Abstract
The nucleus accumbens (NAcc) plays critical roles in healthy motivation and learning, as well as in psychiatric disorders (including schizophrenia and attention deficit hyperactivity disorder). Thus, techniques that confer control of NAcc activity might inspire new therapeutic interventions. By providing second-to-second temporal resolution of activity in small subcortical regions, functional magnetic resonance imaging (fMRI) can resolve online changes in NAcc activity, which can then be presented as “neurofeedback.” In an fMRI-based neurofeedback experiment designed to elicit NAcc activity, we found that subjects could increase their own NAcc activity, and that display of neurofeedback significantly enhanced their ability to do so. Subjects were not as capable of decreasing their NAcc activity, however, and enhanced control did not persist after subsequent removal of neurofeedback. Further analyses suggested that individuals who recruited positive arousal affect were better able to increase NAcc activity in response to neurofeedback, and that NAcc neurofeedback also elicited functionally correlated activity in the medial prefrontal cortex. Together, these findings suggest that humans can modulate their own NAcc activity and that fMRI-based neurofeedback may augment their efforts. The observed association between positive arousal and effective NAcc control further supports an anticipatory affect account of NAcc function.
Introduction
The nucleus accumbens (NAcc) of the ventral striatum plays a critical role in motivation and learning. Animal studies have historically indicated that blocking NAcc dopamine activity decreases motivation for both natural and drug rewards in animal models (Everitt and Robbins, 2005). More recently, human neuroimaging studies have implicated NAcc activity in reward anticipation of healthy as well as disordered individuals (Haber and Knutson, 2009). Both animal and human research show that NAcc activity increases during anticipation of uncertain rewards (Knutson et al., 2001; Schultz et al., 1992), consistent with the argument that NAcc activity can translate motivation to action (Mogenson et al., 1980) by conferring incentive salience or positive arousal affect on potential goal objects (Berridge and Robinson, 1998; Knutson and Greer, 2008).
Due to its deep subcortical location, small size, and dynamic changes in activity, however, researchers have not yet investigated whether humans can voluntarily control their own NAcc activity. Conferral of voluntary control could have important therapeutic applications, since diminished NAcc responsiveness has been implicated in psychiatric disorders (including schizophrenia and attention deficit hyperactivity disorders; e.g., Juckel et al., 2006; Scheres et al., 2007). Since its genesis in the early 1990s (Ogawa et al., 1990), functional magnetic resonance imaging (fMRI) has offered adequate spatial (on the order of millimeters) and temporal (on the order of seconds) resolution to resolve NAcc activity (Haber and Knutson, 2009). Over two decades later, hundreds of fMRI studies confirm that the NAcc plays a critical role in human reward learning (O'Doherty, 2004) and motivation (Knutson and Cooper, 2005). Meanwhile, fMRI-based neurofeedback signals have been extracted from and presented to humans from many brain regions (deCharms, 2008; Sitaram et al., 2009; Sulzer et al., 2013a,b; Weiskopf, 2012), but not yet the NAcc.
Existing fMRI studies suggest that extracting and presenting NAcc neurofeedback might pose unique technical challenges. Beyond its small and irregular subcortical architecture, the NAcc typically shows short bursts of activity, which could impose a number of temporal constraints on study design. First, since NAcc fMRI activity may primarily reflect phasic changes in dopamine release (Knutson and Gibbs, 2007), targeted activity should involve short modulation intervals that afford second-to-second resolution. Second, since human NAcc activity predicts rewards (Knutson and Cooper, 2005), subjects should encounter unpredictable rather than predictable (e.g., aperiodic) instructional cues to increase or decrease activity. Third, since NAcc activity may also respond to reward feedback (O'Doherty, 2004), investigators might initially omit control conditions that imply sustained failure to modulate (e.g., sham neurofeedback), since this could blunt responsiveness to subsequent veridical neurofeedback information (see also Sulzer et al., 2013a,b). Thus, a conservative initial attempt to assess peoples' ability to control their NAcc activity with and without the presence of neurofeedback might ideally incorporate short modulation intervals and unpredictable cues, as well as within-subject control conditions that are unlikely to induce lasting expectations of failure (e.g., by contrasting increase versus decrease instructions).
In this study, we used a neurofeedback task specifically optimized to elicit NAcc activity in order to determine whether people could voluntarily modulate (i.e., increase and decrease) their own NAcc activity, and if so, whether neurofeedback could enhance their efforts. We also examined whether brain activity in the anatomically connected medial prefrontal cortex (MPFC) might show increased functional connectivity with the NAcc during neurofeedback presentation, and whether adoption of strategies associated with increasing positive arousal would most effectively elicit NAcc activity.
Methods
Subjects
31 subjects completed the experiment and 25 (11 female, age range 20–40) were included in the final analysis. Subjects were excluded from analysis if they showed excessive overall motion (i.e., >1.5 mm from one scan to the next; 2 subjects), technical problems causing data loss (2 subjects), or physiological artifacts (e.g. correlated breathing or motion at r > .50; 2 subjects). Prior to participating, applicants were excluded on the basis of a phone interview if they reported a history of neurological or psychiatric disorders, concurrent use of psychotropic or cardiac medications, or metal in the body. Screened subjects included healthy, right-handed individuals with no MRI contraindications. After subjects arrived, they provided written informed consent to participate in the neurofeedback protocol approved by the Institutional Review board of the Stanford University School of Medicine.
Protocol
Before scanning, subjects received a short written description of the goal of neurofeedback, as well as suggested strategies for increasing and decreasing NAcc activity (Supplement 1, Appendix A). Based on an anticipatory affect model (Knutson and Greer, 2008), subjects were informed that activity in the NAcc has been associated with anticipating positive and arousing (e.g., “exciting”) events in past research, so that visualizing exciting events should facilitate activity increases, while visualizing neutral and nonarousing (e.g., “boring”) events should facilitate activity decreases. Subjects then viewed the neurofeedback task at a computer terminal, were allowed to ask questions, and were tested for comprehension as to which cue signaled whether to increase or decrease activity. Distinct circular cues containing different fractal patterns were assigned to increase versus decrease conditions to control for any pre-existing cue associations (e.g., as might occur with words such as “increase” versus “decrease” or arrows pointing up versus down). Subjects also generated and wrote down reminders for up to three personal strategies for inducing feelings of high positive arousal and low positive arousal.
