Abstract
Airborne particulate matter (PM) components from fossil fuel combustion can induce oxidative stress initiated by reactive oxygen species (ROS). Reported associations between worsening asthma and PM2.5 mass could be related to PM oxidative potential to induce airway oxidative stress and inflammation (hallmarks of asthma pathology). We followed 45 schoolchildren with persistent asthma in their southern California homes daily over 10 days with offline fractional exhaled nitric oxide (FENO), a biomarker of airway inflammation. Ambient exposures included daily average PM2.5, PM2.5 elemental and organic carbon (EC, OC), NO2, O3, and endotoxin. We assessed PM2.5 oxidative potential using both an abiotic and an in vitro bioassay on aqueous extracts of daily particle filters: (1) dithiothreitol (DTT) assay (abiotic), representing chemically produced ROS; and (2) ROS generated intracellularly in a rat alveolar macrophage model using the fluorescent probe 2′7′-dicholorohidroflourescin diacetate. We analyzed relations of FENO to air pollutants in mixed linear regression models. FENO was significantly positively associated with lag 1-day and 2-day averages of traffic-related markers (EC, OC, and NO2), DTT and macrophage ROS, but not PM2.5 mass. DTT associations were nearly twice as strong as other exposures per interquartile range: median FENO increased 8.7–9.9% per 0.43 nmole/min/m3 DTT. Findings suggest that future research in oxidative stress-related illnesses such as asthma and PM exposure would benefit from assessments of PM oxidative potential and composition.
Keywords: asthma, exhaled nitric oxide, longitudinal data analysis, oxidative stress, particulate air pollution
INTRODUCTION
Numerous studies have shown generally consistent associations between asthma morbidity and exposure to air pollutants, including mass concentrations of ambient particulate matter (PM) <2.5 and <10 μm in diameter (PM2.5 and PM10) and particular sources of air pollution, especially traffic near the home measured with a variety of markers (e.g., elemental carbon, EC).1–4 However, toxic particle components may not be completely reflected by total mass or by markers of traffic pollution. Specifically, the oxidative potential of PM is expected to be at least partly independent of mass because only a variable and potentially small fraction of chemical components is expected to induce oxidative stress and inflammation in the airways.5 There are many components of aerosols that can have such oxidative potential (e.g., polycyclic aromatic hydrocarbons (PAHs) or transition metals) and components vary temporally and spatially. Therefore, epidemiologic associations may be obscured when analyses rely on uncharacterized mass concentrations (PM2.5 and PM10). The present study addressed this possibility by characterizing the overall oxidative potential of PM2.5, and relating this to daily changes in fractional exhaled nitric oxide (FENO, a biomarker of airway inflammation) in pediatric subjects with asthma.
To assess the oxidative potential of components in PM, the dithiothreitol (DTT) assay has been used to show the capacity of PM to transfer electrons from DTT to oxygen, resulting in the generation of superoxide. This DTT indicator of redox activity is positively correlated with the PAH content,6,7 organic carbon (OC)7–10 fraction of collected PM and the levels of transition metals,11 and is partly inhibited by metal chelators.8,12 DTT consumption is higher in ultrafine PM (<0.15 μm),7,13 all suggesting combustion sources of organic chemicals and transition metals are important to PM oxidative potential. Moreover, an in vitro study showed that the chemical oxidative potential of urban PM assessed by the DTT consumption method is related to its ability to induce oxidative stress responses (heme oxygenase-1 expression).6,13
However, another potentially important mechanism of oxidative stress responses to PM is the intracellular production of reactive oxygen species (ROS) by cells exposed to PM. ROS signaling (e.g., with inflammatory cytokines) is an important inflammatory pathway in airway inflammation.14 This characteristic of PM can be assessed by treating rat alveolar macrophages with particle extracts, and measuring ROS production using a validated method as described below.15 Macrophages are the first line of defense against pulmonary injury, including injury from pollutant particles. The cell-based ROS activity induced by PM extracts in this assay has been positively correlated with transition metal and OC concentrations in studies in Los Angeles16,17 and elsewhere.18,19 We previously reported that repeated weekly measures of FENO in an elderly cohort panel were positively associated with the level of macrophage ROS induced by extracts of quasi-ultrafine particles <0.25 μm in diameter.20
In the present study we measured the overall oxidative potential of ambient PM2.5 using these two abiotic/biotic assays. We hypothesized that FENO in pediatric subjects with persistent asthma would be positively associated with the oxidative potential of particle samples. We also assessed the extent to which previously reported associations of FENO with PM2.5 EC and OC in the present study population21 are related to PM2.5 oxidative potential. Findings using these newer approaches to exposure assessment will encourage further advancements in methods that target a key aspect of pollutant toxicity, oxidative stress.5
METHODS
Population and Design
The study design and measurement methods have been described by us in detail elsewhere.21 Briefly, this is a cohort panel study that was conducted in the Los Angeles air basin with 10 daily repeated measurements of FENO and exposures per subject in groups of 3–4 subjects. The analytic focus is on within-subject exposure–response relationships. Subjects included 45 schoolchildren with confirmed diagnoses of persistent asthma who were ages 9–18 years, nonsmoking, and unexposed to environmental tobacco smoke in the home. Thirteen subjects were followed at their homes from August through December 2003 in Riverside, CA, and 32 subjects were followed at their homes from July through November 2004 in Whittier, CA.
Health Outcome
Exhaled NO was measured using recommended offline procedures,22 with additional improvements suggested by Linn et al.23 Subjects were asked to refrain from performing spirometry, exercise, and food or beverage intake 1 h before sample collection. Subjects inhaled orally to total lung capacity and followed immediately with a slow vital capacity maneuver into an offline device attached to a non-reactive 1.5-l Mylar reservoir bag (Ionics, Boulder, CO, USA). The target flow rate was 100 ml/s.24 To reduce contamination from upper airway NO, around 200 ml of dead-space air was vented before sampling.25 An NO/NO2 chemisorbent filter at the air intake of the offline device controlled for inspired ambient NO. Subjects breathed through it for 15 s (≥2 tidal breaths) before sampling. A nasal clip was used to prevent unfiltered air from entering the lungs. We assessed reliability by collecting two breath samples. Subjects performed once daily collections over a 10-day period at the same time of day each day (late afternoon or early evening) under the direction of field technicians in the home. Although the NO scrubbing filter was expected to control for biased samples, we additionally collected an indoor air sample to assess the influence of indoor NO on FENO and found there was no association.21 We sealed the Mylar bags and refrigerated them at 6 °C after collection. We analyzed bags for NO within 20 h with a chemiluminescence NO analyzer (NOA™ 280i Sievers, GE Analytical Instruments, Boulder, CO, USA). Reliable sample pairs were used as averages in the analysis and were identified if differences were ≤3 ppb or ≤10% of the larger reading. We found 372 pairs (83%) in 45 subjects were reliable by these criteria and used in the analysis.
