Abstract
Objectives
To determine the long-term effects of delayed-release cysteamine bitartrate (DR-CYS) based on our previous work that established the short-term noninferiority of DR-CYS every 12 hours compared with immediate-release cysteamine bitartrate every 6 hours.
Study design
We conducted a prospective, controlled, open label, single-arm study of DR-CYS for 2 years in 40 patients to assess efficacy in depletion of cystine in peripheral white blood cells, to assess the dose required to maintain white blood cell content of cystine <1 nmol ½ cystine/mg protein, to measure quality of life using the Pediatric Quality of Life Inventory, change in estimated glomerular filtration rate, and change in height Z-score.
Results
Through 24 months of study, the mean white blood cell content of cystine was always <1 nmol ½ cystine/mg protein, and the dose of DR-CYS decreased from 43.5-40.1 mg/kg/d (P = .05), and the significant improvement in social function, school function, and in total function scores on the Pediatric Quality of Life Inventory remained. The estimated glomerular filtration rate was maintained and growth velocity was maintained at 24 months compared with the baseline height Z-score.
Conclusions
The use of a DR-CYS administered every 12 hours to patients with cystinosis is of great benefit to their quality of life and to important biomarkers of disease control, when studied in a prospective, controlled fashion. We suggest that DR-CYS should be considered for substrate depletion in patients with cystinosis.
Nephropathic cystinosis (OMIM 219800; 219900) is an autosomal recessive, systemic disease caused by defective cystinosin transport of cystine out of the lysosome. Kidney failure is inevitable with the untreated disease, and multiple organ failures ensue over time as well if the disease is untreated after kidney transplantation. Immediate release cysteamine bitartrate1 treatment is effective but difficult to use long term.
A novel formulation of microspheronized, micro-encapsulated beads of cysteamine bitartrate was developed that allowed extended pharmacokinetics (PK) and pharmacodynamics (PD) such that twice daily administration was possible (delayed-release cysteamine bitartrate [DR-CYS]). A pilot study in 9 patients with cystinosis (data not shown) confirmed both the delayed release (Tmax = 2.78 ± 1.56 and 1.22 ± 0.51 hours for DR-CYS and immediate release cysteamine bitartrate, respectively) and a longer half-life (5.85 ± 2.89 and 1.90 ± 0.58 hours for DR-CYS and immediate release cysteamine bitartrate, respectively) for DR-CYS. In addition, in data not shown, a regression of the blood cysteamine area under the curve from 0–12 hours normalized by dose in milligram/kilogram for DR-CYS vs the area under the curve from 0–6 hours normalized by dose in milligram/kilogram for immediate release cysteamine bitartrate suggested a linear relationship with a slope of 1.4136. Thus, to achieve an equivalent exposure of cysteamine over a 12-hour dosing interval, a single dose of DR-CYS would have to be equal to 2/1.4136 = 1.4148 times a single dose of immediate release cysteamine bitartrate.
Based initially on the observed PK and PD discussed, we demonstrated that in a randomized, controlled, cross-over study of 3 weeks duration in 41 patients with nephropathic cystinosis, DR-CYS (PROCYSBI, Raptor Pharmaceutical Inc, Novato, California), was not inferior to immediate-release cysteamine bitartrate (Castagon, Mylan Pharmaceuticals, Pittsburgh, Pennsylvania), for control of white blood cell content of cystine (WBC [cystine]) (reported as nano-moles of ½ cystine/mg protein), a biomarker of the disease.2
However, in this short-term efficacy study of DR-CYS in nephropathic cystinosis, the ability to evaluate the effect on maintenance of optimal WBC (cystine), native kidney function, somatic growth, and the impact on quality of life was not present. Therefore, we designed an open-label, controlled, prospective study to answer these questions, and provide herein the data for 2 years of treatment.
