SUMMARY
Sensory experience orchestrates the development of cortical circuitry by adaptively modifying neuro-transmission and synaptic connectivity. However, the mechanisms underlying these experience-dependent modifications remain elusive. Here we demonstrate that visual experience suppresses a presynaptic NMDA receptor (preNMDAR)-mediated form of timing-dependent long-term depression (tLTD) at visual cortex layer (L) 4-2/3 synapses. This tLTD can be maintained during development, or reinstated in adulthood, by sensory deprivation. The changes in tLTD are mirrored by changes in glutamate release; visual deprivation enhances both tLTD and glutamate release. These effects require the GluN3A NMDAR subunit, the levels of which are increased by visual deprivation. Further, by coupling the pathway-specific optogenetic induction of tLTD with cell-type-specific NMDAR deletion, we find that visual experience modifies preNMDAR-mediated plasticity specifically at L4-L2/3 synapses.
INTRODUCTION
Sensory manipulations have long been known to sculpt cortical circuits in a developmentally regulated manner (Wiesel and Hubel, 1963), suggesting that the cortical response to a sensory manipulation is shaped by both genetically influenced developmental milestones and previous sensory experience (Espinosa and Stryker, 2012). A fundamental goal of neuroscience is to understand how sensory stimuli adaptively modify neuronal circuits. Hebbian synaptic plasticity provides a mechanism by which sensory experience modifies cortical circuitry. In sensory cortices, Hebbian plasticity can be induced by spike-timing-dependent plasticity (STDP), in which changes in synaptic efficacy are determined by the relative timing of presynaptic and postsynaptic activity (Feldman, 2012). Timing-dependent forms of plasticity are sufficient to alter receptive fields and orientation selectivity in vivo (Meliza and Dan, 2006; Yao and Dan, 2001), indicating that timing-dependent plasticity can powerfully influence the cortical circuits underlying sensory processing.
In early development, timing-dependent long-term depression (tLTD) at L4-L2/3 cortical synapses is expressed presynaptically and occurs independently of postsynaptic NMDAR signaling (Bender et al., 2006; Corlew et al., 2007; Rodríguez-Moreno and Paulsen, 2008). During this early developmental period, tLTD is mediated by a unique mechanism involving astrocytic endocannabinoid signaling (Min and Nevian, 2012), magnesium-insensitive preNMDARs (Banerjee et al., 2009; Larsen et al., 2011), and metabotropic glutamate receptors (Bender et al., 2006; Nevian and Sakmann, 2006). This presynaptically expressed tLTD at L4-L2/3 synapses developmentally shifts to a postsynaptic form following early sensory milestones such as eye opening (Corlew et al., 2007), suggesting that early sensory experience may modify the synaptic proteins underlying tLTD. However, how sensory experience during development and adulthood influences STDP is just beginning to be understood.
Here we sought to understand how sensory experience modifies the induction of STDP at L2/3 synapses. Since mechanisms underlying the expression of plasticity can change through development (Larsen et al., 2010), we determined how sensory experience modulates STDP both during the developmental critical period for heightened ocular dominance plasticity and in adult mice. We found that visual deprivation prevents the developmental loss of presynaptically expressed tLTD, while late-onset visual deprivation during adulthood can reinstate this tLTD. These experience-dependent modifications at L2/3 synapses require preNMDARs in L4 neurons and the NMDAR subunit GluN3A. By optogenetically activating specific intracortical synaptic inputs onto L2/3 neurons, we show that sensory experience differentially regulates tLTD at L4-L2/3 and L2/3-L2/3 synapses. Our results demonstrate a preNMDAR-mediated mechanism by which sensory experience modifies visual cortical circuitry via changes in NMDARs uniquely expressed at presynaptic L4 neurons.
RESULTS
Visual Experience Bidirectionally Modifies the Ability to Induce tLTD
To determine how sensory experience modifies the induction of STDP, we performed whole-cell recordings from L2/3 pyramidal neurons in mouse primary visual cortex (V1) during a period (postnatal days 26–30 [P26–P30]) of heightened ocular dominance plasticity but during which receptive field properties such as orientation selectivity have largely matured (Espinosa and Stryker, 2012; Ko et al., 2013). To examine STDP at L2/3 synapses, we monitored evoked excitatory postsynaptic potentials (EPSPs) before and after multiple pairings of single L4-evoked EPSPs with single L2/3-induced action potentials (APs) (see Experimental Procedures). To influence the polarity of plasticity, we varied whether the EPSP preceded, or followed, the action potential by 10 ms. In developing sensory cortices (<P20), this protocol results in timing-dependent potentiation (tLTP) when the EPSP precedes the AP and tLTD when the EPSP follows it (Markram et al., 1997; Sjöström et al., 2001). In agreement with previous studies performed in the absence of neuromodulators or GABA(A) receptor antagonists (Corlew et al., 2007; Guo et al., 2012; Seol et al., 2007), we failed to induce either tLTP or tLTD in P26–P30 normally reared (NR) mice (Figures 1A–1E). This suggests that developmental mechanisms tightly regulate STDP in the visual cortex.
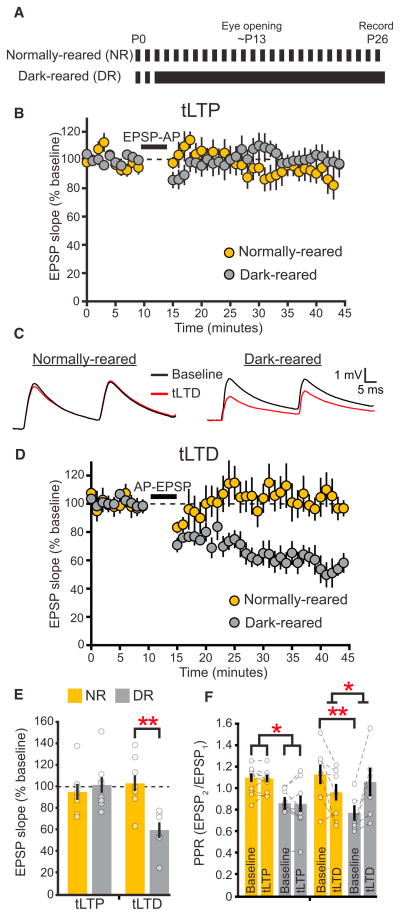
Figure 1. Dark Rearing Prevents the Developmental Loss of L4-L2/3 tLTD but Not tLTP.
(A) Diagram of rearing paradigm for normally reared (NR) and dark-reared (DR) mice.
(B) Neither NR (n = 8) nor DR (n = 9) mice express tLTP in L2/3 pyramidal neurons following EPSP-AP pairings in mice at P26–P30.
(C) Representative averaged recordings of L2/3 EPSPs evoked by a 30 Hz pair of L4 stimuli before and after tLTD induction in NR and DR mice.
(D) AP-EPSP pairings fail to produce tLTD in NR mice (n = 8) but produce significant tLTD in DR mice (n = 5).
(E) Quantification of the last 10 min in (B) and (D). tLTP magnitude does not differ between NR and DR mice (Mann-Whitney test, p = 0.56). However, DR mice exhibit increased tLTD magnitude compared to NR mice (t test, p < 0.004).
(F) EPSP-AP pairings used in tLTP induction protocols do not alter the PPR ratio at 30 Hz in NR mice (2wRmANOVA, p = 0.74), and DR mice have a comparatively lower PPR both before (baseline; post hoc test, p < 0.03) and after (post hoc test, p < 0.02) tLTP induction. The baseline PPR is lower in DR mice compared to NR mice (post hoc test, p < 0.02), and an increase in PPR accompanies tLTD induction in DR mice (2wRmANOVA, p < 0.003). Error bars represent SEM. Gold, NR; gray, DR.
To determine whether sensory experience influences the properties of STDP, we compared STDP in visual cortex slices from age-matched mice that had been dark reared (DR) and those from NR mice (Figure 1A). Similar to NR controls, our tLTP protocol failed to induce plasticity in DR mice at this age (Figure 1B). In contrast, dark rearing prevented the developmental downregulation of tLTD (Figures 1C–1E). We examined whether dark rearing may have altered the ability to induce tLTD by changing L4-L2/3 excitatory drive. This possibility is unlikely because we found that L4-evoked AMPAR-mediated currents in L2/3 pyramidal neurons were similar between NR and DR mice (Figure S1A available online). To determine whether tLTD in DR mice may be expressed presynaptically, we monitored the paired-pulse ratio (PPR) before and after inducing plasticity. PPR typically correlates with initial release probability (Koester and Johnston, 2005) and changes with presynaptically expressed forms of synaptic plasticity at cortical synapses (Rodríguez-Moreno et al., 2013; Sjöström et al., 2003). Dark rearing reduced the baseline PPR of responses evoked at 30 Hz (Figures 1C and 1F), suggesting that dark rearing increased the glutamate release probability. The PPR increased after tLTD induction, consistent with decreased glutamate release. These results suggest that visual experience attenuates high-frequency glutamate release and tLTD at L2/3 synapses in the visual cortex.
Two forms of tLTD are expressed at L2/3 synapses within developing sensory cortices: one that depends on calcium influx through postsynaptic NMDARs (Froemke et al., 2005), and another that depends on preNMDAR-mediated signaling (Banerjee et al., 2009; Bender et al., 2006; Corlew et al., 2007; Rodríguez-Moreno et al., 2011). To determine whether the tLTD induced after visual deprivation requires ionotropic signaling by postsynaptic NMDARs, we repeated tLTD experiments while including the NMDAR open channel blocker MK-801 (1 mM) in the recording pipette (iMK-801) (Corlew et al., 2007). With ion flux through postsynaptic NMDARs blocked, L2/3 synapses activated by L4 stimulation in DR mice still showed substantial tLTD that was accompanied by increases in the PPR (Figure 2). We next determined whether tLTD in DR mice required any NMDAR signaling by testing tLTD induction in the presence of bath applied MK-801 (100 μM). MK-801 prevented tLTD induction and changes in PPR in L2/3 pyramidal neurons of DR mice (Figures 2D and 2F). We also observed similar results when the competitive NMDAR antagonist D-AP5 (50 μM) was included in the bath solution (Figures 2D and 2F). These data suggest that tLTD in DR mice requires glutamate binding and ionotropic signaling through nonpostsynaptic, and putatively presynaptic, NMDARs, similar to what is observed during early visual cortical development (Corlew et al., 2007).
