Abstract
Perfluoroalkyl acids (PFAAs) are persistent, synthetic compounds that are used in a number of consumer products. Perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) have been associated with cardiovascular risk factors, and changes in gene expression and DNA methylation in animals and cellular systems. However, whether PFAA exposure is associated with LINE-1 DNA methylation, a potential marker of cardiovascular risk, in humans remains unknown. We sought to evaluate the cross-sectional associations between serum PFAAs and LINE-1 DNA methylation in a population highly exposed to PFOA. We measured serum PFAAs twice four to five years apart in 685 adult participants (47% male, mean age ± SD=42 ± 11 years). We measured percent LINE-1 DNA methylation in peripheral blood leukocytes at the second time point (follow-up), and estimated absolute differences in LINE-1 methylation associated with an interquartile (IQR) shift in mean PFAA serum levels. IQR increases in mean serum PFOA, PFOS, perfluorononanoic acid (PFNA), and perfluorohexane sulfonate (PFHxS) were associated with differences of −0.04 (p=0.16), 0.20 (p=0.001), 0.06 (p=0.19), and 0.02 (p=0.57), respectively, in % LINE-1 methylation at follow-up after adjustment for potential confounders. We observed a monotonic increase in LINE-1 DNA methylation across tertiles of PFOS and PFNA (ptrend=0.02 for both associations), but not across tertiles of PFOA or PFHxS (ptrend=0.71 and 0.44, respectively). In summary, serum PFOS was associated with LINE-1 methylation, while serum PFOA, PFHxS, and PFNA were not. Additional research is needed to more precisely determine whether these compounds are epigenetically active.
Keywords: perfluoroalkyl acids, perfluorooctanoic acid, perfluorooctane sulfonate, epigenetics, DNA methylation, LINE-1, cardiovascular disease
1. Introduction
Perfluoroalkyl acids (PFAAs) are a class of synthetic compounds that have been widely used in commercial and industrial applications. As PFAAs are extremely stable and repel both oil and water, they have been used in non-stick cooking surfaces, oil-resistant coatings for food contact paper, and stain resistant and waterproofing sprays for fabrics, upholstery, and carpets (Lau et al., 2007). PFAAs are now ubiquitous in the environment (Lindstrom et al., 2011), and over 95% of Americans participating in the 2007-2008 National Health and Nutrition Examination Survey (NHANES) had detectable serum concentrations of four common PFAAs – perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS), perfluorononanoic acid (PFNA), and perfluorohexane sulfonate (PFHxS) (Kato et al., 2011).
Although PFAA exposure has not yet been directly linked to cardiovascular disease (CVD), epidemiological studies do suggest that exposure to PFOA and PFOS may be associated with CVD risk factors such as hyperuricemia (Costa et al., 2009; Shankar et al., 2011; Steenland et al., 2010) and increased total and low density lipoprotein cholesterol (LDL-C) (Costa et al., 2009; Eriksen et al., 2013; Fitz-Simon et al., 2013, Frisbee et al., 2010; Nelson et al., 2010; Olsen et al., 2003; Sakr et al., 2007; Steenland et al., 2009), although studies have not been entirely consistent (e.g. Fisher et al. 2013; Olsen et al. 2012), and have been predominantly cross-sectional, limiting causal inference. Identifying mechanisms by which PFAAs may possibly give rise to adverse health effects, including changes in CVD risk factors, could potentially provide deeper understanding of these relationships. Epigenetic changes have been proposed as one possible mechanism (Tian et al., 2012; Wan et al., 2010), but evidence in humans is largely lacking.
DNA methylation is an epigenetic mechanism that involves the addition of a methyl group to cytosine residues primarily in the context of CpG dinucleotides, a combination which is underrepresented in the human genome but concentrated in the promoter regions of many genes and in DNA repeat regions and known to regulate gene expression (Bird 2002; Tost 2010). In general, methylation of CpG regions suppresses expression of related genes, while demethylation of CpG regions is associated with increased gene expression. One type of repeat region is the long interspersed nuclear element 1 (LINE-1), a group of retrotransposon sequences that are highly methylated (i.e. not expressed) in non-diseased states (Nelson et al., 2011). Methylation of LINE-1 elements may impact expression of surrounding genes depending on the location of the repeat element as well as the specific genes involved. LINE-1 elements make up approximately 17% of the human genome (Lander et al., 2001), and the methylation extent of these regions may provide an indication of whether compounds are epigenetically active (Nelson et al., 2011). Hypomethylation of LINE-1 elements has been associated with genomic instability (Belancio et al., 2009), risk of cancer (Belancio et al., 2010), ischemic heart disease, stroke, and hypertension (Baccarelli et al., 2010), increased LDL-C, and decreased high density lipoprotein cholesterol (HDL-C) (Cash et al., 2011), as well as age, sex, race, ethnicity, and environmental exposures (Nelson et al., 2011).
