SUMMARY
To exert regulatory function, miRNAs guide Argonaute (AGO) proteins to partially complementary sites on target RNAs. Crosslinking and immunoprecipitation (“CLIP”) assays are state-of-the-art to map AGO binding sites, but assigning the targeting miRNA to these sites relies on bioinformatics predictions and is therefore indirect. To directly and unambiguously identify miRNA:target site interactions, we modified our CLIP methodology in C. elegans to experimentally ligate miRNAs to their target sites. Unexpectedly, ligation reactions also occurred in absence of the exogenous ligase. Our in vivo dataset and re-analysis of published mammalian AGO-CLIP data for miRNA-chimeras yielded ~17,000 miRNA:target site interactions. Analysis of interactions and extensive experimental validation of chimera-discovered targets of viral miRNAs suggest that our strategy identifies canonical, noncanonical, and nonconserved miRNA interactions. Our data suggest that ~80% of miRNA interactions have perfect or partial seed complementarity. In summary, analysis of miRNA:target chimeras enables the systematic, context-specific, in vivo discovery of miRNA binding.
INTRODUCTION
miRNAs associate with Argonaute (AGO) proteins to guide the RNA-induced silencing complex (RISC) to transcripts and thereby repress protein production of target mRNAs (Baek et al., 2008; Bartel, 2009; Fabian et al., 2010; Selbach et al., 2008). Consequently, the biological role of a miRNA is mainly specified by its set of targets. To identify miRNA targets remains challenging, because in animals a miRNA has typically hundreds of direct targets under negative selection (Brennecke et al., 2005; Krek et al., 2005; Lewis et al., 2005; Xie et al., 2005), and target recognition occurs through only partial sequence complementarity (Bartel, 2009; Rajewsky, 2006). Of particular importance to target recognition is the miRNA seed sequence, i.e. nucleotides (nts) 2-7 from the 5’end of the miRNA (Bartel, 2009; Lai, 2002; Rajewsky, 2006; Lewis et al., 2005). Perfect complementarity to the seed is often found to be fundamental for binding and a regulatory response. However, in addition to these “canonical” binding sites, numerous noncanonical miRNA target sites have been reported (Bagga et al., 2005; Chi et al., 2012; Didiano and Hobert, 2006; Helwak et al., 2013; Lal et al., 2009; Shin et al., 2010; Vella et al., 2004). The base pairing patterns for noncanonical targets are not well understood due to difficulties in their identification.
Conventional approaches to identify miRNA targets commonly aim to detect perfect seed matches in 3’ untranslated regions (3’UTRs), often by incorporating additional information such as conservation, accessibility, and expression of 3’ UTR sequences. Despite general success of these methods, they do not take into account context-specificity such as binding sites masked by other RNA binding proteins (RBPs) or tertiary structure constraints and are not effective at identifying noncanonical or nonconserved sites. Moreover, false-positives rates are often high unless specificity is boosted at the expense of sensitivity.
Recently, crosslinking and immunoprecipitation (CLIP) methods (Chi et al., 2009; Hafner et al., 2010; Lebedeva et al., 2011) have identified AGO binding sites at a transcriptome-wide scale, generating context-dependent AGO binding maps. These data per se do not reveal the identity of the miRNA(s) bound to a certain site. Therefore, tools have been developed to computationally predict which miRNAs are bound at which AGO sites (Erhard et al., 2013; Khorshid et al., 2013; Liu et al., 2013; Majoros et al., 2013). However, assumptions must be made, such as which miRNAs are loaded into AGO, how miRNAs recognize targets, and regarding the validity of biophysical energy or hidden Markov/Logistics models. It is therefore still difficult to confidently and unambiguously assign which miRNA was bound to a certain site, especially for sites containing none or several different seed matches. The identification of miRNA:targets can be further complicated by sequence similarities between miRNAs. For example, viral miRNAs can share seed sequences with human miRNAs and can thus interfere with human miRNA binding in infected cells (Gottwein et al., 2011; 2007; Manzano et al., 2013; Skalsky et al., 2012; 2007; Zhao et al., 2011).
We set out to complement existing approaches by experimental miRNA:target identification. We recently developed iPAR-CLIP, a method to generate in vivo maps of binding sites for RBPs in C. elegans (Jungkamp et al., 2011). Here we used iPAR-CLIP and mapped 29,000 unique AGO binding sites in the worm, improving resolution and depth of previous studies (Zisoulis et al., 2010). Additionally, we experimentally ligated miRNAs to their binding sites. Our method is similar but not identical to the CLASH protocol recently applied in a human cell line (Helwak et al., 2013). Sequencing and computational analysis of these chimeras revealed thousands of miRNA:targets in C. elegans. Unexpectedly, we also detected thousands of chimeras when no ligase was added, indicating endogenous RNA ligase activty in standard CLIP assays. Indeed, by reanalyzing sequencing data from published AGO-CLIP experiments we succeeded in compiling >13,000 human and mouse miRNA:targets.
We present multiple lines of evidence that these miRNA:targets have features expected from functional interactions. We also tested the functionality of miRNA:targets for viral miRNAs. With the exception of a viral miRNA with poor targeting proficiency (Garcia et al., 2011; Manzano et al., 2013), we confirmed regulation for 87% of tested sites, including noncanonical and nonconserved sites. Computational analyses of our chimera-identified miRNA:targets suggest that ~80% of these interactions have statistically highly significant perfect or imperfect (1 nt mismatch) complementarity to the miRNA seed (nt 2-7). Our data further suggest that mismatches in the seed occur predominantly at positions 2 and 7. Thus, AGO-CLIP sequencing data contain chimeric reads that enable the identification of endogenous, context-specific miRNA:targets on a transcriptome-wide scale. These data allow insights into principles by which miRNA recognize target sites.
RESULTS
Identification of 3,600 miRNA:targets in C. elegans
We adapted our iPAR-CLIP protocol (Jungkamp et al., 2011) to ligate miRNAs to target sites in C. elegans (Figure 1A). Briefly, worms incorporated photoreactive 4-thiouridine nucleosides (4sU) into their RNA, which crosslinks to bound proteins during UV irradiation. After homogenization, the lysate was treated with RNase T1. Argonaute ALG-1 was immunoprecipitated and bound RNAs were treated again with RNase, recovered under stringent conditions and deep-sequenced (Figure S1A,B, Methods). For the miRNA:target ligations, we added T4 RNA ligase to immunopurified and washed AGO complexes. To prevent circularization we prepared the RNA ends leaving the 3’end of target sites blocked (Figure S1C). Thus, T4 RNA ligase solely connects the 3’hydroxyl (3’OH) of full-length miRNAs with the 5’ends of target RNA fragments.
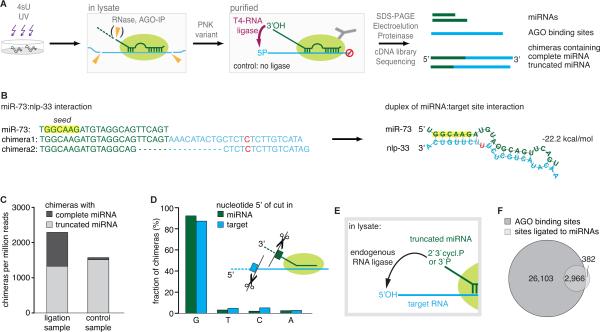
Figure 1. Generation of miRNA:target chimeras via different types of ligations in C. elegans.
(A) C. elegans RNA labeled with photoreactive nucleoside 4-thiouridines (4sU) is crosslinked to bound proteins in vivo. After homogenization of worms, the lysate is treated with RNase T1. Some miRNAs are shortened, others remain complete. Following immunoprecipitation (IP) and washing of AGO, crosslinked RNA is phosphorylated by a PNK variant (leaves 3’ends blocked) and treated with T4 RNA ligase, which ligates the 3’hydroxyl end of complete miRNAs to bound RNA fragments. Crosslinked RNA is recovered and deep sequenced. Computational analysis detects sequence reads of miRNAs and AGO binding sites, along with chimeric reads containing miRNAs connected to their targets.
(B) Example of a miRNA interaction recovered from chimeric reads. Predicted reconstruction of the miRNA:target duplex. green: miRNA sequence; blue: target sequence; red: T to C conversion.
