Abstract
Objective
Intermediate and small conductance KCa channels IK1 (KCa3.1) and SK3 (KCa2.3) are primary targets of endothelial Ca2+ signals in the arterial vasculature and their ablation results in increased arterial tone and hypertension. Activation of IK1 channels by local Ca2+ transients from internal stores or plasma membrane channels promotes arterial hyperpolarization and vasodilation. Here, we assess arteries from genetically altered IK1 knockout mice (IK1−/−) to determine whether IK1 channels exert a positive feedback influence on endothelial Ca2+ dynamics.
Approach and Results
Using confocal imaging and custom data analysis software we found that while the occurrence of basal endothelial Ca2+ dynamics was not different between IK1−/− and wild-type (WT) mice (p > 0.05), the frequency of acetylcholine (ACh 2 µM)-stimulated Ca2+ dynamics was greatly depressed in IK1−/− endothelium (515 ± 153 vs. 1860 ± 319 events; p < 0.01). In IK1−/−/SK3T/T mice, ancillary suppression (+Dox) or overexpression (−Dox) of SK3 channels had little additional impact on the occurrence of events under basal or ACh-stimulated conditions. SK3 overexpression did, however, restore the depressed event amplitudes. Removal of extracellular Ca2+ reduced ACh-induced Ca2+ dynamics to the same level in WT and IK1−/− arteries. Blockade of IK1 and SK3 with the combination of charybdotoxin (0.1 µM) and apamin (0.5 µM) or TRPV4 channels with HC-067047 (1 µM) reduced ACh Ca2+ dynamics in WT arteries to the level of IK1−/−/SK3T/T+Dox arteries. These drug effects were not additive.
Conclusions
IK1, and to some extent SK3 channels, exert a substantial positive feedback influence on endothelial Ca2+ dynamics.
Keywords: Endothelium, Calcium, IK1, SK3, TRPV4
Endothelial Ca2+ activated potassium channels (KCa), including small conductance (SK3 or KCa2.3) and intermediate conductance (IK1 or KCa3.1) isoforms, are important effectors of vasodilation in the arterial circulation. These channels elicit endothelium-derived hyperpolarization (EDH) of vascular smooth muscle, and their pharmacologic inhibition completely blocks nitric oxide- and prostacyclin-independent vasodilation in various arterial beds.1–6 Studies on IK1-deficient mice (IK1−/−) have revealed a pivotal role of this channel in hyperpolarizing the endothelial membrane, dilating resistance arteries, and modulating blood pressure.7 A combined transgenic mouse model (IK1−/−/SK3T/T), which includes conditional doxycycline (Dox)-controlled SK3 channel expression 5, 8 on top of the conventional IK1 knockout genotype, has allowed for detailed elucidation of the combined and complementary roles of these channels.9 This genetic model has demonstrated the pivotal role of IK1channels in agonist (e.g. ACh)-induced dilations of conduit arteries and arterioles and has exposed the supporting role of SK3 channels, both in facilitating nitric oxide-dependent dilations and in compensating for the loss of IK1 channels. Genetic co-suppression of both channels blunts maximal ACh dilations by ~60%.9
Recent findings have implicated IK1 channels as direct targets of dynamic repetitive and short-lived (250 ms – 3 s) Ca2+ events that occur primarily along myoendothelial junctions10, establishing a persistent mechanism for hyperpolarizing vascular smooth muscle and modulating arterial tone. In addition to this basal activation, endothelial stimulation with agonist (e.g. ACh) increases total dynamic events by increasing the number of Ca2+ liberating sites (often multiple sites per cell) along the intima and increasing the frequency of existing active sites.10 Although the primary Ca2+ events (Ca2+ pulsars) emit from IP3R on the endoplasmic reticulum, plasma membrane transient receptor potential (TRP) channels11–13 including TRPA1 in rat cerebral arteries14–16 and TRPV4 in mouse mesenteric arteries17, have recently been implicated as important triggers or potentiators of the endothelial Ca2+ dynamics and vasodilation. These TRP channels have been found to associate closely with KCa channels18, and their direct stimulation promotes KCa-dependent EDH-mediated vasodilation in mice.19 In mouse mesenteric arteries, ACh has been found to evoke spatially restricted Ca2+ sparklets through TRPV4 channels, and these signals augment IK1 channel activation.17 Since Ca2+-induced K+ efflux through IK1 channels and the resulting hyperpolarization might increase the electrochemical driving force for Ca2+ entry, a central question addressed in the current study is whether IK1 channels control endothelial Ca2+ dynamics as a potential positive feedback mechanism.
Quantifying endothelial Ca2+ dynamics is essential for understanding effector recruitment and graded vasodilation. We recently developed and implemented an autodetection and analysis algorithm that allows for comprehensive evaluation of dynamic Ca2+ transients and complex Ca2+ signal distributions in intact tissues, including endothelium.20, 16 Here, we use IK1−/− mice and our automated Ca2+ signal analysis to discern whether IK1 channels, alone or in combination with SK3 channels, enhance endothelial Ca2+ dynamics in mesenteric arteries.