Exposures
Stationary site air monitoring
Harvard Impactors (Air Diagnostics and Engineering, Naples, ME, USA) were used to collect ambient PM and operated at a flow rate of 10 l/min. They were sited at a central site within 12 km of subject homes in Riverside and 5 km of subject homes in Whittier. PM2.5 mass was collected on teflon filters and estimated using the standard gravimetric methods. PM2.5 particulate carbon was collected on quartz filter samples and resolved to EC and OC using the thermal manganese dioxide oxidation technique.26 Teflon and quartz filters were archived at −30 °C in sealed Petri dishes that were additionally sealed in doubled Ziploc bags. Criteria pollutant gases (O3 and NO2) were measured hourly by the South Coast Air Quality Management District at central sites (the Riverside site was the same as the PM monitoring site, but two Whittier sites used differed from the study’s central PM monitoring site, which was 5–8 km from gas monitors). Gas measurements from the two Whittier sites were averaged. Averaging times of all exposures are from late afternoon to late afternoon just before the collection of FENO samples. PM monitoring was limited to 10-day consecutive FENO monitoring periods for each group of 3–4 subjects. Therefore, the analysis focuses on lag day 0 (~last 24 h before FENO measurements), lag 1 day (25–48 h before FENO measurements), and the average of these 2 days. Longer lags would have led to excessive loss of observations in regression models.
PM extraction
Filter-collected PM was extracted with 1.8 ml of high-purity water as previously described.15,18 Briefly, extractions were performed in pre-cleaned polypropylene cryogenic tubes, in the dark, for 16 h on a shaker table. Aliquots of the unfiltered PM suspension were removed for DTT analysis and the remaining suspension filtered with a 0.22-μm polypropylene filter to isolate the water-soluble components (dissolved and colloidal species). If the DTT analysis was not carried out immediately, the aliquots were frozen at −30 °C.
Filter-collected PM samples were stored at −30 °C for 7–8 years before extraction and then immediate chemical/bioassay measurements. We have documented that extractable, potentially very labile, redox-active chemical species (Fe2 + and Fe3 +) in atmospheric PM are stable (in PM) for periods of up to at least 1 year when stored in the dark at −20 °C.27 Furthermore, our unpublished data indicate that the ROS activity of filter-collected atmospheric PM can be stabilized for periods of at least 3 years by storage in the dark at −20 °C. We have also demonstrated that the ROS activity of PM extracts can be preserved for periods of at least 9 months at this temperature. Field sample duplicates were not collected in this study; however, multiple field blanks were characterized.
Water-soluble organic carbon
Secondary organic aerosols and products of biomass burning are comprised of polar and highly oxygenated compounds. As a result of this they are water soluble and as such, water-soluble organic carbon (WSOC) will capture the presence of these components on particle extracts.28–30 WSOC was quantified by performing an assay on an aliquot of the filtered extracts of the filter-collected PM using a Sievers Total Organic Carbon 900 Analyzer (GE Analytical Instruments). Briefly, this involved UV/persulfate oxidation followed by conductance measurement of evolved CO2 in solution. The method limit of detection was 25 ppb C (0.3 μg OC).
DTT assay
This assay measures the oxidative potential of PM by quantifying the capacity of PM to transfer electrons from DTT to oxygen, resulting in the generation of superoxide.12,31,32 DTT (20 μl of a 16 mM solution) was added to the sample extracts and incubated for various times (0, 15, 30, 45, and 60 min) at 37 °C. The remaining thiol reacts with 5,5′-dithiobis-2-nitrobenzoic acid (40 μl of 16 mM), generating 2-nitro-5-mercaptobenzoic acid which is measured spectrophotometrically at 415 nm (Molecular Devices M5e plate reader). The rate of DTT consumption (nmol/min/aerosol extract volume) is proportional to the redox activity in the PM sample (nmol DTT consumed/min/μg of PM mass; and therefore to nmol DTT consumed/min/m3). The assay is performed in 96-well plates, each sample in triplicate (25 μl/well) and to ensure that all measurements were in the linear range, at least three dilutions (in triplicate) were run on each extract. Each analytical batch included eight samples. Positive (Naphthoquinone and an Urban Dust (NIST 1649) extract) and negative (extraction and media method blanks) controls were analyzed along with each set of samples.
Results are filter blank subtracted and reported in nmol DTT consumed/min/m3 of air. The mean DTT measurements on 18 filter blanks was threefold greater than the mean laboratory extraction method blank but <3% of the average PM sample DTT measurement. Positive controls were well within acceptable limits (±20%). Overall method precision based upon both intra- and inter-batch outcomes of reference materials is in the range of 8–11% (RSD) for samples with DTT consumption >10 × the average method blank.
Rat alveolar macrophage assay
Cellular production of ROS induced by the PM samples was measured using rat alveolar macrophage cells (NR8383) cultured from stocks obtained from the American Type Culture Collection. NR8383 cells have all the normal characteristics of primary macrophages.33 Details and validation of the assay have been presented by Landreman et al.15 The teflon filter sample extracts (filtered) described above were buffered in a salts/glucose medium and split to prepare aliquots for a detailed dilution series designed to define the linear ROS-response range of each sample. The aliquots, in triplicate, were mixed with NR8383 cells and 2′7′-dichlorodihydrofluorescein diacetate (DCFH-DA) in 96-well plates and incubated at 37 °C for 2.5 h. DCFH-DA is membrane permeable and is de-acetylated by cellular enzymes. ROS species produced within the cell cytoplasm convert 2′7′-DCFH to the fluorescing species 2′7′-dichlorofluorescein. Fluorescence intensity after the incubation was measured using a Molecular Devices M5e automated fluorescence plate reader and represents the biologically mediated production of ROS within the macrophage cell in response to cell stimulation from “toxic” species, that is, oxidative activity of the PM. Positive (Zymosan suspension and Urban Dust (NIST 1649) extracts), and negative (extraction and media method blanks) controls were analyzed along with each set of samples. A model of microbial particles, un-opsonized Zymosan (a β-1,3-polysachharide of D-glucose) served as a positive control as it binds to Toll-like receptor-2 on macrophage cells and then activates a strong respiratory burst and ROS production.
