Abstract
The clinical features and outcomes of 148 patients with acute myeloid leukemia (AML) and 11q23 chromosomal abnormalities were compared with those of 2640 patients with non-11q23 AML. Patients with t(9;11)), t(6;11), or other 11q23 balanced translocations [t(11;v)(q23;v)] presented at a younger age and with higher percentage of bone marrow blasts. Unbalanced 11q23 abnormalities were commonly associated with deletions of chromosomes 5q, 7q and/or complex karyotypes. In multivariate analysis, when compared to patients with non-11q23 AML and unfavorable risk karyotype, there was a significant difference in overall survival (OS) for patients with t(9;11) (P = .004), whereas there were no difference in OS for patients with t(6;11) (P = .62), t(11;19) (P = 0.20), and unbalanced 11q23 aberrations (P = .85) or t(11;v)(q23;v) (P = 0.59), indicating that t(9;11) has an independent intermediate prognostic factor, with all others poor prognostic factors for OS; this was further confirmed by comparing them with patients with non-11q23 AML and intermediate risk karyotype. Using intention-to treat analysis based on donor availability, we also noted that allogeneic stem cell transplant (SCT) in first remission had a significant benefit towards improving OS (P < 0.001) and relapse-free survival (P<0.001) in patients with AML and 11q23 abnormalities.
Keywords: AML, 11q23 cytogenetic abnormalities, complete remission, prognosis allogeneic stem cell transplantation
INTRODUCTION
Cytogenetic abnormalities in leukemia cells are strongly associated with distinct clinical subgroups and are predictive of both clinical features and therapeutic outcome.1–4 Recurrent cytogenetic abnormalities in chromosome 11q23 involving the mixed-lineage leukemia (MLL) gene have been observed in 3%–4% of adult patients with acute myeloid leukemia (AML), in 3%–7% of adults with acute lymphoblastic leukemia,5 and at even higher rate in infant leukemia.6, 7 To date, more than 60 translocation partner genes of 11q23 have been identified.8
MLL encodes a histone methyltransferase that is critical for maintaining gene expression during embryonic development and hematopoiesis. MLL gene translocations generate chimeric MLL fusion proteins that directly bind to DNA and positively regulate gene transcription. These events result in aberrant expression of downstream MLL targets, including the HOX gene, thereby leading to leukemic transformation.8, 9 Recent studies have shown that the prognosis of 11q23/MLL AML is heterogeneous depending upon the 11q23 fusion partner.10, 11 In addition, the prognosis of AML with 11q23 abnormalities with the same translocation partner differs between adults and children.10–13 As a result, in the 2008 edition of the World Health Organization (WHO) classification of myeloid neoplasms, the category of AML with 11q23/MLL abnormalities was revised to focus on AML with t(9;11) (p22;q23)/MLLT3-MLL, with the notation that rearrangements of MLL with other fusion partners need to be specified upon AML diagnosis.14
Because adult AML cases with 11q23 abnormalities are rare, the clinical features and prognostic significance of the abnormalities other than t(9;11) are not well known. To our knowledge, the prognostic significance of unbalanced 11q23 aberrations has not been reported to date. The prognostic impact of additional chromosomal abnormalities in patients with 11q23 aberrations is also not clear. The existing information about this subgroup is based on data from only a few clinical trials with potential for selection bias. Furthermore, information on the potential therapeutic role of allogeneic stem cell transplantation (allo-SCT) in this subgroup is scarce, as most of the published studies involved groups that were too small to allow definitive conclusions.15, 16
In the present study, to characterize their clinical features, assess their outcomes in response to therapy, and evaluate the significance of allo-SCT performed at first complete remission (CR1), we retrospectively analyzed the data from 148 adult patients with newly diagnosed 11q23 AML between January 1990 and February 2011 who were treated at our institution. We clearly demonstrate that t(6;11), t(11;19), unbalanced 11q23 aberrations and t(11;v)(q23;v) are independent poor prognostic factors whereas patients with t(9;11) have intermediate risk disease. We also demonstrate that allo-SCT in CR1 has a significant benefit for improving overall and relapse-free survival for patients with AML and 11q23 abnormalities.
PATIENTS AND METHODS
Patients
We searched the database of the department of leukemia at the University of Texas – MD Anderson Cancer Center. Between January 1990 and February 2011, 2788 consecutive patients with newly diagnosed AML with available cytogenetic analysis were identified and were the subject of this study [excluding patients with acute promyelocytic leukemia, and those with core binding factor leukemia with t(16;16)/inv(16) or t(8;21)]. Upon initial diagnosis, 148 of the 2788 patients had 11q23 abnormalities detected by conventional cytogenetic analysis; these were confirmed by fluorescence in situ hybridization analysis in 29 patients. AML with MLL partial tandem duplications was not included in this study as it is considered as a distinct entity.14 The remaining 2640 patients were defined as having non-11q23 AML. Among the patients with 11q23 AML, 144 (97%) underwent chemotherapy regimens consisting of high-dose ara-C (HDAC, ≥1 g/ m2 per dose) alone (n=3) or plus idarubicin (n=85), plus fludarabine (n=20), or plus other agents such as clofarabine, topotecan, troxacitabine, or liposomal daunorubicin (n=20);non-HDAC (<1 g/m2 per dose) plus daunorubicin or clofarabine (n=9); 7 patients had other non-ara-C based regimens. Among the 2640 non-11q23 reference group, 131 patients received supportive treatment only (5%). 1838 patients received HDAC based regimens (70%), 297 patients received non-HDAC based regimens (11%), and 374 patients received other non-ara-C based regimens (14%). All patients were treated on prevailing clinical trials and gave written informed consent to participate. The current study was further approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center.
Subgroups of 11q23 abnormalities
11q23 abnormalities were classified into five groups. Group A) t(9;11) (n=65; 44%) included t(9;11)(p22;q23) (n=58) and t(9;11)(p21;q23). Group B) t(6;11) (n=12; 8%) included t(6;11)(q27;q23). Group C) t(11;19) (n=18; 12%) included 12 cases with t(11;19)(q23;p13), 3 with t(11;19) (q23;p13.3), 2 with t(11;19)(q23;p13.1) and one with t(11q23;p13.2). Group D) unbalanced 11q23 aberrations (n=41; 28%) included del(11)(q23) (n=32) and add (11)(q23), inv 11q23, or dic 11q23 (n=9;). Group E) t(11;v)(q23;v) (n=12; 8%) comprised of other balanced 11q23 translocations, including two patients each with t(10;11) and t(11;17), and one each with t(11;15), t(7;11), t(X;11), dic(11;12), t(3;11), t(11;22), t(4;11), and t(11;11). These distributions are similar to those reported in previous studies,17, 18 with the exception of a relatively lower frequency of t(10;11). Additionally, the frequency of unbalanced 11q23 aberrations was relatively higher in our study. Cases of non-11q23 AML were grouped into two cytogenetic risk categories (intermediate and unfavorable) according to the European Leukemia Net criteria.1 The outcomes and clinical features of the cases in the five 11q23 AML subgroups were compared with those of the non-11q23 AML cases.