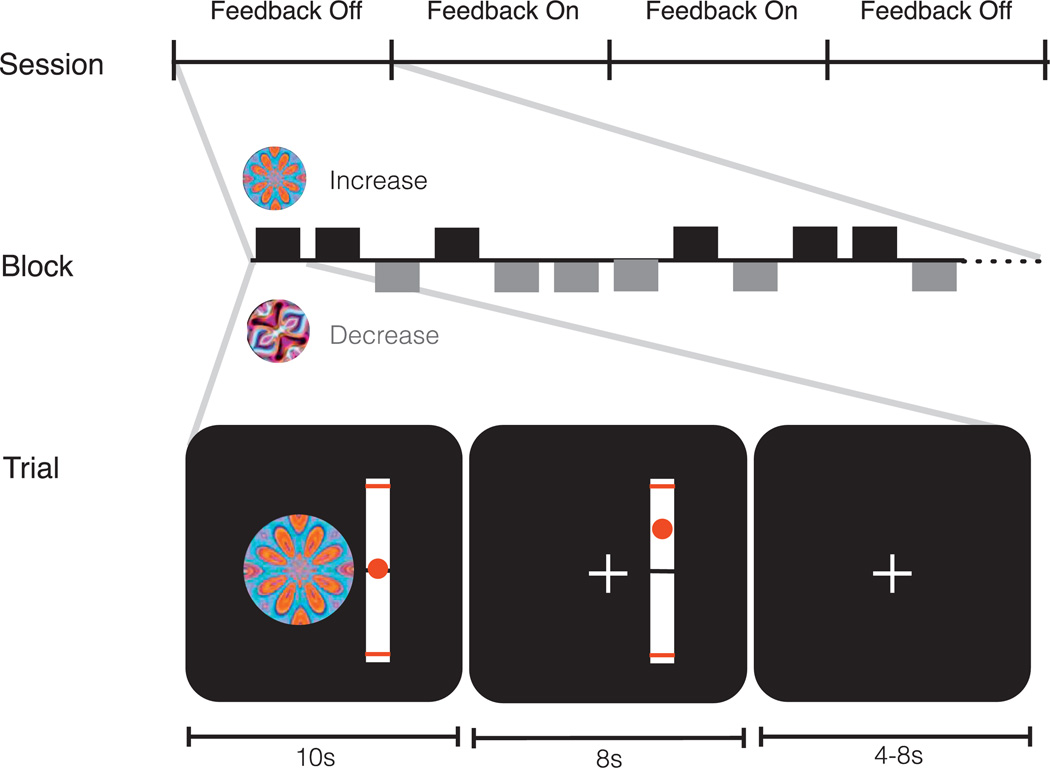
Once in the scanner, in-plane structural scans were acquired and NAcc volumes of interest (VOI) were manually specified on structural scans for extraction and presentation of the neurofeedback signal using customized software (described below). Next, subjects performed the neurofeedback task. Instructions preceded each of four trial blocks informing subjects about whether they would receive feedback or not (i.e., always in the same block order: “no feedback,” “feedback,” “feedback,” “no feedback”). Each trial block was separated by a 30 s rest period. During “feedback” trials, subjects saw cues indicating that they should try to increase or decrease activity (10 s) accompanied by an active thermometer (cue period + 8 additional s) in which a ball began at the 0 marker, but moved vertically as a function of NAcc activity (Fig. 1). Subjects were instructed to modulate their brain activity during the cue period, that the thermometer ball depicted their brain activity lagged by 6 s (i.e., the sum of the hemodynamic lag plus processing time), and that the thermometer would be updated every 2 s (i.e., the length of time required to acquire a whole brain image). During “no feedback” trials, subjects still saw cues indicating that they should try to increase or decrease activity (10 s), but these cues were accompanied by an inactive thermometer whose ball remained stationary at the 0 marker. Between each pseudorandomly ordered increase and decrease trial within each block, subjects fixated on a centrally located cross for a variable interval (4–8 s; Fig. 1). Since each trial took an average of 24 s and each block included 12 trials, each of the four blocks lasted 4 min and 48 s, summing to 20 min and 42 s for the entire task (including three 30 second breaks between blocks).
Fig. 1.
Task sequence and trial structure. Sessions involved Off, On, On, and Off blocks, each consisting of 12 pseudorandomly ordered increase and decrease trials, each of which included periods for cue presentation with neurofeedback (10 s), continuing neurofeedback without the cue (8 s), and a variable intertrial interval (4–8 s).
Neural data acquisition
Functional images were acquired using a General Electric Signa 1.5 Tesla whole body scanner and a standard birdcage quadrature head coil. Head movement was minimized with the use of an adjustable bite bar. Task stimuli were projected on a back-projection screen visible through a mirror mounted on the head coil. Functional images included contiguous 4 mm thick axial slices (no gap) extending from the pons to the top of the skull. Functional images were acquired (TR = 2000 ms, TE = 40 ms, flip = 90°, 3.75 × 3.75 mm in-plane voxel size, 64 × 64 × 24 matrix) with a T2*-sensitive spiral in/out pulse sequence designed to minimize artifacts at the base of the brain (Glover and Law, 2001). Two sets of structural images were also acquired. Before functional scanning, an in-plane structural image was acquired to allow specification of the NAcc volumes of interest (24 contiguous 4 mm thick axial slices; TR = 14 ms, TE = 400 ms, 0.94 × 0.94 mm in-plane resolution, 256 × 256 × 24 matrix). After functional scanning, a high-resolution structural image was additionally acquired to facilitate spatial normalization and visualization of data in a standard (i.e., Talairach) space (3D acquisition; T1-weighted spoiled grass sequence; 0.86 × 0.86 × 1.5 mm voxel size; 256 × 256 × 116 matrix).
