Abstract
Dry eye (DE) is a common ocular disease that results in eye discomfort, visual disturbance and substantially affects the quality of life. It has a multifactorial etiology involving tear film instability, increased osmolarity of the tear film and inflammation of the ocular surface with potential damage to the ocular surface. This review discusses the classification, diagnostic approaches and treatments of DE.
Keywords: Dry eye, Ocular surface disease, Visual disturbance, Diagnostic approaches
Introduction
Dry eye (DE) is a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface, accompanied by increased osmolarity of the tear film and inflammation of the ocular surface.1 Estimated prevalence ranges from about 5% to over 35% in different age groups.2 Despite its high prevalence, DE is frequently under-recognized. Owing to its negative influence on patients’ visual function and quality of life, DE represents a big burden in public healthcare. Therefore, attempts to find better diagnostic approaches and appropriate treatment for DE are worthy of consideration. This review discusses the classification, diagnostic approaches and treatments of DE.
Classification
The major classes of DE, as identified by the International Dry Eye Workshop (DEWS) report are aqueous deficient dry eye (ADDE) and evaporative dry eye (EDE).1 Although both ADDE and EDE present with similar signs of reduced stability and increased tear film osmolarity, ADDE chiefly refers to a failure of lacrimal secretion and EDE is due to excessive water loss from the exposed ocular surface in the presence of normal lacrimal secretory function. It is also important to recognize that ADDE and EDE may coexist. The main etiopathogenic classification is illustrated in Fig. 1.
Figure 1.
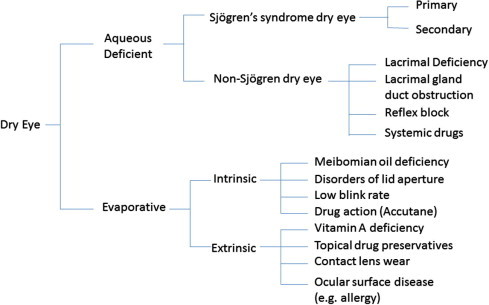
Etiopathogenic classification (modified from 2007 DWES report).1
Diagnostic assessment
Although literature provides an extensive discussion on the role and appropriateness of currently used tests to diagnose DE, there is no gold standard test or even a panel of tests or well-established cutoff values for the available tests.3 The suggested sequence of DE diagnostic tests is: history and examination followed by a symptom questionnaire; tear break-up time and ocular surface fluorescein staining; Schirmer test; lid and meibomian morphology and meibomian expression.2 In Delphi panel, the most frequently cited tests were slit-lamp examination and fluorescein staining (100%) followed by tear breakup time and medical history (both 94%).3 An ideal diagnostic method should be preferably noninvasive, objective, specific, reproducible and sustainable in terms of cost and time. At present, none of the current tests for DE diagnosis altogether meet these features.
Subjective evaluation
The symptoms and history of DE patients vary widely; therefore, validated questionnaires have been developed to ensure consistency in recording symptomatic information. A comparative listing of DE questionnaires is available in the report of the Epidemiology Subcommittee of the International DEWS 2007.2 Previously it was believed that DE can be diagnosed largely on the basis of symptoms; however, recent studies have questioned this opinion as there is often a lack of correlation between the severity of the symptoms and signs of DE.4 This lack of consistency between signs and symptoms presents a problem not only in the diagnosis of the disease, but also in assessment of severity and in the evaluation of the clinical efficacy of treatments.
Objective evaluation
A scientific roundtable on dry eye ranked tear break up time (93%), corneal staining (85%), tear film assessment (76%), conjunctival staining (74%), and the Schirmer test (54%) as the most commonly used diagnostic tests for initial assessment of dry eye.5 Apart from these traditional clinical tests, we will discuss more about the less invasive evaluations based on the recently developed technologies related to tear hyperosmolarity, tear film instability and inflammation.
Tear osmolarity
An increase in tear osmolarity is common to all types of DE. It is suggested that osmolarity values greater than 308 mOsms/l are a sensitive indicator of mild DE and values greater than 312 mOsms/l are indicative of moderate to severe DE (sensitivity 73%; specificity 92%).6 The difference in the tear osmolarity values among normal, mild, moderate or severe dry eye patients is so small that precision is critical. Tear film osmolarity can be measured in three ways: freezing point depression (FPD), (considered to be the gold standard);7 vapor pressure8 and electrical conductivity or impedance.9 Since the electrical impedance of tear samples requires a small sample size (0.05 μl) and short test duration (30 s), it is considered more suitable for clinical use.10 The TearLab system (TearLab Inc., San Diego, CA, USA) uses this method to determine tear osmolarity. While recent studies have demonstrated the correlation between increased osmolarity and DE disease severity, it is also observed that “tear osmolarity cannot be used as the sole indicator of dry eye disease”.11
Assessment of tear stability
The measurement of tear film stability is fundamental to the diagnosis of dry eye.12 A variety of methods are available to assess different aspects of the tear film and provide insights into its “stability”. Tear break-up time (TBUT), introduced by Norn,13 remains the most frequently used diagnostic test to determine tear film instability.14 Generally, the non-invasive tear break-up time (NIBUT) involves the observation of an illuminated grid pattern reflected from the anterior tear surface. NIBUT can be measured by corneal topography, interferometry, aberrometry, functional visual acuity assessment, and confocal microscopy. A regular image of the reflected target indicates a stable tear film. The time (in seconds) from the last blink to the appearance of the first discontinuity or break in the reflected image is recorded.
Tear film particle assessment
Non-invasive tear film particle assessment technique to measure tear film’s upward spread and stability can potentially be used for the precise and objective evaluation of tear film.12,15 Tear film particle velocity is measured as an assessment of tear hydrodynamics by tracking the movement of reflective particles in the tear film. Digital images of the central region of the ocular surface are collected for 10 s to visualize the naturally seen particles in the tear film following a natural blink. Software determines the velocity of the particles as they traverse upwards in the tear film. This technique has been applied to the pre-contact lens tear film to differentiate the wetting characteristics of various contact lens materials, but can potentially be used for clinical evaluation of tear film stability more precisely and objectively.