Statistical analysis and definitions
Complete remission (CR), overall survival (OS), event-free survival (EFS), and relapse-free survival (RFS) were defined on the basis of criteria recommended by the International Working Group.19 A Wilcoxon rank-sum test was used to make pair wise comparisons of continuous variables between cytogenetic subgroups, and a Kruskal-Wallis test was used to compare continuous variables among more than two subgroups. Categorical variables were compared using Fisher’s exact test. The Bonferroni method was used to adjust the type I error when multiple comparisons were used. The Kaplan-Meier method was used to estimate the time-to-event variables. Log rank tests were conducted to compare the survival among the various cytogenetic subgroups. Cox proportional hazards models were used to evaluate the ability of the covariates to predict the time-to-event variables. For each fitted Cox regression model, non-significant variables in univariate analyses were eliminated in a step-down fashion using a P value cutoff of P = 0.05. All computations were carried out using SAS software version 9.1 and S-Plus software version 8.04.
RESULTS
Incidence and clinical characteristics of 11q23 AML
The frequency of 11q23 AML within intermediate and unfavorable cytogenetic risk AML was 5.3% (148/2788). Among the 2640 patients with non-11q23 AML, 1679 had intermediate-risk cytogenetics, and 961 had unfavorable-risk cytogenetics. Among the 148 patients with 11q23 AML, 78(52%) had de novo AML, and 58(40%) had therapy-related AML (t-AML); in 12 (8%) patients the disease was secondary to a prior myelodysplastic syndrome or myeloproliferative neoplasm (s-AML).
The median age of patients with 11q23 AML was 54 years (range, 17 to 84 years), and 61 (41%) patients were male. Of note, 60 of 148 (41%) cases were classified as the French-American-British (FAB) classification M5 (FAB-M5), and 32 (22%) were classified as M4 (FAB-M4). The presenting features of various 11q23/MLL AML subgroups are shown in Table 1. Patients with t(9;11), t(6;11), and t(11;v)(q23;v) presented at a younger age (P<0.001, = 0.002 and <0.001, respectively) and had a higher percentage of bone marrow blasts (P<0.001, <0.001 and = 0.04, respectively) at diagnosis than those in the non-11q23 reference group. Proportion of patients with FAB-M5 was higher among patients with all 11q23 AML subgroups than in the reference group (P<0.001). Patients with t(9;11) were more likely to be female (69%) than the non-11q23 reference group (41%; P<0.001). Patients with t(9;11) were more likely to have t-AML and /or s-AML compared to those with non-11q23 AML (P=0.008),20 whereas patient with t(6;11) commonly presented with de novo AML (P=0.007). No differences in white blood cell counts, platelet counts, hemoglobin levels, FAB-M4 incidence, t-AML or s-AML incidence were observed between the 11q23 subgroups and the non-11q23 reference group (Table 1).
Table 1.
Patient characteristics, response and outcomes by comparison of five 11q23 subgroups with non-11q23 reference group
| Characteristic | Group t(9;11) (n=65) |
Group t(6;11) (n=12) |
Group t(11;19) (n=18) |
Group unbalanced 11q23 (n=41) |
Group t(11;v)(q23;v) (n=12) |
Reference (unfavorable or intermediate , n=2640) |
P* | |
|---|---|---|---|---|---|---|---|---|
| Age, years | Median (range) | 51 (17–77) | 52 (31–62) | 66 (31–81) | 63 (22–84) | 43 (21–65) | 63 (14–93) | <0.001 |
| P# | <0.001 | 0.002 | 1.0 | 0.5 | <0.001 | |||
| WBC, × 109/L | Median (range) | 10.3 (0.6–193.6) | 9.3 (1.0–111.7) | 6.4 (0.8–44.7) | 4.3 (0.7–94.5) | 10.1 (0.6–95.4) | 6.1 (0.2–433) | 0.64 |
| Platelet, × 109/L | Median (range) | 42 (7–194) | 44 (20–155) | 45 (10–112) | 54 (7–199) | 52 (25–192) | 50 (1–2292) | 0.98 |
| HGB, g/dl | Median (range) | 8.4 (4.6–14.4) | 9.1 (6.6–10.5) | 8.0 (4.3–11.2) | 8.0 (5.1–14.1) | 8.8 (4.7–11.8) | 8.2 (2.0–15.1) | 0.46 |
| BM Blast (%) | Median (range) | 81 (20, 98) | 84 (42, 94) | 53 (18, 92) | 50 (17, 92) | 75 (21, 94) | 43 (0, 98) | <0.001 |
| P# | <0.001 | <0.001 | 0.64 | 0.62 | 0.04 | |||
| Race, white | (%) | 51 (79) | 5 (42) | 13 (72) | 33 (81) | 11 (92) | 2131 (81) | 0.04 |
| P# | 0.4 | 0.003 | 0.37 | 1.0 | 0.15 | |||
| t-AML/s-AML | (%) | 33 (51) | 1 (8) | 6 (33) | 16 (39) | 2 (17) | 909 (34) | 0.02 |
| P# | 0.008 | 0.007 | 1 | 0.62 | 0.24 | |||
| FAB | ||||||||
| M4 | (%) | 13 (21) | 3 (25) | 5 (28) | 6 (17) | 2 (18) | 383 (18) | |
| M5 | (%) | 38 (60) | 6 (50) | 5 (28) | 8 (23) | 3 (27) | 159 (8) | <0.001 |
| others | (%) | 12 (19) | 3 (25) | 8 (44) | 21 (60) | 6 (55) | 1582 (75) | |
| P# | <0.001 | <0.001 | 0.004 | 0.009 | <0.001 | |||
| Del(5q)/-5, del(7q)/-7 and/or Complex Karyotype | (%) | 13 (20) | 2(17) | 1 (6) | 27 (66) | 5 (42) | 939 (36) | <0.001 |
| P# | 0.008 | 0.23 | 0.006 | <0.001 | 1.0 | |||
| Trisomy8 | No. (%) | 14 (22) | 1 (8.3) | 1 (6) | 8 (20) | 1 (8) | 222 (8) | 0.004 |
| P# | 0.003 | 1 | 1 | 0.02 | 0.21 | |||
| Gender, male | No. (%) | 20 (31) | 4 (33) | 10 (56) | 22 (54) | 5 (42) | 1562(59) | |
| P# | <0.001 | 0.08 | 0.81 | 0.52 | 0.06 | <0.001 | ||
| CR rate | No. (%) | 44 (68) | 10 (83) | 13(72) | 20 (49) | 11 (92) | [370 (39) and 982 (60), respectively] | <0.001 |
| 5-year OS | % | 27 | 0 | 6 | 5 | 18 | (6 and 19, respectively) | <0.001 |
| 5-year EFS | % | 27 | 0 | 6 | 5 | 0 | (3 and 13, respectively) | <0.001 |
Note: AML with 11q23 abnormalities were defined as 5 groups: t(9;11), t(6;11), t(11;19), unbalanced 11q23 andt(11;v)(q23;v).
WBC indicates white blood cell; HGB, hemoglobin; BM, bone marrow; FAB, French-American-British Classification; CR, complete remission; OS, overall survival; EFS, event-free survival.
Kruskal-Wallis or Fisher exact test.
Wilcoxon rank-sum test or Fisher’s exact test. If the P values in right hand column were significant, we then compared the data from each 11q23 subgroup to the non-11q23 reference group and listed the Pvalues below the row.
