Abstract
Dietary restriction extends lifespan in many organisms, but little is known about how it affects hematophagous arthropods. We demonstrated that diet restriction during either larval or adult stages extends Aedes aegypti lifespan. A. aegypti females fed either single or no blood meals survived 30–40% longer than those given weekly blood meals. However, mosquitoes given weekly blood meals produced far more eggs. To minimize reproduction’s impact on lifespan, adult mosquitoes were fed artificial blood meals containing <10% of the protein in normal human blood, minimizing egg production. A. aegypti fed artificial blood meals containing 25 mg/ml of BSA had significantly shorter lifespans than those fed either 10 or 5 mg/ml. To assess the impact of larval dietary restriction on adult lifespan, we maintained larval A. aegypti on 2X, 1X (normal diet), 0.5X or 0.25X diets. Adult mosquitoes fed 0.5X and 0.25X larval diets survived significantly longer than those fed the 2X larval diet regardless of adult diet. In summary, dietary restriction during both larval and adult stages extends lifespan. This diet-mediated lifespan extension has important consequences for understanding how dietary restriction regulates lifespan and disease transmission.
Keywords: Dietary restriction, Nutrient restriction, Aging, Senescence, Dengue
1. Introduction
One of the most robust mechanisms regulating lifespan across a wide range of organisms is dietary restriction, where nutrient intake is limited to below what an organism would take ad libitum, but above the level of starvation. Lifespan extension from dietary restriction has been observed in a variety of organisms (Mair and Dillin, 2008), including insects as diverse as the fruit fly, the house fly, a butterfly, and even a stick insect (Carey et al., 2002, Cooper et al., 2004, Boggs and Ross, 1993, Roark and Bjorndal, 2009). In Drosophila melanogaster, for example, dietary restriction in adults can extend lifespan by over 50% (Grandison et al., 2009, Partridge et al., 2005). Protein and lipid levels, not calories per se, appear to be especially important in governing this relationship (Mair et al., 2005). Less is known about the impact of dietary restriction at the larval stage. One study demonstrated that while yeast deprived larvae take longer to eclose and adults are smaller, there was no apparent difference in adult mortality between larvae fed a restricted or normal diet (Tu and Tatar, 2003). Given their unique ecology, it is useful to extend this widespread relationship between diet and longevity to blood feeding arthropods such as the mosquito Aedes aegypti.
Wild mosquitoes often experience periods of nutrient restriction. During the aquatic larval stages, container breeding Aedes mosquitoes develop in small transient pools, where they undergo intense inter- and intraspecific competition (Reiskind and Lounibos, 2009) for resources such as bacteria, algae, and other organic matter. Intra- and interspecific competition at the larval stages can impact adult lifespan (Reiskind and Lounibos, 2009), but it is unclear whether nutrient restriction causes these changes. As adults, female A. aegypti largely consume protein-rich blood meals, but will also feed on nectar when a blood source is not readily available or if nectar sources are abundant (Edman, 2003, Martinez-Ibarra et al., 1997). If hosts are in short supply, as is the case in many rural areas, then blood meals and dietary protein will be restricted. Larval competition and the availability of vertebrate blood during adulthood could potentially impact the mosquito’s lifespan in nature.
A. aegypti mosquitoes have a feast and famine feeding behavior. In D. melanogaster, there is conflicting evidence on whether periods of feeding interspersed with periods of starvation can lead to increased lifespan. Some studies using an intermittent feeding schedule showed no signs of lifespan extension in flies intermittently starved (Le Bourg and Médioni, 1991, Le Bourg and Minois, 1996). Studies in medflies and houseflies had similar results (Carey et al., 2002, Cooper et al., 2004). In contrast, a 30% increase in lifespan was observed when D. melanogaster were provided sugar water ad libitum and yeast every sixth day (Partridge et al., 1987). Female A. aegypti adults offer a unique model for examining this effect. After mating, female A. aegypti consume a large (~2 μl) blood meal rich in protein, nearly doubling their body weight. Following this blood meal the female produces a clutch of approximately 100 eggs which are oviposited 48–72 h later. This cyclical pattern of feeding and reproduction is characteristic of many blood feeding arthropods and likely plays an important role in determining overall lifespan. Dietary restriction might be achieved either through limited blood meals due to the lack of a suitable host or through incomplete blood meals caused by disruption of the blood feeding process. In urban environments, where potential hosts are abundant and A. aegypti consume multiple blood meals during a reproductive cycle (Scott et al., 1993), partial blood meals are likely to be a more important source of dietary restriction.
