healthy human donor-derived brain pericytes and human umbilical vein endothelial cells were purchased from ScienCell (Carlsbad, CA, http://www.sciencellonline.com). Primary CD146+ human full-term placenta-derived pericytes were obtained from healthy donors after informed consent as previously described [8]. MSCs were generated from hESCs as described [31] and cultured in α-minimal essential medium supplemented with 20% fetal bovine serum (FBS), 1% penicillin/streptomycin (pen/strep), and 1% l-glutamine.
Pericyte-Peripheral Blood Cell Cocultures
For assessment of pericyte-allostimulated proliferation, peripheral blood mononuclear cells (PBMCs) or CD3+ T cells were prelabeled with carboxyfluorescein succinimidyl ester (CFSE) (10 μM; Life Technologies, Carlsbad, CA, http://www.lifetech.com) and loaded on a monolayer of either brain, placenta, or hPSC pericytes in a 96-well dish at a ratio of 1:100 (PBMCs:pericytes) or 1:50 (T cells:pericytes). Mixed cultures were cultivated in RPMI supplemented with 10% inactivated FBS, 1% pen/strep and 1% l-glutamine for 5 days. PBMCs or T cells were then harvested and colabeled with anti-CD4-antigen-presenting cells (APCs), and proliferation of CFSE cells was estimated by flow cytometry analyses of CFSE dilution of dividing cells. In some proliferation experiments pericytes were pretreated with 5 or 50 ng/ml IFN-γ (R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com) for 72 hours prior to incubation with peripheral blood (PB) cells. PB-allogeneic activated macrophages served as a positive control.
Treg Induction
For Treg induction experiments, pericytes were cocultured with isolated PB CD3+CD25− T cells (3 × 106 cells per 24 wells) for 72 hours in 0.5 ml of RPMI 10% FBS in 24-well dishes. Where indicated, cocultures were supplemented with neutralizing antibodies against both PD-L1 (30 µg/ml; R&D Systems) and PD-L2 (20 µg/ml; R&D Systems) or 10 mM TGF-β receptor kinase inhibitor SB431542 (Tocris Biosciences, Bristol, U.K., http://www.tocris.com). Characterization of induced-Tregs was performed as described in detail in the supplemental online data and supplemental online Table 5.
Induction of Tregs in Basement Membrane Extract Implants
PB CD3+CD25− T cells (15–20 × 106 cells per 200 μl of RPMI only) were mixed with 3–4 × 106 brain, placenta, or hPSC pericytes or allogeneic human PB macrophages and 250 µl of Cultrex reduced growth factor basement membrane extract (BME) (Trevigen, Gaithersburg, MD, http://www.trevigen.com). The BME mixture was then injected subcutaneously into 8–10-week-old NOD/SCID mice. Implants were removed after 3 days and either fixed in 4% formalin, sectioned, and stained with hematoxylin/eosin or enzymatically digested for cell retrieval from BME implants with 2.5 U/ml dispase (Gibco, Grand Island, NY, http://www.invitrogen.com), 0.2% collagenase A (Roche, Indianapolis, IN, http://www.roche.com), and 150 U/ml DNase I (Worthington Biochemical, Lakewood, NJ, http://www.worthington-biochem.com) in phosphate-buffered saline (PBS) for 20 minutes at 37°C. The cell suspension was then washed in PBS filtered through a PBS/0.5% FBS prewashed 45-µm cell strainer and used for flow cytometry analyses. All procedures were approved by the Committee for the Use and Care of Animals of the Technion–Israel Institute of Technology.
Statistical Analyses
Data were analyzed statistically by analysis of variance, single factor, with Excel 2010 software (Microsoft, Redmond, WA, http://www.microsoft.com). p ≤ .05 was considered to be significant.
Results
hPSC Pericytes Exhibit an Immunophenotype Similar to That of Placenta and Brain Pericytes
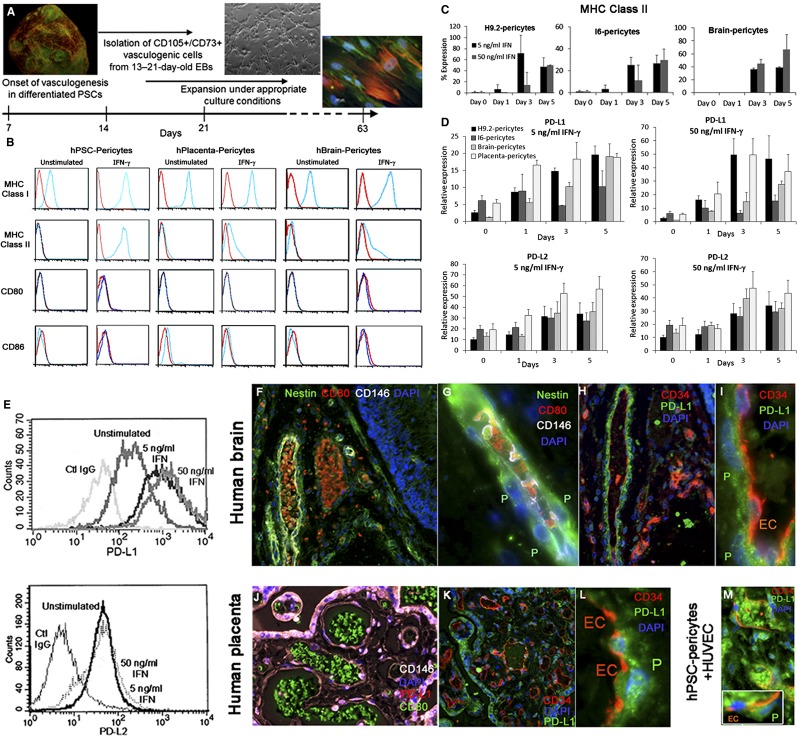
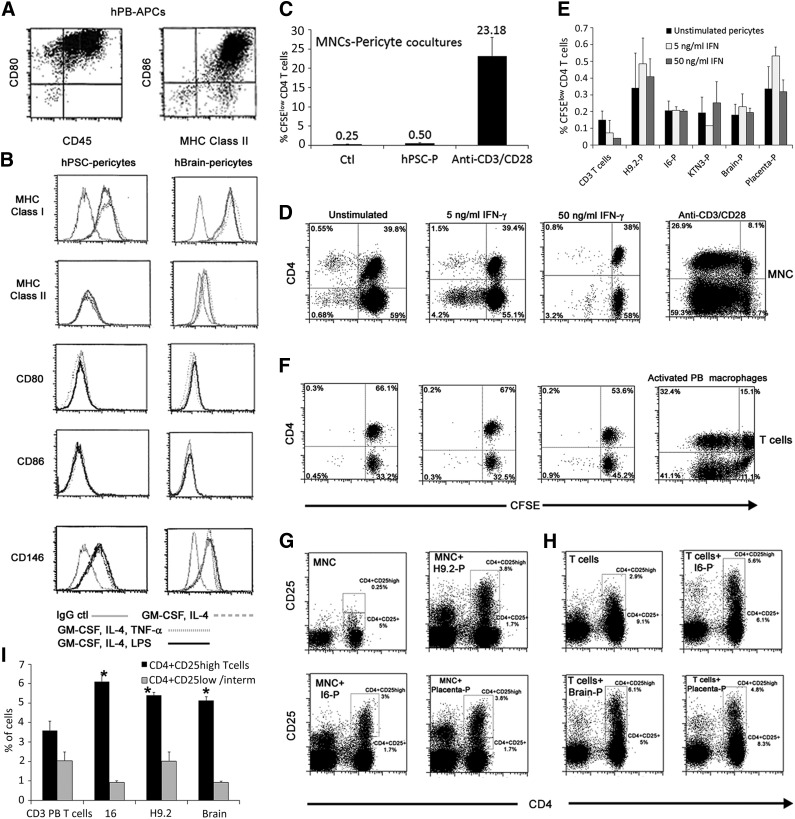
To assess the immunogenic potential of pericytes generated from hPSCs (Fig. 1A), we first compared hPSC pericytes (from hESC H9.2, hESC I6, and hair follicle keratinocyte-iPSC KTN3) with human native tissue-derived pericytes from full-term placenta and brain, for the expression of significant cell surface stimulatory immunological molecules under basal and cytokine-stimulated conditions. Placenta, brain, and hPSC pericytes constitutively expressed MHC class I but not MHC class II or the costimulatory molecules CD80 or CD86 under basal culture conditions. Pretreatment of cultured pericytes with IFN-γ (5 or 50 ng/ml) induced the expression of MHC class II that was maintained for 5 days of stimulation (Fig. 1B, 1C) but did not stimulate the expression of CD80 or CD86 (Fig. 1B). All types of cultivated pericytes in long-term cultures highly expressed the inhibitory molecules PD-L1 (CD274) and PD-L2 (CD273), and when stimulated with IFN-γ they progressively increased the expression of PD-L1 and PD-L2 messengers and cell surface molecules (Fig. 1D, 1E; supplemental online Table 1). Coinciding with the immunophenotype of cultured pericytes, CD146+CD34− microvessel surrounding pericytes of adult normal human brain (Fig. 1F–1I) and term placenta (Fig. 1J–1L) do not express CD80, which was detected on luminal circulating blood cells (Fig. 1F–1G, 1I). In addition, PD-L1 was highly expressed by CD34−CD146+ native tissue pericytes (Fig. 1I–1L) and by transplanted hPSC pericytes, either surrounding human engineered blood vessels or dispersed within the Matrigel implant (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) (Fig. 1M). We further examined whether stimulation of pericytes with granulocyte-macrophage colony-stimulating factor, IL-4, and lipopolysaccharide/TNF-α, which induce the maturation and activation of circulating monocytes and dendritic cell precursors toward professional APCs, would similarly alter the immunophenotype of hPSC pericytes. Whereas stimulated PB-adherent monocytes highly expressed CD80, CD86, MHC class I, and MHC class II (Fig. 2A), hPSC and brain pericytes did not similarly respond to the inflammatory mediators, maintaining MHC class I and CD146 expression (Fig. 2B; supplemental online Table 2). This implied that even under conditions of inflammation, which typically occur at early phase of transplant rejection, pericytes do not adopt conventional features of antigen-presenting cells. Taken together, these findings demonstrate that hPSC pericytes and their native tissue-derived cell counterparts exhibit similar cytokine-dependent and independent expression of immunological molecules in a combination that implies a poor ability of these cells to stimulate allogeneic adaptive immune response.