Del (5q)/-5, del (7q)/-7, complex karyotypes, and additional trisomy 8 with 11q23 abnormalities
The incidence of additional cytogenetic abnormalities including del5/5q-, del7/7q-, and/or complex karyotypes was significantly higher in patients with unbalanced 11q23 aberrations than in the non-11q23 reference group (P<0.001), whereas the incidence of these additional abnormalities was significantly lower in patients with t(9;11) (P=0.008) and t(11;19) (P=0.006) than in the non-11q23 reference group. The incidence of additional trisomy 8 was higher in patients with t(9;11) and unbalanced 11q23 aberrations) than in the non-11q23 reference group (P=0.003 and 0.02, respectively).
Treatment response and clinical outcome
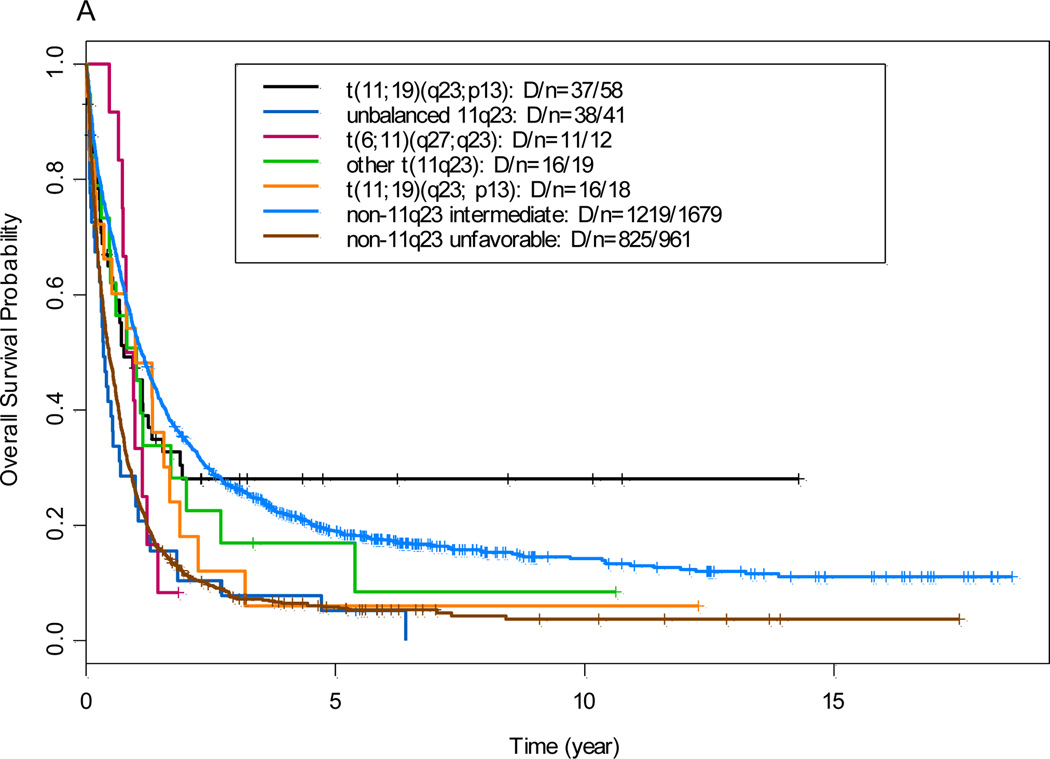
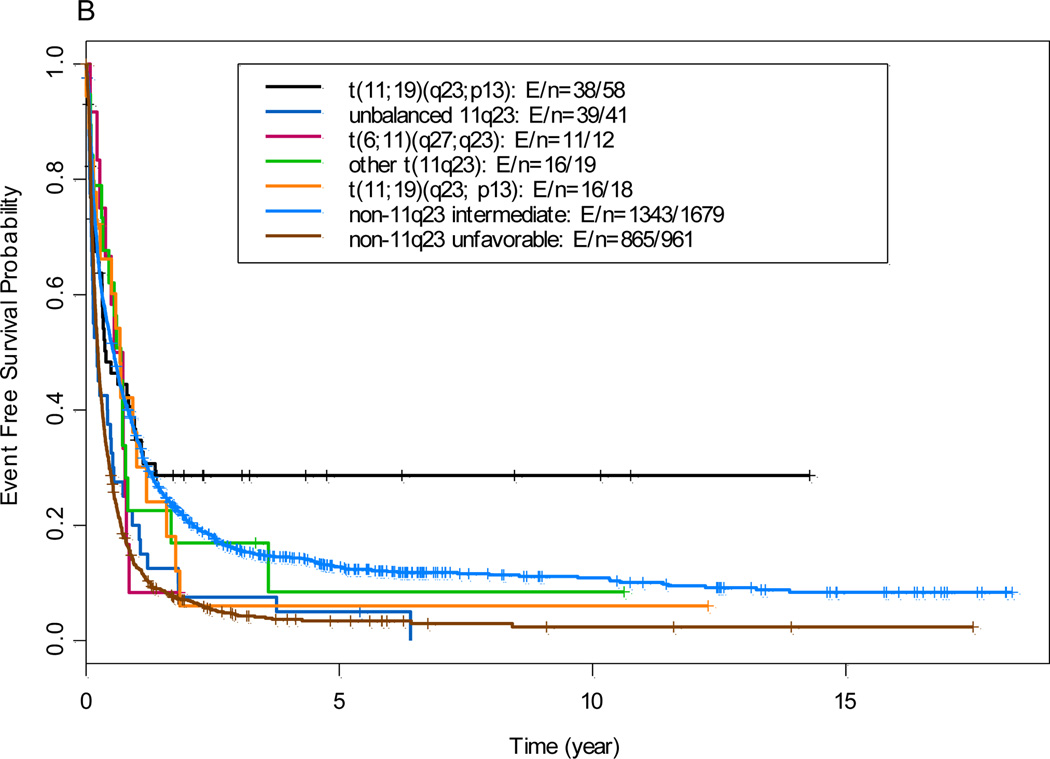
The median follow-up time in all patients (n=2788) was 5 years. The overall CR rate for the 144 patients with 11q23 aberrations who received induction chemotherapy was 68% (n=98); The median OS time in the 11q23 AML group and the non-11q23 AML reference group was 8.5 months (95% confidence interval, 6.4 to 11.9 months) and 9.5 months (95% confidence interval, 9.0 to 10.2 months), respectively (P=0.32; data not shown). Patients with t(9;11) had OS and EFS similar to intermediate non-11q23 reference group, where as all other 11q23 groups (data not shown) had OS and EFS comparable to unfavorable non-11q23 reference group (Figure 1)
Figure 1.