Results are filter blank subtracted and reported in Zymosan equivalent units. The mean ROS activity of 18 filter blanks was not significantly different than the mean laboratory extraction method blank and both were not significantly greater than zero (mean filter blank activity was <1% of average PM sample activity). Positive controls were well within acceptable limits (±20%). Overall method precision based upon both intra- and inter-batch outcomes of reference materials averaged 14% (without zymosan normalization) and 8% with zymosan normalization.
Endotoxin assay
Although ambient endotoxin is not expected to represent major sources of personal exposure, it is potentially important in driving ROS production in the alveolar macrophage assay. To assess this possibility, endotoxin was measured from extracts of archived PM2.5 quartz filters (stored at −30 °C) collected as described above. All quartz filters were baked to remove OC before sampling. Only around 10% of the filters’ surface area was punched out using heat sterilized instruments for EC–OC measurements, leaving sufficient filter media for endotoxin assays. For the endotoxin assay, we developed a rapid and thorough method of extracting endotoxin from quartz PM2.5 filters that is described elsewhere.34 Briefly, the extraction procedure combines the efficient disruption of quartz filter membranes by using a high-speed, reciprocating instrument (FastPrep, MP Biomedicals, Solon, OH, USA) with conventional sonication. First, quartz filters were transferred into pyrogen-free extraction tubes with 4 ml pyrogen-free water. Tubes were then loaded into the FastPrep and processed at 6.5 m/s for 60 s to efficiently homogenize the filter. The extraction tubes were then rotated for 30 min followed by 15-min sonication and clearing of the aqueous extracts of quartz fibers and particles by centrifugation (at 4000 r.p.m. for 5 min, 4 °C). An aliquot of supernatant was then assayed for endotoxin using the Limulus Amoebocyte Lysate kinetic chromogenic assay according to the manufacturer’s protocol (Pyrochrome Associates of Cape Cod, Falmouth, MA, USA). Negative control quartz filters (field blanks) were extracted and analyzed with each set of air samples. The detection limit was estimated at 0.001 endotoxin units (EU)/m3 air. The concentrations in two air samples (with non-detectable endotoxin levels) were set to half this.
Although we do not know what the particle extraction efficiency is or whether it varies by particle size, the multiple mechanical steps described above should produce a thorough removal of collected particles and reduce bias due to particle size.
Analysis
The analytic focus is on within-subject exposure–response relationships, with each subject serving as his/her own control. As each subject was studied during a 10-day phase in groups of 3–4 subjects per session, there are two different exposure–outcome relationships that could affect air pollutant associations with FENO, namely, the between-subject effect and the within-subject effect. The between-subject effect of exposure is the overall outcome levels associated with differences in the air pollutant exposures across subjects. This is potentially confounded by time-independent characteristics of the subjects, including health-related activities. The within-subject effect of exposure is the parameter of interest. This is the association of the overall FENO with differences in the air pollutants across daily measurements for the same subject. We used the following mixed linear regression model as proposed by Janes et al.35
Let the index i indicate the subject (k =1, …, 45) within session j, and t indicate the daily outcome measurement (t = 1, …, 10). Then a given outcome measurement, Yi,j,t will be related to the following three different exposure–outcome relationships:
X̄ij is the average exposure for subject i (between-subject (bs) component).
Xijt − X̄ij is the within-subject (ws) component, which is the assigned exposure at outcome measurement time t for subject i minus the average exposure for the subject.
The mixed model is then:
where ai,j is the random subject intercept nested in session, Zi,j is a vector of subject characteristics specifying such covariates as medication use, and εi,j,t denotes random within-person error in the outcome measurement.
FENO was not normally distributed and was log transformed. As the log-transformed distribution of FENO was symmetric, results are presented as a percentage change in median FENO. A priori adjustments were made for potential time-variant confounders (personal temperature and relative humidity) based on our previous findings.21 We did not find any confounding by respiratory infections (only 13 person-days reported), region of study, sex, cumulative daily use of as-needed β-agonist inhalers, or weekend. Fixed subject characteristics are considered primarily as effect modifiers, and we previously reported that associations were stronger among subjects using inhaled corticosteroids.21 This analysis is not repeated here. We fit an autoregressive-1 correlation structure given the observed variability using the SAS 9.2 procedure Mixed (SAS Institute, Cary, NC, USA). Effect estimates are standardized to exposure interquartile ranges (25th to 75th percentile). Endotoxin data were log-normally distributed so data were natural log transformed for regression analyses.
Residual diagnostics were performed to detect the presence of influential observations and subject clusters, as well as deviations from standard linear mixed-model assumptions. We found that residuals were sufficiently normally distributed, and there were no influential observations, but there was one potentially influential subject cluster with high leverage for modeling FENO. However, after removing the subject from the model, the estimated effects and standard errors changed only nominally and the subject’s data values were plausible. Therefore, this subject was retained in the analysis.
RESULTS
Descriptive Analysis
Subject characteristics are presented in Table 1 and show a diverse population including 58% Hispanics. More than two-thirds were on daily anti-inflammatory medications, reflecting the persistent asthma severity of this population. Exposure data are shown in Table 2 and correlations of PM variables are given in Table 3. The measurements of oxidative potential (DTT and macrophage ROS) are strongly correlated with each other and are moderately to strongly correlated with PM2.5 mass, EC, OC and WSOC, but not endotoxin. NO2 and O3 were not correlated with each other, were weakly correlated with PM2.5, and were moderately correlated with the other variables.
Table 1.
Subject characteristics.
| Subject variables | Data |
|---|---|
| Age (years), mean (range) | 13.5 (9–18) |
| Gender, no. (%) | |
| Female | 14 (31) |
| Male | 31 (69) |
| Race, no. (%) | |
| Hispanic | 26 (58) |
| White | 14 (31) |
| Black | 5 (11) |
| Anti-inflammatory medications, no. (%) | |
| None | 14 (31) |
| Inhaled corticosteroids | 19 (42) |
| Antileukotrienes alone | 2 (4) |
| Antileukotrienes plus inhaled corticosteroids | 10 (22) |
| FENO (ppb), mean±SD | 25.6±25.1 |
Table 2.