Material and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
IK1 channel ablation does not affect the frequency of basal endothelial Ca2+ dynamics
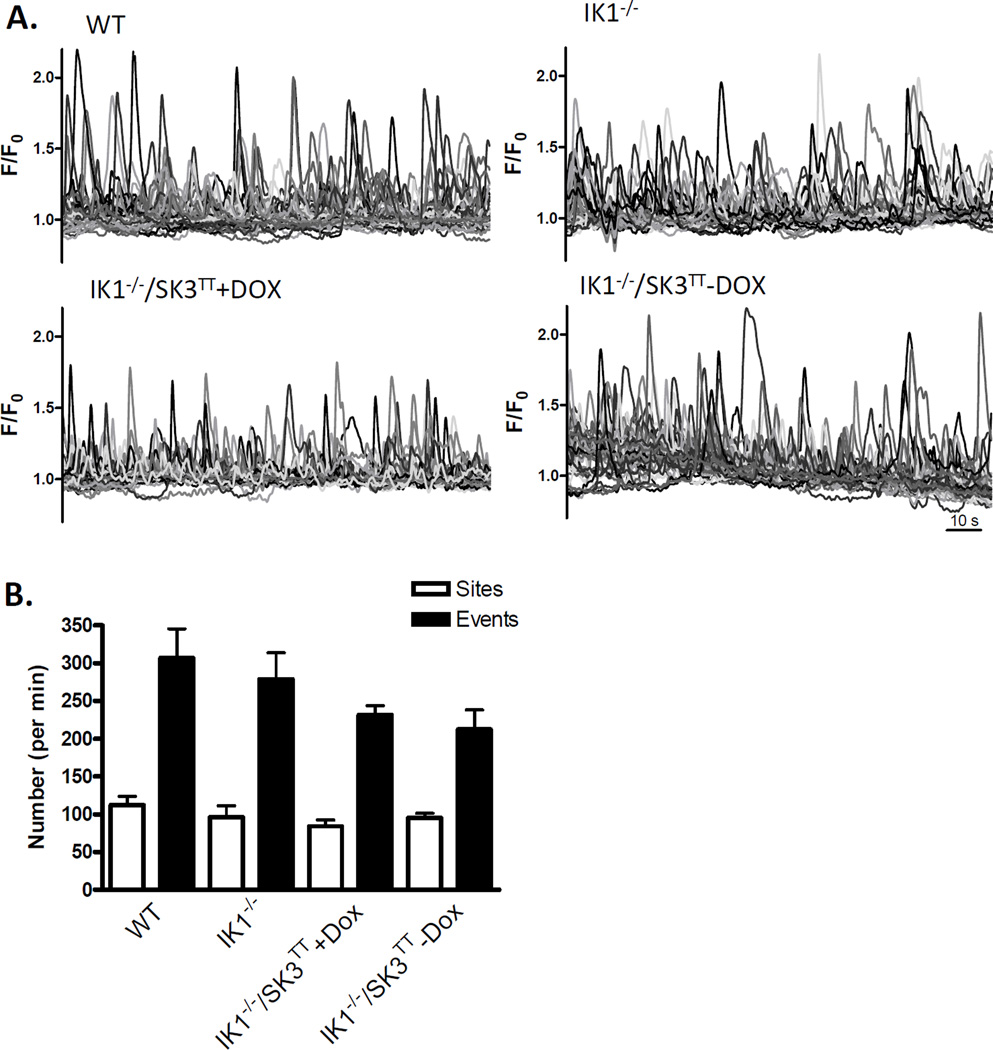
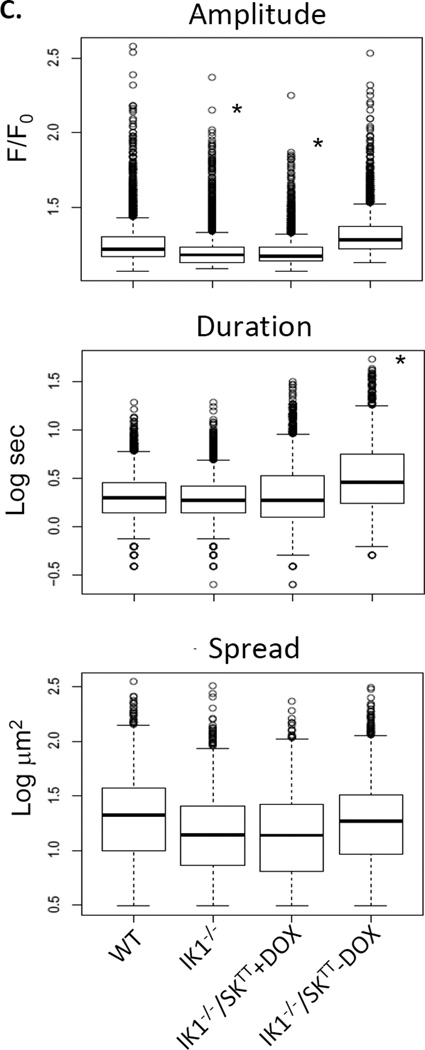
To determine whether IK1 channels provide positive feedback regulation of ongoing Ca2+ dynamics in the endothelium, we evaluated Fluo-4 AM loaded open mesenteric arteries from wild type and IK1-deficient (IK1−/−) transgenic mice using confocal microscopy (Fig 1). The arterial endothelia of all WT, IK1−/− and IK1−/−/SK3T/T mice produced basal Ca2+ transients under resting conditions (Fig 2A). In arteries from WT mice, these events occurred at rate of 307 ± 38 per min from 112 ± 11 sites. Although the number of events and sites trended lower in the IK1−/− mice (279 ± 35 per min from 96 ± 15 sites), no significant differences were discerned (p > 0.05, n = 6; Fig 2B). Evaluation of IK1 knockout mice in which SK3 channel expression was either suppressed (IK1−/−/SK3T/T+Dox) or overexpressed (IK1−/−/SK3T/T−Dox) also showed no significant difference from WT or IK1−/− alone (231 ± 12 per min from 84 ± 8 sites and 212 ± 26 per min from 95 ± 6 sites, respectively). These data suggest no net influence of IK1/SK3 channels on the basal generation of endothelial Ca2+ dynamics, including the number of events, sites and events per site. Assessment of specific Ca2+ event parameters (Fig 2C) revealed that event amplitudes were depressed in IK1−/− as well as IK1−/−/SK3T/T+Dox mice to a similar degree (p < 0.01 vs. WT) and were recovered to WT levels in SK3 overexpressing IK1−/−/SK3T/T−Dox mice. Also, Ca2+ event durations were significantly elevated in IK1−/−/SK3T/T−Dox mice compared to all other genotypes including WT.