In addition to the insights that blood feeding arthropods can provide regarding dietary restriction and lifespan, life history changes in A. aegypti have the potential to impact disease transmission. The capacity of A. aegypti to transmit pathogens such as the four closely related flaviviruses collectively known as dengue is largely contingent on lifespan. In fact, the daily probability of survival is the single most important factor determining the vectorial capacity of a mosquito (Macdonald, 1957). This is due to the fact that many mosquito-borne parasites, including dengue, require an extended developmental period within the mosquito known as the extrinsic incubation period, which can last up to two weeks. In the wild, A. aegypti daily probability of survival is between 70 and 90%, meaning that even under ideal conditions fewer than 20% of wild A. aegypti survive long enough to transmit dengue (Harrington et al., 2001, Costero et al., 1998). However, those mosquitoes that do survive the extrinsic incubation period are infective for life and can potentially infect every subsequent person they bite. Thus, reducing the lifespan of older mosquitoes that transmit a disproportionate amount of the disease could reduce overall dengue transmission. Having a better understanding of how dietary restriction impacts lifespan, both during the aquatic larval stages and blood feeding adult stages, is critical for understanding the epidemiology of dengue and may provide new insights into novel control measures.
One confounding variable in many studies of the relationship between diet and lifespan is that reproduction, a trait often negatively correlated with survival, is directly correlated with food quality and intake (Piper and Partridge, 2007). To address this, Mair et al. (2004) restricted females’ access to males, used mutant genotypes, and eliminated female germlines showed that dietary restriction extended lifespan independent of reproduction in D. melanogaster. A. aegypti reproduction can be easily manipulated, too, in order to reduce the influence of reproduction on lifespan in dietary restriction studies. Because their reproduction is only initiated after they take a protein meal, we can easily manipulate female fecundity by reducing the amount of protein they eat.
In this study we explored the impact of dietary restriction during both larval and adult stages on the lifespan and reproductive capacity of female A. aegypti. At the same time we determined the lifetime fecundity of these dietary restricted mosquitoes when provided with a similar number of discrete protein meals. We also examined how effectively aging mosquitoes produced eggs and whether previous reproductive cycles impact egg production in subsequent gonotrophic cycles.
2. Experimental procedures
2.1. Mosquito rearing
A. aegypti were maintained at 27 °C and 70% RH with a 16 h light and 8 h dark photo period. During the larval stage mosquitoes were reared on a solution containing 125 mg/l of yeast, ground rabbit chow, and lactalbumin (1:1:1 ratio). The standard larval diet was as follows: days 1 and 2 post larval emergence – 400 μl diet/pan, days 3 and 4 – 600 μl/pan, day 5 through pupation – 1.2 ml/pan. Most mosquitoes pupated by day nine. Adult mosquitoes were maintained in 5 l canisters and allowed to feed on 10% dextrose ad libitum using a wick. For egg production mosquitoes were membrane fed on porcine blood containing 1% sodium citrate as an anticoagulant. Mosquitoes given blood for the diet experiments were allowed to feed for 2 h. Pig blood is over 50% protein (520 g/l (Hellwing et al., 2007)), with the remaining fraction containing mostly water.
2.2. Adult blood feeding assay
A. aegypti used in the adult blood feeding assays were fed the standard diet during larval stages. Thirty pans of larvae (200 larvae/l/pan) were established per experiment under identical conditions and pupae were combined to generate a homogenous mixture. Approximately 1000 female mosquitoes and several hundred males were placed in large cages (21 l) to generate three treatment groups. One treatment group received weekly blood meals starting 7 days post-emergence, the second treatment received a single blood meal 7 days post-emergence, and the third treatment received no blood meals. Blood fed treatments were provided with oviposition substrates 48 h after blood feeding and were allowed to oviposit for 72 h. All treatment groups were given unlimited access to a wick soaked in 10% dextrose. Dead female mosquitoes were counted and removed from the cages daily. Experiments were replicated three times.
2.3. Adult dietary restriction
A. aegypti used in the adult dietary restriction tests were fed the standard diet during larval stages. Fifteen pans of larvae (100 larvae/l/pan) were established per experiment under identical conditions and pupae were combined to generate a homogenous mixture. Female mosquitoes emerging on days seven or eight were separated into four cages of 200 females each. Male mosquitoes (50) were added to each cage to ensure that females had mated. Each treatment was given an artificial blood meal consisting of various concentrations of bovine serum albumin (BSA; 1 mg/ml, 5 mg/ml, 10 mg/ml or 25 mg/ml) in feeding buffer (150 mM NaCl, 1 mM ATP at 37 °C). A 10% dextrose solution was provided via wick throughout the assay for all treatments. The first artificial blood meal was given ~5 days after adult emergence. Subsequent feedings were provided twice weekly on Mondays and Thursdays. Oviposition surfaces were provided 48 h after the artificial blood meal for 24–36 h. Eggs laid on the oviposition substrate or the wick of the dextrose source were collected and counted. Dead female mosquitoes were counted and removed from the cages daily. Experiments were replicated five times.