To our knowledge, there has been only one prior epidemiological study evaluating associations between PFAAs and DNA methylation. In a study of newborns, Guerrero-Preston et al. (2010) reported a negative association between PFOA concentrations in cord blood and total methylated cytosine in DNA isolated from cord serum (Guerrero-Preston et al., 2010), but saw no such association with PFOS. Toxicological studies also suggest that exposure to PFOA or PFOS may affect DNA methylation (Tian et al., 2012; Wan et al., 2010) and alter gene expression (Guruge et al., 2006; Rosen et al., 2010; Rosen et al., 2007; Yeung et al., 2007), but information on other PFAAs is limited. In addition, a recent study of a subset of the population studied here found that serum PFOA and PFOS concentrations were associated with the expression of genes related to cholesterol transport and mobilization (Fletcher et al., 2013), although the involvement of epigenetic mechanisms in this relationship has not been established.
Accordingly, we sought to evaluate the epigenetic activity of PFAAs by examining cross-sectional associations between serum PFAA concentrations and LINE-1 DNA methylation in peripheral leukocytes in a population exposed to high levels of PFOA in the environment, but background levels of PFOS, PFNA, and PFHxS.
2. Materials and Methods
2.1 Study Population
Participants included in this analysis are a subset of adults enrolled in the C8 Health Project between August 1, 2005 and August 31, 2006 (Figure 1). The C8 Health Project was a cross-sectional survey of residents in the mid-Ohio River Valley who were exposed to high levels of PFOA via contaminated drinking water, as previously described (Frisbee et al., 2009). A subset of participants in the C8 Health Project who consented to future contact was subsequently invited in 2010 to participate in the Short-Term Follow-Up Study, which was conducted to evaluate changes in several clinical markers including lipids, in relation to changes in serum PFOA and PFOS concentrations (Fitz-Simon et al., 2013). Participation in the follow-up study was restricted to residents in the region affected by PFOA contamination who were between 20 and 60 years of age at the time of the C8 Health Project. Participants were ineligible if they had a history of cancer, were taking anti-inflammatory medications, or had an active or recent infection. The study population for the present analysis is comprised of these participants who provided a blood samples in both the C8 Health Project and the Short-Term Follow-Up Study. For purposes of this analysis, “at enrollment” refers to measurements made upon enrollment in the C8 Health Project in 2005-2006, and “at follow-up” refers to measurements made in 2010 (Figure 1). All participants provided written informed consent prior to enrollment in both the C8 Health Project and the Short-Term Follow-Up Study, and the Brown University IRB approved this analysis.
Figure 1.
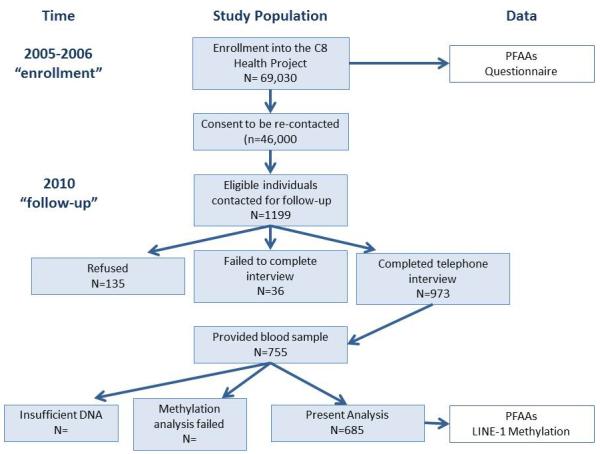
Flow diagram describes our study population and data included in the present analysis. C8 Health Project participants were eligible if they lived in a region affected by PFOA contamination, were between 20 and 60 years of age at the time of the C8 Health, did not have a history of cancer, an active or recent infection, and were not taking anti-inflammatory medications.
2.2 Questionnaires
Upon enrollment in the C8 Health Project, participants completed self-administered questionnaires on demographics, personal health history, and lifestyle habits (Frisbee et al., 2009) and provided a venous blood sample. Information collected included age, gender, race (non-Hispanic white vs. other), household income (≤ $30,000 vs. > $30,000), education (some college or college graduate, yes vs. no), current drinker (yes vs. no), smoking status (ever vs. never) and regular exercise (yes vs. no). At follow-up we administered a questionnaire to obtain current participant information, including health and smoking status.
2.3 Blood Sample Handling
Participants were not required to fast prior to sample collection. Blood samples collected upon enrollment in the C8 Health Project (2005-2006) were centrifuged, divided into aliquots, and refrigerated until shipment to the laboratory for analysis. DNA samples from the time of enrollment into the C8 Health Project are not available. Blood samples for the Short-Term Follow-Up Study, collected between March and July of 2010, were clotted at room temperature for at least 30 minutes, and centrifuged for at least 15 minutes at 2,400 rpm. Serum was transferred to polypropylene Nalgene vials and stored at −30°C for up to 1 week before being shipped on dry ice to the Centers for Disease Control and Prevention (CDC). Blood clots (including buffy coats) were kept in the red-top serum separator tubes in which they were drawn, stored at −30 C until shipment on dry ice to the National Institute for Occupational Safety and Health (NIOSH), and later shipped on dry ice to Brown University for DNA methylation analysis.
2.4 PFAA and Lipid Analysis
Laboratory analysis of PFAAs in blood samples collected at enrollment (Exygen Research Inc., State College, Pennsylvania) has been previously described (Frisbee et al., 2009). In follow-up samples, PFOA and PFOS were measured using online solid phase extraction coupled with reversed-phase high-performance liquid chromatography separation and detection by isotope-dilution tandem mass spectrometry at the CDC (Kato et al., 2011). At both enrollment and follow-up, total cholesterol, HDL cholesterol, and triglycerides were measured enzymatically at a commercial accredited laboratory. LDL-C was calculated by the Friedewald equation when triglycerides were less than 400 mg/dL.