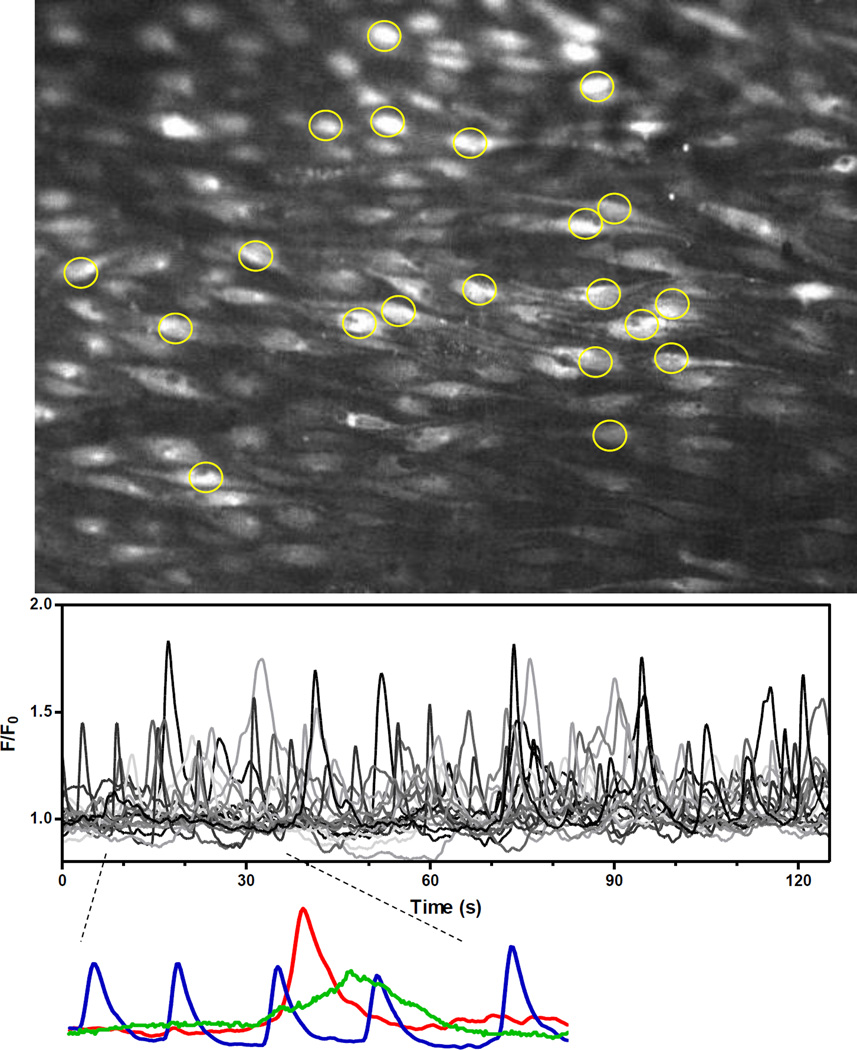
Figure 1.
Detection and analysis of endothelial Ca2+ dynamics in open mouse mesenteric arteries. Opened mesenteric arteries were mounted on silicone blocks (intima-up), loaded with fluo-4 AM and the endothelium imaged with a spinning-disk confocal. Image shows mean fluorescence projection of a 2-minute recording. Image sequences were analyzed offline using LC_Pro, allowing ROIs to be placed automatically at the spatial centers of detected events (20 of the 162 ROIs are shown). Inset shows recordings from three distinct sites (ROIs), emphasizing the variety of basal Ca2+ events with respect to frequency, amplitude and duration.
Figure 2.
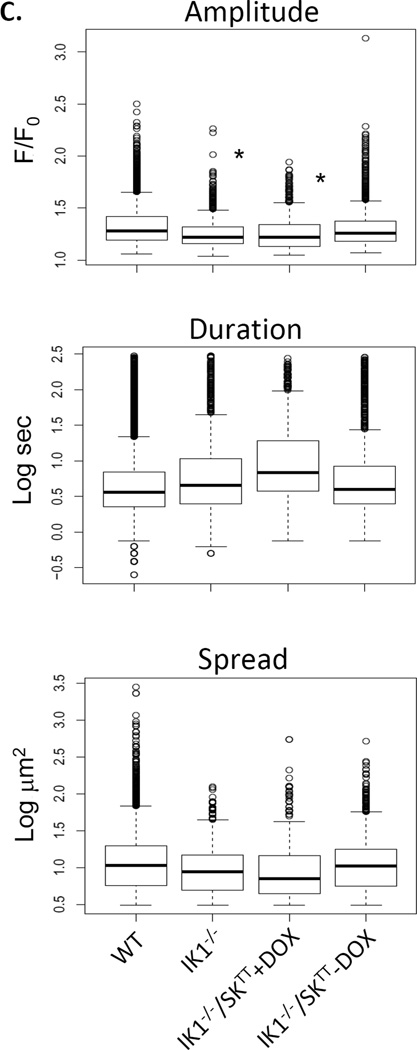
Effect of IK1 and SK3 expression on basal endothelial Ca2+ dynamics. A. Tracings show Ca2+ transients recorded in mesenteric arteries from wild-type (WT) mice, IK1 knockout (IK1−/−) mice, and IK1 knockout mice in which SK3 expression is suppressed (IK1−/−/SK3TT+Dox) or overexpressed (IK1−/−/SK3TT−Dox). B. The number of Ca2+ sites and events occurring per min within sampled fields. Neither sites nor events were different among groups (p > 0.05 for all comparisons, n = 6). C. Individual event parameters (* p < 0.01 vs. WT).
ACh-induced endothelial calcium dynamics are reduced in arteries of IK1-deficient mice
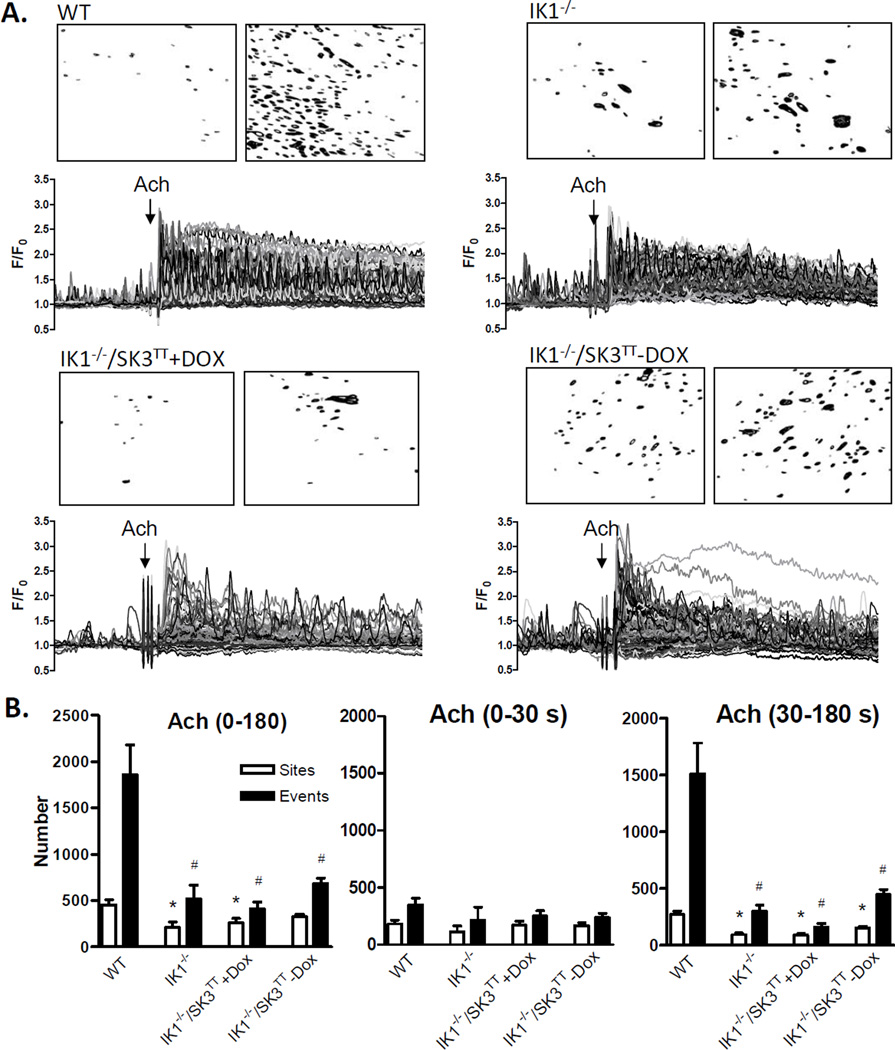
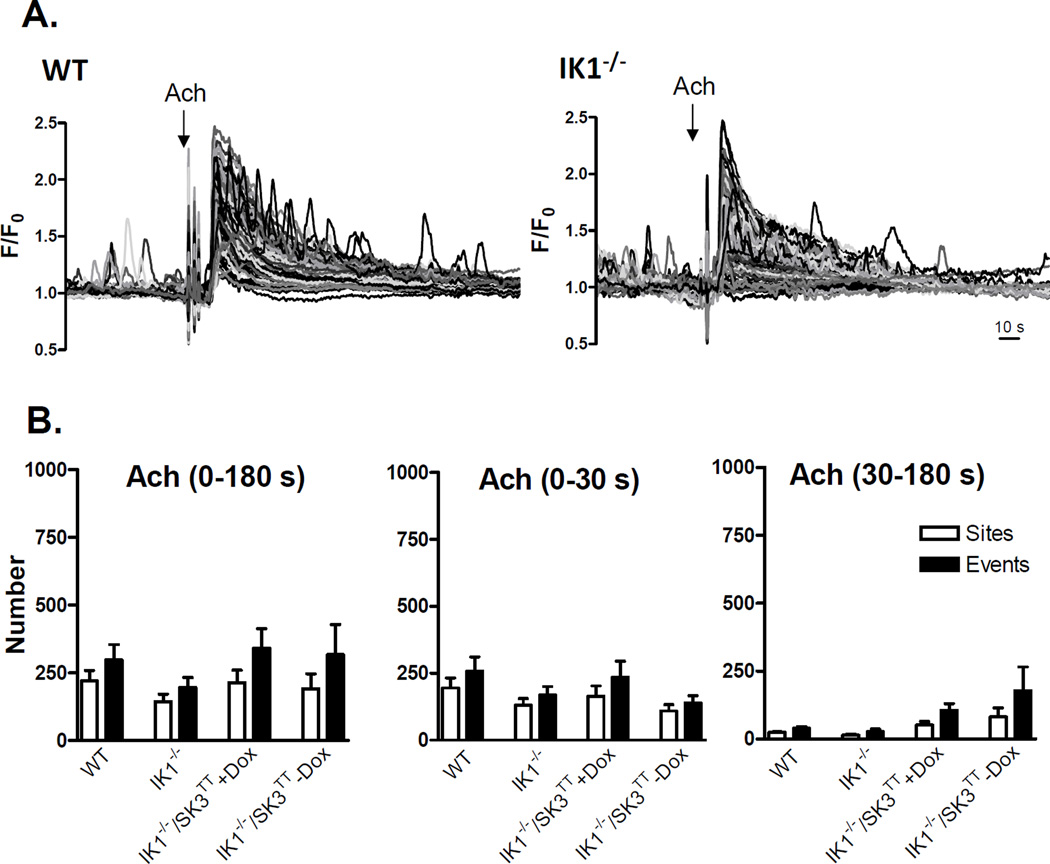
ACh increases Ca2+ dynamics in mesenteric artery endothelial cells by sensitizing release from internal Ca2+ stores through IP3 receptors and by stimulating influx of extracellular Ca2+ through membrane nonselective cation channels, both of which are known to target KCa channels.10, 17 We examined whether a positive influence of KCa channels on Ca2+ dynamics is unmasked under these stimulated conditions. Fig 3A shows Ca2+ recordings from the endothelia of WT, IK1−/−, and IK1−/−/SK3T/T + or − Dox mice before and after addition of 