Structured Abstract
Objective
To determine if a hospital’s ability to rescue patients from major complications underlies variation in outcomes for elderly patients undergoing emergency surgery.
Summary Background Data
Perioperative mortality rates in elderly patients undergoing emergent general/vascular operations are high and vary widely across Michigan hospitals.
Methods
We identified 23,224 patients undergoing emergent general/vascular surgery procedures at 41 hospitals within the Michigan Surgical Quality Collaborative (MSQC) between 2006–2011. Hospitals were ranked by risk- and reliability-adjusted 30-day mortality and grouped into tertiles. We stratified patients by age (<75 and ≥75). Risk-adjusted major complication and failure to rescue (i.e., mortality following major complication) rates were determined for each tertile of hospital mortality.
Results
Risk-adjusted mortality rates in elderly patients varied 2-fold across all hospitals. Complication rates correlated poorly with mortality. Failure-to-rescue rates, however, were markedly higher in high mortality hospitals (29% lowest tertile vs. 41% highest tertile, p<0.01). When compared to younger patients, overall failure to rescue rates were almost 2-fold greater in the elderly (36.1% ≥75 vs. 18.7% <75, p<0.01).
Conclusions
Hospitals’ failure to rescue patients from major complications seems to underlie the variation in mortality across Michigan hospitals following emergent surgery. While higher failure to rescue rates in the elderly may signify their diminished physiological reserve for surviving critical illness, the wide variation across hospitals also highlights the importance of systems aimed at the early recognition and effective management of major complications in this vulnerable population.
Introduction
Surgical mortality increases exponentially with age.[1] Conservative estimates show nearly one third of elderly Americans undergo major inpatient surgery within the last year of their life.[2] With the United States Census Bureau projecting that the percentage of the nation’s population over 65 will increase from 12% in 2010 to over 20% by 2030, this may pose a significant public health crisis.[3] The consequences for hospitals remain unknown, as they prepare for patients who tend to experience higher rates of perioperative morbidity and mortality.[4–6] Potentially the most vulnerable subset of this patient population is those undergoing emergent surgery. Recent evidence demonstrates nearly ten-fold higher mortality rates in the elderly undergoing major emergency surgery when compared to younger patients.[7] Given these concerns, the American College of Surgeons and American Geriatric Society have jointly attempted to mitigate perioperative risk by forming “best practices” for the preoperative assessment and optimization of elderly patients.[8]
However, despite these efforts, it remains unclear how best to reduce surgical mortality in elderly patients. The relative importance of complication prevention versus complication management is not well defined for elderly patients and may be particularly relevant in the emergent setting. Some posit that the elderly’s decreased physiologic reserve underlies patient-level differences in morbidity and mortality.[9, 10] However, others have shown that there is significant hospital variation in outcomes following emergency general surgery in the elderly, thus pointing to differences in the structure and systems of care.[11] Recent efforts to explain hospital-level differences in mortality have focused on the hospital’s ability to respond to major complications (i.e. failure to rescue). [12–14]
The importance of this observation is unknown in the emergent surgical setting where geriatric postoperative management represents an important target for quality improvement. In this context, we used data from the Michigan Surgical Quality Collaborative to examine hospital variation in morbidity, mortality, and failure to rescue after emergency surgery. We then focused on the magnitude of variation in outcomes between the elderly and younger patients. We hypothesize that failure to rescue is a fundamental driver of the inferior outcomes appreciated in the elderly undergoing emergency surgery.
Methods
Data Source and Study Population
We studied data from the Michigan Surgical Quality Collaborative (MSQC) prospective clinical registry from 2006 through 2011. The MSQC represents a partnership between two entities-Blue Cross and Blue Shield of Michigan and 52 Michigan hospitals. This project followed standard data definitions and collection protocols as we have previously described [15, 16]. In brief, data collection occurs at the hospital level by specific MSQC data-collection nurses. Accuracy of data collection and maintenance is ensured by rigorous training of staff and data audits performed at participating sites. All available variables were collected for this analysis including patient demographics, preoperative risk factors, laboratory values, perioperative factors, and 30-day postoperative morbidity and mortality.