2.5 DNA Methylation Analysis
We used quantitative bisulfite PCR pyrosequencing to measure LINE-1 DNA methylation (Cash et al., 2011; Yang et al., 2004) in blood samples collected at follow-up. Genomic DNA was isolated from peripheral blood leukocytes using Puregene Blood Core Kit C with Clotspin Baskets to extract blood clots, and then sodium bisulfite treated using the EZ-96 DNA Methylation Kit, converting non-methylated cytosine residues into uracil (Zymo Research, Orange, CA). Bisulfite converted DNA was PCR amplified using HotStatTaq Polymerase (Qiagen Inc., Valencia, CA) and the product was pyrosequenced in triplicate. Each replicate provided a measure of methyl cytosine relative to the total cytosine and thymine (%) at 4 CpG sites in the LINE-1 region.
2.6 Statistical Analysis
We measured percent LINE-1 DNA methylation in samples from 685 participants. Of these, complete covariate information was available on 671 participants. Average LINE-1 methylation across the 4 CpG sites for each replicate was used to calculate an average of the replicates for each sample. We averaged serum PFAA concentrations measured in samples collected at enrollment and at follow-up as a summary measure of PFAA body burden over the four to five years prior to methylation analysis (2005-2010).
We used linear regression to evaluate the association between mean serum concentrations of PFOA, PFOS, PFNA, and PFHxS and percent LINE-1 methylation. In initial analyses, we modeled serum PFAA concentrations as linear continuous variables and report the results for an interquartile range (IQR) shift of each PFAA. In sensitivity analyses, we repeated these same models considering instead the natural log of serum PFAA levels or tertiles of each PFAA as exposure measures. All models were adjusted for age, gender, body mass index (BMI), smoking status (ever/never), and current drinker (yes/no). In additional sensitivity analyses we stratified all models by gender, since men and women have been found to have differences in percent LINE-1 methylation (Zhu et al., 2012). We also evaluated models that included serum PFAAs measured at either enrollment or follow-up, as opposed to the average measure, as predictors of LINE-1 methylation.
Total cholesterol and LDL-C have been associated with PFAAs (Costa et al., 2009; Frisbee et al., 2010; Nelson et al., 2010; Olsen et al., 2003; Sakr et al., 2007; Steenland et al., 2009) and could also potentially be associated with LINE-1 methylation (Cash et al., 2011). However, the direction of these associations is not clear, such that total cholesterol and LDL-C could be downstream effects of both PFAA and epigenetic changes, in which case should not be included in the model, or they could be potential confounders. Accordingly, we did not adjust for total cholesterol or LDL-C in our primary analyses but then performed sensitivity analyses with additional adjustment for these variables.
In additional sensitivity analyses we excluded participants reporting a history of heart disease, diabetes, or currently taking lipid-lowering or antihypertensive medication. All analyses were conducted using SAS v. 9.3 (Cary, NC) and a 2-sided p-value of <0.05 was considered statistically significant.
3. Results
Characteristics of study participants are presented in Table 1. Serum concentrations of PFOA decreased substantially between enrollment in the C8 Health Project (2005-2006) and follow-up (2010) (Table 2). Serum concentrations of PFOS also decreased during this time period among participants. Consistent with previous studies, LINE-1 methylation differed by gender, with women having on average 0.45 lower percent LINE-1 methylation compared to men (p=<0.0001). LINE-1 methylation was not associated with age, BMI, drinking alcohol, or ever being a smoker (Supplementary Material, Table S1).
Table 1.
Participant characteristics by gender
| Characteristic | Men (n= 322) | Women (n= 363) |
|---|---|---|
| At Enrollment (2005-2006) | ||
| Age, years - mean (SD) | 41.8 (11.3) | 41.5 (11.2) |
| White - n (%) | 315 (97.8) | 357 (98.4) |
| BMI, kg/m2 - mean (SD) | 28.6 (5.2) | 29.1 (7.5) |
| Ever Smoker - n (%) | 149 (46.3) | 135 (37.2) |
| Current Drinker - n (%) | 198 (61.5) | 193 (53.5) |
| Regular Exercise - n (%) | 102 (31.7) | 101 (27.8) |
| Household Income <$30,000 - n (%) | 80 (26.5) | 94 (28.6) |
| At Least Some College - n (%) | 196 (61.3) | 246 (68.3) |
| Total Cholesterol, mg/dl - mean (SD) | 198 (43.6) | 202 (41.0) |
| HDL-C, mg/dl - mean (SD) | 42.8 (10.2) | 54.9 (14.6) |
| LDL-C, mg/dl - mean (SD) | 113 (36.6) | 115 (35.1) |
| Heart Disease - n (%) | 17 (5.3) | 9 (2.5) |
| Diabetes Mellitus - n (%) | 19 (5.9) | 24 (6.6) |
| Lipid Lowering Medication - n (%) | 53 (16.5) | 51 (14.1) |
| Antihypertensive Medication- n (%) | 52 (16.2) | 72 (19.8) |
| Neutrophils, % - mean (SD) | 61.5 (8.3) | 61.8 (8.2) |
| Lymphocytes, % - mean (SD) | 28.8 (7.1) | 29.9 (7.3) |
| At Follow-Up (2010) | ||
| Total Cholesterol, mg/dl - mean (SD) | 189 (41.4) | 200 (40.5) |
| HDL-C, mg/dl - mean (SD) | 41.4 (11.1) | 54.2 (13.9) |
| LDL-C, mg/dl - mean (SD) | 108 (36.5) | 113 (33.7) |
| LINE-1 Methylation, % - mean (SD) | 84.05 (1.27) | 83.61 (1.19) |
Table 2.