2 µM ACh. ACh markedly increased Ca2+ dynamics, which included a rapid phase of multiple synchronous peaks followed by a sustained phase of heterogeneous transients. Overall, approximately 70% of the endothelial cells in WT arteries responded to ACh challenge, compared to approximately 25% in IK1−/−, 20% in IK1−/−/SK3T/T+Dox and 30% in IK1−/−/SK3T/T−Dox arteries. With respect to the total number of events generated over the full 180-second time course of ACh exposure (Fig 3B), responses were blunted in IK1−/− arteries compared to WT (515 ± 153 vs. 1860 ± 319 events; p < 0.001, n = 6). The number of events was seemingly further depressed in SK3-suppressed (IK1−/−/SK3T/T+Dox) arteries (409 ± 74 events) and partially recovered in SK3-overexpressed (IK1−/−/SK3T/T−Dox) arteries (685 ± 57 events), but neither was significantly different from IK1−/− alone, suggesting no additional net impact of SK3 channel suppression or overexpression. Notably, the frequency of events per site was similarly depressed in all IK1-deficient arteries compared to WT (WT 4.2 ± 0.6; IK1−/− 2.4 ± 0.2; IK1−/−/SK3T/T+Dox 1.6 ± 0.06; and IK1−/−/SK3T/T−Dox 2.1 ± 0.1; p<0.05 for all compared to WT), suggesting that loss of IK1 reduces both the number of active sites and the number of events occurring at each site. ACh responses may be divided into two phases, an initial phase primarily attributable to internal store release and a sustained phase that is highly dependent on extracellular Ca2+ entry.21 Partitioning our 3-minute ACh responses into initial (first 30 seconds) and sustained (30 – 180 seconds) phases revealed that IK1 channels primarily influence the occurrence of Ca2+ events in the later phase (Fig 3B). With respect to individual event parameters (Fig 3C), event amplitudes were reduced in IK1−/− and IK1−/−/SK3TT+Dox and restored in IK1−/−SK3TT−Dox arteries, similar to basal data, whereas duration and spatial spread were not different among the groups.