(C) Data from the ligation sample contain chimeras with 3’ truncated (length of miRNA sequence >=13 nts) and with complete miRNAs. A comparable fraction of chimeras with truncated miRNAs was also found in a control sample, to which no ligase was added to generate chimeras.
(D) miRNA and target ends involved in the ligations of the control sample are highly enriched in an upstream G, suggesting that RNase T1 generated the ends used for this type of ligation.
(E) Truncated miRNAs are ligated by the ligase activity of the lysate during IP. (F) 89% of chimera-derived miRNA target sites (mapped to the transcriptome) overlap with AGO binding sites generated from non-chimeric reads. See also Figure S1.
In addition to two standard AGO iPAR-CLIP samples, we generated two biologically and technically independent replicates of ligation samples and control samples. Control samples were generated without the addition of a ligase. Bioinformatic analysis (Methods) of the standard AGO iPAR-CLIP, the ligation and the control samples identified the presence of altogether 13.5 million nonchimeric reads, which had an average read length of 32 nts and mapped uniquely to the transcriptome. In PAR-CLIP, the frequency of T to C conversions in reads reflects the RNA:protein crosslink efficacy. This frequency was very high (14:1) in non-chimeric reads compared to all other possible nucleotide changes.
These data defined a high-resolution AGO binding map (Figure S2A), that includes 2286 C. elegans AGO sites previously identified by Zisoulis and colleagues (Zisoulis et al.: 4,806 unique target sites in 3,093 genes, average length 122 nts; present study: unique 29,000 sites in 8339 genes, average length 42 nts, Table S3). Our bioinformatics analyses (Methods) revealed the presence of thousands of miRNA-chimeric reads in the ligation samples (Figure 1B). When mapping the target sequence in chimeras to the transcriptome, the vast majority mapped to 3’UTRs. Moreover, almost all target sites fell precisely into AGO binding sites (Figure S1D). Consequently, we mapped chimera target sequences directly to AGO sites. This increased the sensitivity of target recovery due to the smaller search space (Methods). In total, we identified 3,627 miRNA:targets for C. elegans (Table S3), 677 of which were supported by more than one chimeric sequence read. Interactions supported by one read showed essentially the same features as interactions recovered by >1 read (see below; Figure S1E,F).
Control samples also contain miRNA:target chimeras
Unexpectedly, we detected substantial numbers of chimeras in our control samples as well as in our AGO iPAR-CLIP samples. These samples have not been treated with T4 RNA ligase to generate chimeras (Figure 1C). While ligation samples had miRNA:target chimeras containing complete or truncated miRNAs, nearly all chimeras detected in control and AGO iPAR-CLIP samples contained 3’-truncated miRNA sequences (Figure 1C, Figure S1G,H).
Truncated miRNAs were strongly enriched in a guanine immediately upstream of the cleavage sites in miRNAs and target RNAs (Figure 1D). RNase T1 cuts with high preference after guanines, strongly suggesting that RNase T1 produced the ends used as substrates for this ligation reaction. The ligation activity required for ligation of 2’,3’-cyclic P (can convert into 3’P) is present in eukaryotic cell lysates, as previously reported (Filipowicz et al., 1983; Martinez et al., 2002; Perkins et al., 1985) (Figure 1E).
But could RNAs in the lysate randomly ligate to AGO-loaded miRNAs? Bacteria are the food source for C. elegans. Therefore, iPAR-CLIP sequencing data usually contain a substantial fraction of bacterial sequences (~30%). In contrast, less than 2% of recovered iPAR-CLIP chimeras contained C. elegans miRNAs together with bacterial sequences, indicating that ligation of random RNA fragments to AGO-loaded miRNAs occurred only rarely. Moreover, miRNA:targets from different samples were highly overlapping (Figure S1I). 76% of interactions derived from chimeras with complete miRNAs were also identified from chimeras with truncated miRNAs (Figure S1J), suggesting for both ligation reactions the recovery of in vivo interactions. Together, these findings prompted us to systematically analyze the ~3,600 identified C. elegans miRNA:target site interactions jointly for known features of miRNA targeting.
C. elegans miRNA:targets recovered from chimeras have features characteristic for miRNA binding
C. elegans miRNA:target chimeras mapped mainly to 3’ UTRs and coding sequences (Figure S2A), similar to AGO binding sites. Also, the sequences ligated to miRNAs are bona fide AGO sites since 89% of mapped chimeras overlapped by at least 80% of their length with AGO binding sites, which is highly statistically significant (p ~ 0, Figure 1F). Consistently, targets in miRNA-chimeras had a T to C conversion rate of 84%, 20-fold enriched over any other type of nucleotide change (Figure S2B). This crosslink-specific signal indicates that sequences ligated to complete or truncated miRNAs were bound by AGO.
We next analyzed if targets found in miRNA chimeras have sequence complementarities to the ligated miRNA. We screened for perfect (non G:U) complementarity to miRNA nts 2-7 (seed), complementarity to miRNA nts 2-7 containing one mismatched or bulged nucleotide and complementarity to miRNA nts 2-8 containing two mismatches. Together these modes were detected in ~80% of interactions and are highly significantly enriched compared to random controls (Figure 2A).
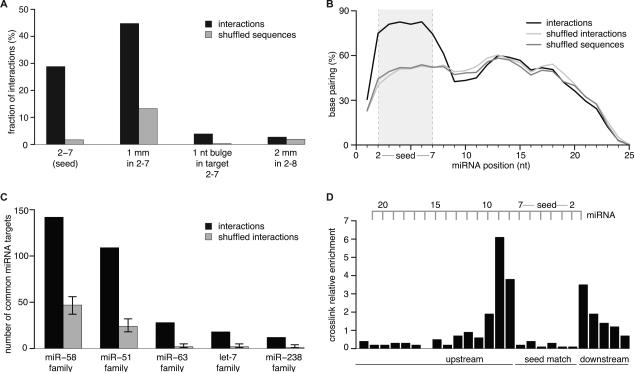
Figure 2. C. elegans miRNA:Targets (3,600) Derived from Chimeras Reflect Endogenous miRNA Targeting.
(A) Target RNAs were analyzed for complementarity to the seed region of their ligated miRNAs. ~80% of interactions possess the tested complementarities. Shuffled sequences (dinucleotides in target sequences are permuted) served as control. mm = mismatch Mismatches were broadly distributed over all types of nucleotides, including G:U.
(B) Hybridization profile summarized over all interactions. The predicted frequency of a miRNA position to be base paired is plotted along the miRNA length. Duplex structures of miRNA:targets were predicted by RNAhybrid allowing G:U pairing. Shuffled sequences (dinucleotides in target sequences are permuted) and shuffled interactions (targets are swapped between miRNAs) served as control.
(C) Target sites derived from miRNA-chimeras are more often found ligated to miRNAs of the same family than expected by chance (p < 0.0001). Shuffling target sites between miRNA families served as control.
(D) Local frequency of crosslink-induced T to C conversions in target RNAs from interactions with a perfect 2-7 seed match (normalized to local thymidine frequency). Nucleotides hybridized to the seed of the miRNA are strongly indisposed to crosslink with the protein. See also Figure S2.
We analyzed miRNA:target hybridization with RNAhybrid (Rehmsmeier et al., 2004). The median free energy was lower (by 3.3 kcal/mol or ~2-3 hybridized nts) for miRNA:targets compared to controls (Figure S2C). Base pairing was as expected for miRNA (Wee et al., 2012; Khorshid et al., 2013): clearly preferred in the seed while reduced at positions 9, 10, and 11 (Figure 2B).
As for interactions with perfect seed matches, analysis of miRNA:targets without detected seed complementarities revealed direct evidence for crosslinking (T-to-C conversions, Figure S2D). However, their binding free energy was less decreased (Figure S2E) and hybridization profiles did not indicate enriched base pairing within the seed region (Figure S2F).
Since miRNA family members have the same seed, they are expected to share some of their targets and indeed, target sites were much more often ligated to members of the same miRNA family than expected by chance. Thus, chimeras can capture multiple endogenous miRNA targeting events at the same target site (Figure 2C, p < 0.0001).