Methods
Patients with nephropathic cystinosis, their native kidneys, and estimated glomerular filtration rate (eGFR) >30 mL/min/1.73 m2 (>0.5 mL/s/1.73 m2) who completed the short-term efficacy study noted above were eligible to enroll in this prospective study, and 40/41 of these patients did so after approval of the study by the ethics boards or institutional review boards of the institutions and signed consent from parents or adolescents and assent from minors according to institutional policies. Patients were seen monthly for the first 6 months of the study and quarterly thereafter. Clinical laboratory assessments (hematology, chemistry, urinalysis), physical examination (including basic neurologic and skin assessments) and vital signs, ECG, body weight, body mass index (BMI), body surface area, and an age-appropriate qualify of life questionnaire (Pediatric Quality of Life Inventory [PedsQL]) were made at each monthly and quarterly visit. During these visits, blood samples for PK (cysteamine) and PD (WBC [cystine]) were collected 0.5 hours after the DR-CYS dose administered at the study visit. Concomitant medications and adverse events (AEs) were collected from a written diary.
Upon entering this study, the initial DR-CYS dose was the same as that used at the end of the short-term study. Subsequent DR-CYS dosing following each study visit was adjusted by each investigator based on their evaluation of interval WBC [cystine], and a clinically meaningful dosing change for purposes of this study was defined by a minimum of ±5% of the baseline dosage. This differentiated trivial changes in either direction based on changes in patient body mass alone.
We determined control of disease by the biomarker, WBC [cystine], and its relationship to plasma [cysteamine], 30 minutes after DR-CYS dosing. We calculated eGFR using the modified Schwartz formula indexed to an international creatinine standard.3 We measured patient height by a stadiometer calibrated to the nearest 0.1 cm, and body mass by a digital scale calibrated to the nearest 0.1 kg. We calculated body surface area by a standard equation. We calculated the height Z-score for age using the Centers of Diseases Control and Prevention growth charts.4 We measured quality of life by using the PedsQL. All data are reported for 24 months of treatment with DR-CYS.
In general, cystine is quantitated from a human white blood cell lysate prepared at each local study center by mixing the clear supernatant obtained after centrifugation with isotope-labeled cystine as an internal standard, and quantitated by liquid chromatography mass spectrometry/mass spectrometry. As reported previously, we converted the amount of protein measured in this manner to a Lowry protein equivalent.5
In general terms, cysteamine is extracted from sodium heparinized human plasma by a protein precipitation extraction with acetonitrile. Before the extraction, isotope-labeled drug is added as an internal standard, and Tris(2-carboxyethyl) phosphine hydrochloride is added as a reducing agent. A supernatant is transferred to a new plate, and diluted with mobile phase. The sample is injected into a liquid chromatography mass spectrometry/mass spectrometry system using a Waters (hydrophilic interaction chromatography column; Waters Corp, Milford, Massachusetts) column with an ammonium formate/acetonitrile/water mobile phase.
Hematology, chemistry, and a urinalysis were provided by Covance Central Laboratory Services.6 We do not report on the specific measurements because there have been no changes in them over time, but we use the serum creatinine for eGFR calculations.
Statistical Analyses
Longitudinal data were analyzed using a mixed model ANOVA. This model allowed for a variable number of repeat evaluations per subject and took into account the variability specific to each subject (intrasubject variance). Linear regressions were then calculated using the least-square means for each time point. Statistical significance is P < .05. Statistical analyses were performed with SAS Software v 9.2 (SAS Institute, Cary, North Carolina).
Results
Forty patients were enrolled (Table I; available at www.jpeds.com). Patients were mostly children, male and female, with an average age of almost 12 years, a height Z-score of −1.15, and with an eGFR = 63 mL/min/1.73 m2 (1.05 mL/s/1.73 m2).
Table I.
Baseline characteristics for enrolled patients
| Baseline | |
|---|---|
| 40 | |
| Age (y) | 11.5 ± 3.6 |
| Children (from age 6 to ≤12) | 25 |
| Adolescents (from age 12 to ≤21) | 15 |
| Adults (>21) | 0 |
| Male, n (%) | 23 (57.5%) |
| Height (cm) | 139.4 ± 19.5 |
| Height (Z-score) | −1.15 ± 0.93 |
| Weight (kg) | 37.1 ± 15.0 |
| BMI (kg/m2) | 18.2 ± 3.1 |
| BSA (m2) | 1.18 ± 0.31 |
| eGFR (mL/min/1.73 m2) | 63 ± 25 (44;58;85) |
| Daily immediate release cysteamine dose (mg/d) | 1807 ± 520 |
| Total daily DR-CYS dose (average % of previous immediate-release cysteamine dose) | 83.7 ± 7.9 |
| WBC (cystine) (nmol ½ cystine/mg protein) | 0.79 ± 1.68 |
| WBC (cystine) ≤1 nmol ½ cystine/mg protein; n (%) | 34 (85%) |
BSA, body surface area.