(A) Overall survival by comparison of 11q23 subgroups with non-11q23 reference groups. (B) Event-free survival by comparison of 11q23 subgroups with non-11q23 reference groups
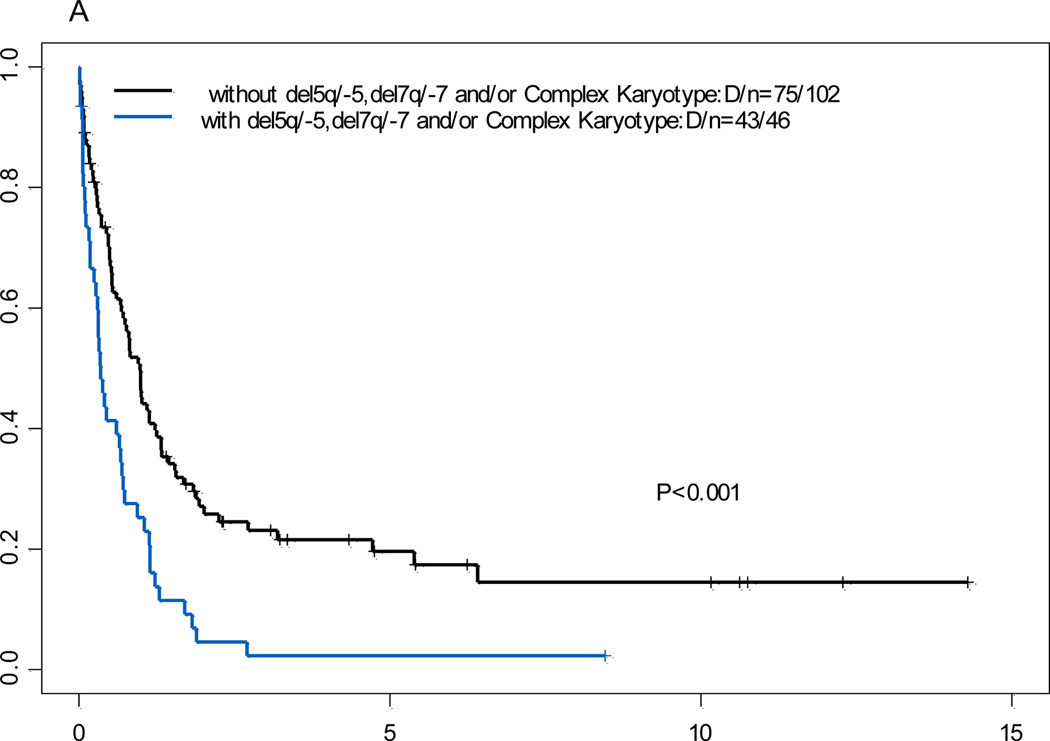
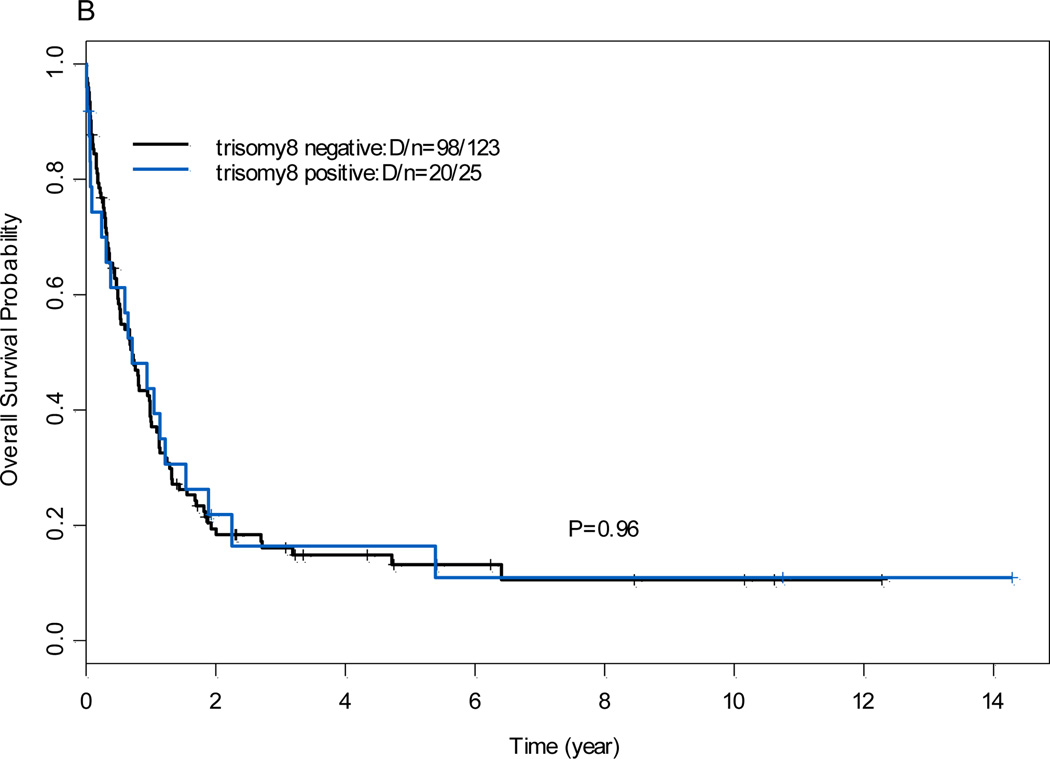
The 5-year OS rates and EFS rates were shown in Table 1. The OS rate was lower in patients who had 11q23 AML with del(5q)/-5, del (7q)/-7, and/or complex karyotypes than in those without del (5q)/-5, del (7q)/-7, and/or complex karyotypes (P<.001; Figure 2). There was no significant difference in OS rates between patients who had 11q23 AML with additional trisomy 8 and those without additional trisomy 8 (P=0.96, data not shown).
Figure 2.
Overall survival for 11q23 acute myeloid leukemia patients with or without del5/5q-, del7/7q- and/or complex karyotype
Risk factor analysis
To determine the prognostic impact of each 11q23 subgroup, we used Cox regression models when age, sex, race, white blood cell counts, platelet counts, hemoglobin levels, lactase dehydrogenase, percentage of bone marrow blasts, type of AML (de novo vs. t-AML/S-AML), type of treatment, performance status, and the five 11q23 subgroups were considered. Cytogenetic abnormalities were excluded because of overlap with five 11q23 subgroups. Multivariate analysis revealed that only t(9;11) was an independent intermediate risk factor for OS when compared to patients with unfavorable non-11q23 AML (hazard ratio [HR] =0.61; P=0.004). There was no significant difference in OS when patients with intermediate non-11q23 were used as the reference group (HR =1.10; P=0.53), further confirming that t(9;11) was an intermediate cytogenetic risk factor. Similar results were observed for EFS when patients with unfavorable or intermediate risk non-11q23 AML were used as the reference group (HR=.52; P<0.001 and HR = 1.04; P=0.80, respectively).
In multivariate analysis, there was no significantly different in O Sin group t(6;11), t(11;19), unbalanced 11q23, ort(11;v)(q23;v) group (HR = 1.17, 0.72, 0.97 and 0.84, respectively; P=0 .62, 0.20, 0.85 and 0.59, respectively; Table 2) when compared to patients with unfavorable non-11q23 AML, indicating they were independent poor prognostic factors for OS. These were further confirmed by comparing to patients with intermediate non-11q23 AML. Of note, the presence of t(11;19) and t(11;v)(q23;v) was a marginally significant factor for poor OS (P=0.07; Table 2) when patients with intermediate non-11q23 AML were used as reference group. Similar results were observed for EFS when patients with unfavorable non-11q23 AML were used as the reference group (Table 2). Of note, when compared to patients with intermediate non-11q23 AML, only t(6;11) and unbalanced 11q23 aberrations were independent poor prognostic features for EFS (HR=2.00, P=0.02 and HR=1.58, P=0.005, respectively).