Daily measurements of exposures to ambient PM2.5 mass, composition and oxidative potential, and to pollutant gases.
| Exposurea | N (missing)b | Mean (SD) | Median | Interquartile range | Minimum/maximum |
|---|---|---|---|---|---|
| PM2.5 mass, composition and oxidative potential | |||||
| PM2.5 mass (μg/m3) | 116 (3) | 23.2 (18.4) | 16.7 | 15.2 | 2.77/87.2 |
| EC (μg/m3) | 111 (8) | 0.96 (0.67) | 0.77 | 0.77 | 0.14/3.64 |
| OC (μg/m3) | 111 (8) | 4.77 (2.04) | 4.36 | 2.85 | 1.64/11.6 |
| WSOC (μg/m3) | 112 (7) | 1.97 (1.23) | 1.75 | 1.29 | 0.24/6.91 |
| Endotoxin (EU/m3) | 107 (12) | 0.50 (0.34) | 0.46 | 0.39 | 0.05/1.72 |
| Macrophage ROS (μg Zymonsan equivalents/m3) | 111 (8) | 224.4 (147.4) | 203.6 | 203.2 | 10.8/863.3 |
| DTT (nmole/min/m3) | 111 (8) | 0.40 (0.25) | 0.32 | 0.32 | 0.04/1.30 |
| Air pollutant gases | |||||
| NO2 (ppb) | 119 (0) | 27.4 (10.5) | 26.1 | 11.7 | 12.1/73.8 |
| O3 (ppb) | 119 (0) | 52.9 (23.7) | 46.8 | 31.2 | 11.1/120.8 |
Data are for daily averages except O3, which is the 8-h maximum.
Results are for one measured sample per day, not person-days of observation (N = 372).
Table 3.
Spearman correlations of ambient PM2.5 mass, composition and oxidative potential, and pollutant gases.a
| Endotoxin | PM2.5 | EC | OC | WSOC | Macrophage ROS | DTT | NO2 | O3 | |
|---|---|---|---|---|---|---|---|---|---|
| Endotoxin | 1.00 | 0.10 | 0.45 | 0.42 | 0.24 | 0.08 | 0.28 | 0.22 | 0.32 |
| PM2.5 | 1.00 | 0.58 | 0.66 | 0.80 | 0.86 | 0.85 | 0.31 | 0.39 | |
| EC | 1.00 | 0.90 | 0.81 | 0.66 | 0.77 | 0.62 | 0.55 | ||
| OC | 1.00 | 0.90 | 0.72 | 0.80 | 0.55 | 0.71 | |||
| WSOC | 1.00 | 0.81 | 0.85 | 0.51 | 0.65 | ||||
| Macrophage ROS | 1.00 | 0.86 | 0.43 | 0.49 | |||||
| DTT | 1.00 | 0.49 | 0.54 | ||||||
| NO2 | 1.00 | 0.07 | |||||||
| O3 | 1.00 |
Data are for daily averages except O3, which is the 8-h maximum.
The relation between endotoxin and macrophage ROS production in ambient PM2.5 samples (N = 100 days) adjusted for ambient temperature was nonsignificantly inverse (P<0.24), suggesting levels of endotoxin in the ambient particle filter extracts were not an important determinant in this assay.
Regression Analysis
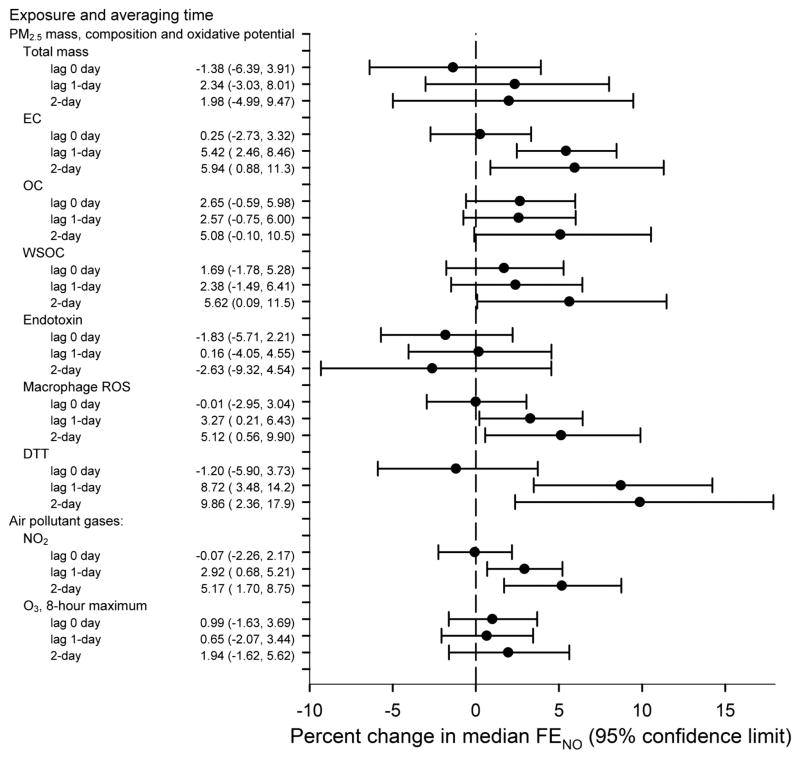
FENO was positively associated with lag 1 day and 2-day moving average PM2.5 EC and was nominally associated with 2-day moving average PM2.5 OC, but was not associated with total PM2.5 mass (Figure 1). Consistent with the OC finding and to a similar magnitude, FENO was positively associated with 2-day moving average WSOC (over 5% increase in FENO per interquartile range increase in WSOC). Endotoxin was not associated with FENO. FENO was positively associated with PM2.5 oxidative potential as measured by both macrophage ROS and DTT for lag 1-day and 2-day moving averages. DTT had an effect size that was nearly twice that of all other exposures with an almost 10% increase in FENO per interquartile range increase in DTT. FENO was also positively associated with lag 1-day and 2-day moving average NO2, but was not associated with O3.
Figure 1.
Relation of fractional exhaled nitric oxide (FENO) to ambient particulate matter (PM2.5) mass, composition and oxidative potential, and to air pollutant gases. The percent change in FENO is in relation to a one-interquartile range change in the concentration of the air pollutant (Table 2), adjusted for personal temperature, personal relative humidity, and exposure run. DTT, dithiothreitol; EC, elemental carbon; OC, organic carbon; ROS, reactive oxygen species; WSOC, water-soluble organic carbon.
Two-pollutant models for various combinations of one of the oxidative potential variables with one of the measured air pollutants were generally not informative (Table 4) in that in most models both variables in the models confounded each other to similar degrees due to their positive correlations. Therefore, for example, it was not possible to assess the extent to which FENO associations with the traffic-related pollutants (EC and NO2) are related to the oxidative potential of PM2.5. Interestingly, the effects of 2-day average OC and WSOC were completely confounded by DTT, although confidence intervals widened considerably. PM2.5 models showed major sign shifts in the regression coefficient from positive (yet nonsignificant) in single pollutant to negative in two-pollutant models for PM2.5. This suggests that strong correlations between PM2.5 and DTT/ROS induced multicollinearity.
