Abstract
Background
Ischemic heart disease (IHD) burden consists of years of life lost from IHD deaths and years of disability lived with 3 nonfatal IHD sequelae: nonfatal acute myocardial infarction, angina pectoris, and ischemic heart failure. Our aim was to estimate the global and regional burden of IHD in 1990 and 2010.
Methods and Results
Global and regional estimates of acute myocardial infarction incidence and angina and heart failure prevalence by age, sex, and world region in 1990 and 2010 were estimated based on data from a systematic review and nonlinear mixed-effects meta-regression methods. Age-standardized acute myocardial infarction incidence and angina prevalence decreased globally between 1990 and 2010; ischemic heart failure prevalence increased slightly. The global burden of IHD increased by 29 million disability-adjusted life-years (29% increase) between 1990 and 2010. About 32.4% of the growth in global IHD disability-adjusted life-years between 1990 and 2010 was attributable to aging of the world population, 22.1% was attributable to population growth, and total disability-adjusted life-years were attenuated by a 25.3% decrease in per capita IHD burden (decreased rate). The number of people living with nonfatal IHD increased more than the number of IHD deaths since 1990, but >90% of IHD disability-adjusted life-years in 2010 were attributable to IHD deaths.
Conclusions
Globally, age-standardized acute myocardial infarction incidence and angina prevalence have decreased, and ischemic heart failure prevalence has increased since 1990. Despite decreased age-standardized fatal and nonfatal IHD in most regions since 1990, population growth and aging led to a higher global burden of IHD in 2010.
Keywords: angina pectoris, epidemiology, heart failure, myocardial infarction, myocardial ischemia, trends, world health
Ischemic heart disease (IHD) was the leading cause of death worldwide in 2010.1 However, many acute myocar-dial infarction (AMI) patients survive, and many adults live with disabling symptoms of stable angina pectoris or ischemic heart failure. Measuring the global burden of IHD requires estimating IHD mortality, prevalence, and disability for men and women, by age and world region. Nonfatal IHD incidence and prevalence do not always correlate with IHD mortality. For example, improved acute and chronic IHD treatments may lead to both decreased IHD mortality and a growing population of chronic IHD survivors. Conversely, even if IHD incidence is high, high case fatality may lead to relatively low prevalence. Regardless of the time trend in age-standardized IHD prevalence, population growth and aging may increase the absolute numbers of people living with nonfatal IHD.1
The Global Burden of Diseases, Injuries, and Risk Factors (GBD) 2010 Study used disability-adjusted life-years (DALYs) to summarize the fatal and nonfatal burden of IHD and 290 other major diseases. IHD DALYs combine years of life lost (YLL) attributable to fatal IHD with years lived with disability (YLD) in persons surviving with chronic IHD. Using a large systematic literature review and meta-regression modeling methods, we estimated YLD attributable to AMI, angina, and ischemic heart failure, and, by combining these estimates with YLL to IHD deaths, estimated the global burden of IHD in 21 world regions in 1990, 2005, and 2010 (region map, Figure I in the online-only Data Supplement).
Methods
Overview
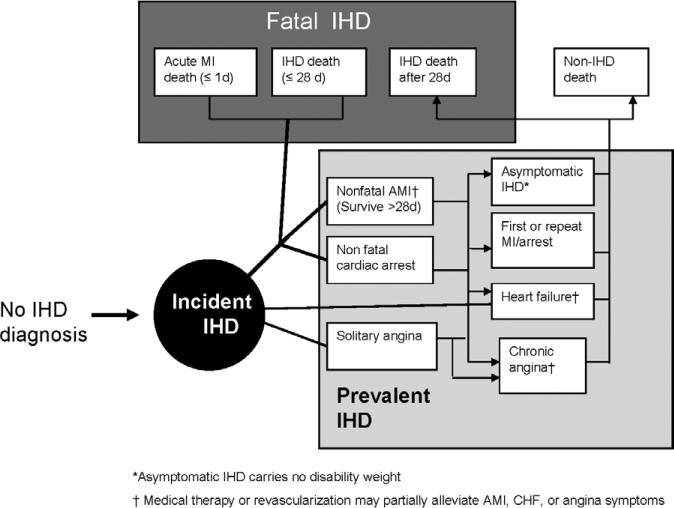
An IHD disease model constructed for the study established the relationships between IHD death, nonfatal AMI, angina, and ischemic heart failure (Figure 1). The GBD 2010 Study only captured stable angina. Because of inconsistent definitions over time and the fact that few low- and middle-income nation studies measured or reported on unstable angina, clinical sequelae of acute, unstable angina were captured in main estimates of either IHD death or AMI. In a sensitivity analysis, we estimated the additional YLD that may be attributable to ≈41% of acute coronary events that are unstable angina observed in the Global Registry of Acute Coronary Events, a 14-nation registry of acute coronary syndrome cases in 95 hospitals in North America, South America, Europe, Australia, and New Zealand.2 Accordingly, we reported the effect on YLD and DALYs of adding an additional 69% to the AMI YLD estimate, assuming that unstable angina has the same symptom severity as AMI.
Figure 1.
Ischemic heart disease (IHD) disease model, the Global Burden of Disease 2010 Study. AMI indicates acute myocardial infarction; CHF, congestive heart failure; and MI, myocardial infarction.