Values presented are the mean ± SD (range) and the (25th percentile; median; 75th percentile) unless otherwise noted.
Control of WBC [cystine]
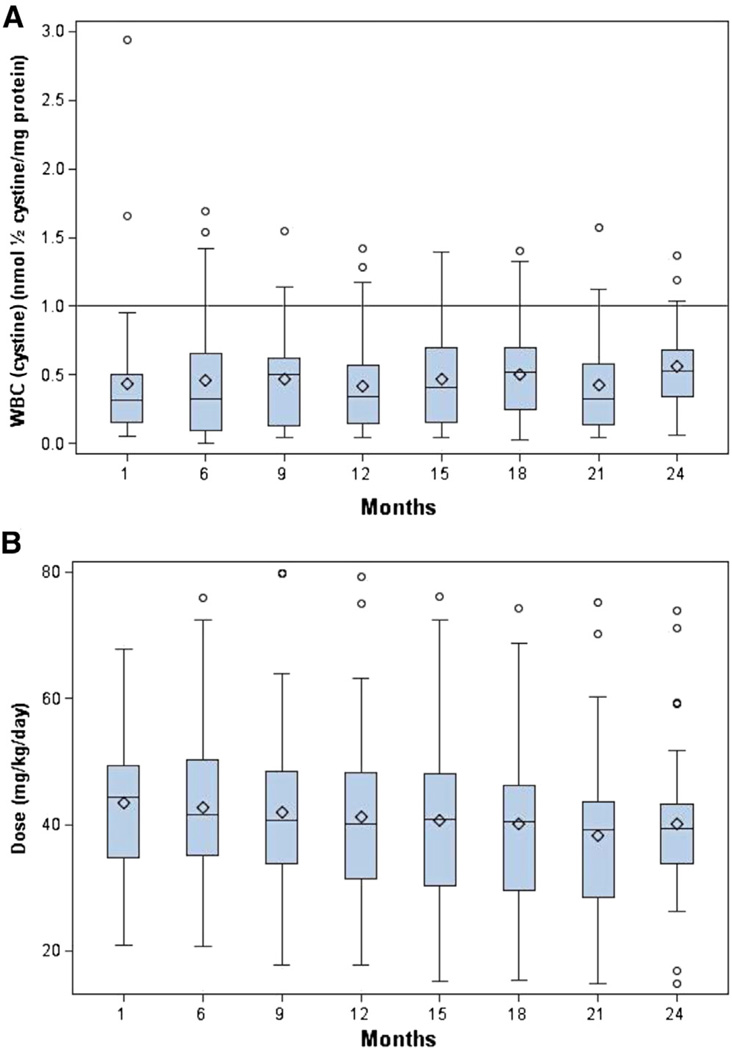
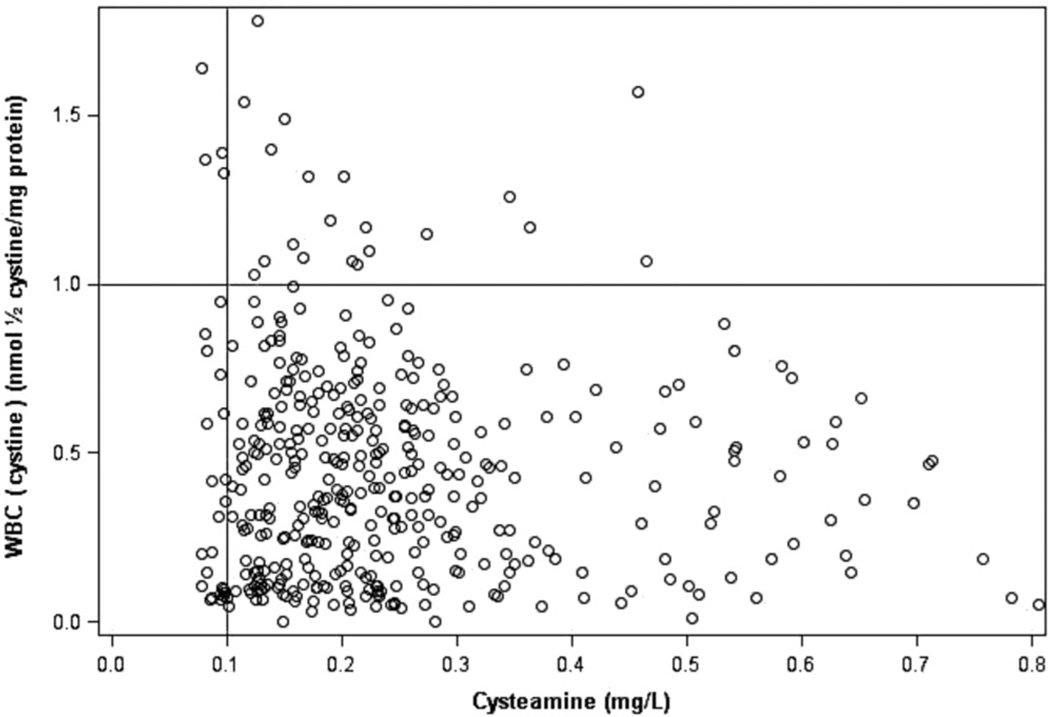
Over 24 months of study, the mean WBC [cystine] was maintained under optimal control (<1 nmol ½ cystine/mg protein) at all times for the study cohort (baseline: 0.43 ± 0.15, mean ± SD; median 0.31; first and third IQR 0.15 and 0.50 nmol ½ cystine/mg protein, respectively; 24 months: 0.55 ± 0.34; median 0.53; first and third IQR 0.34 and 0.68 nmol ½ cystine/mg protein, respectively; P = .38; Figure 1). This was achieved with a mean reduction in DR-CYS dosing over the same time interval from 43.5 ± 10.8 (median, 44.4; first and third IQR 34.8 and 49.3, respectively) to 40.1 ± 13.1 (median 39.4; first and third IQR 33.7 and 43.3 respectively) mg/kg/d (P = .05) (Figure 2). When the patients had a value for plasma (cysteamine) ≥ 0.1 mg/L 0.5 hours after DR-CYS dosing, 94.5% of WBC [cystine] levels were <1 (Figure 3).
Figure 1.
A, Mean WBC (cystine) remained below 1 nmol ½ cystine/mg protein during the entire study. The solid line denotes the optimal upper level of WBC (cystine) to be achieved during substrate reduction therapy with cysteamine. There was no significant change in the WBC (cystine) over the 24-month study. B, The total daily dose (mg/kg/day) of DR-CYS in the study population over the study was reduced significantly. The box plots below show the median (−), mean (♦), and the first and third quartiles denoted by the upper and lower ends of the box, respectively. The lower and upper whiskers denote the minimum and maximum, respectively, and the (○) represent the outliers. The mean dose was reduced from 43.5 to 40.1 mg/kg/d, P = .05, during the 24-month study.
Figure 2.
WBC (cystine) vs plasma (cysteamine) for all study patients who had a WBC (cystine) ≤1 nmol/1/2 cystine/mg protein; 94.5% of measured plasma (cysteamine) values were >0.1 mg/dL when the WBC (cystine) was ≤1 nmol ½ cystine/mg protein.
Figure 3.