Table 2.
Multivariate analysis of prognosis of five 11q23 subgroups
| Variable | Overall Survival | Event-Free Survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Using unfavorable as reference group | ||||||
| AGE | 1.03 | 1.02 to 1.03 | <0.001 | 1.02 | 1.01 to 1.02 | <0.001 |
| HGB | 0.95 | 0.92 to 0.99 | 0.02 | 0.94 | 0.90 to 0.97 | 0.001 |
| Log WBC | 1.09 | 1.03 to 1.15 | 0.004 | 1.14 | 1.08 to 1.20 | <0.001 |
| Log platelet | 0.77 | 0.71 to 0.83 | <0.001 | 0.81 | 0.76 to 0.88 | <0.001 |
| BM blast | 1.00 | 0.99 to 1.00 | 0.007 | 1.00 | 1.00 to 1.00 | 0.11 |
| De novo (yes vs. no) | 0.79 | 0.69 to 0.91 | 0.001 | 0.85 | 0.75 to 0.98 | 0.02 |
| PS (3,4 vs. 0,1,2) | 2.44 | 1.93 to 3.08 | <0.001 | 1.94 | 1.55 to 2.44 | <0.001 |
| Group t(9;11) | 0.61 | 0.43 to 0.85 | 0.004 | 0.52 | 0.37 to 0.73 | <0.001 |
| Group t(6;11) | 1.17 | 0.64 to 2.14 | 0.62 | 0.95 | 0.52 to 1.74 | 0.87 |
| Group t(11;19) | 0.72 | 0.44 to 1.19 | 0.20 | 0.61 | 0.37 to 1.00 | 0.05 |
| Group unbalanced 11q23 | 0.97 | 0.70 to 1.35 | 0.85 | 0.86 | 0.62 to 1.19 | 0.36 |
| Group t(11;v)(q23;v) | 0.84 | 0.45 to 1.58 | 0.59 | 0.69 | 0.37 to 1.30 | 0.25 |
| Treatment type 2 vs. 1 | 0.70 | 0.55 to 0.89 | 0.003 | |||
| Treatment type3 vs. 1 | 0.84 | 0.69 to 1.03 | 0.09 | |||
| Using intermediate as reference group | ||||||
| Age, per 10 years | 1.03 | 1.02 to1.03 | <0.001 | 1.02 | 1.02 to 1.02 | <0.001 |
| Log WBC | 1.08 | 1.03 to 1.13 | 0.001 | 1.11 | 1.06 to 1.16 | <0.001 |
| Log platelet | 0.91 | 0.86 to 0.97 | 0.004 | 0.93 | 0.87 to 0.99 | 0.02 |
| De novo (yes vs. no) | 0.80 | 0.71 to 0.90 | <0.001 | 0.80 | 0.71 to 0.90 | <0.001 |
| PS (3,4 vs. 0,1,2) | 2.03 | 1.63 to 2.53 | <0.001 | 1.82 | 1.46 to 2.26 | <0.001 |
| Group t(9;11) | 1.10 | 0.80 to 1.53 | 0.53 | 1.04 | 0.75 to 1.44 | 0.8 |
| Group t(6;11) | 2.28 | 1.25 to 4.14 | 0.01 | 2.00 | 1.10 to 3.65 | 0.02 |
| Group t(11;19) | 1.59 | 0.97 to 2.61 | 0.07 | 1.19 | 0.73 to 1.96 | 0.49 |
| Group unbalanced 11q23 | 2.01 | 1.45 to 2.79 | <0.001 | 1.58 | 1.15 to 2.19 | 0.005 |
| Group t(11;v)(q23;v) | 1.77 | 0.95 to 3.31 | 0.07 | 1.45 | 0.77 to 2.71 | 0.25 |
| Treatment type 2 vs. 1 | 0.83 | 0.68 to 1.00 | 0.05 | 0.93 | 0.77 to 1.11 | 0.42 |
| Treatment type 3 vs. 1 | 1.29 | 1.09 to 1.52 | 0.003 | 1.55 | 1.32 to 1.82 | <0.001 |
| BM blast | 1.00 | 1.00 to 1.00 | 0.01 | |||
| HGB | 0.93 | 0.91 to 0.96 | <0.001 | |||
Note: AML with 11q23 abnormalities were defined as 5 groups: t(9;11), t(6;11), t(11;19), unbalanced 11q23 and t(11;v)(q23;v). Treatment type1, High-dose ara-C based regimens (HDAC); type2, non-HDAC based regimens; type 3, other non-ara-C based regimens.
WBC, white blood cell; HGB, hemoglobin; BM, bone marrow.
Molecular abnormalities in cases of 11q23 AML
The incidence of RAS mutations was slightly higher in the 11q23 AML group than in the non-11q23 AML reference group [21.8% (12/55) and 18.3% (203/1112), respectively (P=0.48)]. However, FLT3-ITD mutations were detected in 4.8% (3/63) and 16.1% (226/1406; P=0.01) of the 11q23 and non-11q23 groups, respectively; FLT3-D835 mutations were detected in 6.4% (4/63) and 5.0% (70/1405; P=.55), respectively; and NPM1 mutations were detected in 2.9% (1/35) and 20.7% (114/552; P<0.001), respectively. These results are consistent with other reports, thereby indicating that 11q23 AML often harbors gene mutations involved in the RAS pathway,21 whereas mutations in FLT3 and NPM1 are uncommon.21–23
Role of allogeneic SCT in post remission consolidation at CR1
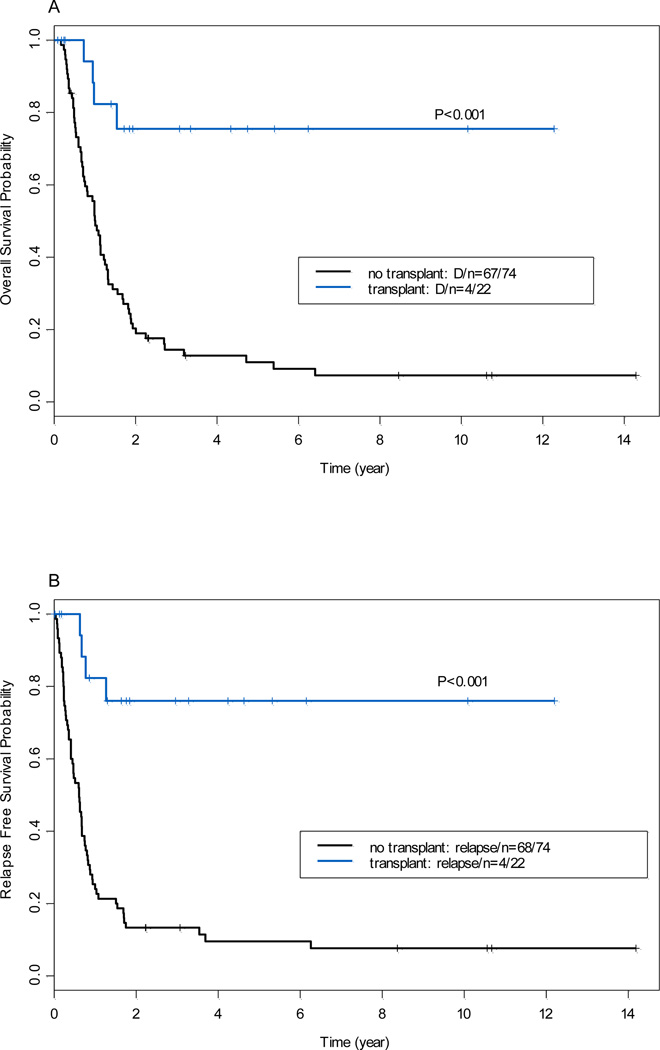
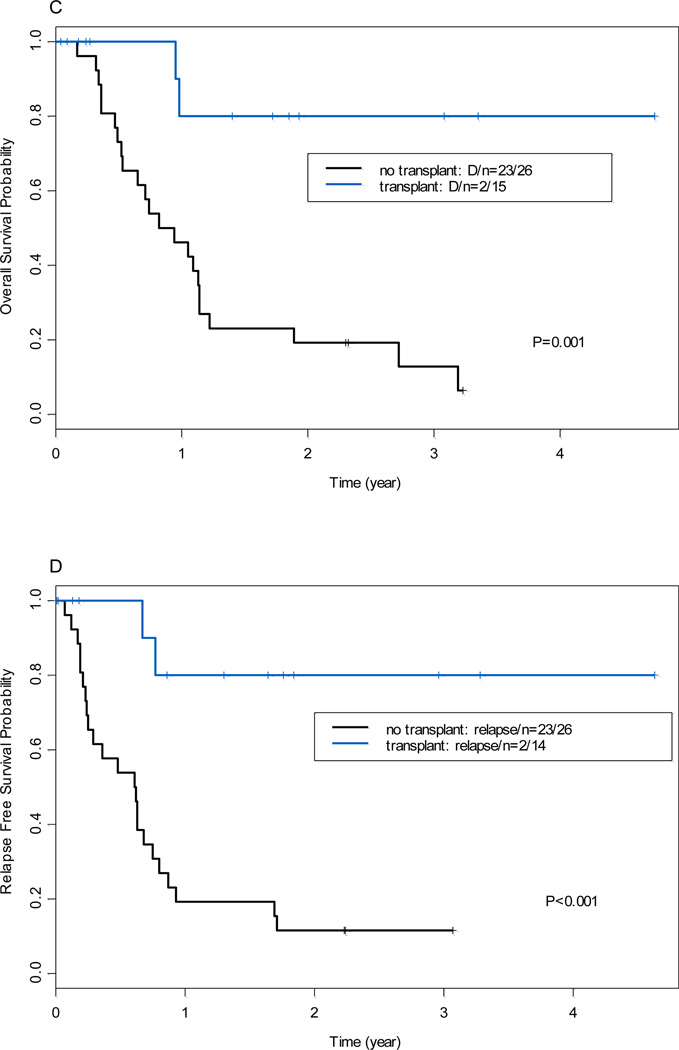
Of the 98 AML patients with 11q23 abnormalities who achieved CR after induction chemotherapy. Donor availability for four patients is unknown. Among remaining 94 patients, 55 patients had no donor or were not screened for donor, 39 patients (41%) had donor available. Patient characteristics by donor vs. non-donor were shown in Table 3. The clinical characteristics of the patients with donor vs. non- donor were well balanced except patient age, bone marrow blast and 11q23 aberrations. Of 39 patients, 24 underwent allogeneic SCT (16 from related donors, 4 from unrelated donors, 3 cord blood, and 1 from a haploidentical donor) in CR1. The conditioning regimens consisted of fludarabine plus busulfan (n=14), fludarabine plus melphalan (n=8), Total body irradiation plus