Table 4.
Two-pollutant models testing relation of FENO to both in vitro particle oxidative potential and direct air pollutant measurements.
| Exposures (2-day averages) | Percent change in median FENO (95% confidence limit) | |
|---|---|---|
| Single pollutant modela | Two-pollutant model | |
| ROS and PM2.5 mass | ||
| ROS | 5.12 (0.55, 9.9) | 11.4 (3.04, 20.5) |
| PM2.5 | 2.86 (−4.24, 10.5) | −10.8 (−21.3, 1.10) |
| DTT and PM2.5 mass | ||
| DTT | 9.85 (2.36, 17.9) | 12.9 (3.21, 23.5) |
| PM2.5 | 2.59 (−4.55, 10.3) | −4.78 (−13.1, 4.27) |
| ROS and EC | ||
| ROS | 5.50 (0.77, 10.4) | 3.28 (−2.42, 9.3) |
| EC | 6.49 (1.06, 12.2) | 4.22 (−2.31, 11.2) |
| DTT and EC | ||
| DTT | 8.87 (1.09, 17.2) | 5.14 (−4.94, 16.3) |
| EC | 6.21 (0.78, 11.9) | 3.69 (−3.44, 11.4) |
| ROS and OC | ||
| ROS | 5.50 (0.77, 10.4) | 4.06 (−2.96, 11.6) |
| OC | 5.57 (0.14, 11.3) | 2.05 (−5.82, 10.6) |
| DTT and OC | ||
| DTT | 8.87 (1.09, 17.2) | 8.33 (−5.91, 24.7) |
| OC | 5.28 (−0.18, 11.0) | 0.37 (−9.25, 11.0) |
| ROS and WSOC | ||
| ROS | 5.12 (0.55, 9.90) | 3.33 (−3.25, 10.4) |
| WSOC | 5.88 (0.34, 11.7) | 2.85 (−5.01, 11.4) |
| DTT and WSOC | ||
| DTT | 9.85 (2.36, 17.9) | 10.5 (−1.54, 24.1) |
| WSOC | 5.61 (0.02, 11.5) | −0.58 (−9.01, 8.62) |
| ROS and NO2 | ||
| ROS | 5.12 (0.55, 9.90) | 2.51 (−3.02, 8.37) |
| NO2 | 4.56 (0.94, 8.32) | 3.35 (−1.11, 8.02) |
| DTT and NO2 | ||
| DTT | 9.85 (2.36, 17.9) | 6.34 (−4.24, 18.1) |
| NO2 | 4.57 (0.92, 8.36) | 2.21 (−3.03, 7.74) |
The percent change in FENO is in relation to a one-interquartile range change in the concentration of the air pollutant (Table 2), adjusted for personal temperature, personal relative humidity, and exposure run.
DISCUSSION
This is the first report to our knowledge of a positive association between FENO in pediatric subjects with asthma and the oxidative potential of PM2.5 as measured with aqueous particle extracts using DTT consumption and the generation of ROS by macrophages. We previously reported and observe here again that FENO was significantly positively associated with the traffic-related air pollutant markers (EC and NO2).21 Our new findings suggest that part of the association with traffic-related pollutants we found may be attributable to particle components that have pro-oxidant effects in the lungs that thereby promote airway inflammation. Both sets of significant associations were found for the same averaging times (lag 1-day and 2-day averages). In a sensitivity analysis, we examined two-pollutant models with oxidative potential (either DTT or macrophage ROS) and one of the traffic markers. However, we were unable to clearly separate independent effects of different pollutants due to their strong correlations (r>0.7, Table 3), particularly oxidative potential and the traffic exposure markers (EC, NO2). DTT, on the other hand, confounded the effects of OC and WSOC, suggesting that effects of these pollutants (and possibly associated SOA or other components) may have been due to the oxidative potential of the mixture.
DTT associations were nearly twice as strong as the other exposure metrics, including macrophage ROS. FENO significantly increased by 8.7–9.9% per interquartile range of 0.43 nmole/min/m3 DTT for lag 1-day and 2-day average, respectively. It is unclear why the DTT metric would reveal stronger associations than the macrophage ROS assay but this needs to be replicated in other studies. However, it is likely that DTT and macrophage assays are sensitive to different components of PM2.5 that were not measured in the present study — including contrasts in sensitivity to insoluble species of PM. Aside from our other report of a positive association between FENO and macrophage ROS in an elderly population,20 there is limited research on the importance of measured particle oxidative potential in human respiratory health. We measured FENO weekly in 60 elderly subjects and measured macrophage ROS as described here using cells exposed to particle extracts from previous 5-day composites of PM filters collecting quasi-ultrafine particles. Our present results contrast a recent semiexperimental study of 31 healthy young adults who were placed for 5 h at intermittent exercise at five different locations in the Netherlands with contrasts in air pollutants and their sources (the pre-exposure site being Utrecht University followed by transport via an air-filtered van).36 They reported that exposure to PM2.5 and PM10 mass and to PM10 oxidative potential was not associated with FENO, whereas FENO was positively associated with EC, NO2/NOx, and particle number concentration (dominated by ultrafine particles). This inconsistency with the present study could be due to the major differences in design, or population characteristics in our study vs theirs (pediatric subjects with asthma vs healthy adults with no history of asthma) or different methods of assessing oxidative potential: they measured in vitro antioxidant depletion of ascorbate and reduced glutathione by particles extracted from PM10 using methanol37, whereas we measured oxidative potential as described (DTT and macrophage ROS generation) using water extraction of PM2.5 filters. Interestingly, these investigators also used concentrated particle samples from eight locations (including the five locations above) for an in vitro assay of murine macrophages. Results showed cellular responses including pro-inflammatory markers that were positively associated with DTT consumption for outdoor PM2.5 and quasi-ultrafine samples from traffic locations characterized by a high EC and OC fractions.38
The importance of traffic emissions to airway inflammation was shown in another longitudinal study of children with asthma in Ciudad Juarez, Mexico, that found FENO was positively associated with road density near the subjects’ homes.39 This finding is consistent with the Children’s Health Study in southern California that showed an association between FENO and road length near the home, although other traffic indexes were nonsignificant.40 We have reported that the risk of recurrent hospital admissions or emergency department visits for asthma in children is increased with higher exposures to traffic-dispersion modeled air pollutants near the home.41 A randomized, crossover field study of adults with asthma showed decreased lung function and increased sputum myeloperoxidase after they were exposed to diesel traffic.42
We found no evidence of an effect on FENO from ambient PM2.5 mass, despite the high correlation (0.85–0.86) of PM2.5 with the ROS and DTT assays. This difference is not driven by influential observations (see analysis, Methods). The high correlations are coming from the low ends of the exposure distributions where both measurements are bounded at 0, whereas there is significant variation above the median for each covariate and this is likely producing the observed difference in the exposure–FENO relationships. Our null findings for PM2.5 mass are in contrast to a longitudinal study of children with asthma in Mexico city with similar PM2.5 levels but positive associations of FENO with PM2.5.43 Another cohort panel study at the US–Mexico border towns of Ciudad Juarez, Mexico, and El Paso, Texas, found positive associations between FENO in children with asthma and both black carbon and PM2.5 measurements at school sites.44 Our null PM2.5 results were consistent with a longitudinal study of FENO in children with asthma in Windsor, Canada, with lower levels of PM2.5 (median 6.5 μg/m3).45 The Canadian study instead found a positive association between a biomarker of oxidative stress in exhaled breath condensates and both PM2.5 and NO2.
