Abstract
Luliconazole is an imidazole antifungal agent with a unique structure, as the imidazole moiety is incorporated into the ketene dithioacetate structure. Luliconazole is the R-enantiomer, and has more potent antifungal activity than lanoconazole, which is a racemic mixture. In this review, we summarize the in vitro data, animal studies, and clinical trial data relating to the use of topical luliconazole. Preclinical studies have demonstrated excellent activity against dermatophytes. Further, in vitro/in vivo studies have also shown favorable activity against Candida albicans, Malassezia spp., and Aspergillus fumigatus. Luliconazole, although belonging to the azole group, has strong fungicidal activity against Trichophyton spp., similar to that of terbinafine. The strong clinical antifungal activity of luliconazole is possibly attributable to a combination of strong in vitro antifungal activity and favorable pharmacokinetic properties in the skin. Clinical trials have demonstrated its superiority over placebo in dermatophytosis, and its antifungal activity to be at par or even better than that of terbinafine. Application of luliconazole 1% cream once daily is effective even in short-term use (one week for tinea corporis/cruris and 2 weeks for tinea pedis). A Phase I/IIa study has shown excellent local tolerability and a lack of systemic side effects with use of topical luliconazole solution for onychomycosis. Further studies to evaluate its efficacy in onychomycosis are underway. Luliconazole 1% cream was approved in Japan in 2005 for the treatment of tinea infections. It has recently been approved by US Food and Drug Administration for the treatment of interdigital tinea pedis, tinea cruris, and tinea corporis. Topical luliconazole has a favorable safety profile, with only mild application site reactions reported occasionally.
Keywords: luliconazole, NND-502, fungal infections, dermatophytes, onychomycosis, clinical trials, review, fungal infections
Core evidence Clinical impact summary for luliconazole
| Outcome measures | Evidence | Implications |
|---|---|---|
| Disease-oriented evidence | Clear evidence from in vitro studies and animal models showing significant antidermatophyte activity. In vitro studies also demonstrate good activity against Candida and Malassezia. |
Effective therapy for management of tinea corporis/cruris and pedis. Results of trials in onychomycosis awaited. Need for clinical studies in patients with candidiasis. |
| Patient-oriented evidence | Multiple clinical trials demonstrate efficacy against dermatophytes, even on short-term use. Few drug induced adverse effects. | May be used as one of the first line therapies for treatment of tinea corporis/cruris and tinea pedis. Short course of treatment effective, leading to greater compliance. |
| Economic evidence | None available currently | – |
Introduction and search strategy
Luliconazole is an imidazole antifungal agent that has been shown to have potent activity against a variety of fungi, especially dermatophytes. This evidence-based review details the pharmacodynamics of topical luliconazole and outlines its place in the treatment of fungal infections. The English language medical literature was searched in October 2013 using the search terms “luliconazole” and “NND-502” in the following databases:
EMBASE (http://www.datastarweb.com)
BIOSIS (http://www.datastarweb.com)
Database of Abstracts of Reviews of Effects (http://www.york.ac.uk/inst/crd/crddatabases.htm)
Cochrane Database of Systematic Reviews (http://www.cochrane.org/index.htm)
Clinical Evidence (BMJ) (http://www.clinicalevidence.com)
National Institute for Health and Clinical Excellence (http://www.nice.org.uk)
National Guideline Clearinghouse (http://www.guideline.gov)
ClinicalTrials.gov (http://www.clinicaltrials.gov)
IndMED (http://www.indmed.nic.in/indmed.html)
Google (google.com).
A total of 17 publications was identified using the above search strategy (Table 1). These included six clinical trials, ten preclinical studies, and one Phase I–II pharmacokinetic study. The search was repeated in March 2014, yielding one more clinical trial. Three meeting abstracts were also included.
Table 1.
Evidence base included in this review
| Category | Number of records
|
|
|---|---|---|
| Full papers | Abstracts | |
| Records included from initial search | 17 | – |
| Additional papers identified | 1 | 3 |
| Level 1 clinical evidence | 6 | – |
| Level 2 clinical evidence | 1 | – |
| Level 3 clinical evidence | – | – |
| Trials other than RCT | – | – |
| Case studies | 2 | – |
| Economic evidence | – | – |
| Total papers included | 18 | 3 |
Abbreviation: RCT, randomized controlled trial.
Disease overview
Fungal infections are a major health problem and an important cause of morbidity.1 Fungal infections may be categorized as superficial or invasive. Superficial fungal infections affect as many as 20%–25% of the world’s population and are associated with interference with daily activities, poor quality of life, and health care expenditure.1 Invasive fungal infections are usually encountered in the presence of one or more predisposing factors, such as in critically ill or immunocompromised patients and those with indwelling catheters and devices, and deep or systemic fungal infections are an important cause of hospitalization and mortality.
Superficial fungal infections can be attributed to dermatophytes, Candida, and Malassezia spp. infection. Dermatophytes are aerobic fungi and the most common offenders in superficial fungal infections. Physiologically, these dermatophytes have the ability to digest keratin for growth and replicate in the superficial layers of the epidermis. Consequently, in clinical practice, the body parts most affected by dermatophytic infection are those rich in keratin, such as the hair, skin, and nails. Survival of embedded arthroconidia for years in scales of hair and skin leads to frequent recurrence or relapse. The causative dermatophytes belong to three genera, ie, Trichophyton, Microsporum, and Epidermophyton. The classic presentation of dermatophytosis is that of an annular or ring-shaped red scaly plaque with central clearing, often associated with severe pruritus. The clinical manifestations of dermatophyte infections vary according to the site of infection and the patient’s immunologic response. Genetic susceptibility is also known to affect the predisposition to dermatophyte infections.2 Tinea pedis, or dermatophytosis of the feet, is the commonest presentation, and is most frequently caused by Trichophyton rubrum. Tinea cruris and tinea corporis are the next most common fungal infections, and are caused by T. rubrum, T. mentagrophytes, and Epidermophyton floccosum. Onychomycosis, or invasion of the nail plate by fungi, can be due to dermatophytes, Candida, or non-dermatophytic molds.