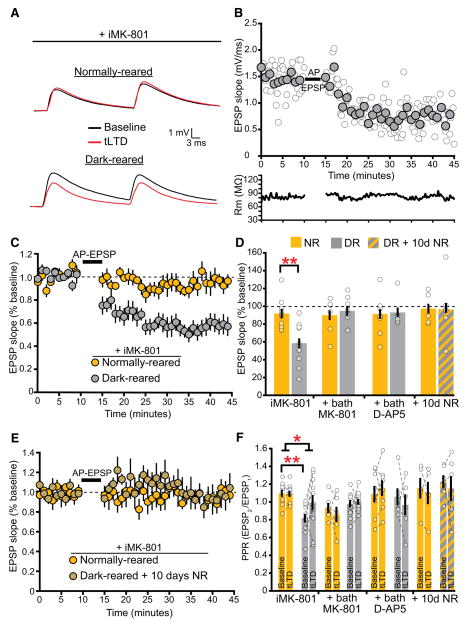
Figure 2. L4-L2/3 tLTD in Dark-Reared Mice Requires PreNMDARs and Is Lost after Visual Experience.
(A) Representative recordings of L2/3 EPSPs before and after tLTD induction in NR and DR mice when the NMDAR antagonist MK-801 is included in the recording pipette (iMK-801).
(B) Sample recording demonstrating tLTD in a DR mouse after AP-EPSP pairings. Filled circles represent 1 min averaged bins of individual EPSP slopes (unfilled circles). Membrane resistance (Rm) did not change significantly across the duration of the experiment (bottom).
(C) L2/3 pyramidal neurons in DR mice (n = 11) exhibit substantial tLTD compared to NR mice (n = 10) when postsynaptic NMDARs are blocked by iMK-801 (t test, p < 0.002).
(D) Quantification of the last 10 min in (C) and (E) as well as from experiments in which D-AP5 or MK-801 was included in the bath. Inclusion of the NMDAR antagonist MK-801 (100 μM) in the bath blocks tLTD in DR mice (DR, n = 9; NR, n = 6, p = 0.60). Inclusion of 50 μM D-AP5 in the bath blocks tLTD in DR mice (DR, n = 6; NR, n = 8; t test, p = 0.87).
(E) DR mice exposed to normal visual experience for 10 days (n = 6) lack tLTD, similar to age-matched NR controls (n = 5; t test, p = 0.98).
(F) PPR at 30 Hz is lower in recordings from DR mice compared to NR controls (post hoc test, p < 0.04). After tLTD, the PPR increases in DR mice (2wRmANOVA, p < 0.03). Inclusion of D-AP5 or MK-801 in the bath blocks tLTD induction and changes in the PPR (D-AP5: 2wRmANOVA, p = 0.32; MK-801: 2wRmANOVA, p = 0.62). Exposure to a normal visual environment after dark rearing increases baseline PPR, which is not altered following tLTD (2wRmANOVA, p = 0.88). In all experiments, 1 mM MK-801 was included in the internal solution. Error bars represent SEM. Gold, NR; gray, DR; striped, DR + 10 days NR.
At L4-L2/3 synapses, tLTD in early development (<P20) requires preNMDARs but in later development requires postsynaptic NMDARs and is gated by GABA(A)-receptor signaling or neuromodulators (Corlew et al., 2007; Guo et al., 2012; Seol et al., 2007). To determine whether GABA(A)-mediated signaling acutely influences tLTD in DR mice, we focally blocked GABA(A)-mediated synaptic transmission by applying 50 μM gabazine (SR95531) near the postsynaptic recording pipette (Figures S1B–S1F). With postsynaptic NMDARs blocked by iMK801, GABA(A) receptor antagonism did not affect the expression of tLTD in DR mice, nor did it enable tLTD in NR mice (Figures S1E–S1I). In contrast to the postsynaptic NMDAR-dependent tLTD observed in NR mice at this age (Corlew et al., 2007), our finding that tLTD in DR mice is readily induced in the presence of GABA(A) antagonists suggests tLTD after visual deprivation is not acutely modulated by fast inhibitory transmission. We next tested whether brief visual experience in DR mice was sufficient to inhibit tLTD induction. We found that 10 days of visual experience suppressed tLTD induction to a level similar to that in age-matched NR controls (Figures 2D–2F). Our findings indicate that visual experience suppresses the ability to induce L4-L2/3 tLTD and that visual deprivation promotes L4-L2/3 tLTD through a mechanism independent of acute GABA(A) activation or postsynaptic ionotropic NMDAR signaling.
Visual Deprivation Increases the Contribution of PreNMDARs to Glutamate Release and tLTD
We sought to determine the mechanism by which sensory experience modifies the properties of tLTD within the visual cortex. Since preNMDARs enhance evoked and spontaneous glutamate release at L2/3 synapses during a restricted developmental window (<P20) (Brasier and Feldman, 2008; Corlew et al., 2007), we tested whether visual deprivation might maintain a role for preNMDARs in mediating glutamate release at L4-L2/3 synapses. To assay neurotransmitter release, we analyzed short-term plasticity at L2/3 synapses by repeatedly evoking glutamate release at various frequencies (5–30 Hz) before and after D-AP5 application (50 μM) (Figure 3). We included MK-801 and the calcium chelator BAPTA in the postsynaptic recording pipette while hyperpolarizing the neuron to minimize the contribution of postsynaptic NMDARs. L4-L2/3 synapses in DR mice exhibited both a higher rate of synaptic depression to 30 Hz trains of six EPSPs and a lower PPR compared to their NR controls (Figures 3A–3C), consistent with visual deprivation increasing high-frequency glutamate release at the L4-L2/3 synapse. Bath application of D-AP5 failed to alter the PPR in NR mice but increased the PPR at 30 Hz in DR mice via a reduction in the first EPSP (Figures 3A–3C). Ten days of normal visual experience reversed the effects of visual deprivation on the PPR (Figure 3C), suggesting that visual experience suppresses glutamate release at L4-L2/3 synapses.
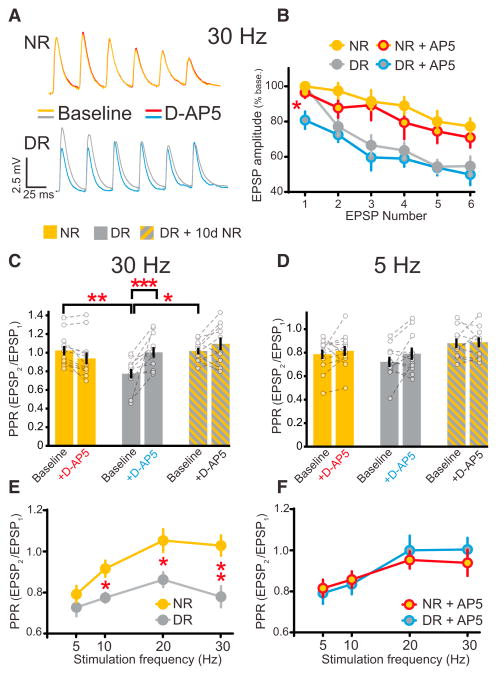
Figure 3. Dark Rearing Enhances PreNMDAR-Mediated Glutamate Release at High Frequencies.
(A) Representative recordings of six EPSPs in NR and DR mice evoked by 30 Hz L4 stimulation before and after D-AP5 application.
(B) Normalized EPSP responses before and after D-AP5 in NR and DR mice. In DR mice, D-AP5 reduces the first EPSP in a train of six EPSPs at 30 Hz (post hoc t test, NR-AP5EPSP1 and DR-AP5EPSP1, p < 0.05).
(C) Dark rearing maintains a low baseline 30 Hz PPR ratio (post hoc test, p < 0.006), and the PPR increases after 10 days of light exposure (post hoc test, p < 0.02). In DR mice, D-AP5 increases the 30 Hz PPR (2wRmANOVA, rearing and AP5 interaction, p < 0.0005).
(D) In contrast, dark rearing and D-AP5 do not alter the initial PPR in response to 5 Hz L4 stimulation (2wRmANOVA, p = 0.32).
(E) Dark rearing alters the baseline PPR ratio at 10 Hz (post hoc tests, p < 0.03), 20 Hz (p < 0.03), and 30 Hz (p < 0.006), but not at 5 Hz (p = 0.2).
(F) Dark rearing occludes the effects of D-AP5 on the PPR at frequencies ≥10 Hz (2wRmANOVA, 5 Hz p = 0.26, 10 Hz p < 0.04, 20 Hz p < 0.0002, 30 Hz p < 0.006). 1 mM MK-801 was included in the internal solution for all experiments. Error bars represent SEM. Gold, NR; gray, DR; red, NR + AP5; blue, DR + AP5.
Experience-dependent changes in PPR and its sensitivity to D-AP5 were dependent on stimulation frequency. The initial PPR at 5 Hz was similar in DR and NR mice, but PPR in DR mice was reduced at higher stimulation frequencies (Figures 3C–3E). Consistent with this frequency dependence, D-AP5 application increased the PPR in DR mice only at frequencies above 5 Hz. PPR at all frequencies tested were the same in recordings from NR and DR mice made in the presence of D-AP5 (Figure 3F). Collectively, these data indicate that visual experience alters presynaptic glutamate release at L2/3 synapses in a frequency- and NMDAR-dependent manner.