Outcomes
The primary outcomes for this study were 30-day in-hospital mortality, major complication, and failure to rescue. We determined various in-hospital postoperative complications such as surgical site infection (superficial, deep, and organ space defined separately), deep venous thrombosis, urinary tract infection, acute renal failure, postoperative bleeding requiring transfusion, stroke, unplanned intubation, fascial dehiscence, prolonged mechanical ventilation over 48 hours, myocardial infarction, pneumonia, pulmonary embolism, sepsis, vascular graft loss, and renal insufficiency. For the purposes of failure to rescue analysis, we excluded urinary tract infection, deep venous thrombosis, renal insufficiency, and superficial surgical site infection from our list of major complications as has been previously described [12]. We defined failure to rescue as death following at least one major complication.
Statistical Analysis
We compared demographic, comorbidity, and operative differences between patients younger than 75 years and patients 75 years of age and older with Student’s t-test, Chi-squared and Fisher’s exact test as appropriate. In order to rank hospitals based on performance, we applied risk-adjusted 30-day mortality as the primary exposure variable. Risk-adjustment models were developed using backward stepwise logistic regression that included variables such as patient age, sex, race, BMI, diabetes, smoking status, alcohol use, dyspnea, do-not-resuscitate (DNR) status, preoperative functional status, chronic obstructive pulmonary disease (COPD), pneumonia, ascites, congestive heart failure, need for dialysis, hemiplegia, transient ischemic attack (TIA), disseminated cancer, steroid use, bleeding disorders, chemotherapy, radiotherapy, sepsis, esophageal varices, prior myocardial infarction, angina, hypertension requiring medication, peripheral vascular disease, prior operations, American Society of Anesthesiologists (ASA) class, operative duration (minutes), surgeon specialty, work Relative Value Units (RVU), and the need for intraoperative transfusion.
The final model included 19 variables with a C statistic of 0.913. Hospital mortality rates were then adjusted for reliability using hierarchical modeling and empirical Bayes techniques.[17] This was done in order to minimize the effects of random variation on observed differences between hospitals. This type of variation observed in surgical outcomes is largely attributable to small sample size (i.e. hospitals with small caseloads).[18] Risk- and reliability-adjusted hospital rankings were used for subsequent analyses.
We grouped hospitals into tertiles based on reliability-adjusted mortality rates. We then evaluated rates of major complications and failure to rescue (i.e. mortality after a major complication) for each tertile of mortality. Results were stratified by patient age (<75 vs. ≥75) in order to compare rates of mortality, major complications, and failure to rescue across different ages at the same hospitals.
Hospitals were then ranked by risk- and reliability-adjusted 30-day mortality for young and elderly patients separately. Hospital rankings for the different age groups were then plotted against each other to assess the correlation between performance within each age strata. We employed Cohen’s kappa coefficient to evaluate agreement between the age-specific hospital rankings. The statistical model retained predictive value and good discriminatory function in patients <75 (C statistic= 0.901) and ≥75 (C statistic= 0.790).
All statistical analyses were performed using Stata statistical software version12.1 (College Station, Texas). This study was approved by the University of Michigan Institutional Review Board.
Results
Study Population
We identified 23,224 patients who underwent emergency surgery at an MSQC member hospital between 2006 and 2011 requiring inpatient hospitalization. The patient population was subsequently divided in to “young” patients <75 years old (n=18,901, 81.2%) and “elderly” patients ≥75 years old (n=4,323, 18.8%). (Table 1) The median age of young patients was 49.2 years, while that of elderly patients was 81.9 years. Elderly patients possessed greater comorbid disease burden. (Table 1) Further, the elderly were more likely to be of higher ASA class at the time of emergency operation (41.8% vs. 14.8% ASA ≥4, p<0.01). Elderly patients also underwent more extensive operations (median work RVU= 19.3) when compared to younger patients (median work RVU= 10.9, p<0.01).
Table 1.
