Abstract
Rationale
Preclinical studies support the hypothesis that endogenous neuroactive steroids mediate some effects of alcohol.
Objectives
The aim of this study was to examine the effect of dutasteride inhibition of 5α-reduced neuroactive steroid production on subjective responses to alcohol in adult men.
Methods
Using a within-subject factorial design, 70 men completed four randomly ordered monthly sessions in which pretreatment with 4 mg dutasteride or placebo was paired with a moderate dose of alcohol (0.8 g/kg) or placebo beverage. The pharmacologic effect of dutasteride was measured by an assay of serum androstanediol glucuronide. Self-reports of alcohol effects were obtained at 40-min intervals following alcohol administration using the Biphasic Alcohol Effects Scale (BAES) and the Alcohol Sensation Scale (SS). We used linear mixed models to examine the effects of dutasteride and alcohol on BAES and SS responses and the interaction of dutasteride with the GABRA2 alcohol dependence-associated polymorphism rs279858. We also examined whether exposure to dutasteride influenced drinking in the weeks following each laboratory session.
Results
A single 4-mg dose of dutasteride produced a 70 % reduction in androstanediol glucuronide. Dutasteride pretreatment reduced alcohol effects on the BAES sedation and SS anesthesia scales. There was no interaction of dutasteride with rs279858. Heavy drinkers had fewer heavy drinking days during the 2 weeks following the dutasteride sessions and fewer total drinks in the first week after dutasteride.
Conclusions
These results provide evidence that neuroactive steroids mediate some of the sedative effects of alcohol in adult men and that dutasteride may reduce drinking, presumably through its effects on neuroactive steroid concentrations.
Keywords: Neuroactive steroids, Human subjects, 5α-Reductase
Introduction
An extensive body of preclinical studies supports the hypothesis that endogenous neuroactive steroids produced in response to alcohol mediate some of the behavioral and electro-physiological effects of alcohol (reviewed in Kumar et al. 2009). 5α- (and 5β-) reduced 3α-pregnane and 3α-androstane neuroactive steroids have anticonvulsant and anxiolytic properties and are potent positive allosteric modulators of GABAA receptors (Holzbauer et al. 1985; Majewska et al. 1986; Morrow et al. 1987; Bitran et al. 1991; Paul and Purdy 1992; Frye et al. 1996; Reddy 2004; Reddy and Jian 2010; review by Mellon and Griffin 2002). In rats, moderate to high doses of alcohol (1–2 g/kg) increase plasma and brain levels of the neuroactive steroid allopregnanolone (VanDoren et al. 2000; Porcu et al. 2010), which are reduced by pretreatment with finasteride, an inhibitor of 5α-reductase (5AR). Finasteride blocks several acute effects of alcohol in rats (VanDoren et al. 2000) and the effects of alcohol on GABA currents in brain slice preparations (Sanna et al. 2004). Blockade of neuroactive steroid production by finasteride attenuates the acquisition of alcohol preference in mice (Ford et al. 2008).
Data from human studies supporting neuroactive steroids as mediators of alcohol effects are limited. In humans, the plasma concentration of allopregnanolone was increased following severe intoxication (Torres and Ortega 2003, 2004), but not moderate intoxication (Nyberg et al. 2005; Holdstock et al. 2006; Pierucci-Lagha et al. 2006; Porcu et al. 2010). To extend preclinical studies with the 5α-reductase inhibitor finasteride to humans, we tested the effects of 200 mg finaste-ride in a human subjects alcohol laboratory paradigm involving 27 subjects (15 males) (Pierucci-Lagha et al. 2005). Although finasteride blocks both type I and type II 5AR in rodents, at clinical dosages in humans, it blocks only type II 5AR, the isoenzyme of 5AR that is most abundant in the prostate and skin. Finasteride's effect on the type I enzyme, which is abundant in the brain, liver, and adrenals, is very limited. Thus, we employed a loading dose that was 40 times the daily therapeutic dose of finasteride. We found that finasteride pretreatment reduced several subjective effects of alcohol in subjects homozygous for a protective allele of the GABRA2 synonymous exon 5 SNP, rs279858, the G-allele of which has been associated with alcohol dependence (Covault et al. 2004).
In the present study, we examined a larger sample of subjects including both nonhazardous (light) drinkers and hazardous (heavy) drinkers pretreated with dutasteride, a second 5AR inhibitor approved by the FDA to treat benign prostatic hyperplasia. In contrast to finasteride, dutasteride inhibits both type I and II 5AR enzymes in humans at clinical dosages, leading to a greater reduction in dihydrotestosterone (DHT) levels than finasteride, without suppressing testosterone (Clark et al. 2004). In the current study, we examined whether a single 4-mg loading dose of dutasteride reduced the acute effects of a moderate dose of alcohol in 70 male nondependent drinkers and whether dutasteride interacted with rs279858 in the GABRA2 gene. As a secondary analysis, based on results using finasteride in animal studies (Ford et al. 2005; Ramaker et al. 2011), we examined the effects of study participation on drinking behavior during the 3-week interval following each laboratory session, on the hypothesis that dutasteride would be associated with reduced drinking.
Methods
Subjects
Men were recruited by advertisement from the Greater Hartford Region, including nearby colleges and universities. To include heavy drinkers in the sample, some advertisements solicited participation by men who drank at least ten drinks per week. Subjects were paid to participate. All subjects gave written informed consent to participate in the study as approved by the University of Connecticut Health Center Institutional Review Board. Following an initial telephone interview, interested participants were screened in person for study eligibility using the Timeline Follow-back Interview (Sobell and Sobell 1992) to quantify alcohol use during the prior 90 days and the Structured Clinical Interview for DSMIV (First et al. 1995) to determine the presence of common psychiatric disorders. Additional screening evaluations included a medical history and physical examination with routine laboratory tests (liver and renal function tests, complete blood count, serum glucose, and urine drug toxicology screen). Finally, to examine the dose effects of the GABRA2 alcohol dependence-associated allele and its interaction with dutasteride treatment on alcohol responses, we oversampled subjects homozygous for each allele at rs279858 by randomly excluding approximately 25 % of heterozygous subjects. We screened 148 subjects of which 70 completed the study. Of the 78 subjects who were excluded, 42 were screen failures or withdrew prior to the first laboratory session, 12 were randomly excluded due to being heterozygous at rs279858, 21 did not complete all four sessions, 2 were excluded due to pharmacy error, and 1 was excluded due to a protocol violation.