2.4. Larval dietary restriction
To test what effects dietary restriction during the larval stage had on the lifespan of adult A. aegypti we modified larval food intake. On day one, newly hatched larvae were evenly distributed into ten pans (100 larvae/l/pan). Five pans were fed half the standard larval diet and the other five pans were fed one quarter the standard diet. Since larvae fed the 1X and 2X diet developed more quickly these treatments were established two days later. This resulted in all of the adult mosquitoes emerging within a 3–4 days window. Three cages containing ~200 adult mosquitoes were established for each larval treatment group (12 cages total). One cage was never bloodfed, one was given a single blood meal 4 days after adult eclosion, and one was given weekly blood meals starting 4 days after adult eclosion. Mosquitoes in all of the cages were given ad libitum access to a wick saturated with 10% dextrose. Dead females were counted and removed from the cages on a daily basis.
2.5. Egg production analysis
To investigate the reproductive success of A. aegypti during successive reproductive cycles, a large cage was established with 1000 newly eclosed females and ~250 males. Mosquitoes were provided with a blood meal 7 days post eclosion and non blood fed females were discarded. Five small cages each containing 20 blood fed females were established. Oviposition substrates were placed in the five treatment groups and the master cage. Mosquitoes were allowed to oviposit for 72 h after which all mosquitoes were returned to the master cage. Eggs from the five treatment groups were dried overnight and counted using ImageJ 1.42 software following the protocol described in Mains et al. (2008). This process was repeated on days 14, 21, and 28. The average number of eggs was calculated by dividing the total number of eggs laid by the number of ovipositing mosquitoes each week. This experiment was replicated three times.
To determine if age impacted egg production during the first reproductive cycle, newly eclosed female (250) and male (100) mosquitoes were established in four separate cages. Each week (7, 14, 21 or 28 days post eclosion), one of the four cages was provided with blood meal. Following blood feeding (48 h), 5 cages of 20 blood fed females each were established and oviposition substrates provided for 72 h. Eggs were counted using ImageJ 1.42 software following the protocol of Mains et al. (2008). The average number of eggs was calculated by dividing the total number of eggs laid by the number of ovipositing mosquitoes each week.
2.6. Statistical analyses
A Cox proportional hazards (CPH) model was used to analyze the survivorship data (Cox, 1972, Therneau et al., 2000). We were particularly interested in investigating the effect of diet on time to death while controlling for the effect of replicate. As such, in the full model, we analyzed the effect of diet on mortality while including replicate as a strata variable (Therneau et al., 2000, Allison, 1995). Additionally, to ask whether the effect of diet was consistently significant over the multiple experimental replicates, we used a CPH to ask whether diet was a significant predictor of time to death for each replicate separately. To ask whether two treatments were significantly different from each other, a full CPH model was analyzed with all other treatments omitted. Significant p-values were interpreted as significant pairwise differences. All survival analyses were performed using PROC TPHREG in SAS version 9.1 (SAS Institute, Cary, NC, USA).
All reproductive data were analyzed using an ANOVA. We initially investigated the effect of multiple reproductive cycles on egg production by fitting a model where reproductive cycle was the predictor variable, replicate was a random effect, and egg count per cage was the response. The number of mosquitoes was included as a covariate in the model because the number of mosquitoes per cage was not constant. Because measurements were taken on the same cage successively (i.e., estimates of the number of eggs per cage per reproductive cycle were not independent across time), the ideal way to analyze these data is a repeated measures analysis where measurements are correlated across reproductive cycles. However, because reproductive cycle was the only predictor variable in the model, we could not use such an analysis. A standard ANOVA was the next best, although not perfect, option. We investigated whether female age at their first blood meal affected the number of eggs laid per cage. Age at first blood meal was the predictor variable and the number of mosquitoes per cage was included as a covariate, as this number was not constant among cages. All ANOVA analyses were conducted using JMP Version 7 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Impact of blood feeding on adult lifespan in female A. aegypti
Female A. aegypti are obligate blood feeders that require a blood meal to produce a clutch of eggs. To examine what impact the consumption of these large protein meals has on A. aegypti’s lifespan we established three treatment groups. One group was allowed to feed on 10% dextrose ad libitum, one group was fed a single blood meal and dextrose ad libitum, and the third group was fed weekly blood meals supplemented with dextrose ad libitum throughout their adult life. Analysis of the full model showed that diet (X2=646.39, p<0.0001) was a significant predictor of time to death. The effect of diet was also a significant predictor of time to death in each replicate treated separately (replicate 1: X2=145.16, p<0.0001, replicate 2: X2=134.00, p<0.0001, replicate 3: X2=581.67, p<0.0001). Female A. aegypti provided with weekly blood meals had the shortest lifespan, surviving an average of eight days or ~30% less than mosquitoes given a single blood meal (X2=418.40; p<0.0001, Fig. 1A) and ten days or ~40% less than mosquitoes fed only sugar (X2=550.90; p<0.0001, Fig. 1A). There was no significant difference between mosquitoes fed a single blood meal versus those fed only sugar (Fig. 1A).