Patient demographics and operative characteristics by age.
| Characteristic | Age <75 (n=18,901) |
Age ≥75 (n=4,323) |
|---|---|---|
| Descriptives | ||
| Median age (years) | 49.2 | 81.9 |
| Male sex (%) | 49.9 | 40.9 |
| Non-white race (%) | 30.2 | 20.8 |
| Mean BMI (Kg/m2) | 29.1 | 26.3 |
| Clinical (%) | ||
| Diabetes mellitus | 13.2 | 20.4 |
| Smoking in past year | 33.3 | 9.4 |
| DNR | 0.49 | 5.5 |
| Chronic obstructive pulmonary disease | 6.1 | 15.4 |
| Dialysis | 2.9 | 3.8 |
| Disseminated cancer | 1.8 | 3.1 |
| Steroid use | 3.6 | 6.1 |
| Sepsis | 28.0 | 29.8 |
| Albumin <3.5 g/dl | 23.1 | 48.3 |
| Independent functional status | 88.6 | 66.2 |
| Hypertension requiring medication | 36.2 | 78.3 |
| Acute renal failure | 1.9 | 3.8 |
| Perioperative | ||
| ASA class ≥ 4 (%) | 14.8 | 41.8 |
| Mean work RVU | 14.3 | 19.1 |
| Median operative duration (minutes) | 57 | 77 |
Morbidity and Mortality Following Emergency Surgery
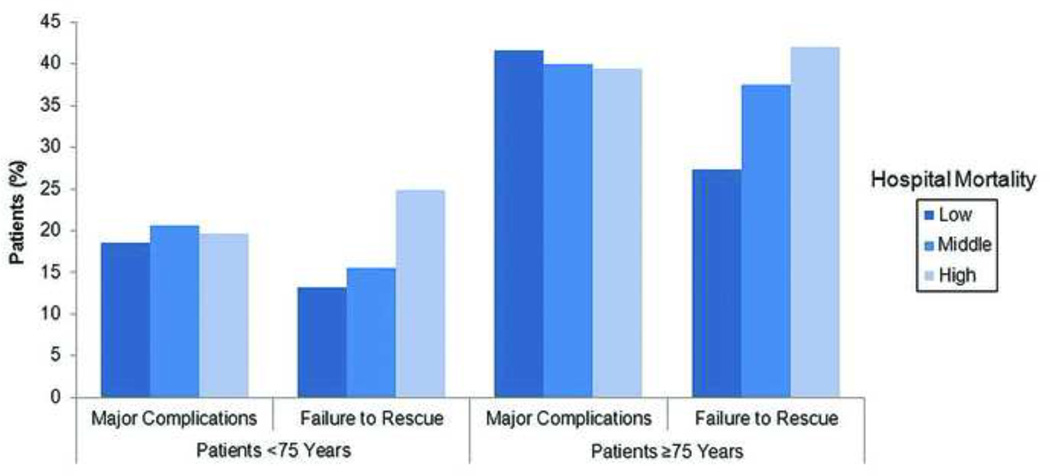
After adjusting for patient clinical factors, operative case mix, and hospital volume, overall 30-day mortality at low, middle, and high mortality hospitals was 6.4%, 7.4%, and 8.9% respectively. The rate of major complications were not significantly different across tertiles of hospital mortality (low= 25.6%, middle= 21.8%, high= 25.9%; p=0.35 low vs. high). Patients were subsequently stratified by age. Mortality for young patients at low, middle, and high mortality hospitals was 4.1%, 4.6%, and 5.4% respectively (p<0.01 low vs. high). Elderly patients experienced higher and more variable mortality ranging from 17.5%, 19.6%, and 22.8% at low, middle, and high mortality centers (p<0.01 low vs. high). The rates of major complications again did not correlate with hospital mortality for young (low= 18.5%, middle= 20.6%, high= 19.7%; p=0.63 low vs. high) or elderly (low= 41.6%, middle= 40.0%, high= 39.4%; p=0.66 low vs. high), though rates were substantially higher in the elderly.
Failure to Rescue
In order to investigate the relationship between major complications and mortality at the hospital level, failure to rescue rates were calculated for each tertile of hospital mortality within each age strata. (Figure 1) While the rates of major complications did not correlate with hospital mortality, failure to rescue rates were higher in high mortality hospitals compared to low mortality hospitals for both young (24.9% vs. 13.2%, p<0.01) and elderly (42.1% vs. 27.4%, p<0.01) patients. Further, at the patient level, failure to rescue rates in the elderly were nearly two-fold higher when compared to younger patients within each tertile of hospital mortality. A subset analysis excluding all DNR patients was also conducted. The differences in failure to rescue rates between high and low mortality hospitals remained for both young (21.0% vs. 16.4%, p<0.01) and elderly (39.0% vs. 27.9%, p<0.01) patients.
