Abstract
Plant phenolics can have applications in pharmaceutical and other industries. To identify and quantify the phenolic compounds in Helianthus tuberosus leaves, qualitative analysis was performed by a reversed phase high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS) and quantitative analysis by HPLC. Ten chlorogenic acids (CGAs) were identified (3-o-caffeoylquinic acid, two isomers of caffeoylquinic acid, caffeic acid, p-coumaroyl-quinic acid, feruloylquinic acid, 3,4-dicaffeoyquinic acid, 3,5-dicaffeoylquinic acid, 1,5-dicaffeoylquinic acid, and 4,5-dicaffeoylquinic acid) by comparing their retention times, UV-Vis absorption spectra, and MS/MS spectra with standards. In addition, four other phenolic compounds, including caffeoyl glucopyranose, isorhamnetin glucoside, kaempferol glucuronide, and kaempferol-3-o-glucoside, were tentatively identified in Helianthus tuberosus leaves for the first time. The 3-o-caffeoylquinic acid (7.752 mg/g DW), 4,5-dicaffeoylquinic acid (5.633 mg/g DW), and 3,5-dicaffeoylquinic acid (4.900 mg/g DW) were the major phenolic compounds in leaves of Helianthus tuberosus cultivar NanYu in maturity. The variations in phenolic concentrations and proportions in Helianthus tuberosus leaves were influenced by genotype and plant growth stage. Cultivar NanYu had the highest concentration of phenolic compounds, in particular 3-o-caffeoylquinic acid and 4,5-dicaffeoylquinic acid compared with the other genotypes (wild accession and QingYu). Considering various growth stages, the concentration of total phenolics in cultivar NanYu was higher at flowering stage (5.270 mg/g DW) than at budding and tuber swelling stages. Cultivar NanYu of Helianthus tuberosus is a potential source of natural phenolics that may play an important role in the development of pharmaceuticals.
1. Introduction
Helianthus tuberosus L. (Jerusalem artichoke), Asteraceae family, is a perennial herb originating from eastern North America. It has been introduced and cultivated widely in the temperate areas for the edible tubers. H. tuberosus has tall stem, large leaves, bright yellow flowers resembling those of sunflowers, and fleshy potato-like tubers. As a source of inulin, the tubers have been used as a folk medicine for the treatment of diabetes and rheumatism with a variety of pharmacological activities, such as aperient, cholagogue, diuretic, spermatogenic, stomachic, and tonic [1]. Additionally, the leaves of H. tuberosus have been utilized as a folk medicine for the treatment of bone fracture, skin wounds, swelling, and pain [2, 3] with antipyretic, analgesic, anti-inflammatory, and antispasmodic effects [4–6]. Moreover, the stalks and leaves of this plant were also found to possess antioxidant, antimicrobial, antifungal, and anticancer activities [1, 6, 7].
The effective compounds in H. tuberosus are coumarins, unsaturatedfatty acids, polyacetylenic derivatives, phenolic compounds, and sesquiterpenes [1]. Recent studies have shown that pharmacological characteristics of H. tuberosus were related to its phenolic compounds with antioxidant and radical-scavenging activity; the main phenolic acids in H. tuberosus leaves were chlorogenic acids [6]. Chlorogenic acids had inhibitory effects on carcinogenesis in the large intestine, liver, and tongue and protective effects against oxidative stress in vivo [8]. More broadly, phenolic acids are widely distributed in plants as the secondary metabolites [9]; some phenolic acids are allelochemicals used to control biological pests [10–12], plant pathogens [13], and weeds [14]. The involvement of phenolics with plant protection and communication makes phenolics pivotal molecules in the responses of plants to their ever-changing environment [15].
Previously, it was demonstrated that the leaves of H. tuberosus contained high concentration of phenolic compounds [5]. Phenolics were separated and identified (such as ferulic acids) from the tubers of H. tuberosus [16]. However, to date, reports on analysis and identification of phenolic compounds from the leaves of H. tuberosus are scarce and only a few phenolics, especially chlorogenic acid and isochlorogenic acids, have been identified and qualitatively analysed [6].
Reversed phase high-performance liquid chromatography coupled to tandem mass spectrometry (HPLC-MS/MS) has been extensively and successfully applied to the online structure elucidation of phenolic compounds in foodstuffs, having advantages of high sensitivity, speed, and low sample consumption [17–24]. In addition, liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) techniques are useful for elucidating the structures of the active compounds (e.g., nonvolatile phenolic compounds) and distinguishing compounds with identical molecular weights [23, 25].
The objectives of the present work were to identify the phenolic compounds in H. tuberosus leaves, using HPLC-MS/MS technique, and to measure the concentration of main phenolics in H. tuberosus leaves of different cultivars at different sampling periods from budding stage to maturity (tuber swelling stage) using HPLC.
2. Materials and Methods
2.1. Chemicals and Materials
Gallic acid was obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China); and 3-o-caffeoylquinic acid was obtained from Aladdin Reagent Co., Ltd. (Shanghai, China). Other standard samples were obtained from Yuanye Biological Technology Co., Ltd. (Shanghai, China). All other analytical grade chemicals were obtained from Shoude Experimental Equipment Co., Ltd. (Nanjing, China).
The leaves of three H. tuberosus cultivars (the wild accession, the southern cultivar NanYu [26], and QingYu originated from northern China) were collected from Dafeng District (Jiangsu, China) in maturity at the end of October 2011. Both cultivars NanYu and QingYu [27, 28] are superior varieties in local areas which have obvious competitive advantages in yield, quality, saline-alkali tolerance, and so on over the wild accession in Dafeng District. The leaves of cultivar NanYu were collected from August to October 2012 at different growth stages including budding, flowering, and tuber swelling stages, respectively.