Topographical analysis systems
Corneal topography systems like TMS-116 and high speed videokeratoscopy (HSV)17 have replaced keratometers in clinical settings to evaluate indices like surface regularity index (SRI), surface asymmetry index (SAI) and topographic pattern – that can be used to evaluate corneal surface regularity and tear film stability. These videokeratoscopy indices enable objective quantification of the quality of the tear film, its breakdown and also consequent effects on image quality.18 According to a 2008 survey, most of the experts chose videokeratoscopy for initial evaluation of post-LASIK dry eye.14
Interferometry
Interferometry is based on the colored fringes that arise from interference between light reflected from the surface of the lipid layer and from the interface between the lipid layer and the aqueous layer of the tear film. These interference patterns could be used to observe the nature, thickness and rupture of the lipid layer.19 Besides lipid layer thickness, interferometry can also measure NIBUT i.e., the time between the last blink and the appearance of the first lipid layer discontinuity. LipiView (TearScience Inc., Morrisville, NC) is a commercially available interferometer that provides quantitative values of the tear-film lipid layer thickness (LLT), and this automated assessment of the LLT might be a suitable screening test for detecting meibomian gland dysfunction (MGD).20 Other instruments, software and prototypes are also being developed.21,22
Aberrometry
Wavefront aberrometry allows the non-invasive assessment of the visual disturbances caused by higher order aberrations arising from tear film instability and break-up. Any local changes in tear film thickness and regularity such as those associated with tear break-up introduce aberrations and subsequently reduce retinal image quality. Changes in tear volume and dynamics induce changes in higher order aberrations, which appear as characteristic patterns in both normal and dry eyes.18,23,24 Low tear volumes in severe DE may cause increased, but stable, aberrations. This may be the result of irregular, damaged epithelium in the optic zone in the absence of dynamic tear film changes.25 The instillation of artificial tears has been shown to reduce aberrations in dry eye when the measurement is taken after several seconds26; however, when measured 2 s after a blink, an initial increase in aberrations was found because of the tear film disturbances induced by increase in tear volume.27 A paper presented at a recent conference suggested that aberrometry could be utilized for not only detecting DE but also for monitoring the efficacy of treatments.28
Functional visual acuity
Despite normal conventional visual acuity, DE patients often complain about decreased visual acuity when driving, reading, and using a computer. Since the ocular surface tends to dry out when normal blinking is suppressed during gazing, patients with dry eye may have problems maintaining clear vision while gazing. To simulate visual acuity while gazing, functional visual acuity (FVA) was conceptualized. FVA is a measure of visual acuity during sustained eye opening without blinking.
FVA was originally defined as the monocular recognition acuity at a specific time point. Later studies used a range of values, as well as the visual maintenance ratio (the ratio between the FVA and the baseline visual acuity) to reflect everyday vision more accurately.29 It has been documented that FVA decreases significantly in both non-Sjögren’s syndrome (NSS) and Sjögren’s syndrome (SS) dry eye patients.30 Despite the criticism that patients are required to keep their eyes open longer than normal, FVA tests have been commonly used to assess visual disturbances in DE patients. To further improve FVA measurements, a new continuous FVA measurement system (FVAM, NIDEK, Gamagori, Japan) was developed, which allows continuous monocular visual acuity measurement during a 30 s blink-free period.31 The methodology has been useful for evaluating patients with tear instability and it has been reported that the assessments correlate with TBUT.32
Optical coherence tomography
The anterior segment optical coherence tomography (OCT) can measure the tear film thickness33 and tear meniscus parameters34 which indicate total tear volume. Due to its high resolution, non-invasiveness, good accuracy, and repeatability, OCT is a useful tool to diagnose DE. Nguyen et al. found that lower tear meniscus measurement with Fourier-domain-OCT correlates well with symptoms of DE and the Schirmer test.35 Shen suggested that lower tear meniscus height and radius were the best indicators of DE with a cut-off meniscus height of 1.64 mm and radius of 1.82 mm.34 It is important to factor in the role of tear secretion, location of the punctum, lacrimal drainage, lid length, eyelid tension, and palpebral aperture while interpreting tear meniscus dimensions. Additionally, OCT has recently been used to grade lid parallel conjunctival folds (LIPCOF),36 to map 3-dimensional corneal epithelial thickness,37 and to investigate meibomian gland structures based on the more developed techniques,38 which would provide deeper insights into DE diagnosis and follow-up.
Non-contact confocal microscopy
A non-contact, tandem-scanning confocal microscope demonstrating real-time images has been used by some researchers to observe the tear film.39. Debris was discovered in the central corneal region of tear film in dry and normal eyes. They concluded that the minimal alteration of tear film function and excellent focusing characteristics makes this a valuable tool for detailed imaging of tear film.
Evaluation of ocular surface and inflammation
Confocal microscopy
Corneal in vivo confocal microscopy (IVCM) is a novel, noninvasive, high-resolution tool that allows imaging the cornea at the cellular level and provides images comparable to histochemical methods. IVCM enables the study of corneal epithelium, corneal stroma and keratocytes, endothelial cells, corneal nerves, corneal immune and inflammatory cells, conjunctiva and meibomian glands in different ocular and systemic diseases that is not possible with direct slit-lamp examination. It may not only be used for diagnostic purposes, but may also serve as a valuable tool to monitor the disease and measure therapeutic efficacy in patients with DE. It is has been suggested that this technique could be used for non-invasive impression cytology in DE evaluation.40
IVCM observations in DE patients demonstrated significantly decreased cell densities in the superficial, intermediate, and basal epithelial layers,41 presumably due to increased desquamation of the superficial cell layer42 and activation of corneal keratocytes in the stroma.43 IVCM also demonstrated a substantial decrease in number and density of sub-basal and stromal nerve cells in DE related conditions. Further, IVCM has demonstrated the abnormal morphology of sub-basal nerves such as increase in bead-like formation, sprouts, tortuosity, irregular branching patterns, and neuromas, which may be explained by nerve degeneration and regeneration, leading to active neural growth.44 More recently, the non-invasive assessment of immune and inflammatory changes has become possible by laser IVCM that allows visualization of the cell components that could not be visualized earlier with previous white light IVCM machines. This is of particular interest in DE patients as over the past decade, the role of inflammation in DE disease has become clearly apparent. This technique could be used for non-invasive impression cytology.40 The increased density of epithelial dendritic cells (Langerhans cells) observed by Lin et al. may indicate the heightened immune status of the cornea in DE.45 Dynamic in vivo assessment of the central corneal inflammatory cell density may serve as an indicator of DE severity and provide new insight for DE treatment. Recently, IVCM has also been applied for the examination of meibomian glands, providing a new tool to assess their morphologic changes.46 Finally, IVCM evaluation of conjunctival epithelium in DE demonstrated conjunctival epithelial cyst formation, decreased density of conjunctival epithelial cells, goblet cells and increased inflammatory cell density.47,48
Meibomian gland evaluation
Meibomian glands are the source of lipids in the lipid layer of the tear film. Since MGD is the most common cause of evaporative dry eye, the International Workshop on MGD recommended performing gland expression routinely for all asymptomatic patients.49 Meibomian glands can be assessed visually using a slit lamp and an appropriate grading system.50 Meiboscopy involves the trans-illumination of the eyelid using a white light, which has been described as “useful, quick and patient-friendly”.51 Meibography is a method of quantification of meibomian gland drop-out that enables masked evaluation and therefore increases objectivity in clinical trials.52 Meibometry involves the use of a plastic tape to blot the central lower lid margin. The lipid blot changes the optical density and can be measured in a photometer to distinguish patients with MGD.53
Corneal and conjunctival staining
Corneal and conjunctival staining is an invasive procedure which enables the assessment of ocular surface damage by instilling a dye such as sodium fluorescein, rose bengal, or lissamine green. Evaluation of ocular surface staining is highly subjective, but the use of charts such as the Oxford, Van Bijsterveld, and CLEK grading scheme can facilitate consistent recording of staining severity.54 The repeatability of staining tests has been found to be poor,55 and they lack discriminatory power in mild to moderate cases of dry eye.56
The SS International Registry modified the Oxford grading scheme to enable concurrent grading of the cornea and bulbar conjunctiva using a combination of one drop of 0.5% fluorescein for corneal staining and one drop of 1% lissamine green for conjunctival staining. On a specially designed form, grades between 0 and 3 are assigned for staining the cornea, the nasal and the temporal conjunctiva. This gives a maximum possible total of 9 points. Three additional points are then allocated for fluorescein only if there are confluent staining (t1), staining in the pupillary area (t1), or if one or more filaments are present (t1), giving a maximum possible score of 12. This study concluded that this would be a suitable test “for diagnosing the ocular component of SS in future classification criteria”.57
Conjunctival impression cytology or brush cytology
Impression cytology is a rapid, minimally invasive, and relatively painless method of harvesting conjunctival epithelial, goblet, and inflammatory cells from the bulbar mucosa.58 Studies have demonstrated that cytokines IL-1a, mature IL-1b, and IL-1Ra are found in a significantly greater percentage of conjunctival cytology specimens from eyes with SS, than in those from normal eyes.59 Further, combined with flow cytometry, dry eye group was found to have a significant difference in the CD4/CD8 ratio compared with normal eyes, and almost double the number of CD14 positive cells (monocytes/macrophages). HLA-DR expression in CK19 positive conjunctival epithelial cells and matrix metalloproteinases levels were also found to be elevated in DE patients.59,60 These studies illustrate how immune cells isolated from the superficial layer of the conjunctiva may influence the pathogenesis of DE. Flow cytometry analysis of epithelial and immune cells of the conjunctiva may emerge as new biomarkers of DE.