Fig. 1.
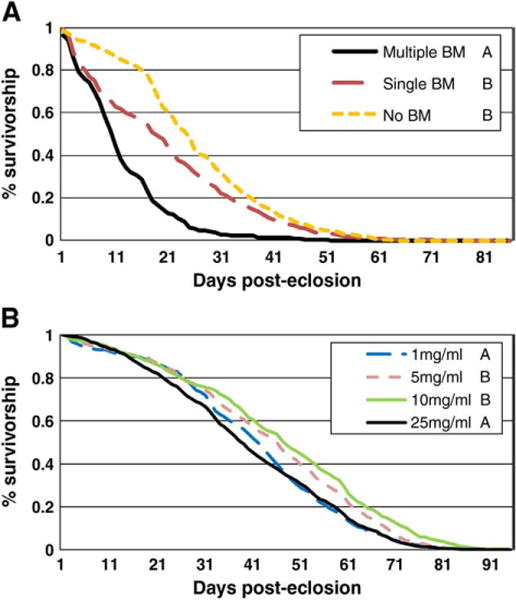
The impact of blood feeding and dietary protein on adult A. aegypti lifespan. A. A representative survivorship curve comparing mosquitoes fed weekly blood meals (BM), a single blood meal, or no blood meals. All treatments were allowed access to 10% dextrose ad libitum. Dead individuals were counted daily. Letters following the figure legend indicate significant differences, with different letters indicating significant differences. B. Combined data showing the lifespan of adult female mosquitoes given access to an artificial blood meal containing 25, 10, 5 or 1 mg/ml of BSA every three days. All treatments were allowed ad libitum access to 10% dextrose. Survivorship was assessed daily. Treatments with the same letters were not different from each other; those with different letters have significant differences.
3.2. Dietary restriction in adult female A. aegypti
We analyzed the effect of dietary restriction during the adult stage on the lifespan of adult A. aegypti. Larval mosquitoes were reared under identical conditions and the resulting adults fed various dilutions of bovine serum albumin (BSA). Human blood contains ~200–250 mg/ml of protein (Bliss, 1928) and a complete blood meal leads to the production of ~100 eggs. Similar concentrations of BSA led to similarly sized egg clutches (data not shown). To minimize egg production, but still observe the effects of dietary restriction, we fed artificial bloomeals containing 25, 10, 5, or 1 mg/ml of BSA to mosquitoes twice weekly. Dextrose (10%) was also provided ad libitum throughout the experiment. Dead females were counted daily to assess their mortality rate and five replicate experiments were performed. During their first reproductive cycle, mosquitoes fed 25 mg/ml produced less than 10% of the eggs achieved with a normal blood meal, and egg production steadily declined during each successive reproductive cycle (Fig. S1). Mosquitoes fed 5 or 1 mg/ml produced virtually no eggs and those fed 10 mg/ml produced an intermediate amount. Adult mosquitoes fed 5 or 10 mg/ml of BSA lived significantly longer than those fed 1 or 25 mg/ml of BSA (Fig. 1B). There was no significant difference between those fed 5 and 10 mg/ml or those fed 25 and 1 mg/ml. Mosquitoes given artificial blood meals containing 10 mg/ml BSA survived an average of nine days longer (~20%) than those fed 25 mg/ml. Those fed 5 mg/ml survived seven days longer. Analysis of the full model showed that diet (X2=145.35, p<0.0001) was a significant predictor of time to death. Diet was a significant predictor of time to death for each replicate analyzed separately (replicate 1: X2=44.16, p<0.0001, replicate 2: X2=65.70, p<0.0001, replicate 3: X2=65.70, p<0.0001, replicate 4: X2=30.06, <0.0001, replicate 5: X2=16.19, p=0.0010).
3.3. Dietary restriction in A. aegypti larvae leads to the extension of adult lifespan
Larval A. aegypti face considerable competition for limited resources in the small pools of water they reside in, and this could affect adult lifespan and dengue transmission. To determine the impact of dietary restriction during the larval stages on adult longevity we established four treatment groups and fed them different larval diets 2X, 1X (standard larval diet), 0.5X and 0.25X. Following emergence, adults were provided with sugar ad libitum and given access to none, one, or weekly blood meals. Each experiment was replicated three times.