An increased T to C conversion frequency directly upstream of seed-matches was previously reported for AGO PAR-CLIP data (Hafner et al., 2010; Kishore et al., 2011). Similarly, miRNA:targets with a perfect or imperfect seed match showed clear conversion patterns with the highest number of conversions at the second position upstream and a strong depletion within seed matches (Figure 2D, S2G). Interestingly, miRNAs found in ligation products had a 3-fold lower T to C conversion rate compared to non-ligated miRNAs (Figure S2H). This is because noncrosslinked miRNAs tend to be lost under the denaturing conditions of protein purification, while ligated, noncrosslinked miRNAs can pass purification due to their covalent connection to AGO.
Published mammalian AGO-CLIP data contain ligated miRNA:targets
If in our C. elegans experiments ligated miRNA:targets are generated through a ligation activity naturally present in the lysate, then existing AGO-CLIP data should also contain miRNA:targets. Therefore, we searched for miRNA-chimeras in published AGO-CLIP data sets across several model systems and CLIP methods, such as HITS-CLIP (high-throughput sequencing of RNA isolated by crosslinking immunoprecipitation; Chi et al., 2009) and PAR-CLIP (Photoactivatable-ribonucleoside-enhanced crosslinking and immunoprecipitation; Hafner et al., 2010) (Table 1; Figure S6; Methods). Our pipeline confidently detected miRNA sequences of 13 nts and longer and mapped targets as short as 16 nts with a FDR < 5%. This sensitivity was essential for analysis since AGO-CLIP sequences from published studies were typically short (16-36 nts).
Table 1.
miRNA:targets from re-analysis of published AGO CLIP data
| publication | CLIP | RNase | Argonaute | model system | miRNA:targets | FDR % |
|---|---|---|---|---|---|---|
| Kishore et al. 2011 | PAR | T1 | Ago2 | human cells (HEK 293) | 6675 | 4 |
| HITS | T1 | Ago2 | human cells (HEK 293) | 2176 | 3 | |
| Memczak et al., 2013 | PAR | T1 | Ago1 | human cells (HEK 293) | 1010 | 4 |
| Lipchina et al. 2011 | PAR | T1 | Ago2 | human embryonic stem cells | 146 | 5 |
| Skalsky et al. 2012 | PAR | T1 | Ago2 | EBV-infected lymphoblastoid cell lines | 74 viral, 997 human | 3 |
| Gottwein et al. 2011 | PAR | T1 | Ago2 | primary effusion lymphoma cell lines (BC-1, BC-3) | 660 viral, 236 human | 4 |
| Chi et al. 2009 | HITS | A | Ago | mouse brain | 565 | 4 |
| Loeb et al. 2012 | HITS | A | Ago2 | mouse T-cells WT | 1269 | 4 |
| HITS | A | Ago2 | mouse T-cells mir-155 KO | 260 | 4 |
Raw sequencing data from the listed AGO PAR-CLIP and HITS-CLIP data sets contain miRNA:target chimeras. BC-1 and BC-3 are primary effusion lymphoma-derived cell lines infected with Kaposi's sarcoma-associated herpesvirus (KSHV) and, in the case of BC-1, also Epstein-Barr virus (EBV). See also Table S3.
In total we recovered ~11,000 human miRNA:targets, ~2,000 mouse miRNA:targets, ~500 for Kaposi's Sarcoma-associated herpesvirus (KSHV) miRNAs and ~300 for Epstein-Barr virus miRNAs. As expected, most chimeras (>80%) contained 3’-truncated miRNAs. Numbers of miRNA:targets varied strongly across studies, presumably due to differences in RNase treatment and RNA size selection.
We subjected miRNA:targets from all seven datasets to the same analyses as our C. elegans miRNA interactions and obtained very similar results, supporting that their vast majority reflect endogenous miRNA:targets (Figure 3, Figure S3A-E). For example, human AGO PAR-CLIP or HITS-CLIP data (Kishore et al.) were enriched for perfect seed matches (43% and 50%, respectively) with an additional fraction of other tested complementarities to the seed region, together comprising ~80% of interactions (Figure 3A).
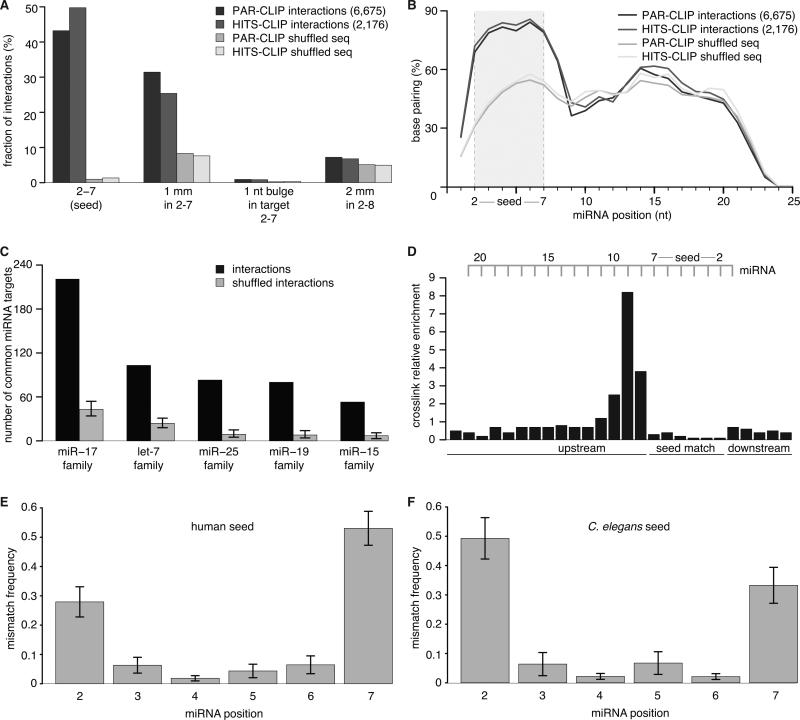
Figure 3. Re-Analysis of published human AGO CLIP data yields miRNA:targets.
(A) AGO2 PAR-CLIP and HITS-CLIP data (HEK 293 cells) from Kishore et al.; analysis as in Figure 2. ~80% of interactions possess the tested complementarities. Mismatches were broadly distributed over all types of nucleotides, including G:U.
(B) Hybridization profiles summarized over all interactions.
(C) Individual target sites are more often ligated to members of the same miRNA family than expected by chance.
(D) Local frequency of crosslink-induced T to C conversions in target RNAs from interactions with a perfect 2-7 seed match (normalized to local thymidine frequency). Positions hybridized to the seed of the miRNA are strongly indisposed to crosslink.
(E) Mismatches in seed sites occur predominantly at position 2 or 7 of the miRNA. Shown is the positional mismatch frequency for interactions with a 2-7 match containing 1 mismatch, averaged over different miRNA families.
(F) As in (E), but in C. elegans. See also Figure S3 and Table S1.
The large numbers of miRNA:targets obtained by chimera analysis allowed us to analyze which seed positions might be more often mismatched than others. Position 2 and 7 of the miRNA showed a significantly increased frequency of mismatches than the positions in between. This pattern was conserved across human, mouse, and C. elegans (Figure 3E,F; Figure S3F).
We analyzed miRNA:targets for complementarity beyond the seed individually for each miRNA (Methods). For the target sites of 18 miRNAs we detected significant stretches of sequence complementarity (p < 0.01, Methods) within the 3’ region of the miRNA (Table S1), amongst them, miR-92, miR-10a, miR-15b and miR-16 have been previously reported (Helwak et al. 2013). Interesting examples include miR-196a and miR-196b, which differ only by one nucleotide at position 12. The targets of these two miRNAs have significant complementarity involving position 13-19 and 12-19, respectively (Table S1), indicating that complementarity within the 3’ region may help to confer target specificity of individual miRNA family members. Our results show partial agreement with the findings of Helwak et al. (2013), a recent study presenting a protocol for the generation of chimeras. Our analysis of human miRNA chimeras in data by Kishore et al. identified 515 genes targeted by the same miRNA family as reported by Helwak et al. This overlap is 4-fold higher than expected by chance (p < 0.0001, random sampling). Our miRNA:targets had a higher fraction of perfect and 1 nt mismatch seed matches (Figure S3G) and scored better according to a state-of-the-art prediction tool (MIRZA; Khorshid et al., 2013) (Figure S3H).