Stable angina diagnosis was primarily defined as Rose questionnaire definite angina.3,4 The GBD also included surveys of physician- diagnosed angina reported by patient (survey respondent) or physician. The GBD captured symptomatic heart failure cases meeting Framingham criteria or inclusive of New York Heart Association class 2 or higher, or hospitalized cases with heart failure as the principal discharge diagnosis (International Classification of Diseases, 9th Revision code 428, International Classification of Diseases, 10th Revision code I50). AMI was defined according to the 2008 World Health Organization definition of myocardial infarction (MI; categories A, B, and C).4,5 Only acute, symptomatic, and clinically recognized AMIs were captured (not silent MI or old Q-wave MI). The gold standard definition of AMI included a positive troponin biomarker test (World Health Organization category A), a highly sensitive and specific marker of myocardial damage that is elevated in all AMIs. Studies of AMI diagnosed with and without troponins in the diagnostic definition and cohort and surveillance studies have demonstrated that the inclusion of troponins leads to increased case finding and incidence.6–10 However, troponins were not routinely measured in high-income region AMI epidemiology studies of until ≈1995 to 2000, and are rarely measured in many low- and middle-income regions to this day. The GBD 2010 Study therefore recorded the use of troponin enzyme measurement in AMI studies and planned a priori to adjust incidence estimates for troponin measurement.
Estimating Nonfatal IHD Incidence and Prevalence
YLL (early deaths) and YLD (nonfatal disability) that make up DALYs were calculated in relation to hypothetical age-specific reference life expectancies (for example, 86.0 years at birth) derived from life-table methods.1,11 IHD mortality and YLL estimation methods and results are reported separately.12,12a IHD YLDs were calculated from AMI incidence and angina and ischemic heart failure prevalence estimated for the year of interest. We used DisMod-MR (Disease Model-Meta Regression, Institute for Health Metrics and Evaluation, Seattle, WA) to estimate the incidence and prevalence of nonfatal IHD outcomes with the use of systematic review study data, adjusting for covariates (Online-only Data Supplement Methods).4 DisMod-MR estimates a generalized negative binomial model for all the epidemiological data with fixed and random effects.13 A negative binomial model regression model was the appropriate model for counts data (prevalent cases). A mixed-effects design was chosen to include not only universal fixed effects associated with IHD, but also random effects capturing heterogeneity at each level of the study's nested geographic design (eg, country within region within super region). Fixed effects included age effects, effects for covariates that predict country variation in the quantity of interest, and effects that predict variation across studies owing to the attributes of the study. Study-level fixed-effects covariates included case definition, diagnostic method, or source of data, and country-level fixed-effects covariates included income per capita, health system performance, and age-standardized IHD mortality rate. Random effects were implemented as super region, region, and country random intercepts. The data were modeled with Bayesian methodology using vague previous distributions. The uncertainty of estimates are described with Bayesian credible intervals as opposed to confidence intervals (Methods in the online-only Data Supplement). Both approaches yield similar numeric results in large samples.14
Covariates entered into the AMI DisMod-MR model were GBD region, country, sex, age-standardized AMI death rate, and, if studies reported on only nonfatal or all MI, only first-ever or both first-ever and repeat AMI, and the measurement of troponin biomarker. Age- standardized AMI death rates were estimated by using the IHD mortality and MI/IHD death rate ratio, the latter conditioned on age, sex, national income, country, and region. IHD risk factors (eg, smoking, mean cholesterol and blood pressure, nutritional factors, income, and health system access) contributed to the AMI mortality covariate and were not directly entered into the AMI model.
Mostly because of the broad geographical coverage of the World Health Survey (WHS), angina prevalence data were obtained for 90 countries and 18 GBD regions. Covariates entered into the DisMod-MR angina model were GBD region, country, sex, age-standardized IHD death rate, and measurement method (self-report versus physician-reported; Rose probable versus Rose definite angina), and source survey. WHS angina prevalence was 21% higher overall than all other surveys.
IHD is only one of several causes of heart failure, so the first step for heart failure estimation was to estimate a total heart failure prevalence envelope. Total heart failure prevalence was estimated by DisMod-MR with the use of systematic review, US Medical Expenditure Panel Survey self-report, and multinational (24 countries) hospital discharge data in a model including covariates for GBD region, nation, sex, age-standardized cardiomyopathy death rate, New York Heart Association classification, body mass index, and self-reported versus physician's diagnosis.4,12 A meta-analysis of clinic-based studies reporting heart failure causes and hospital discharge data from the United States, Canada, Brazil, and Mexico guided region-specific allocation of the heart failure envelope to IHD and 6 other principal causes.12
DALY Weight Estimation and Severity Distributions
GBD disability weights range from no disability (weight=0.00) to death (weight=1.00). Weights for each IHD health state were estimated by using health state valuation of lay health state descriptions in population-based health surveys and an open Web-based survey.15 Lay descriptions for 3 distinct angina and heart failure states of graded severity (mild, moderate, and severe) were based on Canadian Cardiovascular Society class descriptions16 and New York Heart Association symptom classification descriptions, respectively (Table I in the online-only Data Supplement).17 Two study investigators (A.E.M. and G.A.M.) wrote lay descriptions for 2 AMI states (days 1–2 and days 3–28). Distribution of severity for each sequela was based on the distribution of symptom severity in studies of angina patients16 and the distribution of Short Form 12 quality-of-life ratings among US Medical Expenditure Panel Survey patients with heart failure.
The first AMI period started immediately after an AMI attack and lasted, at most, 2 days (10% of duration, disability weight 0.422, 95% uncertainty interval [0.284–0.566]; Table I in the online-only Data Supplement). The second period lasted from 3 to a maximum of 28 days (disability weight 0.056 [0.035–0.082]; 90% of duration). Angina and heart failure disability weights were applied to prevalent cases for the entire year of interest. With the use of Canadian Cardiovascular Society classes,16 angina disability was distributed as mild (27% of angina patients), moderate (19%), and severe (54%). Restricting to New York Heart Association ≥class 2 heart failure, the ischemic heart failure disability was distributed as mild (23% of ischemic heart failure), moderate (22%), and severe (55). Averaging over the severity distributions, the summary disability weight for angina was 0.113, and the summary disability weight for ischemic heart failure was 0.126. Pharmaceutical therapy or revascularization procedures may relieve angina symptoms. Based on the proportion of patients with stable angina who were symptom free (ie, successfully treated) at baseline in the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation trial, we estimated that, in the United States, about one-fifth of patients with stable angina are successfully treated and symptom free.18 In a sensitivity analysis, we reestimated stable angina YLD, assuming one-fifth of patients in high-income regions are treated and symptom free (0.8×DALY weight).