Given the enhanced L4-evoked glutamate release onto L2/3 visual cortical synapses observed in DR mice, we asked whether visual deprivation might alter spontaneous glutamate release onto L2/3 pyramidal neurons. To assay this, we examined the effect of D-AP5 on miniature excitatory postsynaptic currents (mEPSCs) in L2/3 pyramidal neurons in DR and NR mice while postsynaptic NMDARs were blocked by both hyperpolarization (−80 mV) and iMK-801, as previously described (Corlew et al., 2007). In agreement with previous results demonstrating that visual deprivation causes postsynaptic scaling of AMPAR currents (Desai et al., 2002; Goel et al., 2006), mEPSC amplitudes were significantly larger in DR mice, but they were not affected by D-AP5 application (Figures S2A–S2C, S2E, and S2F). In addition, D-AP5 did not alter mEPSC frequency in L2/3 pyramidal neurons from NR mice but significantly reduced the frequency in recordings from DR mice (Figures S2D, S2G, and S2H). This demonstrates that dark rearing increases the contribution of preNMDARs to spontaneous release. Since we did not observe a change in the baseline mEPSC frequency after dark rearing (Figure S2E), our data suggest either that preNMDARs may contribute to spontaneous release only at a subset of synapses onto L2/3 pyramidal neurons following dark rearing or that pre-NMDARs influence spontaneous release in a manner distinct from evoked release. Alternatively, preNMDARs may enhance basal spontaneous release after dark rearing, but the enhancement may be masked in the absence of D-AP5 by the reductions in synapse number that occur after dark rearing (Valverde, 1971).
Our data suggest that dark rearing from birth until ~P30 maintains the contribution of preNMDARs to glutamate release and tLTD at visual cortical L4-L2/3 synapses. Because some forms of experience-dependent plasticity are restricted to early developmental time points, such as during the critical period for rapid ocular dominance plasticity (Espinosa and Stryker, 2012), we next asked whether late-onset visual deprivation (LOVD) could also alter the contribution of preNMDARs to glutamate release. To address this, we measured short-term plasticity at L2/3 synapses in mice that had been normally reared up until adulthood (P60), an age when preNMDAR-mediated effects on neurotransmitter release are normally lost (Corlew et al., 2007), and then visually deprived the mice for 10 days (Figure 4A). Similar to our previous findings (Yashiro et al., 2005), EPSP trains evoked in LOVD mice at 30 Hz, but not 5 Hz, had a lower initial PPR than those of their NR littermates (Figures 4B and 4C). Moreover, D-AP5 increased the initially lower 30 Hz PPR observed in deprived mice without affecting the PPR in NR littermates. These results suggest that LOVD can reverse the developmental loss of preNMDARs that regulate glutamate release at L2/3 synapses.
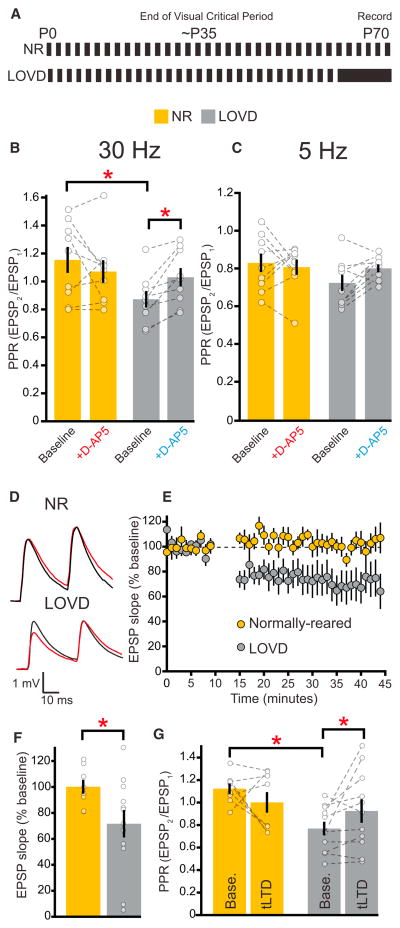
Figure 4. Late-Onset Visual Deprivation during Adulthood Restores the Contribution of PreNMDARs to tLTD and Glutamate Release.
(A) Rearing paradigm for NR and late-onset visual deprivation (LOVD).
(B and C) For recordings testing the effect of D-AP5 on short-term plasticity, mice that underwent LOVD (n = 9) had a lower baseline PPR at 30 Hz (B; p < 0.03), but not 5 Hz (C; p = 0.13), compared to NR controls (n = 9). D-AP5 increases the PPR only at 30 Hz in mice that underwent LOVD (2wRMANOVA, 30 Hz p < 0.02, 5 Hz p = 0.11).
(D) Averaged sample recordings of two EPSPs evoked by 30 Hz L4 stimuli before and after induction of tLTD in NR and LOVD mice.
(E) Recordings from mice that underwent LOVD (n = 11) demonstrate substantial tLTD, whereas their NR littermates (n = 8) have no mean reduction in EPSP slope after AP-EPSP pairings.
(F) Quantification of the last 10 min in (E) demonstrating significant differences in the magnitude of tLTD between NR and LOVD mice (Welch’s t test, p < 0.03).
(G) PPR at 30 Hz is lower in recordings from mice that underwent LOVD compared to NR controls (post hoc test, p < 0.02). The PPR increases after tLTD only in LOVD mice (2wRmANOVA, p < 0.05). Error bars represent SEM. Gold, NR; gray, LOVD.
Given these findings, we asked whether LOVD could reinstate the ability to induce tLTD. Indeed, we were able to induce L4-L2/3 tLTD in adult mice which had undergone LOVD, whereas we observed no significant tLTD in aged-matched NR littermates (Figures 4D–4F). Similar to what we observed in DR mice at P26–P30, tLTD was accompanied by increases in the PPR in LOVD mice (Figures 4D and 4G). This suggests that tLTD was expressed at least in part by a decrease in presynaptic glutamate release. Taken together, our data indicate that sensory deprivation in adulthood (>P60) can restore preNMDAR contributions to glutamate release and tLTD.
Visual Deprivation Increases the Expression of GluN3A, an NMDAR Subunit Required for tLTD at L4-L2/3 Visual Cortical Synapses
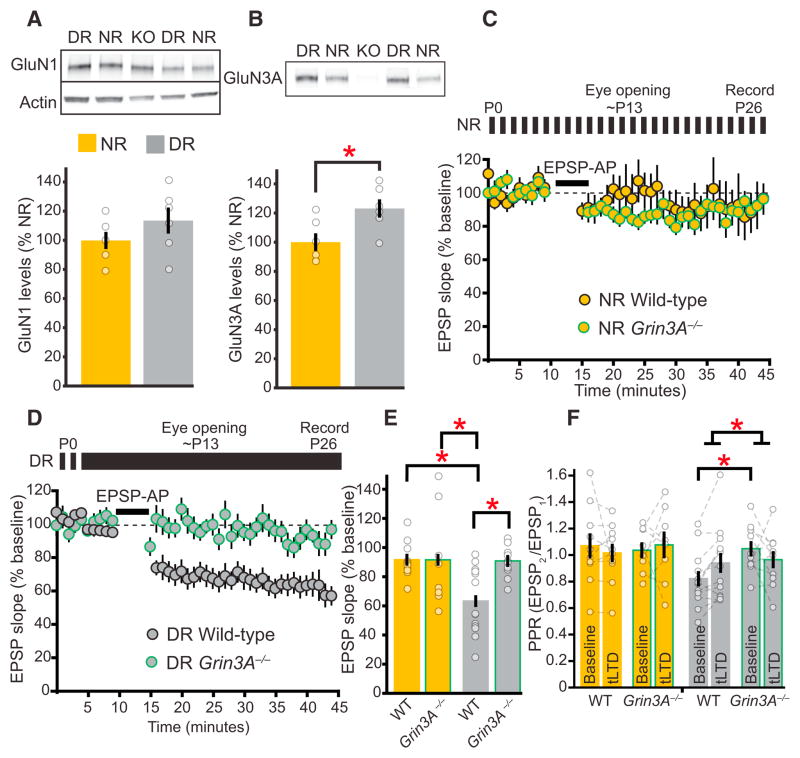
Visual deprivation changes the composition and function of postsynaptic NMDARs in primary visual cortex (Carmignoto and Vicini, 1992; Philpot et al., 2001; Quinlan et al., 1999), and we hypothesized that preNMDAR composition might similarly be changed. We first quantified changes in the expression of candidate NMDAR subunits from the visual cortex of DR and NR mice. Consistent with previous findings (Quinlan et al., 1999; Yashiro et al., 2005), dark rearing did not alter the synaptic expression of the obligatory NMDAR subunit GluN1 (NR1) but decreased the ratio of GluN2A to GluN2B subunits (Figure 5A; Figure S3A). Dark rearing also increased the expression of the GluN3A NMDAR subunit compared to age-matched NR controls (Figure 5B). This suggests a previously unknown regulation of synaptic GluN3A expression by visual experience.
Figure 5. Dark Rearing Upregulates the GluN3A Subunit, which Is Required for tLTD Induction in DR Mice.
(A and B) Representative immunoblots and quantification of GluN1 (A) and GluN3A (B) in synaptosomal fractions from the visual cortex (n = 6 replicates, 2 mice per replicate). Dark rearing does not alter GluN1 expression (t test, p = 0.23) but increases the synaptic expression of GluN3A at P30 (t test, p < 0.03).
(C) P26–P30 NR wild-type (n = 10) and Grin3A−/−(n = 8) mice both lack tLTD.
(D) Wild-type mice show significant tLTD after dark rearing (n = 13), unlike their Grin3A−/− littermates (n = 10).
(E) Quantification of (C) and (D) demonstrating that the tLTD induced in DR wild-type mice is significantly greater than tLTD in NR wild-type mice and is also greater than tLTD induced in DR and NR mice lacking GluN3A (post hoc tests, DR wild-type versus NR wild-type p < 0.02 or versus NR Grin3A−/− p < 0.04, or versus DR Grin3A−/−p < 0.03).
(F) At this age, loss of GluN3A does not alter the PPR in NR mice (post hoc test, p = 0.45). Dark rearing decreases the baseline 30 Hz PPR in wild-type mice compared to DR Grin3A−/− mice (post hoc test, p < 0.03), and the PPR increases following tLTD in recordings from DR wild-type mice (2wRmANOVA, p < 0.02). Error bars represent SEM. Gold, NR; gray, DR; with green border, Grin3A−/−.