Although expected based on other data, the negative results for O3 are not surprising to us, given previous findings46 showing poor prediction of personal O3 exposures by much higher levels of ambient O3 in southern California and given other findings showing significant associations of personal O3 but not ambient O3 with asthma symptoms in children.47
We also observed that FENO was significantly and positively associated with WSOC, which is a tracer of secondary organic aerosols along with biomass burning,28–30 suggesting that these sources are potentially important in inducing airway inflammation. This result is consistent with our previous report of an association between FENO and WSOC in an elderly cohort.20 To our knowledge, there is no direct epidemiologic information on acute asthma outcomes and exposure to markers of outdoor secondary organic aerosols. Most of the relevant data to date on this come from experimental studies of laboratory-generated secondary organic aerosols, showing adverse respiratory effects in mice as reviewed by Mauderly and Chow48 and adverse cellular effects using in vitro cell cultures, for example, Baltensperger et al.49 This characteristic of pollutant particles is important as previously reviewed,20 as secondary organic aerosol chemicals are water soluble (due to being highly oxidized) and they are likely released rapidly from particles deposited in the airways to then induce redox reactions with biomolecules in the respiratory epithelium. Some indirect evidence of the importance of secondary organic aerosols has been provided by large time series studies, showing that associations of asthma emergency department visits or hospital admissions with mean PM10 or PM2.5 are considerably larger in the warm season than in the cold season in New York city,2 in seven Canadian cities,3 and in Atlanta, Georgia.4 These consistent findings are possibly due to greater photochemical generation of secondary organic aerosol in the summer and increased outdoor exposure times.
Limitations include a lack of measurements of specific chemical components that may have been responsible for the level of oxidative potential such as transition metals and PAHs. Associations between risk of wheeze in pediatric subjects with asthma and exposure to particle-bound PAHs have been recently reported and lend support to performing chemical characterization of pollutant particles.50 The present measurements were also performed at a central ambient site and may not be sufficiently representative of personal exposure. The approximate distance of subject homes to the central site ranged from 1.4 to 12 km in Riverside, from 0 to 5 km in Whittier for PM data, and from 1 to 8 km for the two gas-monitoring locations. We previously reported for the same subjects that measured personal exposures to EC and NO2 were also significantly associated with FENO.21 However, personal NO2 was only moderately correlated with ambient NO2 (r =0.46), and personal EC was not correlated with ambient EC (r =0.04), suggesting different sources for personal exposures were important, including local traffic. The previously reported associations with personal EC and NO2 were largely independent of significant associations with personal PM2.5 in two-pollutant models, suggesting that personal PM exposures carried other components relevant to inflammation in the asthmatic airway unrelated to markers of traffic-related sources. In a recently published paper, we also performed an exposure assessment analysis of personal, home, and ambient exposures on a 25% subset of the subjects in the present panel.51 We found correlations between outdoor home and ambient exposures were moderate to strong (r = 0.68–0.97, with the strongest correlations being for PM2.5, followed by OC, and then EC). We used the ratios of outdoor home to central site (ambient) measurements as an approach to predict spatial variation and found little variation in PM2.5 but notable variation of EC and OC in Riverside but not Whittier (where a majority, N =32, of the 45 subjects in the present analysis lived). EC in Riverside showed an influence of freeways, and although the distribution of OC was more homogeneous, we also observed higher ratios in residential areas with denser street networks. In this 25% subset, personal EC and OC were more strongly correlated with outdoor home EC and OC (r =0.57 and 0.55, respectively) than ambient EC and OC (r =0.29 and 0.22, respectively), but correlations for personal PM2.5 were similar (r =0.77 for home and 0.83 for ambient PM2.5).
We conclude that PM oxidative potential is associated with airway inflammation in young subjects with persistent asthma. Associations reported in studies of asthma morbidity events such as hospital admissions that point to clinically adverse effects of ambient PM2.5 could be at least partly attributable to the oxidative potential of particles to induce airway oxidative stress and inflammation. Given that we did not find ambient PM2.5 mass to be associated with FENO, future research in oxidative stress-related illnesses such as asthma and exposure to air pollutant particles would benefit from an assessment of both PM oxidative potential and composition.