Figure 1.
Major complications and failure to rescue rates for young (<75 years) and elderly (≥75 years) patients across hospitals ranked by overall mortality. Major complication rates are not significantly different between tertiles of hospital mortality. Failure to rescue correlates with mortality and is significantly higher in elderly patients.
Evaluating Hospital Performance Across Age Groups
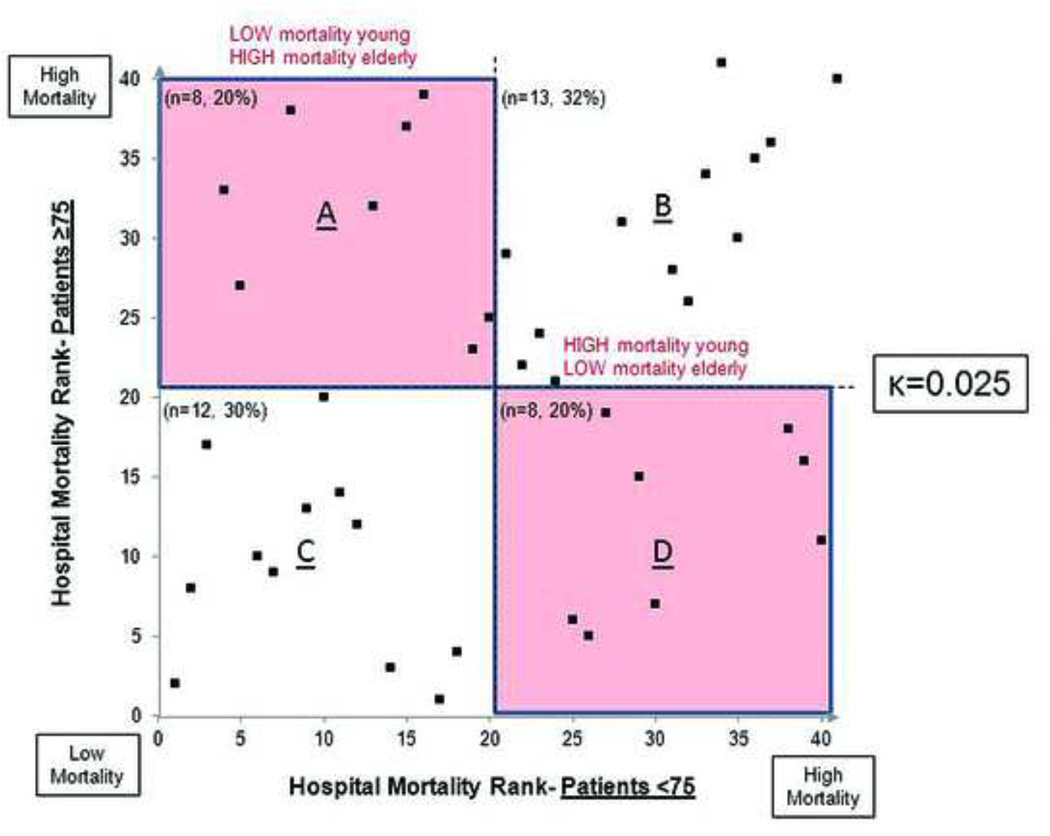
There was evidence of discordance in hospital performance between the two age groups (κ=0.025). (Figure 2) A total of 16 (40%) hospitals differed widely in performance between the two age groups (i.e. low mortality in young and high mortality in elderly or vice versa). (Boxes B and D, Figure 2) When using the age-specific rankings, there was only 30% agreement between those centers included in the high mortality tertiles for young versus elderly patients.
Figure 2.
Relationship between hospital performance in young patient versus elderly patients. There are 8 (20%) hospitals that exhibit good performance (low mortality) in younger patients but poor performance in the elderly. Similarly, there are 8 (20%) hospitals with poor performance in young patients, but good performance in the elderly.