Serum PFAA concentrations at C8 Health Project enrollment (2005-2006) and at follow-up (2010
| Serum PFAAs at Enrollment (2005-2006) |
Serum PFAAs at Follow-Up (2010) |
Mean Serum PFAAs (2005-2010) |
|
|---|---|---|---|
|
|
|||
| GM (GSD)a ng/ml |
GM (GSD) ng/ml |
GM (GSD) ng/ml |
|
|
|
|
||
| All participants (n = 685) | |||
| PFOA | 79.3 (3.2) | 32.9 (3.5) | 57.9 (3.2) |
| PFOS | 19.0 (2.1) | 8.5 (2.3) | 14.1 (2.0) |
| PFNA | 1.4 (1.7) | 1.3 (1.7) | 1.4 (1.6) |
| PFHxS | 3.1 (2.3) | 1.9 (2.3) | 2.6 (2.1) |
| Men (n = 322) | |||
| PFOA | 93.3 (3.2) | 41.9 (3.5) | 70.0 (3.2) |
| PFOS | 22.4 (2.2) | 10.9 (2.3) | 17.1 (2.1) |
| PFNA | 1.5 (1.6) | 1.4 (1.7) | 1.5 (1.6) |
| PFHxS | 4.0 (2.2) | 2.5 (2.2) | 3.3 (2.1) |
| Women (n = 363) | |||
| PFOA | 68.7 (3.1) | 26.5 (3.3) | 48.9 (3.0) |
| PFOS | 16.4 (2.0) | 6.8 (2.1) | 11.9 (1.9) |
| PFNA | 1.2 (1.7) | 1.2 (1.7) | 1.3 (1.5) |
| PFHxS | 2.6 (2.2) | 1.5 (2.2) | 2.1 (2.0) |
GM = geometric mean, GSD = geometric standard deviation
LINE-1 methylation was not significantly associated with PFOA in any analyses (Table 3, Figure 2). For example, an IQR increase in serum PFOA was associated on average with a 0.04 decrease in percent LINE-1 methylation (p=0.16) after adjustment for age, gender, BMI, smoking status, and drinking status.
Table 3.
Associations between mean serum PFAA concentrations and percent LINE-1 methylationa
| Unadjusted (n=685) |
Adjustedb (n=671) |
|||
|---|---|---|---|---|
| Difference in % LINE-1 Methylation (SE) |
p-value | Difference in % LINE-1 Methylation (SE) |
p-value | |
|
|
|
|||
| PFOA (ng/ml) | −0.014 (0.029) | 0.64 | −0.041 (0.029) | 0.16 |
| PFOS (ng/ml) | 0.265 (0.054) | <0.0001 | 0.204 (0.058) | 0.001 |
| PFNA (ng/ml) | 0.102 (0.048) | 0.03 | 0.064 (0.048) | 0.19 |
| PFHxS (ng/ml) | 0.056 (0.035) | 0.11 | 0.020 (0.036) | 0.57 |
Expressed as the difference in % LINE-1 methylation per interquartile range increase (IQR) in mean concentrations of each PFAA. PFOA IQR = 106 ng/ml, PFOS IQR = 12 ng/ml, PFNA IQR = 0.8 ng/ml, PFHxS IQR = 2.6 ng.ml.
Adjusted for age, gender, BMI, smoking status (ever/never), and current drinker (yes/no).
Figure 2.
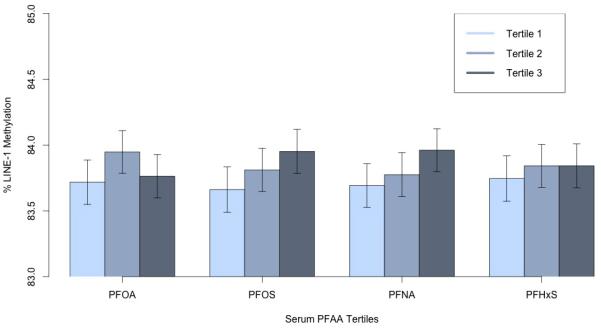
Mean percent LINE-1 methylation by serum PFAA tertiles adjusted for covariates. Error bars represent 95% confidence intervals. P-value for trend: PFOA=0.71, PFOS=0.02, PFNA=0.02, PFHxS=0.44. Minimum and maximum concentrations in each PFAA tertile (T): PFOA: T1=0.8-33.4, T2=33.5-91.1, T3=91.2-1689; PFOS: T1=0.17-11.7, T2=11.8-18.7, T3=18.8-64.1; PFNA: T1=0.15-1.2, T2=1.2-1.6, T3=1.7-10.0; PFHxS: T1=0.15-1.9, T2=2.0-3.5, T3=3.6-68.3.