Figure 3.
ACh -stimulated Ca2+ dynamics in the endothelium of WT, IK1−/−, and IK1−/−/SK3TT ± Dox mouse arteries. A. Each panel shows a continuous 4-minute recording of Ca2+ dynamics with ACh (2 µM) treatment at 1 minute. Images show corresponding binary masks of the sampled intimal fields depicting sites of statistically relevant Ca2+ elevation (black) before (left) and after (right) ACh addition. B. Summary of sites and events following ACh exposure. The left panel shows data for the entire 180-second ACh exposure whereas the middle and right panels show the ACh response separated into the first 30 seconds (0–30 s) and the remaining 150 s (30–180 s) of ACh exposure(* p < 0.05 for sites vs. WT and # p < 0.05 for events vs. WT; n = 6–7). C. Individual event parameters (* p < 0.01 vs. WT).
KCa channel potentiation of endothelial Ca2+ dynamics depends on extracellular Ca2+ entry through TRPV4 channels
Next, we directly tested the role of extracellular Ca2+ by removing it from the bath. ACh-stimulated Ca2+ dynamics were substantially blunted in the absence of extracellular Ca2+ (Ca2+-free + 1 µM EGTA), particularly after the first 30 seconds (Fig 4A and B). Importantly, under Ca2+-free conditions, ACh-stimulated Ca2+ events were not significantly different among the genotypes (WT 256 ± 64; IK1−/− 140 ± 44; IK1−/−/SK3T/T+Dox 340 ± 72; and IK1−/−/SK3T/T−Dox 227 ± 96) at any phase of the response (Fig 4B), indicating that the KCa channels primarily enhance Ca2+ influx.
Figure 4.
ACh-stimulated endothelial Ca2+ dynamics in Ca2+-free solution. A. Continuous recordings of Ca2+ dynamics are shown for arteries from WT and IK1−/− mice with ACh (2 µM) treatment at 1 minute. B. Summary of sites and events following ACh exposure. The left panel shows data for the entire 180-second ACh exposure whereas the middle and right panels show the ACh response separated into the first 30 seconds (0–30 s) and the remaining 150 s (30–180 s) of ACh exposure (n = 5–7, p > 0.05 for all event and site comparisons). C. Individual event parameters (* p < 0.01 vs. WT).
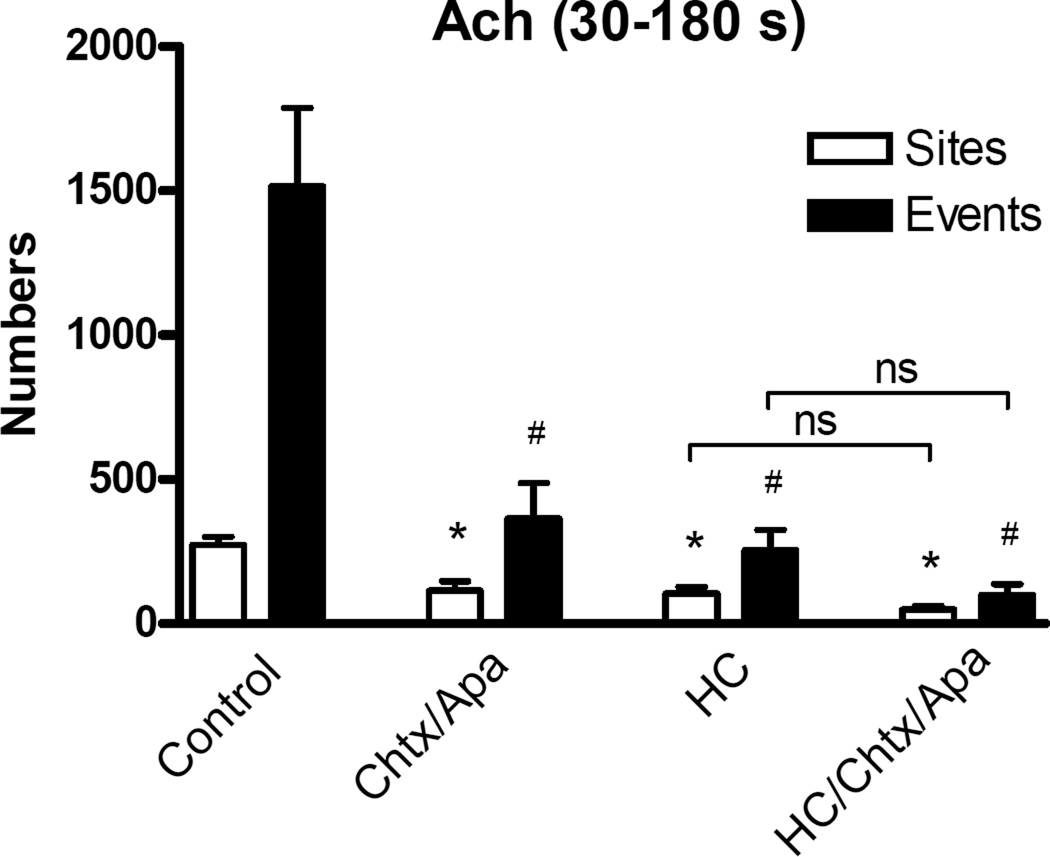
TRPV4 channels are major conduits of Ca2+ entry in the mesenteric artery endothelium.22 They carry discrete ACh-stimulated Ca2+ transients at the plasma membrane that are known to elicit KCa channel activation.17 We assessed whether the IK1 augmentation of endothelial Ca2+ dynamics in normal arteries occurs through potentiation of TRPV4 channel Ca2+ entry. Here, we used WT arteries, where native feedback signaling is preserved. The TRPV4 channel blocker HC-067047 (1 µM) had no significant effect on basal dynamics (data not shown) but it greatly impaired ACh activation of Ca2+ events ( 254 ± 72 vs. 1517 ± 272; Fig 5), supporting a central role for TRPV4 in ACh-stimulated Ca2+ entry. Notably, pharmacologic blockade of IK1 and SK3 channels with the combination of charybdotoxin (0.1 µM) and apamin (0.5 µM) depressed ACh-induced Ca2+ dynamics in WT arteries (to 364 ± 123 events during the 30 to 180-second phase),values comparable to those in IK1−/−/SK3TT+Dox arteries. The reduction of Ca2+ dynamics elicited by charybdotoxin and apamin plus HC-067047 (99 ± 38 events) was not significantly different from that resulting from HC-067047 treatment alone (Fig 5). Notably, Ca2+ events in IK1−/−/SK3T/T+Dox arteries were not significantly altered by addition of charybdotoxin and apamin with or without HC-067047 (data not shown)
Figure 5.