We previously demonstrated that excitatory presynaptic terminals express GluN3A, which is required for preNMDAR function at visual cortical L2/3 synapses during early postnatal life (Larsen et al., 2011). We hypothesized that the upregulation of GluN3A with dark rearing might therefore enhance glutamate release and tLTD induction and that these effects would be absent after genetic deletion of GluN3A. To test this idea, we examined the effects of dark rearing on tLTD and short-term plasticity in L2/3 pyramidal neurons in Grin3A−/− mice and their wild-type littermates (Das et al., 1998). AP-EPSP pairings failed to induce tLTD in both wild-type and Grin3A−/− NR littermates, (Figures 5C and 5E). In contrast, dark rearing preserved tLTD induction in wild-type, but not Grin3A−/−, mice (Figures 5D and 5E). Additionally, DR wild-type mice, but not Grin3A−/− litter-mates, had an initially reduced PPR in response to 30 Hz stimulation, and the PPR increased after tLTD induction (Figure 5F). Finally, D-AP5 increased the 30 Hz PPR in DR wild-type mice, but not DR Grin3A−/− littermates (Figure S3B). These results demonstrate that visual deprivation-induced enhancement of tLTD and glutamate release requires GluN3A.
tLTD at L2/3-L2/3 Synapses Is Not Developmentally Downregulated and Requires Postsynaptic NMDARs
Our results suggest an experience-dependent downregulation of presynaptically expressed tLTD at L4-L2/3 synapses. However, STDP at V1 L2/3 neurons can be induced in vivo in both early development (Meliza and Dan, 2006; Schuett et al., 2001) and in adult cats (Yao and Dan, 2001). This raises the possibility that STDP in adulthood may be shaped by neuromodulatory or inhibitory tone to enable postsynaptic tLTD, mechanisms that have been demonstrated experimentally (Corlew et al., 2007; Seol et al., 2007) or that specific synaptic loci maintain some forms of STDP into adulthood. To determine whether the properties of STDP varied depending on the origin of synaptic inputs in adult mice, we used an optogenetic approach to activate specific intracortical inputs onto L2/3 neurons while selectively manipulating presynaptic gene expression in the same stimulated inputs. Visual cortical L2/3 pyramidal neurons receive excitatory intracortical input predominantly from other L2/3 neurons and L4 neurons (Yoshimura and Callaway, 2005; Yoshimura et al., 2005). Since the ability to express postsynaptic tLTD at horizontal inputs activated by L2/3 extracellular stimulation does not change during development (Froemke and Dan, 2002), we hypothesized that visual experience may downregulate presynaptically expressed tLTD at L4-L2/3 synapses while maintaining postsynaptic tLTD at L2/3-L2/3 synapses.
To determine whether the developmental downregulation in the contribution of preNMDARs to tLTD was specific to L4 inputs onto L2/3 pyramidal neurons, we first addressed whether tLTD at L2/3-L2/3 connections was developmentally downregulated in adult mice. We used an optogenetic approach that allowed us to specifically stimulate a genetically defined population of L2/3 inputs. To accomplish this, we utilized the Wfs1-Tg2:CreErt2 mouse line in which Cre is expressed in a subset of L2/3 cortical neurons after tamoxifen administration (Figure S4A) (Madisen et al., 2010). To generate mice that express channelrhodopsin (ChR2) in a subset of L2/3 neurons, we crossed mice expressing Wfs1-Tg2:CreErt2 with mice expressing stop-floxed-ChR2(H134R)-YFP (Ai32) (Madisen et al., 2012). In these tamoxifen-treated mice (L2/3ChR2 mice; see Experimental Procedures), ChR2 expression was largely confined to L2/3 within V1 at P80 (Figure 6A), consistent with Cre-mediated recombination observed in initial descriptions of this mouse line (Madisen et al., 2010). To selectively activate L2/3 inputs onto neighboring postsynaptic L2/3 neurons, we focally stimulated L2/3 channelrhodopsin-expressing neurons with blue light using a digital micromirror device coupled to an arc illumination source (Figure 6B). Accordingly, ChR2-expressing L2/3 neurons reliably fired APs in response to brief light pulses at 20 Hz and produced corresponding EPSPs in neighboring postsynaptic L2/3 neurons lacking ChR2. To verify that these ChR2-expressing neurons formed monosynaptic glutamatergic synapses onto other L2/3 neurons, we activated ChR2 inputs locally over postsynaptic L2/3 pyramidal neurons in the presence of TTX, 4-AP, and gabazine (Petreanu et al., 2009). ChR2 stimulation produced EPSCs in neurons under these conditions (Figure S4B), demonstrating ChR2-expressing neurons formed monosynaptic glutamatergic synapses onto L2/3 neurons.
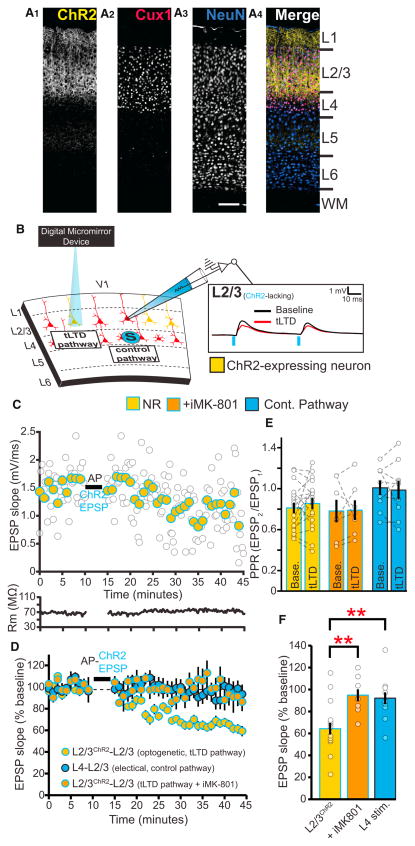
Figure 6. Unlike tLTD at L4-L2/3 Synapses, tLTD at L2/3-L2/3 Synapses Is Not Developmentally Downregulated in Adulthood and Requires Postsynaptic NMDARs.
(A) ChR2-YFP expression is confined to L2/3 neurons in ChR2L2/3 mice. Sections are taken from a P80 mouse and stained with the neuronal marker NeuN and the transcription factor Cux1, which labels the nuclei of L2-4 cortical neurons. Scale bar, 100 μm.
(B) Example recording from a postsynaptic L2/3 neuron lacking ChR2 expression after activation of neighboring ChR2-expressing L2/3 neurons before and after tLTD induction with focal light pulses.
(C) Sample recording demonstrating tLTD in an NR ChR2L2/3 mouse after AP-ChR2 EPSP pairings. Filled circles represent 1 min averaged bins of individual EPSP slopes (unfilled circles).
(D) tLTD at L2/3-L2/3 synapses can be induced in adulthood, is homosynaptic, and requires postsynaptic NMDARs (ChR2 L2/3 n = 18; L4 control pathway n = 9; ChR2 L2/3 with iMK-801 n = 7).
(E) tLTD at L2/3-L2/3 synapses is not correlated with changes in PPR evoked at 20 Hz (paired t test, p = 0.86).
(F) Quantification of the last 10 min in (D), demonstrating AP-EPSP pairings induce postsynaptic NMDAR-dependent tLTD at L2/3 synapses without inducing tLTD at synapses activated by extracellular stimulation L4 (1wANOVA, post hoc test, p < 0.01. With iMK-801, post hoc test, p < 0.01). Error bars represent SEM. Gold, ChR2 L2/3; orange, ChR2 L2/3 + iMK-801; blue = control (L4) pathway.
To determine whether sensory experience downregulated tLTD at L2/3-L2/3 synapses, we next attempted to induce tLTD with light by pairing ChR2-evoked EPSPs with APs in post-synaptic neurons lacking ChR2 from NR L2/3ChR2 adult mice (P85–P95). In contrast to tLTD induced by L4 stimulation (Figure 1), we readily induced tLTD optogenetically at L2/3 horizontal connections in adult NR mice when postsynaptic NMDARs were not blocked (Figures 6C–6F). To determine whether tLTD at L2/3-L2/3 synapses was homosynaptic, in a subset of experiments we also monitored electrically activated L4 inputs that were not paired with APs to induce tLTD. While AP-EPSP pairings resulted in tLTD at L2/3-L2/3 synapses, we did not observe simultaneous depression at control L4 inputs (Figures 6D and 6F). After tLTD at L2/3-L2/3 synapses, the PPR was unchanged (Figure 6E), suggesting that tLTD at these inputs relies on mechanisms distinct from those at L4-L2/3 synapses after visual deprivation. Unlike tLTD at L4-L2/3 synapses, we found that L2/3-L2/3 tLTD required ion flux through postsynaptic NMDARs because it was completely blocked by iMK-801 (Figures 6D and 6F). To further assess possible preNMDAR functions at L2/3-L2/3 synapses in adult mice, we monitored the PPR evoked by ChR2 stimulation at 20 Hz after bath applying D-AP5. D-AP5 application did not alter the 20 Hz PPR, suggesting that preNMDARs do not contribute to L2/3-L2/3 neurotransmission at this frequency in NR adults (Figures S4C and S4C). Collectively, these results demonstrate that tLTD at L2/3-L2/3 synapses occurs homosynaptically, requires ionotropic signaling through postsynaptic NMDARs, and is not developmentally downregulated. These results suggest a synapse-specific segregation in the experience-dependent mechanisms underlying tLTD at L2/3 visual cortical neurons.
Visual Experience Downregulates tLTD Selectively at Excitatory L4-L2/3 Synapses
The lack of a developmental downregulation in the ability to induce tLTD at horizontal L2/3 connections suggests that visual experience may act to restore presynaptic tLTD preferentially at L4-L2/3 synapses. To establish the contribution of L4 NMDARs to experience-dependent modifications in presynaptic release and tLTD, we next deleted NMDARs in L4 neurons by crossing Scnn1a-Tg3:Cre mice (Madisen et al., 2010) to mice expressing a floxed version of the obligatory NMDAR subunit gene, Grin1 (Grin1Fl/Fl mice) (Tsien et al., 1996). We first verified the laminar specificity of the Cre-driver line. As previously described (Madisen et al., 2010), transgenic Scnn1a-Tg3:Cre mice crossed with a stop-floxed-tdTomato reporter line had fluorescence confined to L4 within the visual cortex, demonstrating the intracortical L4-specificity of this line (Figure 7A). We next analyzed tdTomato fluorescence at different stages of development to determine the developmental onset of Cre-mediated recombination within visual cortex. Cre-mediated fluorescence was first observed at P20 in the visual cortex (Figures S5A–S5D). To determine the proportion of L4 visual cortical neurons that express Scnn1a-Tg3:Cre, we quantified the number of tdTomato-expressing GABAergic and glutamatergic (lacking GABA expression) L4 neurons. We observed that approximately 28% of non-GABAergic L4 neurons expressed tdTomato at P20, and we did not observe tdTomato expression in GABAergic L4 neurons (Figures 7B and 7C). This demonstrates that Scnn1a-tg3-Cre expression is confined to a subset of excitatory neurons in L4 of V1.