Subjects were included in the study if they were between 21 and 45 years of age, reported drinking three or more standard drinks (sd) on at least one occasion during the past month, had a body mass index of 18.5–32.5 kg/m2, and weighed 235 lb or less. They were excluded if they had a lifetime DSM-IV diagnosis of alcohol or drug dependence, a diagnosis of alcohol abuse during the past 2 years or current nicotine dependence, or a current untreated medical condition or were currently using benzodiazepines, other psychotropic medications, or medications known to influence steroid hormone levels or metabolism or modify the effects of alcohol. In view of the teratogenic effects of dutasteride, which is not FDA approved for use in women, we enrolled only men.
Study design
The dutasteride/alcohol crossover laboratory study utilized a double-blind, within-subject factorial design, in which each subject participated in four experimental sessions spaced at least 4 weeks apart (average interval 36 days) in which they received either 0 or 4 mg of dutasteride 2–4 days prior to participating in an alcohol laboratory session where they received three drinks containing either an alcohol mask or 0.8 g/kg ethanol. Vodka was used as the ethanol source and was mixed with the participant's choice of martini mixer. Placebo drinks had a small volume of 1 % ethanol floated on top to provide the odor of alcohol. Drinks were mixed immediately before serving, so that subjects could taste and smell the alcohol floated on the placebo drink before it was diluted by the addition of juice. To control for expectancy effects, subjects were told that they might or might not be given alcohol (Martin and Sayette 1993). They were told to abstain from alcohol, nicotine, and other drugs (caffeine not included) during the 24 h prior to each laboratory session. Subjects arrived at approximately 10:30 AM on the day of the laboratory session. At 11:00 AM, they ate a low-fat light lunch, and at 11:30, they completed the prealcohol subjective ratings, and baseline physiologic measures were obtained. The first standard drink of alcohol or placebo beverage was administered at noon, and subjects had 36 min to consume the three drinks (i.e., one drink administered every 12 min). Breath alcohol concentration (BrAC), heart rate, and subjective response assessments were repeated at approximately 40-min intervals beginning 40 min after starting to drink. The order of drug (placebo or 4 mg dutasteride) and alcohol (placebo or 0.8 mg/kg) was randomly assigned to provide a balanced assignment of the 24 possible administration sequences for light and heavy drinker groups and by GABRA2 genotype.
Subjective effects were measured using two self-report questionnaires, described below, which were administered on a computer, programmed using SPSS Data Builder version 3.0. A monitor was placed approximately 22 in. in front of the subject at a normal viewing level. Subjects were instructed to read each question carefully and to answer it to the best of their ability based on how they felt at the time. Subjects used a computer mouse to click on their responses and to scroll down the screen answering the self-report questions until they reached the end. Study personnel monitored their progress.
The Biphasic Alcohol Effects Scale (BAES) (Martin et al. 1993) is a 14-item unipolar adjective rating scale used to measure both the stimulant and sedative effects of alcohol (seven items each for the stimulant and sedative subscales). The Alcohol Sensation Scale (SS) (Maisto et al. 1980) consists of 26 items divided into six subscales measuring somatic sensations produced by alcohol. The six subscales are central-stimulant, sensations associated with light-headedness and dizziness (four items); anesthetic, numbed sensations (five items) and sedation (four items); dynamic-peripheral, sensations associated with increased heart rate and breathing (three items); warmth-glow, blushing sensations (three items); gastrointestinal, stomach sensations (four items); and impaired function, perceived changes in psychomotor performance (three items). Prior to alcohol ingestion, as baseline measures, subjects were asked to rate both measures on a scale of 0 (not at all) to 10 (extremely) the extent to which they experienced “alcohol-like” feelings. After alcohol ingestion, subjects were asked to rate the extent to which drinking alcohol produced these feelings.
Hormone assay
We measured serum levels of 3α-androstanediol glucuronide (3α-diolG) as a biochemical indicator of dutasteride inhibition of 5AR enzyme activity. 3α-diolG is the major excreted metabolite of DHT via 3α reduction of DHT to 5α-androstane-3α,17β-diol (aka 3α,5α-androstanediol) and 17β reduction of 3α,5α-androsterone (androsterone) to 5α-androstane-3α,17β-diol. 3α-diolG has been shown to decrease in parallel with DHT levels following finasteride treatment in men (Rittmaster et al. 1989; Gormley et al. 1990) and has been used to monitor the reduction of 5AR activity in women treated with finasteride (Wong et al. 1995; Falsetti et al. 1999). Serum was collected prior to dutasteride (or placebo) administration and again at the beginning of each laboratory session 2–4 days following drug administration to confirm dutasteride inhibition of 5AR. In a separate pharmacokinetic study described below, subjects received 2-, 3-, or 4-mg doses of dutasteride, and serum was collected at 1, 3, 7, 14, 21, 28, and 42 days after drug administration. Serum 3α-diolG was assayed in duplicate using an enzyme-linked immunoassay (ALPCO Diagnostics, Salem, NH).
Genotyping
DNAwas purified from blood samples using the PureGene kit (GentraSystems, Minneapolis, MN) according to the manufacturer's instructions. Rs279858, a synonymous substitution in exon 5 of the GABRA2 gene, was genotyped using primers and TaqMan probes described previously (Covault et al. 2004).