We analyzed the overlap between miRNA:target interactions identified by our analysis of chimeras (detected in data published by Kishore et al., 2011) with MIRZA predictions. For each AGO site, MIRZA ranks all miRNAs by the estimated binding energy using energy constants fitted on AGO PAR-CLIP data. We recorded how often the miRNA that we had found ligated to a site corresponded to the top-ranking miRNA predicted for this site. In MIRZA predictions we found the majority (~81%) of perfect seed interactions to be top-ranking, whereas most of the 1 nt mismatch seed interactions (~86%) were not (Figure S3I). These results highlight the value of unambiguous identification of imperfect seed binding by biochemical methods.
The majority of identified miRNA:targets are functional
First evidence that chimera-identified miRNA:targets are functional comes from the recovery of known miRNA:targets. Of the miRNA interactions annotated in miRTarBase (Hsu et al., 2011), 148 were identified by chimera analysis, an overlap of high statistical significance (p < 0.0001, Table S2). For instance, in C. elegans, chimeras recovered well-known miRNA:target interactions such as let-7:daf-12, let-7:hbl-1, let-7:lin41, let-7:lin28, lin-4:lin-28 and lin-4:daf-12.
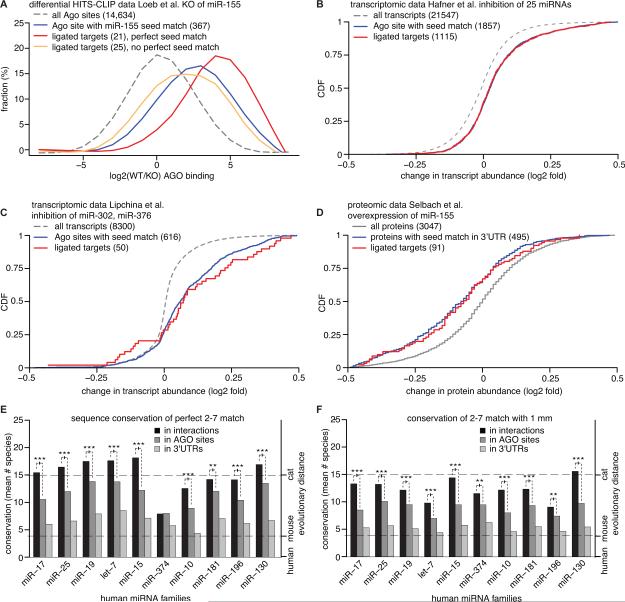
We next asked whether chimera-identified miRNA:targets are likely to be biologically relevant by analyzing expression data from published miRNA perturbation experiments. Loeb and colleagues performed a differential HITS-CLIP study and identified AGO2 targets in WT and miR-155 knock out (KO) mouse embryonic stem cells (Loeb et al., 2012). We searched these data for chimeras and discovered 46 miR-155 interactions in the WT sample and, as expected, none in the miR-155 KO sample. These 46 target sites had substantially lower CLIP read coverage in the KO cells (Figure 4A), suggesting that they are indeed target sites of miR-155. 25 of the 46 sites contained a miR-155 seed match. These 25 targets were even more frequently recovered in the WT sample than all AGO-target sites with miR-155 seed matches (p < 0.004, KS test). We note that the remaining 21 ligated sites without a perfect miR-155 seed match are likely true miR-155 sites, since they were more frequently found in WT cells compared to miR-155 KO (p < 0.003, KS test). Loeb and colleagues already verified two of these interactions (TRIB1 and ZC3H11A).
Figure 4. Functional miRNA interactions derived from chimeras.
(A) HITS-CLIP sequencing data from WT and miR-155 KO cells (Loeb et al.) were analyzed for chimeras containing miR-155. miR-155 ligated target sites with a perfect 2–7 seed match (red) were targeted by AGO2 in WT cells more often than in miR-155 KO cells compared to all transcripts (dashed) and all clusters with a seed match (blue) (p < 0.004; KS test). miR-155 ligated target sites without a perfect 2–7 match (orange) are AGO2 bound in WT significantly more often than in KO cells (p < 0.003; KS test).
(B,C,D) miRNA perturbation data demonstrates functionality of chimera-identified miRNA interactions. Changes in transcript abundance after inhibition of 25 miRNAs in HEK293 cells (Hafner et al.) (B) and miR-302a/b/c/d, miR-367 in mouse embryonic stem cells (Lipchina et al.) (C) and changes in protein abundance after overexpression of miR-155 in a human cell line (Selbach et al.) (D). Targets recovered in chimeras with these miRNAs (from all HEK293 data and human embryonic stem cell data, respectively; Table 1) were upregulated upon miRNA inhibition on the transcript level (B,C) and downregulated on the protein level upon miRNA overexpression (D).
(E and F) Conservation across 31 vertebrate species of perfect seed (2–7) matches (E) and seed matches with 1 nt mismatch (1 mm) (F) from human miRNA: targets recovered by analysis of chimeras. Conservation of other seed matches for the same miRNA served as a control. A perfect seed match in human was counted as conserved if present at the same position in the alignment. A seed match with 1mm was deemed conserved if the identical 1mm seed match or the perfect seed match was present at the same position in the alignment. On average, 100 miRNA interactions (median) were included per miRNA family. miRNA:targets with a mismatch in the 2-7 seed were significantly conserved (***: p < 0.005; **: p < 0.01, Mann-Whitney-U test) but to a lower degree than perfect seed matches. See also Figure S4 and Table S2.
To study functionality of our miRNA:targets we correlated them with mRNA expression changes from miRNA perturbation experiments. Hafner et al. simultaneously inhibited the 25 top expressed miRNAs in a human cell line (HEK 293) and monitored changes in mRNA abundance (Hafner et al., 2010). A similar experiment was performed in human embryonic stem cells for the miR-302 family and miR-367 (Lipchina et al., 2011). The majority of mRNAs with chimera-supported interactions to these miRNAs were indeed significantly derepressed upon miRNA inhibition (Figures 4B, p < 7.63*10^-25, and 4C, p < 9.8*10^-8; KS test). The majority of these interactions (590 out of 1115 for data by Hafner et al.; 41 out of 68 for data by Lipchina et al.) did not posses perfect seed complementarity and consequently could not have been easily predicted bioinformatically.
To assay the impact of miRNA:target interactions on protein production from target mRNAs and not solely on transcript levels, we re-analyzed pulsed SILAC data (Selbach et al., 2008) which were obtained after ectopic miR-155 expression in a human cell line (HeLa). Indeed, protein synthesis for 91 miR-155 targets identified by chimera analysis was highly significantly downregulated (Figure 4D).
Finally, we analyzed evolutionary conservation of miRNA:targets, since conservation indicates negative selection and thus functionality. Our finding that chimera-discovered perfect seed matches (Figure 4E, S4A), as well as seed matches containing one mismatched nucleotide (Figure 4F, S4B), were significantly more conserved than other seed matches for the corresponding miRNAs in 3’UTRs or AGO sites, strongly suggests their functional importance. It should be noted that the 2-7 matches containing one mismatched nucleotide have a lower degree of conservation than perfect matches, which also holds true for C. elegans (Figure S4A,B).
Taken together, analysis of miRNA perturbation experiments and of target site conservation provides evidence that our miRNA:target interactions are enriched in functionally important sites. However, we decided to experimentally test the functionality of identified miRNA:targets, e.g. those found in virus-infected human cells.
Identification of Nonconserved, Noncanonical, and Viral miRNA Binding Sites
The identification of miRNA:targets through the analysis of miRNA chimeras finds a particularly valuable application in the study of human pathogenic herpesviruses, e.g., KSHV and Epstein-Barr virus (EBV) (Cai and Cullen, 2006; Grundhoff et al., 2006; Kincaid and Sullivan, 2012; Pfeffer et al., 2004; 2005; Samols et al., 2005). For most viral miRNAs, target identification is severely complicated by the fact that these viruses encode mostly evolutionary novel and unique miRNAs and infect only humans. Therefore viral miRNAs are not expected to preferentially bind conserved sites. The use of computational target prediction is difficult in this case. Moreover, the seeds of viral and human miRNAs can be similar, which causes difficulties in unambiguously assigning the targeting miRNA to a site. Previous studies have suggested that these viral miRNAs can regulate the same binding sites as the corresponding host miRNA and therefore function to mimic the host miRNA (Gottwein et al., 2007; 2011; Manzano et al., 2013; Skalsky et al., 2007). The most prominent example is KSHV miR-K11, which shares sequence identity of nts 1-8 with cellular miR-155 and is known to mimic the oncogenic properties of miR-155 in B cells (Boss et al., 2011; Dahlke et al., 2012; Gottwein et al., 2007; Linnstaedt et al., 2010; Skalsky et al., 2007). We indeed recovered 11 shared binding sites of miR-K11 and miR-155 when searching for miRNA chimeras in AGO2-CLIP data from KSHV-infected B cell lines expressing KSHV miR-K11 (Gottwein et al., 2011) and lymphoblastoid cell lines expressing cellular miR-155 (Skalsky et al., 2012) (Figure S5A). This overlap between the miR-K11 and miR-155 binding sites is significantly greater than expected to occur by chance (p < 4.3*10^-8; hypergeometric test), expands the repertoire of known shared targets and includes the previously verified common target BACH1 (Gottwein et al., 2007; Skalsky et al., 2007).