Candidosis is an infection caused by yeasts of the genus Candida. Superficial infections of the mucous membranes and skin are most frequent, but Candida can also cause deep invasive disease, including septicemia, endocarditis, and meningitis. Candida albicans is the member of the genus most commonly isolated from cutaneous infections, while others such as C. tropicalis, C. pseudotropicalis, C. parapsilosis, and C. krusei are occasional causes of human infection, seen more commonly in disseminated infections and in immunocompromised hosts. Oral candidiasis, or oral thrush, is an infection of the oral mucosa with the yeast. Most cases of cutaneous candidosis occur in the skin folds or where occlusion by clothing or medical dressings produces abnormally moist conditions. Periorificial areas and fingers that are frequently contaminated with saliva are also at risk. Candidal intertrigo and vulvovaginal candidiasis are the common presentations.3
The genus Malassezia includes multiple lipid-dependent species, the most common being M. sympodialis, M. globosa, M. restricta, M. slooffiae, M. furfur, and M. obtus. Colonization by these species is especially dense in the scalp, upper trunk, and flexures, areas rich in sebaceous glands and their secretions. Malassezia spp. are the cause of pityriasis versicolor and Malassezia folliculitis, and are also believed to have a role in seborrheic dermatitis.3
Antifungal agents
Treatment strategies to deal with fungal infections involve use of a systemic or topical antifungal agent. Ergosterol is an integral part of the fungal cell membrane. All the currently available antifungals interfere with the biosynthesis of ergosterol, an important component of the fungal cell wall, thus causing inhibition of fungal growth and replication. However, their action on different enzymes in the same pathway potentially results in different properties and degrees of efficacy. Allylamines are squalene epoxidase inhibitors and act early in the course of ergosterol biosynthesis, with resultant accumulation of squalene that is toxic to the fungal cell membrane and responsible for the fungicidal activity of allylamines. They have very good efficacy against Trichophyton spp. but only fungistatic action against C. albicans and M. furfur. Amorolfine, a morpholine antifungal compound that inhibits both C14 reductase and C7–C8 isomerase activity, has potent activity against Trichophyton spp., C. albicans, and M. furfur.4 The azole antifungals inhibit 14α-lanosterol demethylase, and have potent activity against C. albicans and Trichophyton spp. Accumulation of lanosterol has a less toxic effect than squalene, so imidazoles have a fungistatic action only.5 The efficacy of topical agents in the treatment of superficial mycoses depends not only on the type of lesion and the actual mechanism of action of the drug, but also on the viscosity, hydrophobicity, and acidity of the formulation and its distribution and retention in the stratum corneum. Regardless of the type of formulation, penetration of topical agents in hyperkeratotic lesions is often uncertain.6
Unmet needs
Adequate treatment of cutaneous mycoses with most of the currently used antifungals requires prolonged treatment for complete clearance of the fungal elements. Noncompliance with the prolonged topical treatment usually required is frequent once the clinical features begin to subside. It is possible that a small number of dermatophytes below the detection limit can survive in these partially treated lesions and/or surrounding tissues. This often leads to poor compliance, as patients frequently discontinue treatment once clinical improvement starts to show. As a result, the high rate of relapse in patients who were previously considered cured is one of the biggest challenges in the treatment of fungal infections. To tackle this, it is desirable to develop antifungals with fungicidal activity that attain mycologic negativity even after short-term use.
The ideal topical antifungal agent for superficial dermatophytosis should have broad-spectrum activity, efficacy at low concentrations, fungicidal activity with convenient dosing schedules, keratinophilic and lipophilic effects, high mycologic and clinical cure rates, a reservoir effect in the stratum corneum, lack of development of fungal resistance, low relapse rates, a low incidence of adverse effects, and a low cost.7
The use of orally administered drugs, which are the backbone of therapy for onychomycosis, is limited by the risk of hepatotoxicity and possible drug–drug interactions with other systemic medications. An ideal formulation for onychomycosis needs to penetrate through the nail plate as well as maintain high levels at the infection site in the nail bed for a prolonged duration to achieve eradication of fungus and produce a cure. However, currently available topical therapies have the drawbacks of low efficacy, recurrence, and need for prolonged treatment.
Azole antifungals
Development of the imidazole group of antifungals was a turning point in the treatment of superficial and deep mycoses, due to their high efficacy and low toxicity, as well as their immunomodulatory activity.6 Miconazole is the first member of the conazole pedigree. However, problems associated with the use of topical miconazole included the need for twice-daily application, a prolonged treatment duration, and the presence of fungistatic and not fungicidal activity, leading to frequent recurrences. Bifonazole has potent antifungal activity and is retained well in the stratum corneum, and was the first topical agent to be used in a once-daily regimen.8
Azoles can be either imidazoles (two nitrogen atoms in the azole ring) or triazoles (three nitrogen atoms in the azole ring). They are fungistatic, except in high concentrations, when they can also be fungicidal. As the triazoles have greater affinity for fungal compared with mammalian P450 enzymes, their safety profile is significantly improved over the imidazoles. The use of imidazoles is limited to treating superficial mycoses. Currently available topical imidazoles include clotrimazole, econazole, ketoconazole, miconazole, oxiconazole, isoconazole, bifonazole, sertaconazole, tioconazole, butoconazole, eberconazole, and luliconazole.
In the late 1970s, Niwano et al found that introduction of an imidazole moiety onto a ketene dithioacetal structure increased its antifungal activity manifold. Lanoconazole, the compound thus generated, has been shown to have activity against a variety of fungi, including yeast, dermatophytes, and dematiaceous fungi, and has significant fungicidal activity against Trichophyton spp. It has also been shown that a sufficient amount of lanoconazole is retained in the skin for long periods after application. Lanoconazole is a racemic mixture, and further studies revealed that its antifungal activity is attributed to the R-enantiomer, and the latter has at least two-fold more potent antifungal activity when compared with the racemic compound.9
Chemistry and pharmacokinetics
Luliconazole, also known as NND-502, is an imidazole anti-fungal first synthesized by Nihon Nohyaku Co Ltd (Osaka, Japan). It has a unique structure as the imidazole moiety is incorporated into the ketene dithioacetate structure. It is an optically related compound of lanoconazole, with a 2,4-dichlorophenyl group on the ketene dithioacetal structure. The chemical structure of luliconazole, ie, (−)-(E)-[4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene]-1-imidazolylacetonitrile, is shown in Figure 1. Similar to lanoconazole, the S-enantiomer is inactive, so luliconazole, being the active R-enantiomer, has more potent antifungal activity than lanoconazole. It has been reported to have strong in vitro antifungal activity against Trichophyton spp., C. albicans, and Aspergillus fumigatus.10,11 Luliconazole 1% cream was approved in Japan in 2005 for the treatment of tinea infections, followed by approval in November 2013 by the US Food and Drug Administration for the treatment of interdigital tinea pedis, tinea cruris, and tinea corporis caused by the organisms T. rubrum and E. floccosum, in patients 18 years of age and older. It is indicated for once-daily application for one week in tinea corporis/cruris and for 2 weeks in tinea pedis. In June 2009, the 1% cream was approved for marketing in India.
Figure 1.
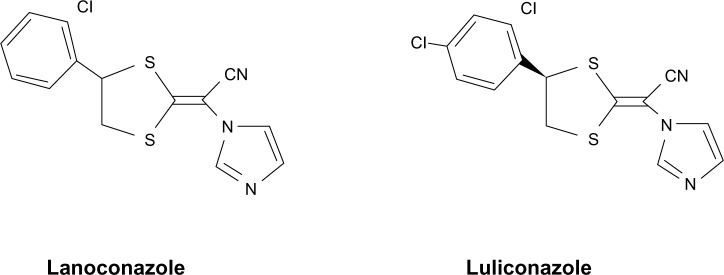
Chemical structure of lanoconazole and luliconazole.