Data analysis
Paired t tests were used to examine the change in 3α-diolG following dutasteride and the change in drinking in the weeks following laboratory sessions. Chi-square tests were used to compare genotype frequencies between light and heavy drinkers. Linear mixed-effects models were used to detect the effect of alcohol and dutasteride on BrAC, heart rate, and subjective responses. Seven time points were included in the analysis relative to the first alcohol drink: 40, 80, 120, 160, 210, 240, and 300 min, with the time after beginning alcohol administration included as a covariate. Main and interaction effects were examined for statistical significance with a focus on the interaction of alcohol×dutasteride. Additional models were run including GABRA2 rs279858 genotype, both as a three-level genotype coded 0, 1, or 2 copies of the alcohol dependence-associated G-allele or as a two-level AA vs. G-carrier variable. To examine potential confounding effects of prior month dutasteride exposure and heavy vs. light baseline drinking status, these variables were added independently in additional mixed-effects models. For the primary analysis of dutasteride moderation of alcohol effects on HR, BAES, and SS scales (nine outcomes), we controlled for multiple testing by using p<0.006 (=0.05/9) to reflect statistical significance. All statistical analyses were conducted with SPSS v15.
Results
Choice of dutasteride dose
Although dutasteride is a competitive and specific inhibitor of both type I and type II steroid 5AR enzymes, it is less potent for type I than type II 5AR (Gisleskog et al. 1998). In humans, type I 5AR is present in the skin, liver, adrenal gland, and brain. Type II 5AR is the predominant form in the prostate and skin, is also present in the liver but not in the brain, and is responsible for approximately 70 % of circulating DHT (Imperato-McGinley 1991). Dutasteride has complex elimination kinetics with parallel linear and nonlinear pathways. Although single low doses (0.5–1 mg) are metabolized with a 3–4-day half-life, repeated daily dosing or single doses above 5–10 mg produce delayed elimination with a half-life of 5 weeks due to substrate saturation of the rapid elimination pathway (Gisleskog et al. 1999). Limited pharmacokinetic data comparing 1, 2.5, 5, and 10 mg dutasteride in four subjects at each dose (Gisleskog et al. 1998) suggested that a dose in the range of 2–5 mg would balance goals of a significant inhibition of 5AR activity 2–4 days after a single dose together with moderate carryover effect in the subsequent session of a within-subjects crossover design. To identify an appropriate dutasteride dose for the laboratory study, prior to conducting the main alcohol laboratory study, we randomly assigned 24 healthy men [average age=27.9 years (SD=7.6)] to receive 2-, 3-, or 4-mg single doses of dutasteride in a 6-week pharmacokinetic study (Two participants in this pharmacokinetic study subsequently participated in the alcohol/ dutasteride main study with an interval of 9–10 months between participation in the two studies). After 3 days, the three doses produced similar reductions in levels of the 5α-reduced androstane metabolite 3α-diolG (73, 71, and 74 %, respectively). The suppression of enzyme activity was maintained for 1 week (71, 70, and 76 %, respectively), followed by a slow recovery, such that at 28 days, 3α-diolG levels were suppressed relative to baseline by 23, 34, and 42 %, respectively (Fig. 1). Based on these results, we employed a 4-mg loading dose administered 2–4 days prior to the alcohol laboratory sessions for the dutasteride×alcohol crossover laboratory study to maximize the inhibition of brain type I enzyme, recognizing that this dose could result in subjects continuing to have reductions in peripheral type II 5AR activity after 4 weeks. Recovery of type I 5AR was expected to occur before type II 5AR, as dutasteride is a 60-fold less potent inhibitor of type I than type II 5AR (Gisleskog et al. 1998).
Fig. 1.
Pharmacokinetic comparison of 2-, 3-, or 4-mg single doses of dutasteride in 24 healthy men. Change relative to predrug baseline for serum 3α-diol G, a measure of relative 5AR activity, at 1, 3, 7, 14, 21, 28, and 42 days after drug administration (mean±SEM)
Baseline characteristics of subjects completing the dutasteride/alcohol crossover study
Of the 70 participants who completed all four laboratory sessions in the dutasteride×alcohol study, 61 (87 %) were non-Hispanic Caucasians, 4 (6 %) were Hispanic Caucasians, 2 (3 %) were African American, 2 (3 %) were mixed race (Caucasian and African American), and 1 (1.5 %) was Asian. The average age of the subjects was 26.1 years (SD=6.5). Subjects’ mean weight was 84.7 kg (SD=10.3). Most subjects were currently employed (80 %), and 50 % of subjects were college graduates. During the 90 days prior to study enrollment, subjects drank on an average of 2.2 days (DD) per week, with 0.7 heavy drinking days (HDD) per week (i.e., >4 standard drinks), and consumed an average of 8.1 sd per week. Because subjective responses to alcohol have been reported to differ in heavy vs. light drinkers (King et al. 2002; Gilman et al. 2012), we subdivided the sample into nonhazardous or light drinkers (LD), n=37 (<15 sd/week and not more than 1 HDD per month during the past 90 days consistent with safe drinking guidelines) and hazardous or heavy drinkers (HD), n=33, who either drank heavily more than once per month or consumed ≥15 drinks per week. Drinking measures from the 90-day timeline follow-back (TLFB) are shown in Table 1. Twenty-six subjects (LD=16) were rs279858 A-allele homozygotes, 30 (LD=14) were heterozygotes, and 14 (LD=7) were G-allele homozygotes. There was no distortion in the distribution of GABRA2 genotypes comparing light vs. heavy drinkers [χ2(2)=0.06; p=0.97].
Table 1.