Angina and heart failure may coexist or occur along with other comorbid illnesses. Because of the lack of data on comorbid illness disability weighting, country-specific microsimulations were used to estimate the probability of comorbidity (eg, angina and heart failure together), assuming independence (Online-only Data Supplement Methods). Disability from multiple comorbid conditions was assumed to be a multiplicative effect of disability from the individual diseases and was calculated as one minus the product of one minus each disability weight. The method was validated by repeating the procedure with US Medical Expenditure Panel Survey participants by using International Classification of Diseases–coded conditions, and resulting distribution of DALY weights matched well with the distribution of self-reported quality of life on the Short Form 12 scale in persons with multiple comorbid conditions (correlation coefficient=0.999).
AMI incidence, angina and heart failure prevalence, YLDs and DALYs for 21 GBD regions from 1990 to 2010 are reported either as absolute numbers or by dividing these quantities by the regional populations. Age standardization and dividing by population at risk allowed the analysis of changes in IHD rates and prevalence independent of changes in population size and age structure. Age standardization was done by the direct method, by using 5-year GBD age categories and the World Health Organization standard population. The independent influences of population aging and population growth on change in total DALYs between 1990 and 2010 were assessed by (1) computing the numbers of DALYs expected assuming a scenario of the same population growth to 2010 but with a 1990 age structure, and (2) computing the number of DALYs expected in scenario with the real 2010 age structure assuming no population growth since 1990.
Results
Nonfatal IHD in 21 World Regions in 1990 and 2010
In 2010, the Eastern Europe and Central Asia regions—the regions of the former Soviet Union—had the highest age- standardized AMI incidence rates in the world (>340 per 100 000 in males and >180 in females; Table II in the online- only Data Supplement). Lowest AMI incidence was estimated for the High Income Asia/Pacific and East Asia regions (both <140 AMI per 100 000 in males and <80 in females).
Globally, age-standardized AMI incidence in all ages decreased from 1990 to 2010, from 222.7 to 195.3 per 100 000 in males and from 136.3 to 115.0 in females. Age-standardized AMI incidence declined the most in Australasia, Western and Central Europe, and North America High Income (all decreases of >87 per 100 000 in males and >35 in females). AMI incidence increased the most in Eastern Europe (62 per 100 000 increase in males and 17 in females). In the majority of other regions, AMI incidence decreased modestly.
Age-standardized angina prevalence decreased globally, from 21.9 to 20.3 per 100 000 in males and from 17.7 to 15.9 in females. Prevalence decreased ≥5.0 percentage points in males and ≥3.9 percentage points in females in Australasia, Western Europe, and North America (Table III in the online-only Data Supplement). Other regions had modest decreases in angina, with the exceptions of small increases in South Asia, Central Asia, Western sub-Saharan Africa (males and females), and Eastern Europe (males only).
Global ischemic heart failure prevalence increased from 2.4 to 2.7 per 100 000 in males and remained at 1.9 in females between 1990 and 2010. Small increases in age-standardized ischemic heart failure prevalence were almost uniform across the 21 GBD regions (Table IV in the online-only Data Supplement).
Average Age at First IHD Event and Years Lived With IHD Disability in 1990 and 2010
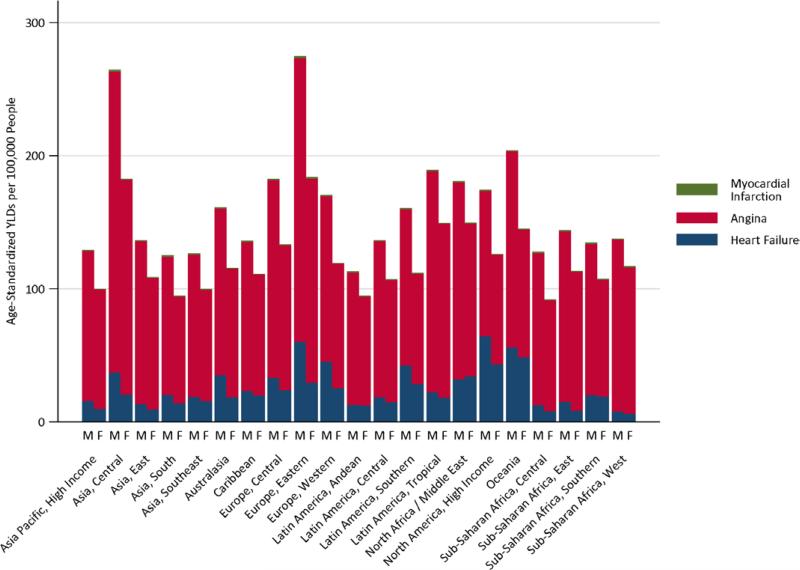
In 2010, the average age of first AMI was >70 years and the average age at incident angina was >60 years in all high-income regions (Table V in the online-only Data Supplement). For several low- and middle-income regions (South Asia, North Africa/Middle East, and the sub-Saharan Africa), the average age at first AMI was <65 years, and the average age at first angina was <55 years. The highest rates of IHD disability in 2010 were found in Eastern Europe and Central Asia (Figure 2). Prevalent stable angina contributed the most to IHD disability, followed by smaller contributions from ischemic heart failure and nonfatal AMI (Figure 3). Absolute numbers of IHD YLDs increased in all regions from 1990 to 2010, ranging from a 15% to 33% increase in high-income regions and from a 13% to 100% increase in low- and middle-income regions (Table VI in the online-only Data Supplement). Owing to fewer data and uncertainty around estimates in the source epidemiological data, the uncertainty intervals around IHD YLD estimates (based on nonfatal IHD) were much wider than those for YLL (based on IHD mortality; Methods and Figure II in the online- only Data Supplement).