Online fMRI data processing and analysis
Real-time fMRI data was acquired using custom modified software (“BrainView”), which was developed at Stanford University. Right and left NAcc volumes of interest were defined in one anatomical slice according to previously published anatomical specifications (Breiter et al., 1997; Knutson et al., 2008; 10 mm diameter on each side) using the VOISelect subroutine of BrainView applied to axial in-plane structural images (Supplement 2, Appendix A). Functional data were averaged across spiral in and out acquisitions (using weights derived from a previous five-minute functional scan during which subjects saw affective pictures, not further described in this report), corrected for motion in six dimensions (i.e., x, y, z, pitch, roll, yaw; Genovese et al., 1997), and averaged bilaterally across NAcc masks prior to being presented as neurofeedback.
Neurofeedback signal was calculated in real time using the following digital signal processing algorithm: Feedbackt = (Xt − mean(Xt − 20..t))/(mean(Xt − 20..t)). In this algorithm, Xt indicates the current averaged mask data from the NAcc, and Xt − 20..t indicates the preceding 20 TRs (or 40 s) of NAcc data. The 40 s baseline was used to reduce the influence of slow signal changes and maintain comparability with post-processed data, while also minimizing undue short-term influences of activity fluctuations during the previous trial. Neurofeedback was presented to subjects in the form of a ball marker superimposed on a vertical graduated thermometer. The ball's position indicated the level increase or decrease in activity relative to the rolling baseline. The thermometer was initialized at 0% signal change (middle black line) and ranged upward to 1% and downward to −1% signal change (signal changes that exceeded these limits remained at the maximum or minimum, and the range was based on signal changed observed in previous experiments that elicited robust NAcc activity; e.g., Knutson et al., 2001). The thermometer appeared adjacent to modulation cues, and was continuously updated every 2 s, reflecting activity changes 6 s prior to the update. This delay constituted the sum of the lag in the hemodynamic response of the blood oxygen level dependent (BOLD) signal (4 s) plus time needed for signal processing (2 s). To control for visual stimulation associated with presentation of neurofeedback, “no feedback” trials also presented a thermometer with a static ball 6 s after cue presentation (Fig. 1).
Post-scan fMRI data processing and analysis
For post-scan analyses, data were submitted to standard preprocessing protocols using AFNI software (Cox, 1996). First, spiral in and out acquisitions were merged using weighting based on whole scan average (Glover and Law, 2001). Next, data were corrected for the timing of slice acquisition and motion in six dimensions. Finally, time-series data were high-pass filtered to remove slow temporal trends (>0.01 Hz) and slightly spatially smoothed with a Gaussian kernel (4 mm FWHM; e.g., Sacchet and Knutson, 2012). These preprocessed data were then submitted to whole brain analysis, volume of interest analysis, functional connectivity analysis, and individual difference analyses.
Whole brain analyses were conducted to verify whether the increase versus decrease instructions specifically elicited differential activity in the NAcc during each block. Multiple regressions were conducted using boxcar regressors that contrasted neural activity during cue periods of increase versus decrease trials. These regressors were convolved with a single gamma function modeling the hemodynamic response prior to inclusion in the model (Cohen, 1997). Six motion-related covariates were also included to account for potential motion confounds, as well as terms that modeled baseline, linear and quadratic trends.
Volume of interest analyses were conducted by averaging trial-based activity time courses across all increase and decrease trials in each block for each subject with custom Python code. These averaged activity time courses were extracted using the same individualized NAcc mask files used in online analysis. Data from control volumes of interest were also averaged and extracted from spheres (8 mm diameter) centered on foci showing maximal activation during anticipation of monetary incentives in a previous meta-analysis of studies of incentive processing (Knutson and Greer, 2008). These volumes of interest specifically focused on Talairach coordinates±30, 20, 2 for the anterior insula and ±4, 48,−2 for the medial prefrontal cortex. Averaged activity time courses indicated that the most robust differences in activity occurred during acquisition of the first two brain images during the modulation period (i.e., the two 2-s volume acquisitions immediately following an initial 4 s hemodynamic lag). Thus, these two acquisitions were averaged to provide measures of peak activity for each individual during each trial, which were then submitted to regression analyses and posthoc comparisons. Finally, individual difference measures of neurofeedback efficacy were calculated by subtracting peak activity for the initial neurofeedback “off” block from peak activity for the average of both neurofeedback “on” blocks (activity from the last neurofeedback “off” block was not included in this calculation to avoid incorporating data susceptible to influence by previous neurofeedback experience).
Finally, functional connectivity analyses were conducted to identify whether other brain regions might show increased functional connectivity to the NAcc during neurofeedback. Specifically, we implemented psycho physiological interaction analyses (Friston et al., 1997) that combined activity from the NAcc seed region and a contrast of cued instructions (i.e. increase versus decrease) to construct the interaction term. Multiple regressions were conducted for each block which included reconvolved regressors modeling (1) deconvolved activity from bilateral NAcc regions of interest, (2) a contrast of increase versus decrease trials (by applying a boxcar regressor to the cue period), and (3) the interaction of these two regressors (providing the regressor of interest). Six motion-related covariate regressors were also included in these models to account for potential motion confounds, as well as models of baseline, linear and quadratic trends, activity in white matter volumes of interest, and activity in ventricular volumes of interest (Chang and Glover, 2009). Regression coefficients for the interaction term were used to evaluate the prediction that the medial prefrontal cortex (MPFC) might show increased functional connectivity with NAcc activity during feedback (versus no feedback) blocks.
Self-report measures
After scanning, subjects completed a questionnaire in which they indicated whether the instructions were clear, whether cues and feedback were visible, and whether task timing was too short or long. Subjects also indicated their perceived success at controlling their NAcc activity for each block on scales indexing motivation, alertness, confidence, lack of difficulty, and memorial clarity (i.e., on 1–7 point Likert scales running from “very untrue” to “very true”). Since “motivated” and “alert” ratings were robustly correlated within each block (r's > 0.62), these ratings were averaged within each subject to form a composite index of motivation for each block. Subjects also rated their affective reactions to increase and decrease cues in terms of valence and arousal (i.e., on 1–9 point Likert scales running from “bad” to “good” for valence and “not at all” to “extremely” for arousal). Affect ratings were then mean-centered and rotated forty-five degrees through two-dimensional space to derive measures of cue-elicited “positive arousal” and “negative arousal” (i.e., positive arousal = ((arousal − 4) / sqrt(2)) + ((valence − 4) / sqrt(2)); negative arousal = ((arousal − 4) / sqrt(2)) − ((valence − 4) / sqrt(2)); (Knutson et al., 2005). Affect ratings were transformed, since measures that include both valence and arousal have correlated most robustly with NAcc activity in previous research.