In contrast, we observed 0.20 higher percent LINE-1 methylation per IQR increase in mean serum PFOS (p=0.001), after adjusting for covariates (Table 3). Results were qualitatively similar when we instead considered natural log-transformed serum PFOS (see Supplementary Material, Table S2). We also observed a monotonic increase in LINE-1 methylation in tertiles of serum PFOS after controlling for covariates (p for trend=0.02; Figure 2).
There was inconsistent evidence of PFNA being positively associated with LINE-1 methylation. In the primary analysis modeled as continuous variables (Table 3) the adjusted coefficient was associated with a p-value of 0.19, but after log-transformation (see Supplementary Material, Table S2), the association was slightly stronger and in analyses considering tertiles of serum PFNA we observed a monotonic increase in LINE-1 methylation (p-value for trend = 0.02, Figure 2). Serum concentrations of PFHxS were not associated with LINE-1 methylation in any analysis.
Results were similar in sensitivity analyses additionally adjusting for total cholesterol or LDL-C, percent lymphocytes, percent neutrophils, or excluding participants reporting a history of heart disease, or taking lipid lowering medication (data not shown). Gender stratified results (see Supplementary Material, Table S3) and results from models evaluating serum PFAAs measured at either enrollment or follow-up (data not shown), or models evaluating each of the four measured CpG sites idividually (data not shown), were not substantially different from those observed in our main analyses.
4. Discussion
We examined cross-sectional associations between PFAAs measured in serum at two different time points and LINE-1 DNA methylation among participants in the C8 Health Project, a large epidemiologic study of a community highly exposed to PFOA. In summary, we observed consistent positive associations between percent LINE-1 methylation and serum PFOS concentrations, but no significant association with PFOA, PFNA, or PFHxS.
The fall in serum PFOA and PFOS by a little over 50% between enrollment and follow-up may have played a role in the observed associations. Local population intake of PFOA fell during the study period, presumably due to efforts to reduce plant emissions of PFOA, provision of bottled drinking water and the installation of filtration for local water supplies beginning in 2007. The decrease in serum PFOS concentrations is similar to that observed in the US population overall (Kato et al., 2011), likely due to the cessation of production of PFOS by US manufacturers during this time. If there were a relationship between either PFOA and PFOS and methylation, and it was reversible, this pattern over time might complicate observed relationships depending on delays in the response to changing serum levels. However, since results were more or less identical using the measures at enrollment or follow up, this suggests that the falling serum levels do not have an impact on LINE-1 methylation.
We are aware of only one prior study to consider the association between PFAAs and DNA methylation in humans. Guerrero-Preston et al., (2010) reported a negative association between PFOA concentrations and a marker of global DNA methylation in cord blood, while we observed some evidence of a negative, non-significant association between LINE-1 methylation and serum PFOA level (Table 3). However, Guerrero-Preston et al., (2010) measured total methylated cytosine while we evaluated LINE-1 DNA methylation, two methods which are not directly comparable (Nelson et al., 2011). In addition, Guerrero-Preston et al., (2010) examined cord blood serum from newborns and selected study participants based on smoking status, while we looked at peripheral blood leukocytes from adults in a community-based study, further complicating comparisons between these studies.
A recent analysis of gene expression among a subset of the population studied here suggests that serum PFOA and PFOS concentrations are associated with gender-specific changes in the expression of genes related to cholesterol transport (Fletcher et al., 2013). However, whether these associations may be mediated by changes in DNA methylation or other epigenetic mechanisms remains unknown.
We observed associations between percent LINE-1 methylation and other covariates in our data similar to those previously reported in other studies. For example, women in our study had approximately 0.45 lower percent LINE-1 methylation compared to men (Table S1), consistent with gender differences reported in prior studies (Cash et al., 2011; Cash et al., 2012; Zhu et al., 2012). Our observed weak, non-significant associations between LINE-1 methylation and age, BMI, smoking status, drinking status, and the proportion of specific leukocyte types present (data not shown) were also consistent with previous reports (Cash et al., 2011; Jintaridth and Mutirangura 2010; Terry et al., 2011; Zhu et al., 2012).
Interestingly, although our study population was highly exposed to PFOA, LINE-1 methylation was not associated with PFOA concentrations in serum, but was positively associated with serum PFOS. Differences in the magnitude of exposure to the different PFAAs among this population could potentially explain these observations. For example, if PFAAs affect DNA methylation only at relatively low serum concentrations, there would be little variability in LINE-1 methylation in relation to PFOA since many of the participants were highly exposed. Differences in the chemical properties of PFOA and PFOS may be a possible explanation, since studies suggest that PFOA and PFOS may differentially bind to plasma lipoproteins (Butenhoff et al., 2012), activate PPARs (Bjork et al., 2011; Bjork and Wallace 2009), and affect gene expression (Fletcher et al., 2013). It is also interesting that we observed a positive relationship between PFOS and LINE-1 methylation, whereas in many studies, decreased methylation of LINE-1 regions has generally been associated with poorer health outcomes and various environmental exposures. We are unable to provide an explanation for this inconsistency since so little is known regarding potential relationships between PFAAs and DNA methylation.