To investigate whether chimera-discovered viral miRNA:targets are indeed functional, we directly tested a subset of these sites for regulation by the ligated viral miRNAs in luciferase reporter assays. We constructed WT 3’UTR reporter constructs and reporters in which the miRNA binding site of interest was mutated. This experimental setup directly reports the regulatory potential of the identified miRNA binding site, rather than that of the entire 3’UTR.
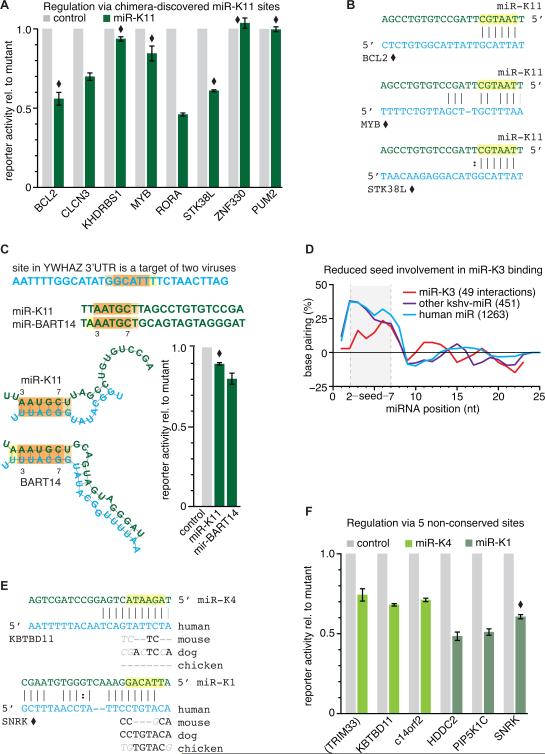
Our initial experiments focused on miR-K11, due to the likely importance of this miRNA to KSHV pathogenesis (Boss et al., 2011; Dahlke et al., 2012; Gottwein, 2012). The sites we selected included canonical seed matches that are likely to result in robust regulation (RORA, CLCN3), but also several “noncanonical” interactions (BCL2, STK38L, MYB, ZNF330, KHDRBS1, PUM2, YWHAZ). Here we defined “canonical” interactions as those that involve perfect complementarity to miRNA nts 2-7 with an A opposing nt 1 of the miRNA and/or perfect complementarity to miRNA nts 2-8. 5 out of 9 chimera-discovered miR-K11 target sites (Figure 5A and C) indeed conferred reporter repression. Functional noncanonical interactions included those with complementarity to nts 2-7 only (BCL2 and STK38L) or with a mismatch within the seed region (MYB) (Figure 5B). All sites that mediated reporter repression by miR-K11 were also responsive to miR-155 (Figure S5B), suggesting that nts 1-8 of miR-K11 are important for the observed regulation. The importance of the miR-K11 seed, also for the noncanonical interactions, is further highlighted by the abrogation of regulation when the seed region was disrupted by a two nucleotide substitution (Figure S5C). Thus, analysis of chimeric reads enabled us to identify functional canonical and noncanonical interactions.
Figure 5. Validation of canonical, noncanonical and nonconserved sites targeted by viral miRNAs.
(A) The majority of tested, chimera-identified KSHV miR-K11 interactions resulted in specific reporter repression, including sites with weak seed matches. miRNA interactions were tested in dual luciferase reporter assays using wt and binding site mutant 3’UTR reporters and either control miRNA or viral miRNA mimics. Noncanonical interactions are marked with a diamond. (canonical: perfect match to miRNA position 2-7 with an A opposing the first miRNA nucleotide and/or perfect complementarity to at least miRNA positions 2-8); numbers are mean ± SEM (n >= 3).
(B) Predicted base pairing for noncanonical miR-K11 sites that were responsive in the reporter assay.
(C) EBV miRNA BART-14 and KSHV miRNA-K11 are identical in only five positions (nt 3–7), but these might bind the same nucleotides in the target (noncanonical binding for miR-K11, canonical for miR-BART14). Duplex structures were predicted by RNAhybrid, G:U allowed. Shortening of the miRNA by 4 nt from the 3’end enabled an in silico hybridization in which the GC-poor 5’region of the miRNA is predicted to base pair.
(D) Hybridization profile of KSHV miR-K3 interactions compared to all other KSHV and human miRNA interactions identified by analyzing CLIP data by Gottwein et al. 2011 and Skalsky et al. 2012; miR-K3 interactions display reduced binding in the 5’region of the miRNA. RNAhybrid, G:U allowed, controls (permutation of dinucleotides in target sequences) were subtracted.
(E) Predicted base pairing of tested interactions at nonconserved sites; lack of conservation in seed matches shown for mouse, dog, chicken (continued in Figure S5F).
(F) KSHV miR-K4 and miR-K1 repress targets via nonconserved sites. Of the six sites tested, only the miR-K1 binding site in the 3’UTR of TRIM33 is conserved. Its localization in a conserved region that extends over ~500 nts is not indicative of evolutionary selection specific to the miRNA binding site. See also Figure S5.
In the conventional analysis of AGO-CLIP data, the bioinformatic assignment of the targeting miRNA is often complicated by ambiguities if AGO-bound regions bear seed matches to several different miRNAs and the general difficulties of identifying noncanonical sites. The situation becomes even trickier in the context of a viral infection, when either cellular or viral miRNAs may mediate targeting. This scenario is exemplified by our identification of one specific site in the 3’UTR of YWHAZ, which was ligated to either miR-K11 or the EBV miRNA miR-BART14 in a B cell line co-infected with KSHV and EBV. Interestingly, miR-K11 and miR-BART14 share identical nts 3-7, which may bind the same nucleotides of the target (Figure 5C). Only miR-BART14 resulted in significant reporter repression within the sensitivity of the assay (Figure 5C and Figure S5D). The analysis of chimeric reads can therefore eliminate ambiguities in the assignment of the targeting miRNA(s).
We recently reported that the KSHV miRNA miR-K3 mimics miR-23, through offset seed homology (Manzano et al., 2013). Like human miR-23, miR-K3 has a low regulatory potency when tested for regulation of canonical target sites that were predicted from PAR-CLIP data using bioinformatic assignment (Garcia et al., 2011; Manzano et al., 2013). We therefore examined chimera-identified miRK3:targets for obvious peculiarities by screening for seed complementarity and predicting hybridization patterns in silico (Figure 5D). To our surprise we found that only 7% of miR-K3 targets have perfect seed matches, a considerably lower fraction compared to all other identified interactions of the remaining KSHV miRNAs or human miRNAs (30% and 35% of interactions derived from data by Gottwein et al. 2011 have a perfect seed match, respectively). Furthermore, we observed an increased fraction of 1 nt off-set seed matches (complementarity to miRNA nts 3-8), which represented ~15% of miR-K3 interactions but only 5% and 6% of all interactions from other KSHV or human miRNAs, respectively. We selected 5 noncanonical miR-K3 interactions for further examination of their regulatory potential in reporter assays, but did not detect reporter repression mediated by any of these sites (Figure S5E). Thus, even chimera-identified binding sites for the special case of miR-K3 appear to have poor regulatory capacity, at least in the experimental system employed here.