Subjects were also asked to provide open-ended written descriptions of strategies that they used during each block of the study. Words from these strategy descriptions were classified with the Linguistic Inquiry and Word Count dictionary (Pennebaker et al., 2001) with respect to positive and negative words, which were then calculated as percentages of the total words in each description (since three subjects did not provide self-report data, a total of 22 subjects' self-report data were analyzed).
Results
Neural activity
Whole brain analyses
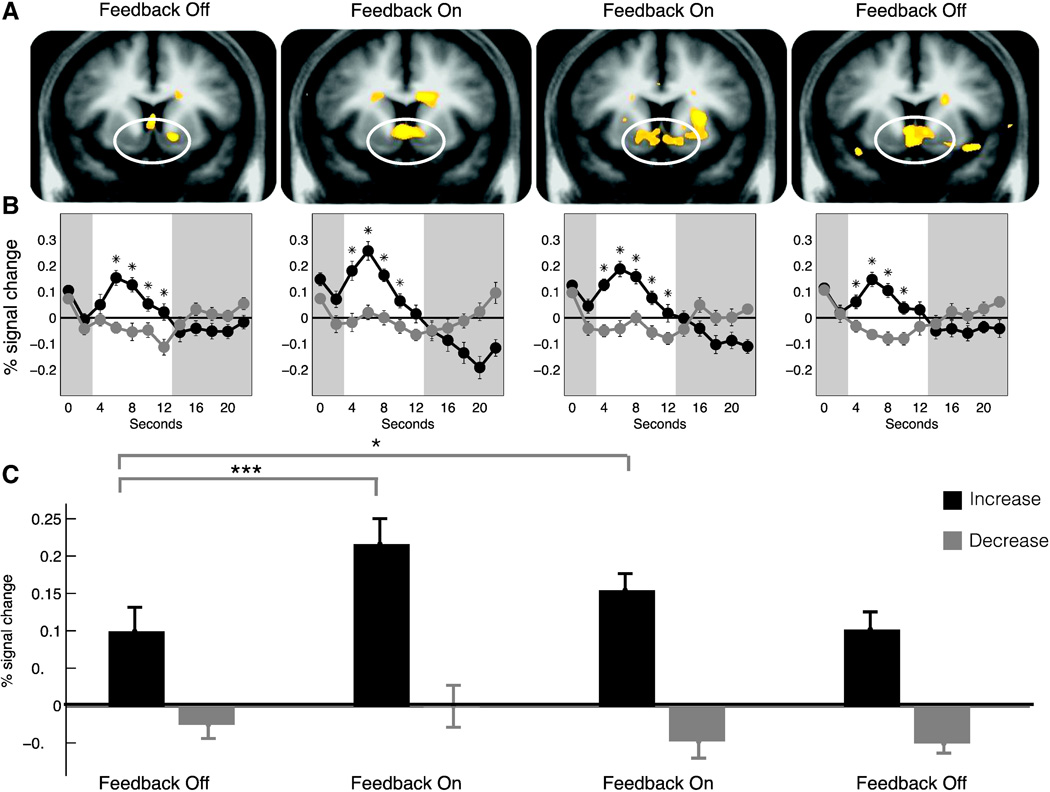
Whole brain contrasts of increase versus decrease instructions activated a broad range of brain regions implicated in reward processing including the NAcc during all four blocks of the experiment (all Z's > 4.2, p's < .05, corrected; Fig. 2A, Supplement 3, Appendix A).
Fig. 2.
NAcc activity in response to increase versus decrease cues across blocks. A. Whole brain maps of activity for increase versus decrease contrasts across blocks (thresholded at p < .0001, uncorrected). B. Activation time courses from bilateral NAcc volumes of interest for increase versus decrease trials across blocks (mean ± SEM; cue presentation period highlighted). C. Peak activation for increase versus decrease trials across blocks (mean ± SEM; *p < .05; ***p < .001).
Volume of interest analyses
A general linear model tested the effects of (1) cued instruction (increase versus decrease); (2) feedback (on/off); (3) time (i.e., four consecutive blocks); and their interactions on NAcc volume of interest activity, where fixed effects of subjects were modeled with dummy regressors. We predicted that cued instruction would have a main effect on NAcc activity, and that feedback might enhance cued instruction, as reflected by an interaction of cued instruction by feedback. Consistent with these predictions, analyses revealed significant main effects of cued instruction (t(169) = 11.40, p < 0.0001), feedback (t(169) = 3.20, p < 0.01), and time (t(169) = −2.50, p = 0.014), as well as significant interactions of cued instruction by feedback (t(169) = 2.32, p < 0.05), and feedback by time (t(169) = −2.01, p < 0.05), but not of cued instruction by time (t(169) = −0.02, p = 0.99) or the three-way interaction of these factors (t(169) = −0.56, p = 0.56; Table 1). Posthoc comparisons revealed greater NAcc activity for all increase versus decrease trials (t(24)= 8.09, p < 0.0001; Fig. 2B), and even greater NAcc activity during feedback periods than during no feedback periods for increase trials (t(24) = 4.60, p < 0.001), but no difference for decrease trials (t(24)= 0.57, p = 0.60; Fig. 2C). Together, these findings indicated that subjects could increase NAcc activity when presented with the increase cue, and further that these elevations were significantly augmented by presentation of feedback (see also Supplement 4, Appendix A).