2.2. Extraction of Phenolic Compounds
The air-dried (room temperature) and milled [6] leaves (10 g) of H. tuberosus were refluxed under vacuum at 50°C using 70% v/v ethanol (EtOH) for three hours. After evaporation under reduced pressure, the dry residue was redissolved in 25 mL of methanol and used for colorimetric and chromatographic analyses. For HPLC analysis, all samples were filtered through a 0.22 μm cellulose acetate filter (Millipore Corp., Bedford, MA, USA) before injections.
2.3. Measurement of Total Phenolic Concentration
The total phenolic concentration (TPC) was determined using the Folin-Ciocalteu reagent with gallic acid as standard [19, 29, 30]. The reaction mixture contained 0.5 mL of test sample, 0.5 mL of the Folin-Ciocalteu reagent freshly prepared in our laboratory, 2.0 mL of 10% w/v sodium carbonate solution, and 3.0 mL of distilled water. After 2 h of reaction under ambient temperature in the dark, the absorbance at 760 nm was measured. A calibration curve with equation: y = 0.0029x + 0.0107 (R 2 = 0.9991) was constructed using gallic acid solutions in the range of 1.470–294 mg/L. Results were expressed in milligram gallic acid equivalents per gram of dried sample.
2.4. HPLC-MS/MS Analysis
HPLC-MS/MS analysis of phenolics in H. tuberosus extracts was performed using an Agilent 1200 series HPLC system (Agilent Technology Co. Ltd., USA), composed of a diode array detector and an Agilent 6400 series triple quadrupole (QQQ) mass spectrometer equipped with an electrospray ionization (ESI) source. Data were collected and processed via a personal computer running Agilent MassHunter workstation (Micromass, Qualitative Analysis Version B.01.03 of Agilent Technology Co. Ltd., USA). A reverse-phase Eclipse XDB-C18 column (250 mm × 4.6 mm, 5 µm, Agilent Technology Co. Ltd., USA) was used for separation. The mobile phases consisted of methanol (A) and 1.0% v/v formic acid aqueous solution (B). Gradient elution was started with 30% of A and ascended to 50% of A in 45 min. The flow rate was kept at 0.8 mL/min, and the column temperature was 30°C. Samples were filtered through a 0.22 µm filter prior to HPLC injection. The injection volume was 5 µL. UV-Vis absorption spectra were recorded online from 200 to 600 nm during HPLC analysis. Phenolics were detected at the wavelength of 330 nm.
Mass and MS/MS spectra were achieved by electrospray ionization (ESI) in negative modes. The voltages used were 4000 V for the source capillary and 10 V for the extraction cone: the source temperature was 150°C and the desolvation temperature was 350°C. The electrospray probe flow was adjusted to 70 mL/min. The ESI-MS and ESI-MS/MS spectra were obtained by scanning from 200 to 1200 m/z. The MS/MS fragmentations were carried out with 10%–50% energy.
2.5. Identification and Quantification of Phenolic Compounds
The phenolic compounds in H. tuberosus leaves extracts were identified by comparing their UV-Vis absorption spectra, matching their molecular ions (m/z) obtained by ESI-MS and ESI-MS/MS chromatographic characteristics with the literature data reported [5, 6] or with available reference standards. The external standard method was used for the quantification of main phenolic acids. Concentrations of 3-o-caffeoylquinic acid, caffeic acid, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, 1,5-dicaffeoylquinic acid, 4,5-dicaffeoylquinic acid, and so on were calculated with the regression equations from the standard curves. Concentrations were expressed as mg/g dried weight sample (DW).
2.6. Statistical Analysis
The TPC and concentration of phenolic compounds in H. tuberosus leaves of different cultivars and different growth stages were sources of variation. These data were reported as mean ± SD from triplicate determinations. Statistical analysis was performed with analysis of variance (ANOVA) and statistical significance specified at P ≤ 0.05.
3. Results
3.1. Identification of the Chromatographic Peaks
The examination of the chromatograms in a full-scan mode revealed the presence of several compounds, which were positively identified by comparison with available standards. Figure 1 showed the HPLC chromatogram of ethanol extract from H. tuberosus leaves. There were 15 phenolic peaks separated in extracts using the reversed phase C-18 column. As shown in Table 1, peak identification was performed by comparing retention times (t R), UV-Vis spectra, mass, and MS/MS spectra with those of reference standards or literature data.
Figure 1.
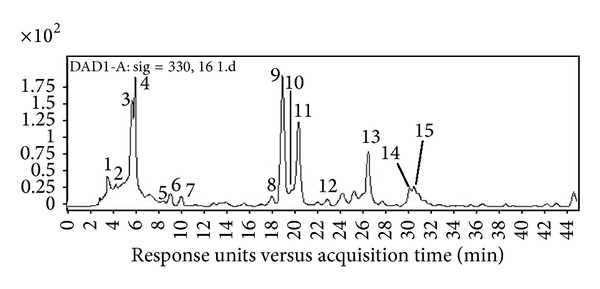
HPLC chromatogram of the ethanol extract of H. tuberosus leaves detected at 330 nm. Peak numbers were consistent with those shown in Table 1.
Table 1.
