Abstract
Objective
Resection cavity diameter of <40 mm is required to be eligible for stereotactic radiosurgery (SRS), following gross total resection of brain metastasis at our institution. Our study evaluates the correlation between vasogenic edema and change in cavity size over 30 days.
Methods
Cavity size was measured on the post-operative and follow-up MRIs. Vasogenic edema was quantified as the largest axial measurement of T2 hyperintensity surrounding the resection cavity (post-operative MRI).
Results
Thirty-nine resection cavities (37 patients) were reviewed. There was statistically significant (Pearson coefficient=-0.35, P=0.02) negative correlation between edema and change in cavity size. An arbitrary cut-off value of 15 mm edema yielded a sensitivity of 96% and specificity of 65% (P<0.001) to predict 10% decrease in cavity size.
Conclusions
In patients with cavity size close to the size cutoff for SRS, rescanning closer to the date of SRS should be considered, especially if there is significant edema surrounding the cavity.
Introduction
Whole brain radiotherapy (WBRT) is the standard of care following resection of a single brain metastasis1,2 but is often withheld in practice due to its impact on cognition3-5. In light of the estimated 170,000 to 200,000 new cases of metastatic brain disease annually1,6 and an aging population, it is clinically important to have alternatives to WBRT in selected candidates. Stereotactic radiosurgery (SRS) is frequently used after surgical resection to maximize local control7 and avoid the negative cognitive effects of whole brain radiation thus also improving the cost effectiveness of treatment8.
The size of the radiation port used for SRS varies in the literature and among institutions7,9. The eligibility size criteria to treat with SRS vary and depend on factors such as tumor type, location, experience of operator and the SRS delivery system10,11. Some reports use sizes such as 3-3.5 cm for intra- axial brain metastasis9, while others have treated vestibular schwannomas with tumor volumes up to 24 cubic cm12.
An on-going, IRB approved, clinical research protocol at our institution is comparing local control in patients randomized to SRS versus observation, following gross total resection (GTR) of brain metastasis. SRS is administered to the operative bed within 30 days, provided the cavity size is <40 mm in diameter. The prospective study is being performed to test an alternative treatment strategy to WBRT.
Current thinking is that cavity size remains stable; and the immediate post-operative MRI determines SRS eligibility. However, our own experience, and that of others, indicates that the cavity size in the early post-operative period is not constant13. Jarvis et al.13recently reported on the dynamic nature of the post-operative resection cavity, and resulting implications on the timing of subsequent stereotactic radiosurgery. Their review included cases with gross total tumor resection as well as some cases with radiographic evidence for residual. They found that while some resection cavities collapsed, others stayed constant or even increased in size. Factors that might help predict which resection cavities would be expected to decrease in size and by how much have not been evaluated.
In the current study, we assessed the correlation of T2 hyperintensity surrounding the postoperative resection cavity and the change in resection cavity diameter within 30 days.
Methods and Materials
Between October 2009 and August 2010, 45 patients with gross total resection (GTR) of brain metastasis had enrolled in the IRB-approved, prospective clinical trial at our institution. Thirty-seven of these 45 patients had follow up imaging data within 30 days of surgery and were included in our review. All others were excluded.
All MRI data were acquired on 1.5 or 3.0 T GE MRI scanners (Excite HD or HDxt MR scanners; GE Healthcare, Waukesha, WI) using 8-channel phased-array head coils. Diffusion weighted imaging (DWI), T2 weighted imaging (T2WI), T2 fluid attenuated inversion recovery (FLAIR), Gradient Echo (GRE), and pre-contrast T1 weighted imaging (T1WI) in the axial plane as well as multi-planar post-contrast T1WI were performed. (typical 1.5T MRI protocol is given in Supplemental Table number 1).
GTR of brain metastasis was confirmed and cavity size was measured in consensus by two board-certified neuroradiologists. Cavity size was measured on both the immediate postoperative MRI (within 24 hours) and on the follow-up/treatment planning study performed within 30 days of surgery. Cavity size was measured on the axial T2WI or post-contrast T1WI, depending on which optimally showed the cavity margins, using the largest axial diameter of the cavity. The maximum axial dimension of T2/FLAIR- hyper-intensity (vasogenic edema) surrounding the resection cavity on the immediate post-operative MRI was also recorded. Extent of edema on the follow up MRI was not evaluated, as frequently only a volumetric T1 post-contrast sequence was obtained for radiation planning.