Table 1.
| NAcc |
MPFC |
Ant. Insula |
||||
|---|---|---|---|---|---|---|
| t(169) | p | t(169) | p | t(169) | p | |
| Instruction (Increase/decrease) | 11.35 | <0.0001 | 5.03 | <0.0001 | 7.94 | <0.0001 |
| Time (blocks 1–4) | −2.48 | 0.0143 | 0.45 | 0.6536 | −1.93 | 0.0553 |
| Feedback (on/off) | 3.21 | 0.0016 | 1.67 | 0.0961 | −0.23 | 0.8195 |
| Feedback * Time | −2.01 | 0.0460 | 0.41 | 0.6807 | −0.84 | 0.4031 |
| Feedback * Instruction | 2.32 | 0.0218 | −1.42 | 0.1572 | −0.09 | 0.9283 |
| Instruction * Time | −0.02 | 0.9860 | 0.16 | 0.8712 | −0.54 | 0.5915 |
| Feedback * Instruction * Time | −0.59 | 0.5588 | −0.36 | 0.7190 | −1.05 | 0.2947 |
| Constant | 4.94 | <0.0001 | 3.51 | 0.0006 | 4.91 | <0.0001 |
| Full Model F(168) | 7.71 | <0.0001 | 4.31 | <0.0001 | 2.14 | 0.001 |
| Full Model R2 | 0.59 | 0.44 | 0.28 | |||
To rule out nonspecific effects of neurofeedback, we also analyzed activity in medial prefrontal cortex (MPFC) and anterior insula volumes of interest using the same general linear model analysis described above. These regions were chosen for comparison since they have previously been implicated in affect and valuation (Knutson and Greer, 2008), but were not specifically targeted by neurofeedback in this experiment. Consistent with their role in affect and valuation, while instruction (increase versus decrease) showed a significant main effect on both MPFC (t(169) = 5.03, p < 0.0001) and anterior insula activity (t(169) = 7.94, p < 0.0001), there were no significant main effects of feedback, time, or any interactions of these factors on activity in either comparison region (Table 1).
Functional connectivity
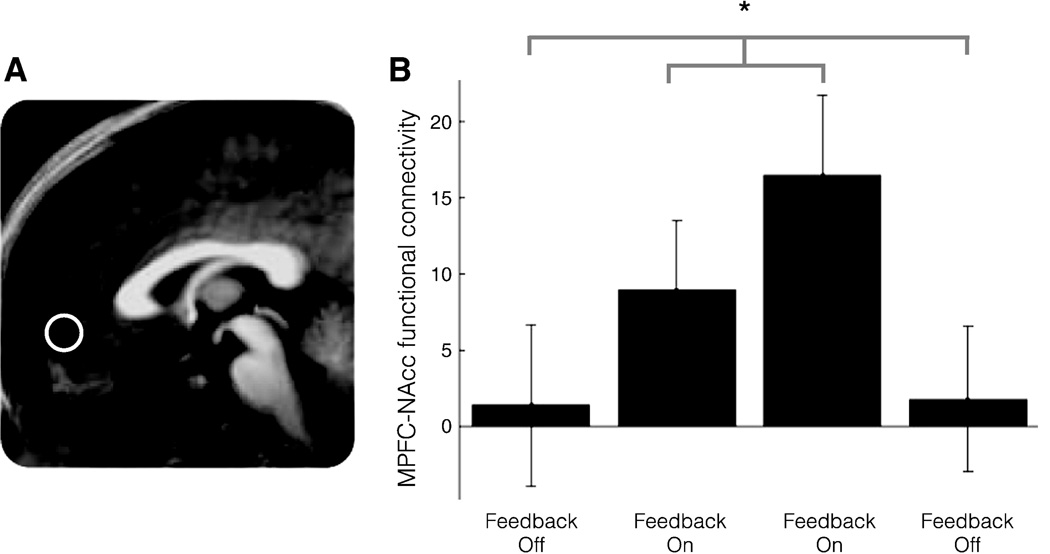
Consistent with the notion that the MPFC might utilize neurofeedback information to modulate NAcc function via unidirectional projections, activity in the MPFC and NAcc were not functionally correlated during the two nonfeedback blocks, but were significantly more functionally correlated during the two neurofeedback blocks, as confirmed by a t-test contrasting connectivity coefficients for feedback on versus off blocks in the MPFC volume of interest (t(24) = 2.40, p < 0.05). These findings suggest that providing neurofeedback increased functional connectivity between the MPFC and NAcc (Fig. 3).
Fig. 3.
MFPC–NAcc functional connectivity across blocks. A. MPFC volume of interest. B. Difference in functional connectivity of MPFC–NAcc during feedback on versus feedback off blocks (*p < .05).
Self-reported motivation and affect
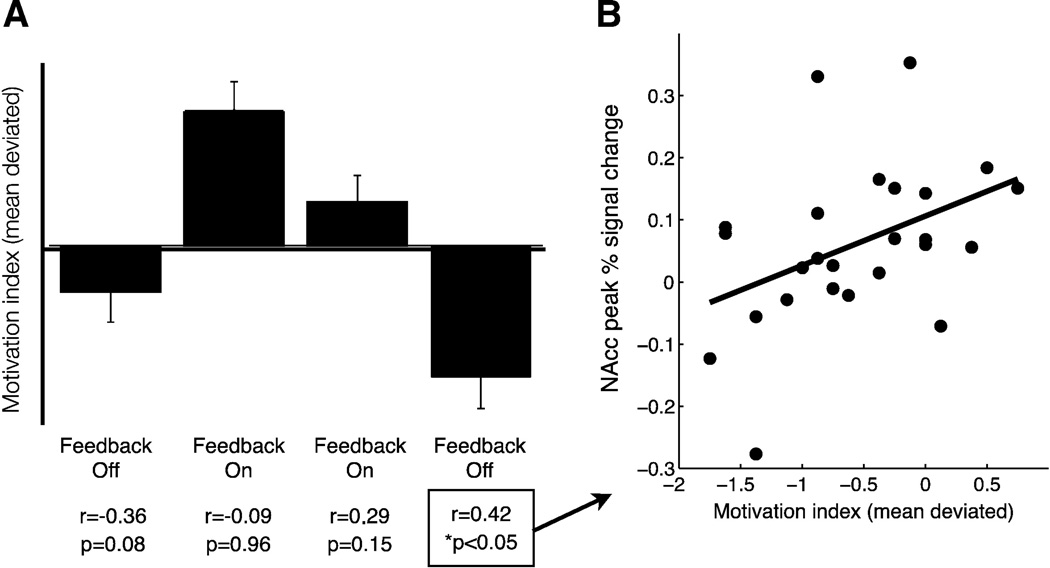
A general linear model analyzed the effects of (1) feedback (on/off); (2) time (i.e., four consecutive blocks); and (3) their interaction on the motivation index. This analysis revealed significant main effects of feed-back (t(95) = 5.64, p < 0.001) and time (t(95) = −2.86, p < 0.01), but no interaction of feedback by time (t(95) = −1.08, p = 0.28) on motivation. Individual differences in motivation only correlated with subjects' ability to increase NAcc activity during the final block when feedback was not presented (r(25) = 0.42, p < 0.05) but not during other blocks (i.e., block 1: r(25) = −0.36, p = 0.08; block 2: r(25) = −0.09, p = 0.96; block 3: r(25) = 0.29, p = 0.15; Fig. 4).