An important difficulty when investigating viral miRNAs with evolutionary novel seed sequences, i.e. those that do not bear similarity to any conserved cellular miRNAs, is that sequence conservation has limited use in predicting binding sites. The KSHV miRNAs miR-K1 and miR-K4 constitute such cases and we therefore decided to test some of their chimera-identified target sites for their regulatory potential. Six interactions, including 5 for which the seed match was not conserved (Figure 5E and Figure S5F), were tested for reporter repression. Upon site mutation, a strong reporter derepression of 40-60% could be detected for all of them (Figure 5F), including one noncanonical interaction with sequence complementarity to position 3-10 of miR-K1. Leaving the exceptional case of miR-K3 aside, our results strongly support the notion that the analysis of miRNA:target chimeras uncovers canonical interactions with high confidence (7/7 sites tested), but also successfully identifies functional noncanonical (4/8) and nonconserved (4/4, considering only canonical sites) miRNA interactions, which are much more challenging to identify using conventional approaches.
DISCUSSION
Our initial key aim was to ligate miRNAs to their target sites in order to identify these interactions from sequencing data. We show for the model system C. elegans that the T4 RNA ligase can be used to carry out this task and generated the first comprehensive map of directly defined miRNA:target interactions in vivo.
Our data and analyses demonstrate that ligation reactions seem to occur specifically between miRNAs and bound targets. The reasons for this are (a) almost all target sites within chimeras have the crosslink specific T-C conversion (b) although bacterial RNAs from E.coli are present in the course of the experiment we find almost no bacterial RNA in miRNA chimeras (c) the overlap between chimeras and miRNA target sites from AGO-CLIP data is extremely high (d) miRNA family members had much higher chance to be ligated to the same target site than expected by chance and (e) miRNA:target chimeras had sequence and base pairing properties which are characteristic for genuine miRNA target interactions.
However, to our surprise we discovered that chimeras were also present in experiments in which we did not perform the additional ligation step via the T4 RNA ligase. More detailed analyses of these chimeras revealed that the vast majority of them contained miRNAs sequences, which were shortened at their 3’ ends. We can explain the generation of these chimeras with the endogenous ligase activity of cell lysates, which can act on RNase T1-produced RNA ends (Filipowicz et al., 1983; Martinez et al., 2002; Perkins et al., 1985). The most likely candidate for this ligase is the highly conserved tRNA ligase (Popow et al., 2011), which cannot ligate the miRNA without the biochemical modifications introduced by the RNase, explaining why we rarely observe full-length miRNAs in these chimeras. Although in C. elegans we recovered more than 3,600 unique miRNA:targets, the ligation efficacies are relatively low (0.14% of all reads), similarly to a recent study by Helwak et al. where the authors presented a protocol for generating miRNA chimeras from human cells using the T4 RNA ligase. However, the ligation reactions are not identical, as they are performed by different ligases acting on different RNA end-modifications at different steps in the experiment. There are probably pros and cons for either reaction, e.g. fewer experimental steps in our case. On the other hand, reactions in the lysate are perhaps less controllable and prone to biases of the RNase. Taken together, our data argue that future optimization of the ligation reaction in the lysate might be a potent way to routinely and comprehensively identify endogenous miRNA interactions.
We developed a computational pipeline which detects miRNA:targets in CLIP data, systematically applied it to published AGO-CLIP data sets generated in several model systems and discovered large numbers of these chimeras. Detailed analyses, again demonstrated that they have all the features expected, such as a highly enhanced seed match frequencies. To test functionality, we reanalyzed published miRNA perturbation data and show that chimeras are highly significantly enriched in functional miRNA:target interactions. Strikingly, we also found that ligated target sites, including those with imperfect seed matches, are highly conserved.
How can the ligation reaction preferentially occur at miRNA:targets which are functionally important? We believe that the recovery of miRNA chimeras is more frequent for stable and well-expressed interactions, as those have a higher chance of being crosslinked. This is also in line with the fact that we recover miRNA-chimeras from interactions in the 3’UTR more often than ligation products from miRNA-interactions in the coding sequence (Figure S2A), which were reported to have overall less regulatory potency (Fang and Rajewsky, 2011) or to be less stable (Gu et al., 2009). Thus, boosting the efficacy of the ligation reaction as discussed above might actually improve sensitivity at the cost of functional importance.
To independently prove functionality we carried out extensive validation for interactions of viral miRNAs that we had identified from CLIP data of human cells infected with Kaposi Sarcoma-associated herpesvirus (KSHV). In this system it is particularly important to be able to unambiguously identify the miRNA bound to target sites because viral miRNAs and human miRNAs can compete for the same sites, and some viral miRNAs are similar in sequence to human miRNAs. Our reporter assays demonstrated that the majority of tested viral miRNA binding sites, including noncanonical and nonconserved ones, conferred repression of the reporter (Figure 5A,C,F). Although the validation rate of our miRNA interactions by reporter assays was high, we did not discover regulation for every tested interaction. An intriguing case was the one of KSHV miR-K3. In line with previous reports of low proficiency (Manzano et al., 2013), even chimera-identified interactions of this miRNA did not prove functional in reporter assays. However, we note that the validation assay is an artificial system in which miRNA sites are tested in HEK cells instead of primary lymphoma cells, and only one of the viral miRNAs is present. It is entirely possible, that functionality of the miRNA interaction depends on cell type specific factors, or on cooperating viral miRNAs. Alternatively, KSHV miR-K3 might indeed not function to downregulate its targets but instead exert another, potentially non-repressive function. In any case, we identified a substantial subset of its targets and revealed imperfect seed binding. In conclusion, our data and analyses show that we obtained an unambiguous, in vivo, context specific, and large-scale map of miRNA:target interactions in human, mouse, C. elegans, and virus-infected human cells.
From AGO crystal studies it is known that nucleotides of the miRNA seed are exposed to the solvent and thereby well positioned for target binding (Elkayam et al., 2012; Parker et al., 2009). It is further well established that perfect seed complementarity is a major mode of miRNA targeting. Based on the large number of miRNA interactions unambiguously discovered by the analysis of chimeras, we are now able to explore miRNA binding across mouse, worm, and human at unprecedented resolution. Our data suggest that ~45% of human miRNA interactions have perfect seed complementarity (nts 2-7 from miRNA 5’end). The majority of all other miRNA-interactions without a perfect seed match have a single mismatch in the seed (additional ~30%). These cases include binding patterns that were previously reported, for example complementarity to miRNA nts 3-8 and 2-7 matches with a G:U wobble pair (Yekta et al., 2004; Wu and Belasco et al., 2005; Didiano and Hobert, 2006). We remark that the sequence information contents in perfect or imperfect hexamer seeds is so low that a purely computational approach for identifying miRNA targets will produce an overwhelming number of putative sites with many false positives. Using conservation as a filter will remove functionally important nonconserved sites and will still retain many false positives that are conserved for other reasons than being miRNA target sites. Our in vivo data further suggest that the seed is more tolerant to mismatches at positions 2 and 7, which is in line with previous studies investigating the effect of mismatches within the seed on target binding in vitro (Wee et al., 2012). These findings improve the understanding of miRNA targeting.
Interactions for which we could not detect complementarities to the seed (~20%) are enriched in crosslinks and we think that it is unlikely that they constitute false positives. Some of these chimeras might originate from AGO binding independent of sequence complementarity, perhaps similar to AGO-recruitment via the Smaug protein (Pinder and Smibert, 2013). Others might reflect miRNA binding that does not involve, or to a very limited extent involves, base pairing of seed nucleotides (Shin et al., 2010; Helwak et al., 2013). Nevertheless, ~80% of miRNA:targets show clear complementarity to the seed, suggesting the relevance of this hexamer for miRNA binding in general.
Since the sensitivity of our method is currently limited, computational approaches will remain important for the transcriptome-wide identification of miRNA targets. It seems clear that our data will be important for complementing and improving other methods to predict miRNA targets within AGO binding sites (Erhard et al., 2013; Khorshid et al., 2013; Liu et al., 2013; Majoros et al., 2013). In any case, our directly identified miRNA:targets can be used to systematically explore different modes of seed complementarity or other recognition patterns, potentially dependent on the miRNA sequence or binding sites context.
Finally, we remark that it is entirely possible that the ligation reaction in the lysate occurs also for other RNA:RNA interactions during CLIP experiments, for example for other regulatory small RNAs such as piRNAs or, more generally, for double stranded RNAs bound by RNA binding proteins (RBPs). Thus, our approach may also help to uncover binding sites and function of other RNAs or RBPs.