Figure 2.
Years of life lived with disability attributable to ischemic heart disease in 2010, in 21 Global Burden of Disease Study regions. YLD indicates years lived with disability.
Figure 3.
Contributions of AMI, angina, and ischemic heart failure to years lived with ischemic heart disease disability in 2010, by sex, in 21 Global Burden of Disease Study regions. AMI indicates acute myocardial infarction.
Disability Adjusted Life-Years Attributable to IHD in 1990 and 2010
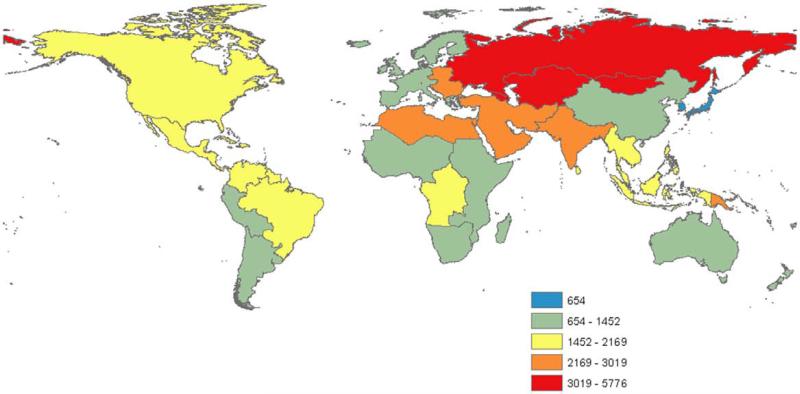
Four regions—Western Europe, Central Europe, Australasia, and North America—experienced a decrease in absolute IHD DALYs and age-standardized IHD DALYs per 100 000 population between 1990 and 2010, despite demographic changes in the interval (Table; Table VII in the online-only Data Supplement). With the exception of Eastern Europe and Central Asia, the regions with the highest IHD mortality rates, the rest of the world had larger relative 20-year increases in YLD than in DALYs. Eastern Europe, Central Asia, North Africa/Middle East, and South Asia had the highest age- standardized DALYs per 100 000 in the world in 2010 (Figure 4). DALY rates trended upward in these and other low- and middle-income regions after 1990, but appeared to flatten in most, but not all regions after 2005 (Table).
Table.
Age-Standardized DALYs Lost per 100 000 Persons Because of IHD by Region in 1990, 2005, and 2010, the Global Burden of Disease 2010 Study
| GBD 2010 Super Region |
1990 |
2005 |
2010 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| GBD 2010 Region | Mean | Upper | Lower | Mean | Upper | Lower | Mean | Upper | Lower |
| High income | |||||||||
| Asia Pacific, high income | 1031 | 1137 | 931 | 688 | 772 | 627 | 654 | 743 | 582 |
| Europe, Western | 2311 | 2433 | 2156 | 1312 | 1471 | 1239 | 1160 | 1308 | 1088 |
| Australasia | 2636 | 2787 | 2451 | 1228 | 1402 | 1134 | 1118 | 1288 | 1028 |
| North America, high income | 2889 | 3041 | 2716 | 1849 | 2061 | 1758 | 1636 | 1839 | 1528 |
| Latin America, Southern | 2169 | 2350 | 2063 | 1578 | 1735 | 1495 | 1452 | 1603 | 1367 |
| Eastern Europe/Central Asia | |||||||||
| Europe, Central | 3936 | 4111 | 3699 | 2960 | 3183 | 2855 | 2608 | 2829 | 2513 |
| Europe, Eastern | 4972 | 5331 | 4816 | 7329 | 7600 | 6699 | 5776 | 6025 | 5291 |
| Asia, Central | 5316 | 5672 | 5156 | 6038 | 6289 | 5679 | 5459 | 5798 | 5055 |
| Latin America/Caribbean | |||||||||
| Latin America, Tropical | 2621 | 2820 | 2435 | 1940 | 2155 | 1838 | 1811 | 2017 | 1706 |
| Latin America, Central | 1969 | 2124 | 1851 | 1658 | 1765 | 1516 | 1675 | 1792 | 1523 |
| Latin America, Andean | 1512 | 1683 | 1406 | 1256 | 1357 | 1105 | 1144 | 1252 | 1014 |
| Caribbean | 2762 | 2935 | 2597 | 2210 | 2408 | 2118 | 2169 | 2670 | 2049 |
| East Asia/Pacific | |||||||||
| Asia, East | 1152 | 1450 | 1047 | 1302 | 1401 | 1133 | 1242 | 1341 | 1057 |
| Asia, Southeast | 1850 | 2011 | 1700 | 1711 | 1975 | 1618 | 1711 | 1941 | 1611 |
| Oceania | 2399 | 3352 | 2060 | 2357 | 3319 | 1986 | 2324 | 3131 | 1946 |
| North Africa / Middle East | |||||||||
| North Africa / Middle East | 3957 | 4328 | 3748 | 3221 | 3417 | 3024 | 3019 | 3199 | 2773 |
| South Asia | |||||||||
| Asia, South | 2685 | 3009 | 2541 | 2897 | 3080 | 2458 | 2728 | 3022 | 2316 |
| Sub-Saharan Africa | |||||||||
| Sub-Saharan Africa, Southern | 1855 | 2021 | 1516 | 1371 | 1638 | 1258 | 1246 | 1493 | 1135 |
| Sub-Saharan Africa, East | 1524 | 1662 | 1296 | 1198 | 1336 | 1088 | 1154 | 1301 | 1050 |
| Sub-Saharan Africa, Central | 2055 | 2389 | 1699 | 1882 | 2172 | 1636 | 1981 | 2294 | 1717 |
| Sub-Saharan Africa, West | 1340 | 1530 | 1211 | 1309 | 1497 | 1175 | 1320 | 1528 | 1189 |
Data are means and upper and lower limits of 95% credible intervals. DALYs indicates disability-adjusted life years; and IHD, ischemic heart disease.