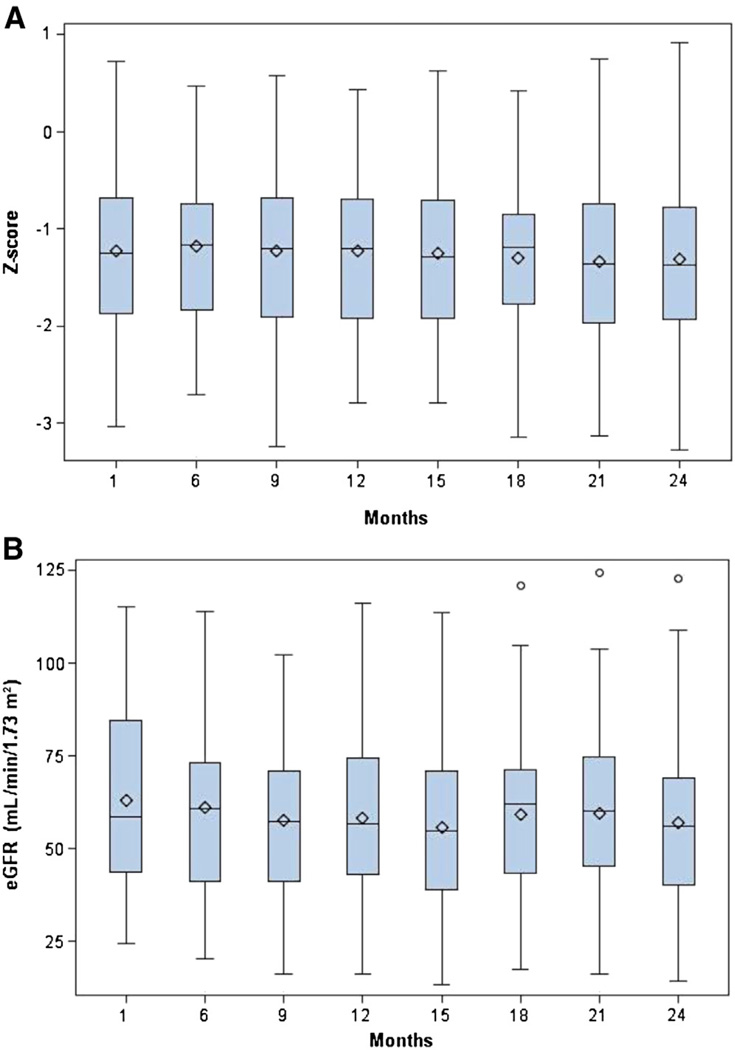
A, Evolution of height (Z-score) over the 24-month study. There was no significant change in height Z-socre during the 24-month study. B, The eGFR of the study population over time. The box plots below show the median (−), mean (♦), and the first and third quartiles denoted by the upper and lower ends of the box, respectively. The lower and upper whiskers denote the minimum and maximum, respectively, and the (○) represent the outliers. The mean eGFR did not decline significantly during the course of the 24-month study.
Patient Growth
No patient using growth hormone during the study were evaluated for growth change with the other members of the study. Compared with baseline height Z-score (−1.15 ± −0.93; median −1.22; first and third IQR −1.87 and −0.64, respectively), we found that the height Z-score did not change over the 24-month period of study (−1.21 ± −0.96; median −1.24; first and third IQR −1.90 and −0.70, respectively; P = .46). Similarly, the patient’s BMI did not change significantly during the study (baseline: 18.2 ± 3.1 vs 18.3 ± 3.2 kg/m2, respectively; P = .27).
Evolution of eGFR
eGFR was maintained for 24 months of study (baseline: 63 ± 25; median 58.5; first and third IQR, 43.8 and 84.5 mL/min/1.73 m2 (baseline: 1.05 ± 0.42; median, 0.98; first and third IQR, 0.73 and 1.40 mL/s/1.73 m2) respectively; 24-months: 57 ± 25; median, 56.2; first and third IQR, 40.4 and 69.1 mL/min/1.73 m2 (24-months: 0.95 mL/s/1.73 m2; median, 0.94; first and third IQR, 0.67 and 1.15 mL/s/1.73 m2), respectively; P = .32). During this 24-month period, 1 patient went to transplantation at 17 months, and 1 patient withdrew from the study at 21 months with a decline in eGFR and was placed on maintenance dialysis.