Identification of phenolic compounds in H. tuberosus leaves by HPLC-MS/MS.
| Peaks number | t R (min) | UV λ max (nm) | MW | MS− | MS/MS | Identification |
|---|---|---|---|---|---|---|
| 1 | 3.58 | 246.0, 263.0 | 360 | 359.4 | 297.3, 281.6, 230.9, 135.2 | Unknown |
| 2 | 4.36 | 214.3, 323.4 | 354 | 353.4 | 191.1, 179.1, 161.1, 135.1, 85.1 | Caffeoylquinic acid (isomer of chlorogenic acid) |
| 3 | 5.78 | 214.4, 327.0 | 354 | 353.4 | 191.1, 127.0, 85.0 | 3-o-Caffeoylquinic acid (3-CQA) |
| 4 | 6.07 | 237.9, 324.4 | 354 | 353.4 | 191.2, 127, 93.1, 85.0 | Caffeoylquinic acid |
| 5 | 8.57 | 323.0 | 180 | 179.1 | 136.0, 107.9 | Caffeic acid (CA) |
| 6 | 9.19 | 239.1, 311.5 | 338 | 337.3 | 191.1, 173.0, 93.0 | p-Coumaroyl-quinic acid |
| 7 | 10.12 | 241.4, 325.8 | 368 | 367.3 | 191.0, 173.1, 134.0, 93.0 | Feruloylquinic acid |
| 8 | 18.09 | 243.8, 327.0 | 516 | 515.5 | 354.3, 191.1, 173.1, 179.1, 135.0 | Dicaffeoylquinic acid (3,4-DiCQA) |
| 9 | 19.03 | 242.6, 327.0 | 516 | 515.5 | 354.1, 191.2 | Dicaffeoylquinic acid (3,5-DiCQA) |
| 10 | 20.04 | 327 | 342 | 341.3 | 179.1, 161.1 | Caffeoyl glucopyranose |
| 11 | 20.43 | 243, 329.4 | 516 | 515.5 | 354.1, 191.1, 179.1, 173.1, 135.1 | Dicaffeoylquinic acid (1,5-DiCQA) |
| 12 | 22.97 | 253.3, 349.7 | 478 | 477.4 | 315.3, 300.1, 270.9, 180.2 | Isorhamnetin glucoside |
| 13 | 26.57 | 327.0 | 516 | 515.5 | 191.1, 179.1, 173.1, 135.0 | Dicaffeoylquinic acid (4,5-DiCQA) |
| 14 | 30.20 | 263.9, 341.3 | 462 | 461.4 | 315.2, 284.8, 161.0, 132.7, 85.1 | Kaempferol glucuronide |
| 15 | 30.58 | 263.0, 333.0 | 448 | 447.4 | 285.4, 190.8, 153.1, 96.9 | Kaempferol-3-o-glucoside |
Classically, chlorogenic acids (CGAs) are a family of esters formed between quinic acid and certain trans-cinnamic acids, most commonly caffeic, p-coumaric, and ferulic acids [31]. Fragment ions m/z 191 and m/z 179, corresponding to deprotonated quinic acid and caffeic acid fragments, were characteristics of the MS/MS spectra of quinic or caffeic acid derivatives [32].
Among all the peaks in the chromatogram (Figure 1), peak 9 was quite prominent, indicating a predominant phenolic compound in H. tuberosus leaves. This peak presented spectral characteristics of the dicaffeoylquinic acid [5, 6] with UV λ max at 242.6 and 327.0 nm and t R of 19.03 min. The ESI-MS/MS spectra showed [M-H]− at m/z 515.5, fragment ion [M-C9H6O3]− at m/z 354.1, and fragment ion [M-H-2C9H6O3]− at m/z 191.2 (Figures 2(c), 2(d), 2(e), and 2(f)). Compared with the standard, this compound was unambiguously identified as 3,5-dicaffeoylquinic acid. Peaks 8, 11, and 13 had the same spectral characteristics as peak 9 (Table 1), with UV λ max at 243.8 and 327.0 nm (peak 8), 243.0 and 329.4 nm (peak 11), and 327.0 nm (peak 13). Based on the MS/MS analyses, the caffeoylquinic acid m/z 354.1 ion further fragmented to form characteristic m/z 173.1 [M-H-2C9H6O3-H2O]−, 135.0 [M-C7H10O5-C9H6O3-COOH]−, and 179.0 [M-H-C9H6O3-C7H10O5]− ions [33]. Compared with the standard, these compounds were identified as 3,4-dicaffeoylquinic acid, 1,5-dicaffeoylquinic acid, and 4,5-dicaffeoylquinic acid, respectively [34].
Figure 2.
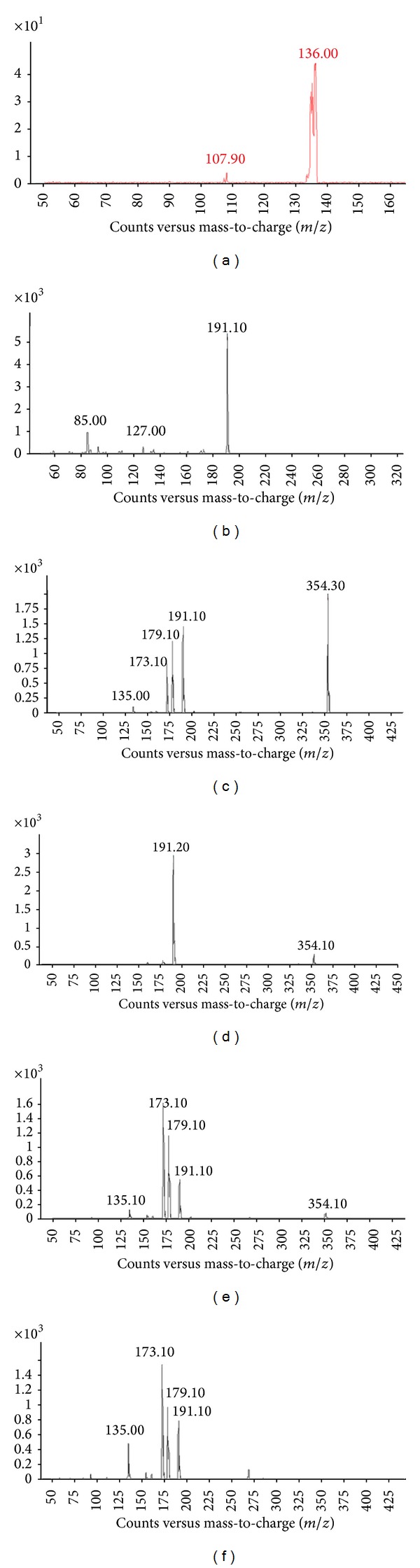
HPLC-MS-MS spectra of phenolic acids (m/z 179 of caffeic acid (a), m/z 353 of 3-o-caffeoylquinic acid (b), m/z 515 of 3,4-dicaffeoylquinic acid (c), m/z 515 of 3,5-dicaffeoylquinic acid (d), m/z 515 of 1,5-dicaffeoylquinic acid (e), and m/z 515 of 4,5-dicaffeoylquinic acid (f)).