Role of TRPV4 channels in KCa-potentiated endothelial Ca2+ dynamics. Bar graphs show ACh -stimulated Ca2+ dynamics (sites and events over 30–180 seconds) in the endothelium of WT arteries following blockade of IK1 and SK3 channels with 0.1 µM charybdotoxin (Chtx) and 0.5 µM apamin (Apa), blockade of TRPV4 channels with 1 µM HC-067047 (HC), or the combination of all three drugs (* p < 0.05 for sites vs. control and # p < 0.05 for events vs. control; ns, not significant; n = 4–6).
Discussion
Small/intermediate conductance KCa channels have been identified as primary targets of basal and ACh-stimulated Ca2+ dynamics in arterial endothelium.10, 17, 23 Previous work has shown that genetic ablation of IK1 and SK3 channels impairs vascular hyperpolarization and ACh-induced vasodilation, and promotes hypertension.5, 7, 9 Here, we used IK1 deficient mice, including those with suppressed or overexpressed SK3, to assess whether these channels exert a positive feedback influence on the endothelial Ca2+ dynamics themselves. Our data indicate that while IK1 channels do not significantly influence ongoing basal Ca2+ dynamics in mesenteric artery endothelium, they do strongly potentiate the occurrence of Ca2+ dynamics following endothelial stimulation with acetylcholine. Increasing or decreasing SK3 expression had little additional effect on the occurrence of events but did promote increased event amplitudes and durations. We found that KCa-promoted Ca2+ dynamics were completely dependent on extracellular Ca2+ entry through TRPV4 channels. Together, these findings suggest that IK1 channels may play an important role in amplifying vasodilation by expanding TRPV4-triggered dynamic Ca2+ signals along the intima while SK3 channels may play a supporting role in adjusting event size
Recent studies have revealed the importance of spatially and temporally distinct Ca2+ transients, rather than global Ca2+ changes, in tuning the specificity and magnitude of endothelial responses in intact arteries.10, 17, 16, 18, 24 These inherent Ca2+ dynamics are clearly discernible in the endothelia of both pressurized and opened mesenteric arteries.20 Confocal imaging in pressurized arteries is limited to very few endothelial cells due to narrow viewable fields and movement artifact. Employing open artery preparations in the current study allowed us to comprehensively quantify event parameters in broad intact endothelial fields using our custom software (LC_Pro). Designed to detect all Ca2+ deflections above noise without user bias, this approach reveals a heterogeneous assortment of intrinsic events occurring along the vascular intima. In mesenteric arteries, basal endothelial Ca2+ dynamics emit intermittently from internal stores through IP3Rs.10 These signals elicit hyperpolarization by engaging nearby IK1 channel clusters in the endothelial membrane, particularly at myoendothelial junction sites.10 At these sites, EDH is communicated to smooth muscle through gap junctions 4, 25–27 or via K+ activation of inward rectifier K+ channels (KIR) or Na+/K+ ATPase.28, 29 Endothelial stimulation with Gq-coupled receptor agonist (e.g. ACh) amplifies this hyperpolarization and hence vasodilation by recruiting new Ca2+-liberating sites along the intima and by increasing the frequency of dynamic Ca2+ events at pre-existing sites.10 New data suggests that in addition to IP3 elevation, this Ca2+ recruitment depends on stimulation of membrane TRPV4 channels, which increases the occurrence of focal Ca2+ sparklet events along the plasma membrane.17 These transients are known to both directly activate IK1 channels and to provoke large increases in endothelial Ca2+ dynamics. The latter effect likely involves Ca2+ induced Ca2+ release from IP3-sensitized stores. In fact, isolated TRPV4 sparklets are only discernible when internal stores are depleted.17 Overall, the agonist-augmented Ca2+ dynamics support further vasodilation through expanded KCa-mediated hyperpolarization as well as eNOS dependent NO production.30
The pivotal functional role of IK1 channels has been well demonstrated in pharmacologic studies and is clearly evident in IK1-deficient mice that exhibit blunted ACh-induced hyperpolarization and dilation (in both conduit and resistance arteries) as well as hypertension.7, 9 SK3 suppression further exacerbates these dilator and blood pressure changes while SK3 overexpression partially recovers them. 9 It should be noted that endothelial Ca2+ dynamics persistently recruit IK1 and/or SK3 channels in the vasculature,10, 16, 17 regulating membrane potential and tone (i.e. via direct communication of the membrane potential to smooth muscle via gap junctions). This effect may constantly modify blood pressure with or without altering the Ca2+ signals themselves. However, the lack of voltage-gated Ca2+ channels in non-excitable endothelial cells allows IK1 channels to act not only as Ca2+ detectors but as Ca2+ amplifiers, whereby Ca2+-activated K+ efflux and hyperpolarization increases the driving force for Ca2+ entry. We found that in arteries from IK1-deficient mice, basal endothelial Ca2+ dynamics are not significantly altered whereas ACh-stimulated Ca2+ dynamics are substantially muted. This suggests that endothelial stimulation is needed to drive sufficient IK1-dependent positive feedback Ca2+ entry to enhance dynamics. The specific impact of IK1 channels on Ca2+ entry is supported by our observations that IK1 knockout depressed sustained ACh Ca2+ dynamics without affecting the initial Ca2+ release (first 30 seconds21, 31) and ACh responses in the absence of extracellular Ca2+ were indistinguishable between WT and IK1−/− arteries.