Conjunctival brush cytology using a soft brush obtains superficial cells (as in impression cytology) as well as basal cells. The sample can then be assessed for the presence of squamous metaplasia, inflammatory cells, and the expression of surface markers on the ocular surface epithelium.61,62 This is often combined with flow cytology, which gives a highly sensitive and specific analysis of epithelial cell markers, inflammatory cells, and goblet cells.62
Other tests
Fluorophotometry measures the uptake of fluorescein at the center of the cornea and is considered “a sensitive measure of epithelial integrity”.63 As such, patients with DE demonstrate an increased corneal permeability and a slower rate of fluorescein elimination compared to patients with normal eyes. A correlation between tear cytokine levels and the severity of symptoms and ocular surface signs in all forms of DE has been found.64 Tear protein analysis and tear lipid analysis may provide more information on the etiology of DE as different tear proteins are present in DE with or without meibomian gland disease.64,65 While the mild cases of DE exhibit normal tear production levels with no fluorescein staining, an increase in inflammatory cells could still be present. This indicates that inflammatory mediators in the tears are perhaps a better indicator of DE disease than the measure of tear production or staining. Recent advances in tear lipid analysis technology and instrumentation have seen huge progress in the field of lipidomics, which involves the identification and quantification of lipid molecular species and their interactions with other molecules, which can be used to diagnose MGD.66
Treatment
Only a handful of therapies are available for DE patients and are used according to the disease severity.67 Artificial tears provide palliative relief to eye irritation in patients with aqueous tear deficiency, but do not prevent the underlying inflammation or reverse conjunctival squamous metaplasia in chronic DE. Combinations of artificial tears, oral omega-3 essential fatty acid supplements, mucin secretagogues, short-term steroids, and daily cyclosporine A (CsA) are used to combat underlying inflammation and restore normal tear film in patients with mild-to-moderate disease. Use of more aggressive treatment options, such as autologous serum, oral tetracyclines, prosthetic lens, and systemic immune-suppressants is restricted to patients with more severe forms of DE. The most severe forms of chronic DE, often associated with systemic diseases – SS and Stevens–Johnson syndrome, may benefit from surgical intervention, including tarsorrhaphy and amniotic membrane transplant. The Delphi panel suggested that the severity of disease (categorized according to patient’s signs and symptoms, not tests) should be the primary determinant for the therapeutic strategy chosen. Additionally, a stepwise guide to approach the best combination of medications to avoid symptoms of DE was also recommended.3
Anti-inflammatory treatments
Cyclosporine A
Inflammation is a key pathogenic factor in DE. Cyclosporine A (CsA) exerts immunosuppressive and anti-inflammatory activity through several pathways. Since the 1980s, several reports highlighted that topical CsA can be used to treat a variety of ocular inflammatory conditions including DE, high-risk corneal transplants, autoimmune uveitis, and vernal keratoconjunctivitis. Recent studies have shown that topical administration of CsA not only controls ocular surface inflammation, but is also effective in increasing tear secretion and tear film stability (possibly by promoting the local release of parasympathetic nervous system and through an increase in goblet cell density). Consequently, CsA may help in restoring epithelial damage, and reducing disease recurrences over the long term.68
Topical CsA significantly alleviates the signs and symptoms of DE and is often prescribed for long-term use by eye care practitioners. The cumulative findings of several clinical trials using 0.05% CsA ophthalmic emulsion for long-term have indicated improvement in both objective (like corneal surface staining and Schirmer test with anesthesia) and, subjective findings (like blurred vision and frequency of artificial tear application).69 In addition, topical CsA treatment may be associated with a significant improvement in many of the cellular and molecular markers of disease severity.70 Although higher dosing frequencies may increase treatment efficacy, some patients experience bothersome adverse effects (e.g., burning or irritation) that impair medication tolerability. The beneficial effects of CsA treatment in DE are well established; however, many patients with DE do not show a consistent therapeutic response to topical CsA.