Figure 1.
hPSC-derived pericytes exhibit an immunophenotype similar to that of human brain and full-term placenta pericytes. (A): Illustrated protocol for derivation of multipotent perivascular progenitor cells from spontaneously differentiating hPSCs. (B): Representative flow cytometry analyses of the expression of key immunological molecules on cultivated hPSC pericytes (hESC: H9.2 and I6; human induced pluripotent stem cell: KTN), primary human term placenta, and brain pericytes in the presence or absence of IFN-γ. Red lines: control IgG. Blue lines: indicated antibody. (C, D): Flow cytometry analyses of cell surface expression of MHC class II (C) and quantitative polymerase chain reaction gene expression analyses of PD-L1 and PD-L2 (D) in cultured IFN-γ stimulated (5 or 50 ng/ml) or unstimulated hPSC, brain, and placenta pericytes at the indicated time points. The average expression normalized to GAPDH is shown. (E): Cell surface expression of PD-L1 and PD-L2 by IFN-γ treated (3 days) or untreated pericytes. A similar pattern of expression was detected for hPSC, brain, and placenta pericytes by flow cytometry. (F–M): Immunophenotyping of hPSC pericytes in Matrigel implant and human tissues. Sections from human brain (F–I), human term placenta (J–L), and Matrigel implant containing hPSC pericytes and human umbilical vein endothelial cells (M) were indirectly immunolabeled. CD146+ (white, [F, G, J]) Nestin+ (green, [F, G]), and CD34-negative pericytes (red endothelial cells, [H, I, K–M]) coexpressed PD-L1 (G, I, J, L, M [inset]) but not CD80 (F, G, J). CD80 immunolabeling was abundant in circulating blood vessel leukocytes in the brain (red, [F, G]) and in full-term placenta (green, [J]). Original magnifications, ×100 (K), ×200 (F, H, K, M), ×400 (J), ×630 (G, I, L). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; EBs, embryoid bodies; EC, endothelial cell; hBrain, human brain; hPlacenta, human placenta; hPSC, human pluripotent stem cell; HUVEC, human umbilical vein endothelial cell; IFN, interferon; MHC, major histocompatibility complex; P, pericyte; PD-L1/2, programmed cell death ligand 1/2; PSC, pluripotent stem cell.
Figure 2.
Allostimulation of peripheral blood mononuclear cells (PBMCs) or PB T cells with hPSC pericytes does not induce T-cell activation and increases the frequency of the CD4+CD25high T-cell subset. (A, B): Representative expression patterns of MHC class I, MHC class II, CD80, and CD86 by mature PB-derived macrophages (A) compared with hPSC and brain pericytes (B) under culture conditions (interleukin-4, granulocyte-macrophage colony-stimulating factor, and LPS/tumor necrosis factor-α) that gradually induce maturation of professional antigen presenting cells. (C–F): Proliferation of carboxyfluorescein succinimidyl ester-labeled CD4+ T cells after 72 hours in cocultures of PBMCs (C, D) or resting CD3+ PB T cells (E, F) with pericytes from the indicted sources. Activation of PBMCs with soluble antibodies against CD3/CD28 (C, D) and activation of PB T cells by allogeneic PB-derived APCs (F) served as positive controls. Data are mean ± SEM of three independent experiments and at least two different PB cells per experiment. (G, H): Representative fluorescence-activated cell sorting dot plots of expression of CD4 and CD25 on PBMCs (G) or PB T cells (H) following cocultivation with pericytes for 5 days. The frequencies of gated CD4+CD25+ and CD4+CD25high subsets are displayed. (I): Proportions of CD4+CD25+ or CD4+CD25high cell populations out of CD3+ PB T cells after 72 hours in pericyte cocultures compared with nonstimulated CD3+ PB T cells. Data are mean ± SEM of three independent experiments per pericytic line. ∗, p < .0001 compared with unstimulated T cells of the matched subset. Abbreviations: APC, antigen-presenting cell; Ctl, control; hPB, human peripheral blood; hPSC, human pluripotent stem cell; IFN, interferon; LPS, lipopolysaccharide; MHC, major histocompatibility complex; MNC, mononuclear cell; P, pericyte; PB, peripheral blood.
Tissue and hPSC Pericytes Do Not Stimulate Allogeneic PBMCs and T Cells
To assess the ability of hPSC pericytes to induce allogeneic response, human PBMCs from healthy donors were cocultured with hPSC pericytes. In parallel, PBMCs from the same donors were used for multiple coculture combinations with pericytes from human brain or term placenta to directly compare the alloimmunogenicity of hPSC pericytes with that of native tissue-derived pericytes. Following 5 days of cultivation, less than 1% of CFSElow proliferating CD4+ T cells were detected in cocultures of hPSC pericytes and PBMCs in comparison with massive proliferation of CFSE-labeled CD4+ T cells upon stimulation with soluble antibodies against CD3 and CD28 (Fig. 2C, 2D). It has been demonstrated previously that suppression of the cytotoxicity of unstimulated CD8+ T cells or activated T cells is dependent on the induction of tolerogenic CD14+ monocytes by MSCs [27, 32, 33]. Therefore, we further tested whether the poor activation of T cells by pericytes in cocultures with PBMCs is essentially mediated through PB-derived monocytes. To address this possibility, circulating T cells (PB CD3+ T cells > 98%) were isolated, CFSE-labeled, and incubated with unstimulated and IFN-γ-stimulated pericytes. We found that the depletion of monocytes and other PBMC subsets did not affect the lack of allogeneic T-cell response to hPSC, brain, and placenta pericytes (Fig. 2E, 2F). Prestimulation of hPSC, brain, or placenta pericytes with IFN-γ (5 or 50 ng/ml) constantly induced MHC class II expression by all types of pericytes as illustrated in Figure 1B and 1C but did not significantly affect the proliferation of CFSE-labeled CD4+ T cells in comparison with untreated pericytes (Fig. 2E, 2F), although we observed a mild increase in T-cell proliferation in cocultures of 5 ng/ml IFN-γ-prestimulated placenta pericytes (Fig. 2E).