Overall, dietary restriction during the larval stages significantly extended adult lifespan. Adult mosquitoes provided with sugar only achieved maximal lifespan when fed half of the normal diet as larvae (2X diet: 50% survival=38 days, 10% survival=58 days; 1X diet: 50% survival=41 days, 10% survival =59 days; 0.5X diet: 50% survival=43 days, 10% survival=61 days; 0.25X diet: 50% survival=41 days, 10% survival=63 days; Fig. 2A). Analysis of the full model showed that larval diet (X2=27.33, p<0.0001) was a significant predictor of time to death. Larval diet was a significant predictor of time to death for the first two but not the third replicate (replicate 1: X2=12.10, p=0.0070, replicate 2: X2=27.84, p<0.0001, replicate 3: X2=4.59, p=0.20). Adult mosquitoes fed a single blood meal lived longest when fed the 0.25X diet, and had the shortest lifespan when fed a nutrient rich larval diet (2X diet: 50% survival=34 days, 10% survival = 57 days; 1X diet: 50% survival =42 days, 10% survival=61 days; 0.5X diet: 50% survival=37 days, 10% survival=62 -days; 0.25X diet: 50% survival=43 days, 10% survival=67d; Fig. 2B). The full model analysis showed that larval diet (X2 = 29.00, p<0.0001) was a significant predictor of time to death. Larval diet was a significant predictor of time to death in two of the three replicates (replicate 1: X2=5.21, p=0.1572, replicate 2: X2=40.69, p<0.0001, replicate 3: X2=8.10, p=0.044). Mosquitoes provided with weekly blood meals reached maximal lifespan with the most restricted larval diet (2X diet: 50% survival=25 days, 10% survival=46 days; 1X diet: 50% survival=28 days, 10% survival=49 days; 0.5X diet: 50% survival=28 days, 10% survival=53 days; 0.25X diet: 50% survival=28 days, 10% survival=52 days; Fig. 2C). Under a full model, larval diet (X2=80.69, p<0.0001) was a significant predictor of time to death. Larval diet was a significant predictor of time to death for all three replicates (replicate 1: X2=12.13, p = 0.0070, replicate 2: X2 = 24.46, p<0.0001, replicate 3: X2=71.93, p<0.0001). A slight, but significant, decrease in body size was observed in both males and females given different diets as larvae, with those mosquitoes receiving the least food producing the smallest adults based on wing length (Fig. S2).
Fig. 2.
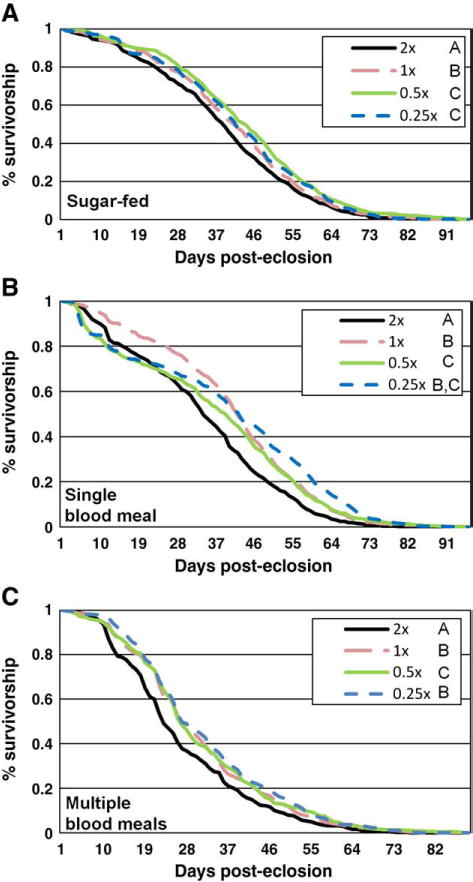
The effect of dietary restriction during the larval stages. A. Survivorship of mosquitoes fed 2X, 1X, 0.5X or 0.25X their normal diet as larvae and strictly sugar as adults. Letters following the figure legend indicate whether the differences between treatments were significant. Treatments with the same letters were not different from each other, while those with different letters had significant differences. B. Survivorship of mosquitoes fed 2X, 1X, 0.5X or 0.25X their normal diet as larvae and a single blood meal and 10% dextrose ad libitum as adults. Letters following the figure legend indicate significant differences, with different letters indicating significant differences. C. Survivorship of mosquitoes fed 2X, 1X, 0.5X or 0.25X their normal diet as larvae and multiple blood meals and 10% dextrose as adults. Letters following the figure legend indicate significant differences, with different letters indicating significant differences.