Steroids
Topical steroids are also used to dampen inflammation on the ocular surface in DE, often in combination with CsA. The effect of corticosteroids on the inflammatory cascade, specifically the blockade of cyclooxygenase, production of prostanoids from arachidonic acid and stimulation of the apoptosis of lymphocytes, is well known and is likely the reason this form of therapy has been efficacious in practice.71 Corticosteroids also exert local immuno-modulatory activity through the inhibition of certain transcription factor activity. Clinical trials have demonstrated the efficacy of topical corticosteroid treatment at diminishing symptom severity and minimizing ocular surface staining.72 Unfortunately, long term topical or systemic corticosteroid use is associated with deleterious adverse effects, such as ocular hypertension, cataracts, and opportunistic infections. Repetitive short-term pulsatile administration of topical corticosteroids is a promising method of harnessing their beneficial effects, while minimizing the risk of adverse events.73
Hormonal therapy
Receptors for androgens, estrogens, progesterone and prolactin have been identified in several ocular tissues, including the lacrimal gland and meibomian glands.74–76 Experimental and human studies have demonstrated that adequate androgen, prolactin and estrogen levels are essential for normal lacrimal gland function and structural organization.77–79 Administration of topically applied androgen and estrogen steroid hormones for 3–4 months has also been found to show clinical improvement in the form of increased tear production TBUT and lipid layer thickness with corresponding symptomatic relief. 80,81 Systemic replacement with combined esterified estrogen and methyl-testosterone for 4–24 months was found to reduce symptoms and promote clinical improvement in postmenopausal women with DE.82
Antibiotics
In addition to the antibacterial effect, macrolide antibiotics (azithromycin) and tetracycline derivatives (tetracycline, doxycycline, and minocycline) have immunomodulatory properties which have been noted to decrease ocular surface inflammation and normalize lipid production by the meibomian glands. These may be particularly useful in dry eye secondary to ocular rosacea and blepharitis.83 Experimental investigations have demonstrated that the tetracycline derivative, doxycycline, can inhibit c-Jun N-terminal kinase, extracellular signal-related kinase and mitogen-activated protein kinase signaling in epithelial cells of the ocular surface exposed to hyperosmolar stress. Additionally, doxycycline is also known to down-regulate the expression of CXCL8 and proinflammatory cytokines IL-1β and TNF84 and inhibit the activity of MMPs (e.g., MMP-9).85 Similarly, the tetracycline derivative minocycline inhibits the expression of cell associated pro-inflammatory molecules.86 Despite extensive evidence from experimental trials indicating the potential benefits of administration of tetracycline derivatives in the treatment of DE, clinical evidence of their efficacy remains limited.87
Supplementary treatments
Essential Fatty Acids (EFAs)
Omega-3 (alpha-linolenic acid) and omega-6 (linoleic acid) are biologically necessary fatty acids that must be ingested because they cannot be synthesized de novo by the human body. EFAs are the precursors of eicosanoids (prostaglandins, prostacyclins, thromboxanes, and leukotrienes) that modulate immune responses; while omega-3 FAs are generally classified as anti-inflammatory, omega-6 FAs are considered proinflammatory.88 Investigations on the use of EFAs in the treatment of DE have produced conflicting results; however, most of the available evidence suggests that systemic administration of anti-inflammatory omega-3 FAs, can lessen DE severity.89,90 Topical EFAs have also been evaluated in murine DE models and showed potential therapeutic effect in the form of decreased ocular surface staining, cytokine expression, and immune cell infiltration.91 Similarly, topical administration of resolvin E1, an omega-3 FA derivative increased tear production, helped maintain ocular surface integrity, decreased cyclooxygenase 2 expression, and decreased immune cell infiltration in experimental dry eye.92 Available data suggest that using EFAs to treat dry eye disease is a promising frontier in ocular surface therapeutics and worthwhile subject for future research in DE; however, more evidence is needed to identify the most efficacious forms and doses of EFAs.
Nerve growth factor (NGF)
NGF has been observed to increase ocular surface sensitivity, inhibit inflammatory reactions and regulate tear film production.93 Thus, NGF seems to a play a pivotal role in the pathophysiology of DE and may be a promising therapeutic option.94 Data from several studies seem to be supporting this hypothesis. For instance, tear concentration of NGF has been observed to be increased as a compensatory mechanism in DE,94 particularly under hyperosmolar stress,95 suggesting that NGF may be involved in reducing the apoptosis of corneal epithelial cells triggered by hyperosmolarity. Additionally, NGF has been shown to regulate conjunctival epithelial differentiation into MUC5AC-secreting goblet cells.96. Therefore, exogenous NGF administration may be beneficial in recovering ocular surface damage due to chronic hyperosmolarity; for example, topical NGF administration has been observed to increase tear production and conjunctival goblet cell density in a dog experimental model of dry eye.97
Autologous serum
Autologous and umbilical cord serum contains substances that support the proliferation, differentiation, and maturation of the normal ocular surface epithelium98 and therefore, finds application in the treatment of severe DE.99 In 1984, Fox and colleagues reported the beneficial effects of autologous serum in SS.100 Documenting similar findings, Tsubota and Bradley attributed the improvements to the presence of EGF, vitamin A, lysozyme, fibronectin and TGF-beta.101,102 Autologous serum eye drops have been found to be better than conventional therapy utilizing artificial tears in less severe cases of DE as well. For instance, a prospective randomized, controlled, crossover study comparing 50% autologous serum eye drops with conventional therapy utilizing artificial tear solutions confirmed that ocular surface vital staining score and cytological improvements were due to serum drops, as the effects were reversed when treatment was reverted to conventional therapy.103 Similarly, another double-blind randomized clinical trial reported that a short-term treatment with 20% autologous serum eye drops achieved better symptomatic improvement than conventional artificial tears in DE patients.104
Acupuncture
The use of acupuncture as a treatment for eye disease is based on the claims that acupuncture modulates autonomic nervous system and immune system,105,106 which in turn might regulate lacrimal gland function. It therefore seems pertinent to evaluate the effectiveness of acupuncture as a treatment for DE. To date, more than 70 papers have examined the effect of acupuncture in treating DE. While some authors have suggested that acupuncture can influence lacrimal gland secretions,107 others have postulated that it can alleviate pain intensity (or increase pain threshold).108
Surgical treatment
Punctal occlusion
Punctal occlusion reduces drainage, preserves natural tears and prolongs the effect of lubricants. It is indicated in patients refractory to medical treatment, having a Schirmer test (with anesthesia) result of less than 5 mm at 5 min, and showing the evidence of ocular surface dye staining.109 Several techniques of punctal occlusion have been studied. For instance, temporary occlusion that dissolves in 1 or 2 weeks can be achieved by inserting collagen plugs into the canaliculi. Long-lasting collagen plugs that take 2–6 months to dissolve are also available. Silicon plugs are commonly used as reversible prolonged occlusion. Punctal occlusion using atelocollagen causes fewer complications when compared to insoluble plugs.110 Permanent punctal occlusion may be achieved surgically using cauterization. Combined use of punctal plugs and cyclosporine 0.05% demonstrated better improvement in Schirmer scores and rose bengal staining, and reduced overall artificial tear use compared to either treatment alone. 111
Salivary gland procedures
Surgical procedures involving salivary glands for the management of DE have been explored since 1951, when Filatov and Chevalijev described the parotid duct transfer to the conjunctival fornix.112 Murube described the transfer of the submandibular salivary gland to the temporal region and implant of the Wharton duct into the upper fornix.113 Studies have also reported the use of a graft of labial mucosa and minor salivary glands to treat severe dry eye.114 Soares and Franca have routinely performed this surgery in their clinic in Brazil between 2000 and 2004115 on patients with severe dry eye caused by Stevens–Johnson syndrome, chemical burns, pemphigoid, SS, and surgical removal of the lacrimal gland. The patients reported a subjective relief in DE symptoms immediately after surgery.
Subcutaneous abdominal artificial tear pump-reservoir
The artificial tear pump-reservoir was suggested by Murube for the treatment of severe dry eye.116 It was implanted into a subcutaneous pocket of the anterolateral abdominal wall and the silicon tube catheter is passed via chest, neck and face to the upper conjunctival fornix.
Conclusion
In conclusion, the understanding of the pathogenesis and specific cellular responses involved in different forms of DE could result in the development of other treatment strategies for a better management and long lasting results. The evidence implicating inflammation in the pathogenesis of DE has opened up new avenues for the treatment of this complex disorder. Development of additional treatment options in the form of compounds targeting specific components such as the epithelial barrier, corneal nerves, conjunctival goblet cells, or immune cells and cytokines involved in the ocular inflammatory reaction would provide hope for the millions of individuals who daily experience this deleterious condition.
Disclosure
-
a.
Financial Support: An unrestricted grant from Research to Prevent Blindness.
-
b.