We further assessed the expression of activation markers on PBMCs, which were allostimulated with pericytes in the presence or absence of IFN-γ. The early activation marker CD69 was absent on freshly isolated PBMCs and T cells or PBMCs and T cells cocultured with stimulated or unstimulated pericytes (data not shown). In contrast, pericytes from hPSC, placenta, or brain induced a slight elevation in the frequency of the CD25-expressing CD4+ T-cell subset within PBMCs (Fig. 2G) or T cells (Fig. 2H) in comparison with unstimulated PBMCs or T cells from the same donor, independent of IFN-γ stimulation (data not shown). Within pericyte induced-CD4+CD25+ T cells, two subsets could be distinguished based on high or intermediate coexpression of CD25 as detected by flow cytometry analyses (Fig. 2G, 2H). When PBMCs were cocultured with matched hESC-MSCs [31], which are CD146-negative (supplemental online Fig. 1), or hBM-MSCs, we observed similar effects on CD25 expression on PBMCs (supplemental online Fig. 2). Moreover, when circulating T cells were isolated from PBMCs and were further allostimulated with all types of pericytes, the distinction between CD4+CD25high and CD4+CD25+ subpopulations was more pronounced with a significant elevation only in the frequency of the CD25high subset over CD25low/intermediate (Fig. 2I).
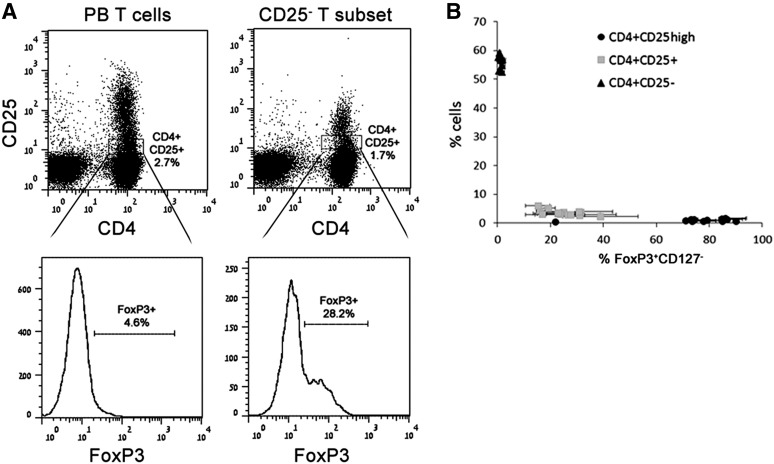
Pericytes From hPSCs, Brain, and Placenta Mediate the Formation of Functional CD4+CD25highCD127−Foxp3+ Tregs
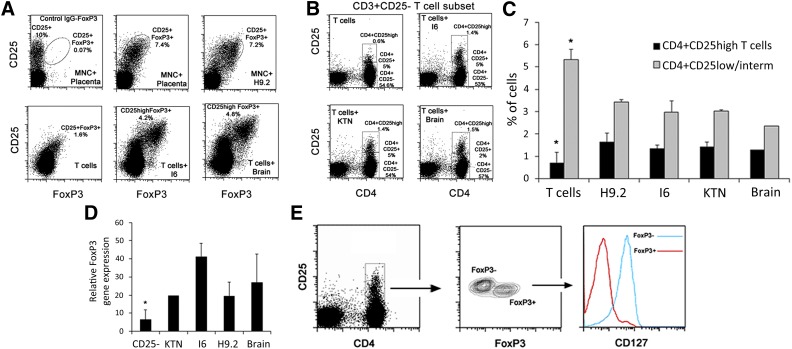
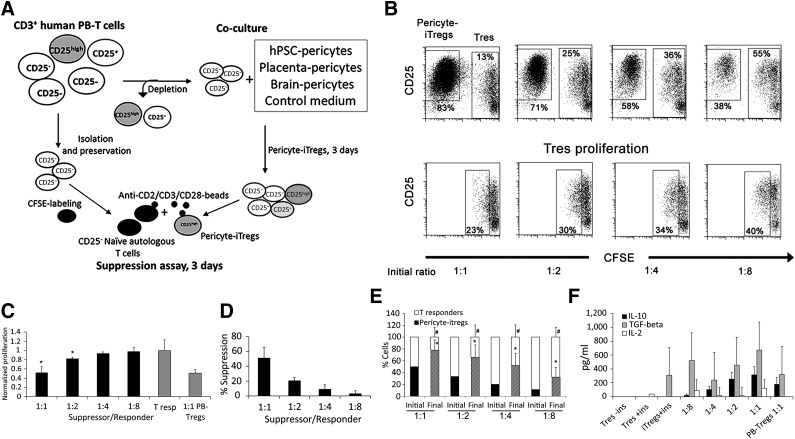
Given the lack of expression of MHC class II or costimulatory molecules, the inability to increase T-cell activation, and the observed increase in the frequency of the CD25+ T-cell subset together with the demonstrated perivascular origin of multipotent MSCs [6], we further examined whether pericytes share another proven feature of MSCs as immunomodulators, mediating the induction of Tregs. To address this possibility, PBMCs or PB CD3+ T cells were cultured in the presence or absence of hPSC pericytes. In addition, brain and placenta pericytes were cocultured in parallel with PB cells from the same donors for direct comparison with hPSC pericytes. In human CD4+ T cells, high expression of CD25 together with the transcription factor forkhead box P3 (FoxP3) and the lack of CD127 expression imply Treg identity. Therefore, flow cytometry analyses were performed based on this set of markers, demonstrating an increase in the proportion of CD4+CD25highFoxP3+ within PBMCs or T cells cocultured with CD146+ hPSC-derived or tissue-derived pericytes within 72 hours compared with matched donor-derived control PB T cells or PBMCs (Fig. 3A). In humans, the proportion of CD4+CD25highFoxP3+CD127− cells ranges between 0.5% and 2% of circulating PBMCs and consists of Tregs, transiently activated nonregulatory T cells, and memory T cells [34, 35]. Therefore, the observed increased proportion of CD4+CD25high population can be attributed to either expansion of the pre-existing population or de novo induction of these cells. To distinguish between these two possibilities, PB CD3+ T cells were separated into two subsets: (a) CD25+ T cells (6.5% ± 4.2%), consisting of activated CD4+CD25+FoxP3−/low T cells and CD4+CD25highFoxP3+ Tregs, and (b) CD4+CD25−CD127+ circulating naïve T cells (53% ± 10.1%). Allostimulation of CD25− T cells with hPSC or native tissue pericytes resulted in reappearance of CD4+CD25+ and CD4+CD25high populations within 72 hours, wherein the CD4+CD25high subset was either very rare or totally absent in unstimulated PB CD3+CD25− T cells (Fig. 3B, 3C). The ability to induce the formation of putative CD4+CD25highFoxP3+ Tregs was also proven for brain- and placenta-derived pericytes (Fig. 3A–3C). In accordance with this, quantitative real-time polymerase chain reaction analyses demonstrated that messenger expression of the transcription factor FoxP3 was significantly increased in pericyte-induced CD25−CD3+ T-cell subsets (Fig. 3D). CD4+CD25+ hPSC pericyte-induced T cells could be divided into two subpopulations based on FoxP3 and CD127 expression, wherein the CD4+CD25high subset exhibited the Treg phenotype, with high expression of CD25, positive for FoxP3, and lacking CD127 expression (Fig. 3E). Given that FoxP3 is not an exclusive marker of Tregs and that in humans the CD4+CD25highFoxP3+CD127− combination can represent other circulating subsets, we tested the functionality of pericyte-induced Tregs (pericyte-iTregs) by in vitro suppression assays, as well as ELISA for detection of suppressive soluble factors. Since it was not possible to distinguish between PB-derived, expanded pre-existing Tregs and pericyte-iTregs within the CD25+ T-cell subset, we tested the suppressive capabilities of putative CD25+ pericyte-iTregs that were generated from the CD25− T-cell subset. Isolated CD25+ pericyte-iTregs were mixed with autologous resting, CD3+CD25− CFSE-labeled, responder T cells in the presence or absence of Treg suppression inspector reagent (Fig. 4A). Pericyte-iTregs exhibited efficient suppressive activity, reducing the proliferation of activated responder T cells in a Treg/Tresponder ratio-dependent manner (Fig. 4B–4D). Suppression of activated T-cell proliferation was accompanied by a significant expansion of pericyte-iTregs, inverting initial loading ratios in favor of pericyte-iTregs versus effector T cells (Fig. 4B–4E). In agreement with previous studies and the classic definition of Treg identity [36], anti-CD2/CD3/CD28-bead activated pericyte-iTregs secreted IL-10 at concentrations correlating to their frequency in mixed Treg/Tresponder cultures as well as TGF-β (Fig. 4F), both known to inhibit proliferation of effector T cells in an IL-2-dependent manner, with the latter being produced and secreted only from primed CD4 effector T cells over Tregs, regardless of their physiological origin. Accordingly, secreted IL-2 was detected in the suppression assay-conditioned medium only in the presence of activated T responders (Fig. 4F). Together these results demonstrate that, like native tissue-derived pericytes, hPSC pericytes have the ability to exert immunosuppressive effects by favoring activation and propagation of Tregs.