Discussion
This study enhances the limited body of literature detailing the importance of failure to rescue in evaluating hospital quality for emergency surgery. It further highlights that elderly patients are uniquely vulnerable to poor outcomes following emergent procedures. Mortality rates for these patients are significantly higher and more variable across hospitals when compared to younger patients. This observation is not correlated with morbidity, as major complication rates did not differ across tertiles of hospital mortality in either age group, though absolute complication rates were markedly higher in the elderly. Rather, death after major complication, or failure to rescue, is a major driver of mortality differences between hospitals. An age disparity persists as failure to rescue rates in elderly patients are significantly higher than younger patients after emergency surgery. Finally, hospital mortality in young patients is poorly correlated with mortality in the elderly, indicating variability in appropriate management of patients across different ages. Our findings are consistent with the published literature, and highlight disproportionately poor outcomes in the elderly compared to younger emergent surgical patients independent of comorbid disease burden [1, 11]. Further study by Ingraham et al. described substantial hospital variation in the care of young and elderly emergent general surgical patients, with elderly patients experiencing nearly three-fold higher postoperative mortality.[11] Recent work has attempted to address questions brought forth by these observational studies regarding actionable avenues for quality improvement. Current practice measures effectively mitigate the occurrence of cardiovascular complications in elderly surgical patients but may not adequately address pulmonary and renal events [19]. Despite this, relatively little work has been done to promote attention to complication rescue in addition to prevention.
This work illuminates several critical issues regarding surgical care in the elderly. A large proportion of emergency surgery cases are performed in patients over 75 years of age. This is likely to increase as the baby boom generation ages.[3] Implications of this surge will be appreciated by providers at the point of care and by policymakers nationally. First, for surgeons, it is important that care teams establish practice measures specifically for complication rescue. In the elderly emergent surgical patient this requires prompt identification of major complications and appropriate management thereafter. It is likely that the elderly patients possess diminished physiologic reserve and are more likely to exhibit the frailty phenotype attributed to poor surgical outcomes [9]. Within this context, early recognition and management (rescue) may be particularly high-yield quality improvement targets. For hospitals, it is unclear which factors related to complication rescue are amenable to regional quality improvement initiatives.[20] More importantly, the coordination of care, tailored to geriatric patients, may be more important that the mere availability of advanced resources.[21] Collaborative efforts between surgeons, hospital administrators, and payers will be necessary for program implementation within our State.
There are several limitations to this study. First, this is a retrospective cohort study performed on existing data. The main purpose of the prospectively collected, audited MSQC dataset is identification of quality improvement initiatives across Michigan hospitals. As such, while we are unable to establish causality between the clinical factors collected in the dataset and postoperative events, our findings demonstrate important associations for future research. Second, the MSQC dataset does not collect information on all possible postoperative complications, despite being comprehensive in the nature and severity of events recorded. Thus, we could be missing other complications in our analysis, but our failure to rescue analysis is limited to patients with a recorded postoperative complication. Finally, the data collected are patient and operation level variables. There are relatively few hospital-level factors available for application in statistical analysis. Despite these limitations, attempts were made to correct for case mix and procedural volume that undoubtedly influence surgical outcomes. Understanding failure to rescue necessarily requires the evaluation of hospital-level variables, though this is beyond the scope of the current study and represents an active field of current investigation. Hospital variation in mortality after emergent general and vascular surgery is explained in part by failure to rescue. More importantly, the elderly are substantially harder to rescue once a major complication has occurred and high performance in younger patients does not translate into better rescue rates in the elderly. Hospitals and surgical teams must develop age-specific care practices for emergency surgical patients that address complication prevention in addition to rescue. As surgeons continue to operate on older, sicker patients, practice measures that address complication rescue will prove important across hospitals.
Appendix
DISCUSSANT
DR. L.D. BRITT (Norfolk, VA): Let me first thank the authors for asking me to review their manuscript and commend them for contributing this important work.