etoposide and cyclophosphamide (n=1), or unknown regimen (n=1). The median time from CR1 to SCT was 3 months (range, 1 to 27 months). Patients who underwent allo-SCT at CR1 (n=39) had a significantly longer OS (P<0.001) and RFS (P<0.001) than those who did not undergo allo-SCT at CR1 (n=55; Figure 3A and 3B). This superior outcome was also evident when considering only patients with t(9;11) (Figure 3C and 3D). Multivariate analysis using allo-SCT as a time-dependent covariable after controlling for other risk factors as described above revealed a significant positive impact of allo-SCT at CR1 on OS (HR=0.56; P=0.02) and RFS (HR=0.51; P=0.01; data not shown).
Table 3.
Patient characteristics and outcomes by donor availability in first remission
| Parameter | No donor available (n= 55) |
Donor available (n=39) |
P | |
|---|---|---|---|---|
| Age, years | Median (range) | 54 (20, 82) | 49 (17, 67) | 0.02 |
| WBC, × 109/L | Median (range) | 7.2 (0.6, 111.7) | 3.8 (0.6, 73.7) | 0.29 |
| Platelet, × 109/L | Median (range) | 39 (7, 199) | 58 (8, 189) | 0.17 |
| HGB, g/dl | Median (range) | 8.1 (4.3, 14.1) | 8.5 (6, 14.4) | 0.32 |
| BM Blast (%) | Median (range) | 64 (21, 96) | 83 (20, 98) | 0.03 |
| t-AML/s-AML | 24(43.6%) | 15(38.5%) | 0.67 | |
| Del(5q)/-5, del(7q)/-7 and/or Complex Karyotype | 19(34.5%) | 6(15.4%) | 0.06 | |
| Gender, male | 25(45.5%) | 14(35.9%) | 0.4 | |
| 11q23 aberrations | 0.03 | |||
| t(9;11) | 23(41.8%) | 21(53.8%) | ||
| t6;11) | 3(5.5%) | 7(17.9%) | ||
| All other 11q23 aberrations | 29(52.7%) | 11(28.2%) | ||
| 5-year overall survival | Median (range) | 0.13 (0.06, 0.25) | 0.39 (0.26, 0.58) | <0.001 |
| 5-year relapse-free survival | Median (range) | 0.10 (0.05, 0.23) | 0.40 (0.27, 0.59) | <0.001 |
Note: WBC, white blood cell; HGB, hemoglobin.
Figure 3.
(A) Overall survival by donor availability at first complete remission (all patients with 11q23). (B) Relapse-free survival by donor availability at first complete remission (all patients with 11q23). (C) Overall survival by donor availability [patients with t(9;11) only]. (D) Relapse-free survival by donor availability [patients with t(9;11) only]
DISCUSSION
The results of the current study of a large cohort of patients confirm that AML with t(9;11) is indeed a distinct entity with a better clinical outcome than AML with other 11q23 abnormalities. In multivariate analysis, t(9;11) was the only independent predictor of survival with the OS and EFS of these patients being comparable to those of patients with intermediate cytogenetic risk (HR 1.10 and 1.04, respectively; Table 2). These results are in agreement with those reported in previous studies of 47 adult patients with de novo AML by the Cancer and Leukemia Group B18 and of 180 adult patients with AML by the German AML Intergroup16 and in a more recent study by the Medical Research Council, which showed that the prognosis of these patients was better than that of patients with other balanced t(11q23).4 Our data conflict with another study of 54 adult patients with AML that demonstrated no significant difference in prognosis between cases with t(9;11) (n=19) and cases with other balanced t(11q23) (unfavorable prognosis).17 The reasons for the potential superior outcome of patients with t(9;11) compared to those with other MLL translocations are unknown and may be related to the differences of proteins encoded by MLL fusion genes derived from various translocations. Of note, t(9;11) is more commonly associated with t-AML/s-AML, but has the highest OS rate among 11q23 AML subgroups and non-11q23 AML reference group, this could be due to t(9;11) is less associated with del (5q)/-5, del (7q)/-7 and /or complex karyotype and also suggests that, at least in this population, t-AML/s-AML is a weak prognostic factor.24
In our study, patients with t(6;11), like those with t(9;11), presented at a younger age and with a higher percentage of bone marrow blasts than patients with non-11q23. However, t(6;11) was shown to be an independent poor prognostic feature in adult AML (Tables 2). Adverse outcomes of patients with t(6;11) have been reported in pediatric AML12 and adult AML by Cancer and Leukemia Group B15 and the German AML Intergroup.16 Given the dismal outcomes of these patients, clinical trials using novel strategies should be encouraged for this group.