Figure 4.
Disability-adjusted life-years lost owing to ischemic heart disease (IHD DALYs) in 2010, in 21 Global Burden of Disease Study regions.
The absolute global burden of IHD increased by 29 million DALYs (29%) between 1990 and 2010 (Table VIII in the online-only Data Supplement). IHD DALYs increased 32.4% globally between 1990 and 2010 because of the aging of the world population and increased 22.1% because of increased population. These increases were attenuated by a 25.3% decrease in combined age-standardized, per capita IHD DALY rate. In general, population growth contributed most to IHD DALY increases in low- and middle-income regions, and aging contributed the most in high-income regions. However, within low- and middle-income regions, population growth was the main driver of DALY increases in South Asia, North Africa/Middle East, and sub-Saharan Africa, but aging drove DALY increases more in the former Soviet Union regions and in Latin America and the Caribbean.
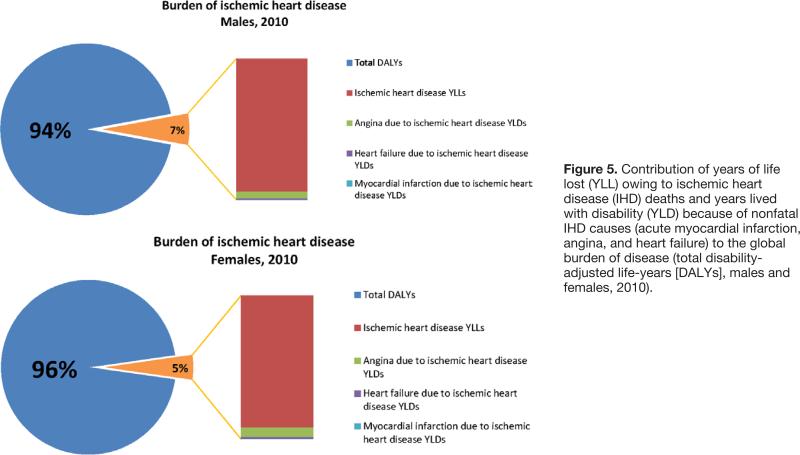
Despite higher age-standardized rates of fatal and nonfatal IHD in males, the proportion of male global burden of disease attributable to IHD (7%) was only slightly higher than the female IHD proportion (5%; Figure 5). Overall, nonfatal IHD (YLDs) contributed much less than IHD mortality (YLL) to global IHD DALYs (YLL attributable to premature IHD deaths constituted 94% of IHD DALYs in men and 92% of IHD DALYs in women).
Figure 5.
Contribution of years of life lost (YLL) owing to ischemic heart disease (IHD) deaths and years lived with disability (YLD) because of nonfatal IHD causes (acute myocardial infarction, angina, and heart failure) to the global burden of disease (total disability-adjusted life-years [DALYs], males and females, 2010).
Sensitivity Analyses
When acute unstable angina disability was added to AMI disability, total IHD YLD increased by 0.4% in males and 0.3% in females, and acute coronary syndromes went from accounting for 0.5% to 0.9% of YLD in males and from 0.4% to 0.7% of YLD in females; overall IHD DALYs were increased by 0.02%. When we assumed that one-fifth of angina patients in high-income regions were successfully treated and asymptomatic, overall IHD YLD decreased by 3.7% in males and females, and IHD DALYs decreased by 0.2% in males and 0.3% in females.
Discussion
The global burden of IHD in 2010 presents a mixed picture: progress is evident in the reduced age-standardized IHD burden since 1990, especially in high-income regions; however, at the same time, high IHD burden was found in a geographic band including the former Soviet Union territories, North Africa, the Middle East, and South Asia. Aging populations and population growth in many regions have increased both YLD and IHD burden worldwide. This finding is consistent with the ascendance of chronic, noncommunicable diseases found in the GBD 2010 Study as a whole.1 Since 1990, the number of years of disability attributable to nonfatal IHD has grown faster than IHD mortality, although the absolute numbers of nonfatal disability years were dwarfed by YLL attributable to IHD deaths.
Although nonfatal IHD contributed little to DALYs, the relatively steeper increase in YLD in comparison with mortality and YLL suggests the need for a strategy shift in IHD treatment. IHD death rate and case fatality will no longer be the sole public health benchmarks; improved quality of life in patients with chronic IHD will be an important secondary outcome. Interventions designed to relieve symptoms and improve fitness and vitality in patients with chronic angina and heart failure are needed. The mainstays of chronic IHD treatment are standard, low-cost medications that are sadly insufficiently used in patients who have chronic cardiovascular disease worldwide, especially in low- and middle-income regions. The Prospective Urban Rural Epidemiological (PURE) study found that among patients with cardiovascular disease, 11% of high-income region patients were not taking any standard secondary prevention drugs and 80% of lower- income region patients were taking none of the medications.19 Overall in PURE, countries’ health expenditure per capita correlated positively with the percentage of use of standard secondary prevention medications, although, in several cases, better medication coverage was achieved by countries at the same or lower health expenditures.