Financial Disclosure(s): The authors have no financial interests in the topic of this manuscript. No conflicting relationship exists for any author.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgement
The authors thank the Grant from Research to Prevent Blindness, New York, NY to the Wilmer Eye Institute for research support.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5(2):179–93. [DOI] [PubMed]
- 2.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5(2):93–107. [DOI] [PubMed]
- 3.Behrens A., Doyle J.J., Stern L. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25(8):900–907. doi: 10.1097/01.ico.0000214802.40313.fa. [DOI] [PubMed] [Google Scholar]
- 4.McGinnigle S., Naroo S.A., Eperjesi F. Evaluation of dry eye. Surv Ophthalmol. 2012;57(4):293–316. doi: 10.1016/j.survophthal.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Serin D., Karsloglu S., Kyan A., Alagoz G. A simple approach to the repeatability of the Schirmer test without anesthesia: eyes open or closed? Cornea. 2007;26(8):903–906. doi: 10.1097/ICO.0b013e3180950083. [DOI] [PubMed] [Google Scholar]
- 6.Lemp M.A., Bron A.J., Baudouin C. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151(5):792–798. doi: 10.1016/j.ajo.2010.10.032. e1. [DOI] [PubMed] [Google Scholar]
- 7.Gilbard J.P. Tear film osmolarity and keratoconjunctivitis sicca. CLAO J. 1985;11(3):243–250. [PubMed] [Google Scholar]
- 8.Pensyl C.D., Benjamin W.J. Vapor pressure osmometry: minimum sample microvolumes. Acta Ophthalmol Scand. 1999;77(1):27–30. doi: 10.1034/j.1600-0420.1999.770106.x. [DOI] [PubMed] [Google Scholar]
- 9.Ogasawara K., Mitsubayashi K., Tsuru T., Karube I. Electrical conductivity of tear fluid in healthy persons and keratoconjunctivitis sicca patients measured by a flexible conductimetric sensor. Graefes Arch Clin Exp Ophthalmol. 1996;234(9):542–546. doi: 10.1007/BF00448797. [DOI] [PubMed] [Google Scholar]
- 10.Versura P., Profazio V., Campos E.C. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr Eye Res. 2010;35(7):553–564. doi: 10.3109/02713683.2010.484557. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki M., Massingale M.L., Ye F. Tear osmolarity as a biomarker for dry eye disease severity. Invest Ophthalmol Vis Sci. 2010;51(9):4557–4561. doi: 10.1167/iovs.09-4596. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney D.F., Millar T.J., Raju S.R. Tear film stability: a review. Exp Eye Res. 2013;117:28–38. doi: 10.1016/j.exer.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Norn M.S. Desiccation of the precorneal film. I. Corneal wetting-time. Acta Ophthalmol (Copenh) 1969;47(4):865–880. doi: 10.1111/j.1755-3768.1969.tb03711.x. [DOI] [PubMed] [Google Scholar]
- 14.Smith J., Nichols K.K., Baldwin E.K. Current patterns in the use of diagnostic tests in dry eye evaluation. Cornea. 2008;27(6):656–662. doi: 10.1097/QAI.0b013e3181605b95. [DOI] [PubMed] [Google Scholar]
- 15.Varikooty J., Keir N., Simpson T. Estimating tear film spread and stability through tear hydrodynamics. Optometry Vision Sci. 2012;89(8):E1119–E1124. doi: 10.1097/OPX.0b013e3182644cb7. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z., Pflugfelder S.C. Corneal surface regularity and the effect of artificial tears in aqueous tear deficiency. Ophthalmology. 1999;106(5):939–943. doi: 10.1016/S0161-6420(99)00513-8. [DOI] [PubMed] [Google Scholar]
- 17.Szczesna D.H., Alonso-Caneiro D., Iskander D.R. Predicting dry eye using noninvasive techniques of tear film surface assessment. Invest Ophthalmol Vis Sci. 2011;52(2):751–756. doi: 10.1167/iovs.10-5173. [DOI] [PubMed] [Google Scholar]
- 18.Montes-Mico R., Caliz A., Alio J.L. Wavefront analysis of higher order aberrations in dry eye patients. J Refract Surg. 2004;20(3):243–247. doi: 10.3928/1081-597X-20040501-08. [DOI] [PubMed] [Google Scholar]
- 19.McDonald J.E. Surface phenomena of tear films. Trans Am Ophthalmol Soc. 1968;66:905–939. [PMC free article] [PubMed] [Google Scholar]
- 20.Finis D., Pischel N., Schrader S., Geerling G. Evaluation of lipid layer thickness measurement of the tear film as a diagnostic tool for meibomian gland dysfunction. Cornea. 2013;32(12):1549–1553. doi: 10.1097/ICO.0b013e3182a7f3e1. [DOI] [PubMed] [Google Scholar]
- 21.Yokoi N., Komuro A. Non-invasive methods of assessing the tear film. Exp Eye Res. 2004;78(3):399–407. doi: 10.1016/j.exer.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Resua C., Fernandez M.J.G., Penedo M.F.G. New software application for clarifying tear film lipid layer patterns. Cornea. 2013;32(4):538–546. doi: 10.1097/ICO.0b013e31824d0d04. [DOI] [PubMed] [Google Scholar]
- 23.Koh S., Maeda N., Hirohara Y. Serial measurements of higher-order aberrations after blinking in normal subjects. Invest Ophthalmol Vis Sci. 2006;47(8):3318–3324. doi: 10.1167/iovs.06-0018. [DOI] [PubMed] [Google Scholar]
- 24.Lin Y.Y., Carrel H., Wang I.J. Effect of tear film break-up on higher order aberrations of the anterior cornea in normal, dry, and post-LASIK eyes. J Refract Surg. 2005;21(5):S525–S529. doi: 10.3928/1081-597X-20050901-21. [DOI] [PubMed] [Google Scholar]
- 25.Koh S., Maeda N., Hirohara Y. Serial measurements of higher-order aberrations after blinking in patients with dry eye. Invest Ophthalmol Vis Sci. 2008;49(1):133–138. doi: 10.1167/iovs.07-0762. [DOI] [PubMed] [Google Scholar]
- 26.Montes-Mico R., Alio J.L., Charman W.N. Postblink changes in the ocular modulation transfer function measured by a double-pass method. Invest Ophthalmol Vis Sci. 2005;46(12):4468–4473. doi: 10.1167/iovs.05-0609. [DOI] [PubMed] [Google Scholar]
- 27.Ridder W.H., LaMotte J., Hall J.Q. Contrast sensitivity and tear layer aberrometry in dry eye patients. Optometry Vision Sci. 2009;86(9):1059–1068. doi: 10.1097/OPX.0b013e3181b599bf. [DOI] [PubMed] [Google Scholar]
- 28.Dieckow J. 6th International Conference on the Tear Film & Ocular Surface: basic science and clinical relevance (Florence, Italy, September 2010) Ocul Surf. 2011;9(1):3–12. doi: 10.1016/s1542-0124(11)70004-0. [DOI] [PubMed] [Google Scholar]
- 29.Kaido M., Dogru M., Ishida R., Tsubota K. Concept of functional visual acuity and its applications. Cornea. 2007;26(9 Suppl. 1):S29–S35. doi: 10.1097/ICO.0b013e31812f6913. [DOI] [PubMed] [Google Scholar]