With an aging population and the fact that it takes twice the time and effort for a general surgeon to take care of an elderly patient, any emphasis on improving mortality in this vulnerable special population in the emergency setting is noteworthy. Using data from the Michigan Surgical Quality Collaborative that has a 41-hospital consortium, the authors found that hospitals with overall higher mortality rates for this cohort of patients also had higher failure to rescue rates—the inability to recover from post-operative complications and concluded that the variation in mortality across Michigan hospitals after emergency surgery was, in part, the hospital's failure to rescue patients from major complications.
This retrospective cohort study supports the hypothesis that failure to rescue is a fundamental driver of inferior outcomes seen in elderly patients. However, this study fails to examine hospital resources, protocols, quality improvement initiatives, or other indices that might be in place at the various institutions. I would like the authors to fully address this criticism. In addition, the authors failed to adequately present the makeup of the hospitals with respect to important variables such as not having a surgeon or anesthesiologist immediately available or the ready availability of blood products. In the population analysis, there was no control for chronic medical conditions, pre-existing functional status, or socio-economic class. Because all these factors would impact patient outcomes across hospitals (especially the elderly population), I would like the authors to address this noticeable oversight.
Perhaps, a more informative investigation would be an analysis of specific system-based approaches in the more successful hospitals. In a prospective fashion, one could take those quality improvements and protocols and implement them at a low-performing hospital and determine if such practice improvement initiatives actually affect patient outcomes. Specifically, would it lower the failure to rescue rate in this special population?
CLOSING DISCUSSANT
KYLE H. SHEETZ: We would first like to thank Dr. Britt for agreeing to serve as our primary discussant for this paper.
You raised several important points. First, given the constraints of the data we were working with, we were unable to assess the impact of specific hospital resources, protocols, or other quality improvement initiatives on rescue rates. Second, we did adjust for patient comorbidities and hospital procedure mix in determining the risk- and reliability-adjusted mortality rates. Finally, we appreciate your interest in the future steps of this work. We do in fact have an excellent Collaborative in Michigan where we can learn, pilot, and implement best practices across hospitals to better care for elderly patients undergoing emergency surgery.
DISCUSSANT
DR. RICHARD J. MULLINS (Portland, OR): Forty years from now, when you are a senior surgeon, you may find that one of your primary responsibilities when doing emergency surgery on an elder patient is providing them, or often their family, a prognosis. You need to give them your expectation how the operation will turn out. There are many elder patients who are fearful of being rescued. They do not want the aggressive care and prolong treatment in a University ICU, which is distant from their home. They want to stay close to their family members. If their time has come to die, they are willing to accept that. They do not want tube feedings. They do not want prolonged mechanical ventilation.
I have two questions. How can you justify a 30-day mortality rate as a measure of outcome, when, in fact, elders continue to die postoperatively over 90 days or 180 days? In other words what message are your observations sending: "Make sure they survive 30 days and get the patient discharged from the hospital, so my rescue numbers look good."? Is it a rescue to discharge a severely disabled patient to a nursing facility where they soon die?
Another way to ask that question is the following: Which is a better hospital; a hospital where the length of stay for those who die, the decedents, is 30 days or the hospital where the length of stay before death is 10 days?
CLOSING DISCUSSANT
KYLE H. SHEETZ: We acknowledge that 30 day outcomes are somewhat limited in their scope. Clearly, 90 day or one year outcomes could be more informative particularly given that many of these patients are discharged to sub-acute rehabilitation or other secondary care facilities where they eventually may die. We acknowledge this limitation and examine it further, perhaps in the Medicare population.
Regarding your question about length of stay, we have actually started to look at this question in more depth. There are several factors that may influence this beyond patient factors, including resource utilization, surgeon preferences, and the availability of nursing homes or other secondary care facilities. Right now, we do not have all of this information, but it is something we are looking into further.
DISCUSSANT
DR. KEITH D. LILLEMOE (Boston, MA): I would like to thank you and your colleagues in the Michigan Collaborative for again bringing to this audience, as well as to the surgical community, the results of the fine work that you are doing with your state-wide organization.
Having worked at three tertiary care hospitals, not in Michigan, I recognize that the concept of rescue for many smaller hospitals is transferring the patient to a tertiary care hospital. You talked about high mortality and low mortality hospitals, but you gave no information related to whether, as Dr. Britt asked, where do tertiary care hospitals with great ICUs and acute care surgery services fall with respect to your mortality categorization?