3.4. The effect of multiple reproductive cycles on egg production
We observed that the number of reproductive cycles a mosquito undergoes impacts the mosquito’s lifespan (Fig. 1A). However, we were interested in whether the number of reproductive cycles or the age of the mosquito affected the number of eggs produced during each cycle. To assess this, we provided a weekly blood meal to a large pool of mosquitoes, discarded those that failed to blood feed and determined total egg production from 20 mosquitoes during their first four reproductive cycles. During their first reproductive cycle the pool of 20 mosquitoes produced ~1100 eggs (Fig. 3A). Egg production decreased by ~30% during each of the next two cycles before stabilizing during the fourth cycle. The number of successive reproductive cycles was a significant predictor of the number of eggs laid (F3,34=23.31, p<0.0001). This indicates that A. aegypti mosquitoes, at least under laboratory conditions, devote a large portion of their resources to their initial reproductive efforts. To assess whether this observed reduction in egg production was due to the mosquitoes’ age we provided pools of mosquitoes with their first blood meal when they were either 1, 2, 3, or 4 weeks of age. Mosquitoes aged 1 to 3 weeks laid a similar number of eggs and age only impacted egg production as females reached 4 weeks of age (Fig. 3B). Age at first blood meal was a significant predictor of the number of eggs laid per cage (F3,15=6.20, p=0.0060).
Fig. 3.
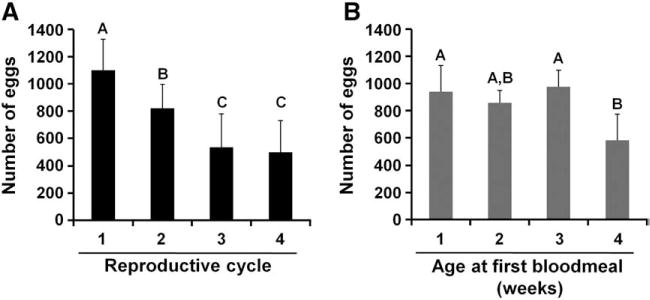
Egg production during successive reproductive cycles in A. aegypti and during the first reproductive cycles of aged mosquitoes. A. The number of eggs per cage laid by fully-fed females assayed for reproductive output on successive reproductive cycles. Different letters indicate a significant difference between treatments. B. Cohorts of adult mosquitoes aged 1, 2, 3, or 4 weeks old were given an identical blood meal and the number of eggs per cage was counted. Different letters indicate significant differences between treatments.
4. Discussion
Dietary restriction extends the lifespan of many different organisms. We have demonstrated that dietary restriction during both the larval and adult stages also impacts the adult lifespan of a haematophagic insect, the mosquito A. aegypti. Adult female A. aegypti consume large, nutrient rich meals determined by the availability of vertebrate hosts. Therefore, limited access to vertebrate hosts and their blood may actually increase lifespan and extend their ability to reproduce and transmit diseases such as dengue.
Our studies indicate that mosquitoes fed only sugar survive nearly as well as those given a single blood meal, and significantly longer than those given multiple blood meals. Previous studies examining the impact of blood feeding versus sugar feeding on various species of Aedes mosquitoes give conflicting results. While some suggest blood feeding is detrimental, others suggest it is beneficial. For example, A. aegypti mosquitoes given daily human blood meals with or without sugar both survived better than those given only sugar (Styer et al., 2007a). In the case of daily blood meals, the protein that is consumed while a reproductive cycle is already underway could be devoted towards somatic maintenance or stored as reserve nutrients, potentially extending lifespan. In urban areas where dense host populations exist it has been shown that A. aegypti mosquitoes routinely feed multiple times during a reproductive cycle. However, in rural areas where hosts are not as abundant this may not be the case. A second study by the same group on A. aegypti mosquitoes recently collected from the field showed that mosquitoes fed both sugar and blood survived significantly longer than those fed either sugar or blood alone (Styer et al., 2007b). Interestingly, mosquitoes in the blood only treatments given access to blood every day survived much better than those given blood every other day, providing some evidence that frequent blood feeding between reproductive cycles can enhance survivorship. Similarly, A. aegypti and Aedes albopictus mosquitoes given daily blood meals both survived significantly longer when they had access to sugar, although lifetime fecundity was similar (Braks et al., 2006). In contrast, Aedes communis maintained on 25% or 50% sucrose or fructose survived significantly longer than those maintained on 10% sucrose or those given a blood meal supplemented with either water or 10% sucrose (Andersson, 1992). These conflicting results suggest that the frequency of blood feeding, availability of nectar sources, and host density can have a large impact on mosquito survivorship.
All of the dietary restriction studies in mosquitoes to date compare the impact of blood feeding on the mosquito’s lifespan by limiting the mosquito’s ability to consume a blood meal. However, because each blood meal leads to the production of a complete clutch of eggs, reproduction itself might impact lifespan. Lifespan and reproduction are often negatively correlated, and so dietary restriction could extend lifespan by reducing reproduction. To uncouple reproduction and lifespan in our assays, we provided mosquitoes with artificial blood meals containing low protein concentrations that reduced egg production. Egg production was reduced by more than 85%, but we could not eliminate egg production altogether or equally among all treatment groups. We therefore cannot completely rule out that reproduction influenced lifespan. Nonetheless, reproduction was significantly reduced in all of the treatments, which strongly suggests that dietary restriction and not egg production is the major factor influencing lifespan extension in A. aegypti.