Although AMI incidence remains high in many regions, nonfatal AMI burden was lower than angina and ischemic heart failure burden owing to the assumption that, absent associated angina or heart failure, AMI symptoms do not persist beyond 30 days. Assuming an added nonfatal IHD burden owing to acute unstable angina (and symptom severity the same as AMI), YLD increased only 0.3% to 0.4%, and acute unstable angina plus AMI (acute coronary syndromes) increased overall IHD burden by 0.02%. Prevalent cases of stable angina contributed the most to the burden of nonfatal IHD. All angina diagnoses in the source studies relied on a subjective, self-reported measure. Studies using a gold standard of objectively measured exercise-induced ischemia accompanying chest pain found that the Rose questionnaire had 40% to 67% sensitivity and 56% to 80% specificity and a much lower positive predictive value in women than in men.19a,20 However, Rose questionnaire definite angina has been associated with higher IHD mortality,21 and there is a growing body of evidence that many cases of angina, especially in women, are associated with microvascular coronary atherosclerosis not identifiable with coronary angiography.22–24 More enigmatic was the pattern of generally higher angina prevalence relative to regional IHD mortality elicited by using the Rose questionnaire in the WHS, especially in the sub-Saharan African WHS sites. It is unknown whether this pattern represents a systematic bias (due, for example, to using a measurement tool not properly validated in diverse populations), or an emerging higher prevalence of IHD in sub-Saharan Africa preceding changes in IHD mortality. Unlike the largest meta-analysis of Rose questionnaire angina published to date, we did not find angina was more prevalent in women than in men.25 IHD measurement problems would best be addressed by improved methods of health surveillance, including an investment in better interview or objective diagnostic screening methods applicable to low- income settings.
The global pattern of ischemic heart failure prevalence estimated by the GBD 2010 Study was similar to the few past studies of regional differences in heart failure causes.26 To adjust for the effect of the advent of troponin biomarker on AMI incidence around the turn of the 21st century, we adjusted for troponin measurement. This adjustment standardized comparisons of AMI incidence between 1990 and 2010, but may have biased AMI incidence estimation for regions that continue without access to troponin and other AMI diagnostic tools. The case mix of ST- and non-ST-elevation AMI and the potential impact of implementing improved diagnostic tools in many regions remain unknown.
For nonfatal IHD incidence, prevalence, and case fatality, we depended predominantly on data gathered from a large systematic literature review. Inevitably, these studies usually sampled only a subset of the populations studied, and portions of the population went unrepresented. This was particularly true of the usually small studies from lower-income regions. For example, despite an extensive literature review of data published over a 28-year period, no studies of AMI incidence or case fatality were included for sub-Saharan Africa regions, and almost all sub-Saharan Africa estimates of angina prevalence come from 1 study (the WHS, 2002–2004).4 DisMod-MR used a Bayesian method that allowed us to start with a priori general epidemiological characteristics of IHD obtained from the aggregate study-level data compiled from the systematic review (pattern of prevalence over the lifespan, consistent relationships between incidence, prevalence, and mortality), and allow geographic, temporal, and age-related heterogeneity to revise these previous assumptions because it is applied to specific countries, ages, and years. A mixed, multilevel model reflected the geographical nesting of the study design. However, DisMod-MR has limitations. Bayesian methods run the risk of relying on expert opinion to set the previous assumptions, and these assumptions may be false, particularly for countries with sparse data.
For the estimation of prevalence for each IHD sequela, adjustment was made for known sources of bias. For AMI, adjustment was made for the outcome definition (incidence rate versus event rate) and measurement method (use of troponin biomarker or not). For angina, adjustment was made for the measurement method (Rose questionnaire or other method) and for the WHS versus other surveys. For heart failure, adjustment was made for the case definition and range of symptom severity used in the study. Sources of uncertainty surrounding nonfatal IHD source data were propagated forward to GBD summary estimates, resulting in much larger credible intervals around IHD YLD (nonfatal) estimates than for IHD YLL (mortality) estimates (Figure II in the online- only Data Supplement). Although the geographic coverage for angina prevalence was broader than for the other IHD sequelae—largely because of the WHS—angina estimation was the most prone to bias. This vulnerability stemmed from the subjective nature of self-report, and perhaps the cultural context in which the questions were interpreted by survey participants. AMI cases are likely systematically underdiagnosed in lower- resource regions because of less access to health facilities and scarcity of diagnostic technology. Parts of countries and regions with higher IHD rates may have been more likely to be studied and contribute data. Despite our efforts to adjust estimates to account for bias, we could not completely compensate for regions with sparse data, nor could we adjust for unmeasured sources of bias
Our results showed that, with the use of the DALY metric, global IHD burden was dominated by fatal IHD (YLL), non-fatal IHD made only a minor contribution, and acute nonfatal IHD (AMI or unstable angina) made the least contribution. It is important to note that, as a metric of societal disease burden, DALYs are a measure of health loss only, not financial or productivity loss because of disease (eg, financial costs of hospitalization or chronic medications, lost work time, and family or other caregiver burden related to nonfatal IHD).27
Implications
Despite decreasing age-standardized IHD incidence and mortality rates in most regions, population growth and aging has increased the global burden of IHD since 1990. Nonfatal IHD (nonfatal AMI, angina, and ischemic heart failure) has increased more than IHD deaths since 1990, but IHD mortality remains the greatest contributor to disease burden. Underlying global IHD burden trends are substantial regional differences in rates and time trends of AMI, angina, ischemic heart failure, and IHD mortality.
Supplementary Material
CLINICAL PERSPECTIVE.