The peaks 2, 3, and 4 were identified as three isomers of caffeoylquinic acids (chlorogenic acid) based on the detailed fragmentation, UV absorption, and also [35]. Previously, the presence of cis derivatives of chlorogenic acids was reported in coffee leaves, Rudbeckia hirta, Carlina acaulis, H. tuberosus, Symphyotrichum novae-angliae, maté tea (Ilex paraguariensis), and leaves of other Asteraceae plants [31, 36–38]. In a negative ion ESI mode, the deprotonated molecule [M-H]− at m/z 353 and fragment ion [M-H-C9H6O3]− formed from deprotonated quinic acid at m/z 191 were observed (Figure 2(b)). Fragment ions m/z 85 and m/z 93, characteristic of the quinic acid moiety of monoacyl and diacyl chlorogenic acids, defined the parent ions of putative chlorogenic acids [36]. Other fragment ions with different energies such as the caffeic acid unit (m/z 179.1) and [M-H-C7H12O6]− (m/z 161.1) were used to distinguish the three isomers [38]. Compared with the standard, peak 3 was identified as 3-o-caffeoylquinic acid.
The peak 5 was pseudomolecular ion [M-H]− at m/z 179.1 and fragment ions at m/z 136.0 [M-COO]− and 107.9 [M-COO-CO]− (Figure 2(a)), which were the typical masses of caffeic acid in the negative mode [39]. Fragment ions at m/z 191 and 179 were also observed in ESI-MS/MS− spectra of peaks 6, 7, and 10 (Table 1) indicating that they were derivatives of quinic acid or caffeic acid. Peak 6 was eluted at 9.19 min (Figure 1), with the molecular ion at m/z 337.3 [M-H]− and the main fragment ions at m/z 191.1 [quinic acid-H]− (UV λ max at 239.1 nm) and 173.0 [quinic acid-H-H2O]− (UV λ max at 311.5 nm); this peak was identified as p-coumaroyl-quinic acid [31, 36, 40]. Peak 7 (Table 1) was identified as feruloylquinic acid ([M-H]− at m/z 367 and UV λ max at 241.4 and 325.8 nm) [5, 32].
The MS/MS spectrum of peak 10 (Table 1) suggested caffeoyl glucopyranose that possesses both molecular ion m/z 341.3 and fragmental ion m/z 179.1 formed by loss of one dehydrated molecule of glucose (Glc) [M-H-(Glc-H2O)]−, 161.1 [(Glc-H2O)-H]− [33, 41]. To our knowledge, caffeoyl glucopyranose has not been previously reported in H. tuberosus leaves.
The MS/MS analysis of peaks 12, 14, and 15 (Table 1) showed fragment ions at m/z 315, 301, and 285, corresponding to methyl quercetin or methoxy kaempferol, quercetin aglycone, and kaempferol, suggesting that they were kaempferol and quercetin glycoside derivatives [33]. Peak 12 had a molecular ion [M-H]− at m/z 477 and fragment ions at m/z 315 [M-H-(Glc-H2O)]−, 300.1 [M-H-(Glc-H2O)-CH3]−, and 270.9 [M-H-(Glc-H2O)-CH3-CO]−, which proved to be isorhamnetin glucoside [23, 33]. Peaks 14 and 15 were possibly kaempferol glucuronide and kaempferol-3-o-glucoside, which have similar fragment ion 285 [kaempferol-H]− and different parent ions 461 [M-H]− and 447 [M-H]− [23, 32, 33, 42–44]. These kaempferol and quercetin glycoside derivatives (peaks 12, 14, and 15) are also the first ever reports in Helianthus tuberosus leaves. Their exact structures need further confirmation and additional NMR data will be required.
Phenolics in peak 1 (Table 1) in the HPLC chromatogram were not identified.
3.2. Quantification of Phenolics
Concentration of phenolic compounds in H. tuberosus leaves of cultivar NanYu was determined by the HPLC method, whereas the concentration of total phenolics was calculated as the sum of the individual phenolic compounds and was also estimated by using the Folin-Ciocalteu method (Table 2). The 3-o-caffeoylquinic acid (7.752 mg/g DW), 4,5-dicaffeoylquinic acid (5.633 mg/g DW), and 3,5-dicaffeoylquinic acid (4.900 mg/g DW) were the major phenolic compounds in H. tuberosus leaves, and their concentrations accounted for 33%, 24%, and 21% of the total phenolics, respectively. Among all the quantified phenolics, chlorogenic acids (CGAs) including 3-o-caffeoylquinic acid, caffeoylquinic acids (peaks 2 and 4), caffeic acid, p-coumaroyl-quinic acid, feruloylquinic acid, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, 1,5-dicaffeoylquinic acid, and 4,5-dicaffeoylquinic acid contributed to the total of 22.015 mg/g DW (93% of the total phenolics).