Preclinical studies
The MIC of luliconazole against Trichophyton spp. has been shown to be 2–4 times lower than that of lanoconazole, and the lowest amongst a wide variety of drugs tested, including terbinafine, liranaftate, butenafine, amorolfine, ketoconazole, clotrimazole, neticonazole, miconazole, bifonazole, and sertaconazole.9,12–15
The MIC of luliconazole against Candida spp. has been reported to be higher than that against filamentous fungi; however, it is similar to lanoconazole and greater than that of bifonazole, terbinafine, and amorolfine.12,13,15 The MIC against C. albicans was higher than that of ketoconazole, clotrimazole, neticonazole, and miconazole.12 Luliconazole has been shown to be many times more effective than lanoconazole and bifonazole in inhibiting 14α demethylase of C. albicans.16 In one study, the MIC of luliconazole was shown to be similar to that of flucytosine against the C. albicans IFO 1270 strain and 1–4 times lower than that of flucytosine against other strains of C. albicans; however, in vivo susceptibility of systemic infection with C. albicans strain IFO 1270 was much lower for oral luliconazole than for flucytosine. Luliconazole was found to be 150 times less potent than flucytosine in controlling systemic C. albicans infection. The authors attributed this difference to the different pharmacokinetic properties of the two compounds. Good oral absorption, metabolic stability, and low protein binding in animals/humans possibly translated into lower in vitro activity of luliconazole despite its higher in vitro activity when compared with flucytosine.11
Uchida et al17 reported the MIC range of luliconazole against M. restricta (considered a pathogenic factor in seborrheic dermatitis) to be almost comparable to or lower than ketoconazole, a drug that is most commonly employed in the management of Malassezia infections.17–19 The activity of luliconazole has been found to be almost equal to lanoconazole and 3–4 times more potent than that of bifonazole and terbinafine against M. furfur, M. sympodialis, and M. slooffiae.17
Brown et al recently demonstrated that therapeutic levels of luliconazole were achieved across full-thickness human nail plate within 7 days of daily dosing with 10% luliconazole solution in an in vitro T. rubrum-infected nail model.20 They also reported that luliconazole 12.5% solution resulted in a significant reduction (<90%) of viable dermatophytes recovered from the nail when compared with that from an infected non-dosed control nail at 7 days. It was successful in eradicating the fungal load within 21 days of application.21 They also demonstrated that luliconazole significantly reduced the amount of adenosine triphosphate measured when compared directly with a commercially available formulation of ciclopirox nail lacquer at equivalent time points (P<0.001). Reduction in the amount of adenosine triphosphate was a marker of antifungal activity.21 Low binding affinity for keratin allows luliconazole to be released from the keratinous nail plate and be transported across the nail bed. In contrast with many other azoles, its potency remains unaffected by keratin.20,22
Animal studies
Because of their susceptibility to dermatophytosis and their large body surface area for performing experiments to determine clinical and mycologic drug efficacy against dermatophytes, guinea pigs are the first choice as test subjects for in vivo studies. The in vivo studies have used the guinea pig tinea pedis model developed by Fujita and Matsuyama, in which dermatophyte infection of the foot is sustained for a long period without spontaneous regression and the clinical and histopathologic features resemble those of naturally occurring tinea pedis in humans.23–25 This is in contrast with the previously used hairy skin model produced by inoculating T. mentagrophytes into the dorsal hairy skin of the guinea pig, producing pathologic features similar to those of kerion celsi, a known deep-seated form of tinea affecting the human scalp. This model has the drawback of spontaneous healing within 2–3 weeks of infection, which interferes with evaluation of the drug in question.26
In the first in vivo experiment conducted by Niwano et al (using the abovementioned guinea pig model), 0.5% of luliconazole, lanoconazole, or terbinafine were applied for 3 or 7 days. Treatment with 0.5% luliconazole resulted in negative cultures (5 days after last treatment) in 70% and 100% of animals in the 3-day and 7-day treatment groups, respectively. Although terbinafine and lanoconazole were both highly effective, mycologic negativity was achieved in only 30% and 50% animals, respectively, even after 7 days of treatment.10 In the second experiment, the procedure used for infection was identical to the method described by Uchida et al.27 Three concentrations of luliconazole (0.25%, 0.5%, and 1%) were compared with each other and with reference drugs, ie, lanoconazole and terbinafine. The therapeutic efficacy was noted to increase with concentration of the drug, and 100% mycologic negativity was attained with 1% luliconazole. However, negative cultures were not obtained in any of the animals treated with terbinafine or lanoconazole.10
Topical application of luliconazole for one week has also been shown to achieve a complete mycologic cure in a guinea pig model of tinea pedis.28 These authors also used the guinea pig model developed by Fujita and Matsuyama, and all animals were culture-positive even 20 weeks post inoculation, thereby ruling out any possibility of spontaneous healing. None of the animals showed culture positivity 16 weeks after completion of one week of treatment with topical 1% luliconazole cream. The authors suggested that short-term treatment with the drug offers a high possibility of complete cure.28
Ghannoum et al have also compared the efficacy of 1% luliconazole cream with that of lanoconazole and terbinafine using the guinea pig model earlier optimized by them.29,30 Untreated infected animals showed hair loss, marked ulceration, and occasional scabbing. Three parameters were assessed, ie, reduction of erythema, disappearance of skin lesions, and regrowth of hair. They found the clinical response in guinea pigs treated with luliconazole (50.8%) to be higher than that with lanoconazole (26.2%) but somewhat lower than with terbinafine (56.4%, no statistically significant difference versus terbinafine). However, mycologic assessment showed 100% efficacy and clearance of the dermatophyte from the test area using the hair root invasion assay in all the groups. The authors attributed the difference in clinical efficacy to the different mechanisms of action of the study drugs, with terbinafine being primarily fungicidal while azoles are fungistatic.29
Koga et al recently conducted a dose-response study evaluating the in vivo clinical efficacy of different concentrations (0.02%, 0.1%, 0.5%, 1%) of once-daily luliconazole and 1% bifonazole in the management of tinea corporis in the guinea pig. Luliconazole cream was demonstrated to have concentration-dependent clinical efficacy, with concentrations of 0.5% and above achieving a complete mycologic cure. They also conducted a short-term study evaluating the efficacy of 1% luliconazole applied for 4 days or 8 days for tinea corporis and applied for 7 days or 14 days for tinea pedis. In the tinea corporis group, the 4-day regimen of 1% luliconazole cream eliminated fungi from the skin at the site of infection and the 8-day regimen completely cured the infection, with 100% culture negativity achieved in both groups after 14 days of treatment with 1% luliconazole. In contrast, terbinafine 1% cream and bifonazole 1% cream displayed only moderate and slight efficacy, respectively. Superiority of luliconazole over terbinafine and bifonazole was similarly demonstrated in the tinea pedis group.31
Oral administration of luliconazole for 7 days was shown to prevent death in a dose-dependent manner in both immunocompetent and immunocompromised mice with systemic aspergillosis. In contrast, fluconazole had no protective effect, even at a dose of 50 mg/kg/day, while itraconazole 20 mg/kg/day prolonged survival only slightly. Likewise, 7 days of treatment with intravenous luliconazole was more effective than amphotericin B in prolonging survival in immunocompromised mice with pulmonary aspergillosis.11
The MIC of luliconazole has been reported to be many times lower than that of terbinafine. Further, luliconazole has been shown to have favorable pharmacokinetic properties in the skin when compared with terbinafine.32 The drug concentration in the stratum corneum of skin with tinea in guinea pigs treated with 1% luliconazole cream was significantly higher than that found in those treated with 1% terbinafine cream. Luliconazole has also been shown to be easier to release from the stratum corneum as compared with terbinafine.31 Thus, the strong clinical antifungal activity of luliconazole may be attributable to a combination of strong in vitro antifungal activity and favorable pharmacokinetic properties in the skin.