Secondly, how did you handle your mortality statistics with respect to patients transferred from smaller hospitals to the University of Michigan or other academic medical centers to provide complex tertiary care?
Finally, a bigger ethical question that perhaps should be directed to one of the more senior members of your group, is how does a hospital that is interested in preserving their reputation, their overall mortality statistics, and their reportable outcomes, deal with the fact that we, on an almost daily basis, accept transfer patients who are told that the only chance of saving them is to be transferred to the MGH or the University of Michigan or Indiana University, or any other AMC, full well recognizing the chances for survival are extraordinarily low and that they will likely end up showing on our mortality statistics and making us appear to be a higher mortality hospital? Should we just say no?
CLOSING DISCUSSANT
KYLE H. SHEETZ: We did not explicitly look at hospital resources as they pertained to emergency surgical care within the hospitals included in this analysis. We have some of this data available, and in general, there are no gross differences in overall hospital size, ICU bed numbers, or level-1 trauma status between high and low mortality hospitals. We believe that how resources are structured and implemented is most relevant to rescue. Unfortunately, this is difficult to study with our clinical registry data. We did account for transfer status for this analysis data, though we acknowledge that this does not fully account for patient acuity on presentation or transfer, it is a strong predictor of patient outcome. This is taken into account when determining the risk-adjusted mortality rates of hospitals. As such, the quaternary institutions to which you referred, may not be as adversely affected by a death that occurs post transfer as one may suspect.
DISCUSSANT
DR. WILLIAM B. INABNET (New York, NY): I would like to expand upon Dr. Britt's comments about the nature of the hospitals with better outcomes.
Many hospitals around the country are starting to develop geriatric emergency rooms where they have a dedicated section of the emergency room that has been earmarked specifically for geriatric patients. Institutions are also implementing palliative care services, where geriatricians work with the primary team to care for the critically ill patient when end-of-life decisions need to be made.
Can you comment on the presence of either of those two programs in your low mortality hospitals? In other words, did the presence of those services in any way influence your results?
CLOSING DISCUSSANT
KYLE H. SHEETZ: Unfortunately, we did not explicitly look at any hospital based resources. The presence of geriatric emergency rooms or geriatric specialists within the emergency setting is not readily available in our registry data. This is fertile ground for future work. We need to understand what hospitals do particularly well, or poorly, in caring for elderly patients.
End-of-life care is an important consideration for this project. While we accounted for preoperative DNR status in our primary analysis, we also conducted a sub-set analysis of our data excluding all of these patients and found identical results. Unfortunately, we are unable to glean from the data which patients had care withdrawn at any given point during their hospitalization.
CLOSING DISCUSSANT
DR. AMIR A. GHAFERI: I also want to echo Dr. Britt's comments and commend Kyle on presenting this paper very nicely and handling the audience's questions with poise. I would like to make a closing remark to address the overall theme of the questioning I am hearing from the audience. Your questions are all very insightful and I am excited to hear the enthusiasm for this work and its potential future steps. We are in the midst of an NIH funded study evaluating failure to rescue amongst the elderly in the State of Michigan. Our study focuses on acquiring data about hospital resources, safety attitudes, and safety behaviors that may affect failure to rescue rates. I am confident that the results of this study will inform future implementation projects aimed at improving rescue rates across hospitals in an effective and cost-efficient manner.