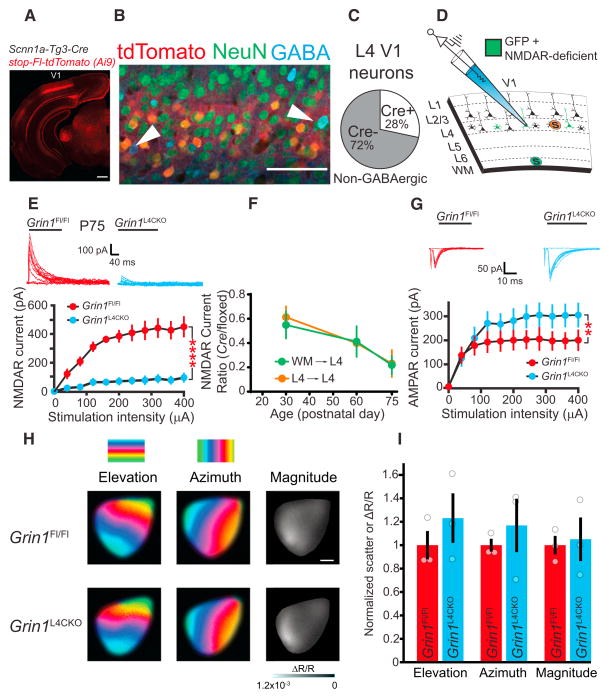
Figure 7. Targeted Genetic Deletion of L4 NMDARs in Grin1L4CKO Mice Reduces L4 NMDAR Currents, Increases AMPAR Neuro-transmission, but Does Not Significantly Disrupt In Vivo V1 Retinotopy or Visual Response Magnitude.
(A) At P30, Scnn1a-tg3:cre-mediated recombination of stop-floxed Td-tomato labels L4 neurons within the visual cortex. Scale bar, 500 μm.
(B) Image from a P20 Scnn1a-Tg3:Cre:Ai9 mouse that has also been stained with anti-GABA to mark cortical interneurons. Note the lack of colocalization of tdTomato-positive neurons with GABAergic somata (arrows). Scale bar, 50 μm.
(C) Scnn1a-tg3:cre labels ~30% of excitatory (non-GABAergic) neurons in visual cortical layer 4.
(D) Schematic of L4 and white-matter (WM) stimulation paradigm used to evoke NMDAR or AMPAR currents in Zsgreen-expressing (Cre-positive) L4 neurons.
(E) NMDAR currents are sharply reduced at inputs activated by WM stimulation in Grin1L4CKO mice (n = 4) as compared to their Grin1Fl/Fl littermates at P75 (n = 7; 2wRmANOVA p < 0.0001).
(F) Developmental profile of the Cre-mediated loss of NMDARs demonstrating gradual reductions in NMDAR currents through postnatal development (quantified from Figure S4).
(G) Macroscopic AMPAR currents evoked by WM stimulation are larger in L4 neurons lacking NMDARs compared to recordings from Fl-only slices at P75-85 (Grin1L4CKO, n = 16; Grin1Fl/Fl, n = 10; 2wRmANOVA p < 0.01).
(H) Representative intrinsic signal optical images of V1 responses measured in vivo from Grin1L4CKO and Grin1Fl/Fl littermates. Retinotopic maps were obtained in response to a white bar drifting in the elevation or azimuth direction (n = 3 mice/group). The color scales indicate the position of the stimulus bar on the monitor that activated the corresponding cortical region. The response magnitude maps within V1 were measured using a drifting vertical grating patch.
(I) Phase scatter measurements of the retinotopic maps are similar in Grin1L4CKO and Grin1Fl/FL mice (t tests, elevation, p = 0.51; azimuth, p = 0.40). Additionally, the V1 response to a grating patch is similar between Grin1L4CKO and Grin1Fl/FL mice (t test, p = 0.81). Values were normalized to mean Grin1Fl/FL measurements. Error bars represent SEM. Blue, Grin1Fl/Fl; red, Grin1L4CKO.
To disrupt expression of L4 NMDARs, we crossed Scnn1a-Tg3:Cre mice to Grin1Fl/Fl mice, hereafter referred to as Grin1L4CKO mice. To identify L4 neurons that expressed Cre, we generated triple-transgenic mice that also expressed stop-floxed-Zsgreen. We then recorded pharmacologically isolated NMDAR currents from green fluorescently labeled L4 V1 neurons in Grin1L4CKO mice (Figure 7D) or from their Grin1Fl/Fl littermates. We used increasing stimulations in vertical (white matter) or horizontal (L4) pathways to test for loss of L4 NMDARs. There was a slow developmental loss of NMDAR currents in fluorescent Cre-positive neurons, with peak NMDAR currents being reduced by ~40% at P30 and by ~60% at P60 as compared to currents in Grin1Fl/Fl littermates (Figure 7F; Figures S5E–S5L). Mean NMDAR currents were reduced by approximately 80% at P75, and ~30% of the neurons lacked detectable NMDAR currents even at maximal stimulation intensities (Figure 7E; Figures S5M–S5O). The slow time course for loss of GluN1 we observed is likely influenced by the long distance between loxP sites (Tsien et al., 1996), which contributes to inefficient Cre-mediated recombination (Zheng et al., 2000). We therefore focused on determining the effects of L4 NMDAR deletion on visual deprivation in adult mice (>P85).
We first sought to address whether the loss of L4 NMDARs altered non-NMDAR-mediated excitatory synaptic transmission onto L4 neurons or gross visual cortical function. To determine whether loss of NMDARs altered postsynaptic AMPAR currents onto L4 neurons, we recorded AMPAR currents measured in response to increasing WM stimulation in Zsgreen-positive neurons in P85–P95 Grin1L4CKO mice and their Grin1Fl/Fl-only litter-mates. AMPAR currents recorded from GluN1-deleted L4 neurons were slightly strengthened compared to neurons with intact NMDAR expression (Figure 7G), consistent with previous studies examining the effects of NMDAR deletion on synaptic transmission (Adesnik et al., 2008).
Having determined that L4 neurons lacking NMDARs receive excitatory synaptic transmission, we next asked whether postnatal deletion of NMDARs from a subset of L4 excitatory neurons would affect visual cortical retinotopy or the gross response of V1 to visual stimuli. To accomplish this, we performed in vivo intrinsic signal optical imaging of the visual cortex of Grin1L4CKO mice and their littermate Grin1Fl/Fl controls. Retino-topic maps of azimuth and elevation showed topographically structured primary visual cortices (Figure 7H). Neither the retino-topic organization (measured as phase scatter) nor the response magnitude evoked by grating patches was substantially different between Grin1L4CKO and Grin1Fl/Fl littermates (Figure 7I). This indicates the postnatal deletion of NMDARs from ~28% of excitatory L4 V1 neurons does not grossly impair retinotopic organization and visual cortical function.
We next evaluated how loss of L4 (presynaptic) NMDARs influenced synaptic transmission and experience-dependent plasticity at L4-L2/3 synapses. Compared to the use of NMDAR antagonists that lack cell-type specificity and have distinct pharmacological properties (Nabavi et al., 2013), the conditional deletion of NMDARs from a subset of L4 neurons allowed us to delete preNMDARs from L4-L2/3 synapses at an age when preNMDARs no longer contribute significantly to tLTD or glutamate release (Corlew et al., 2007). We first assessed extracellularly evoked short-term plasticity at L4-L2/3 synapses from NR Grin1L4CKO mice and their Grin1Fl/Fl littermates at P85–P95. Consistent with previous evidence for a negligible contribution of preNMDARs to synaptic transmission in NR adult mice, deletion of L4 NMDARs in Grin1L4CKO mice did not grossly alter pre-synaptic release at L2/3 synapses as assayed by measuring the PPR across several stimulation frequencies (Figure S6A). Loss of L4 NMDARs in NR mice also did not alter mEPSC frequency or amplitude at these synapses (Figures S6B and S6C). We next visually deprived Grin1L4CKO and their Grin1Fl/Fl littermates for 10–15 days using the LOVD paradigm to determine whether loss of L4 NMDARs affected preNMDAR contributions to glutamate release after visual deprivation. Genetic deletion of GluN1 occluded the reduction in mEPSC frequency by D-AP5 normally observed in L2/3 pyramidal neurons of LOVD mice, without affecting the amplitude of mEPSCs (Figures S6D–S6I). This suggests that the preNMDARs contributing to spontaneous release onto L2/3 pyramidal neurons after visual deprivation predominately reside in L4.
We hypothesized that if L4 preNMDARs contribute to spontaneous release at L2/3 synapses following LOVD, then L4 pre-NMDARs might also underlie the ability to express tLTD after LOVD. Our previous experimental design could not definitively identify the presynaptic cell types contributing to preNMDAR-mediated tLTD after visual deprivation, due to the possible activation of non-L4 fibers of passage with extracellular stimulation. To overcome this limitation, we used an optogenetic approach to selectively stimulate L4 excitatory neurons. For this, we crossed mice expressing Scnn1a-Tg3:Cre with mice expressing stop-floxed-ChR2(H134R)-YFP to express channelrhodopsin in L4 neurons. We confirmed that expression of channelrhodopsin was confined to L4 neurons within V1 in ChR2L4 mice (Figure 8A). Focal light pulses (2–4 ms) delivered at 20 Hz over channelrhodopsin-positive somata reliably produced L4 action potentials and postsynaptic L2/3 EPSPs (Figure 8B). As expected, ChR2-expressing L4 neurons formed monosynaptic glutamatergic synapses onto L2/3 neurons that could be activated by light stimulation over postsynaptic L2/3 neurons in the presence of TTX, 4-AP, and gabazine (Figure S7A). In contrast, focal stimulation of ChR2-expressing L4 somata in the presence of AMPA/kainate and NMDAR antagonists produced no postsynaptic L2/3 response (Figure S7B). These findings suggest that ChR2-expressing visual cortical L4 neurons form monosynaptic glutamatergic synapses onto postsynaptic L2/3 pyramidal neurons.