Ninety-day TLFB measures of alcohol use, mean (SD)
| Group | Number | DD/week | HDD/week | Drinks/week | Drinks/HDD |
|---|---|---|---|---|---|
| Entire sample | 70 | 2.2 (1.5) | 0.7 (0.9) | 8.1 (7.2) | 6.4 (1.6) |
| Light drinkers | 37 | 2.1 (1.8) | 0.08 (0.09) | 4.3 (3.5) | 5.9 (0.9) |
| Heavy drinkers | 33 | 2.3 (1.2) | 1.4 (0.9) | 12.3 (7.8) | 6.8 (1.9) |
DD drinking days, HDD heavy drinking days
Effect of dutasteride pretreatment on 5AR enzyme activity
We measured serum 3α-diolG as a biochemical indicator of dutasteride inhibition of 5AR enzyme activity before dutasteride/placebo administration and again at the beginning of each of the 280 laboratory sessions 2–4 days later [average 2.75 days (SD=0.74)]. As shown in Fig. 2a, a single 4-mg dose of dutasteride reduced 3α-diolG levels by 73 % [(SD= 10 %); t(107)=−17.1, p<0.001] from baseline when examining either the first laboratory session or sessions that followed placebo drug exposure, indicating complete blockade of 5AR type II and partial blockade of 5AR type I. As expected from our pharmacokinetic study, there was a carryover suppression of baseline 3α-diolG for sessions following a dutasteride session (Fig. 2b). However, the mean levels of 3α-diolG following dutasteride were not different between laboratory sessions immediately following a prior month dutasteride exposure (e.g., reflecting two consecutive months of dutasteride pretreatment) and those without a prior month dutasteride exposure [2.8 ng/ml (SD=0.9) vs. 3.1 ng/ml (SD=1.4); t(138)=0.3, ns]. Data from all four laboratory sessions were included for each subject.
Fig. 2.
Serum 3α-diolG (ng/ml) before and 2–4 days following placebo or 4 mg dutasteride (mean±SEM) for the 280 laboratory sessions completed by 70 participants in the dutasteride/ alcohol laboratory study. Comparison of results for sessions with no dutasteride exposure during the prior month (a) or for sessions with 4 mg dutasteride during the prior month (b)
Effect of dutasteride on the acute effects of alcohol
BrAC
There was a main effect of time on BrAC (F(1,838)= 4,499.7; p<0.001), which ascended steeply after the initiation of drinking, with an average peak of 0.073 g/l (SD=0.017) at 40 min, after which it descended steadily to an average of 0.02 g/l (SD=0.010) at 300 min. There was no significant main effect or interaction with time of dutasteride pretreatment on BrAC [F(1,838)=3.1; p=0.08 and F(1,838)=2.1; p=0.15, respectively; Fig. 3a].
Fig. 3.
a Breath alcohol concentration (mean±SEM) as a function of time relative to beginning the first drink. BrAC peaked at the first time point, 40 min following initiation of drinking, and was not affected by dutasteride pretreatment. b Change in heart rate (mean±SEM) as a function of time relative to the beginning of drinking. Heart rate significantly increased following alcohol administration (p<0.001); there was no effect of dutasteride pretreatment on heart rate change following alcohol
Heart rate
Linear mixed model analysis revealed main effects of alcohol (F(1,1792)=18.6; p<0.001), time (F(1,1792)= 136.3; p<0.001), and an interaction of alcohol×time (F(1,1792)=9.4; p=0.002) on the change in heart rate from baseline, with no effect of dutasteride pretreatment or interaction of dutasteride with alcohol (Fig. 3b).
BAES stimulation
There were main effects of alcohol (F(1,1878)=214.4; p<0.001) and interaction of alcohol×time (F(1,1878)=96.3; p<0.001), but no significant interaction of dutasteride with alcohol (Fig. 4a).
Fig. 4.
Acute alcohol effects displayed as the sum (mean± SEM) of the seven BAES stimulation (a) and sedation (b) items. Dutasteride pretreatment significantly reduced BAES sedation following alcohol, p<0.001, in a mixed model that included all time points following alcohol. Post hoc testing *p<0.05 for 0 vs. 4 mg dutasteride in the alcohol condition for individual time points
BAES sedation
There were main effects of alcohol (F(1,1878)=304.9 p<0.001), the interactions of alcohol×time (F(1,1878)=70.9; p<0.001) and alcohol ×dutasteride (F(1,1878)=11.4; p=0.001), and a trend for significance in the three-way interaction of alcohol×dutasteride×time (F(1,1878)=3.6; p=0.059). The form of the interactions is shown in Fig. 4b; dutasteride significantly reduced the sedative effects of alcohol as measured by the BAES, with greater differences present at earlier times following alcohol administration.
SS
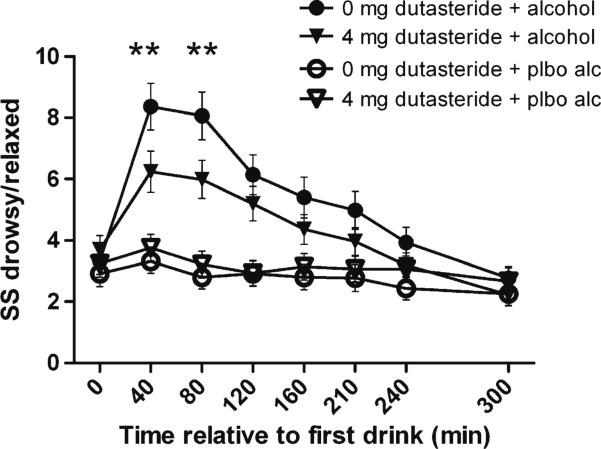
There were significant alcohol and alcohol×time interactions for all of the SS subscales. There was a significant alcohol×dutasteride interaction for the SS anesthesia subscale (F(1,1878)=9.8; p=0.002), which was driven by the four sedation items “drowsy,” “relaxed,” “limbs heavy,” and “heavy” (F(1,1878)=15.6; p<0.001), such that dutasteride pretreatment reduced self-reported sedation/relaxation (Fig. 5). After correction for multiple testing, there were no other significant alcohol×dutasteride interactions for the remaining SS subscales.