The present study sheds some light on the relationship between larval diet and adult lifespan. Previous studies in D. melanogaster did not find a relationship between larval diet and adult lifespan (Tu and Tatar, 2003). However, we find that in all cases where larval diet was restricted, individuals with a restricted diet during the larval stages showed a modest, but significant, increase in adult lifespan compared to those given twice the normal amount of food. Interestingly, mosquitoes fed 25% of their normal larval diet and given only sugar during adulthood had a reduced lifespan, likely due to depletion of protein reserves. However, if those same mosquitoes received even a single blood meal during adulthood they survived as well as or better than any of the other treatment groups. Therefore, although blood meals can decrease lifespan, they might also be beneficial when individuals experience restricted diets as larvae, as they often do in the wild. To our knowledge, this is the first study to show that dietary restriction at the larval stages can extend lifespan and is especially relevant given the ecology of these insects.
Mosquitoes are important vectors of disease parasites, including malaria, filariasis, dengue, and West Nile virus. Lifespan is the most important factor affecting how efficiently a mosquito can transmit these parasites and even modest changes can greatly impact disease spread (Macdonald, 1957). Our work suggests that dietary restriction during both the aquatic larval stages and adulthood might increase the opportunity for disease transmission. This important link between diet, lifespan, and disease transmission might be used to identify areas where A. aegypti transmitted diseases might increase. For example, if inter- and intraspecific competition for food at the larval stages is high where pools of water are especially small, dry climates might be more disease-prone than originally thought. Similarly, if mosquitoes with restricted access to protein-rich blood meals live longer, A. aegypti transmitted disease might increase in rural areas where hosts are limited.
As in other organisms, the lifespan of adult A. aegypti mosquitoes can be extended through dietary restriction. In contrast to other invertebrate models of aging, such as D. melanogaster and C. elegans, A. aegypti’s feeding and reproductive behavior are characterized by bursts of nutrient intake directly linked to the production of a clutch of eggs. By limiting the amount of protein in the adult females’ diet, we were able to reduce the impact of reproduction in the dietary restriction assays. Overall, our data suggest that we might find new opportunities for controlling mosquito-borne diseases by understanding the interplay between larval nutrition, adult blood feeding, reproduction and lifespan. This work opens intriguing avenues into controlling mosquito and other vector borne disease transmission through dietary restriction.
Supplementary Material
Acknowledgments
We would like to thank Frank Ramberg for helping with mosquito rearing. This work was supported by the Ellison Medical Foundation and the BIO5 Institute.
Appendix A. Supplemental data
Supplementary data associated with this article can be found, in the online version, at doi: 10.1016/j.exger.2010.04.009.
References
- Allison PD. Survival Analysis Using the SAS System: a Practical Guide. SAS Institute Inc; Cary, NC: 1995. [Google Scholar]
- Andersson IH. The effect of sugar meals and body size on fecundity and longevity of female Aedes communis (Diptera: Culicidae) Physiol Entomol. 1992;17:203–207. [Google Scholar]
- Bliss S. The amide nitrogen of blood. Science. 1928;67:515–516. doi: 10.1126/science.67.1742.515. [DOI] [PubMed] [Google Scholar]
- Boggs CL, Ross CL. The effect of adult food limitation on life history traits in Speyeria mormonia. Ecology. 1993;74:433–441. [Google Scholar]
- Braks MA, Juliano SA, Lounibos LP. Superior reproductive success on human blood without sugar is not limited to highly anthropophilic mosquito species. Med Vet Entomol. 2006;20:53–59. doi: 10.1111/j.1365-2915.2006.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Harshman L, Zhang Y, Muller HG, Partridge L, Wang JL. Life history response of Mediterranean fruit flies to dietary restriction. Aging Cell. 2002;1:140–148. doi: 10.1046/j.1474-9728.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- Cooper TM, Mockett RJ, Sohal BH, Sohal RS, Orr WC. Effect of caloric restriction on life span of the housefly, Musca domestica. FASEB J. 2004;18:1591–1593. doi: 10.1096/fj.03-1464fje. [DOI] [PubMed] [Google Scholar]