The symptoms and disability experienced by acute myocardial infarction survivors and patients who have chronic angina pectoris and ischemic heart failure contribute to the overall burden of ischemic heart disease (IHD). The Global Burden of Disease 2010 Study estimated years lived with nonfatal IHD disability for 21 world regions and combined these with life-years lost from fatal IHD to estimate the global burden of IHD. Global acute myocardial infarction incidence and angina prevalence decreased per capita between 1990 and 2010. Despite those favorable trends, ischemic heart failure prevalence increased, and the number of people living with IHD increased in all regions, including low- and middle-income regions. Recent surveys suggest that the basic treatments that alleviate symptoms and extend survival in chronic IHD survivors—such as daily standard oral medications—are underused globally, but especially in low- and middle-income regions. Despite reductions in fatal and nonfatal IHD rates in most world regions, population growth and aging increased the overall burden of IHD between 1990 and 2010.
Acknowledgments
We sincerely thank the many study participants and investigators of the many studies contributing data to this analysis.
Sources of Funding
This research was supported by the Bill and Melinda Gates Foundation, an American Heart Association Postdoctoral Fellowship to Dr Roth, and US National Heart, Lung, and Blood Institute award K08 HL089675-01A1, and a Columbia University Irving Scholarship to Dr Moran.
Footnotes
Disclosures
The perspectives expressed in this article are those of the authors and do not necessarily represent the views of the National Institutes of Health, Department of Health and Human Services, or any other government entity.
References
- 1.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Steg PG, Goldberg RJ, Gore JM, Fox KA, Eagle KA, Flather MD, Sadiq I, Kasper R, Rushton-Mellor SK, Anderson FA, GRACE Investigators Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE). Am J Cardiol. 2002;90:358–363. doi: 10.1016/s0002-9149(02)02489-x. [DOI] [PubMed] [Google Scholar]
- 3.ROSE GA. Ischemic Heart Disease. Chest Pain Questionnaire. Milbank Mem Fund Q. 1965;43:32–39. [PubMed] [Google Scholar]
- 4.Moran AE, Oliver JT, Mirzaie M, Forouzanfar MH, Chilov M, Anderson L, Morrison JL, Khan A, Zhang N, Haynes N, Tran J, Murphy A, Degennaro V, Roth G, Zhao D, Peer N, Pichon-Riviere A, Rubinstein A, Pogosova N, Prabhakaran D, Naghavi M, Ezzati M, Mensah GA. Assessing the global burden of ischemic heart disease, Part 1: methods for a systematic review of the global epidemiology of ischemic heart disease in 1990 and 2010. Glob Heart. 2012;7:315–329. doi: 10.1016/j.gheart.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendis S, Thygesen K, Kuulasmaa K, Giampaoli S, Mahonen M, Ngu Blackett K, Lisheng L. World Health Organization definition of myocar-dial infarction: 2008–09 revision. Int J Epidemiol. 2011;40:139–146. doi: 10.1093/ije/dyq165. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson JL, Beckett GJ, Stoddart M, Walker SW, Fox KA. Myocardial infarction redefined: the new ACC/ESC definition, based on cardiac troponin, increases the apparent incidence of infarction. Heart. 2002;88:343–347. doi: 10.1136/heart.88.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin JC, Apple FS, Murakami MM, Luepker RV. Rates of positive cardiac troponin I and creatine kinase MB mass among patients hospitalized for suspected acute coronary syndromes. Clin Chem. 2004;50:333–338. doi: 10.1373/clinchem.2003.026708. [DOI] [PubMed] [Google Scholar]
- 8.Roger VL, Killian JM, Weston SA, Jaffe AS, Kors J, Santrach PJ, Tunstall-Pedoe H, Jacobsen SJ. Redefinition of myocardial infarction: prospective evaluation in the community. Circulation. 2006;114:790–797. doi: 10.1161/CIRCULATIONAHA.106.627505. [DOI] [PubMed] [Google Scholar]
- 9.Roger VL, Weston SA, Gerber Y, Killian JM, Dunlay SM, Jaffe AS, Bell MR, Kors J, Yawn BP, Jacobsen SJ. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121:863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh NI, Gona P, Larson MG, Fox CS, Benjamin EJ, Murabito JM, O'Donnell CJ, Vasan RS, Levy D. Long-term trends in myocardial infarction incidence and case fatality in the National Heart, Lung, and Blood Institute's Framingham Heart study. Circulation. 2009;119:1203–1210. doi: 10.1161/CIRCULATIONAHA.108.825364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Dwyer-Lindgren L, Lofgren KT, Rajaratnam JK, Marcus JR, Levin-Rector A, Levitz CE, Lopez AD, Murray CJ. Age-specific and sex-specific mortality in 187 countries, 1970-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2071–2094. doi: 10.1016/S0140-6736(12)61719-X. [DOI] [PubMed] [Google Scholar]