Clinical studies
Various clinical studies have provided evidence of the efficacy of luliconazole in cutaneous dermatophyte infections (Table 2). A multicenter, randomized, single-blind, two-way, parallel-group study compared the clinical efficacy of 1% luliconazole cream applied once daily for 2 weeks with that of bifonazole 1% cream applied once daily for 4 weeks in patients with tinea pedis. Pregnant patients and those with severe hyperkeratosis, contact dermatitis, or secondary infection in the affected skin area, and those with a serious systemic disorder or a history of intake of systemic antimycotics within the previous 8 weeks or topical antimycotics in the previous 4 weeks were excluded from the study. A total of 489 patients were included. In the luliconazole group, the patients applied placebo cream for 2 weeks after 2 weeks of treatment with the active drug. Mycologic efficacy was assessed by KOH microscopy and post treatment fungal cultures were performed in patients who had positive microscopy after 2 weeks of therapy. Clinical efficacy at the end of 4 weeks was found to be similar for both groups, with 91.5% of patients in the luliconazole group and 91.7% patients in the bifonazole group showing at least moderate improvement. Both study drugs showed similar efficacy in achieving negative KOH microscopy (76.1% for luliconazole versus 75.9% for bifonazole). Fungal cultures were performed in patients with positive KOH (luliconazole 112 patients, bifonazole 117 patients) at week 2. Luliconazole 1% cream demonstrated superior efficacy over bifonazole in achieving mycologic cure (negative culture), with 73% of patients found to be culture-negative in the luliconazole group in contrast with 50% of patients in the bifonazole group. The clinical and mycologic efficacy of short-term (2-week) treatment with luliconazole was found to be at par with the standard 4 weeks of treatment with bifonazole for tinea pedis.33
Table 2.
Summary of clinical studies on use of topical luliconazole cream in dermatophyte infections
| Subtype of tinea | Patients (n) | Study design | Clinical outcome | Mycologic outcome | Type of study | Reference |
|---|---|---|---|---|---|---|
| Tinea pedis | 489 | LLCZ 1% (OD) for 2 weeks versus BFZ 1% (OD) for 4 weeks | Clinical efficacy LLCZ 91.5% BFZ 91.7% |
Negative microscopy LLCZ 76.1%, BFZ 75.9% Negative culture LLCZ 73%, BFZ 50% |
Multicenter, randomized, single-blind, parallel, two-group comparison | Watanabe et al33 |
| Tinea pedis | 213 | Comparison of various concentrations of luliconazole (0.1%, 0.5%, and 1%) applied OD for 2 weeks | Global improvement at 4 weeks 0.1% LLCZ 90.5%, 0.5% LLCZ 91%, 1% LLCZ 95.8% |
Negative microscopy at 6 weeks 0.1% LLCZ 87.7%, 0.5% LLCZ 94%, 1% LLCZ 88.9% |
Dose-finding comparison | Watanabe et al34 |
| Tinea corporis and cruris | 83 | Comparison of SER 2% (BD for 4 weeks), TBF 1% (OD for 2 weeks), LLCZ 1% (OD for 2 weeks) | ↓ in mean total composite score SER 97.1%, TBF 91.2%, LLCZ 92.9% |
Successful outcome (clinical cure plus negative KOH) SER 100%, TBF 86.4%, LLCZ 95% |
Randomized, multicenter, open-label, parallel | Jerajani et al35 |
| Interdigital tinea pedis | 147 | LLCZ 1% (OD) versus vehicle for 2 or 4 weeks | Clinical and mycological cure LLCZ (2 weeks) 6.8%, LLCZ (4 weeks) 45.7%, Vehicle (2/4 weeks) ∼10% |
Negative microscopy at 4 weeks from baseline LLCZ (2 weeks) 78%, LLCZ (4 weeks) 77.1%, Vehicle 8%–10% |
Randomized, double-blind vehicle controlled | Jarratt et al36 |
| Tinea cruris | 256 | LLCZ 1% (OD) versus vehicle for one week | Complete clearance LLCZ 21.2%, vehicle 4.4% Effective treatment LLCZ 43%, vehicle 18.7% |
Both microscopy and culture negative LLCZ 78.2%, Vehicle 45.1% | Randomized, multicenter, double-blind, vehicle-controlled | Jones et al37 |
| Cutaneous mycoses | 150 | Comparison of LLCZ, SER, AMO, TBF, EBR (OD) For one week in tinea corporis/cruris and 2 weeks in tinea pedis |
Efficacious treatment SER 93.3%, LLCZ 86.6%, AMO 83.3%, TBF 80%, EBR 73.3% |
NA | Multicenter, randomized, open-label, comparative | Selvan et al38 |
| Tinea corporis/cruris | 60 | TBF 1% versus LLCZ 1% for 2 weeks | Day 15 and day 30 composite score – zero in both groups | KOH microscopy negative in all patients at day 15 (n=60) and at day 30 (n=51) | Prospective, parallel | Lakshmi et al39 |
Abbreviations: LLCZ, luliconazole; TBF, terbinafine; BFZ, bifonazole; SER, sertaconazole, EBR, eberconazole; AMO, amorolfine; KOH, potassium hydroxide; NA, not available; OD, once daily; BD, twice daily.