- Costero A, Edman JD, Clark GG, Scott TW. Life table study of Aedes aegypti(Diptera: Culicidae) in Puerto Rico fed only human blood versus blood plus sugar. J Med Entomol. 1998;35:809–813. doi: 10.1093/jmedent/35.5.809. [DOI] [PubMed] [Google Scholar]
- Edman JD. Fitness advantages in multiple blood-feeding: the Aedes aegypti example. In: Takken W, Scott TW, editors. Ecological Aspects for Application of Genetically Modified Mosquitoes. Kluwer Academic Publishers Dordrecht; Boston: 2003. p. 63. [Google Scholar]
- Grandison RC, Wong R, Bass TM, Partridge L, Piper MD. Effect of a standardised dietary restriction protocol on multiple laboratory strains of Drosophila melanogaster. PLoS One. 2009;4:e4067. doi: 10.1371/journal.pone.0004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LC, Buonaccorsi JP, Edman JD, Costero A, Kittayapong P, Clark GG, Scott TW. Analysis of survival of young and old Aedes aegypti (Diptera: Culicidac) from Puerto Rico and Thailand. J Med Entomol. 2001;38:537–547. doi: 10.1603/0022-2585-38.4.537. [DOI] [PubMed] [Google Scholar]
- Hellwing ALF, Tauson AH, Skrede A. Blood parameters in growing pigs fed increasing levels of bacterial protein meal. Acta Vet Scand. 2007;49:33. doi: 10.1186/1751-0147-49-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klowden MJ, Lea AO. Blood meal size as a factor affecting continued host-seeking by Aedes aegypti(L.) Am J Trop Med Hyg. 1978;27:827–831. doi: 10.4269/ajtmh.1978.27.827. [DOI] [PubMed] [Google Scholar]
- Le Bourg E, Minois N. Failure to confirm increased longevity in Drosophila melanogaster submitted to a food restriction procedure. J Gerontol A Biol Sci Med Sci. 1996;51:B280–B283. doi: 10.1093/gerona/51a.4.b280. [DOI] [PubMed] [Google Scholar]
- Macdonald G. The Epidemiology and Control of Malaria. Oxford University Press; London: 1957. [Google Scholar]
- Mains JW, Mercer DR, Dobson SL. Digital image analysis to estimate numbers of Aedes eggs oviposited in containers. J Am Mosq Control Assoc. 2008;24:496–501. doi: 10.2987/5740.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- Mair W, Sgro CM, Johnson AP, Chapman T, Partridge L. Lifespan extension by dietary restriction in female Drosophila melanogaster is not caused by a reduction in vitellogenesis or ovarian activity. Exp Gerontol. 2004;39:1011–1019. doi: 10.1016/j.exger.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Mair W, Piper MDW, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Ibarra JA, Rodriguez MH, Arredondo-Jimenez JI, Yuval B. Influence of plant abundance on nectar feeding by Aedes aegypti (Diptera: Culicidae) in southern Mexico. J Med Entomol. 1997;34:589–593. doi: 10.1093/jmedent/34.6.589. [DOI] [PubMed] [Google Scholar]
- Partridge L, Green A, Fowler K. Effects of egg-production and exposure to males on female survival in Drosophila melanogaster. J Insect Physiol. 1987;33:745–749. [Google Scholar]
- Partridge L, Piper MD, Mair W. Dietary restriction in Drosophila. Mech Ageing Dev. 2005;126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Piper MD, Partridge L. Dietary restriction in Drosophila: delayed aging or experimental artifact? PLoS Genet. 2007;3:e57. doi: 10.1371/journal.pgen.0030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiskind MH, Lounibos LP. Effects of intraspecific larval competition on adult longevity in the mosquitoes Aedes aegypti and Aedes albopictus. Med Vet Entomol. 2009;23:62–68. doi: 10.1111/j.1365-2915.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roark AM, Bjorndal KA. Metabolic rate depression is induced by caloric restriction and correlates with rate of development and lifespan in a parthenogenetic insect. Exp Gerontol. 2009;44:413–419. doi: 10.1016/j.exger.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Scott TW, Chow E, Strickman D, Kittayapong P, Wirtz RA, Lorenz LH, Edman JD. Blood-feeding patterns of Aedes aegypti (Diptera: Culicidae) collected in a rural Thai village. J Med Entomol. 1993;30:922–927. doi: 10.1093/jmedent/30.5.922. [DOI] [PubMed] [Google Scholar]
- Styer LM, Carey JR, Wang JL, Scott TW. Mosquitoes do senesce: departure from the paradigm of constant mortality. Am J Trop Med Hyg. 2007a;76:111–117. [PMC free article] [PubMed] [Google Scholar]
- Styer LM, Minnick SL, Sun AK, Scott TW. Mortality and reproductive dynamics of Aedes aegypti (Diptera: Culicidae) fed human blood. Vector Borne Zoonotic Dis. 2007b;7:86–98. doi: 10.1089/vbz.2007.0216. [DOI] [PubMed] [Google Scholar]
- Therneau TM, Grambsch P, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer-Verlag; New York, NY: 2000. [Google Scholar]
- Tu MP, Tatar M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell. 2003;2:327–333. doi: 10.1046/j.1474-9728.2003.00064.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