Fig. 5.
Sum of response to four alcohol sensation anesthesia subscale drowsy/relaxed items (mean±SEM). Dutasteride pretreatment reduced the alcohol-associated increase in response for these items, p<0.001, in a mixed model that included all time points following alcohol. Post hoc testing **p<0.01 for 0 vs. 4 mg dutasteride in the alcohol condition for individual time points
Effects of baseline drinking and prior month dutasteride exposure
The significant linear mixed model dutasteride×alcohol interactions for BAES sedation and SS anesthesia and drowsy/ relaxed items, and lack of significant dutasteride×alcohol interactions for other measures, remained when high vs. low baseline drinking status or prior month dutasteride exposure were included as factors in the models. The three-way interaction of dutasteride×alcohol×baseline drinking group was not significant (p>0.2). Inclusion of session order (i.e., placebo vs. dutasteride pretreatment×placebo alcohol vs. 0.8 g/kg alcohol) as a factor in linear mixed models also did not change the statistical outcomes.
There was an interaction of alcohol×baseline drinking group on three of the SS subscales such that light drinkers reported greater alcohol-related body sensations [central- stimulant (F(1,1879) = 22.3; p < 0.001), anesthesia (F(1,1879)=8.6; p=0.003), and warmth-glow (F(1,1879)= 12.1; p=0.001) than heavy drinkers.
Test of the interaction of GABRA2 polymorphism and dutasteride on alcohol effects and of the moderation by GABRA2 genotype of alcohol effects
The addition of GABRA2 genotype to the mixed models (either as the three-level or two-level genotypes) showed no interactive effects of genotype with dutasteride for the effects of alcohol on heart rate, BAES stimulation, BAES sedation, or any of the six SS subscales. The interaction of genotype× dutasteride on alcohol effects was also not significant when high vs. low baseline drinking or prior month dutasteride exposure were included in the models.
The moderation of alcohol effects by GABRA2 genotype was also examined in the absence of dutasteride pretreatment. Mixed model results showed an alcohol×genotype interaction, such that G-allele carriers reported higher subjective effects on four of the SS subscales [central-stimulant (F(1,900)=9.1; p=0.03), anesthesia (F(1,900)=9.5; p= 0.002), impaired function (F(1,900)=15.6; p<0.001), and gastrointestinal (F(1,900)=16.8; p<0.001)]. The three-way interactions of alcohol×G-carrier×baseline drinking were not significant. We did not observe alcohol×genotype effects on heart rate or response on the BAES stimulation or sedation subscales (p>0.2).
Effects of dutasteride exposure on naturalistic drinking following laboratory sessions
In a secondary analysis, we examined drinking data obtained using the TLFB method at each monthly laboratory session and by mail 4 weeks after the last laboratory session, both to evaluate a potential increase in hazardous drinking following alcohol laboratory sessions and to investigate whether dutasteride exposure changed subjects’ alcohol use in a natural setting. In the heavy drinker group, the number of HDDs during the first and second weeks following dutasteride pre-treatment sessions was reduced from the 90-day baseline period [week 1: t(45)=−4.3, p<0.001; week 2: t(45)=−2.9, p=0.007]. In contrast, there was no such effect following laboratory sessions with placebo pretreatment (Fig. 6a). The total number of drinks in the first week after dutasteride was also reduced from baseline [t(45)=−3.5, p=0.001] for the heavy-drinker group (Fig. 6c). No significant effect of dutasteride treatment on drinking was seen for the 37 light drinkers, perhaps reflecting a floor effect for this group (Fig. 6b, d).
Fig. 6.
Weekly TLFB data following laboratory sessions with dutasteride or placebo pretreatment for heavy vs. light male drinkers. a, b Heavy drinking days per week (mean± SEM) were significantly reduced in the heavy drinking group for 2 weeks following dutasteride pretreatment but not placebo pretreatment, paired t test for post-laboratory drinking compared with 90-day TLFB baseline data, ***p≤0.001; **p<0.01. c, d Total standard drinks per week (mean±SEM) were significantly reduced for the heavy drinking group during week 1 following dutasteride but not placebo, ***p≤0.001
Adverse effects of dutasteride paired with alcohol
Dutasteride was well tolerated by study participants. No subject dropped out of the study due to adverse experiences related to the study medication. We collected a total of 903 medication adverse event reports from subjects over three time points: 2–4 days after receiving dutasteride or placebo and prior to receiving alcohol or placebo drinks and again 1 and 7 days after the alcohol (or placebo alcohol) laboratory session. For 842 of these occasions, subjects reported no adverse effects. A total of 61 reports indicated a potential medication-related adverse event, with 48 of these reports occurring during the interval between medication dose and alcohol administration. Only for “stomach discomfort” during the 2–4-day interval between medication administration and alcohol sessions were there more reports of adverse events after dutasteride than after placebo [8 vs. 2 % χ2(1)=6.0; p= 0.014]. The frequency of reports of “stomach discomfort” following dutasteride was similar in the light- vs. heavy-drinker groups [χ2(1)=1.2; p=0.28].
We collected adverse event data for a total of 323 occasions during subjects’ alcohol administration laboratory sessions. Reports of any drink-related adverse effects were more common on days in which alcohol was administered [34 vs. 21 % of occasions (χ2(1)=7.4; p=0.007)]. Sleepiness/being tired was the most common adverse effect reported during alcohol sessions. There was a trend for dutasteride pretreatment prior to alcohol to be associated with fewer reports of any adverse effect than placebo pretreatment [dutasteride 28 % vs. placebo 41 % (χ2(1)=2.9; p=0.09)].