Group t(11;19) had the lowest incidence of del5/5q-, del7/7q- and/or complex karyotypes (6%) among 11q23 subgroups and the non-11q23 reference group, and is not commonly associated with t-AML/s-AML. However, t(11;19) was also identified as an independent adverse prognostic feature in adult AML, a finding that is consistent with the results reported in the majority of other such studies16, 17, 25 but that conflicts with the findings of a recent study by the Medical Research Council.4
The various other unbalanced 11q23 aberrations were the second most common 11q23 abnormalities, and the majority of the cases in this group had del(11)(q23). This group was commonly associated with del5/5q-, del7/7q-, and/or complex karyotypes and a poor prognosis. Del(11)(q23) and inv 11q23 aberrations have been described involving the human MLL gene and several translocation partner genes have been identified for each of them.26–30 The prognostic significant of unbalanced 11q23 aberrations was first reported in a smaller study.31 In present study, we confirmed that the presence of unbalanced 11q23 aberrations is an independent poor prognostic factor. The subset of cases with other balanced translocations involving 11q23 was small, and the prognosis of this group was similar to that of the patients with unfavorable risk in the non-11q23 reference group.
In this study of allo-SCT for AML patients with 11q23 abnormalities, we demonstrated that all-SCT in CR1 results in sustained remission and long-term survival for approximately 40% of patients. Allo-SCT significantly improved OS and RFS compared with no allo-SCT. A majority of AML patients with 11q23 abnormalities who received consolidation with chemotherapy relapsed and died within a year and 5-year OS and RFS is 13% and 10% respectively. We also observe that there is a plateau in the OS and RFS curves, suggesting the allo-SCT could be a curative for these rare diseases. In multivariate analysis, we revealed a significant impact of allogeneic SCT in CR1 on OS (P=0.02) and RFS (P=0.01) in patients with 11q23 aberrations. In the last decade, the number of allo-SCT has increased significantly.32 In a recent study of 3638 patients with AML using meta-analysis, investigators revealed a significant survival benefit of allo-SCT from matched sibling donor for AML patients with unfavorable and intermediate cytogenetic risk groups.33 Two other studies showed that allo-SCT from HLA-matched unrelated donor and marched sibling donor yield similar clinical outcomes in patients with high risk AML.34, 35 Our results are in line with these studies, but contradict a recent report of 49 patients with AML and 11q23 abnormalities who received allo-SCT at CR1, in which allogeneic SCT had only borderline significant impact on OS.16
In summary, we found characteristic differences among patients with different 11q23 aberrations, which may suggest differences in etiology; we confirmed that in adult AML, t(9;11) is an intermediate risk factor, whereas, t(6;11), t(11;19), unbalanced 11q23 aberrations, and t(11;v)(q23;v) all had dismal outcomes; the presence of del5/5q-, del7/7q-, and/or complex karyotypes confers a poorer prognosis in patients with 11q23 AML. Most importantly, we revealed that allo-SCT provides significant OS and RFS benefits for patients with 11q23 AML in CR1. Innovative treatment strategies should be investigated in adults with these cytogenetic abnormalities.
Footnotes
Disclosure of Conflict of Interest: The Authors declare no competing financial interests.
REFERENCES
- 1.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European Leukemia Net. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 2.Lowenberg B. Diagnosis and prognosis in acute myeloid leukemia--the art of distinction. N Engl J Med. 2008;358(18):1960–1962. doi: 10.1056/NEJMe0802379. [DOI] [PubMed] [Google Scholar]
- 3.Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100(13):4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 4.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 5.Cox MC, Panetta P, Lo-Coco F, Del Poeta G, Venditti A, Maurillo L, et al. Chromosomal aberration of the 11q23 locus in acute leukemia and frequency of MLL gene translocation: results in 378 adult patients. Am J Clin Pathol. 2004;122(2):298–306. doi: 10.1309/RX27-R8GJ-QM33-0C22. [DOI] [PubMed] [Google Scholar]
- 6.Pui CH, Behm FG, Downing JR, Hancock ML, Shurtleff SA, Ribeiro RC, et al. 11q23/MLL rearrangement confers a poor prognosis in infants with acute lymphoblastic leukemia. J Clin Oncol. 1994;12(5):909–915. doi: 10.1200/JCO.1994.12.5.909. [DOI] [PubMed] [Google Scholar]
- 7.Satake N, Maseki N, Nishiyama M, Kobayashi H, Sakurai M, Inaba H, et al. Chromosome abnormalities and MLL rearrangements in acute myeloid leukemia of infants. Leukemia. 1999;13(7):1013–1017. doi: 10.1038/sj.leu.2401439. [DOI] [PubMed] [Google Scholar]
- 8.Muntean AG, Hess JL. The pathogenesis of mixed-lineage leukemia. Annu Rev Pathol. 2012;7:283–301. doi: 10.1146/annurev-pathol-011811-132434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer. 2007;7(11):823–833. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- 10.Balgobind BV, Zwaan CM, Pieters R, Van den Heuvel-Eibrink MM. The heterogeneity of pediatric MLL-rearranged acute myeloid leukemia. Leukemia. 2011;25(8):1239–1248. doi: 10.1038/leu.2011.90. [DOI] [PubMed] [Google Scholar]
- 11.Tamai H, Inokuchi K. 11q23/MLL acute leukemia : update of clinical aspects. J Clin Exp Hematop. 2010;50(2):91–98. doi: 10.3960/jslrt.50.91. [DOI] [PubMed] [Google Scholar]
- 12.Balgobind BV, Raimondi SC, Harbott J, Zimmermann M, Alonzo TA, Auvrignon A, et al. Novel prognostic subgroups in childhood 11q23/MLL-rearranged acute myeloid leukemia: results of an international retrospective study. Blood. 2009;114(12):2489–2496. doi: 10.1182/blood-2009-04-215152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coenen EA, Raimondi SC, Harbott J, Zimmermann M, Alonzo TA, Auvrignon A, et al. Prognostic significance of additional cytogenetic aberrations in 733 de novo pediatric 11q23/MLL-rearranged AML patients: results of an international study. Blood. 2011;117(26):7102–7111. doi: 10.1182/blood-2010-12-328302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 15.Blum W, Mrozek K, Ruppert AS, Carroll AJ, Rao KW, Pettenati MJ, et al. Adult de novo acute myeloid leukemia with t(6;11)(q27;q23) - Results from cancer and leukemia group B study 8461 and review of the literature. Cancer. 2004;101(6):1420–1427. doi: 10.1002/cncr.20489. [DOI] [PubMed] [Google Scholar]