Table 2.
Concentration of total phenolics and phenolic compounds in H. tuberosus leaves (cv. NanYu).
| Phenolic compounds | Concentrationa (mg/g dry weight) |
|---|---|
| Caffeoylquinic acid (peak 2)b | 0.063 ± 0.008d |
| 3-o-Caffeoylquinic acid | 7.752 ± 2.872b |
| Caffeoylquinic acid (peak 4)b | 0.538 ± 0.081d |
| Caffeic acid | 0.098 ± 0.052d |
| p-Coumaroyl-quinic acid | 0.153 ± 0.061d |
| Feruloylquinic acid | 0.527 ± 0.199d |
| 3,4-Dicaffeoylquinic acid | 0.618 ± 0.215d |
| 3,5-Dicaffeoylquinic acid | 4.900 ± 1.492c |
| Caffeoyl glucopyranosec | 0.001 ± 0.319d |
| 1,5-Dicaffeoylquinic acid | 1.733 ± 0.567d |
| Isorhamnetin glucosided | 0.348 ± 0.057d |
| 4,5-Dicaffeoylquinic acid | 5.633 ± 2.990bc |
| Kaempferol glucuronided | 0.186 ± 0.034d |
| Kaempferol-3-o-glucosided | 1.020 ± 0.379d |
| Total phenolicse | 23.570 |
| Total phenolicsf | 30.159 ± 4.410a |
aValues are expressed as mean ± SD of triplicate measurements; the means in a column followed by the same letters represent values that are not significantly different according to Duncan's test (P ≤ 0.05); bquantified as 3-o-caffeoylquinic acid; cquantified as caffeic acid; dquantified as glucoside; esum of the individual phenolic compounds; and fquantified as gallic acid equivalents.
As shown in Table 2, the content of total phenolics calculated as the sum of the individual phenolic compounds was 23.570 mg/g DW, whereas the value obtained by the Folin-Ciocalteu method was 30.159 mg/g DW. The substantial difference between the two values was likely due to the interference of other reducing substances in phenolic extracts, leading to overestimation of total phenolic contents in the Folin-Ciocalteu colorimetric analysis [45, 46].
Concentration of six main phenolic compounds (peaks 3, 5, 8, 9, 11, and 13 in Table 1) in leaves of different H. tuberosus cultivars sampled at different periods from budding to tuber swelling stages was presented in Figure 3. Among the tested genotypes of H. tuberosus (Figure 3(a)), NanYu had the highest concentration of phenolic compounds in leaves (around 7-fold higher than the wild accession and 3-fold higher than QingYu).
Figure 3.
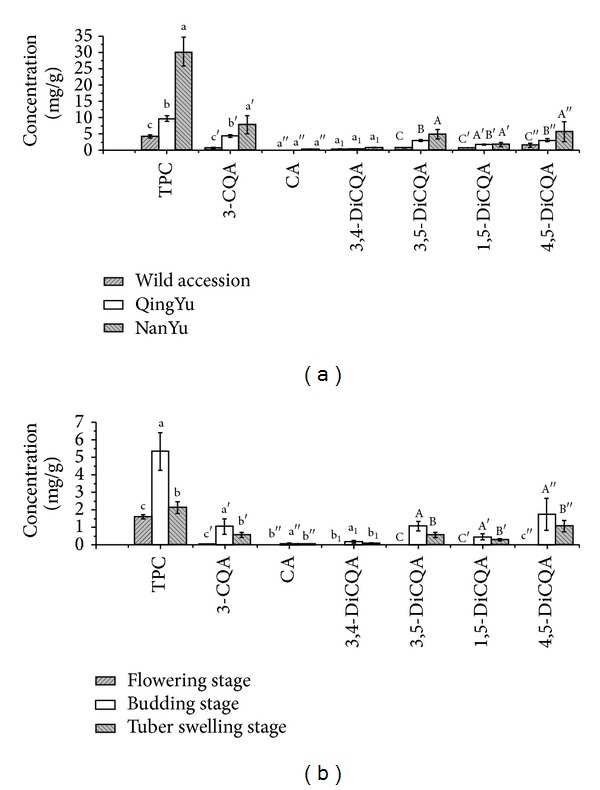
Concentration of phenolics in H. tuberosus leaves of different genotypes: (a) in 2011 and growth stages of cultivar NanYu (flowering stage, budding, and tuber swelling stages) and (b) from August to October in 2012. Concentrations were in mg/g dry weight of leaves. Values are expressed as mean ± SD of triplicate measurements; columns with the same letters are not significantly different according to Duncan's test (P ≤ 0.05).
Caffeic acid was detected in low concentration in all genotypes, whereas concentrations of 3-o-caffeoylquinic acid and 4,5-dicaffeoylquinic acid were considerably higher in all cultivars investigated (Figure 3(a)).
Concentration of total phenolics in leaves of cultivar NanYu was higher at flowering stage (5.270 mg/g DW) than budding and tuber swelling stages (Figure 3(b)), frombudding, flowering to tuber swelling stages.
4. Discussion
Phenolic acids are secondary metabolites that are commonly found in plant-derived foods. They have attracted considerable interest due to their many potential health benefits, which are powerful antioxidants and have been reported to demonstrate antibacterial, antiviral, anticarcinogenic, anti-inflammatory, and vasodilatory actions [47]. As allelochemicals, the phenolic acids might play an important role in plant defense against pathogens [48], pests, and weeds [14, 49]. The mechanism of a phenolic with defense, communication, and protection roles was considered as a pivotal molecule in the responses of plants to their ever-changing environment [15].