Discussion
The primary results of this study are that acute inhibition of 5AR activity by dutasteride in adult males was associated with (1) reduced self-reported sedative (but not stimulating) effects of a moderate dose of alcohol in a laboratory setting and (2) reduced alcohol self-administration for 1–2 weeks following a single 4-mg dose in the natural environment. Reductions in sedative effects were observed using two different assessments, the BAES and the SS. Our results, when considered in the context of prior reports that a moderately intoxicating dose of alcohol similar to that used here does not elevate plasma levels of the 5α-reduced neuroactive steroid allopregnanolone (Nyberg et al. 2005; Holdstock et al. 2006; Pierucci-Lagha et al. 2006; Porcu et al. 2010), suggest that alcohol is directly affecting the local generation of 5α-reduced neuroactive steroids in the brain, which is reduced by dutasteride.
The lack of an interaction of dutasteride with GABRA2 genotype on subjective alcohol effects contrasted with our prior findings in a smaller sample of an interaction of geno-type with finasteride on several SS subscale measures (Pierucci-Lagha et al. 2005). Our prior study included both men and women but only 7 A-allele homozygotes and 20 G-allele carriers, so the results may be less reliable than those from the 26 A-allele homozygotes and 44 G-allele carriers in the present study. Differences in findings may also have resulted from unanticipated pharmacologic effects of the high dose of finasteride used previously (which was 40 times the clinically effective dose) compared with a moderate 4-mg dose of dutasteride in the present study (due to the slow metabolism and accumulation over time, the standard chronic daily 0.5-mg dose of dutasteride yields a blood level equivalent to a single 10–20-mg dutasteride dose).
We also observed alcohol×GABRA genotype interactions on four of the SS scales (central-stimulant, anesthesia, impaired-function, and gastrointestinal), with carriers of the alcohol dependence-associated G-allele reporting greater subjective effects on these measures. The direction of this effect is opposite that reported previously in a smaller sample, in which carriers of the G-allele had reduced responses to alcohol (Pierucci-Lagha et al. 2005). The results of the earlier study also contrast with other more recent reports that GABRA2 alleles, which are overrepresented in alcohol dependence subjects, were associated with a greater “high” following alcohol administration (Kareken et al. 2010; Arias et al. 2014). The lack of a GABRA2 genotype×alcohol interaction on BAES stimulation and sedation subscales reported herein is consistent with the lack of GABRA2 genotype moderation of alcohol effects on the BAES reported by three other groups examining moderately large samples (Haughey et al. 2007; Roh et al. 2011; Uhart et al. 2013).
Although not anticipated based on results from the laboratory study of the acute effects of alcohol, the observed reduction of alcohol consumption during the 1–2 weeks following dutasteride exposure in heavy drinkers is consistent with preclinical reports that finasteride reduces alcohol self-administration (Ford et al. 2005; Ramaker et al. 2011) and acquisition of alcohol preference (Ford et al. 2008) in male mice. The reduction of sedative effects by dutasteride pretreatment, as measured by self-report questionnaires, would seem more likely to promote increased alcohol intake in the natural environment. The reduction in drinking following dutasteride, but not placebo drug sessions, suggests that dutasteride (and by inference its effects on neuroactive steroid concentrations) moderates the psychological or reinforcing properties of alcohol in ways not measured by the questionnaires used here.
Of note, in relation to the observed effect on drinking, a recent case series described that among 83 men who developed persistent sexual side effects from finasteride treatment of male pattern hair loss, 65 % of the men reported reduced alcohol use during or after discontinuation of finasteride (Irwig 2013). These patients took finasteride for an average of 26 months and retrospectively reported a decrease in average drinks per week from 5.2 prior to finasteride to 2.0 drinks per week after discontinuing finasteride. Although these findings are suggestive, subjects with persistent sexual side effects of finasteride represent a small minority of men treated with the drug, and the cases described were light drinkers. We are aware of no other studies reporting long-term effects of finasteride or dutasteride on alcohol use in human subjects. Although it is also possible that, in our study, dutasteride produced a general aversive reaction that transiently reduced alcohol use, the paucity of side effects reported at 1 and 7 days after the alcohol laboratory sessions (n=13) does not support this explanation.
Strengths of this study include a randomized, double-blind, crossover design with placebo controls for both dutasteride and alcohol, a robust sample size for the primary subjective outcomes, selection for rs279858 homozygotes, and biochemical evaluation of the pharmacologic inhibition of 5AR by dutasteride. A primary limitation of the study is that the single 4-mg dutasteride dosing strategy used likely resulted in only a partial inhibition of the brain type I 5AR. Additionally, the long half-life of dutasteride created a carryover effect in the month following dutasteride exposure which may have reduced the magnitude of dutasteride vs. placebo pretreatment contrasts. Therefore, the effects observed (or not observed) may not fully reflect the role of brain-derived neuroactive steroids in alcohol's effects. Although chronic daily use of dutasteride for clinical purposes results in nearly complete blockade of both types I and II 5AR, with reductions of DHT of 95 % compared with 70 % for finasteride (Clark et al. 2004), our within-subjects design limited the dose of dutasteride that we could use without producing high levels of 5AR inhibition in subsequent sessions [e.g., a single 10-mg dose of dutasteride produces a 94 % reduction in DHT at 1– 3 days and 90 % at 28 days; Gisleskog et al. 1998]. An alternate study design using a fully inhibitory dose of dutasteride could not practically be done using a placebo crossover design. An alternative to pharmacological inhibition of 5AR as a tool to test the potential involvement of neuroactive steroids in acute alcohol effects would be to examine the moderation of alcohol's effects by polymorphisms in genes encoding neuroactive steroid metabolic enzymes. A second limitation of using 5AR inhibitors to probe the involvement of neuroactive steroids in alcohol's effects is that 5β-reduced 3α-pregnane and androstane steroids are also potent neuroactive GABA receptor modulators (Bitran et al. 1991), but whose production by the 5β-reductase encoded in humans by AKR1D1 (Jez et al. 1997) is not blocked by 5AR inhibitors. Finally, this study was limited to men due to the potential teratogenic reproductive effects of dutasteride in women, so any sex effects of dutasteride were not detectable.