Figure 3.
Pericytes from hPSC, brain, and placenta promote the formation of CD4+CD25highFoxP3+CD127− T cells. (A): Representative dot plots of CD25 and FoxP3 expression of gated CD4 T cells from cocultures of peripheral blood mononuclear cells or peripheral blood (PB) CD3+ T cells with hPSC, brain, and placenta pericytes after 3 days of induction. (B): CD3+ T cells were separated into CD3+CD25+ and CD3+CD25− subsets, and then the CD3+CD25− subset was used for pericyte induction experiments. The expression of CD4 and CD25 and the formation of CD4+CD25high populations among the allostimulated CD25− T-cell subset are shown in representative dot plots. (C): Percentages of CD4+CD25+ and CD4+CD25high cell populations generated from the CD3+CD25− T-cell subset in hPSC and brain pericyte cocultures. Mean ± SEM of triplicate samples is shown from seven independent experiments with seven different PB CD3+CD25− T cells and at least four pericyte cell lines per experiment. ∗, p < .05 compared with pericyte-stimulated T cells of a matched subset. (D): Quantitative polymerase chain reaction gene expression of FoxP3 in the CD3+CD25− T-cell subset compared with the CD3+CD25− T-cell subset after 3 days of cultivation with hPSC and brain pericytes. Data are mean ± SEM of 3 independent experiments with PB cells from 2 different donors. ∗, p = .05 compared with pericyte-stimulated CD25− T cells. (E): Representative expression of FoxP3 in gated CD4+CD25+ population, which was induced by pericyte from the CD3+CD25− subset within 72 hours. CD127 expression was evaluated on CD4+CD25+FoxP3+ and CD4+CD25+FoxP3− subpopulations. Abbreviation: MNC, mononuclear cell.
Figure 4.
Pericytes from hPSCs, placenta, and brain induce functional suppressive Tregs. (A): Schematic illustration of experiment. Following depletion of CD3+CD25+ T cells from CD3+ PB T cells, the CD3+CD25− T-cell subset was either incubated with hPSC, brain, or placenta pericytes or preserved for further use as autologous responder T cells. After 3 days in coculture, CD25+ derivatives were isolated and mixed with autologous CD25− T responder cells in the presence or absence of anti-CD2/CD3/CD28 beads during the 3-day suppression assay. (B): Representative dot plots of the frequencies of pericyte-iTregs and CFSE T responders that were mixed at the indicated initial ratios, stained with anti-CD25, and analyzed by flow cytometry. CFSE−CD25high, CFSElow, and CFSEhigh T-cell subsets represent pericyte-iTregs, proliferating T responders, and nonproliferating T responders, respectively (top panel). CFSE+/lowCD25+ cells (bottom panel) represent activated proliferating effector T cells. (C, D): Flow cytometry-based determination T responder proliferation (C) or Treg-mediated suppression (D). Isolated donor-derived PB Tregs were used for positive controls. (E): Proportions of CD25highCFSE− pericyte-iTregs and CFSE+/low T responders at the beginning of the experiment (initial) and after 3 days of stimulation (final). (F): Concentrations of secreted IL-10, TGF-β1, and IL-2 within the indicated conditioned media. (C–F): Data are mean ± SEM of three independent experiments with three different donors with duplicates or triplicates. p < .005 compared with normalized proliferation of T responders (C) and p < .05 compared with initial frequency of T responders (#) and Tregs (∗). Abbreviations: CFSE, carboxyfluorescein succinimidyl ester; IL, interleukin; iTreg, induced regulatory T cell; PB, peripheral blood; TGF, transforming growth factor; Tres, Tresponder.
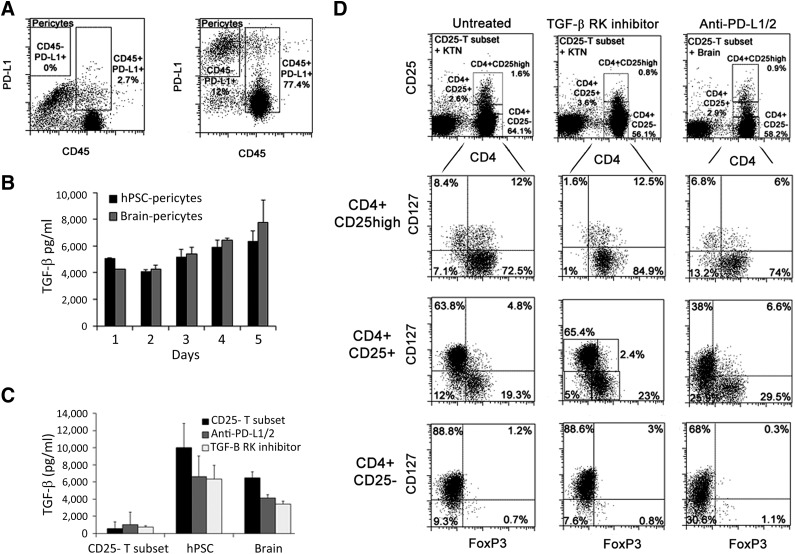
Pericyte-Mediated Induction of CD25highFoxP3+ Tregs Is Regulated by TGF-β and PD-L1
It has previously been demonstrated by numerous murine and human experimental models that Treg induction can be attributed to cell surface expression of the inhibitory molecules programmed death ligand-1 (PD-L1/CD274) and PD-L2 (CD273) [37] or stimulation by secreted TGF-β [22, 38, 39]. Therefore, we verified that PD-L1 is still expressed on pericytes in coculture with PB T cells (Fig. 5A) and further demonstrated by ELISA that substantial amounts of soluble TGF-β were secreted by hPSC and brain pericytes, peaking after 24 hours of cultivation in either pericyte growth medium or 10% FBS RPMI (coculture medium), and were maintained for 5 days of culture (Fig. 5B). High concentrations of TGF-β were also secreted from pericytes in the presence of T cells (Fig. 5C). All types of cultured pericytes did not secret IL-2 at the level of detection similarly to nonactivated CD4+CD25− T cells. However, IL-2 was detected in the conditioned medium of the mixed cultures when hPSC pericytes or tissue-derived pericytes were cocultured with allogeneic CD4+CD25− T cells at concentrations of 393 ± 90 and 377 ± 192 pg/ml, respectively. Of note, pretreatment of pericytes with IFN-γ had no effect on TGF-β secretion (data not shown). To identify whether PD-L1 and PD-L2 are involved in Treg induction, neutralizing antibodies to PD-L1 and PD-L2 were added to the cocultures of hPSC pericytes and CD25− T-cell subsets, as well as to matched placenta and brain pericyte cocultures. Blockade of PD-L1/2 significantly reduced the induction of pericyte-CD4+CD25highFoxP3+CD127− Tregs from CD25− PB T subsets (Fig. 5D; supplemental online Fig. 3). Independently of PD-L1/2, inhibition of TGF-β signaling using specific inhibitor of TGF-β receptor kinase (SB431542) significantly repressed the generation of pericyte-induced CD4+CD25highFoxP3+CD127− Treg from the CD25− T-cell subset (Fig. 5D; supplemental online Fig. 3). In addition, secretion of TGF-β was reduced as a consequence of neutralization of PD-L1/2 or blockage of TGF-β signaling (Fig. 5C). We further characterized whether PD-L1/2 or TGF-β mediated inhibition affected the immunophenotype of CD4+ pericyte-iTregs based on the commonly used markers FoxP3 and CD127 over a range of CD25 expression levels, divided into negative, positive, and high CD25 expression, compared with untreated iTregs, generated in the absence or presence of pericytes (Fig. 5D; supplemental online Fig. 3). Although only CD4+CD25high subpopulation was affected by PD-L1/2 blockage or TGF-β inhibition, the frequency of FoxP3+CD127− was significantly reduced within both the CD4+CD25− and CD4+CD25high subsets and was not affected in the CD4+CD25+ subset (Fig. 5D; supplemental online Fig. 3). Of note, in the absence of TGF-β secreting pericytes, the expression of FoxP3 in CD25high T cells was dramatically reduced, whereas neutralization of PD-L1/2 on T cells in the absence of pericytes resulted in opposite effect, increasing the generation of CD4+CD25highFoxP3+CD127− Tregs from CD3+CD25− PB T cells (supplemental online Fig. 3), implying an intrinsic role for endogenous TGF-β in the maintenance of FoxP3 expression in T cells and implying that the interactions between PD-L1/2 on pericytes and T cell PDs are those that promote Treg induction.
