Abstract
In the last decades, there has been an increase of cholera epidemics caused by multidrug resistant strains. Particularly, the integrative and conjugative element (ICE) seems to play a major role in the emergence of multidrug resistant Vibrio cholerae. This study fully characterized, by whole genome sequencing, new ICEs carried by multidrug resistant V. cholerae O1 strains from Nigeria (2010) (ICEVchNig1) and Nepal (1994) (ICEVchNep1). The gene content and gene order of these two ICEs are the same, and identical to ICEVchInd5, ICEVchBan5 and ICEVchHai1 previously identified in multidrug resistant V. cholerae O1. This ICE is characterized by dfrA1, sul2, strAB and floR antimicrobial resistance genes, and by unique gene content in HS4 and HS5 ICE regions. Screening for ICEs, in publicly available V. cholerae genomes, revealed the occurrence and widespread distribution of this ICE among V. cholerae O1. Metagenomic analysis found segments of this ICE in marine environments far from the direct influence of the cholera epidemic. Therefore, this study revealed the epidemiology of a spatio-temporal prevalent ICE in V. cholerae O1. Its occurrence and dispersion in V. cholerae O1 strains from different continents throughout more than two decades can be indicative of its role in the fitness of the current pandemic lineage.
Background
The burden of cholera has grown strikingly during the last years, and has spread to countries previously spared of this disease. An increasing number of multidrug resistant Vibrio cholerae strains have been reported in recent cholera outbreaks [1], and this resistance phenotype is mainly associated with the presence of mobile and mobilized elements, such as integrative and conjugative elements (ICEs). ICEs are self-transmissible mobile elements, able to integrate into the host bacterial chromosome, excise and transfer to a new host genome through conjugation [2]. To date, a dozen related ICEs belonging to the SXT/R391 family have been identified, which are characterized by a SXT integrase gene (int SXT) that enables site-specific integration of the ICE into the 5′ end of prfC gene. There are 52 core genes present in all SXT/R391 ICEs required for integration/excision, conjugative transfer, regulation, as well as many genes of unknown function. There are five regions considered hotspots (HS) for insertion of foreign DNA that afford specific characteristics to the bacterial host [3] [4] [5]. The HS content is variable but some ICEs occasionally share these contents. Additional variable DNA inserts (named variable regions) outside the HS are also present [5]. Differences in the HS and variable regions are responsible for ICE size variation, but mainly for specific traits, such as antimicrobial resistance [5] and fitness [6], that characterize the distinct SXT/R391 ICEs.
According to Mutreja et al [7], the 7th cholera pandemic spread from the Bay of Bengal in three independent waves of global transmission, in which waves 2 and 3 were characterized by the acquisition of SXT/R391 ICEs.
The V. cholerae O1 lineage, responsible for the Haitian cholera epidemic in 2010, harbored an ICE (ICEVchHai1) characterized by the antimicrobial resistance genes dfrA1 (trimethoprim), sul2 (sulfamethoxazole), strAB (streptomycin) and floR (chloramphenicol) [8]. V. cholerae O1 strains harboring ICEs with this same set of antimicrobial resistance genes have also been reported in India (ICEVchInd5, 1994–2005) [9] [5] and Bangladesh (ICEVchBan5, 1998) [5].
Here, we fully characterized, by whole genome sequencing, new ICEs carried by multidrug resistant V. cholerae O1 strains from Nigeria (2010) (ICEVchNig1) and Nepal (1994) (ICEVchNep1). It was demonstrated that these two ICEs shared the same gene content and gene order with ICEs (ICEVchInd5, ICEVchBan5, ICEVchHai1) previously found in a set of multidrug resistant V. cholerae O1 strains isolated in different countries throughout more than two decades. These elements corresponded to a single ICE type, named here group 1, characterized by dfrA1, sul2, strAB and floR antimicrobial resistance genes, and by unique gene content in HS4 and HS5 regions. Segments of this ICE were found in marine metagenome datasets. Therefore, here, we compiled their genomic information, revealing the real scenario of this ICE group distribution, and proposed that HS4 and HS5 regions would be useful to trace the group 1 ICE.