Quality of Life
Upon entering this study, and using the PedsQL, we documented a significant change in the patients as they switched from immediate-release cysteamine to DR-CYS in 3 measures: the social function (P = .049), school function (P = .004), and in total function (P = .048). We assessed the subsequent change from all baseline values in the PedsQL, and demonstrated that after 2 years of DR-CYS therapy, the 3 significant changes persisted, and there were no significant losses of quality of life in the other 2 measures (physical, emotional) (Table II).
Table II.
PedsQL percent change from baseline over the 24-month study
| Functionality parameter | |||||
|---|---|---|---|---|---|
| Physical | Emotional | Social | School | Total | |
| Intercept (LSM) | 6.62 | 6.62 | 11.23 | 14.27 | 5.99 |
| P value | .160 | .136 | .049 | .004 | .048 |
| Slope | 0.302 | 0.019 | 0.492 | 0.126 | 0.184 |
| P value | .054 | .890 | .201 | .598 | .072 |
LSM, least squares mean.
To summarize the analyses of percent change in PedsQL item scores from baseline, regression analyses with a mixed model of ANOVA were performed. The intercept value (LSM) represents the change from the value under immediate release cysteamine from the prior study2 with its accompanying P value for that change. The slope represents the change from that new baseline during the 24 months of the current study using DR-CYS. There was no loss of the significant changes gained in the social, school, and total function parameters and no decline in the other 2 measured parameters (physical and emotional) during the 24-month study.
Safety
There were no unexpected or serious safety concerns experienced by subjects in the current study attributable to DR-CYS. The incidence of AEs during this study was reduced to 0.059 AEs per individual per month at 24 months compared with 0.30 AEs per individual per month as determined during the period of treatment under immediate-release cysteamine bitartrate in our prior study.2 More specifically, all 40 (100.0%) of the subjects have experienced 1 or more treatment-emergent AEs. Gastrointestinal disorders were the highest subject treatement emergent adverse event incidence experienced by 35 (87.5%) subjects. Expressed in terms of specific symptoms, emesis was experienced by 28 (70.0%) subjects, followed by headache in 14 (35.0%) subjects, upper respiratory tract symptoms in 9 (22.5%) subjects, and diarrhea in 8 (20.0%) subjects.
Discussion
Nephropathic cystinosis is a systemic disease that results in kidney failure at the end of the first decade of life when untreated,7–9 or even when treatment is initiated after 5 years of age with immediate release cysteamine therapy.10 To date, the only studies evaluating such therapy in patients with the disorder have been observational and retrospective.9,10 Such studies have suggested that immediate release cysteamine improves ultimate outcomes by reducing the frequency of hypothyroidism, diabetes, neuromuscular disorders, or death long-term.10–12 Treatment before age 5 years may preserve kidney function in some patients.10 Such outcomes may be linked to WBC [cystine] itself, a marker of disease control, but such observations have not rigorously controlled for this exposure.9,10 However, optimal control of the disease, a WBC [cystine] <1 nmol ½ cystine/mg protein, has never been reported in a cohort of patients because of the inherent difficulties in having patients remain adherent to the every six hours regimen required for immediate release therapy to achieve such control.13
In contrast, we provide 2 years of data with optimal control of the disease under a controlled protocol and demonstrate that with DR-CYS, there was preservation of kidney function, stable somatic growth, and BMI. Importantly, quality of life improved in several subscales and in total function as patients switched to delayed release cysteamine compared with immediate release cysteamine, and these changes were maintained for the entire 24-month period. None of the other subscales were lower at the end of the study. In addition, we found no untoward side effects or safety concerns with DR-CYS and an overall slight decrease in overall AEs (data not reported).
Two large series of patients have examined retrospectively the relationship between the age of treatment initiation with immediate release cysteamine therapy and subsequent kidney functional outcomes in nephropathic cystinosis.10,14 When used either before 2 years of age, and with a median WBC (cystine) ≤2 nmol ½ cystine/mg protein in 1 study (adequate treatment, representative of only 25.4% of the reported study population),14 or when used before 5 years of age and with a mean WBC (cystine) ≤2 nmol ½ cystine/mg protein (representative of only 29.3% of the study population reported),10 there was preservation of reduced kidney function. This attests to the inability to maintain optimal disease control with immediate-release cysteamine for most patients with nephropathic cystinosis, and that progressive chronic kidney disease is inevitable.