Figure 5.
De novo generation of Tregs by pericytes is mediated through PD-L1/2 and TGF-β. (A): Expression of PD-L1 by CD45− brain pericytes and CD45+ T cells in cocultures. Left panel: CD45-labeled, IgG control cells. The dot plot is representative of the CD25− T-cell subset cultured with hPSC or placenta pericytes for n = 4 independent experiments. (B, C): Concentrations of secreted TGF-β in the conditioned medium of cultivated brain and hPSC pericytes (B) or cocultures of pericytes and CD3+CD25− T cells (C) in the indicated treatments. (D): Representative dot plot analyses of the expression of CD127 and FoxP3 by CD4+ T cells as a dependency on the level of expression of CD25 in the presence or absence of TGF-β tyrosine kinase inhibitor (SB431542) or blocking antibodies against PD-L1/2. Abbreviations: hPSC, human pluripotent stem cell; PD-L1/2, programmed cell death ligand 1/2; TGF, transforming growth factor.
Considering that CD4+CD25+ T cells were depleted from PB CD3+ T cells, a subset that represents CD4+CD25high Tregs and CD4+CD25+ TCR-activated effector T cells, we further compared the expression of the key regulator FoxP3 in circulating CD4+CD25+ T cells and pericyte-induced CD4+CD25+ T cells from the CD4+CD25− subset. As seen in Figure 6A, PB CD4+CD25+ T cells contained only a minor population expressing FoxP3 compared with an approximately 10-fold increase in FoxP3 expression within induced CD4+CD25+ mediated by all type of pericytes. In addition, the expression levels of CD25 directly correlated to the FoxP3+CD127− phenotype, with T cells forming three matching clusters (Fig. 6B).
Figure 6.
Positive correlation between pericyte allostimulated expression of CD25 and FoxP3 in converted CD4 T cells. (A): Representative flow cytometry analyses of the expression of FoxP3 in gated CD4+CD25+ subsets that were derived from either PB CD3 T cells (left panels) or isolated CD4+CD25− subset (right panels), which was allostimulated for 72 hours with pericytes. (B): CD25 and FoxP3 expression-based clustering of pericyte-stimulated CD4+CD25− T cells. The nonclustered CD4+CD25high black dot represents T cells that were treated with transforming growth factor-β RK inhibitor in the absence of pericytes. n = 3 lines of hPSC, brain, and placenta pericytes with duplicates and 5 different donors. Abbreviation: PB, peripheral blood.
Implanted hPSC Pericytes Induce Peripheral Tregs From CD25− PB-Derived T Cells
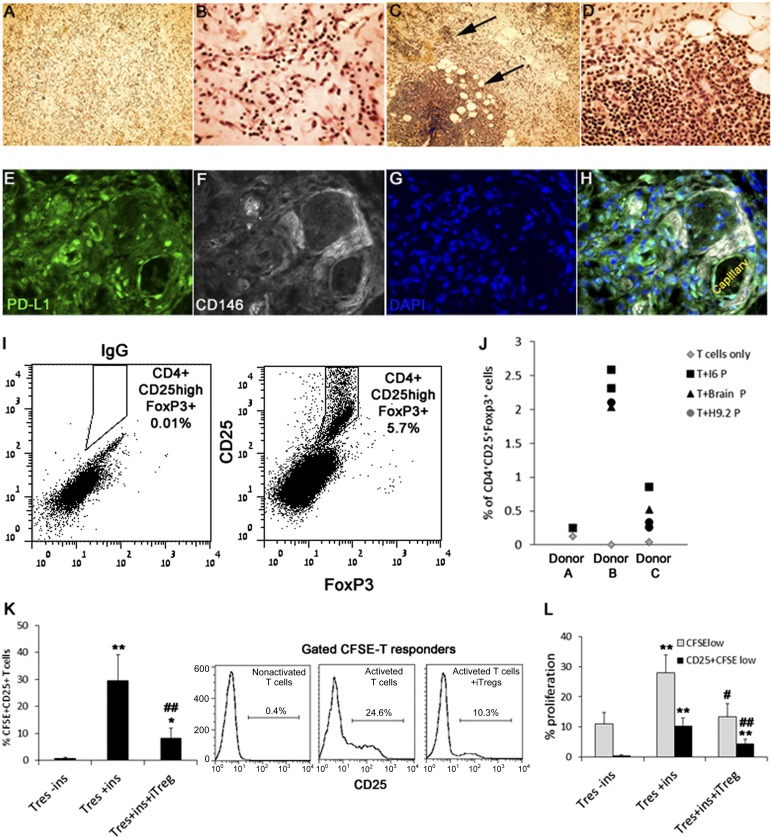
We used the BME implant as an in vivo model for evaluation of a short-term alloimmune modulation of T-cell response by transplanted pericytes. Isolated PB CD4+CD25− T cells were mixed with either brain, placenta, or hPSC pericytes and implanted subcutaneously into NOD/SCID immunodeficient mice. Mixed implants containing PB-allogeneic macrophages served as positive controls for assessments of T-cell activation. In addition, to exclude the possible contribution of host vasculature to the immunomodulatory effects of transplanted pericytes, implants were removed 3 days after transplantation, before the substantial generation of chimeric vascular networks and while pericytes are still viable [8, 40]. Examination of sectioned implants containing T cells and pericytes (Fig. 7A, 7B) or T cells and macrophages (Fig. 7C, 7D) by hematoxylin and eosin staining confirmed that transplanted T cells could be subjected to activation by professional APCs within implants, which is seen as clusters of dense proliferating T cells (Fig. 7C, 7D). In contrast, T cells were approximately evenly distributed between coimplanted pericytes, with no occurrences of local proliferation (Fig. 7A, 7B). Immunohistochemistry staining revealed that hPSC pericytes and brain pericytes maintained PD-L1 expression within BME implants. High expression of PD-L1 was detected in CD146+CD34− hPSC pericytes and CD146+CD34− perivascular placental and brain pericytes in BME T cell mixed implants (Fig. 7E–7H), coinciding with the observed constitutive high expression of PD-L1 by pericyte in cocultures of CD25− T cells and pericytes (Fig. 5A). Consistent with their immunogenicity in vitro, we found that implanted pericytes from hPSCs, brain, or placenta did not prime coimplanted CD4+CD25− T cells to express intermediate levels of CD25 (Fig. 7I) but mediated the generation of CD4+CD25highFoxP3+ Tregs 3 days following implantation (Fig. 7I, 7J). CD25+ pericyte-iTregs were then isolated from enzymatically dissociated BME and tested for their ability to suppress primed T cells using autologous suppression assay. Implant-derived pericyte-iTregs inhibited the activation of primed T cells, as indicated by significant reduction in the frequency of CFSE+CD4+CD25+ responder T cells, which represent primed T responders (Fig. 7K). Additionally, extramedullary pericyte-iTregs efficiently suppressed responder T-cell proliferation as evaluated by CFSE dilution in comparison with matched controls from the same donor (Fig. 7L).