Demographic information including sex, age, and primary tumor type were recorded. Statistical correlation was assessed using Pearson's correlation, unpaired T-test, and a post-hoc analysis using calculation for cut-off values of sensitivity and specificity (ROC analysis and Chi squared two tailed test; using S-PLUS 8.0 for Windows, Insightful Corporation Palo Alto, CA and Microsoft Excel 2007, Microsoft Corporation, Redmond, WA ).
Results
Among the 37 patients included in this study, two patients had had two lesions each resected, for a total of 39 resection cavities reviewed. Of the 37 patients, 22 were males and 15 were females. The median age was 54.5 years (range, 20-79 years). The patients had the following types of primary cancer metastatic to the brain: 10 malignant melanomas, 7 non-small cell lung, 6 genitourinary (renal cell and ovarian), 5 colorectal, 5 breast, 1 oral cavity, 1 esophageal, and 2 unknown primary cancer (see Supplemental Table 2 for patient demographics, tumor types, and corresponding cavity sizes).
One patient had a cavity <=10 mm, 12 cavities were 11-20 mm, 16 cavities were 21-30 mm, and 6 cavities were 31-40 mm. Four patients had cavity size >40 mm on the immediate postoperative MRI (and would not have qualified for SRS). The pericavitary vasogenic edema (extent of surrounding T2 signal) measured >30 mm in 6 patients, 21-30 mm in 18 patients, 11-20 mm in 5 patients, and 0-10 mm in 10 patients. No residual enhancing tumor was noted on any of the post-operative exams.
Of the 39 resection cavities evaluated, only 1 of 10 patients (10%) with vasogenic edema from 0-10 mm had a decrease of >10% in the cavity size, while 3 of 5 (60%) of patients with 10-20 mm edema and 19 of 24 (80%) with >20 mm edema had such decreases. The correlation between extent of pericavitary edema and change in cavity size was statistically significant (Pearson coefficient of -0.35, P=0.02). The edema (in mm) versus percent change in post-operative cavity size is shown in Figure 1. Representative cases of significant edema with decrease in cavity size (Figure 2), and minimal edema without a decrease in cavity size (Figure 3) are given. Receiver operating curve (ROC) analysis of sensitivity and specificity values demonstrated an area under the curve of 0.76 ± 0.076 (Figure 4). Using a post-hoc arbitrary cut off value of 15 mm (near ROC curve inflection point), 1 of 12 patients (8%) with vasogenic edema from 0-15 mm had a decrease of >10% in the cavity size, while 21 of 27 (78%) of patients with >15 mm edema had such decreases (P<0.001, chi-squared test; Table 1). Accordingly, using edema ≤15 mm to predict a decrease of > 10% in cavity size had an estimated sensitivity of 96% and an estimated specificity of 65%.
Figure 1. Extent of edema (in mm) versus percentage change in cavity size (immediate post-operative study versus follow-up imaging within 30 days).
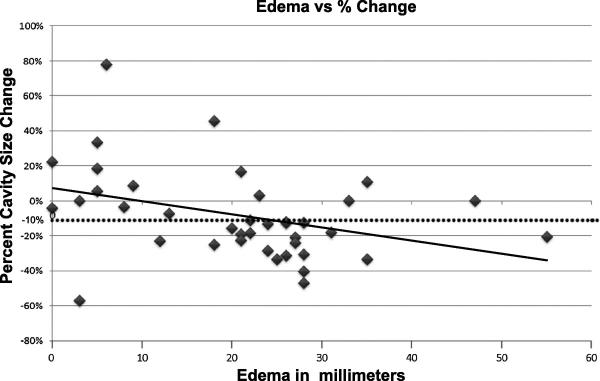
The solid line is an R2 fit (R2=0.15) showing the negative correlation. The dotted line denotes a 10% decrease in cavity size, which was used as the post-hoc cut-off value in Table 1 and Figure 4.
Figure 2. 61-year-old-male with adenocarcinoma of the oral tongue metastatic to the brain. A.
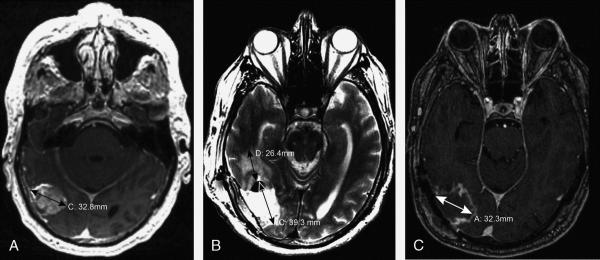
Axial post-contrast T1WI shows a 33-mm solid enhancing mass in the right temporo-occipital region. B. T2WI from the post-operative MRI obtained within 24 hours shows a resection cavity diameter of 39 mm. Extent of vasogenic edema (surrounding T2 abnormality) is 26 mm. C. Axial post-contrast T1WI obtained 17 days post-surgery shows the resection cavity diameter decreased by 18%, to 32 mm.