Our study has other limitations. First, we did not have DNA samples, and thus information on LINE-1 methylation, from participants at the time of enrollment into the C8 Health Project. As a result, we were not able to evaluate temporal changes in LINE-1 methylation, relationships with PFAA exposure over time, or cross-sectional relationships with participant information collected at enrollment. Second, if PFAAs affect DNA methylation primarily through activation of PPARs, DNA methylation in the promoter regions of specific genes activated by PPARs may provide a more precise measure of changes related to PFAA exposure and should be explored in future studies. Third, LINE-1 methylation differs across various types of leukocytes (Zhu et al., 2012). If PFAA exposure is associated with changes in the types of leukocytes present in peripheral blood, then leukocyte type may confound associations between serum PFAAs and LINE-1 methylation. Unfortunately, differential leukocyte information for the DNA samples used in this analysis, which were collected at follow-up, was not available. As an alternative, we controlled for the percent lymphocytes or percent neutrophils measured in samples collected at enrollment and our results were not materially different. Finally, we had limited health information from the time of follow-up, and most covariates were self-reported, potentially leading to some measurement error and residual confounding.
Nonetheless, strengths of this study includes the evaluation of a novel hypothesis in a large, community-based sample of individuals exposed to high levels of PFOA and background levels of other PFAAs.
5. Conclusion
In this community-based sample we found that mean serum levels of PFOS were associated with LINE-1 DNA methylation, suggesting that this compound may be epigenetically active. More research is needed to further elucidate the mechanisms by which epigenetics may be involved in PFAA exposure related disease, such as exploring gene-specific methylation in the affected tissues and studying the effects of PFAA exposure on DNA methylation in different populations.
Supplementary Material
Acknowledgements
We would like to thank Dr. David Savitz for facilitating this research. This research was funded in part by the C8 class action settlement agreement [Jack W. Leach, et al. v. E.I. du Pont de Nemours & Company (no. 01-C-608 W.Va., Wood County Circuit Court, West Virginia, USA] between DuPont and plaintiffs. Funds were administered by the Garden City Group (Melville, New York) that reports to the court. Our work and conclusions are independent of either party to the lawsuit. This work was also funded by grant R00-ES015774 from NIEHS, grants R01-CA126939 and R01-CA121147 from NCI, and seed funds from the Brown University Office of International Affairs. Dr. Watkins was funded by 5T32HL094300. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the study sponsors.
Abbreviations
- BMI
body mass index
- C8
8 carbon chain, referring to perfluorooctanoic acid
- CDC
Centers for Disease Control and Prevention
- CVD
cardiovascular disease
- HDL-C
high density lipoprotein cholesterol
- IQR
interquartile range
- IRB
Institutional Review Board
- LDL-C
low density lipoprotein cholesterol
- LINE-1
long interspersed nuclear element 1
- NHANES
National Health and Nutrition Examination Survey
- NIOSH
National Institute for Occupational Safety and Health
- PFAA
perfluoroalkyl acid
- PFHxS
perfluorohexane sulfonate
- PFNA
perfluorononanoic acid
- PFOA
perfluorooctanoic acid
- PFOS
perfluorooctane sulfonate
- PPAR
peroxisome-proliferator activated receptor
Footnotes
The authors declare they have no competing financial interests.
References
- Baccarelli A, Wright R, Bollati V, Litonjua A, Zanobetti A, Tarantini L, et al. Ischemic Heart Disease and Stroke in Relation to Blood DNA Methylation. Epidemiology. 2010;21(6):819–828. doi: 10.1097/EDE.0b013e3181f20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ Health Perspect. 2010;118(2):222–228. doi: 10.1289/ehp.0901252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belancio VP, Deininger PL, Roy-Engel AM. LINE dancing in the human genome: transposable elements and disease. Genome Med. 2009;1(10):97–97. doi: 10.1186/gm97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belancio VP, Roy-Engel AM, Deininger PL. All y’all need to know ’bout retroelements in cancer. Seminars in Cancer Biology. 2010;20(4):200–210. doi: 10.1016/j.semcancer.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16(1):6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Bjork JA, Butenhoff JL, Wallace KB. Multiplicity of nuclear receptor activation by PFOA and PFOS in primary human and rodent hepatocytes. Toxicology. 2011;288(1-3):8–17. doi: 10.1016/j.tox.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Bjork JA, Wallace KB. Structure-Activity Relationships and Human Relevance for Perfluoroalkyl Acid-Induced Transcriptional Activation of Peroxisome Proliferation in Liver Cell Cultures. Toxicol Sci. 2009;111(1):89–99. doi: 10.1093/toxsci/kfp093. [DOI] [PubMed] [Google Scholar]