Acknowledgments
We thank staff in the Department of Epidemiology and the General Clinical Research Center, University of California Irvine. Funding Support: This study was supported by National Institute of Environmental Health Sciences, U.S. National Institutes of Health (R01 ES11615 and R21 ES019711), General Clinical Research Center University of California Irvine (National Institutes of Health grant MO1-RR00827), and South Coast Air Management District, through the University of California Los Angeles Asthma and Outdoor Air Quality Consortium (Contract No. UCLA-35692).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Sarnat JA, Holguin F. Asthma and air quality. Curr Opin Pulm Med. 2007;13:63–66. doi: 10.1097/MCP.0b013e3280117d25. [DOI] [PubMed] [Google Scholar]
- 2.Silverman RA, Ito K. Age-related association of fine particles and ozone with severe acute asthma in New York City. J Allergy Clin Immunol. 2010;125:367–373. doi: 10.1016/j.jaci.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 3.Stieb DM, Szyszkowicz M, Rowe BH, Leech JA. Air pollution and emergency department visits for cardiac and respiratory conditions: a multi-city time-series analysis. Environ Health. 2009;8:25. doi: 10.1186/1476-069X-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strickland MJ, Darrow LA, Klein M, Flanders WD, Sarnat JA, Waller LA, et al. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med. 2010;182:307–316. doi: 10.1164/rccm.200908-1201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayres JG, Borm P, Cassee FR, Castranova V, Donaldson K, Ghio A, et al. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential--a workshop report and consensus statement. Inhal Toxicol. 2008;20:75–99. doi: 10.1080/08958370701665517. [DOI] [PubMed] [Google Scholar]
- 6.Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho AK, Sioutas C, Miguel AH, Kumagai Y, Schmitz DA, Singh M, et al. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ Res. 2005;99:40–47. doi: 10.1016/j.envres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Lin P, Yu JZ. Generation of reactive oxygen species mediated by humic-like substances in atmospheric aerosols. Environ Sci Technol. 2011;45:10362–10368. doi: 10.1021/es2028229. [DOI] [PubMed] [Google Scholar]
- 9.Rattanavaraha W, Rosen E, Zhang H, Li Q, Pantong K, Kamens RM. The reactive oxidant potential of different types of aged atmospheric particles: an outdoor chamber study. Atmos Environ. 2011;45:3848–3855. [Google Scholar]
- 10.Verma V, Rico-Martinez R, Kotra N, King L, Liu J, Snell TW, et al. Contribution of water-soluble and insoluble components and their hydrophobic/hydrophilic sub-fractions on the ROS-generating potential of fine ambient aerosols. Environ Sci Technol. 2012;46:11384–11392. doi: 10.1021/es302484r. [DOI] [PubMed] [Google Scholar]
- 11.Charrier JG, Anastasio C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: evidence for the importance of soluble transition metals. Atm Chem Phys. 2012;12:9321–9333. doi: 10.5194/acpd-12-11317-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinyashiki M, Eiguren-Fernandez A, Schmitz DA, DiStefano E, Li N, Linak WP, et al. Electrophilic and redox properties of diesel exhaust particles. Environ Res. 2009;109:239–244. doi: 10.1016/j.envres.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Li N, Wang M, Bramble LA, Schmitz DA, Schauer JJ, Sioutas C, et al. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential. Environ Health Perspect. 2009;117:1116–1123. doi: 10.1289/ehp.0800319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee IT, Yang CM. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem Pharmacol. 2012;84:581–590. doi: 10.1016/j.bcp.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Landreman AP, Shafer MM, Hemming JC, Hannigan MP, Schauer JJ. A macrophage-based method for the assessment of reactive oxygen species (ROS) activity of atmospheric particulate matter (PM) and application to routine (daily 24-hour) aerosol monitoring studies. Aerosol Sci Technol. 2008;42:946–957. [Google Scholar]
- 16.Hu S, Polidori A, Arhami M, Shafer MM, Schauer JJ, Cho A, et al. Redox activity and chemical speciation of size fractioned PM in the communities of the Los Angeles-Long Beach harbor. Atmos Chem Physics. 2008;8:6439–6451. [Google Scholar]
- 17.Verma V, Polidori A, Schauer JJ, Shafer MM, Cassee FR, Sioutas C. Physicochemical and toxicological profiles of particulate matter in Los Angeles during the October 2007 southern California wildfires. Environ Sci Technol. 2009;43:954–960. doi: 10.1021/es8021667. [DOI] [PubMed] [Google Scholar]
- 18.Shafer MM, Perkins DA, Antkeweitz DS, Stone EA, Quraishi T, Schauer JJ. Reactive oxygen species activity and chemical speciation of size-fractionated atmospheric particulate matter from Lahore Pakistan: an important role for transition metals. J Environ Monit. 2010;12:704–715. doi: 10.1039/b915008k. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Schauer JJ, Shafer MM, Hannigan MP, Dutton SJ. Source apportionment of in vitro reactive oxygen species bioassay activity from atmospheric particulate matter. Environ Sci Technol. 2008;42:7502–7509. doi: 10.1021/es800126y. [DOI] [PubMed] [Google Scholar]
- 20.Delfino RJ, Staimer N, Tjoa T, Arhami M, Polidori A, George SC, et al. Associations of primary and secondary organic aerosols with airway and systemic inflammation in an elderly panel cohort. Epidemiology. 2010;21:892–902. doi: 10.1097/EDE.0b013e3181f20e6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delfino RJ, Staimer N, Gillen D, Tjoa T, Sioutas C, Fung K, et al. Personal and ambient air pollution is associated with increased exhaled NO in children with asthma. Environ Health Perspect. 2006;114:1736–1743. doi: 10.1289/ehp.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Thoracic Society (ATS) and European respiratory Society (ERS) ATS/ERS Recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 23.Linn WS, Avila M, Gong H., Jr Exhaled nitric oxide: sources of error in offline measurement. Arch Environ Health. 2004;59:385–391. doi: 10.3200/AEOH.59.8.385-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroesbergen A, Jobsis Q, Bel EH, Hop WC, de Jongste JC. Flow-dependency of exhaled nitric oxide in children with asthma and cystic fibrosis. Eur Respir J. 1999;14:871–875. doi: 10.1034/j.1399-3003.1999.14d24.x. [DOI] [PubMed] [Google Scholar]
- 25.Jöbsis Q, Raatgeep HC, Hop WC, de Jongste JC. Controlled low flow off line sampling of exhaled nitric oxide in children. Thorax. 2001;56:285–289. doi: 10.1136/thorax.56.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fung K, Chow JC, Watson JG. Evaluation of OC/EC speciation by thermal manganese dioxide oxidation and the IMPROVE method. J Air Waste Manage Assoc. 2002;52:1333–1341. doi: 10.1080/10473289.2002.10470867. [DOI] [PubMed] [Google Scholar]