Figure 7.
Generation of Tregs in BME implants from CD3+CD25− PB T cells by transplanted pericytes. (A–D): Hematoxylin and eosin staining of 3-day-old BME implants containing isolated CD3+CD25− PB T cells coimplanted with either pericytes (A, B) or PB macrophages (C, D). Clusters of macrophage-activated proliferating T cells are indicted by arrows. Magnification, ×100 (A, C), ×200 (B, D). (E–H): Representative immunofluorescence labeling of H9.2 pericytes mixed with PB T cells in BME implant, PD-L1+ (green, [E]) and CD146+ (white, [F]) are coexpressed by dispersed and capillary surrounding pericytes (H). Nuclear staining by DAPI (blue, [G, H]). (I, J): Pericyte-induced CD4+CD25+FoxP3+ Tregs from the CD4+CD25− subset in BME implants. (I): Gated CD4+ representative dot plots of IgG control (left) and CD25−, FoxP3- labeled cells from 3-day-old mixed implants. (J): Proportions of BME implanted CD4+CD25− T cells, converted into CD4+CD25+ and coexpressing FoxP3, in the presence and absence of pericytes. (K, L): Pericyte iTregs were isolated from implants and mixed with CFSE-labeled T responders at a ratio of 1:1. Matched controls included incubation of CD25−CFSE+ T responders in the presence or absence of suppression reagent (ins). The effect of pericyte-iTregs on stimulation of CD25 expression (K) or proliferation of CFSE-T responders (L) was evaluated by flow cytometry. Data are mean ± SEM of pericyte-iTregs from four pericytic cell lines with T cells from two different donors. ∗, p < .005 and ∗∗, p < .0005 compared with matched population of untreated T responders. #, p < .05 and ##, p < .005 compared with matched population of activated T responders. Abbreviations: CFSE, carboxyfluorescein succinimidyl ester; DAPI, 4′,6-diamidino-2-phenylindole; ins, inspector reagent; iTreg, induced regulatory T cell; P, pericyte; Tres, Tresponder.
Discussion
Our studies were focused on the immunogenicity of human pericytes from various hPSC lines, brain, and placenta in steady-state homeostasis. We demonstrate here that pericytes are not just poorly immunogenic cells but also actively modulate immune response through induction of functional suppressive regulatory T cells in a PD-L1 (cell-cell contact)-dependent and secreted TGF-β (soluble mediator)-dependent manner. Importantly, long-term cultured pericytes exhibited a stable immunophenotype that resembled that of tissue-embedded or implanted pericytes. It is now well accepted that microvessel-residing and hPSC pericytes share similarities with multipotent mesenchymal precursors, in terms of phenotype and gene expression, and display mesodermal developmental potential in long-term cultures following disengagement from their vascular niche [6, 41]. Based on immunological aspects, our findings extend this notion, demonstrating an additional common feature shared by MSCs and pericytes from multiple sources. Although much knowledge was gathered in past years relating to the modes of action through which autologous and/or allogeneic MSCs suppress adaptive and innate immunity [9, 19, 27], the immunological features of pericytes are just beginning to be described. Together with the fact that pericytes are a heterogeneous population with complex ontogeny and are distributed within multiple tissues, it is important to understand whether these characteristics can be generalized, similarly to that shown for the MSC-like performance of pericytes, regardless of their tissue origin [6]. With this context, it is conceivable to refer to hPSC pericytes as tissue-extrinsic, archetypical cells that are devoid of the variable domestic cues to which tissue-residing pericytes are exposed. In agreement with this assumption, we demonstrate here that hPSC pericytes and pericytes from human brain and term placenta possess characteristics identical to those of poorly immunogenic cells, which directly alloinduce the formation of Tregs from CD4+ naïve circulating T cells. In this respect, it should be confirmed that pericytes from all vascular beds exhibit equivalent immunological features.
In vascularized allografts, ECs of microvessel and capillaries constitutively express MHC class II and a milieu of cytokines and interleukins that make them “semiprofessional” APCs, which in turn participate in alloactivation of circulating memory but not naïve cells [42, 43]. Presently, much effort is invested in the generation and expansion of ECs for the achievement of vascularized engineered tissues. However, similar to MSCs [44], transplanted tissue pericytes [45–48] and hPSC pericytes [8] exhibit the ability to rapidly and efficiently promote de novo formation of vasculature, which is based on the recruitment of recipient-derived, autologous ECs into the nascent blood vessels. The lack of an immediate alloadaptive immune response to hPSC pericytes, together with their ability to promote Treg formation in vivo, places these cells as promising candidates in the field of vascular therapy.
We show here that unlike APC precursors, hPSC pericytes are incapable of maturing into professional APCs even under inflammatory conditions. However, pericytes respond to IFN-γ stimulation by expression of MHC class II, which in the absence of costimulatory molecules induces T-cell anergy over T-cell activation [12]. The immunophenotype of pericytes in vitro and in vivo in various acute or chronic inflammation conditions (in the presence or absence of ECs) and their involvement in functional regulation of innate and adaptive immune responses are still poorly defined. Related studies have demonstrated that pericytes upregulate the expression of intercellular adhesion molecule-1 (ICAM-1) to facilitate and ameliorate T-cell migration into inflamed tissue via haptotactic signals [5, 49]. Together, these findings suggest that physiologically, pericytes participate in amelioration and surveillance of immune responses.
The conversion of subsets of human circulating T cells into suppressive Tregs by stromal cells was shown to be regulated by numerous key mechanisms, including (a) secretion of soluble mediators such as human leukocytes antigen-G5 [50] and TGF-β [33], (b) cell-cell interactions through PD-L1 [23, 43, 51], and (c) pushing monocytes toward an anti-inflammatory/tolerogenic phenotype [27, 33]. In another model, IFN-γ induced expression of MHC class II on human placenta or brain pericytes and resulted in TCR-dependent, clonal alloantigen anergy of allostimulated CD4+ PB T cells [12]. Using unstimulated pericytes, we demonstrate here that brain, placenta, and hPSC pericytes induce the conversion of PB CD4+CD25− T cells to Tregs by a mechanism that is independent of TCR activation or tolerogenic monocytes.
TGF-β has a central role in maintenance and induction of FoxP3 expression in vitro, activating signaling pathways involving SMADs and NFAT, which bind the TGF-β sensitive element (enhancer) within the FoxP3 locus [52, 53]. In accordance with this, we demonstrate here that inhibition of TGF-β signaling was accompanied with a parallel and significant reduction in the frequency of two T-cell subpopulations, CD4+CD25−Foxp3+ and CD4+CD25highFoxp3+, implying that the first may represent a subset of TGF-β-dependent Treg precursors. An experimental model of knockout mice for the Smad3 binding site was used to reveal a fundamental difference between TGF-β/SMAD3-dependent development of Tregs in vitro and in vivo. Whereas SMAD3 was dispensable for the development of Tregs in vivo, it was essential for TGF-β induction of Tregs in vitro [54]. However, in this model, TCR stimulation was performed in parallel with treatments with TGF-β in vitro, similarly to the native development of Tregs in the thymus. In support of the notion that TGF-β regulate peripheral Tregs, TGF-β−/− mice and mice bearing T cell-specific deletion of TGF-βRII have reduced numbers of peripheral Tregs compared with thymic Tregs [38, 39]. Therefore, the question of whether TGF-β-dependent SMAD3 signaling in the absence of TCR activation is requisite for the conversion of naïve T cells into Tregs or for survival of peripheral Tregs remains open. Furthermore, differing from the murine system [55, 56], induction of expression of FoxP3 through TCR activation does not necessarily confer a regulatory phenotype in equivalent human experimental models [57], which can be attributed to transient expression, low levels, or post-translational acetylation of FoxP3 [34, 52, 58]. In this contest, our model serves as a useful tool to study the development of human Tregs in the absence of TCR stimulation. Whereas TGF-β requires TCR signaling for Foxp3 expression by naïve CD4+Foxp3− T cells [51, 57, 59], the induction of Tregs from the CD4+CD25− subset is mediated by pericytes in the absence of MHC class II. This can be attributed to pericytic-PD-L1 interactions with PD1 on T cells. PD1 activation of naïve T cells was shown to induce Treg formation by promoting phosphatase and tensin homolog (PTEN) expression and limiting downstream mTOR activation in a TCR-independent manner [37]. In addition, PD-L1/PD1 interactions, which impair TCR signaling, also recruit SHP1/2 to inactivate the FoxP3 negative regulator STAT1 [60], to induce the conversion of human Th1 cells into Tregs [51]. Accordingly, we demonstrated here that the blockade of PDL-1/2 ligands partially inhibits the preferential induction of Tregs by hPSC and native tissue pericytes in the presence of TGF-β, implying that the mechanisms that govern Treg induction may be different and should be targeted based on the putative phenotypic and epigenetic heterogeneity of Treg precursors within the CD4+CD25− T-cell subset.