A subsequent multiclinic, randomized, double-blind, three-way, parallel-group comparative study evaluated different strengths of luliconazole cream (1%, 0.5%, and 0.1%) applied once daily for 2 weeks, and 213 participants with tinea pedis completed the study.34 The exclusion criteria were similar to those in the study described above.33 Follow-up was undertaken 4 weeks after the end of topical treatment. An overall assessment was done for all patients, based on both clinical symptoms and mycologic evaluation (by KOH microscopy). All patients reached a plateau at week 4 in terms of efficacy as compared with efficacy at week 6. Rates for improvement of skin lesions in the groups treated with luliconazole 1%, 0.5%, or 0.1% cream assessed at week 4 were 90.5%, 91.0%, and 95.8%, respectively. Achievement of mycologic negativity (by KOH microscopy) at week 4 was 79.7%, 76.1%, and 72.2% and at week 6 (4 weeks after the end of topical treatment) was 87.7%, 94%, and 88.9%, respectively. Achievement of negative fungal microscopy when assessed separately for interdigital tinea pedis was found to be concentration-dependent and indicated a statistically significant difference – 81.1% (1% luliconazole), 62.9% (0.5% luliconazole), and 58.3% (0.1% luliconazole). The authors concluded that luliconazole 1% cream is an effective treatment for tinea pedis, even when used in the short term.34
In India, Jerajani et al conducted a prospective, randomized, multicenter, open-label, parallel-group study in adults aged 18–70 years with a clinical diagnosis and at least positive KOH microscopy for tinea corporis and tinea cruris. Patients with a clinical diagnosis of tinea pedis/manuum, those who had received topical or oral antimycotic therapy one or four weeks, respectively, prior to initiation of the study, those with a history of hypersensitivity to the study drugs, immunocompromised status, or bacterial superinfection, and pregnant or lactating women were excluded from the study. The patients were randomized into three groups to receive: sertaconazole 2% cream applied topically twice daily for 4 weeks, terbinafine 1% cream once daily for 2 weeks, or luliconazole 1% cream once daily for 2 weeks. In addition to assessment in the treatment phase, patients were also assessed clinically and mycologically for relapse 2 weeks after the end of treatment. In the 62 patients who completed the study, mycologic response was based on KOH microscopy. At baseline, the composite score (maximum possible score =10) for all clinical symptoms and signs of tinea infection (total of pruritus, erythema, vesicles, and desquamation graded as 0 to 3 based on severity) was 6.80 in the sertaconazole group, 6.73 in the terbinafine group and 7.05 in the luliconazole group. At the end of the treatment phase, there was a greater reduction in the mean total composite score in the sertaconazole group (97.1%) when compared with the terbinafine group (91.2%) and the luliconazole group (92.9%). At the end of the follow-up phase, the mean total composite score was zero in the sertaconazole and luliconazole groups and 0.05 in the terbinafine group. The successful treatment outcome rate (defined as a clinical cure plus negative KOH microscopy at end of the treatment phase) was 100% in the sertaconazole group when compared with the terbinafine group (86.4%) and the luliconazole group (95%). Four weeks of treatment with twice-daily sertaconazole was concluded to be slightly more effective than 2 weeks of treatment with once-daily luliconazole for tinea corporis/cruris.35
A double-blind, randomized, vehicle-controlled study was conducted by Jarratt et al to compare the efficacy of topical 1% luliconazole cream used for either 2 or 4 weeks. Patients aged older than 12 years with a clinical diagnosis of tinea interdigitalis and presence of fungal hyphae on KOH microscopy were included. Participants in whom culture was positive and in whom species identification was possible were continued on treatment. Participants with negative culture for dermatophytes were excluded from the efficacy assessment and included only in the safety population. Patients with moccasin-type tinea pedis, concurrent onychomycosis, severe dermatophytosis, or concurrent bacterial skin infection were excluded, as were immunocompromised, pregnant, or lactating patients and those with hypersensitivity to imidazole compounds. A minimum washout period of 30 days for topical antifungals and topical or systemic steroids, 8 months for oral terbinafine, and 8 weeks for all other systemic antifungal agents was mandatory. Patients were randomly allocated into four groups to receive luliconazole 1% cream or vehicle applied once daily for 2 or 4 weeks. One hundred and eighteen patients were evaluated at weeks 0, 2, 4, and 6 in the 2-week treatment groups and at weeks 0, 2, 4, 6 and 8 in the 4-week treatment groups. At the primary efficacy assessment (2 weeks post treatment), complete clearance, defined as both clinical and mycologic cure (negative KOH microscopy and fungal culture), was seen in 26.8% and 45.7% of participants in the 2-week and 4-week luliconazole groups, respectively. In contrast, approximately 10% patients in each vehicle group showed complete clearance. In the 2-week active treatment group, 58.5% (24/41) of participants demonstrated a mycologic cure at the end of treatment, which increased to 78.0% (32/41) at 2 weeks post treatment and 82.9% (34/41) at 4 weeks post treatment (day 42). Similarly, in the 4-week active treatment group, 77.1% (27/35) of participants demonstrated a mycologic cure at the end of treatment, which increased to 88.6% (31/35) at 2 weeks post treatment and 91.4% (32/35) at 4 weeks post treatment (day 56). The proportion of participants with a clinical cure in the 2-week and 4-week vehicle groups remained approximately the same at 2 weeks and 4 weeks post treatment, but the proportion of participants with a mycologic cure decreased by 40% in the 2-week vehicle group and remained the same in the 4-week vehicle group. Antifungal-induced fungal clearance and clinical improvement would be expected to increase and be maintained over a period of time, even after cessation of treatment because of fungal eradication. However, the response observed early on with the use of vehicle is likely to be nonspecific and unsustainable. Doubling the duration of treatment with luliconazole cream 1% from 2 to 4 weeks only modestly increased mycologic and/or clinical cure rates when measured at the end of treatment, suggesting that the potent fungicidal activity of luliconazole is rapid and does not require prolonged treatment. The mycologic cure rates in the 4-week active treatment group were 77.1%, 88.6%, and 91.4% at the end of treatment and 2 and 4 weeks post treatment, respectively. The respective figures were 58.5%, 78.0%, and 82.9% in the 2-week active treatment group. The authors suggested that the post treatment duration to be of greater significance than the actual length of treatment. Four weeks after initiation of therapy, mycologic cure rates were 77.1% and 78% in the 4-week and 2-week treatment groups, respectively. Likewise, 88.6% and 82.9% of those in the 4-week and 2-week treatment groups, respectively, had a mycologic cure 6 weeks after initiation of treatment. Therefore, the authors proposed 2 weeks to be the optimum duration of treatment for interdigital tinea pedis when using 1% luliconazole cream once daily.36
A recently published, randomized, double-blind, parallel-group, vehicle-controlled, multicenter study included 256 patients with a clinical diagnosis of tinea cruris confirmed on KOH microscopy and fungal culture. Participants applied either 1% luliconazole cream or vehicle once daily for 7 days. Dermatophytes isolated from the patients were used to estimate the MICs for luliconazole, terbinafine, and itraconazole. Complete clearance was defined as both clinical and mycologic cure (negative KOH and culture). Clinical cure was defined as no clinical signs of tinea cruris, while effective treatment was defined as negative KOH and fungal culture with mild erythema and/or scaling without pruritus. In total, 21.2% of patients treated with luliconazole and 4.4% of patients treated with vehicle had complete clearance. Similarly, clinical cure (24.2% versus 6.6%), mycologic cure (78.2% versus 45.1%), and effective treatment (43% versus 18.7%) rates were all significantly higher in luliconazole-treated patients as compared with vehicle-treated patients. Luliconazole 1% cream applied once daily for 7 days was concluded to be more effective than vehicle for the treatment of tinea cruris.37