- 12.Forouzanfar MH, Moran AE, Flaxman AD, Roth G, Mensah GA, Ezzati M, Naghavi M, Murray CJL. Assessing the global burden of ischemic heart failure, Part 2: analytic methods and estimates of the global epidemiology of ischemic heart disease in 2010. Global Heart. 2012;7:331–342. doi: 10.1016/j.gheart.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Murray CJL, Naghavi M. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: The Global Burden of Disease 2010 Study. Circulation. 2014;129:1483–1492. doi: 10.1161/CIRCULATIONAHA.113.004042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barendregt JJ, Van Oortmarssen GJ, Vos T, Murray CJ. A generic model for the assessment of disease epidemiology: the computational basis of DisMod II. Popul Health Metr. 2003;1:4. doi: 10.1186/1478-7954-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bland JM, Altman DG. Bayesians and frequentists. BMJ. 1998;317:1151–1160. doi: 10.1136/bmj.317.7166.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, Begum N, Shah R, Karyana M, Kosen S, Farje MR, Moncada G, Dutta A, Sazawal S, Dyer A, Seiler J, Aboyans V, Baker L, Baxter A, Benjamin EJ, Bhalla K, Bin Abdulhak A, Blyth F, Bourne R, Braithwaite T, Brooks P, Brugha TS, Bryan-Hancock C, Buchbinder R, Burney P, Calabria B, Chen H, Chugh SS, Cooley R, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, Davis A, Degenhardt L, Díaz-Torné C, Dorsey ER, Driscoll T, Edmond K, Elbaz A, Ezzati M, Feigin V, Ferri CP, Flaxman AD, Flood L, Fransen M, Fuse K, Gabbe BJ, Gillum RF, Haagsma J, Harrison JE, Havmoeller R, Hay RJ, Hel-Baqui A, Hoek HW, Hoffman H, Hogeland E, Hoy D, Jarvis D, Karthikeyan G, Knowlton LM, Lathlean T, Leasher JL, Lim SS, Lipshultz SE, Lopez AD, Lozano R, Lyons R, Malekzadeh R, Marcenes W, March L, Margolis DJ, McGill N, McGrath J, Mensah GA, Meyer AC, Michaud C, Moran A, Mori R, Murdoch ME, Naldi L, Newton CR, Norman R, Omer SB, Osborne R, Pearce N, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Pourmalek F, Prince M, Rehm JT, Remuzzi G, Richardson K, Room R, Saha S, Sampson U, Sanchez-Riera L, Segui-Gomez M, Shahraz S, Shibuya K, Singh D, Sliwa K, Smith E, Soerjomataram I, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Taylor HR, Tleyjeh IM, van der Werf MJ, Watson WL, Weatherall DJ, Weintraub R, Weisskopf MG, Whiteford H, Wilkinson JD, Woolf AD, Zheng ZJ, Murray CJ, Jonas JB. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemingway H, Fitzpatrick NK, Gnani S, Feder G, Walker N, Crook AM, Magee P, Timmis A. Prospective validity of measuring angina severity with Canadian Cardiovascular Society class: the ACRE study. Can J Cardiol. 2004;20:305–309. [PubMed] [Google Scholar]
- 17.The Criteria Committee of the New York Heart Association . Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Blood Vessels. Little Brown; Boston: 1964. [Google Scholar]
- 18.Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, Bowen J, Dunbar SB, Deaton C, Kaufman S, O'Rourke RA, Goeree R, Barnett PG, Teo KK, Boden WE, Mancini GB, COURAGE Trial Research Group Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008;359:677–687. doi: 10.1056/NEJMoa072771. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S, Islam S, Chow CK, Rangarajan S, Dagenais G, Diaz R, Gupta R, Kelishadi R, Iqbal R, Avezum A, Kruger A, Kutty R, Lanas F, Lisheng L, Wei L, Lopez-Jaramillo P, Oguz A, Rahman O, Swidan H, Yusoff K, Zatonski W, Rosengren A, Teo KK, Prospective Urban Rural Epidemiology (PURE) Study Investigators Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet. 2011;378:1231–1243. doi: 10.1016/S0140-6736(11)61215-4. [DOI] [PubMed] [Google Scholar]
- 19a.Garber CE, Carleton RA, Heller GV. Comparison of “rose questionnaire angina” to exercise thallium scintigraphy: different findings in males and females. J Clin Epidemiol. 1992;45:715–720. doi: 10.1016/0895-4356(92)90048-r. [DOI] [PubMed] [Google Scholar]
- 20.Bass EB, Follansbee WP, Orchard TJ. Comparison of a supplemented Rose Questionnaire to exercise thallium testing in men and women. J Clin Epidemiol. 1989;42:385–394. doi: 10.1016/0895-4356(89)90126-1. [DOI] [PubMed] [Google Scholar]
- 21.Hart CL, Watt GC, Davey Smith G, Gillis CR, Hawthorne VM. Pre-existing ischaemic heart disease and ischaemic heart disease mortality in women compared with men. Int J Epidemiol. 1997;26:508–515. doi: 10.1093/ije/26.3.508. [DOI] [PubMed] [Google Scholar]
- 22.Lanza GA, Buffon A, Sestito A, Natale L, Sgueglia GA, Galiuto L, Infusino F, Mariani L, Centola A, Crea F. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol. 2008;51:466–472. doi: 10.1016/j.jacc.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 23.Buchthal SD, den Hollander JA, Merz CN, Rogers WJ, Pepine CJ, Reichek N, Sharaf BL, Reis S, Kelsey SF, Pohost GM. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med. 2000;342:829–835. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- 24.Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ, WISE Investigators Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–741. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 25.Hemingway H, Langenberg C, Damant J, Frost C, Pyörälä K, Barrett-Connor E. Prevalence of angina in women versus men: a systematic review and meta-analysis of international variations across 31 countries. Circulation. 2008;117:1526–1536. doi: 10.1161/CIRCULATIONAHA.107.720953. [DOI] [PubMed] [Google Scholar]
- 26.Khatibzadeh S, Farzadfar F, Oliver J, Ezzati M, Moran A. Worldwide risk factors for heart failure: a systematic review and pooled analysis. Int J Cardiol. 2013;168:1186–1194. doi: 10.1016/j.ijcard.2012.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray CJ, Ezzati M, Flaxman AD, Lim S, Lozano R, Michaud C, Naghavi M, Salomon JA, Shibuya K, Vos T, Wikler D, Lopez AD. GBD 2010: design, definitions, and metrics. Lancet. 2012;380:2063–2066. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.