- 30.Goto E., Yagi Y., Matsumoto Y., Tsubota K. Impaired functional visual acuity of dry eye patients. Am J Ophthalmol. 2002;133(2):181–186. doi: 10.1016/s0002-9394(01)01365-4. [DOI] [PubMed] [Google Scholar]
- 31.Ishida R., Kojima T., Dogru M. The application of a new continuous functional visual acuity measurement system in dry eye syndromes. Am J Ophthalmol. 2005;139(2):253–258. doi: 10.1016/j.ajo.2004.08.075. [DOI] [PubMed] [Google Scholar]
- 32.Kaido M., Ishida R., Dogru M. Efficacy of punctum plug treatment in short break-up time dry eye. Optometry Vision Sci. 2008;85(8):758–763. doi: 10.1097/OPX.0b013e3181819f0a. [DOI] [PubMed] [Google Scholar]
- 33.Wang J., Fonn D., Simpson T.L., Jones L. Precorneal and pre- and postlens tear film thickness measured indirectly with optical coherence tomography. Invest Ophthalmol Vis Sci. 2003;44(6):2524–2528. doi: 10.1167/iovs.02-0731. [DOI] [PubMed] [Google Scholar]
- 34.Shen M., Li J., Wang J. Upper and lower tear menisci in the diagnosis of dry eye. Invest Ophthalmol Vis Sci. 2009;50(6):2722–2726. doi: 10.1167/iovs.08-2704. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen P., Huang D., Li Y. Correlation between optical coherence tomography-derived assessments of lower tear meniscus parameters and clinical features of dry eye disease. Cornea. 2012;31(6):680–685. doi: 10.1097/ICO.0b013e3182261577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veres A., Tapaszto B., Kosina-Hagyo K. Imaging lid-parallel conjunctival folds with OCT and comparing its grading with the slit lamp classification in dry eye patients and normal subjects. Invest Ophthalmol Vis Sci. 2011;52(6):2945–2951. doi: 10.1167/iovs.10-5505. [DOI] [PubMed] [Google Scholar]
- 37.Kanellopoulos A.J., Asimellis G. In vivo 3-dimensional corneal epithelial thickness mapping as an indicator of dry eye: preliminary clinical assessment. Am J Ophthalmol. 2014;157(1):63–68. doi: 10.1016/j.ajo.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 38.Ju M.J., Shin J.G., Hoshi S. Three-dimensional volumetric human meibomian gland investigation using polarization-sensitive optical coherence tomography. J Biomed Opt. 2014;19(3):30503. doi: 10.1117/1.JBO.19.3.030503. [DOI] [PubMed] [Google Scholar]
- 39.Mathers W.D., Daley T.E. In-vivo observation of the human tear film by tandem scanning confocal microscopy. Scanning. 1994;16(5):316–319. [PubMed] [Google Scholar]
- 40.Wakamatsu T.H., Sato E.A., Matsumoto Y. Conjunctival In Vivo Confocal Scanning Laser Microscopy in Patients with Sjogren Syndrome. Invest Ophthalmol Vis Sci. 2010;51(1):144–150. doi: 10.1167/iovs.08-2722. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X.B., Chen Q., Chen W. Tear dynamics and corneal confocal microscopy of subjects with mild self-reported office dry eye. Ophthalmology. 2011;118(5):902–907. doi: 10.1016/j.ophtha.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 42.Pflugfelder S.C., Solomon A., Stern M.E. The diagnosis and management of dry eye: a twenty-five-year review. Cornea. 2000;19(5):644–649. doi: 10.1097/00003226-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Efron N. Contact lens-induced changes in the anterior eye as observed in vivo with the confocal microscope. Prog Retin Eye Res. 2007;26(4):398–436. doi: 10.1016/j.preteyeres.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Tuominen I.S., Konttinen Y.T., Vesaluoma M.H. Corneal innervation and morphology in primary Sjogren’s syndrome. Invest Ophthalmol Vis Sci. 2003;44(6):2545–2549. doi: 10.1167/iovs.02-1260. [DOI] [PubMed] [Google Scholar]
- 45.Lin H., Li W., Dong N. Changes in corneal epithelial layer inflammatory cells in aqueous tear-deficient dry eye. Invest Ophthalmol Vis Sci. 2010;51(1):122–128. doi: 10.1167/iovs.09-3629. [DOI] [PubMed] [Google Scholar]
- 46.Ibrahim O.M., Matsumoto Y., Dogru M. The efficacy, sensitivity, and specificity of in vivo laser confocal microscopy in the diagnosis of meibomian gland dysfunction. Ophthalmology. 2010;117(4):665–672. doi: 10.1016/j.ophtha.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 47.Hong J., Zhu W., Zhuang H. In vivo confocal microscopy of conjunctival goblet cells in patients with Sjogren’s syndrome dry eye. Br J Ophthalmol. 2010;94(11):1454–1458. doi: 10.1136/bjo.2009.161059. [DOI] [PubMed] [Google Scholar]
- 48.Wakamatsu T.H., Sato E.A., Matsumoto Y. Conjunctival in vivo confocal scanning laser microscopy in patients with Sjogren syndrome. Invest Ophthalmol Vis Sci. 2010;51(1):144–150. doi: 10.1167/iovs.08-2722. [DOI] [PubMed] [Google Scholar]
- 49.Nichols K.K., Foulks G.N., Bron A.J. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922–1929. doi: 10.1167/iovs.10-6997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bron A.J., Benjamin L., Snibson G.R. Meibomian gland disease. Classification and grading of lid changes. Eye (Lond) 1991;5(Pt 4):395–411. doi: 10.1038/eye.1991.65. [DOI] [PubMed] [Google Scholar]
- 51.Arita R., Itoh K., Inoue K. Noncontact meibography detects changes in meibomian glands in the aging process in a normal population and patients with meibomian gland dysfunction. Cornea. 2009;28(9):S75–S79. [Google Scholar]
- 52.Tomlinson A., Bron A.J., Korb D.R. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):2006–2049. doi: 10.1167/iovs.10-6997f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chew C.K., Jansweijer C., Tiffany J.M. An instrument for quantifying meibomian lipid on the lid margin: the meibometer. Curr Eye Res. 1993;12(3):247–254. doi: 10.3109/02713689308999470. [DOI] [PubMed] [Google Scholar]
- 54.Bron A.J., Evans V.E., Smith J.A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–650. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Nichols K.K., Mitchell G.L., Zadnik K. The repeatability of clinical measurements of dry eye. Cornea. 2004;23(3):272–285. doi: 10.1097/00003226-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 56.Sullivan B.D., Whitmer D., Nichols K.K. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci. 2010;51(12):6125–6130. doi: 10.1167/iovs.10-5390. [DOI] [PubMed] [Google Scholar]