Figure 3. 41-year-old female with breast cancer metastatic to the brain A.
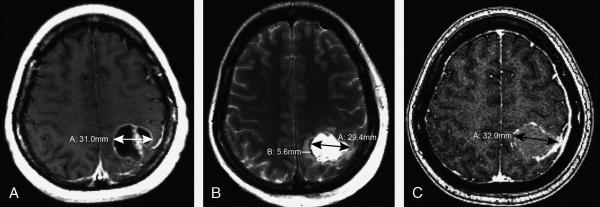
Axial post-contrast T1-weighted image shows a 31-mm enhancing, partially cystic, left parietal mass. B. Axial T2WI from the post-operative MRI obtained within 24 hours shows a 29-mm resection cavity. Minimal surrounding edema measures 6 mm. C. Axial post-contrast T1WI obtained 18 days post-surgery shows the resection cavity diameter increased to 32 mm.
Figure 4. Sensitivity and specificity relationship for the correlation between extent of edema and 10% decrease in cavity size by 30 days post-surgery.

The area under the receiver operator curve (ROC) is 0.76 ± 0.076. The inflection point corresponds to approximately 15 mm of edema with a sensitivity of 96% and specificity of 65%.
Table 1.
Comparison of edema to cavity size change for specific values. Chart demonstrates the number of cavities with or without 15 mm of edema, versus if the cavity diameter decreased by 10% from the post-operative exam to a follow-up study within 30 days. Both of these cut-off values were determined by post-hoc analysis.
| Edema | Cavity Size Decrease, ≥10% | <10% |
|---|---|---|
| ≥ 15 mm | 22 | 6 |
| < 15 mm | 1 | 11 |
Table 1 demonstrates the number of cavities with or without 15 mm of edema versus if the cavity diameter decreased by 10% from the post-operative examination to a follow-up study within 30 days. Both of these cutoff values were determined using the post hoc analysis.
Seven of the 39 surgical cavities did not follow the correlation for a cut-off of 15 mm of edema predicting a 10% change in size. Six of 39 surgical cavities had edema >15 mm on the immediate post-operative MRI, and failed to demonstrate a 10% decrease in cavity size within the 1-month follow up period.
Discussion
In our study, we found a significant correlation between extent of post-operative vasogenic edema and the subsequent cavity contraction. We found that 23 of 39 cavities decreased by >10% of their initial dimensions, and that vasogenic edema >15 mm was the determining factor for cavity involution in 22 out of 23 cases for which it occurred (Table 1). Four patients with cavity size > 40 mm, decreased to a size below the threshold on follow up imaging within 30 days, and would not have qualified for SRS had the immediate post-operative cavity size been used to determine eligibility. This finding is important as local control rates achieved by stereotactic radiosurgery to the operative bed compare favorably with the results obtained by postoperative WBRT, and avoid the neurotoxicity associated with WBRT14. SRS is typically delivered within 4 weeks after surgery, but the optimal time after resection for radiosurgery to the resection cavity is not known. In cases where gross tumor is left behind, the risk of progression must be balanced with allowing for the acute surgical changes to resolve.
Edema surrounding a resection cavity is a result of the inflammatory reaction of the brain in response to the metastasis and surgery15. Unlike primary brain tumors, metastatic brain tumors tend to displace and destroy brain structure rather than infiltrate the parenchyma16. Therefore, metastases are usually confined to the area of T1 contrast-enhancement, which is removed during GTR. In cases with gross residual tumor following resection (not applicable to our study), mass effect and persistent edema from tumor progression can contrinute to collapse of the resection cavity13.
The surgical cavity may fail to contract (or enlarge) due to an increase in mass effect within the cavity itself, which can be caused by development of a hematoma, abscess, or by communication with CSF pulsation with a one-way valve mechanism. Resolution of an adjacent extra-axial collection or hematoma can also result in expansion of the surgical cavity. Of the 6 patients with edema >15 mm that failed to show decrease is cavity of greater than 10%, we noted that 3 of these 6 patients had resection cavities that were peripherally located with large direct communication with the extra-axial space. Two of these 6 patients had post-operative collections adjacent to the cavity which resolved on the follow up study, One of the 6 patients with edema >15 mm and <10% decrease in cavity size had the follow up MRI performed at 11 days post-surgery. This patient also had a subsequent study performed at 45 days post-surgery which demonstrated a 27% decrease in cavity size from the initial post-operative MRI.