Another multicenter, randomized, open-label comparative study from India included adult patients with clinical evidence of skin mycoses (the commonest presentation of tinea corporis) and a combined score >5 (total of erythema, scaling, and pruritus on a scale of 1–3). The clinical diagnosis was confirmed by KOH microscopy. Pregnant or lactating women, patients with clinical evidence of severe cardiac, pulmonary, gastrointestinal, renal, hepatic, or neurologic disease, and those with uncontrolled diabetes mellitus were excluded, as were patients with known hypersensitivity to allylamine/benzylamine agents, those treated with systemic antifungals in the previous month, itraconazole in the previous 6 months, systemic antibiotics in the previous 2 weeks, or corticosteroids or immunosuppressants in the previous 6 weeks. Patients were randomly assigned to receive luliconazole, sertaconazole, amorolfine, eberconazole, or terbinafine cream in blocks of 30. All drugs were applied once daily for one week in patients with tinea cruris/tinea corporis and for 2 weeks in patients with tinea pedis. Efficacy, as assessed by change in clinical signs and symptoms and by negative KOH microscopy, was seen in 93.3%, 86.6%, 83.3%, 80%, and 73.3% in the groups treated with sertaconazole, luliconazole, amorolfine, terbinafine, and eberconazole, respectively. Adverse effects were seen in 16.6% of patients, and included burning, irritation, peeling of skin, itching, and hyperpigmentation. None of the patients had to discontinue treatment because of adverse effects. Sertaconazole was shown to be the most efficacious, followed by luliconazole, for the treatment of cutaneous dermatophytosis.38
In a prospective, parallel-group study by Laxmi et al, 60 patients with a clinical diagnosis of tinea corporis/cruris confirmed by KOH microscopy were alternately assigned to receive either terbinafine or luliconazole once daily for 2 weeks. Pregnant/lactating females, immunocompromised patients, and those with other clinical types of tinea infection or a history of intolerance or hypersensitivity to imidazole or allylamine compounds were excluded. Patients who had used topical antifungal agents/topical corticosteroids in the treatment area(s) in the previous 30 days, systemic antifungals in the previous 8 weeks (8 months for oral terbinafine), or systemic corticosteroids in the previous 30 days were also excluded. Clinical examination and KOH microscopy was performed at the end of treatment (day 15) and after 2 weeks (day 30). The total composite score and KOH mount was negative by day 15, with similar improvement in symptoms and signs in both groups (P>0.05). Luliconazole and terbinafine applied once daily for 2 weeks were found to be equally efficacious for tinea corporis/cruris.39
Onychomycosis
Jones and Tavakkol have recently reported their previously unpublished observation that luliconazole readily crosses both healthy and dermatophyte-infected toenails. In the only Phase I–II clinical study evaluating the safety and tolerability of topical 10% luliconazole solution for the treatment of moderate to severe distal subungual onychomycosis, they recruited 24 patients who underwent application of 20 mg of the drug to all ten toenails and periungual areas for 29 days and were then followed up for a further 7 days. The dose used was twice the clinical dose anticipated to be necessary. Systemic exposure to the drug was shown to be very low, with the highest individual peak plasma concentration (0.314 ng/mL) observed on day 29 (ie, at the end of treatment). The mean (standard deviation) concentration of luliconazole measured 7 days after the last application of the study drug was 40.8 (30.7) mg/g of nail plate, suggesting presence of the drug for an extended period of time at levels hundreds of times higher than the MIC against the two common nail pathogens, T. rubrum and T. mentagrophytes. No significant side effects or laboratory abnormalities requiring interruption of treatment with the study drug or discontinuation from the study were reported. There were no reports of local application site reactions (burning/stinging, erythema, pruritus, erosions). This Phase I/IIa study demonstrated excellent local tolerability and a lack of systemic side effects.40
Another randomized, double-blind, vehicle-controlled study (ClinicalTrials.gov identifier NCT01431820) is currently being conducted by Topica Pharmaceuticals (Los Altos, CA, USA) to assess the safety and efficacy of two dosing regimens of luliconazole 10% solution in distal subungual onychomycosis of the toenails. Patients with mild to moderate distal subungual onychomycosis of the great toenail with positive KOH microscopy and fungal culture for dermatophytes have been randomized into two dosing regimens and control groups as follows: luliconazole 10% solution applied once daily for 48 weeks; luliconazole 10% solution applied once daily for 12 weeks followed by luliconazole 10% solution applied once weekly for 36 weeks; vehicle solution applied once daily for 48 weeks; or vehicle solution applied once daily for 12 weeks followed by vehicle solution applied once weekly for 36 weeks. Recruitment for this study is now closed at 334 patients. Participants were evaluated at baseline and will be re-evaluated every 4 weeks for 48 weeks, with an additional 4 weeks off drug. The primary efficacy variable is the proportion of subjects achieving complete cure of the target great toenail at week 52.41 Preliminary analysis of the results of this study (until August 30, 2013) indicate no significant local or systemic side effects.42 The estimated primary completion date of this Phase IIb/III study is July 2014.41 It is likely that luliconazole may emerge in the future as a promising addition to the topical drug therapies for onychomycosis.
Limitations of existing trials
The clinical studies evaluating the efficacy of luliconazole have some limitations. First, only four studies have directly compared the efficacy of luliconazole with at least one other agent, such as bifonazole, terbinafine, sertaconazole, or amorolfine. The number of subjects included in three of the studies is too small to reach definite conclusions. Second, some of these studies have compared different dosages (once daily application of luliconazole with twice daily application of sertaconazole) and different durations of application (luliconazole applied for 2 weeks compared with bifonazole applied for 4 weeks).33,35 Such design is usually based on the standard dosing frequency and duration of different drugs. However, assessment of efficacy in such cases does not take into account the marked difference in the amount of drug applied. In such cases, the response to treatment should be judged in light of higher compliance rates and the lower costs associated with the drug when used once daily or for a shorter duration. It is likely that a once-daily or shorter regimen may be associated with greater compliance and produce similar or probably higher efficacy in clinical practice. Lastly, due to the differing costs of the various antifungal agents available, the absence of any pharmacoeconomic studies comparing the cost-effectiveness of luliconazole with that of other topical antifungals makes it difficult to establish overall superiority of one drug over the others.
Safety
Topical luliconazole cream in different strengths has generally been well tolerated in the clinical studies. Jones et al reported fewer side effects with luliconazole 1% cream (11.3%) as compared with vehicle (16.9%). Three application site reactions, namely general application site reactions, pruritus, and pain were reported in two patients treated with 1% luliconazole cream, but did not require discontinuation of therapy.37 Watanabe et al also reported only mild side effects with 1% luliconazole cream. These were seen in 2% of patients (n=253) and included itching, heat sensation, irritation, pain, and redness.33 In their comparative study using different concentrations of luliconazole, local adverse effects were seen in five patients (n=224), and included eczema, contact dermatitis, pruritus, erythema, and pain.34 Four patients in the terbinafine group showed mild contact dermatitis versus none in the luliconazole group in a prospective, parallel-group study from India.39 No application site reactions or systemic events were reported in the other trials.35,36,38,40 Allergic contact dermatitis with luliconazole 1% cream, with positive patch test reactions to both luliconazole and lanoconazole, has been reported.43,44 Patch test responses to other imidazoles with a β-substituted 1-phenethyl imidazole (β-SPI) structure were negative in these patients. The same patients did not crossreact with neticonazole, a vinyl imidazole. The authors suggested that the dithioacetal structure present in luliconazole and lanoconazole may be the main culprit in inducing the allergic contact dermatitis seen in patients receiving these drugs.