The variation in concentration of phenolic acids reported here and in the literature was probably due to the isomerisation of chlorogenic acids (CGAs) [50] and different H. tuberosus parts considered (tubers, leaves, or whole plants) [6, 9, 31]. In addition, the phenolic profiles of Helianthus tuberosus leaves of cultivar NanYu (Dafeng District, Jiangsu, China) were different from previous studies in which the major phenolic compounds were 3-o-caffeoylquinic acid and 1,5-dicaffeoylquinic acid [6] in H. tuberosus leaves from Yulin District (Shannxi, China), probably due to different cultivars of H. tuberosus, different sampling periods, or different origins.
However, this was to be expected as there were so many environmental factors such as pedoclimatic (soil type, sun exposure, and rainfall) and agronomic factors (growth in greenhouses or fields, biological culture, hydroponic culture, fruit yield per tree, etc.) that could affect phenolics concentration in plants [51]. A degree of ripeness also considerably affected the concentrations and proportions of various phenolics [52]. Thus, cultivar NaYu can be a potential source of natural phenolics, which could have multiple functions (e.g., pharmaceuticals) and could play an important role in plant interactions and ecosystem patterning [14].
5. Conclusions
Reversed phase high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS) was successfully employed in the qualitative analysis of phenolic compounds in H. tuberosus leaves. Ten chlorogenic acids (CGAs) were identified (3-o-caffeoylquinic acid, two isomers of caffeoylquinic acids, caffeic acid, p-coumaroyl-quinic acid, feruloylquinic acid, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, 1,5-dicaffeoylquinic acid, and 4,5-dicaffeoylquinic acid), and four others (caffeoyl glucopyranose, isorhamnetin glucoside, methoxy kaempferol glucoside, and kaempferol-3-o-glucoside) were tentatively identified for the first time. Quantitative analysis of phenolics indicated that 3-o-caffeoylquinic acid, 4,5-dicaffeoylquinic acid, and 3,5-dicaffeoylquinic acid were the three major phenolic compounds in H. tuberosus leaves. The variation in phenolic concentrations and proportions in H. tuberosus leaves was characterised in different genotypes and at different sampling periods from budding to tuber swelling stages. H. tuberosus cultivar NaYu had the highest concentration of total phenolics and might be a potential source of natural phenolics, which could play an important role in the development of pharmaceuticals.
Acknowledgments
The authors are grateful for the financial support of Jiangsu Agricultural Science and Technology Independent Innovation Fund Project (no. CX(12)1005-6), National Natural Science Foundation of China (no. 31201692; 41171216), the National Key Projects of Scientific and Technical Support Programs funded by the Ministry of Science and Technology of China (no. 2011BAD13B09), the Ministry of Science and Technology of Jiangsu Province (no. BE2011368), Fundamental Research Funds for Central Universities (no. Y0201100249), the CAS/SAFEA International Partnership Program for Creative Research Teams, Yantai Double-hundred High-end Talent Plan (XY-003-02), and the Project of a Special Fund for Public Welfare Industrial (Agriculture) Research of China (no. 200903001-5).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publishing of this paper.
Authors' Contribution
Fujia Chen and Xiaohua Long contributed to the work of the paper equally.
References
- 1.Pan L, Sinden MR, Kennedy AH, et al. Bioactive constituents of Helianthus tuberosus (Jerusalem artichoke) Phytochemistry Letters. 2009;2(1):15–18. [Google Scholar]
- 2.Baba H, Yaoita Y, Kikuchi M. Sesquiterpenoids from the leaves of Helianthus tuberosus L. Journal of Tohoku Pharmaceutical University. 2005;52:21–25. [Google Scholar]
- 3.Talipova M. Lipids of Helianthus tuberosus. Chemistry of Natural Compounds. 2001;37(3):213–215. [Google Scholar]
- 4.Stange RR, Jr., Midland SL, Holmes GJ, Sims JJ, Mayer RT. Constituents from the periderm and outer cortex of Ipomoea batatas with antifungal activity against Rhizopus stolonifer. Postharvest Biology and Technology. 2001;23(2):85–92. [Google Scholar]
- 5.Yuax X, Gao M, Wang K, Xiao H, Tan C, Du Y. Analysis of chlorogenic acids in Helianthus tuberosus Linn leaves using high performance liquid chromatography-mass spectrometry. Chinese Journal of Chromatography. 2008;26(3):335–338. [PubMed] [Google Scholar]
- 6.Yuan X, Gao M, Xiao H, Tan C, Du Y. Free radical scavenging activities and bioactive substances of Jerusalem artichoke (Helianthus tuberosus L.) leaves. Food Chemistry. 2012;133(1):10–14. [Google Scholar]
- 7.Ahmed MS, El-Sakhawy FS, Soliman SN, Abou-Hussein DMR. Phytochemical and biological study of Helianthus tuberosus L. Egyptian Journal of Biomedical Science. 2005;18:134–147. [Google Scholar]