Fig. 4.
Individual differences in motivation correlate with neurofeedback transfer. A. Motivation increased for feedback on versus off blocks, but correlated with peak activity only during the last off block, B. correlation of individual differences in motivation with peak NAcc activity during the last off block.
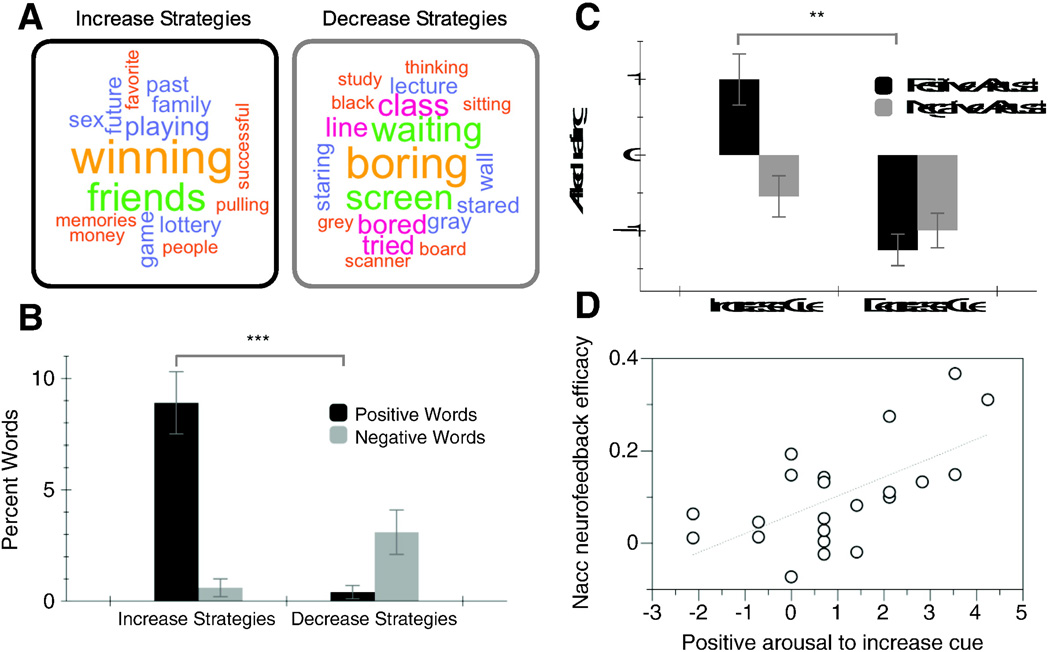
Qualitative semantic analysis of words that subjects used to describe strategies for increasing and decreasing NAcc activity after scanning also suggested that words associated with high positive arousal were used more often to describe strategies for increasing NAcc activity, but words associated with low positive arousal were used more often to describe strategies for decreasing NAcc activity (i.e., represented by word size in Fig. 5). These qualitative impressions were confirmed by quantitative word counts, since a greater percentage of positive emotional words used to describe strategies for increasing versus decreasing NAcc activity (t(21) = 5.77, p < .001). Percentage of negative emotional words used to describe strategies for increasing versus decreasing NAcc activity did not, however, significantly differ (t(21)=−2.02, p= .06). Consistent with instructions, subjects reported that the increase cue-elicited greater positive arousal than the decrease cue (t(24) = 5.30, p < 0.001; Fig. 4), but that the increase and decrease cues did not differentially elicit negative arousal (t(24) = 1.40, p = 0.20). Finally, individuals also varied in their NAcc neurofeedback efficacy — or their ability to increase NAcc activity in response to feedback versus no feedback (during the first session). Individuals with higher NAcc neurofeedback efficacy also reported experiencing greater positive arousal in response to the increase cue (r(22) = .62, p < .005; Fig. 5), but not greater negative arousal in response to the increase cue (r(22)=.25, n.s.), consistent with facilitation by positive arousal rather than general arousal.
Fig. 5.
Affective impact of neurofeedback. A. Words used to describe strategies for increasing versus decreasing NAcc activity (size indicates use frequency); B. percentage of positive and negative words used to describe strategies for increasing versus decreasing NAcc activity (***p < .001); C. affective responses to increase and decrease cues (**p < .01); D. association of individual differences in positive arousal response to increase cue with NAcc neurofeedback efficacy (i.e., neurofeedback on minus neurofeedback off peaks; r = .62, p < .01).
Discussion
By combining a targeted procedure with fMRI scanning, we found that people could modulate their nucleus accumbens (NAcc) activity, and that NAcc neurofeedback significantly enhanced this ability. Subjects were more able to increase NAcc activity than decrease it, however, and enhanced control did not persist after subsequent removal of neurofeedback. Individuals who recruited positive arousal affect were better able to increase NAcc activity in response to neurofeedback, and NAcc neurofeedback also elicited functionally correlated activity in the medial prefrontal cortex.