EXPERIMENTAL PROCEDURES
Modified iPAR-CLIP for generation of miRNA-chimeras
C. elegans Argonaute ALG-1 iPAR-CLIP was performed in L3 staged, 4sU-labled worms largely as described by Jungkamp et al. A ligation reaction using T4 RNA ligase was performed on purified ALG-1 complexes, for which RNA ends were prepared to prevent circularization. Sequencing with 100 cycles was carried out on a Genome Analyzer II (Illumina). (detailed description: Supplemental Experimental Procedures)
Computational identification and characterization of miRNA chimeras
AGO-CLIP sequencing reads were preprocessed. Low quality reads were removed, adapter sequences trimmed, identical reads collapsed. Reads were mapped first to the miRNA mature sequences (miRBase version 19) and then to the AGO binding sites. Both mapping steps were filtered by several cutoffs in alignment scores and the relative positions of mappings inside the chimeric reads. For each dataset, false discovery rates (FDR) were estimated using shuffled sequences. Cutoffs were adjusted such that all chimeras reported have a FDR < 5% (detailed description of the entire pipeline: Supplemental Experimental Procedures).
Reporter assays
Reporter assays were essentially conducted as described (Gottwein et al., 2011), with minor modifications. Briefly, WT or miRNA binding site mutant firefly luciferase reporters were co-transfected with an internal control vector expressing Renilla luciferase and 10pmols/24 well of either negative control mimic 1 or specific miRNA mimics (mirVana Mimics, Life Technologies). Firefly luciferase activities obtained for the WT 3’UTR reporters were sequentially normalized to the Renilla luciferase activities obtained for the internal control vector, and the normalized values obtained for the control mimic and the miRNA binding site mutant, which was set at 1. (detailed description: Supplemental Experimental Procedures)
Supplementary Material
HIGHLIGHTS.
Comprehensive AGO binding map and thousands of miRNA:target interactions in C. elegans
AGO-CLIP samples contain miRNA:target chimeras generated by an endogenous ligase
17,000 miRNA interactions discovered in human and other systems are largely functional
~80% of interactions contain seed sites, roughly half of them are imperfect
ACKNOWLEDGEMENTS
We thank F. Slack for the GFP::ALG-1 transgenic strain and M. Simard for the ALG-1 antibody. We thank all members of the Rajewsky lab for discussions and support. C. Langnick and M. Feldkamp (W. Chen lab, MDC) performed sequencing runs. A.F. thanks the DFG Graduate School “Computational Systems Biology” CBS-GRK 1772 for a fellowship. F.K. acknowledges funding from DEEP (Deutsches Epigenom Programm), and M.S. acknowledges funding from the e:Bio program. Validation experiments for viral miRNA interactions in this publication were supported by the National Cancer Institute of the National Institutes of Health under Award Number U54CA143869. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. S.G. designed and performed the ALG-1 iPAR-CLIP ligation and control experiments; M.H. contributed technical assistance. A.F. designed and performed the computational experiments. S.G., A.F. and N.R. analyzed and interpreted the data on miRNA chimeras. M.S. performed conservation analysis, F.K. compared chimeras with computational predictions. E.G. and M.M. performed the validation experiments for viral miRNA interactions and we acknowledge P. Shamulailatpam for technical assistance. N.R. conceived and supervised the project. S.G., E.G. and N.R. wrote the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ACCESSION NUMBERS
The sequencing data have been deposited in the GEO database under the accession number GSE56180.
SUPPLEMENTAL INFORMATION
Supplemental Information includes six figures, three tables, Supplemental Experimental Procedures and Supplemental References. Table S3 contains C.elegans iPAR-CLIP Argonaute sites and miRNA interactions listed per species.
REFERENCES
- Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–563. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss IW, Nadeau PE, Abbott JR, Yang Y, Mergia A, Renne R. A Kaposi's sarcoma-associated herpesvirus-encoded ortholog of microRNA miR-155 induces human splenic B-cell expansion in NOD/LtSz-scid IL2Rγnull mice. Journal of Virology. 2011;85:9877–9886. doi: 10.1128/JVI.05558-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS Biol. 2005;3:e85. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Cullen BR. Transcriptional origin of Kaposi's sarcoma-associated herpesvirus microRNAs. Journal of Virology. 2006;80:2234–2242. doi: 10.1128/JVI.80.5.2234-2242.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi SW, Hannon GJ, Darnell RB. An alternative mode of microRNA target recognition. Nature Structural & Molecular Biology. 2012;19:321–327. doi: 10.1038/nsmb.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479–486. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlke C, Maul K, Christalla T, Walz N, Schult P, Stocking C, Grundhoff A. A microRNA encoded by Kaposi sarcoma-associated herpesvirus promotes B-cell expansion in vivo. PLoS ONE. 2012;7:e49435. doi: 10.1371/journal.pone.0049435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didiano D, Hobert O. Perfect seed pairing is not a generally reliable predictor for miRNA-target interactions. Nature Structural & Molecular Biology. 2006;13:849–851. doi: 10.1038/nsmb1138. [DOI] [PubMed] [Google Scholar]
- Elkayam E, Kuhn C-D, Tocilj A, Haase AD, Greene EM, Hannon GJ, Joshua-Tor L. The structure of human argonaute-2 in complex with miR-20a. Cell. 2012;150:100–110. doi: 10.1016/j.cell.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhard F, Dölken L, Jaskiewicz L, Zimmer R. PARma: identification of microRNA target sites in AGO-PAR-CLIP data. Genome Biology. 2013;14:R79. doi: 10.1186/gb-2013-14-7-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- Fang Z, Rajewsky N. The impact of miRNA target sites in coding sequences and in 3′UTRs. PLoS ONE. 2011;6:e18067. doi: 10.1371/journal.pone.0018067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W, Konarska M, Gross HJ, Shatkin AJ. RNA 3′-terminal phosphate cyclase activity and RNA ligation in HeLa cell extract. Nucleic Acids Res. 1983;11:1405–1418. doi: 10.1093/nar/11.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia DM, Baek D, Shin C, Bell GW, Grimson A, Bartel DP. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nature Structural & Molecular Biology. 2011;18:1139–1146. doi: 10.1038/nsmb.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwein E. Kaposi's Sarcoma-Associated Herpesvirus microRNAs. Front Microbiol. 2012;3:165. doi: 10.3389/fmicb.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwein E, Corcoran DL, Mukherjee N, Skalsky RL, Hafner M, Nusbaum JD, Shamulailatpam P, Love CL, Dave SS, Tuschl T, et al. Viral microRNA targetome of KSHV-infected primary effusion lymphoma cell lines. Cell Host Microbe. 2011;10:515–526. doi: 10.1016/j.chom.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwein E, Mukherjee N, Sachse C, Frenzel C, Majoros WH, Chi J-TA, Braich R, Manoharan M, Soutschek J, Ohler U, et al. A viral microRNA functions as an orthologue of cellular miR-155. Nature. 2007;450:1096–1099. doi: 10.1038/nature05992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundhoff A, Sullivan CS, Ganem D. A combined computational and microarray-based approach identifies novel microRNAs encoded by human gamma-herpesviruses. Rna. 2006;12:733–750. doi: 10.1261/rna.2326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Jin L, Zhang F, Sarnow P, Kay MA. Biological basis for restriction of microRNA targets to the 30 untranslated region in mammalian mRNAs. Nat. Struct. Mol. Biol. 2009;16:144–150. doi: 10.1038/nsmb.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, Rothballer A, Ascano M, Jungkamp A-C, Munschauer M, et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helwak A, Kudla G, Dudnakova T, Tollervey D. Mapping the Human miRNA Interactome by CLASH Reveals Frequent Noncanonical Binding. Cell. 2013;153:654–665. doi: 10.1016/j.cell.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S-D, Lin F-M, Wu W-Y, Liang C, Huang W-C, Chan W-L, Tsai W-T, Chen G-Z, Lee C-J, Chiu C-M, et al. miRTarBase: a database curates experimentally validated microRNA-target interactions. Nucleic Acids Res. 2011;39:D163–D169. doi: 10.1093/nar/gkq1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungkamp A-C, Stoeckius M, Mecenas D, Grün D, Mastrobuoni G, Kempa S, Rajewsky N. In vivo and transcriptome-wide identification of RNA binding protein target sites. Mol Cell. 2011;44:828–840. doi: 10.1016/j.molcel.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorshid M, Hausser J, Zavolan M, Van Nimwegen E. A biophysical miRNA-mRNA interaction model infers canonical and noncanonical targets. Nat Methods. 2013;10:253–255. doi: 10.1038/nmeth.2341. [DOI] [PubMed] [Google Scholar]