Conclusion
As discussed here, terbinafine has fungicidal activity against dermatophytes, while the azole antifungals are known to be only fungistatic. However, luliconazole, which belongs to the azole group, has strong fungicidal activity against Trichophyton spp., similar to that of terbinafine. The potent antifungal activity of luliconazole is possibly attributable to a combination of strong in vitro antifungal activity and favorable pharmacokinetic properties in the skin. Luliconazole has also been shown to have extremely potent activity against dermatophytes while retaining significant activity against C. albicans. The frequency of application (once daily) and duration of treatment (one week for tinea corporis/cruris and 2 weeks for interdigital tinea pedis) is also favorable when compared with other topical regimens used for the treatment of tinea pedis, such as 2–4 weeks of twice-daily treatment with econazole, up to 4 weeks of twice-daily treatment with sertaconazole, 1–2 weeks of twice-daily treatment with terbinafine, 4 weeks of once-daily application of naftifine, and 4–6 weeks of once-daily treatment with amorolfine.6,45 The efficacy of luliconazole in the treatment of dermatophyte infections appears to be at par with terbinafine and the other azoles. Although, there are no clinical trials evaluating the efficacy of luliconazole in candidal infections at present, preclinical studies have supported such a role. It is possible that luliconazole may emerge as an effective and broad-spectrum antifungal agent in the future.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51(Suppl 4):2–15. doi: 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 2.Zaias N, Tosti A, Rebell G, et al. Autosomal dominant pattern of distal subungual onychomycosis caused by Trichophyton rubrum. J Am Acad Dermatol. 1996;34:302–334. doi: 10.1016/s0190-9622(96)80142-3. [DOI] [PubMed] [Google Scholar]
- 3.Hay RJ, Ashbee HR. Mycology. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook’s Textbook of Dermatology. 8th ed. Oxford, UK: Blackwell Science; 2010. [Google Scholar]
- 4.Nishiyama Y, Asagi Y, Hiratani T, Yamaguchi H, Yamada N, Osumi M. Morphological changes associated with growth inhibition of Trichophyton mentagrophytes by amorolfine. Clin Exp Dermatol. 1992;17(Supp 1):13–17. doi: 10.1111/j.1365-2230.1992.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 5.Nimura K, Niwano Y, Ishiduka S, Fukumoto R. Comparison of in vitro anti-fungal activities of topical antimycotics launched in 1990s in Japan. Int J Antimicrob Agents. 2001;18:173–178. doi: 10.1016/s0924-8579(01)00365-x. [DOI] [PubMed] [Google Scholar]
- 6.Dias MF, Bernardes-Filho F, Quaresma-Santos MV, Amorim AG, Schechtman RC, Azulay DR. Treatment of superficial mycoses: review. Part II. An Bras Dermatol. 2013;88:937–944. doi: 10.1590/abd1806-4841.20132018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singal A. Butenafine and superficial mycoses: current status. Expert Opin Drug Metab Toxicol. 2008;4:999–1005. doi: 10.1517/17425255.4.7.999. [DOI] [PubMed] [Google Scholar]
- 8.Lücker PW, Beubler E, Kukovetz WR, Ritter W. Retention time and concentration in human skin of bifonazole and clotrimazole. Dermatologica. 1984;169:51–55. doi: 10.1159/000249639. [DOI] [PubMed] [Google Scholar]
- 9.Niwano Y, Ohmi T, Seo A, Kodama H, Koga H, Sakai A. Lanoconazole and its related optically active compound nnd-502: novel anti-fungal imidazoles with a ketene dithioacetal structure. Curr Med Chem. 2003;2:147–160. [Google Scholar]
- 10.Niwano Y, Kuzuhara N, Kodama H, Yoshida M, Miyazaki T, Yamaguchi H. In vitro and in vivo antidermatophyte activities of NND-502, a novel optically active imidazole antimycotic agent. Antimicrob Agents Chemother. 1998;42:967–970. doi: 10.1128/aac.42.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niwano Y, Kuzuhara N, Goto Y, et al. Efficacy of NND-502, a novel imidazole antimycotic agent, in experimental models of Candida albicans and Aspergillus fumigatus infections. Int J Antimicrob Agents. 1999;12:221–228. doi: 10.1016/s0924-8579(99)00076-x. [DOI] [PubMed] [Google Scholar]
- 12.Koga H, Nanjoh Y, Makimura K, Tsuboi R. In vitro anti-fungal activities of luliconazole, a new topical imidazole. Med Mycol. 2009;47:640–647. doi: 10.1080/13693780802541518. [DOI] [PubMed] [Google Scholar]
- 13.Koga H, Tsuji Y, Inoue K, et al. In vitro anti-fungal activity of luliconazole against clinical isolates from patients with dermatomycoses. J Infect Chemother. 2006;12:163–165. doi: 10.1007/s10156-006-0440-4. [DOI] [PubMed] [Google Scholar]
- 14.Pfaller MA, Sutton DA. Review of in vitro activity of sertacon azole nitrate in the treatment of superficial fungal infections. Diagn Microbiol Infect Dis. 2006;56:147–152. doi: 10.1016/j.diagmicrobio.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Uchida K, Nishiyama Y, Yamaguchi H. In vitro anti-fungal activity of luliconazole (NND-502), a novel imidazole anti-fungal agent. J Infect Chemother. 2004;10:216–219. doi: 10.1007/s10156-004-0327-1. [DOI] [PubMed] [Google Scholar]
- 16.Niwano Y, Koga H, Kodama H, et al. Inhibition of sterol 14 alpha-demethylation of Candida albicans with NND-502, a novel optically active imidazole antimycotic agent. Med Mycol. 1999;37:351–355. doi: 10.1046/j.1365-280x.1999.00243.x. [DOI] [PubMed] [Google Scholar]
- 17.Uchida K, Nishiyama Y, Tanaka T, Yamaguchi H. In vitro activity of novel imidazole anti-fungal agent NND-502 against Malassezia species. Int J Antimicrob Agents. 2003;21:234–238. doi: 10.1016/s0924-8579(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 18.Sugita T, Tajima M, Ito T, et al. Anti-fungal activities of tacrolimus and azole agents against the eleven currently accepted Malassezia species. J Clin Microbiol. 2005;43:2824–2829. doi: 10.1128/JCM.43.6.2824-2829.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tajima M, Sugita T, Nishikawa A, Tsuboi R. Molecular analysis of Malassezia microflora in seborrheic dermatitis patients: Comparison with other diseases and healthy subjects. J Invest Dermatol. 2008;128:345–351. doi: 10.1038/sj.jid.5701017. [DOI] [PubMed] [Google Scholar]
- 20.Brown M, Turner R, Nakamura N. Nail permeation and depth profiling of luliconazole, a novel imidazole anti-fungal agent, for the topical treatment of onychomycosis; Poster presented at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy; September 10–13, 2013; Denver, CO, USA. [Google Scholar]