These findings thus provide an initial demonstration that people can voluntarily increase their NAcc activity, and further suggest that neurofeedback may promote their efforts. Several issues, however, remain unresolved. First, consistent with previous research (Knutson and Cooper, 2005), and as indicated by time course analyses, subjects could only increase NAcc activity for a brief period of time (i.e., on the order of seconds). This brief duration may reflect time-limited neurochemical events, such as the phasic availability of dopamine in the NAcc synapse (Knutson and Gibbs, 2007). Further research will be necessary to determine how long increased NAcc activity can be sustained. Methodologically, these findings imply that without sufficient temporal resolution, neurofeedback protocols risk missing detection of fast changes in neural activity. Second, subjects were more successful at increasing NAcc activity above baseline than decreasing NAcc activity below baseline, consistent with the notion that reward feedback related to successfully decreasing NAcc activity could have paradoxical effects, potentially even canceling out earlier decreases. Future research will need to comprehensively characterize whether subjects can significantly decrease NAcc activity and to what extent, possibly by introducing greater temporal separation between modulation cues and feedback. Third, although neurofeedback enhanced subjects' abilities to increase NAcc activity, this enhancement did not significantly carry over into subsequent trials when feedback was no longer available (consistent with neurofeedback studies of other deep brain structures including the subgenual cingulate in Hamilton et al., 2011 and ventral tegmental area in Sulzer et al., 2013a,b). With more extensive or repeated training, however, enhancements might transfer into subsequent nonfeedback periods (e.g., consistent with learning effects) — a possibility worthy of future exploration.
The findings also illuminate psychological moderators of peoples' ability to increase their NAcc activity. Importantly, individuals who reported experiencing more positive arousal in response to the increase cue also showed greater NAcc neurofeedback efficacy, indexed by their ability to further increase their NAcc signal after encountering feedback. Motivation also changed over the course of the experiment in a pattern that mirrored neurofeedback success. Although motivation was highest during neurofeedback trials, it remains unclear whether this increase represented time-dependent tradeoffs between increasing proficiency followed by increasing fatigue or actual effects of the neurofeedback. Increasing fatigue would be consistent with the observed significant effects of time on NAcc activity and may suggest limits on the length of training sessions. Individual differences in motivation were only correlated with success at increasing NAcc activity during the final block of trials, when neurofeedback was not presented. This finding suggests that transfer effects may be strongest for individuals who maintain their motivation after neurofeedback practice, but more extensive research will be necessary to test the robustness and stability of these individual differences in transfer.
Functional connectivity analyses suggested that MPFC activity significantly correlated with NAcc activity during neurofeedback trials, but not during trials without neurofeedback. This finding is consistent with research implicating the MPFC in processing reward feedback (e.g., Knutson and Greer, 2008; Knutson et al., 2003). Thus, neurofeedback may have enhanced subjects' ability to increase their own NAcc activity by recruiting unidirectional glutamatergic projections from the MPFC. These pathways, which are well-characterized in primates (Haber, 2003), have recently been verified in humans by researchers using diffusion tensor imaging (Cohen et al., 2008; Draganski et al., 2008), and their structural integrity has been shown to correlate with individual differences in reward learning (Samanez-Larkin et al., 2012). Future research may determine whether more extensive neurofeedback training enhances functional or even structural connectivity between the MPFC and NAcc.
The present findings rule out a number of alternative accounts for subjects' success in increasing their NAcc activity. First, mere presentation of instructions did not cause increases in NAcc activity, since presentation of decrease cues did not significantly change NAcc activity. Second, mere exposure to feedback did not cause increases in NAcc activity, since presentation of neurofeedback after decrease cues did not significantly change NAcc activity. Third, increases in NAcc activity could not be attributed to changes in the activity of other theoretically relevant brain regions, since activity in the MPFC and anterior insula did not show similar responsiveness to instruction cues or feedback. Fourth, increases in NAcc activity were more strongly associated with changes in positive arousal than with general or negative arousal, as implied both by changes in motivation during the session and positive arousal (but not negative arousal) elicited by the increase cues (Fig. 5).
By demonstrating that people can modulate their own NAcc activity, these findings contribute to a growing fMRI-based neurofeedback literature. Recent fMRI neurofeedback studies have begun to venture beyond primary sensory and motor cortices to target deep evolutionarily-conserved circuits implicated in motivation and affect (Caria et al., 2007; Posse et al., 2003; Sitaram et al., 2011). In perhaps the most relevant recent demonstration, investigators focused on a reward-related region housing dopamine neurons that project to the NAcc — the ventral tegmental area (VTA) of the midbrain (Sulzer et al., 2013a,b). As in the present experiment, Sulzer and colleagues also found evidence for enhanced modulation of VTA activity during presentation of neurofeedback, but did not find evidence for transfer after the withdrawal of feedback. Still, when seeking to control both VTA and NAcc activity, repeated and spaced training might eventually improve subjects' chances of transferring skills learned during feedback to contexts without feedback (e.g., de Charms et al., 2004). Once the ability to modulate activity in a specific brain region has been established, the affordances and boundaries of skill transference can then be explored and mapped.
Because our procedure was optimized to elicit NAcc activity, this study contributes a number of advances beyond previous research. For example, we sought to present and elicit phasic changes rather than tonic changes in NAcc activity, used a randomized order of instructional cues to minimize anticipatory confounds, and did not include control conditions likely to induce lasting expectations of failure (e.g., sham feedback manipulations). Additionally, with respect to processing NAcc neurofeedback, we did not covary out whole brain signals, since activity in many other brain regions correlates with NAcc activity, but instead computed a rolling difference between current and preceding NAcc activity to resolve second-to-second dynamic fluctuations. This within-subjects design, however, also has some weaknesses. Although we included the within-subject comparison of instructions to decrease (versus increase) NAcc activity, which allowed us to address several alternative explanations for the observed activity, we did not include a between-group control experiment involving only sham feedback. Future studies should test such a control condition, which understandably may elicit pervasive and lasting expectations of failure. Also, as mentioned previously, conditions conducive to transfer effects remain to be established.