- 16.Krauter J, Wagner K, Schafer I, Marschalek R, Meyer C, Heil G, et al. Prognostic Factors in Adult Patients up to 60 Years Old With Acute Myeloid Leukemia and Translocations of Chromosome Band 11q23: Individual Patient Data-Based Meta-Analysis of the German Acute Myeloid Leukemia Intergroup. Journal of Clinical Oncology. 2009;27(18):3000–3006. doi: 10.1200/JCO.2008.16.7981. [DOI] [PubMed] [Google Scholar]
- 17.Schoch C, Schnittger S, Klaus M, Kern W, Hiddemann W, Haferlach T. AML with 11q23/MLL abnormalities as defined by the WHO classification: incidence, partner chromosomes, FAB subtype, age distribution, and prognostic impact in an unselected series of 1897 cytogenetically analyzed AML cases. Blood. 2003;102(7):2395–2402. doi: 10.1182/blood-2003-02-0434. [DOI] [PubMed] [Google Scholar]
- 18.Mrozek K, Heinonen K, Lawrence D, Carroll AJ, Koduru PR, Rao KW, et al. Adult patients with de novo acute myeloid leukemia and t(9; 11)(p22; q23) have a superior outcome to patients with other translocations involving band 11q23: a cancer and leukemia group B study. Blood. 1997;90(11):4532–4538. [PubMed] [Google Scholar]
- 19.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Cortes J, O'Brien S, Kantarjian H, Cork A, Stass S, Freireich EJ, et al. Abnormalities in the long arm of chromosome 11 (11q) in patients with de novo and secondary acute myelogenous leukemias and myelodysplastic syndromes. Leukemia. 1994;8(12):2174–2178. [PubMed] [Google Scholar]
- 21.Balgobind BV, Van Vlierberghe P, van den Ouweland AM, Beverloo HB, Terlouw-Kromosoeto JN, van Wering ER, et al. Leukemia-associated NF1 inactivation in patients with pediatric T-ALL and AML lacking evidence for neurofibromatosis. Blood. 2008;111(8):4322–4328. doi: 10.1182/blood-2007-06-095075. [DOI] [PubMed] [Google Scholar]
- 22.Goemans BF, Zwaan CM, Miller M, Zimmermann M, Harlow A, Meshinchi S, et al. Mutations in KIT and RAS are frequent events in pediatric core-binding factor acute myeloid leukemia. Leukemia. 2005;19(9):1536–1542. doi: 10.1038/sj.leu.2403870. [DOI] [PubMed] [Google Scholar]
- 23.Hollink IH, Zwaan CM, Zimmermann M, Arentsen-Peters TC, Pieters R, Cloos J, et al. Favorable prognostic impact of NPM1 gene mutations in childhood acute myeloid leukemia, with emphasis on cytogenetically normal AML. Leukemia. 2009;23(2):262–270. doi: 10.1038/leu.2008.313. [DOI] [PubMed] [Google Scholar]
- 24.Ostgard LS, Kjeldsen E, Holm MS, Brown Pde N, Pedersen BB, Bendix K, et al. Reasons for treating secondary AML as de novo AML. Eur J Haematol. 2010;85(3):217–226. doi: 10.1111/j.1600-0609.2010.01464.x. [DOI] [PubMed] [Google Scholar]
- 25.Tamai H, Yamaguchi H, Hamaguchi H, Yagasaki F, Bessho M, Kobayashi T, et al. Clinical features of adult acute leukemia with 11q23 abnormalities in Japan: a co-operative multicenter study. Int J Hematol. 2008;87(2):195–202. doi: 10.1007/s12185-008-0034-2. [DOI] [PubMed] [Google Scholar]
- 26.Meyer C, Burmeister T, Strehl S, Schneider B, Hubert D, Zach O, et al. Spliced MLL fusions: a novel mechanism to generate functional chimeric MLL-MLLT1 transcripts in t(11;19)(23; p13.3) leukemia. Leukemia. 2007;21(3):588–590. doi: 10.1038/sj.leu.2404542. [DOI] [PubMed] [Google Scholar]
- 27.Meyer C, Kowarz E, Hofmann J, Renneville A, Zuna J, Trka J, et al. New insights to the MLL recombinome of acute leukemias. Leukemia. 2009;23(8):1490–1499. doi: 10.1038/leu.2009.33. [DOI] [PubMed] [Google Scholar]
- 28.Meyer C, Schneider B, Reichel M, Angermueller S, Strehl S, Schnittger S, et al. Diagnostic tool for the identification of MLL rearrangements including unknown partner genes. Proc Natl Acad Sci U S A. 2005;102(2):449–454. doi: 10.1073/pnas.0406994102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wechsler DS, Engstrom LD, Alexander BM, Motto DG, Roulston D. A novel chromosomal inversion at 11q23 in infant acute myeloid leukemia fuses MLL to CALM, a gene that encodes a clathrin assembly protein. Genes Chromosomes Cancer. 2003;36(1):26–36. doi: 10.1002/gcc.10136. [DOI] [PubMed] [Google Scholar]
- 30.Kourlas PJ, Strout MP, Becknell B, Veronese ML, Croce CM, Theil KS, et al. Identification of a gene at 11q23 encoding a guanine nucleotide exchange factor: Evidence for its fusion with MLL in acute myeloid leukemia. P Natl Acad Sci USA. 2000;97(5):2145–2150. doi: 10.1073/pnas.040569197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Archimbaud E, Charrin C, Magaud JP, Campos L, Thomas X, Fiere D, et al. Clinical and biological characteristics of adult de novo and secondary acute myeloid leukemia with balanced 11q23 chromosomal anomaly or MLL gene rearrangement compared to cases with unbalanced 11q23 anomaly: confirmation of the existence of different entities with 11q23 breakpoint. Leukemia. 1998;12(1):25–33. doi: 10.1038/sj.leu.2400853. [DOI] [PubMed] [Google Scholar]
- 32.Gupta V, Tallman MS, Weisdorf DJ. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: myths, controversies, and unknowns. Blood. 2011;117(8):2307–2318. doi: 10.1182/blood-2010-10-265603. [DOI] [PubMed] [Google Scholar]
- 33.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301(22):2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basara N, Schulze A, Wedding U, Mohren M, Gerhardt A, Junghanss C, et al. Early related or unrelated haematopoietic cell transplantation results in higher overall survival and leukaemia-free survival compared with conventional chemotherapy in high-risk acute myeloid leukaemia patients in first complete remission. Leukemia. 2009;23(4):635–640. doi: 10.1038/leu.2008.352. [DOI] [PubMed] [Google Scholar]
- 35.Gupta V, Tallman MS, He W, Logan BR, Copelan E, Gale RP, et al. Comparable survival after HLA-well-matched unrelated or matched sibling donor transplantation for acute myeloid leukemia in first remission with unfavorable cytogenetics at diagnosis. Blood. 2010;116(11):1839–1848. doi: 10.1182/blood-2010-04-278317. [DOI] [PMC free article] [PubMed] [Google Scholar]