Results and Discussion
Using whole genome sequencing, ICEs were identified and characterized in multidrug-resistant V. cholerae O1 clinical strains from Nigeria (2010) and Nepal (1994). Surprisingly, comparing their entire genomic content and structure, we concluded that they are siblings of ICEVchHai1, ICEVchInd5 and ICEVchBan5, which were previously identified and characterized in multidrug-resistant strains causing outbreaks/epidemics in Haiti [8] [10], India [9] and Bangladesh [5], respectively. Moreover, searches in publicly available V. cholerae genomes revealed the widespread presence of this sibling ICE in strains from cholera outbreaks/epidemics in three continents over a period of more than two decades. We also identified int SXT, HS4 and HS5 sequences, belonging to these ICEs, in marine metagenome datasets.
ICEs characterization
Thirteen multidrug-resistant strains from cholera outbreaks in Nigeria (n = 12, 2009–2010) and a strain from Nepal (n = 1, 1994) were identified harboring ICEs (Table S1). V. cholerae O1 strains from Nigeria (n = 12, 2009–2010) and Nepal (n = 1, 1994) showed susceptibility to tetracycline (MIC 1 mg/L) and florfenicol (MIC≤8 mg/L), reduced susceptibility to chloramphenicol (MIC 6–8 mg/L), and resistance to both streptomycin (MIC>1024 mg/L) and trimethoprim/sulfamethoxazole (MIC>32 mg/L), which represents an increased resistance profile compared with early seventh-pandemic isolates and resembles a resistance phenotype encoded by some ICEs, such as the one harbored by epidemic V. cholerae O1 lineage from Haiti [8] [11]. These resistant strains carried the int SXT gene, indicating the presence of an ICE. Class 1 and class 2 integrons were not found in the strains carrying ICE and mutations in gyrA and parC, associated with resistance to quinolones, have been previously observed only in the strains from Nigeria [12].
A strain from Nigeria (VC833) and a strain from Nepal (VC504) were selected for ICE characterization by the whole genome sequencing approach. In general the ICEs have been named based on the species and country where they were identified [4] therefore, the ICEs characterized here were named ICEVchNig1 and ICEVchNep1. They represent the first ICEs characterized in V. cholerae O1 strains from these countries, where cholera has been epidemic and endemic for decades [12] [13].
The ICEVchNig1 and ICEVchNep1 contained the same set of 94 open reading frames (ORFs), including the core genes, the five HS and variable regions. These ICEs differed from ICEVchHai1 by only two (ICEVchNig1) and eight (ICEVchNep1) single-nucleotide polymorphism along the entire sequence. Such SNPs occurred in the intergenic regions, except by one that corresponds to a silent mutation in the pol gene coding for a DNA polymerase V. Therefore, these polymorphisms did not impact the transmissibility of these ICEs.
Aiming to access the gene content and diversity of the complete ICE sequences, a comparative genomic analysis of ICEVchNig1 and ICEVchNep1 with all ICE sequences available (db-mml.sjtu.edu.cn/ICEberg/) was performed. Considering the ICEs structure and gene content, three groups were observed distributed within epidemic V. cholerae O1 strains. The ICEs within each of these groups had similar gene content and organization (Figure 1A). There are 58 ICEs described in V. cholerae but only eight have a complete sequence available (db-mml.sjtu.edu.cn/ICEberg/). Since ICEVchBan8 does not encode an int SXT orthologous, it is not considered a member of the SXT/R391 family [5] and was not included in our analysis.
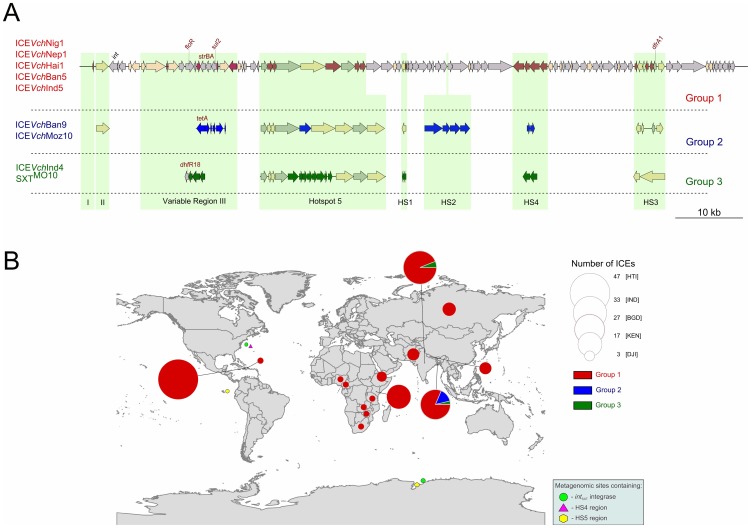
Figure 1. Structure and worldwide distribution of Integrative and Conjugative Elements (ICEs) in epidemic V. cholerae strains.