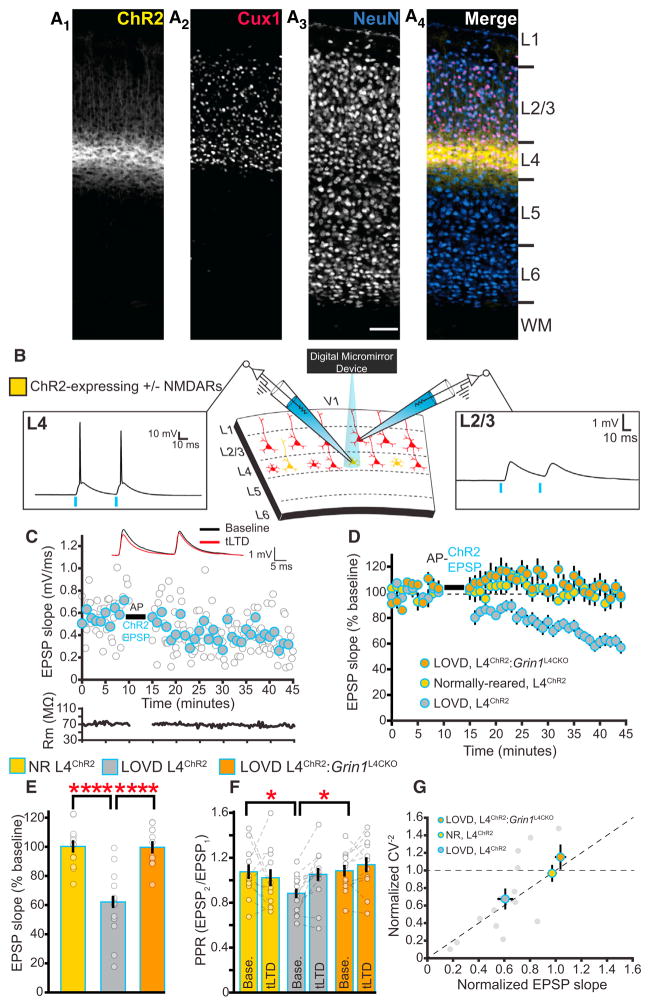
Figure 8. The Ability to Induce L4-L2/3 tLTD after Late-Onset Visual Deprivation Requires Presynaptic L4 NMDARs.
(A) ChR2-YFP expression is confined to L4 neurons in ChR2L4 mice. Sections are taken from a P100 mouse and stained with the neuronal marker NeuN and the transcription factor Cux1. Scale bar, 75 μm.
(B) Example recordings from an L4 and a post-synaptic L2/3 neuron after activation of ChR2-expressing L4 neurons with focal light pulses at 20 Hz.
(C) Sample recording demonstrating optically induced tLTD in a LOVD ChR2L4 mouse after AP-ChR2 EPSP pairings. Filled circles represent 1 min averaged bins of individual EPSP slopes (unfilled circles). Averaged traces before and after tLTD for the depicted neuron are within the inset.
(D) LOVD restores the ability to optically induce tLTD at L4-L2/3 synapses in ChR2L4 mice. In Grin1L4CKO:ChR2 mice, however, LOVD does not restore the ability to induce tLTD.
(E) Quantification of the last 10 min in (C) demonstrating larger reductions in EPSP slope after the induction of tLTD in LOVD ChR2L4 mice (n = 15) compared to NR ChR2L4 mice (n = 12; post hoc test, p < 0.0001). LOVD does not enable optically induced tLTD in Grin1L4CKO:ChR2 mice (n = 12, post hoc test versus LOVD ChR2L4, p < 0.0001).
(F) Visual deprivation decreases the baseline 20 Hz PPR in LOVD ChR2L4 mice as compared to both NR ChR2L4 mice (post hoc test, p = 0.05) and Grin1L4CKO:ChR2 mice (post hoc test, p < 0.05). After tLTD, the PPR increases in recordings from LOVD ChR2L4, but not their NR littermates or Grin1L4CKO:ChR2 mice, consistent with a presynaptic expression of tLTD (2wRmANOVA, p < 0.03).
(G) Normalized plot demonstrating relationship between CV−2 versus mean EPSP slope after the tLTD induction protocol (gray circles represent individual recordings from LOVD ChR2L4 mice). In all experiments, 1 mM MK-801 was included in the internal solution. Error bars represent SEM. Yellow, NR L4ChR2; gray, LOVD L4ChR2; orange, LOVD L4ChR2:Grin1L4CKO; blue border, tLTD w/ChR2.
We utilized this pathway-specific optogenetic approach to selectively stimulate L4 neurons and to determine whether LOVD altered tLTD specifically at L4-L2/3 synapses. To accomplish this, we recorded ChR2-evoked EPSPs in L2/3 pyramidal neurons and attempted to induce tLTD with light with repeated pairings of single ChR2-evoked EPSPs, followed 10–12 ms later by a postsynaptic action potential. Consistent with our previous results (Figure 4), NR adult ChR2L4 mice lacked light-induced tLTD. However, LOVD restored light-induced tLTD in ChR2L4 littermates (Figures 8C–8E). Changes in PPR at 20 Hz (Figure 8F) and fluctuation analysis (Figure 8G) were both consistent with a presynaptic locus for tLTD in LOVD ChR2L4 mice. To determine whether GABAergic inhibition modulated optically induced tLTD in adults, we repeated tLTD experiments in NR and LOVD ChR2L4 mice and included GABA(A) and GABA(B) antagonists in the bath to eliminate feedforward and feedback inhibition. However, GABA antagonists did not affect the expression of tLTD in LOVD ChR2L4 mice, nor did the antagonists enable tLTD in NR ChR2L4 mice (Figures S7C and S7D).
Fluctuation analysis and changes in PPR after tLTD both suggest that presynaptic mechanisms contribute to tLTD after LOVD. We sought to localize the site of plasticity more definitively by selectively disrupting NMDAR expression only at pre-synaptic L4 inputs. Accordingly, we generated triple transgenic mice by crossing Grin1L4CKO mice to stop-floxed-ChR2(H134R)-YFP mice, resulting in channelrhodopsin expression selectively in L4 neurons that also lacked NMDARs. We then compared optically induced tLTD in ChR2L4 and Grin1L4CKO:ChR2 mice. We found that Grin1L4CKO:ChR2 mice lacked tLTD after LOVD, demonstrating that L4 NMDARs were required for tLTD after visual deprivation (Figure 8D). Loss of L4 NMDARs also occluded both the initial decrease in PPR observed in LOVD mice as well as any increase in PPR following tLTD induction protocols (Figures 8E and 8F). Similar light pulse parameters (e.g., area and pulse length) were used across groups (Figures S7E and S7H). Accordingly, the postsynaptic L2/3 AMPAR response to varying L4 ChR2 stimulation spot sizes or light power was not different among experimental groups, and optically induced response magnitudes were similar to those evoked by electrical L4 stimulation (Figure S8). Jitter in the onset of EPSPs was higher with ChR2 stimulation than electrical stimulation, but this jitter was similar among experimental groups (Figure S7I). These results suggest that differences in light stimulation parameters did not underlie experience-dependent modifications in the ability to induce tLTD. Overall, these findings indicate that LOVD enhances preNMDAR-mediated glutamate release and tLTD induction predominately at L4 inputs onto L2/3 pyramidal neurons.
DISCUSSION
Our results demonstrate that visual experience dictates a form of timing-dependent metaplasticity. In contrast to the effects of visual deprivation on frequency-dependent LTD (Cooper and Bear, 2012), we find that sensory deprivation upregulates mechanisms favoring tLTD at L4-L2/3 excitatory synapses. Visual deprivation from early life maintains a form of presynaptically expressed tLTD that is normally lost during maturation, and subsequent visual experience downregulates the ability to induce this tLTD. The effect of visual deprivation is not restricted to early developmental periods, as late-onset visual deprivation reinstates the ability to induce tLTD. These results demonstrate that preNMDARs modulate the expression of presynaptic tLTD at specific intracortical synapses through their regulation by sensory experience.
Brief and competitive forms of sensory deprivation are typically driven by deprived-input depression during critical periods of development but by spared-input response potentiation in the adult sensory cortex (Feldman, 2009; Fox and Wong, 2005). However, the history of sensory experience also has a dramatic and age-dependent impact on sensory-driven cortical rearrangements. For example, brief (~10 days) binocular deprivation during adulthood can restore subsequent monocular deprived-eye-driven depression that is normally only observed during a developmental critical period for ocular dominance plasticity (He et al., 2006). The extent to which preNMDARs contribute to such experience-driven changes in synaptic plasticity and cortical rearrangements is poorly understood, but a role for preNMDARs is supported by the observation that monocular deprivation can shift the contribution of preNMDARs from slow-wave LTP to slow-wave LTD at L4-L4 synapses (Wang et al., 2012). Because STDP is sufficient to modify V1 receptive fields and orientation selectivity (Meliza and Dan, 2006; Yao and Dan, 2001), we propose that sensory-induced changes in preNMDAR-mediated tLTD provide another mechanism by which cortical circuitry can adapt to alterations in the visual environment. Our results demonstrate that visual deprivation, either during development or adulthood, restores preNMDAR-mediated tLTD at L4-L2/3 synapses and thus provides a mechanism and glutamatergic synaptic locus that may contribute to sensory experience-driven cortical rearrangements.