Our findings support TRPV4 channels being the primary targets of both ACh-stimulated Ca2+ influx and the IK1 Ca2+ feedback. In WT arteries, selective inhibition of TRPV4 channels greatly blunted the occurrence of sustained stimulated Ca2+ dynamics, similar to that achieved with Ca2+ free solution (see Fig 4) or general inhibition of nonselective cation channel influx with Gd3+ (data not shown). Indeed, ACh-induced vasorelaxation is greatly impaired in arteries of TRPV4-deficient mice (TRPV4−/−), including a general loss of EDH.32 However, intact ACh-induced dilation has been observed in carotid arteries of TRPV4−/− mice, suggesting regional differences among beds.19 Notably, in the current study, specific pharmacologic blockade of IK1/SK3 channels in WT arteries mimicked the effect of genetic IK1/SK3 knockdown on ACh-stimulated Ca2+ dynamics. Moreover, IK1/SK3 inhibition was not additive with TRPV4 inhibition, suggesting this impact of KCa activation requires TRPV4-mediated Ca2+-entry. Importantly, we have also found that the TRPV4 and IK1/SK3 effects on Ca2+ dynamics are preserved at 37°C (Supplemental Fig I), supporting their contribution under physiologic conditions.
The impact of IK1 on Ca2+ dynamics appears to be multifaceted. While they modestly augment Ca2+ event amplitude, under both basal and agonist-stimulated conditions, their most notable impact is acute recruitment of new events, which involves soliciting de novo Ca2+ firing sites along the intima as well as increasing firing frequencies at pre-existing sites (see Fig 3). This influence is highly dependent on TRPV4, implying that IK1 channels can tune endothelial responses by critically expanding subtle TRPV4 Ca2+ entry transients into robust repetitive Ca2+ events. Physiologically, these triggered events may be further amplified by Ca2+ store release, expanding recruitment of KCa channels as well as other Ca2+-dependent effectors. We recently reported a similar “triggering” role of TRP channels in rat cerebral arteries, where direct activation of ankyrin-associated TRPA1 channels ignited new wave-like endothelial cell Ca2+ events16, and recruitment of these new Ca2+ events corresponded precisely with IK1/SK3-mediated vasodilation. Although the physiologic role of TRPV4 - IK1 coupling is not yet clear, recent findings from Bagher et al suggest this association may effectively tune pressure-dependent responses in cremaster muscle arterioles, whereby increased endothelial TRPV4 Ca2+ dynamics at low intravascular pressure enhance EDH and contribute to autoregulation.18 Findings from Ma et al also suggest a role for TRPV4 in flow-induced endothelial Ca2+ entry.33 Our current findings suggest that IK1 channels may be particularly crucial in controlling the capacity of physiologic responses (i.e. to pressure, agonist and shear) not only by directly evoking Ca2+-dependent hyperpolarization but also by expanding the spatial and temporal range of the Ca2+ signals along the intima.
Previous evaluation suggests that while SK3 channels are not as essential as IK1 channels for ACh vasodilation, their suppression augments the effects of IK1 knockout on ACh dilations, and overexpression of SK3 partially rescues these dilations.9 Overall, IK1 knockout reduces ACh dilation of pressurized arteries to ~65% of control which is further reduced to ~45% by SK3 suppression and recovered to ~80% with SK3 overexpression.9 Distinct distributions of IK1 and SK3 channels within endothelial cell plasma membranes support their differential targeting.34 In the current study, we assessed whether SK3 channels might supplement IK1 impacts on endothelial Ca2+ dynamics. SK3 suppression had little additional effect on the occurrence of endothelial Ca2+ events. Moreover, SK3 overexpression failed to recover normal Ca2+ dynamics in IK1-deficient mice, indicating that SK3 channels cannot functionally replace IK1 channels. However, with respect to event parameters, SK3 overexpression tended to augment basal Ca2+ durations and effectively recovered depressed event amplitudes associated with IK1 deficiency under both basal and stimulated conditions. This suggests SK3 channels may play a role in positive feedback Ca2+ regulation by shaping the size and time course of individual events, even under basal conditions. Protraction of Ca2+ events may be particularly important in tuning stimulation of cellular effectors such as eNOS30, 35–37 as increased SK3 expression was previously found to enhance NO-mediated dilation of cremaster arterioles.9 Further study is warranted to elucidate the functional implications of differential IK1 and SK3 tuning of endothelial Ca2+ dynamics with graded stimuli, including other receptor agonists and shear stress.
Our current findings reveal a new mechanistic role of KCa channels in expanding the very Ca2+ signals they detect. IK1 channels are particularly pivotal in tuning real-time endothelial Ca2+ signaling and physiologic vasodilator responses. A limitation of the current study is that our comprehensive assessment of Ca2+ dynamics along the vascular intima cannot be obtained simultaneously with diameter measurements within individual pressurized arteries. However, the broad discriminating evaluation afforded by our algorithm exposes distinct profiles of physiologic signaling not previously recognized. Overall, our data fit well with an emerging model of endothelial vasoregulation based on close associations of TRP and KCa channels, and suggest relative expression, spatial proximity and differential trafficking of TRPV4 and IK1 might underlie variable levels of positive Ca2+ feedback, and hence EDH, in different vascular beds. Moreover, this feedback provides a mechanism through which KCa channels might predictably influence other Ca2+-dependent endothelial effectors such as eNOS and CaM kinases. Future studies will focus on how altered tuning of Ca2+ feedback by KCa channels contributes to endothelial dysfunction in disease and whether pharmacological manipulation of this mechanism by specific channel activators38 improves endothelial function.
Supplementary Material
Significance.
The intermediate and small conductance Ca2+-activated K+ channels, IK1 (KCa3.1) and SK3 (KCa2.3), have been established as key players in endothelium dependent vasodilation, particularly through endothelium derived hyperpolarization of vascular smooth muscle. These channels are activated by dynamic endothelial Ca2+ signals that increase with endothelial stimulation. Using genetically altered mouse models with differential IK1 and SK3 expression, the current study shows for the first time that these channels exert their influence not only through unidirectional signaling to smooth muscle but also by enhancing the cytosolic endothelial Ca2+ dynamics themselves. Dependent on augmented Ca2+ influx through membrane cation channels (TRPV4), this positive feedback influence is substantial under stimulated conditions. The work suggests an expanded role of endothelial KCa channels in arterial function and heightens interest in these channels as therapeutic targets to improve endothelial function.
Acknowledgements
Sources of funding
This work was supported by grants of the National Institutes of Health (Grant HL085887 to MST) and of the Deutsche Forschungsgemeinschaft (KO1899-10/11 to RK).