We studied patients at a mean age and a similar level of reduced kidney function to those reported by Markello et al.9 In their study, the slope of the line describing subsequent years of kidney function in the ‘adequate treatment’ group was downward trending, but not as steep as those untreated or poorly treated. In distinction, in the current study using DR-CYS, we demonstrated that prospective, optimal control of disease activity (WBC [cystine] < 1 nmol ½ cystine/mg protein) did not lead to a downward slope change in eGFR during the 2 years of treatment.
What might account for the differences in outcomes of kidney function between immediate release cysteamine and DR-CYS? First, we targeted patients with an optimal level of disease control, and by having a prospective, controlled study protocol, could maintain our target values, rather than relying on observational data alone. In fact, our mean WBC [cystine] was always below 1 nmol ½ cystine/mg protein, and the patients reported in the retrospective study as using immediate release cysteamine and having ‘adequate treatment’ had a median value of≤2 nmol ½ cystine/mg protein, defining a population well above the levels we achieved.9 Second, we would suggest that the adherence was superior with DR-CYS in our study compared with immediate-release cysteamine in observational studies, as we were able to maintain optimal disease control through measured WBC [cystine] at a lower dose of DR-CYS over time in the entire cohort. This likely is due to the ability to have the drug taken every 12 hours compared with the need to have every 6-hour dosing with immediate release cysteamine. Consistent with our hypothesis that increased adherence was an important component of outcome in our patients treated with DR-CYS for 2 years, are the additional observations we made of preservation of statural growth, stability of BMI, and improved measures of quality of life.
Our study provided a new insight into the management of nephropathic cystinosis, based on the measurements of plasma [cysteamine]. Given the observation that 94.5% of measured WBC [cystine] values demonstrated optimal control (<1 nmol ½ cystine/mg protein) when the plasma [cysteamine] was >0.1 mg/L 0.5 hours after the DR-CYS dose, patients might have only plasma [cysteamine] measured in follow-up care. Importantly, this measure of plasma [cysteamine] does not reflect long-term drug adherence, but rather that the dose administered to the patient is sufficient for optimal disease control. Furthermore, until more data are available, we would suggest that such means of patient follow-up be limited to those having demonstrated optimal control for a period of time.
Our study had limitations. We did not directly compare a 2-year treatment period between DR-CYS and immediate release cysteamine. This was purposeful, following the observation we made of noninferiority of DR-CYS compared with immediate release cysteamine for optimal disease control measured by WBC (cystine).2 As has been well documented many times, patients in clinical trials may have outcomes based on higher adherence than in real world care. However, given the ability to dose every 12 hours and avoid a middle of the night dose required for immediate release cysteamine, real world adherence may more closely mimic that which we observed in the current study. Lastly, we studied only previously, optimally controlled patients under immediate release cysteamine therapy, which by historical record represents a minority of patients with nephropathic cystinosis. Current ongoing studies with DR-CYS in both treatment naïve patients and in those not optimally controlled patients with immediate release cysteamine will answer additional questions about the utility of DR-CYS in a broader population of patients with cystinosis. In addition, longer-term outcomes using DR-CYS should be done to obtain further evidence of the important effects we observed after 2 years of treatment.
The blood level of WBC [cystine] maintained below 1 nmol ½ cystine/mg protein for 2 years is associated with preservation of native kidney function, stable statural growth, stable body mass, and improved quality of life. Although the longer-term consequences of DR-CYS use remain unknown at present, it is highly encouraging to suggest that increased adherence may result from this new therapeutic agent’s every 12 hour dosing regimen, and the improvements we documented on patient-centered quality of life measures.
Acknowledgments
Supported by Raptor Pharmaceuticals Inc and National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR000150, UL1TR000454, and UL1 TR000093). Raptor Pharmaceuticals also supported the patients’ travel during the study. M.B. and P.R. are employees of Raptor Pharmaceuticals Inc.