- Kincaid RP, Sullivan CS. Virus-encoded microRNAs: an overview and a look to the future. PLoS Pathog. 2012;8:e1003018. doi: 10.1371/journal.ppat.1003018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore S, Jaskiewicz L, Burger L, Hausser J, Khorshid M, Zavolan M. A quantitative analysis of CLIP methods for identifying binding sites of RNA-binding proteins. Nat Methods. 2011;8:559–564. doi: 10.1038/nmeth.1608. [DOI] [PubMed] [Google Scholar]
- Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- Lai EC. Micro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet. 2002;30:363–364. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- Lal A, Navarro F, Maher CA, Maliszewski LE, Yan N, O'Day E, Chowdhury D, Dykxhoorn DM, Tsai P, Hofmann O, et al. miR-24 Inhibits cell proliferation by targeting E2F2, MYC, and other cell-cycle genes via binding to “seedless” 3′UTR microRNA recognition elements. Mol Cell. 2009;35:610–625. doi: 10.1016/j.molcel.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedeva S, Jens M, Theil K, Schwanhäusser B, Selbach M, Landthaler M, Rajewsky N. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol Cell. 2011;43:340–352. doi: 10.1016/j.molcel.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Linnstaedt SD, Gottwein E, Skalsky RL, Luftig MA, Cullen BR. Virally induced cellular microRNA miR-155 plays a key role in B-cell immortalization by Epstein-Barr virus. Journal of Virology. 2010;84:11670–11678. doi: 10.1128/JVI.01248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipchina I, Elkabetz Y, Hafner M, Sheridan R, Mihailovic A, Tuschl T, Sander C, Studer L, Betel D. Genome-wide identification of microRNA targets in human ES cells reveals a role for miR-302 in modulating BMP response. Genes & Development. 2011;25:2173–2186. doi: 10.1101/gad.17221311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Mallick B, Long D, Rennie WA, Wolenc A, Carmack CS, Ding Y. CLIP-based prediction of mammalian microRNA binding sites. Nucleic Acids Res. 2013;41:e138. doi: 10.1093/nar/gkt435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb GB, Khan AA, Canner D, Hiatt JB, Shendure J, Darnell RB, Leslie CS, Rudensky AY. Transcriptome-wide miR-155 binding map reveals widespread noncanonical microRNA targeting. Mol Cell. 2012;48:760–770. doi: 10.1016/j.molcel.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoros WH, Lekprasert P, Mukherjee N, Skalsky RL, Corcoran DL, Cullen BR, Ohler U. MicroRNA target site identification by integrating sequence and binding information. Nat Methods. 2013;10:630–633. doi: 10.1038/nmeth.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano M, Shamulailatpam P, Raja AN, Gottwein E. Kaposi's Sarcoma-Associated Herpesvirus Encodes a Mimic of Cellular miR-23. Journal of Virology. 2013;87:11821–11830. doi: 10.1128/JVI.01692-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Patkaniowska A, Urlaub H, Lührmann R, Tuschl T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell. 2002;110:563–574. doi: 10.1016/s0092-8674(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- Parker JS, Parizotto EA, Wang M, Roe SM, Barford D. Enhancement of the seed-target recognition step in RNA silencing by a PIWI/MID domain protein. Mol Cell. 2009;33:204–214. doi: 10.1016/j.molcel.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KK, Furneaux H, Hurwitz J. Isolation and characterization of an RNA ligase from HeLa cells. Proc Natl Acad Sci USA. 1985;82:684–688. doi: 10.1073/pnas.82.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S, Sewer A, Lagos-Quintana M, Sheridan R, Sander C, Grässer FA, van Dyk LF, Ho CK, Shuman S, Chien M, et al. Identification of microRNAs of the herpesvirus family. Nature Publishing Group. 2005;2:269–276. doi: 10.1038/nmeth746. [DOI] [PubMed] [Google Scholar]
- Pfeffer S, Zavolan M, Grässer FA, Chien M, Russo JJ, Ju J, John B, Enright AJ, Marks D, Sander C, et al. Identification of virus-encoded microRNAs. Science. 2004;304:734–736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- Pinder BD, Smibert CA. microRNA-independent recruitment of Argonaute 1 to nanos mRNA through the Smaug RNA-binding protein. EMBO Rep. 2013;14:80–86. doi: 10.1038/embor.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popow J, Englert M, Weitzer S, Schleiffer A, Mierzwa B, Mechtler K, Trowitzsch S, Will CL, Lührmann R, Söll D, et al. HSPC117 is the essential subunit of a human tRNA splicing ligase complex. Science. 2011;331:760–764. doi: 10.1126/science.1197847. [DOI] [PubMed] [Google Scholar]
- Rajewsky N. microRNA target predictions in animals. Nat Genet. 2006;38(Suppl):S8–S13. doi: 10.1038/ng1798. [DOI] [PubMed] [Google Scholar]
- Rehmsmeier M, Steffen P, Hochsmann M, Giegerich R. Fast and effective prediction of microRNA/target duplexes. Rna. 2004;10:1507–1517. doi: 10.1261/rna.5248604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samols MA, Hu J, Skalsky RL, Renne R. Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi's sarcoma-associated herpesvirus. Journal of Virology. 2005;79:9301–9305. doi: 10.1128/JVI.79.14.9301-9305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- Shin C, Nam J-W, Farh KK-H, Chiang HR, Shkumatava A, Bartel DP. Expanding the microRNA targeting code: functional sites with centered pairing. Mol Cell. 2010;38:789–802. doi: 10.1016/j.molcel.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalsky RL, Corcoran DL, Gottwein E, Frank CL, Kang D, Hafner M, Nusbaum JD, Feederle R, Delecluse H-J, Luftig MA, et al. The Viral and Cellular MicroRNA Targetome in Lymphoblastoid Cell Lines. PLoS Pathog. 2012;8:e1002484. doi: 10.1371/journal.ppat.1002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalsky RL, Samols MA, Plaisance KB, Boss IW, Riva A, Lopez MC, Baker HV, Renne R. Kaposi's sarcoma-associated herpesvirus encodes an ortholog of miR-155. Journal of Virology. 2007;81:12836–12845. doi: 10.1128/JVI.01804-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella MC, Choi E-Y, Lin S-Y, Reinert K, Slack FJ. The C. elegans microRNA let-7 binds to imperfect let-7 complementary sites from the lin-41 3′UTR. Genes & Development. 2004;18:132–137. doi: 10.1101/gad.1165404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee LM, Flores-Jasso CF, Salomon WE, Zamore PD. Argonaute divides its RNA guide into domains with distinct functions and RNA-binding properties. Cell. 2012;151:1055–1067. doi: 10.1016/j.cell.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Belasco JG. Micro-RNA regulation of the mammalian lin-28 gene during neuronal differentiation of embryonal carcinoma cells. Mol. Cell. Biol. 2005;25:9198–9208. doi: 10.1128/MCB.25.21.9198-9208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Lu J, Kulbokas EJ, Golub TR, Mootha V, Lindblad-Toh K, Lander ES, Kellis M. Systematic discovery of regulatory motifs in human promoters and 3′ UTRs by comparison of several mammals. Nature. 2005;434:338–345. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekta S, Shih I-H, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Xu H, Yao Y, Smith LP, Kgosana L, Green J, Petherbridge L, Baigent SJ, Nair V. Critical role of the virus-encoded microRNA-155 ortholog in the induction of Marek's disease lymphomas. PLoS Pathog. 2011;7:e1001305. doi: 10.1371/journal.ppat.1001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisoulis DG, Lovci MT, Wilbert ML, Hutt KR, Liang TY, Pasquinelli AE, Yeo GW. Comprehensive discovery of endogenous Argonaute binding sites in Caenorhabditis elegans. Nature Structural & Molecular Biology. 2010;17:173–179. doi: 10.1038/nsmb.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.