- 21.Brown M, Turner R, Nakamura N. In vitro fungicidal activity of luliconazole in an infected nail model using full thickness human toenails and T Rubrum; Poster presented at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy; September 10–13, 2013; Denver, CO, USA. [Google Scholar]
- 22.Scher RK, Nakamura N, Tavakkol A. Luliconazole: a review of a new anti-fungal agent for the topical treatment of onychomycosis. Mycoses. 2014;57:389–393. doi: 10.1111/myc.12168. [DOI] [PubMed] [Google Scholar]
- 23.Fujita S, Matsuyama T. Experimental tinea pedis induced by non-abrasive inoculation of Trichophyton mentagrophytes arthrospores on the plantar part of a guinea pig foot. J Med Vet Mycol. 1987;25:203–213. doi: 10.1080/02681218780000541. [DOI] [PubMed] [Google Scholar]
- 24.Fujita S. Conditions required for successful experimental dermatophytosis and histopathogenesis. Jpn J Med Mycol. 1992;33:115–125. [Google Scholar]
- 25.Fujita S, Matsuyama T, Sato Y. Experimental tinea pedis in guinea pig feet-scanning electron microscopic and histological study of the infection. Jpn J Med Mycol. 1988;29:163–168. [Google Scholar]
- 26.Takahashi H. New method for the in-vivo animal evaluation of topical fungicides. Jpn J Med Mycol. 1983;24:230–233. [Google Scholar]
- 27.Uchida K, Kudoh M, Yamaguchi H. A study on effectiveness of treatment and prevention of relapse using topical administration of terbinafine in a guinea pig model for tinea pedis. Jpn J Antibiot. 1994;47:1407–1412. Japanese. [PubMed] [Google Scholar]
- 28.Uchida K, Tanaka T, Yamaguchi H. Achievement of complete mycological cure by topical anti-fungal agent NND-502 in guinea pig model of tinea pedis. Microbiol Immunol. 2003;47:143–146. doi: 10.1111/j.1348-0421.2003.tb02797.x. [DOI] [PubMed] [Google Scholar]
- 29.Ghannoum MA, Long L, Kim HG, Cirino AJ, Miller AR, Mallefet P. Efficacy of terbinafine compared to lanoconazole and luliconazole in the topical treatment of dermatophytosis in a guinea pig model. Med Mycol. 2010;48:491–497. doi: 10.3109/13693780903373811. [DOI] [PubMed] [Google Scholar]
- 30.Ghannoum MA, Hossain MA, Long L, et al. Evaluation of anti-fungal efficacy in an optimized animal model of Trichophyton mentagrophytes-dermatophytosis. J Chemother. 2004;16:139–144. doi: 10.1179/joc.2004.16.2.139. [DOI] [PubMed] [Google Scholar]
- 31.Koga H, Nanjoh Y, Kaneda H, Yamaguchi H, Tsuboi R. Short-term therapy with luliconazole, a novel topical anti-fungal imidazole, in guinea pig models of tinea corporis and tinea pedis. Antimicrob Agents Chemother. 2012;56:3138–3143. doi: 10.1128/AAC.05255-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koga H, Nanjoh Y, Inoue K, et al. Luliconazole, a novel topical imidazole: results of the preclinical studies; Poster presented at 16th Congress of the International Society of Human and Animal Mycology; June 25–29, 2006; Paris, France. [Google Scholar]
- 33.Watanabe S, Takahashi H, Nishikawa T, et al. A comparative clinical study between 2 weeks of luliconazole 1% cream treatment and 4 weeks of bifonazole 1% cream treatment fortinea pedis. Mycoses. 2006;49:236–241. doi: 10.1111/j.1439-0507.2006.01218.x. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe S, Takahashi H, Nishikawa T, et al. Dose-finding comparative study of 2 weeks of luliconazole cream treatment for tinea pedis – comparison between three groups (1%, 0.5%, 0.1%) by a multi-center randomised double-blind study. Mycoses. 2007;50:35–40. doi: 10.1111/j.1439-0507.2006.01305.x. [DOI] [PubMed] [Google Scholar]
- 35.Jerajani H, Janaki C, Kumar S, Phiske M. Comparative assessment of the efficacy and safety of sertaconazole (2%) cream versus terbinafine cream (1%) versus luliconazole (1%) cream in patients with dermatophytoses: a pilot study. Indian J Dermatol. 2013;58:34–38. doi: 10.4103/0019-5154.105284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jarratt M, Jones T, Kempers S, et al. Luliconazole for the treatment of interdigital tinea pedis: a double-blind, vehicle-controlled study. Cutis. 2013;91:203–210. [PubMed] [Google Scholar]
- 37.Jones TM, Jarratt MT, Mendez-Moguel I, et al. A randomized, multi-center, double-blind, vehicle-controlled study evaluating the efficacy and safety of luliconazole cream 1% once daily for 7 days in patients aged $12 years with tinea cruris. J Drugs Dermatol. 2014;13:32–38. [PubMed] [Google Scholar]
- 38.Selvan A, Girisha G, Vijaybhaskar, Suthakaran R. Comparative evaluation of newer topical anti-fungal agents in the treatment of superficial fungal infections (tinea or dermatophytic) Int Res J Pharm. 2013;4:224–228. [Google Scholar]
- 39.Lakshmi VC, Bengalorkar GM, Shiva Kumar V. Clinical efficacy of topical terbinafine versus topical luliconazole in treatment of tinea corporis/tinea cruris patients. Br J Pharm Res. 2013;3:1001–1014. [Google Scholar]
- 40.Jones T, Tavakkol A. Safety and tolerability of luliconazole solution 10 percent in patients with moderate to severe distal sub-ungual onychomycosis. Antimicrob Agents Chemother. 2013;57:2684–2689. doi: 10.1128/AAC.02370-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ClinicalTrials.gov Topica Pharmaceuticals. Safety and efficacy of luliconazole solution, 10% in subjects with mild to moderate onychomycosis (SOLUTION) [Accessed March 15, 2013]. Available from: http://clinicaltrials.gov/ct2/show/NCT01431820.
- 42.Stein L, Pollak R, Kempers S, et al. A preliminary update on the ongoing solution study, a phase 2b/3 study of two dosing regimens of a novel 10% luliconazole solution for treatment of onychomycosis; Poster presented at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy; September 10–13, 2013; Denver, CO, USA. [Google Scholar]
- 43.Shono M. Allergic contact dermatitis from luliconazole. Contact Dermatitis. 2007;56:296–297. doi: 10.1111/j.1600-0536.2006.01023.x. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka T, Satoh T, Yokozeki H. Allergic contact dermatitis from luliconazole: implication of the dithioacetal structure. Acta Derm Venereol. 2007;87:271–272. doi: 10.2340/00015555-0200. [DOI] [PubMed] [Google Scholar]
- 45.Gupta AK, Einarson TR, Summerbell RC, Shear NH. An overview of topical anti-fungal therapy in dermatomycoses. A North American perspective. Drugs. 1998;55:645–674. doi: 10.2165/00003495-199855050-00004. [DOI] [PubMed] [Google Scholar]