(A) The upper line represents the genetic organization of the 2010 Nigeria V. cholerae O1 ICE (Group 1). Regions of variability among previously sequenced ICEs (variable regions), and previously identified hotspots of homologous recombination (HS1–HS5) are indicated in light green shading. The core and dispensable genes are indicated by gray and cream arrows, respectively. Unique genes of type 1, 2 and 3 groups are indicated in red, blue and green arrows, respectively. Genes associated with antimicrobial drug resistance floR (chloramphenicol), strAB (streptomycin), sul2 (sulfamethoxazole), and dfrA1/dhfR18 (trimethoprim) are written in red. The ICE group 1 have the same structure and gene content in ICEVchInd5, ICEVchBan5, ICEVchNig1 and ICEVchNep1; similar occurs with group 2 (including ICEVchBan9 and ICEVchMoz10), and group 3 (including ICEVchInd4 and SXTMO10) have the same gene content and structure. (B) Spatial distribution of ICEs siblings found in epidemic V. cholerae. The circles are proportional to abundance found in epidemic V. cholerae genomes and the colors are accord to ICE group. The metagenome sites where were obtained BLAST hits are depicted.
Most of the ICEs (ICEVchNig1, Nep1, Hai1, Ban5 and Ind5) belong to group 1; the group 2 and group3 contain two other ICEs each. These groups are distinguished by different gene content in the variable regions (VR) I, II and III as well as in the HS 1–5 (Figure 1A). The variable region III and HS3 tend to accumulate most of the antimicrobial resistance genes harbored by these ICEs. The ICEs from group 1 contain in the VR-III the floR; strAB; sul2 antimicrobial resistance genes. The ICEs from group 2 and group 3 contain this same set of genes in addition to tet(A) and dfrA18, respectively (Table 1). ICEs from group 1 contain a dfrA1 gene in the HS3 region (Figure 1A). BLASTN searches were performed against the GenBank and this analysis revealed that the HS4 and HS5 nucleotide region is unique and characteristic of group 1 ICEs. The HS4 and HS5 gene content are represented by four ORFs (∼5.4 kb) and nine ORFs (15 kb), respectively and assigned by RAST [14] and PGAAP [15] mainly as hypothetical proteins. These regions could be used as markers to identify, specifically, ICEs from group 1 distribution.
Table 1. Groups of integrative and conjugative elements with similar gene content found in epidemic V. cholerae O1.
| ICE name | Host strain | Country and year of isolation | Resistance profile | Accession | References | |
| Group 1 | ||||||
| ICEVchNig1 | VC833, O1 | Nigeria, 2010 | floR, strAB, sul2, dfrA1 | KC886258 | This study | |
| ICEVchNep1 | VC504, O1 | Nepal, 1994 | floR, strAB, sul2, dfrA1 | KC886257 | This study | |
| ICEVchInd5 | Ind5, O1 | India, 1994 | floR, strAB, sul2, dfrA1 | GQ463142 | [5] | |
| ICEVchBan5 | Ban5, O1 | Bangladesh, 1998 | floR, strAB, sul2, dfrA1 | GQ463140 | [5] | |
| ICEVchHai1 | 2010EL-1786,O1 | Haiti, 2010 | floR, strAB, sul2, dfrA1 | JN648379 | [8] | |
| Group 2 | ||||||
| ICEVchBan9 | MJ1236, O1 | Bangladesh, 1994 | floR, strAB, sul2, dfrA1 a,tet(A) | CP001485 | [5] | |
| ICEVchMoz10 | B33, O1 | Mozambique, 2004 | floR, strAB, sul2, tet(A′) | ACHZ00000000 | [5] | |
| Group 3 | ||||||
| SXTMO10 | MO10, O139 | India, (1992) 2002 | dfrA18 b, floR, strAB, sul2 | AY055428 | [25] | |
| ICEVchInd4 | Ind4, O139 | India, 1997 | floR, strAB, sul2 | GQ463141 | [5] |
dfrA1 is absent in ICEVchMoz10;
dfrA18 is absent in ICEVchInd4;
NA, not available.