From a clinical and physiological perspective, the therapeutic potential of isolated pericytes has been demonstrated in various mammalian pathological models. Intramuscular injection of hPSC pericytes restores blood perfusion and muscle regeneration of murine ischemic limb [8]. Pericytes from human leg veins are already advancing to first-in-human clinical trials for treatment of myocardial ischemia [47]. Human adipose tissue pericytes mediate bone formation and vascularization when combined with Nel-like molecule 1 [61] and protect from retinal vasculopathy in murine model of induced retinopathy [62]. In addition, the MSC features of pericytes imply that they have the potential to prevent graft rejection. Still, health condition, age, and tissue availability can result in a shortage of autologous pericytes in numbers and/or quality suitable for clinical transplantations [63].
It has become increasingly evident that extrinsic environmental cues or the intrinsic FoxP3 expression/epigenetic signature regulates Tregs stability [64, 65]. In effort to integrate reported discrepancies and various new insights related to Treg fate, Sawant and Vignali have recently proposed a three-signal hypothesis of Treg stability, which distinguishes between fate responses of thymic Tregs and peripheral Tregs [65]. These differences are suggested to be dependent on phenotypic heterogeneity of Tregs and stabilizing/destabilizing signals [65]. In this context, we demonstrate here that pericytes deliver stabilizing cues that favor induction of stable bone fide Tregs via secreted TGF-β and increased Foxp3 expression, which in turn correlates with suppressive functionality. However, it is essential to further correlate the epigenetic signature (5′ enhancer and TDSR in the FoxP3 locus) of pericyte-induced Tregs in correlation with CD25 and/or Foxp3 expression and strength of functional suppression in the absence of pericytes. Accordingly, conversions of conventional CD4+CD25−/dim effector T cells into Tregs by human adipose MSCs or Treg induction from CD25− memory T cells by rapamycin-pretreated ECs were accompanied by demethylation of the TSDR region in the FoxP3 gene [23, 66]. In addition, studying the responsiveness of pericyte-induced Tregs to stabilizing/destabilizing factors in the absence of pericytes or their identity in vivo using experimental animal models will assist in exposing their heterogeneity if it exists, their lineage commitment, and their potential capacity to retain Treg characteristics or reacquire a Treg phenotype after conversion into “exTregs.”
Finally, induced and PB Tregs have been proven to maintain their regulatory properties following ex vivo expansion, as well as preventing allograft rejection and providing protection from autoimmune disease, in preclinical experimental models [22, 33, 67]. The potential possibility of further combining the methodology for generation of Tregs from allogeneic or autologous pericytes, either isolated from native tissue or generated from iPSCs, together with existing protocols for Treg expansion, holds great promise for the use of hPSC- and tissue-derived pericytes in stem cell-based therapy.
Conclusion
Using an in vitro coculture model and an extramedullary in vivo model, we demonstrated here that under steady-state conditions, hPSC pericytes maintain poor immunogenicity and immunomodulation capabilities, favoring allostimulation of Tregs over T-cell activation, similarly to their native tissue-derived counterparts.
These findings constitute an important step forward in preclinical characterization of hPSC pericytic derivatives and support the idea that, with further characterization, hPSC pericytes can be applied to allogeneic cell therapy in the clinic, not only without provoking immediate immune responses but also actively modulating suppressive immunity.
Supplementary Material
Acknowledgments
We thank Dr. Ofir Goldenberg and Yakov Sakuri for assistance with flow cytometry and Oren Ben-Yosef for technical assistance. We greatly appreciate the valuable review of Dr. Sonia Berrih-Aknin. This work was conducted in the Berlin Family Laboratory for Stem Cell and Tissue Regeneration Research at the Sohnis and Forman Families Center for Stem Cell and Tissue Regeneration Research, Ruth and Bruce Rappaport Faculty of Medicine, Technion–Israel Institute of Technology, Haifa, Israel.
Author Contributions
H.D.: conception and design, collection and/or assembly of data, data analysis and interpretation; I.M.: collection and/or assembly of data; J.I.-E.: conception and design, data analysis and interpretation, financial support, administrative support, final approval of manuscript; A.D.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Corselli M, Chen CW, Crisan M, et al. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30:1104–1109. doi: 10.1161/ATVBAHA.109.191643. [DOI] [PubMed] [Google Scholar]
- 2.Dore-Duffy P, Cleary K. Morphology and properties of pericytes. Methods Mol Biol. 2011;686:49–68. doi: 10.1007/978-1-60761-938-3_2. [DOI] [PubMed] [Google Scholar]
- 3.Alon R, Shulman Z. Chemokine triggered integrin activation and actin remodeling events guiding lymphocyte migration across vascular barriers. Exp Cell Res. 2011;317:632–641. doi: 10.1016/j.yexcr.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Pober JS, Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol. 2012;33:49–57. doi: 10.1016/j.it.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stark K, Eckart A, Haidari S, et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol. 2013;14:41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 6.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Tormin A, Li O, Brune JC, et al. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011;117:5067–5077. doi: 10.1182/blood-2010-08-304287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dar A, Domev H, Ben-Yosef O, et al. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation. 2012;125:87–99. doi: 10.1161/CIRCULATIONAHA.111.048264. [DOI] [PubMed] [Google Scholar]
- 9.English K, Mahon BP. Allogeneic mesenchymal stem cells: Agents of immune modulation. J Cell Biochem. 2011;112:1963–1968. doi: 10.1002/jcb.23119. [DOI] [PubMed] [Google Scholar]
- 10.Kimbrel EA, Kouris NA, Yavanian GJ, et al. Mesenchymal stem cell population derived from human pluripotent stem cells displays potent immunomodulatory and therapeutic properties. Stem Cells Dev. 2014;23:1611–1624. doi: 10.1089/scd.2013.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.English K, Tonlorenzi R, Cossu G, et al. Mesoangioblasts suppress T cell proliferation through IDO and PGE-2-dependent pathways. Stem Cells Dev. 2013;22:512–523. doi: 10.1089/scd.2012.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maier CL, Pober JS. Human placental pericytes poorly stimulate and actively regulate allogeneic CD4 T cell responses. Arterioscler Thromb Vasc Biol. 2011;31:183–189. doi: 10.1161/ATVBAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu Z, Li Y, Smith DS, et al. Retinal pericytes inhibit activated T cell proliferation. Invest Ophthalmol Vis Sci. 2011;52:9005–9010. doi: 10.1167/iovs.11-8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochs K, Sahm F, Opitz CA, et al. Immature mesenchymal stem cell-like pericytes as mediators of immunosuppression in human malignant glioma. J Neuroimmunol. 2013;265:106–116. doi: 10.1016/j.jneuroim.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Ren G, Su J, Zhang L, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 16.Carrade Holt DD, Wood JA, Granick JL, et al. Equine mesenchymal stem cells inhibit T cell proliferation through different mechanisms depending on tissue source. Stem Cells Dev. 2014;23:1258–1265. doi: 10.1089/scd.2013.0537. [DOI] [PubMed] [Google Scholar]