The reduction in heavy drinking during the first 2 weeks following exposure to dutasteride by non-treatment-seeking hazardous drinkers enrolled in the study has potential clinical relevance. The time course of these findings suggests that dutasteride's inhibition of 5AR in hazardous drinkers may have reduced the reinforcing effects of alcohol in a time-limited fashion, decreasing over time as the inhibition of 5AR waned. These results parallel preclinical studies showing a reduction in alcohol self-administration in mice following 5AR inhibition with finasteride (Ford et al. 2005; Ramaker et al. 2011). The findings reported here, together with the favorable side effect profile of dutasteride, argue for the investigation of the utility of dutasteride to reduce heavy drinking in treatment-seeking men.
Acknowledgments
Supported by NIH grants R01 AA015606 (to JC), K24 AA13736 (to HRK), P60 AA03510 (Alcohol Research Center), and M01 RR06192 (University of Connecticut General Clinical Research Center). The authors thank Linda Burian and Pamela Fall for their expert technical assistance in the conduct of this study.
Footnotes
Conflict of interest JC, RF, AJA, and TP have no disclosures to make. HK has been a consultant or advisory board member for the following pharmaceutical companies: Alkermes, Lilly, Lundbeck, Pfizer, and Roche. He is also a member of the American Society of Clinical Psychopharmacology's Alcohol Clinical Trials Initiative, which is supported by Lilly, Lundbeck, AbbVie, and Pfizer. CO has received study supplies from Pfizer Pharmaceuticals for a smoking cessation study.
Contributor Information
Jonathan Covault, Alcohol Research Center, Department of Psychiatry, University of Connecticut School of Medicine, Farmington, CT, USA; Department of Psychiatry, University of Connecticut Health Center, 263 Farmington Avenue, Farmington, CT 06030-1410, USA.
Timothy Pond, Department of Psychiatry, University of Pennsylvania Perelman School of Medicine and Philadelphia VAMC, Philadelphia, PA, USA.
Richard Feinn, Department of Medical Sciences, Frank Netter School of Medicine, Quinnipiac University, North Haven, CT, USA.
Albert J. Arias, Department of Psychiatry, Yale University and VA CT Healthcare, West Haven, CT, USA
Cheryl Oncken, Department of Medicine, University of Connecticut School of Medicine, Farmington, CT, USA.
Henry R. Kranzler, Department of Psychiatry, University of Pennsylvania Perelman School of Medicine and Philadelphia VAMC, Philadelphia, PA, USA
References
- Arias AJ, Covault J, Feinn R, Pond T, Yang BZ, Ge W, Oncken C, Kranzler HR. A GABRA2 variant is associated with increased stimulation and ‘high’ following alcohol administration. Alcohol Alcohol. 2014;49(1):1–9. doi: 10.1093/alcalc/agt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3 alpha-hydroxy-5 alpha[beta]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561(1):157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab. 2004;89(5):2179–2184. doi: 10.1210/jc.2003-030330. [DOI] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004;129(1):104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Falsetti L, Gambera A, Legrenzi L, Iacobello C, Bugari G. Comparison of finasteride versus flutamide in the treatment of hirsutism. Eur J Endocrinol. 1999;141(4):361–367. doi: 10.1530/eje.0.1410361. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV Axis I disorders—patient edition (SCID - I/P, version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Ford MM, Nickel JD, Finn DA. Treatment with and withdrawal from finasteride alter ethanol intake patterns in male C57BL/6J mice: potential role of endogenous neurosteroids? Alcohol. 2005;37(1):23–33. doi: 10.1016/j.alcohol.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Yoneyama N, Strong MN, Fretwell A, Tanchuck M, Finn DA. Inhibition of 5alpha-reduced steroid biosynthesis impedes acquisition of ethanol drinking in male C57BL/6J mice. Alcohol Clin Exp Res. 2008;32(8):1408–1416. doi: 10.1111/j.1530-0277.2008.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Van Keuren KR, Erskine MS. Behavioral effects of 3 alpha-androstanediol. I: Modulation of sexual receptivity and promotion of GABA-stimulated chloride flux. Behav Brain Res. 1996;79(1–2):109–118. doi: 10.1016/0166-4328(96)00004-6. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Crouss T, Hommer DW. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology. 2012;37(2):467–477. doi: 10.1038/npp.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisleskog PO, Hermann D, Hammarlund-Udenaes M, Karlsson MO. A model for the turnover of dihydrotestosterone in the presence of the irreversible 5 alpha-reductase inhibitors GI198745 and finasteride. Clin Pharmacol Ther. 1998;64(6):636–647. doi: 10.1016/S0009-9236(98)90054-6. [DOI] [PubMed] [Google Scholar]
- Gisleskog PO, Hermann D, Hammarlund-Udenaes M, Karlsson MO. The pharmacokinetic modelling of GI198745 (dutasteride), a compound with parallel linear and nonlinear elimination. Br J Clin Pharmacol. 1999;47(1):53–58. doi: 10.1046/j.1365-2125.1999.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley GJ, Stoner E, Rittmaster RS, Gregg H, Thompson DL, Lasseter KC, Vlasses PH, Stein EA. Effects of finasteride (MK-906), a 5 alpha-reductase inhibitor, on circulating androgens in male volunteers. J Clin Endocrinol Metab. 1990;70(4):1136–1141. doi: 10.1210/jcem-70-4-1136. [DOI] [PubMed] [Google Scholar]
- Haughey HM, Ray LA, Finan P, Villanueva R, Niculescu M, Hutchison KE. Human gamma-aminobutyric acid A receptor alpha2 gene moderates the acute effects of alcohol and brain mRNA expression. Genes Brain Behav. 2007;7:447–454. doi: 10.1111/j.1601-183X.2007.00369.x. [DOI] [PubMed] [Google Scholar]