With emerging studies supporting the role of SRS to post-operative resection cavities and with the ongoing neurotoxicity associated with WBRT, the number of patients receiving SRS is expected to grow. Changes in cavity size are important clinically because they represent a shifting target for SRS. Such change underscores the importance of concordant imaging with SRS planning as the cavity size is dynamic, especially in the early post-operative period. As part of the SRS planning team at our institution the radiologist works closely with the neurosurgeons and radiation oncologists to 1) determine extent of resection, 2) identify additional lesions that were not approached surgically, and 3) determine eligibility for SRS to the resection cavity.
In summary, if SRS is being considered in the immediate post-operative period for a patient with a cavity size close to the size cutoff for the procedure, rescanning closer to the planned date of SRS should be considered, especially if there is significant edema surrounding the resection cavity. This change in protocol would be anticipated to increase the number of patients considered eligible for SRS.
A larger sample size or multicenter study conducted prospectively with evaluation of other factors also contributing to changes in cavity size would be useful in validating these findings, and in determining an optimal window to administer SRS.
Supplementary Material
References
- 1.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–9. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 2.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 3.Aoyama H, Tago M, Kato N, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007;68:1388–95. doi: 10.1016/j.ijrobp.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 4.DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789–96. doi: 10.1212/wnl.39.6.789. [DOI] [PubMed] [Google Scholar]
- 5.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–44. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 6.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–91. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 7.Hartford AC, Paravati AJ, Spire WJ, et al. Postoperative Stereotactic Radiosurgery Without Whole-Brain Radiation Therapy for Brain Metastases: Potential Role of Preoperative Tumor Size. Int J Radiat Oncol. 2013;85:650–5. doi: 10.1016/j.ijrobp.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 8.Lal LS, Byfield SD, Chang EL, et al. Cost-effectiveness analysis of a randomized study comparing radiosurgery with radiosurgery and whole brain radiation therapy in patients with 1 to 3 brain metastases. Am J Clin Oncol. 2012;35:45–50. doi: 10.1097/COC.0b013e3182005a8f. [DOI] [PubMed] [Google Scholar]
- 9.Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:45–68. doi: 10.1007/s11060-009-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith ML, Lee JY. Stereotactic radiosurgery in the management of brain metastasis. Neurosurg Focus. 2007;22:E5. doi: 10.3171/foc.2007.22.3.6. [DOI] [PubMed] [Google Scholar]
- 11.Tsao MN, Lloyd NS, Wong RK, Rakovitch E, Chow E, Laperriere N. Radiotherapeutic management of brain metastases: a systematic review and meta-analysis. Cancer Treat Rev. 2005;31:256–73. doi: 10.1016/j.ctrv.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Williams BJ, Xu Z, Salvetti DJ, McNeill IT, Larner J, Sheehan JP. Gamma Knife surgery for large vestibular schwannomas: a single-center retrospective case-matched comparison assessing the effect of lesion size. J Neurosurg. 2013;119:463–71. doi: 10.3171/2013.4.JNS122195. [DOI] [PubMed] [Google Scholar]
- 13.Jarvis LA, Simmons NE, Bellerive M, et al. Tumor bed dynamics after surgical resection of brain metastases: implications for postoperative radiosurgery. Int J Radiat Oncol Biol Phys. 2012;84:943–8. doi: 10.1016/j.ijrobp.2012.01.067. [DOI] [PubMed] [Google Scholar]
- 14.Jagannathan J, Yen CP, Ray DK, et al. Gamma Knife radiosurgery to the surgical cavity following resection of brain metastases. J Neurosurg. 2009;111:431–8. doi: 10.3171/2008.11.JNS08818. [DOI] [PubMed] [Google Scholar]
- 15.Wick W, Kuker W. Brain edema in neurooncology: radiological assessment and management. Onkologie. 2004;27:261–6. doi: 10.1159/000077976. [DOI] [PubMed] [Google Scholar]
- 16.Lu S, Ahn D, Johnson G, Cha S. Peritumoral diffusion tensor imaging of high-grade gliomas and metastatic brain tumors. AJNR Am J Neuroradiol. 2003;24:937–41. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