- 17.Issa F, Robb RJ, Wood KJ. The where and when of T cell regulation in transplantation. Trends Immunol. 2013;34:107–113. doi: 10.1016/j.it.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 19.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 20.Yamane H, Paul WE. Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets. Immunol Rev. 2013;252:12–23. doi: 10.1111/imr.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kingsley CI, Karim M, Bushell AR, et al. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 22.Lu L, Zhou X, Wang J, et al. Characterization of protective human CD4CD25 FOXP3 regulatory T cells generated with IL-2, TGF-β and retinoic acid. PLoS ONE. 2010;5:e15150. doi: 10.1371/journal.pone.0015150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Yi T, Qin L, et al. Rapamycin-treated human endothelial cells preferentially activate allogeneic regulatory T cells. J Clin Invest. 2013;123:1677–1693. doi: 10.1172/JCI66204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 25.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 27.Akiyama K, Chen C, Wang D, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–555. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 29.Amit M, Itskovitz-Eldor J. Derivation and spontaneous differentiation of human embryonic stem cells. J Anat. 2002;200:225–232. doi: 10.1046/j.1469-7580.2002.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novak A, Shtrichman R, Germanguz I, et al. Enhanced reprogramming and cardiac differentiation of human keratinocytes derived from plucked hair follicles, using a single excisable lentivirus. Cell Reprogram. 2010;12:665–678. doi: 10.1089/cell.2010.0027. [DOI] [PubMed] [Google Scholar]
- 31.Domev H, Amit M, Laevsky I, et al. Efficient engineering of vascularized ectopic bone from human embryonic stem cell-derived mesenchymal stem cells. Tissue Eng Part A. 2012;18:2290–2302. doi: 10.1089/ten.TEA.2011.0371. [DOI] [PubMed] [Google Scholar]
- 32.Hof-Nahor I, Leshansky L, Shivtiel S, et al. Human mesenchymal stem cells shift CD8+ T cells towards a suppressive phenotype by inducing tolerogenic monocytes. J Cell Sci. 2012;125:4640–4650. doi: 10.1242/jcs.108860. [DOI] [PubMed] [Google Scholar]
- 33.Melief SM, Schrama E, Brugman MH, et al. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31:1980–1991. doi: 10.1002/stem.1432. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Ioan-Facsinay A, van der Voort EI, et al. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 35.Bacchetta R, Passerini L, Gambineri E, et al. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006;116:1713–1722. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbas AK, Benoist C, Bluestone JA, et al. Regulatory T cells: Recommendations to simplify the nomenclature. Nat Immunol. 2013;14:307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 37.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marie JC, Letterio JJ, Gavin M, et al. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Melero-Martin JM, De Obaldia ME, Allen P, et al. Host myeloid cells are necessary for creating bioengineered human vascular networks in vivo. Tissue Eng Part A. 2010;16:2457–2466. doi: 10.1089/ten.tea.2010.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng J, Mantesso A, Sharpe PT. Perivascular cells as mesenchymal stem cells. Expert Opin Biol Ther. 2010;10:1441–1451. doi: 10.1517/14712598.2010.517191. [DOI] [PubMed] [Google Scholar]
- 42.Al-Lamki RS, Bradley JR, Pober JS. Endothelial cells in allograft rejection. Transplantation. 2008;86:1340–1348. doi: 10.1097/TP.0b013e3181891d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taflin C, Charron D, Glotz D, et al. Regulation of the CD4+ T cell allo-immune response by endothelial cells. Hum Immunol. 2012;73:1269–1274. doi: 10.1016/j.humimm.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Lasala GP, Silva JA, Minguell JJ. Therapeutic angiogenesis in patients with severe limb ischemia by transplantation of a combination stem cell product. J Thorac Cardiovasc Surg. 2012;144:377–382. doi: 10.1016/j.jtcvs.2011.08.053. [DOI] [PubMed] [Google Scholar]
- 45.Kim JH, Jung M, Kim HS, et al. Adipose-derived stem cells as a new therapeutic modality for ageing skin. Exp Dermatol. 2011;20:383–387. doi: 10.1111/j.1600-0625.2010.01221.x. [DOI] [PubMed] [Google Scholar]
- 46.Chen CW, Okada M, Proto JD, et al. Human pericytes for ischemic heart repair. Stem Cells. 2013;31:305–316. doi: 10.1002/stem.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katare RG, Madeddu P. Pericytes from human veins for treatment of myocardial ischemia. Trends Cardiovasc Med. 2013;23:66–70. doi: 10.1016/j.tcm.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janebodin K, Zeng Y, Buranaphatthana W, et al. VEGFR2-dependent angiogenic capacity of pericyte-like dental pulp stem cells. J Dent Res. 2013;92:524–531. doi: 10.1177/0022034513485599. [DOI] [PubMed] [Google Scholar]
- 49.Ayres-Sander CE, Lauridsen H, Maier CL, et al. Transendothelial migration enables subsequent transmigration of neutrophils through underlying pericytes. PLoS ONE. 2013;8:e60025. doi: 10.1371/journal.pone.0060025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 51.Amarnath S, Mangus CW, Wang JC, et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3003130. 111ra120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: The key to a stable regulatory T-cell lineage? Nat Rev Immunol. 2009;9:83–89. doi: 10.1038/nri2474. [DOI] [PubMed] [Google Scholar]
- 53.Frazier TP, McLachlan JB, Gimble JM, et al. Human adipose-derived stromal/stem cells induce functional CD4+CD25+FoxP3+CD127- regulatory T cells under low oxygen culture conditions. Stem Cells Dev. 2014;23:968–977. doi: 10.1089/scd.2013.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schlenner SM, Weigmann B, Ruan Q, et al. Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. J Exp Med. 2012;209:1529–1535. doi: 10.1084/jem.20112646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davidson TS, DiPaolo RJ, Andersson J, et al. Cutting edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 56.Zheng SG, Wang J, Wang P, et al. IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 57.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li B, Samanta A, Song X, et al. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci USA. 2007;104:4571–4576. doi: 10.1073/pnas.0700298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leibowitz MS, Srivastava RM, Andrade Filho PA, et al. SHP2 is overexpressed and inhibits pSTAT1-mediated APM component expression, T-cell attracting chemokine secretion, and CTL recognition in head and neck cancer cells. Clin Cancer Res. 2013;19:798–808. doi: 10.1158/1078-0432.CCR-12-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Askarinam A, James AW, Zara JN, et al. Human perivascular stem cells show enhanced osteogenesis and vasculogenesis with Nel-like molecule I protein. Tissue Eng Part A. 2013;19:1386–1397. doi: 10.1089/ten.tea.2012.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mendel TA, Clabough EB, Kao DS, et al. Pericytes derived from adipose-derived stem cells protect against retinal vasculopathy. PLoS ONE. 2013;8:e65691. doi: 10.1371/journal.pone.0065691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu KR, Kang KS. Aging-related genes in mesenchymal stem cells: A mini-review. Gerontology. 2013;59:557–563. doi: 10.1159/000353857. [DOI] [PubMed] [Google Scholar]
- 64.da Silva Martins M, Piccirillo CA. Functional stability of Foxp3+ regulatory T cells. Trends Mol Med. 2012;18:454–462. doi: 10.1016/j.molmed.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Sawant DV, Vignali DA. Once a Treg, always a Treg? Immunol Rev. 2014;259:173–191. doi: 10.1111/imr.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Engela AU, Hoogduijn MJ, Boer K, et al. Human adipose-tissue derived mesenchymal stem cells induce functional de-novo regulatory T cells with methylated FOXP3 gene DNA. Clin Exp Immunol. 2013;173:343–354. doi: 10.1111/cei.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu DC, Hester J, Nadig SN, et al. Ex vivo expanded human regulatory T cells can prolong survival of a human islet allograft in a humanized mouse model. Transplantation. 2013;96:707–716. doi: 10.1097/TP.0b013e31829fa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.