ICEs distribution
Most studies infer the presence of ICEs just by the antimicrobial resistance profile and identification of the int SXT gene [16] [17] [18] [19] without showing the complete ICE gene content, which actually characterizes an ICE. In order to establish the scenario of the distribution of group 1, 2 and 3 ICEs, we performed a screening of 206 V. cholerae genomes (GenBank, Mutreja et al [7] and Hendriksen et al [20] using as queries the int SXT sequence (marker of SXT/R391 ICEs), and group 1 HS4 and HS5 regions.
We identified 140 V. cholerae O1 genomes harboring the entire sequence of group 1 ICE (Table S2). So far, the group 1 ICEs are composed by the siblings: ICEVchInd5 (India, 1994–2005) [9] [5], ICEVchBan5 (Bangladesh, 1998) [5], ICEVchHai1 (Haiti, 2010) [8], ICEVchNig1 (Nigeria, 2010) and ICEVchNep1 (Nepal,1994) (this study).
The 140 group 1 ICEs are distributed among strains from India (1984–2009), Bangladesh (1991–2011), Nepal (1994–2010), Zimbabwe (2003), Zambia (2004), Kenya (2005–2009), Russia (2005–2012), Djibouti (2007), Tanzania (2009), South Africa (2009), Pakistan (2009–2010), Cameroon (2010), Nigeria (2010), Haiti (2010–2011) and the Dominican Republic (2011) (Figure 1B, Table S2), showing a widespread distribution as well as their persistence.
Of note, recently, Valia et al, 2013 [21] suggested that siblings of ICEVchInd5, prevalent in altered V. cholerae O1 strains causing epidemics in the Indian subcontinent and Haiti had spread to Africa, once they identified ICEVchAng3, sibling of ICEVchInd5, in two V. cholerae O1 from Angola (2006) [21]. Herein, the identification of ICEVchNig1, sibling of ICEVchInd5, ICEVchAng3 and ICEVchHai1, in epidemic V. O1 from Nigeria (2009–2010) shows that, in fact, West Africa is resembling the scenario that has been occurring in the Indian subcontinent and in East African countries [9] [22].
One notable factor in the ongoing evolution of pandemic cholera was the acquisition of the SXT/R391 ICE family [7]. Although the genetic relatedness of some of these ICEs were previously inferred [5] [8], our results showed a dispersion of siblings of group 1 ICE over three continents and their persistence for more than two decades. Their persistence and widespread distribution could be due to their presence in a V. cholerae O1 lineage and/or because this mobile element can easily be transferred and maintained by positive selection among V. cholerae populations. Therefore, we speculate that beyond their multidrug resistance conferred by a set of antimicrobial resistance genes, several hypothetical proteins, encoded in the HS4 and HS5 regions, could have a role in the fitness of the strains.
Metagenomic analyzes
In order to gain insights into the possible origin of HS4 and HS5 sequences, unique and exclusive regions of siblings of group 1 ICE, we searched for their presence in environmental metagenomic datasets. Hits were obtained for HS4 and HS5 ORFs as well as the int SXT gene in the Sargasso sea, a mangrove in the Fernandina Island (Galapagos) and in the Ace Lake Vestfold Hills (Antarctic) (Figure 1B and Table 2). These hits could be due to the presence of epidemic V. cholerae O1 harboring this group 1 ICEs. To test this hypothesis, we searched for the presence of the major virulence determinants of epidemic V. cholerae O1 (CTXΦ and TCP) in these environmental metagenomic datasets and no hits were obtained. Thus, SXT/R391 ICEs, HS4 and HS5 are present in marine environments away from the direct influence of a cholera pandemic area.