References
- 1.Turrentine FE, Wang H, Simpson VB, et al. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg. 2006;203:865–877. doi: 10.1016/j.jamcollsurg.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 2.Kwok AC, Semel ME, Lipsitz SR, et al. The intensity and variation of surgical care at the end of life: a retrospective cohort study. Lancet. 2011;378:1408–1413. doi: 10.1016/S0140-6736(11)61268-3. [DOI] [PubMed] [Google Scholar]
- 3.Hobbs FaNS. U.S. Administration on Aging; 2008. U.S. Census Bureau, Census 2000 Special Reports, Series CENSR-4, Demographic Trends in the 20th Century. [Google Scholar]
- 4.Hamel MB, Henderson WG, Khuri SF, et al. Surgical outcomes for patients aged 80 and older: morbidity and mortality from major noncardiac surgery. J Am Geriatrics Soc. 2005;53:424–429. doi: 10.1111/j.1532-5415.2005.53159.x. [DOI] [PubMed] [Google Scholar]
- 5.Finlayson E, Fan Z, Birkmeyer J. Outcomes in octogenarians undergoing high-risk cancer operation: a national study. J Am Coll Surg. 2007;205:729–734. doi: 10.1016/j.jamcollsurg.2007.06.307. [DOI] [PubMed] [Google Scholar]
- 6.Finlayson EV, Birkmeyer JD. Operative mortality with elective surgery in older adults. Eff Clin Pract. 2001;4:172–177. [PubMed] [Google Scholar]
- 7.Lidsky ME, Thacker JK, Lagoo-Deenadayalan SA, et al. Advanced age is an independent predictor for increased morbidity and mortality after emergent surgery for diverticulitis. Surgery. 2012;152:465–472. doi: 10.1016/j.surg.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 8.Chow BK, Rosenthal C, Esnaola RN. ACS-NSQIP/AGS Best Practice Guidelines: Optimal Preoperative Assessment of the Geriatric Surgical Patient. 2012 doi: 10.1016/j.jamcollsurg.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Englesbe MJ, Lee JS, He K, et al. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg. 2012;256:255–261. doi: 10.1097/SLA.0b013e31826028b1. [DOI] [PubMed] [Google Scholar]
- 11.Ingraham AM, Cohen ME, Raval MV, et al. Variation in quality of care after emergency general surgery procedures in the elderly. J Am Coll Surg. 2011;212:1039–1048. doi: 10.1016/j.jamcollsurg.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in Hospital Mortality Associated with Inpatient Surgery. New Eng J Med. 2009;361:1368–1375. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 13.Ghaferi AA, Birkmeyer JD, Dimick JB . Hospital volume and failure to rescue with high-risk surgery. Med Care. 2011;49:1076–1081. doi: 10.1097/MLR.0b013e3182329b97. [DOI] [PubMed] [Google Scholar]
- 14.Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in medicare patients. Ann Surg. 2009;250:1029–1034. doi: 10.1097/sla.0b013e3181bef697. [DOI] [PubMed] [Google Scholar]
- 15.Campbell DA, Englesbe MJ, Kubus JJ, et al. Accelerating the pace of surgical quality improvement: the power of hospital collaboration. Arch Surg. 2010;145:985–991. doi: 10.1001/archsurg.2010.220. [DOI] [PubMed] [Google Scholar]
- 16.Englesbe MJ, Brooks L, Kubus J, et al. A statewide assessment of surgical site infection following colectomy: the role of oral antibiotics. Ann Surg. 2010;252:514–519. doi: 10.1097/SLA.0b013e3181f244f8. discussion 519-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimick JB, Ghaferi AA, Osborne NH, et al. Reliability adjustment for reporting hospital outcomes with surgery. Ann Surg. 2012;255:703–707. doi: 10.1097/SLA.0b013e31824b46ff. [DOI] [PubMed] [Google Scholar]
- 18.Dimick JB, Welch HG, Birkmeyer JD. Surgical mortality as an indicator of hospital quality: the problem with small sample size. JAMA. 2004;292:847–851. doi: 10.1001/jama.292.7.847. [DOI] [PubMed] [Google Scholar]
- 19.Bentrem DJ, Cohen ME, Hynes DM, et al. Identification of specific quality improvement opportunities for the elderly undergoing gastrointestinal surgery. Arch Surg. 2009;144:1013–1020. doi: 10.1001/archsurg.2009.114. [DOI] [PubMed] [Google Scholar]
- 20.Ghaferi AA, Osborne NH, Birkmeyer JD, et al. Hospital characteristics associated with failure to rescue from complications after pancreatectomy. J Am Coll Surg. 2010;211:325–330. doi: 10.1016/j.jamcollsurg.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 21.Mangram AJ, Shifflette VK, Mitchell CD, et al. The creation of a geriatric trauma unit "G-60". Am Surg. 2011;779:1144–1146. doi: 10.1177/000313481107700925. [DOI] [PubMed] [Google Scholar]