- 57.Whitcher J.P., Shiboski C.H., Shiboski S.C. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjogren’s Syndrome International Registry. Am J Ophthalmol. 2010;149(3):405–415. doi: 10.1016/j.ajo.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reddy M., Reddy P.R., Reddy S.C. Conjunctival impression cytology in dry eye states. Indian J Ophthalmol. 1991;39(1):22–24. [PubMed] [Google Scholar]
- 59.Solomon A., Dursun D., Liu Z. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42(10):2283–2292. [PubMed] [Google Scholar]
- 60.Barabino S., Montaldo E., Solignani F. Immune response in the conjunctival epithelium of patients with dry eye. Exp Eye Res. 2010;91(4):524–529. doi: 10.1016/j.exer.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 61.Tsubota K., Kajiwara K., Ugajin S., Hasegawa T. Conjunctival brush cytology. Acta Cytol. 1990;34(2):233–235. [PubMed] [Google Scholar]
- 62.Wakamatsu T.H., Okada N., Kojima T. Evaluation of conjunctival inflammatory status by confocal scanning laser microscopy and conjunctival brush cytology in patients with atopic keratoconjunctivitis (AKC) Mol Vis. 2009;15:1611–1619. [PMC free article] [PubMed] [Google Scholar]
- 63.Nelson J.D. Simultaneous evaluation of tear turnover and corneal epithelial permeability by fluorophotometry in normal subjects and patients with keratoconjunctivitis sicca (KCS) Trans Am Ophthalmol Soc. 1995;93:709–753. [PMC free article] [PubMed] [Google Scholar]
- 64.Lam H., Bleiden L., de Paiva C.S. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;47(2):198–205. doi: 10.1016/j.ajo.2008.08.032. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Enriquez-de-Salamanca A., Castellanos E., Stern M.E. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16:862–873. [PMC free article] [PubMed] [Google Scholar]
- 66.Butovich I.A. Lipidomics of human meibomian gland secretions: chemistry, biophysics, and physiological role of meibomian lipids. Prog Lipid Res. 2011;50(3):278–301. doi: 10.1016/j.plipres.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5(2):163–78. [DOI] [PubMed]
- 68.Utine C.A., Stern M., Akpek E.K. Clinical review: topical ophthalmic use of cyclosporin A. Ocul Immunol Inflammation. 2010;18(5):352–361. doi: 10.3109/09273948.2010.498657. [DOI] [PubMed] [Google Scholar]
- 69.Sall K., Stevenson O.D., Mundorf T.K. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology. 2000;107(4):631–639. doi: 10.1016/s0161-6420(99)00176-1. [DOI] [PubMed] [Google Scholar]
- 70.Kunert K.S., Tisdale A.S., Gipson I.K. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120(8):1099. doi: 10.1001/archopht.120.3.330. [DOI] [PubMed] [Google Scholar]
- 71.Aksoy M.O., Li X., Borenstein M. Effects of topical corticosteroids on inflammatory mediator-induced eicosanoid release by human airway epithelial cells. J Allergy Clin Immunol. 1999;103(6):1081–1091. doi: 10.1016/s0091-6749(99)70183-1. [DOI] [PubMed] [Google Scholar]
- 72.Pflugfelder S.C., Maskin S.L., Anderson B. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138(3):444–457. doi: 10.1016/j.ajo.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 73.Hong S., Kim T., Chung S.H. Recurrence after topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjogren’s syndrome. J Ocul Pharmacol Ther. 2007;23(1):78–82. doi: 10.1089/jop.2006.0091. [DOI] [PubMed] [Google Scholar]
- 74.Rocha E.M., Wickham L.A., da Silveira L.A. Identification of androgen receptor protein and 5alpha-reductase mRNA in human ocular tissues. Br J Ophthalmol. 2000;84(1):76–84. doi: 10.1136/bjo.84.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khandelwal P., Liu S., Sullivan D.A. Androgen regulation of gene expression in human meibomian gland and conjunctival epithelial cells. Mol Vis. 2012;18:1055–1067. [PMC free article] [PubMed] [Google Scholar]
- 76.Sullivan D.A., Rocha E.M., Ullman M.D. Androgen regulation of the meibomian gland. Adv Exp Med Biol. 1998;438:327–331. doi: 10.1007/978-1-4615-5359-5_46. [DOI] [PubMed] [Google Scholar]
- 77.Sullivan D.A. Tearful relationships? Sex, hormones, the lacrimal gland, and aqueous-deficient dry eye. Ocul Surf. 2004;2(2):92–123. doi: 10.1016/s1542-0124(12)70147-7. [DOI] [PubMed] [Google Scholar]
- 78.Greenblatt R.B., Colle M.L., Mahesh V.B. Ovarian and adrenal steroid production in the postmenopausal woman. Obstet Gynecol. 1976;47(4):383–387. [PubMed] [Google Scholar]
- 79.Gagliano C., Caruso S., Napolitano G. Low levels of 17-beta-oestradiol, oestrone and testosterone correlate with severe evaporative dysfunctional tear syndrome in postmenopausal women: a case-control study. Br J Ophthalmol. 2014;98(3):371–376. doi: 10.1136/bjophthalmol-2012-302705. [DOI] [PubMed] [Google Scholar]
- 80.Worda C., Nepp J., Huber J.C., Sator M.O. Treatment of keratoconjunctivitis sicca with topical androgen. Maturitas. 2001;37(3):209–212. doi: 10.1016/s0378-5122(00)00181-x. [DOI] [PubMed] [Google Scholar]
- 81.Sator M.O., Joura E.A., Golaszewski T. Treatment of menopausal keratoconjunctivitis sicca with topical oestradiol. Br J Obstet Gynaecol. 1998;105(1):100–102. doi: 10.1111/j.1471-0528.1998.tb09358.x. [DOI] [PubMed] [Google Scholar]
- 82.Scott G., Yiu S.C., Wasilewski D. Combined esterified estrogen and methyltestosterone treatment for dry eye syndrome in postmenopausal women. Am J Ophthalmol. 2005;139(6):1109–1110. doi: 10.1016/j.ajo.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 83.Stone D.U., Chodosh J. Oral tetracyclines for ocular rosacea: an evidence-based review of the literature. Cornea. 2004;23(1):106–109. doi: 10.1097/00003226-200401000-00020. [DOI] [PubMed] [Google Scholar]
- 84.Solomon A., Rosenblatt M., Li D. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Am J Ophthalmol. 2000;130(5):688. doi: 10.1016/s0002-9394(00)00755-8. [DOI] [PubMed] [Google Scholar]
- 85.De Paiva C.S., Corrales R.M., Villarreal A.L. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83(3):526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 86.Nikodemova M., Watters J.J., Jackson S.J. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)alpha/betaII. J Biol Chem. 2007;282(20):15208–15216. doi: 10.1074/jbc.M611907200. [DOI] [PubMed] [Google Scholar]
- 87.Stevenson W., Chauhan S.K., Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130(1):90–100. doi: 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rosenberg E.S., Asbell P.A. Essential fatty acids in the treatment of dry eye. Ocul Surf. 2010;8(1):18–28. doi: 10.1016/s1542-0124(12)70214-8. [DOI] [PubMed] [Google Scholar]