- 27.Majestic BJ, Schauer JJ, Shafer MM, Turner JR, Fine PM, Singh M, et al. Development of a wet-chemical method for the speciation of iron in atmospheric aerosols. Environ Sci Technol. 2006;40:2346–2351. doi: 10.1021/es052023p. [DOI] [PubMed] [Google Scholar]
- 28.Snyder DC, Rutter AP, Collins R, Worley CA, Schauer JJ. Insights into the origin of water soluble organic carbon in atmospheric fine particulate matter. Aerosol Sci Technol. 2009;43:1099–1107. [Google Scholar]
- 29.Docherty KS, Stone EA, Ulbrich IM, DeCarlo PF, Snyder DC, Schauer JJ, et al. Apportionment of primary and secondary aerosols, in Southern California during the 2005 Study of Organic Aerosols in Riverside (SOAR) Environ Sci Technol. 2008;42:7655–7662. doi: 10.1021/es8008166. [DOI] [PubMed] [Google Scholar]
- 30.Stone EA, Snyder DC, Sheesley RJ, Sullivan A, Weber RJ, Schauer JJ. Source apportionment of fine organic aerosol in Mexico City during the MILAGRO Experiment 2006. Atmos Chem Phys. 2008;8:1249–1259. [Google Scholar]
- 31.Kumagai Y, Koide S, Taguchi K, Endo A, Nakai Y, Yoshikawa T, et al. Oxidation of proximal protein sulfhydryls by phananthraquinone, a component of diesel exhaust particles. Chem Res Toxicol. 2002;5:483–489. doi: 10.1021/tx0100993. [DOI] [PubMed] [Google Scholar]
- 32.Shima H, Koike E, Shinohara R, Kobayashi T. Oxidative ability and toxicity of n-hexane insoluble fraction of diesel exhaust particles. Toxicol Sci. 2006;61:218–226. doi: 10.1093/toxsci/kfj119. [DOI] [PubMed] [Google Scholar]
- 33.Lane KB, Egan B, Vick S, Abdolrasulnia R, Shepherd VL. Characterization of a rat alveolar macrophage cell line that expresses a functional mannose receptor. J Leukoc Biol. 1998;64:345–350. doi: 10.1002/jlb.64.3.345. [DOI] [PubMed] [Google Scholar]
- 34.Delfino RJ, Staimer N, Tjoa T. Personal endotoxin exposure in a panel study of school children with asthma. Environ Health. 2011;10:69. doi: 10.1186/1476-069X-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janes H, Sheppard L, Shepherd K. Statistical analysis of air pollution panel studies: an illustration. Ann Epidemiol. 2008;18:792–802. doi: 10.1016/j.annepidem.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Strak M, Janssen NA, Godri KJ, Gosens I, Mudway IS, Cassee FR, et al. Respiratory health effects of airborne particulate matter: the role of particle size, composition, and oxidative potential-the RAPTES Project. Environ Health Perspect. 2012;120:1183–1189. doi: 10.1289/ehp.1104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Godri KJ, Duggan ST, Fuller GW, Baker T, Green D, Kelly FJ, et al. Particulate matter oxidative potential from waste transfer station activity. Environ Health Perspect. 2010;118:493–498. doi: 10.1289/ehp.0901303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steenhof M, Gosens I, Strak M, Godri KJ, Hoek G, Cassee FR, et al. In vitro toxicity of particulate matter (PM) collected at different sites in the Netherlands is associated with PM composition, size fraction and oxidative potential--the RAPTES project. Part Fibre Toxicol. 2011;8:26. doi: 10.1186/1743-8977-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holguin F, Flores S, Ross Z, Cortez M, Molina M, Molina L, et al. Traffic-related exposures, airway function, inflammation, and respiratory symptoms in children. Am J Respir Crit Care Med. 2007;176:1236–1242. doi: 10.1164/rccm.200611-1616OC. [DOI] [PubMed] [Google Scholar]
- 40.Eckel SP, Berhane K, Salam MT, Rappaport EB, Linn WS, Bastain TM, et al. Residential traffic-related pollution exposures and exhaled nitric oxide in the children’s health study. Environ Health Perspect. 2011;119:1472–1477. doi: 10.1289/ehp.1103516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delfino RJ, Chang J, Wu J, Ren C, Tjoa T, Nickerson B, et al. Repeated hospital encounters for asthma in children and exposure to traffic-related air pollution near the home. Ann Allergy Asthma Immunol. 2009;102:138–144. doi: 10.1016/S1081-1206(10)60244-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCreanor J, Cullinan P, Nieuwenhuijsen MJ, Stewart-Evans J, Malliarou E, Jarup L, et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med. 2007;357:2348–2358. doi: 10.1056/NEJMoa071535. [DOI] [PubMed] [Google Scholar]
- 43.Barraza-Villarreal A, Sunyer J, Hernandez-Cadena L, Escamilla-Nuñez MC, Sienra-Monge JJ, Ramírez-Aguilar M, et al. Air pollution, airway inflammation, and lung function in a cohort study of Mexico City schoolchildren. Environ Health Perspect. 2008;116:832–838. doi: 10.1289/ehp.10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarnat SE, Raysoni AU, Li WW, Holguin F, Johnson BA, Flores Luevano S, et al. Air pollution and acute respiratory response in a panel of asthmatic children along the U.S-Mexico border. Environ Health Perspect. 2012;120:437–444. doi: 10.1289/ehp.1003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu L, Poon R, Chen L, Frescura AM, Montuschi P, Ciabattoni G, et al. Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ Health Perspect. 2009;117:668–674. doi: 10.1289/ehp11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L-JS, Delfino RJ, Koutrakis P. Ozone exposure assessment in a southern California community. Environ Health Perspect. 1997;105:58–65. doi: 10.1289/ehp.9710558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delfino RJ, Coate B, Zeiger RS, Seltzer JM, Street DH, Koutrakis P. Daily asthma severity in relation to personal ozone exposure and outdoor fungal spores. Am J Respir Crit Care Med. 1996;154:633–641. doi: 10.1164/ajrccm.154.3.8810598. [DOI] [PubMed] [Google Scholar]
- 48.Mauderly JL, Chow JC. Health effects of organic aerosols. Inhal Toxicol. 2008;20:257–288. doi: 10.1080/08958370701866008. [DOI] [PubMed] [Google Scholar]
- 49.Baltensperger U, Dommen J, Alfarra MR, Duplissy J, Gaeggeler K, Metzger A, et al. Combined determination of the chemical composition and of health effects of secondary organic aerosols: the POLYSOA project. J Aerosol Med Pulm Drug Deliv. 2008;21:145–154. doi: 10.1089/jamp.2007.0655. [DOI] [PubMed] [Google Scholar]
- 50.Gale SL, Noth EM, Mann J, Balmes J, Hammond SK, Tager IB. Polycyclic aromatic hydrocarbon exposure and wheeze in a cohort of children with asthma in Fresno, CA. J Expo Sci Environ Epidemiol. 2012;22:386–392. doi: 10.1038/jes.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ducret-Stich R, Delfino RJ, Tjoa T, Gemperli A, Wu J, Phuleria HC, et al. Examining representativeness of home outdoor models for PM2.5, EC, and OC estimates for daily personal exposures in Southern California. Air Qual Atmos Health. 2012;5:335–351. doi: 10.1007/s11869-010-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]