- Bollati V, Schwartz J, Wright R, Litonjua A, Tarantini L, Suh H, et al. Decline in genomic DNA methylation through aging in a cohort of elderly subjects. Mech Ageing Dev. 2009;130(4):234–239. doi: 10.1016/j.mad.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenhoff JL, Pieterman E, Ehresman DJ, Gorman GS, Olsen GW, Chang SC, et al. Distribution of perfluorooctanesulfonate and perfluorooctanoate into human plasma lipoprotein fractions. Toxicol Lett. 2012;210(3):360–365. doi: 10.1016/j.toxlet.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Cash HL, McGarvey ST, Houseman EA, Marsit CJ, Hawley NL, Lambert-Messerlian GM, et al. Cardiovascular disease risk factors and DNA methylation at the LINE-1 repeat region in peripheral blood from Samoan Islanders. Epigenetics. 2011;6 doi: 10.4161/epi.6.10.17728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash HL, Tao L, Yuan JM, Marsit CJ, Houseman EA, Xiang YB, et al. LINE-1 hypomethylation is associated with bladder cancer risk among nonsmoking Chinese. Int J Cancer. 2012;130(5):1151–1159. doi: 10.1002/ijc.26098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa G, Sartori S, Consonni D. Thirty years of medical surveillance in perfluooctanoic acid production workers. J Occup Environ Med. 2009;51(3):364–372. doi: 10.1097/JOM.0b013e3181965d80. [DOI] [PubMed] [Google Scholar]
- Eriksen KT, Raaschou-Nielsen O, McLaughlin JK, Lipworth L, Tjonneland A, Overvad K, et al. Associations between plasma PFOA and PFOS levels and total cholesterol in a middle-aged Danish population. Plos One. 2013;8(2):e56969. doi: 10.1371/journal.pone.0056969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Arbuckle TE, Wade M, Haines DA. Do perfluoroalkyl substances affect metabolic function and plasma lipids?-Analysis of the 2007-2009, Canadian Health Measures Survey (CHMS) Cycle 1. Environ Res. 2013;121:95–103. doi: 10.1016/j.envres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Fitz-Simon N, Fletcher T, Luster M, Steenland K, Calafat AM, Kato K, et al. Reductions in serum lipids in relation to a 4.4-year decrease in serum concentrations of perfluorooctanoic acid and perfluorooctanesulfonic acid. Epidemiology. 2013;24(4):569–576. doi: 10.1097/EDE.0b013e31829443ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher T, Galloway TS, Melzer D, Holcroft P, Cipelli R, Pilling LC, et al. Associations between PFOA, PFOS and changes in the expression of genes involved in cholesterol metabolism in humans. Environ Int. 2013;57-58:2–10. doi: 10.1016/j.envint.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Frisbee SJ, Brooks Jr AP, Maher A, Flensborg P, Arnold S, Fletcher T, et al. The C8 health project: design, methods, and participants. Environ Health Perspect. 2009;117(12):1873–1882. doi: 10.1289/ehp.0800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee SJ, Shankar A, Knox SS, Steenland K, Savitz DA, Fletcher T, et al. Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: results from the C8 Health Project. Arch Pediatr Adolesc Med. 2010;164(9):860–869. doi: 10.1001/archpediatrics.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Preston R, Goldman LR, Brebi-Mieville P, Ili-Gangas C, Lebron C, Witter FR, et al. Global DNA hypomethylation is associated with in utero exposure to cotinine and perfluorinated alkyl compounds. Epigenetics. 2010;5(6):539–546. doi: 10.4161/epi.5.6.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruge KS, Yeung LW, Yamanaka N, Miyazaki S, Lam PK, Giesy JP, et al. Gene expression profiles in rat liver treated with perfluorooctanoic acid (PFOA) Toxicol Sci. 2006;89(1):93–107. doi: 10.1093/toxsci/kfj011. [DOI] [PubMed] [Google Scholar]
- Jintaridth P, Mutirangura A. Distinctive patterns of age-dependent hypomethylation in interspersed repetitive sequences. Physiol Genomics. 2010;41(2):194–200. doi: 10.1152/physiolgenomics.00146.2009. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999-2008. Environ Sci Technol. 2011;45(19):8037–8045. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405(6785):421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- Lander ES, Int Human Genome Sequencing C, Linton LM, Birren B, Nusbaum C, Zody MC, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Langevin SM, Houseman EA, Christensen BC, Wiencke JK, Nelson HH, Karagas MR, et al. 2011;6(7):908–919. doi: 10.4161/epi.6.7.16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99(2):366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Leonard RC, Kreckmann KH, Sakr CJ, Symons JM. Retrospective cohort mortality study of workers in a polymer production plant including a reference population of regional workers. Ann Epidemiol. 2008;18(1):15–22. doi: 10.1016/j.annepidem.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL. Polyfluorinated compounds: past, present, and future. Environ Sci Technol. 2011;45(19):7954–7961. doi: 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- Lundin JI, Alexander BH, Olsen GW, Church TR. Ammonium perfluorooctanoate production and occupational mortality. Epidemiology. 2009;20(6):921–928. doi: 10.1097/EDE.0b013e3181b5f395. [DOI] [PubMed] [Google Scholar]
- Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS. Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the U.S. National Health and Nutrition Examination Survey. Environ Health Perspect. 2010;118(5):686–692. doi: 10.1289/ehp.0901584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HH, Marsit CJ, Kelsey KT. Global Methylation in Exposure Biology and Translational Medical Science. Environ Health Perspect. 2011;119(11):1528–1533. doi: 10.1289/ehp.1103423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JW, Hatch EE, Webster TF. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect. 2010;118(2):197–202. doi: 10.1289/ehp.0901165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Burlew MM, Mandel JH. Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. J Occup Environ Med. 2003;45(3):260–270. doi: 10.1097/01.jom.0000052958.59271.10. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Ehresman DJ, Buehrer BD, Gibson BA, Butenhoff JL, Zobel LR. Longitudinal assessment of lipid and hepatic clinical parameters in workers involved with the demolition of perfluoroalkyl manufacturing facilities. J Occup Environ Med. 2012;54(8):974–983. doi: 10.1097/JOM.0b013e31825461d2. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Schmid JR, Corton JC, Zehr RD, Das KP, Abbott BD, et al. Gene Expression Profiling in Wild-Type and PPARalpha-Null Mice Exposed to Perfluorooctane Sulfonate Reveals PPARalpha-Independent Effects. PPAR Res. 2010 doi: 10.1155/2010/794739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MB, Thibodeaux JR, Wood CR, Zehr RD, Schmid JE, Lau C. Gene expression profiling in the lung and liver of PFOA-exposed mouse fetuses. Toxicology. 2007239(1-2):15–33. doi: 10.1016/j.tox.2007.06.095. [DOI] [PubMed] [Google Scholar]
- Sakr CJ, Leonard RC, Kreckmann KH, Slade MD, Cullen MR. Longitudinal study of serum lipids and liver enzymes in workers with occupational exposure to ammonium perfluorooctanoate. J Occup Environ Med. 2007;49(8):872–879. doi: 10.1097/JOM.0b013e318124a93f. [DOI] [PubMed] [Google Scholar]
- Sakr CJ, Symons JM, Kreckmann KH, Leonard RC. Ischaemic heart disease mortality study among workers with occupational exposure to ammonium perfluorooctanoate. Occup Environ Med. 2009;66(10):699–703. doi: 10.1136/oem.2008.041582. [DOI] [PubMed] [Google Scholar]
- Shankar A, Xiao J, Ducatman A. Perfluoroalkyl chemicals and elevated serum uric acid in US adults. Clinical epidemiology. 2011;3:251–258. doi: 10.2147/CLEP.S21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A, Xiao J, Ducatman A. Perfluorooctanoic Acid and Cardiovascular Disease in US Adults. Arch Intern Med. 2012;172(18):1397–1403. doi: 10.1001/archinternmed.2012.3393. [DOI] [PubMed] [Google Scholar]
- Steenland K, Tinker S, Frisbee S, Ducatman A, Vaccarino V. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am J Epidemiol. 2009;170(10):1268–1278. doi: 10.1093/aje/kwp279. [DOI] [PubMed] [Google Scholar]
- Steenland K, Tinker S, Shankar A, Ducatman A. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with uric acid among adults with elevated community exposure to PFOA. Environ Health Perspect. 2010;118(2):229–233. doi: 10.1289/ehp.0900940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugii S, Evans RM. Epigenetic codes of PPAR gamma in metabolic disease. FEBS Lett. 2011;585(13):2121–2128. doi: 10.1016/j.febslet.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs ML, Abbott BD. Activation of mouse and human peroxisome proliferator-activated receptors (alpha, beta/delta, gamma) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol Sci. 2007;95(1):108–117. doi: 10.1093/toxsci/kfl135. [DOI] [PubMed] [Google Scholar]
- Terry MB, Delgado-Cruzata L, Vin-Raviv N, Wu HC, Santella RM. DNA methylation in white blood cells Association with risk factors in epidemiologic studies. Epigenetics. 2011;6(7):828–837. doi: 10.4161/epi.6.7.16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Peng S, Martin FL, Zhang J, Liu L, Wang Z, et al. Perfluorooctanoic acid induces gene promoter hypermethylation of glutathione-S-transferase Pi in human liver L02 cells. Toxicology. 2012;296(1-3):48–55. doi: 10.1016/j.tox.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Tost J. DNA methylation: an introduction to the biology and the disease-associated changes of a promising biomarker. Mol Biotechnol. 2010;44(1):71–81. doi: 10.1007/s12033-009-9216-2. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and - gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol Sci. 2006;92(2):476–489. doi: 10.1093/toxsci/kfl014. [DOI] [PubMed] [Google Scholar]
- Wan YJ, Li YY, Xia W, Chen J, Lv ZQ, Zeng HC, et al. Alterations in tumor biomarker GSTP gene methylation patterns induced by prenatal exposure to PFOS. Toxicology. 2010;274(1-3):57–64. doi: 10.1016/j.tox.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Wu HC, Wang Q, Delgado-Cruzata L, Santella RM, Terry MB. Genomic Methylation Changes Over Time in Peripheral Blood Mononuclear Cell DNA: Differences by Assay Type and Baseline Values. Cancer Epidemiol Biomarkers Prevent. 2012;21(8):1314–1318. doi: 10.1158/1055-9965.EPI-12-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang AS, Estecio MRH, Doshi K, Kondo Y, Tajara EH, Issa JPJ. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32(3) doi: 10.1093/nar/gnh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung LWY, Guruge KS, Yamanaka N, Miyazaki S, Lam PKS. Differential expression of chicken hepatic genes responsive to PFOA and PFOS. Toxicology. 2007;237(1-3):111–125. doi: 10.1016/j.tox.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Youngson NA, Morris MJ. What obesity research tells us about epigenetic mechanisms. Philos Trans R Soc B-Biol Sci. 2013;368(1609) doi: 10.1098/rstb.2011.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZZ, Hou LF, Bollati V, Tarantini L, Marinelli B, Cantone L, et al. Predictors of global methylation levels in blood DNA of healthy subjects: a combined analysis. Int J Epidemiol. 2012;41(1):126–139. doi: 10.1093/ije/dyq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