- 8.Jin U, Lee J, Kang S, et al. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: isolation and identification from methanol extract of Euonymus alatus. Life Sciences. 2005;77(22):2760–2769. doi: 10.1016/j.lfs.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 9.Mattila P, Hellström J. Phenolic acids in potatoes, vegetables, and some of their products. Journal of Food Composition and Analysis. 2007;20(3-4):152–160. [Google Scholar]
- 10.Friedman M. Chemistry, biochemistry, and dietary role of potato polyphenols. A review. Journal of Agricultural and Food Chemistry. 1997;45(5):1523–1540. [Google Scholar]
- 11.Percival GC, Karim MS, Dixon GR. Pathogen resistance in aerial tubers of potato cultivars. Plant Pathology. 1999;48(6):768–776. [Google Scholar]
- 12.Sinden SL, Sanford LL, Cantelo WW, Deahl KL. Bioassays of segregating plants—a strategy for studying chemical defenses. Journal of Chemical Ecology. 1988;14(10):1941–1950. doi: 10.1007/BF01013487. [DOI] [PubMed] [Google Scholar]
- 13.Wen A, Delaquis P, Stanich K, Toivonen P. Antilisterial activity of selected phenolic acids. Food Microbiology. 2003;20(3):305–311. [Google Scholar]
- 14.Tesio F, Weston LA, Ferrero A. Allelochemicals identified from Jerusalem artichoke (Helianthus tuberosus L.) residues and their potential inhibitory activity in the field and laboratory. Scientia Horticulturae. 2011;129(3):361–368. [Google Scholar]
- 15.Ferreres F, Figueiredo R, Bettencourt S, et al. Identification of phenolic compounds in isolated vacuoles of the medicinal plant Catharanthus roseus and their interaction with vacuolar class III peroxidase: an H2O2 affair? Journal of Experimental Botany. 2011;62(8):2841–2854. doi: 10.1093/jxb/erq458. [DOI] [PubMed] [Google Scholar]
- 16.Tchoné M, Bärwald G, Annemüller G, Fleischer LG. Separation and identification of phenolic compounds in Jerusalem artichoke (Helianthus tuberosus L.) Sciences des Aliments. 2006;26(5):394–408. [Google Scholar]
- 17.Alcalde-Eon C, Saavedra G, De Pascual-Teresa S, Rivas-Gonzalo JC. Identification of anthocyanins of pinta boca (Solanum stenotomum) tubers. Food Chemistry. 2004;86(3):441–448. [Google Scholar]
- 18.Charrouf Z, Hilali M, Jauregui O, Soufiaoui M, Guillaume D. Separation and characterization of phenolic compounds in argan fruit pulp using liquid chromatography-negative electrospray ionization tandem mass spectroscopy. Food Chemistry. 2007;100(4):1398–1401. [Google Scholar]
- 19.Fang Z, Zhang M, Wang L. HPLC-DAD-ESIMS analysis of phenolic compounds in bayberries (Myrica rubra Sieb. et Zucc.) Food Chemistry. 2007;100(2):845–852. [Google Scholar]
- 20.Longo L, Vasapollo G. Extraction and identification of anthocyanins from Smilax aspera L. berries. Food Chemistry. 2006;94(2):226–231. [Google Scholar]
- 21.Pedreschi R, Cisneros-Zevallos L. Phenolic profiles of Andean purple corn (Zea mays L.) Food Chemistry. 2007;100(3):956–963. doi: 10.1021/jf0531050. [DOI] [PubMed] [Google Scholar]
- 22.Rauter AP, Martins A, Borges C, et al. Liquid chromatography-diode array detection-electrospray ionisation mass spectrometry/nuclear magnetic resonance analyses of the anti-hyperglycemic flavonoid extract of Genista tenera: structure elucidation of a flavonoid-C-glycoside. Journal of Chromatography A. 2005;1089(1-2):59–64. doi: 10.1016/j.chroma.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 23.Seeram NP, Lee R, Scheuller HS, Heber D. Identification of phenolic compounds in strawberries by liquid chromatography electrospray ionization mass spectroscopy. Food Chemistry. 2006;97(1):1–11. [Google Scholar]
- 24.Zu Y, Li C, Fu Y, Zhao C. Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. Journal of Pharmaceutical and Biomedical Analysis. 2006;41(3):714–719. doi: 10.1016/j.jpba.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 25.Hu P, Liang Q, Luo G, Zhao Z, Jiang Z. Multi-component HPLC fingerprinting of Radix Salviae Miltiorrhizae and its LC-MS-MS identification. Chemical and Pharmaceutical Bulletin. 2005;53(6):677–683. doi: 10.1248/cpb.53.677. [DOI] [PubMed] [Google Scholar]
- 26.LONG X, CHI J, LIU L, LI Q, LIU Z. Effect of seawater stress on p hysiological and biochemical responses offive Jerusalem a rtichoke ecotypes. Pedosphere. 2009;19(2):208–216. [Google Scholar]
- 27.Long XH, Tian J, Zhong QW, Huang ZR, Li L, Liu ZP. Study the cultivars comparison of Helianthus Tuberosus L. and planting technology of low cost and high quality in non-infield of Qinghai and Xinjiang Provinces. Chinese Agricultural Science Bulletin. 2010;26(13):354–358. [Google Scholar]
- 28.Huang Z, Long X, Wang L, et al. Growth, photosynthesis and H+-ATPase activity in two Jerusalem artichoke varieties under NaCl-induced stress. Process Biochemistry. 2012;47(4):591–596. [Google Scholar]
- 29.Tawaha K, Alali FQ, Gharaibeh M, Mohammad M, El-Elimat T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chemistry. 2007;104(4):1372–1378. [Google Scholar]
- 30.Zhou K, Yu L. Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in Colorado. LWT—Food Science and Technology. 2006;39(10):1155–1162. [Google Scholar]
- 31.Jaiswal R, Deshpande S, Kuhnert N. Profling the chlorogenic acids of Rudbeckia hirta, Helianthus tuberosus, Carlina acaulis and symphyotrichum novae-angliae leavesby LC-MSn . Phytochemical Analysis. 2011;22(5):432–441. doi: 10.1002/pca.1299. [DOI] [PubMed] [Google Scholar]