- 89.Wojtowicz J.C., Butovich I., Uchiyama E. Pilot, prospective, randomized, double-masked, placebo-controlled clinical trial of an omega-3 supplement for dry eye. Cornea. 2011;30(3):308–314. doi: 10.1097/ICO.0b013e3181f22e03. [DOI] [PubMed] [Google Scholar]
- 90.Barabino S., Rolando M., Camicione P. Systemic linoleic and gamma-linolenic acid therapy in dry eye syndrome with an inflammatory component. Cornea. 2003;22(2):97–101. doi: 10.1097/00003226-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 91.Rashid S., Jin Y.P., Ecoiffier T. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126(2):219–225. doi: 10.1001/archophthalmol.2007.61. [DOI] [PubMed] [Google Scholar]
- 92.Li N., He J.C., Schwartz C.E. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J Ocul Pharmacol Ther. 2010;26(5):431–439. doi: 10.1089/jop.2010.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee H.K., Lee K.S., Kim H.C. Nerve growth factor concentration and implications in photorefractive keratectomy vs laser in situ keratomileusis. Am J Ophthalmol. 2005;139(6):965–971. doi: 10.1016/j.ajo.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 94.Lambiase A., Mantelli F., Sacchetti M. Clinical applications of NGF in ocular diseases. Archives Italiennes De Biologie. 2011;149(2):283–292. doi: 10.4449/aib.v149i2.1363. [DOI] [PubMed] [Google Scholar]
- 95.Chang E.J., Im Y.S., Kay E.P. The role of nerve growth factor in hyperosmolar stress induced apoptosis. J Cell Physiol. 2008;216(1):69–77. doi: 10.1002/jcp.21377. [DOI] [PubMed] [Google Scholar]
- 96.Lambiase A., Aloe L., Centofanti M. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: implications for glaucoma. Proc Natl Acad Sci USA. 2009;106(32):13469–13474. doi: 10.1073/pnas.0906678106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coassin M., Lambiase A., Costa N. Efficacy of topical nerve growth factor treatment in dogs affected by dry eye. Graefes Arch Clin Exp Ophthalmol. 2005;243(2):151–155. doi: 10.1007/s00417-004-0955-2. [DOI] [PubMed] [Google Scholar]
- 98.Celebi A.R., Ulusoy C., Mirza G.E. The efficacy of autologous serum eye drops for severe dry eye syndrome: a randomized double-blind crossover study. Graefes Arch Clin Exp Ophthalmol. 2014;252(4):619–626. doi: 10.1007/s00417-014-2599-1. [DOI] [PubMed] [Google Scholar]
- 99.Yoon K.C., Heo H., Im S.K. Comparison of autologous serum and umbilical cord serum eye drops for dry eye syndrome. Am J Ophthalmol. 2007;144(1):86–92. doi: 10.1016/j.ajo.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 100.Fox R.I., Chan R., Michelson J.B. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum. 1984;27(4):459–461. doi: 10.1002/art.1780270415. [DOI] [PubMed] [Google Scholar]
- 101.Tsubota K., Goto E., Fujita H. Treatment of dry eye by autologous serum application in Sjogren’s syndrome. Br J Ophthalmol. 1999;83(4):390–395. doi: 10.1136/bjo.83.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bradley J.C., Bradley R.H., McCartney D.L., Mannis M.J. Serum growth factor analysis in dry eye syndrome. Clin Exp Ophthalmol. 2008;36(8):717–720. doi: 10.1111/j.1442-9071.2008.01895.x. [DOI] [PubMed] [Google Scholar]
- 103.Noble B.A., Loh R.S., MacLennan S. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. Br J Ophthalmol. 2004;88(5):647–652. doi: 10.1136/bjo.2003.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Urzua C.A., Vasquez D.H., Huidobro A. Randomized double-blind clinical trial of autologous serum versus artificial tears in dry eye syndrome. Curr Eye Res. 2012;37(8):684–688. doi: 10.3109/02713683.2012.674609. [DOI] [PubMed] [Google Scholar]
- 105.Kavoussi B., Ross B.E. The neuroimmune basis of anti-inflammatory acupuncture. Integr Cancer Ther. 2007;6(3):251–257. doi: 10.1177/1534735407305892. [DOI] [PubMed] [Google Scholar]
- 106.Backer M., Grossman P., Schneider J. Acupuncture in migraine: investigation of autonomic effects. Clin J Pain. 2008;24(2):106–115. doi: 10.1097/AJP.0b013e318159f95e. [DOI] [PubMed] [Google Scholar]
- 107.Gong L., Sun X. Treatment of intractable dry eyes: tear secretion increase and morphological changes of the lacrimal gland of rabbit after acupuncture. Acupunct Electrother Res. 2007;32(3–4):223–233. doi: 10.3727/036012907815844011. [DOI] [PubMed] [Google Scholar]
- 108.Nepp J., Jandrasits K., Schauersberger J. Is acupuncture an useful tool for pain-treatment in ophthalmology? Acupunct Electrother Res. 2002;27(3–4):171–182. doi: 10.3727/036012902816025988. [DOI] [PubMed] [Google Scholar]
- 109.Baxter S.A., Laibson P.R. Punctal plugs in the management of dry eyes. Ocul Surf. 2004;2(4):255–265. doi: 10.1016/s1542-0124(12)70113-1. [DOI] [PubMed] [Google Scholar]
- 110.Miyata K., Otani S., Miyai T. Atelocollagen punctal occlusion in dry eye patients. Cornea. 2006;25(1):47–50. doi: 10.1097/01.ico.0000164783.10667.a4. [DOI] [PubMed] [Google Scholar]
- 111.Roberts C.W., Carniglia P.E., Brazzo B.G. Comparison of topical cyclosporine, punctal occlusion, and a combination for the treatment of dry eye. Cornea. 2007;26(7):805–809. doi: 10.1097/ICO.0b013e318074e460. [DOI] [PubMed] [Google Scholar]
- 112.Tavares Fde P., Fernandes R.S., Bernardes T.F. Dry eye disease. Semin Ophthalmol. 2010;25(3):84–93. doi: 10.3109/08820538.2010.488568. [DOI] [PubMed] [Google Scholar]
- 113.Geerling G., Sieg P., Bastian G.O., Laqua H. Transplantation of the autologous submandibular gland for most severe cases of keratoconjunctivitis sicca. Ophthalmology. 1998;105(2):327–335. doi: 10.1016/s0161-6420(98)93406-6. [DOI] [PubMed] [Google Scholar]
- 114.Guerrissi J.O., Belmonte J. Surgical treatment of dry eye syndrome: conjunctival graft of the minor salivary gland. J Craniofac Surg. 2004;15(1):6–10. doi: 10.1097/00001665-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 115.Soares E.J., Franca V.P. Transplantation of labial salivary glands for severe dry eye treatment. Arq Bras Oftalmol. 2005;68(4):481–489. doi: 10.1590/s0004-27492005000400012. [DOI] [PubMed] [Google Scholar]
- 116.Murube J., Murube E., ChenZhuo L., Rivas L. Subcutaneous abdominal artificial tears pump-reservoir for severe dry eye. Orbit. 2003;22(1):29–40. doi: 10.1076/orbi.22.1.29.14012. [DOI] [PubMed] [Google Scholar]