Synapse-Specific Mechanisms Contribute to the Developmental Regulation of tLTD
In combination with our previous findings (Corlew et al., 2007), our results demonstrate that tLTD at L4-L2/3 synapses requires preNMDARs in early development but that these synapses undergo an experience-dependent shift and require postsynaptic NMDARs for tLTD in more mature V1 (>P20) (Corlew et al., 2007). However, postsynaptic tLTD at mature L4 inputs also requires exogenous neuromodulators or blockade of GABA(A) signaling (Corlew et al., 2007; Kuhlman et al., 2010; Seol et al., 2007). This suggests that tLTD induction at L4-L2/3 synapses becomes increasingly dependent on the coordinated activity of multiple synaptic inputs with maturation. Visual deprivation for 2 days extends the temporal window for this postsynaptic tLTD in the presence of exogenous adrenergic agonists, but this brief visual deprivation is not sufficient to restore presynaptically expressed tLTD (Guo et al., 2012). Combined with our data showing that a longer (10 day) period of deprivation can reinstate presynaptically expressed tLTD, it is clear that the duration and nature of the deprivation dictate experience-dependent changes in the properties of tLTD, similar to other sensory deprivation-induced synaptic modifications (Maffei and Turrigiano, 2008). Perhaps not coincidently, preNMDARs contribute to tLTD at L4-L2/3 synapses during periods of immature or altered inhibition (Morales et al., 2002), which itself is thought to developmentally constrain STDP to favor temporal coherence (Kuhlman et al., 2010).
In contrast to L4-L2/3 synapses, L2/3-L2/3 synapses in V1 appear to express postsynaptic tLTD throughout development, because the ability to induce tLTD at these inputs is not developmentally regulated and requires postsynaptic NMDARs (Figure 6 and Froemke and Dan, 2002). STDP induced by the focal stimulation of L2/3 apical dendrites is similarly expressed postsynaptically and probably results from the recruitment of either horizontal L2/3 or extracortical inputs that target the apical dendrite (Froemke et al., 2005; Petreanu et al., 2009). The post-synaptic expression of tLTD at L2/3-L2/3 synapses is consistent with observations that preNMDARs do not enhance glutamate release at these inputs in the visual (Figure S4) or somatosensory cortex (Brasier and Feldman, 2008). Indeed, the synapse-specific expression of preNMDARs may be a general property of these receptors and probably contributes to difficulty in functionally localizing them to axonal compartments (Buchanan et al., 2012; Christie and Jahr, 2009). Such distinct pathway-specific mechanisms underlying STDP induction may be important for allowing experience to distinctly alter feedforward and recurrent synapses, as is thought to occur during visual cortical development (Ko et al., 2013).
Contributions of preNMDARs to tLTD after Visual Deprivation
We used selective optogenetic stimulation of presynaptic L4 neurons that lack NMDARs to demonstrate directly that preNMDARs mediate tLTD at L4-L2/3 synapses within visual cortex after visual deprivation. While a presynaptic locus for synaptic plasticity can be difficult to discern (Kerchner and Nicoll, 2008), five lines of evidence suggest that L4-L2/3 tLTD is expressed presynaptically, and not postsynaptically, after visual deprivation: (1) tLTD induction requires NMDAR activity but can be induced independently of postsynaptic NMDAR signaling (Figure 2), (2) tLTD consistently increases the PPR after tLTD induction, but not when the induction protocol fails to induce depression (Figures 1, 2, 4, 5, and 6), (3) tLTD requires the GluN3A subunit that is expressed presynaptically in early V1 development (Larsen et al., 2011), (4) the magnitude of tLTD correlates with trial-to-trial EPSP variability (correlates with CV−2; Figure 8G), and (5) the loss of presynaptic NMDARs prevents tLTD induction (Figure 8). In combination with previous studies demonstrating that NMDARs influence synaptic transmission at presynaptic neurons (Buchanan et al., 2012; Rodríguez-Moreno et al., 2011), our findings that genetic deletion of L4 preNMDARs prevents tLTD induction at L2/3 pyramidal neurons after LOVD demonstrates that NMDARs influence synaptic transmission at specific presynaptic sites.
We utilized a unique genetic strategy to selectively disrupt the expression of preNMDARs at L4 inputs while simultaneously expressing ChR2 at these inputs for optogenetic stimulation. This approach allowed us to assess the synapse-specific contributions of preNMDARs to tLTD and to overcome the possibility that channel-blocking NMDAR antagonists may not always block LTD induction (Nabavi et al., 2013; but see Babiec et al., 2014). While our genetic approach demonstrated a role for preNMDARs in L4-L2/3 tLTD and avoided the potential confound of using an open-channel NMDAR antagonist, we also found that reducing ion flux through NMDARs with MK-801 blocked tLTD mediated by preNMDARs at L4-L2/3 synapses (Figure 2) as well as tLTD mediated by postsynaptic NMDARs at L2/3-L2/3 synapses (Figure 6). Our results demonstrate that timing-dependent forms of visual cortical LTD are not mediated by metabotropic NMDAR activity, in agreement with previous findings from somatosensory cortex (Rodríguez-Moreno and Paulsen, 2008), suggesting that this might be a general property of LTD in sensory neocortices.
In agreement with a synapse-selective expression of preNMDARs (Buchanan et al., 2012), our results suggest that sensory experience maintains or restores the high-pass filtering of glutamate release by preNMDARs at a subset of synaptic inputs. In support of our findings, we previously found that visual deprivation can either reduce or fail to alter the PPR at L4-L2/3 synapses, the key difference being whether preNMDARs are blocked (Philpot et al., 2001; Yashiro et al., 2005). Our findings are broadly consistent with an experience-dependent reduction in presynaptic glutamate release at L4-L2/3 synapses during development, an effect that coincides with a reduction in preNMDAR function, GluN3A expression, and GluN1 labeling at excitatory presynaptic terminals (Cheetham and Fox, 2010; Corlew et al., 2007; Larsen et al., 2011). Collectively, our results suggest that sensory deprivation alters preNMDAR functions via changes confined to a restricted number of synaptic sites and that these effects are only revealed at certain activation frequencies.
While it has long been known that visual deprivation increases NMDAR synaptic responses (Carmignoto and Vicini, 1992; Fox et al., 1991), our results demonstrate a role for preNMDARs, and specifically the NMDAR subunit GluN3A, in experience-dependent plasticity in the visual cortex. We previously demonstrated that GluN3A is developmentally downregulated in the visual cortex at a time that corresponds with the loss of both tLTD and the contribution of preNMDARs to glutamate release (Larsen et al., 2011). Reminiscent of the developmental upregulation of postsynaptic GluN2A, which is partially reversed by dark rearing (Quinlan et al., 1999), our results demonstrate that dark rearing increases the synaptic expression of GluN3A. Since deprivation-induced enhancements in both tLTD induction and glutamate release require GluN3A, our observations suggest that visual deprivation prevents the developmental downregulation or reinstates the expression of GluN3A subunits and their associated functions. Consistent with this idea, chronic activity blockade in neuronal cultures prevents clathrin-mediated endocytosis of GluN3A, leading to enhanced surface expression of GluN3A-containing NMDARs (Chowdhury et al., 2013). The alterations in neuronal activity induced by visual deprivation may engage similar mechanisms to prevent the developmental downregulation of GluN3A.
In summary, our data demonstrate a form of experience-dependent plasticity that requires preNMDARs and is thus functionally distinct from the canonical postsynaptic NMDAR signaling typically associated with rate-dependent forms of Hebbian plasticity. Overall, these findings indicate that sensory stimuli regulate synaptic proteins, such as GluN3A, involved in STDP at specific synapses to expand plasticity outcomes and shape cortical circuitry. Sensory-driven reductions in tLTD induction may be one of several important factors for increasing the barrier to synaptic modification in favor of information storage (Feldman, 2012).
EXPERIMENTAL PROCEDURES
Experimental mice were raised under the animal care guidelines for University of North Carolina at Chapel Hill and were provided food and water ad libitum. Normally reared (NR) mice were raised on a 12:12 light:dark cycle until P26–P30, whereas dark-reared (DR) mice were raised in complete darkness from P2–P3 until P26–P30. In some instances, DR mice were returned to a 12:12 light:dark cycle for 10–12 days beginning at P30. Late-onset visual deprivation (LOVD) was achieved by placing P60–P63 NR mice into complete darkness for 10–12 days. However, in experiments in which L4 neurons expressed ChR2 or were NMDAR deficient, LOVD was achieved by placing these transgenic mice and their littermate controls into complete darkness at P75–P85 for 10–15 days, after which recordings were performed between P85–P95. Descriptions of transgenic mice are provided in Supplemental Experimental Procedures.
Electrophysiology
Spike-Timing-Dependent Plasticity
Monophasic and fixed latency response EPSPs were recorded for a 10 min stable baseline. When attempting to induce tLTP, baseline periods were minimized and induction of tLTP was always in the first 12 min after break in to reduce washout effects. The spike-timing-dependent plasticity induction period consisted of 100 single action potentials at 0.2 Hz, each preceded (tLTP) or followed (tLTD) within 10–12 ms by a single EPSP generated by L4 or L2/3 stimulation. Postsynaptic action potentials were produced by a brief (<5 ms) depolarization of the postsynaptic L2/3 cell and EPSPs generated in L4 or L2/3 were produced in an identical manner as the baseline period. At P21–P30 in the absence of neuromodulators or GABA(A) antagonists, the pairing of single APs (Corlew et al., 2007) or multiple APs (Guo et al., 2012; Seol et al., 2007) with L4-generated EPSPs fails to induce tLTP or tLTD, suggesting that failure to observe STDP at these synapses is not due to the number of postsynaptic APs used during tLTD induction. Changes in EPSP slope, CV−2, or PPR (mean EPSP2 amplitude/EPSP1 amplitude) were calculated as the decrease in the EPSP slope or the PPR from the last 10 min post-LTD induction period compared to the last 5 min of the baseline. Further description of electrophysiology parameters is provided in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Jesper Sjöström, Richard Weinberg, Mike Wallace, and Janet Berrios for critical readings of the manuscript and members of Paul Manis’s and Spencer Smith’s laboratories for technical input. We also thank Matt Judson and Wilmer Del Cid for microscopy and immunohistochemistry assistance. We thank Nobuki Nakanishi and Stuart Lipton for generating the Grin3A−/− mice, which were supplied to us via Isabel Pérez-Otaño. We thank Susumu Tonegawa for providing Floxed-Grin1 mice and The Allen Institute for Brain Science for providing Ai9, Ai6, Ai32, Wfs1-tg2-CreErt2, and Scnn1a-Tg3-Cre mice. We also thank Bram Kuijer for mouse genotyping. This work was supported by NRSA predoctoral fellowships F31 MH091817 (to R.S.L) and GM080162 (to R.J.C.), the UNC Department of Cell Biology and Physiology’s Dr. Susan Fellner fellowship (to R.S.L), a Helen Lyng White Fellowship (to I.T.S), a Whitehall Foundation grant (to S.L.S.), and NARSAD, NEI R01 EY018323, NINDS RO1 NS085093, and National Science Foundation grants 0822969 (to B.D.P.). The confocal imaging core was funded by grants from NINDS (P30NS045892) and NICHD (P30HD03110).