- Holdstock L, Penland SN, Morrow AL, de Wit H. Moderate doses of ethanol fail to increase plasma levels of neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one-like immunoreactivity in healthy men and women. Psychopharmacology (Berl) 2006;186(3):442–450. doi: 10.1007/s00213-005-0187-0. [DOI] [PubMed] [Google Scholar]
- Holzbauer M, Birmingham MK, De Nicola AF, Oliver JT. In vivo secretion of 3 alpha-hydroxy-5 alpha-pregnan-20-one, a potent an-aesthetic steroid, by the adrenal gland of the rat. J Steroid Biochem. 1985;22(1):97–102. doi: 10.1016/0022-4731(85)90147-5. [DOI] [PubMed] [Google Scholar]
- Imperato-McGinley J. 5 Alpha-metabolism in finasteride-treated subjects and male pseudohermaphrodites with inherited 5 alpha-reductase deficiency. A review. Eur Urol. 1991;20(Suppl 1):78–81. doi: 10.1159/000471751. [DOI] [PubMed] [Google Scholar]
- Irwig MS. Decreased alcohol consumption among former male users of finasteride with persistent sexual side effects: a preliminary report. Alcohol Clin Exp Res. 2013;37(11):1823–1826. doi: 10.1111/acer.12177. [DOI] [PubMed] [Google Scholar]
- Jez JM, Flynn TG, Penning TM. A new nomenclature for the aldoketo reductase superfamily. Biochem Pharmacol. 1997;54(6):639–647. doi: 10.1016/s0006-2952(97)84253-0. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Liang T, Wetherill L, Dzemidzic M, Bragulat V, Cox C, Talavage T, O'Connor SJ, Foroud T. A polymorphism in GABRA2 is associated with the medial frontal response to alcohol cues in an fMRI study. Alcohol Clin Exp Res. 2010;34(12):2169–2178. doi: 10.1111/j.1530-0277.2010.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26(6):827–835. [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205(4):529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, Connors GJ, Tucker JA, McCollam JB, Adesso VJ. Validation of the Sensation Scale, a measure of subjective physiological responses to alcohol. Behav Res Ther. 1980;18(1):37–43. doi: 10.1016/0005-7967(80)90067-4. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232(4753):1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Martin CS, Sayette MA. Experimental design in alcohol administration research: limitations and alternatives in the manipulation of dosage-set. J Stud Alcohol. 1993;54(6):750–761. doi: 10.15288/jsa.1993.54.750. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17(1):140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13(1):35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142(3):483–485. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Andersson A, Zingmark E, Wahlstrom G, Backstrom T, Sundstrom-Poromaa I. The effect of a low dose of alcohol on allopregnanolone serum concentrations across the menstrual cycle in women with severe premenstrual syndrome and controls. Psychoneuroendocrinology. 2005;30(9):892–901. doi: 10.1016/j.psyneuen.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6(6):2311–2322. [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken C, Morrow AL, Kranzler HR. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30(6):1193–1203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Khisti RT, Morrow AL, Marx CE, Shampine LJ, Kranzler HR. Subjective effects and changes in steroid hormone concentrations in humans following acute consumption of alcohol. Psychopharmacology (Berl) 2006;186(3):451–461. doi: 10.1007/s00213-005-0231-0. [DOI] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Alward SE, Song SC, Grant KA, de Wit H, Leslie Morrow A. Differential effects of ethanol on serum GABAergic 3alpha,5alpha/3alpha,5beta neuroactive steroids in mice, rats, cynomolgus monkeys, and humans. Alcohol Clin Exp Res. 2010;34(3):432–442. doi: 10.1111/j.1530-0277.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Ford MM, Fretwell AM, Finn DA. Alteration of ethanol drinking in mice via modulation of the GABA(A) receptor with ganaxolone, finasteride, and gaboxadol. Alcohol Clin Exp Res. 2011;35(11):1994–2007. doi: 10.1111/j.1530-0277.2011.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3alpha-androstanediol and 17beta-estradiol. Neuroscience. 2004;129(1):195–207. doi: 10.1016/j.neuroscience.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Jian K. The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors. J Pharmacol Exp Ther. 2010;334(3):1031–1041. doi: 10.1124/jpet.110.169854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittmaster RS, Stoner E, Thompson DL, Nance D, Lasseter KC. Effect of MK-906, a specific 5 alpha-reductase inhibitor, on serum androgens and androgen conjugates in normal men. J Androl. 1989;10(4):259–262. doi: 10.1002/j.1939-4640.1989.tb00097.x. [DOI] [PubMed] [Google Scholar]
- Roh S, Matsushita S, Hara S, Maesato H, Matsui T, Suzuki G, Miyakawa T, Ramchandani VA, Li TK, Higuchi S. Role of GABRA2 in moderating subjective responses to alcohol. Alcohol Clin Exp Res. 2011;35(3):400–407. doi: 10.1111/j.1530-0277.2010.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24(29):6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Humana Press; Totowa: 1992. pp. 41–42. [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in female adolescent humans. Neuropsychopharmacology. 2003;28(6):1207–1209. doi: 10.1038/sj.npp.1300170. [DOI] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in male adolescent humans. Psychopharmacology (Berl) 2004;172(3):352–355. doi: 10.1007/s00213-003-1662-0. [DOI] [PubMed] [Google Scholar]
- Uhart M, Weerts EM, McCaul ME, Guo X, Yan X, Kranzler HR, Li N, Wand GS. GABRA2 markers moderate the subjective effects of alcohol. Addict Biol. 2013;18(2):357–369. doi: 10.1111/j.1369-1600.2012.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20(5):1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong IL, Morris RS, Chang L, Spahn MA, Stanczyk FZ, Lobo RA. A prospective randomized trial comparing finasteride to spironolactone in the treatment of hirsute women. J Clin Endocrinol Metab. 1995;80(1):233–238. doi: 10.1210/jcem.80.1.7829618. [DOI] [PubMed] [Google Scholar]