Footnotes
Disclosures
None
References
- 1.Coleman HA, Tare M, Parkington HC. EDHF is not K+but may be due to spread of current from the endothelium in guinea pig arterioles. Am J Physiol Heart Circ Physiol. 2001;280:H2478–H2483. doi: 10.1152/ajpheart.2001.280.6.H2478. [DOI] [PubMed] [Google Scholar]
- 2.Burnham MP, Bychkov R, Félétou M, Richards GR, Vanhoutte PM, Weston AH, Edwards G. Characterization of an apamin-sensitive small-conductance Ca2+-activated K+channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bychkov R, Burnham MP, Richards GR, Edwards G, Weston AH, Félétou M, Vanhoutte PM. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+channel in porcine coronary endothelium: relevance to EDHF. Br J Pharmacol. 2002;137:1346–1354. doi: 10.1038/sj.bjp.0705057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dora KA, Sandow SL, Gallagher NT, Takano H, Rummery NM, Hill CE, Garland CJ. Myoendothelial gap junctions may provide the pathway for EDHF in mouse mesenteric artery. J Vasc Res. 2003;40:480–490. doi: 10.1159/000074549. [DOI] [PubMed] [Google Scholar]
- 5.Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, Adelman JP, Nelson MT. Altered expression of small-conductance Ca2+-activated K+(SK3) channels modulates arterial tone and blood pressure. Circ Res. 2003;93:124–131. doi: 10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- 6.Edwards G, Félétou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch. 2010;459(6):863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 7.Si H, Heyken WT, Wölfle SE, Tysiac M, Schubert R, Grgic I, Vilianovich L, Giebing G, Maier T, Gross V, Bader M, de Wit C, Hoyer J, Köhler R. Impaired endothelium derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+channel. Circ Res. 2006;99:537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- 8.Bond CT, Sprengel R, Bissonnette JM, Kaufmann WA, Pribnow D, Neelands T, Storck T, Baetscher M, Jerecic J, Maylie J, Knaus HG, Seeburg PH, Adelman JP. Respiration and parturition affected by conditional overexpression of the Ca2+-activated K+channel subunit, SK3. Science. 2000;289:1942–1946. doi: 10.1126/science.289.5486.1942. [DOI] [PubMed] [Google Scholar]
- 9.Brähler S, Kaistha A, Schmidt VJ, et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119:2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- 10.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA. 2008;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwan HY, Huang Y, Yao X. TRP channels in endothelial function and dysfunction. Biochim Biophys Acta. 2007;1772:907–914. doi: 10.1016/j.bbadis.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di A, Malik AB. TRP channels and the control of vascular function. Curr Opin Pharmacol. 2010;10:127–132. doi: 10.1016/j.coph.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+channels. Circ Res. 2009;104:987–994. doi: 10.1161/CIRCRESAHA.108.189530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan MN, Francis M, Pitts NL, Taylor MS, Earley S. Optical Recording and Automated Analysis of Ca2+-Permeable Ion Channel Activity in Primary Human Endothelial Cells. Mol Pharmacol. 2012;82(3):464–472. doi: 10.1124/mol.112.078584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian X, Francis M, Solodushko V, Earley S, Taylor MS. Recruitment of dynamic endothelial Ca2+signals by the TRPA1 channel activator AITC in rat cerebral arteries. Microcirculation. 2013;20(2):138–148. doi: 10.1111/micc.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland CJ, Dora KA. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc Natl Acad Sci U S A. 2012;109(44):18174–18179. doi: 10.1073/pnas.1211946109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Köhler R. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS One. 2007;2(9):e827. doi: 10.1371/journal.pone.0000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francis M, Qian X, Charbel C, Ledoux J, Parker JC, Taylor MS. Automated region of interest analysis of dynamic Ca2+ signals in image sequences. Am J Physiol Cell Physiol. 2012;303(3):C236–C243. doi: 10.1152/ajpcell.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter TD, Ogden D. Acetylcholine-stimulated changes of membrane potential and intracellular Ca2+ concentration recorded in endothelial cells in situ in the isolated rat aorta. Pflugers Arch. 1994;428(5–6):476–484. doi: 10.1007/BF00374568. [DOI] [PubMed] [Google Scholar]
- 22.Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol. 2004;286:C195–C205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- 23.Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res. 2008;102:1247–1255. doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kansui Y, Garland CJ, Dora KA. Enhanced spontaneous Ca2+events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell Calcium. 2008;44:135–146. doi: 10.1016/j.ceca.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- 26.Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. Involvement of myoendothelial gap junction in the actions of endothelium-derived hyperpolarizing factor. Circ Res. 2002;90:1108–1113. doi: 10.1161/01.res.0000019756.88731.83. [DOI] [PubMed] [Google Scholar]
- 27.Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res. 2005;97:399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- 28.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 29.Ulusoy HB, Kaya MG. Potassium induced dilation in bovine coronary artery involves both inward rectifier potassium channels and Na+/K+ATPase. Acta Physiol Hung. 2009;96:427–436. doi: 10.1556/APhysiol.96.2009.4.3. [DOI] [PubMed] [Google Scholar]
- 30.Busse R, Mulsch A. Calcium-dependent nitric oxide synthesis in endothelial cytosol is mediated by calmodulin. FEBS Lett. 1990;265:133–136. doi: 10.1016/0014-5793(90)80902-u. [DOI] [PubMed] [Google Scholar]
- 31.Lückhoff A, Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflugers Arch. 1990;416(3):305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- 32.Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge ZD, Li R, Warltier DC, Suzuki M, Gutterman DD. Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension. 2009;53(3):532–538. doi: 10.1161/HYPERTENSIONAHA.108.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma X, Qiu S, Luo J, Ma Y, Ngai CY, Shen B, Wong CO, Huang Y, Yao X. Functional role of vanilloid transient receptor potential 4-canonical transient receptor potential 1 complex in flow-induced Ca2+influx. Arterioscler Thromb Vasc Biol. 2010;30:851–858. doi: 10.1161/ATVBAHA.109.196584. [DOI] [PubMed] [Google Scholar]
- 34.Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function? J Anat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem. 1997;272:15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- 37.Yi FX, Magness RR, Bird IM. Simultaneous imaging of [Ca2+]i and intracellular NO production in freshly isolated uterine artery endothelial cells: effects of ovarian cycle and pregnancy. Am J Physiol Regul Integr Comp Physiol. 2005;288:R140–R148. doi: 10.1152/ajpregu.00302.2004. [DOI] [PubMed] [Google Scholar]
- 38.Köhler R, Kaistha BP, Wulff H. Vascular KCa-channels as therapeutic targets in hypertension and restenosis disease. Expert Opin Ther Targets. 2010;14(2):143–155. doi: 10.1517/14728220903540257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.