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and eight figures and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2014.07.039.
References
- Adesnik H, Li G, During MJ, Pleasure SJ, Nicoll RA. NMDA receptors inhibit synapse unsilencing during brain development. Proc Natl Acad Sci USA. 2008;105:5597–5602. doi: 10.1073/pnas.0800946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiec WE, Guglietta R, Jami SA, Morishita W, Malenka RC, O’Dell TJ. Ionotropic NMDA receptor signaling is required for the induction of long-term depression in the mouse hippocampal CA1 region. J Neurosci. 2014;34:5285–5290. doi: 10.1523/JNEUROSCI.5419-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Meredith RM, Rodríguez-Moreno A, Mierau SB, Auberson YP, Paulsen O. Double dissociation of spike timing-dependent potentiation and depression by subunit-preferring NMDA receptor antagonists in mouse barrel cortex. Cereb Cortex. 2009;19:2959–2969. doi: 10.1093/cercor/bhp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier DJ, Feldman DE. Synapse-specific expression of functional presynaptic NMDA receptors in rat somatosensory cortex. J Neurosci. 2008;28:2199–2211. doi: 10.1523/JNEUROSCI.3915-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan KA, Blackman AV, Moreau AW, Elgar D, Costa RP, Lalanne T, Tudor Jones AA, Oyrer J, Sjöström PJ. Target-specific expression of presynaptic NMDA receptors in neocortical microcircuits. Neuron. 2012;75:451–466. doi: 10.1016/j.neuron.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Cheetham CE, Fox K. Presynaptic development at L4 to l2/3 excitatory synapses follows different time courses in visual and somatosensory cortex. J Neurosci. 2010;30:12566–12571. doi: 10.1523/JNEUROSCI.2544-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury D, Marco S, Brooks IM, Zandueta A, Rao Y, Haucke V, Wesseling JF, Tavalin SJ, Pérez-Otaño I. Tyrosine phosphorylation regulates the endocytosis and surface expression of GluN3A-containing NMDA receptors. J Neurosci. 2013;33:4151–4164. doi: 10.1523/JNEUROSCI.2721-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Jahr CE. Selective expression of ligand-gated ion channels in L5 pyramidal cell axons. J Neurosci. 2009;29:11441–11450. doi: 10.1523/JNEUROSCI.2387-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper LN, Bear MF. The BCM theory of synapse modification at 30: interaction of theory with experiment. Nat Rev Neurosci. 2012;13:798–810. doi: 10.1038/nrn3353. [DOI] [PubMed] [Google Scholar]
- Corlew R, Wang Y, Ghermazien H, Erisir A, Philpot BD. Developmental switch in the contribution of presynaptic and postsynaptic NMDA receptors to long-term depression. J Neurosci. 2007;27:9835–9845. doi: 10.1523/JNEUROSCI.5494-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, et al. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393:377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012;75:230–249. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. The spike-timing dependence of plasticity. Neuron. 2012;75:556–571. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K, Wong RO. A comparison of experience-dependent plasticity in the visual and somatosensory systems. Neuron. 2005;48:465–477. doi: 10.1016/j.neuron.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Fox K, Daw N, Sato H, Czepita D. Dark-rearing delays the loss of NMDA-receptor function in kitten visual cortex. Nature. 1991;350:342–344. doi: 10.1038/350342a0. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Dan Y. Spike-timing-dependent synaptic modification induced by natural spike trains. Nature. 2002;416:433–438. doi: 10.1038/416433a. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Poo MM, Dan Y. Spike-timing-dependent synaptic plasticity depends on dendritic location. Nature. 2005;434:221–225. doi: 10.1038/nature03366. [DOI] [PubMed] [Google Scholar]
- Goel A, Jiang B, Xu LW, Song L, Kirkwood A, Lee HK. Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nat Neurosci. 2006;9:1001–1003. doi: 10.1038/nn1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Huang S, de Pasquale R, McGehrin K, Lee HK, Zhao K, Kirkwood A. Dark exposure extends the integration window for spike-timing-dependent plasticity. J Neurosci. 2012;32:15027–15035. doi: 10.1523/JNEUROSCI.2545-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Hodos W, Quinlan EM. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci. 2006;26:2951–2955. doi: 10.1523/JNEUROSCI.5554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Cossell L, Baragli C, Antolik J, Clopath C, Hofer SB, Mrsic-Flogel TD. The emergence of functional microcircuits in visual cortex. Nature. 2013;496:96–100. doi: 10.1038/nature12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester HJ, Johnston D. Target cell-dependent normalization of transmitter release at neocortical synapses. Science. 2005;308:863–866. doi: 10.1126/science.1100815. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, Lu J, Lazarus MS, Huang ZJ. Maturation of GABAergic inhibition promotes strengthening of temporally coherent inputs among convergent pathways. PLoS Comput Biol. 2010;6:e1000797. doi: 10.1371/journal.pcbi.1000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RS, Rao D, Manis PB, Philpot BD. STDP in the developing sensory neocortex. Front Synaptic Neurosci. 2010;2:9. doi: 10.3389/fnsyn.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RS, Corlew RJ, Henson MA, Roberts AC, Mishina M, Watanabe M, Lipton SA, Nakanishi N, Pérez-Otaño I, Weinberg RJ, Philpot BD. NR3A-containing NMDARs promote neurotransmitter release and spike timing-dependent plasticity. Nat Neurosci. 2011;14:338–344. doi: 10.1038/nn.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ, 3rd, Gu X, Zanella S, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. J Neurosci. 2008;28:4377–4384. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Meliza CD, Dan Y. Receptive-field modification in rat visual cortex induced by paired visual stimulation and single-cell spiking. Neuron. 2006;49:183–189. doi: 10.1016/j.neuron.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Min R, Nevian T. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat Neurosci. 2012;15:746–753. doi: 10.1038/nn.3075. [DOI] [PubMed] [Google Scholar]
- Morales B, Choi SY, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci. 2002;22:8084–8090. doi: 10.1523/JNEUROSCI.22-18-08084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi S, Kessels HW, Alfonso S, Aow J, Fox R, Malinow R. Metabotropic NMDA receptor function is required for NMDA receptor-dependent long-term depression. Proc Natl Acad Sci USA. 2013;110:4027–4032. doi: 10.1073/pnas.1219454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevian T, Sakmann B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J Neurosci. 2006;26:11001–11013. doi: 10.1523/JNEUROSCI.1749-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci. 1999;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Moreno A, Paulsen O. Spike timing-dependent long-term depression requires presynaptic NMDA receptors. Nat Neurosci. 2008;11:744–745. doi: 10.1038/nn.2125. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Moreno A, Kohl MM, Reeve JE, Eaton TR, Collins HA, Anderson HL, Paulsen O. Presynaptic induction and expression of timing-dependent long-term depression demonstrated by compartment-specific photorelease of a use-dependent NMDA receptor antagonist. J Neurosci. 2011;31:8564–8569. doi: 10.1523/JNEUROSCI.0274-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Moreno A, González-Rueda A, Banerjee A, Upton AL, Craig MT, Paulsen O. Presynaptic self-depression at developing neocortical synapses. Neuron. 2013;77:35–42. doi: 10.1016/j.neuron.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuett S, Bonhoeffer T, Hübener M. Pairing-induced changes of orientation maps in cat visual cortex. Neuron. 2001;32:325–337. doi: 10.1016/s0896-6273(01)00472-x. [DOI] [PubMed] [Google Scholar]
- Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee HK, Kirkwood A. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–929. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–1164. doi: 10.1016/s0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Valverde F. Rate and extent of recovery from dark rearing in the visual cortex of the mouse. Brain Res. 1971;33:1–11. doi: 10.1016/0006-8993(71)90302-7. [DOI] [PubMed] [Google Scholar]
- Wang L, Fontanini A, Maffei A. Experience-dependent switch in sign and mechanisms for plasticity in layer 4 of primary visual cortex. J Neurosci. 2012;32:10562–10573. doi: 10.1523/JNEUROSCI.0622-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Yao H, Dan Y. Stimulus timing-dependent plasticity in cortical processing of orientation. Neuron. 2001;32:315–323. doi: 10.1016/s0896-6273(01)00460-3. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Corlew R, Philpot BD. Visual deprivation modifies both presynaptic glutamate release and the composition of perisynaptic/extrasynaptic NMDA receptors in adult visual cortex. J Neurosci. 2005;25:11684–11692. doi: 10.1523/JNEUROSCI.4362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y, Callaway EM. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat Neurosci. 2005;8:1552–1559. doi: 10.1038/nn1565. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Dantzker JL, Callaway EM. Excitatory cortical neurons form fine-scale functional networks. Nature. 2005;433:868–873. doi: 10.1038/nature03252. [DOI] [PubMed] [Google Scholar]
- Zheng B, Sage M, Sheppeard EA, Jurecic V, Bradley A. Engineering mouse chromosomes with CreloxP: range, efficiency, and somatic applications. Mol Cell Biol. 2000;20:648–655. doi: 10.1128/mcb.20.2.648-655.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.