We thank the patients, their parents, and the study personnel at each site for their sustained interest, participation, and expertise in carrying out this study.
Glossary
- AE
Adverse event
- BMI
Body mass index
- DR-CYS
Delayed-release cysteamine bitartrate
- eGFR
Estimated glomerular filtration rate
- PD
Pharmacodynamics
- PedsQL
Pediatric Quality of Life Inventory
- PK
Pharmacokinetics
- WBC [cystine]
White blood cell content of cystine
Footnotes
The other authors declare no conflicts of interest.
References
- 1.Cystagon-PI. Cystagon Package Insert. Package Insert Labeling. 2007 [Google Scholar]
- 2.Langman CB, Greenbaum LA, Sarwal M, Grimm P, Niaudet P, Deschênes G, et al. A randomized controlled crossover trial with delayed-release cysteamine bitartrate in nephropathic cystinosis: effectiveness on white blood cell cystine levels and comparison of safety. Clin J AmSoc Nephrol. 2012;7:1112–1120. doi: 10.2215/CJN.12321211. Erratum in Clin J AmSoc Nephrol 2013; 8:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4:1832–1843. doi: 10.2215/CJN.01640309. [DOI] [PubMed] [Google Scholar]
- 4.CDC. A SAS Program for the CDC Growth Charts. [Accessed December 15, 2013];2000 Available at: http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm.
- 5.Powell KL, Langman CB. An unexpected problem in the clinical assessment of cystinosis [Letter to the Editor] Pediatr Nephrol. 2012;27:687–688. [Google Scholar]
- 6.Covance. Biomarker and Bioanalytical Anlayte List. 2011 [Google Scholar]
- 7.Seegmiller JE, Friedmann T, Harrison HE, Wong V, Schneider JA. Cystinosis. Combined clinical staff conference at the National Institutes of Health. Ann Intern Med. 1968;68:883–905. doi: 10.7326/0003-4819-68-4-883. [DOI] [PubMed] [Google Scholar]
- 8.Mahoney CP, Striker GE, Hickman RO, Manning GB, Marchioro TL. Renal transplantation for childhood cystinosis. N Engl J Med. 1970;283:397–402. doi: 10.1056/NEJM197008202830804. [DOI] [PubMed] [Google Scholar]
- 9.Markello TC, Bernardini IM, Gahl WA. Improved renal function in children with cystinosis treated with cysteamine. N Engl J Med. 1993;328:1157–1162. doi: 10.1056/NEJM199304223281604. [DOI] [PubMed] [Google Scholar]
- 10.Brodin-Sartorius A, Tete MJ, Niaudet P, Antignac C, Guest G, Ottolenghi C, et al. Cysteamine therapy delays the progression of nephropathic cystinosis in late adolescents and adults. Kidney Int. 2012;81:179–189. doi: 10.1038/ki.2011.277. [DOI] [PubMed] [Google Scholar]
- 11.Gahl WA, Balog JZ, Kleta R. Nephropathic cystinosis in adults: natural history and effects of oral cysteamine therapy. Ann Intern Med. 2007;147:242–250. doi: 10.7326/0003-4819-147-4-200708210-00006. [DOI] [PubMed] [Google Scholar]
- 12.Kleta R, Bernardini I, Ueda M, Varade WS, Phornphutkul C, Krasnewich D, et al. Long-term follow-up of well-treated nephropathic cystinosis patients. J Pediatr. 2004;145:555–560. doi: 10.1016/j.jpeds.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 13.Levtchenko EN, van Dael CM, de Graaf-Hess AC, Wilmer MJ, van den Heuvel LP, Monnens LA, et al. Strict cysteamine dose regimen is required to prevent nocturnal cystine accumulation in cystinosis. Pediatr Nephrol. 2006;21:110–113. doi: 10.1007/s00467-005-2052-0. [DOI] [PubMed] [Google Scholar]
- 14.Gahl WA, Thoene JG, Schneider JA. Cystinosis. N Engl J Med. 2002;347:111–121. doi: 10.1056/NEJMra020552. [DOI] [PubMed] [Google Scholar]