- 32.Bravo L, Goya L, Lecumberri E. LC/MS characterization of phenolic constituents of mate (Ilex paraguariensis, St. Hil.) and its antioxidant activity compared to commonly consumed beverages. Food Research International. 2007;40(3):393–405. [Google Scholar]
- 33.Wang X, Sun W, Sun H, et al. Analysis of the constituents in the rat plasma after oral administration of Yin Chen Hao Tang by UPLC/Q-TOF-MS/MS. Journal of Pharmaceutical and Biomedical Analysis. 2008;46(3):477–490. doi: 10.1016/j.jpba.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Križman M, Baričevič D, Prošek M. Determination of phenolic compounds in fennel by HPLC and HPLCMS using a monolithic reversed-phase column. Journal of Pharmaceutical and Biomedical Analysis. 2007;43:481–485. doi: 10.1016/j.jpba.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 35.Tolonen A, Joustamo T, Mattlla S, Kämäräinen T, Jalonen J. Identification of isomeric dicaffeoylquinic acids from Eleutheracoccus senticosus using HPLC-ESI/TOF/MS and H-NMR methods. Phytochemical Analysis. 2002;13(6):316–328. doi: 10.1002/pca.663. [DOI] [PubMed] [Google Scholar]
- 36.Clifford MN, Zheng W, Kuhnert N. Profiling the chlorogenic acids of aster by HPLC-MSn. Phytochemical Analysis. 2006;17(6):384–393. doi: 10.1002/pca.935. [DOI] [PubMed] [Google Scholar]
- 37.Clifford MN, Kirkpatrick J, Kuhnert N, Roozendaal H, Salgado PR. LC-MSn analysis of the cis isomers of chlorogenic acids. Food Chemistry. 2008;106(1):379–385. [Google Scholar]
- 38.Jaiswal R, Kiprotich J, Kuhnert N. Determination of the hydroxycinnamate profile of 12 members of the Asteraceae family. Phytochemistry. 2011;72(8):781–790. doi: 10.1016/j.phytochem.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 39.Pan J, Cheng Y. Identification and analysis of absorbed and metabolic components in rat plasma after oral administration of “Shuangdan” granule by HPLC-DAD-ESI-MS/MS. Journal of Pharmaceutical and Biomedical Analysis. 2006;42(5):565–572. doi: 10.1016/j.jpba.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Tian C, Xu X, Liao L, Zhang J, Liu J, Zhou S. Separation and identification of chlorogenic acid and related impurities by high performance liquid chromatography-tandem mass spectrometry. Chinese Journal of Chromatography. 2007;25(4):496–500. [PubMed] [Google Scholar]
- 41.Zhang L, Fan C, Zhang X, Yin Z, Ye W. A new steroidal glycoside from Lygodium japonicum . Journal of China Pharmaceutical University. 2006;37(6):491–493. [Google Scholar]
- 42.Ablajan K, Abliz Z, Shang X, He J, Zhang R, Shi J. Structural characterization of flavonol 3,7-di-O-glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry. Journal of Mass Spectrometry. 2006;41(3):352–360. doi: 10.1002/jms.995. [DOI] [PubMed] [Google Scholar]
- 43.Kachlicki P, Einhorn J, Muth D, Kerhoas L, Stobiecki M. Evaluation of glycosylation and malonylation patterns in flavonoid glycosides during LC/MS/MS metabolite profiling. Journal of Mass Spectrometry. 2008;43(5):572–586. doi: 10.1002/jms.1344. [DOI] [PubMed] [Google Scholar]
- 44.Sánchez-Rabaneda F, Jáuregui O, Casals I, Andrés-Lacueva C, Izquierdo-Pulido M, Lamuela-Raventós RM. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao) Journal of Mass Spectrometry. 2003;38(1):35–42. doi: 10.1002/jms.395. [DOI] [PubMed] [Google Scholar]
- 45.He Z, Xia W. Analysis of phenolic compounds in Chinese olive (Canarium album L.) fruit by RPHPLC-DAD-ESI-MS. Food Chemistry. 2007;105(3):1307–1311. [Google Scholar]
- 46.Schieber A, Keller P, Carle R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. Journal of Chromatography A. 2001;910(2):265–273. doi: 10.1016/s0021-9673(00)01217-6. [DOI] [PubMed] [Google Scholar]
- 47.Duthie GG, Duthie SJ, Kyle JAM. Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants. Nutrition Research Reviews. 2000;13(1):79–106. doi: 10.1079/095442200108729016. [DOI] [PubMed] [Google Scholar]
- 48.Doughari JH, Human IS, Bennade S, Ndakidemi PA. Phytochemicals as chemotherapeutic agents and antioxidants: possible solution to the control of antibiotic resistant verocytotoxin producing bacteria. Journal of Medicinal Plants Research. 2009;3(11):839–848. [Google Scholar]
- 49.Lu CH, Liu XG, Xu J, et al. Enhanced exudation of DIMBOA and MBOA by wheat seedlings alone and in proximity to wild oat (Avena fatua) and flixweed (Descurainia sophia) Weed Science. 2012;60(3):360–365. [Google Scholar]
- 50.Schrader K, Kiehne A, Engelhardt UH, Maier HG. Determination of chlorogenic acids with lactones in roasted coffee. Journal of the Science of Food and Agriculture. 1996;71(3):392–398. [Google Scholar]
- 51.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. The American Journal of Clinical Nutrition. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 52.Duan X, Wu G, Jiang Y. Evaluation of the antioxidant properties of litchi fruit phenolics in relation to pericarp browning prevention. Molecules. 2007;12(4):759–771. doi: 10.3390/12040759. [DOI] [PMC free article] [PubMed] [Google Scholar]