Beyond methodological contributions, the current findings have implications for theory. With respect to psychological function, the findings support an anticipatory affect account of NAcc activity (Knutson and Greer, 2008), since cues for increasing NAcc activity evoked positive arousal but not negative arousal, motivated subjects seemed best able to maintain enhancements from NAcc neurofeedback, and subjects' self-reported strategies for effectively increasing NAcc activity involved strategies that invoked positive arousal. If positive arousal can increase NAcc activity, this knowledge may help subjects to most effectively implement neurofeedback control of this region. These findings also complement affect regulation findings in which subjects were able to decrease their NAcc activation to exciting monetary cues (Delgado et al., 2008) or drug cues (Kober et al., 2010). In the present experiment, however, neurofeedback-based control more potently increased than decreased NAcc activity, which could critically inform clinical applications. Beyond its association with affect in healthy individuals, blunted NAcc activity has been associated with troublesome psychiatric symptom profiles, including a lack of motivation in the context of schizophrenia (e.g., Juckel et al., 2006), and hyperactivity in the context of ADHD (e.g., Scheres et al., 2007). If disordered patients can also be taught to modulate their NAcc activity, this might have a transient or even lasting impact on relevant psychiatric symptoms, and so could complement existing therapeutic techniques.
Conclusion
These findings provide an initial demonstration that humans can voluntarily increase their NAcc activity, and that fMRI neurofeedback may enhance this control. The findings also imply that positive arousal may promote NAcc neurofeedback and that neurofeedback may enhance NAcc connectivity with the medial prefrontal cortex. NAcc neurofeedback may eventually facilitate healthy motivation and learning, as well as target and treat troublesome psychiatric symptoms.
Supplementary Material
Acknowledgments
We thank Maureen S. Chalfin and Robbie Ruelas for help with data acquisition and analysis; Christian Buchel, Matthew Sacchet, and an anonymous reviewer for feedback on preliminary drafts; and the National Institute on Drug Abuse (DA R03-020615 to BK) and National Institute of Biomedical Imaging and Bioengineering (P41 EB-01589 to GG) for support.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neuroimage.2014.03.073.
References
- 1.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Rev. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 2.Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 3.Caria A, Veit R, Sitaram R, Lotze M, Weiskopf N, Grodd W, Birbaumer N. Regulation of anterior insular cortex activity using real-time fMRI. Neuroimage. 2007;35(3):1238–1246. doi: 10.1016/j.neuroimage.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6(2):93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MX, Schoene-Bake JC, Elger CE, Weber B. Connectivity-based segregation of the human striatum predicts personality characteristics. Nat. Neurosci. 2008;12(1):32–34. doi: 10.1038/nn.2228. [DOI] [PubMed] [Google Scholar]
- 7.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 8.deCharms CR. Applications of real-time fMRI. Nat. Rev. Neurosci. 2008;9(9):720–729. doi: 10.1038/nrn2414. [DOI] [PubMed] [Google Scholar]
- 9.Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nat. Neurosci. 2008;11(8):880–881. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Draganski B, Kherif F, Klöppel S, Cook PA, Alexander DC, Parker GJ, Frackowiak RS. Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J. Neurosci. 2008;28(28):7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 12.Friston KJ, Price CJ, Buechel C, Frackowiak RSJ. A taxonomy of study design. Hum. Brain Funct. 1997:141–159. [Google Scholar]
- 13.Genovese CR, Noll DC, Eddy WF. Estimating test retest reliability in functional MR imaging I: Statistical methodology. Magn. Reson. Med. 1997;38(3):497–507. doi: 10.1002/mrm.1910380319. [DOI] [PubMed] [Google Scholar]
- 14.Glover GH, Law CS. Spiral in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn. Reson. Med. 2001;46(3):515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 15.Haber SN. The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat. 2003;26(4):317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2009;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton JP, Glover GH, Hsu JJ, Johnson RF, Gotlib IH. Modulation of subgenual anterior cingulate cortex activity with real time neurofeedback. Hum. Brain Mapp. 2011;32(1):22–31. doi: 10.1002/hbm.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr. Opin. Neurol. 2005;18(4):411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- 19.Knutson B, Gibbs SE. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology. 2007;191(3):813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- 20.Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philos. Trans R. Soc. B Biol. Sci. 2008;363(1511):3771–3786. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J. Neurosci. 2005;25(19):4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal–striatal pathway underlies cognitive regulation of craving. Proc. Natl. Acad. Sci. 2010;107(33):14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog. Neurobiol. 1980;14(2):69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 25.O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr. Opin. Neurobiol. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pennebaker JW, Francis ME, Booth RJ. Linguistic Inquiry and Word Count: LIWC 2001. Mahway: Lawrence Erlbaum Associates; 2001. p. 71. [Google Scholar]
- 28.Posse S, Fitzgerald D, Gao K, Habel U, Rosenberg D, Moore GJ, Schneider F. Real-time fMRI of temporolimbic regions detects amygdala activation during single-trial self-induced sadness. Neuroimage. 2003;18(3):760–768. doi: 10.1016/s1053-8119(03)00004-1. [DOI] [PubMed] [Google Scholar]
- 29.Sacchet MD, Knutson B. Spatial smoothing systematically biases the localization of reward-related brain activity. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samanez-Larkin GR, Levens SM, Perry LM, Dougherty RF, Knutson B. Frontostriatal white matter integrity mediates adult age differences in probabilistic reward learning. J. Neurosci. 2012;32(15):5333–5337. doi: 10.1523/JNEUROSCI.5756-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J. Neurosci. 1992;12(12):4595–4610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sitaram R, Caria A, Birbaumer N. Hemodynamic brain–computer interfaces for communication and rehabilitation. Neural Netw. 2009;22(9):1320–1328. doi: 10.1016/j.neunet.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Sitaram R, Lee S, Ruiz S, Rana M, Veit R, Birbaumer N. Real-time support vector classification and feedback of multiple emotional brain states. Neuroimage. 2011;56(2):753–765. doi: 10.1016/j.neuroimage.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Sulzer J, Haller S, Scharnowski F, Weiskopf N, Birbaumer N, Blefari ML, Sitaram R. Real-time fMRI neurofeedback: progress and challenges. Neuroimage. 2013a doi: 10.1016/j.neuroimage.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sulzer J, Sitaram R, Blefari ML, Kollias S, Birbaumer N, Stephan KE, Gassert R. Neurofeedback-mediated self-regulation of the dopaminergic midbrain. Neuroimage. 2013b doi: 10.1016/j.neuroimage.2013.05.115. [DOI] [PubMed] [Google Scholar]
- 36.Weiskopf N. Real-time fMRI and its application to neurofeedback. Neuroimage. 2012;62(2):682–692. doi: 10.1016/j.neuroimage.2011.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.