Table 2. BLAST search results using HS4 and HS5 ORFs from the group 1 ICE in metagenomic datasets.
| Hotspot | ORF | Length (bp) | Function | Metagenomic project | Metagenomic site | No. of hits | Hit size (bp) | Identity (%) | E-value |
| HS5 | |||||||||
| 1 | 801 | hypothetical protein | AAMa | Station 365 (Antartic) | 1 | 233 | 96 | 10−100 | |
| 2 | 549 | hypothetical protein | - | - | - | - | - | - | |
| 3 | 3690 | FIG00912888: hypothetical protein | AAM | Station 354 (Antartic) | 3 | 364 | 96 | 10−157 | |
| Station 365 (Antartic) | 348 | 93 | 10−133 | ||||||
| Station 365 (Antartic) | 530 | ||||||||
| 4 | 3741 | putative type II restriction enzyme methylase subunit | AAM | Station 236 (Antartic) | 2 | 251 | 94 | 10−102 | |
| Station 365 (Antartic) | 530 | ||||||||
| GOSb | Fernandina Island (Galapagos) | 1 | 930 | 85 | 0.0 | ||||
| 5 | 2304 | hypothetical protein | GOS | Fernandina Island (Galapagos) | 1 | 996 | 83 | 0.0 | |
| 6 | 2043 | ATP-dependent protease La | - | - | - | - | - | - | |
| 7 | 771 | hypothetical protein | - | - | - | - | - | - | |
| 8 | 816 | hypothetical protein | - | - | - | - | - | - | |
| HS4 | |||||||||
| 1 | 1617 | ATPase involved in DNA repair | - | - | - | - | - | - | |
| 2 | 762 | Transposase ISTB | GOS | Sargasso Station 11 | 1 | 756 | 97 | 0.0 | |
| 3 | 1455 | Transposase IstA | GOS | Sargasso Station 11 | 1 | 1017 | 99 | 0.0 | |
| 4 | 1080 | ATPase involved in DNA repair | - | - | - | - | - | - |
AAM, Antatic Aquatic Metagenome.
GOS, Global Ocean Sampling Expedition.
Conclusions
In the present study, the complete sequences of ICEs from Nigeria and Nepal were determined and compared to other complete ICEs from V. cholerae O1 strains. We identified three types of ICEs within epidemic V. cholerae O1, which had a similar gene content. In the further analysis, performing BLASTn searches against V. cholerae O1 genomes, we found that a type of ICEs showed widespread distribution and persistence over two decades.
There is a spatio-temporal prevalence of ICE in V. cholerae O1, carrying a set of antimicrobial resistance determinants and exclusive gene content. This element had already been identified and addressed in V. cholerae strains from four countries but our study revealed its presence in strains from more ten countries. The prevalence and dispersion of this ICE can be indicative of its role in the fitness of the current pandemic lineage. Moreover, this study revealed the scenario showing the occurrence of this ICE since the current V. cholerae ICE nomenclature is based on their geographic origin not considering their gene content. Therefore, these sibling ICEs are in epidemic V. cholerae O1, lasting for more than two decades and spreading worldwide.
Materials and Methods
Forty-one clinical V. cholerae O1 strains from the Bacteria Culture Collection of Environment and Health at the Oswaldo Cruz Foundation, FIOCRUZ were included in this study. These strains were storage in MicroBank beads (ProLab Diagnostics) at −70°C. All of them were screened for the presence of genetic elements associated with antimicrobial resistance, such as ICEs, class 1 and class 2 integrons [12].
Susceptibility testing was performed by the disc diffusion method according to the CLSI guidelines [23] using the following commercial antibiotics: erythromycin (15 µg), gentamicin (10 µg), amikacin (30 µg), sulfonamide (300 µg), sulfamethoxazole (25 µg), trimethoprim/sulfamethoxazole (30 µg), trimethoprim (5 µg), chloramphenicol (30 µg), tetracycline (30 µg), ampicillin (10 µg), cefoxitin (30 µg), cefuroxime (30 µg), ceftazidime (30 µg), streptomycin (10 µg), spectinomycin (100 µg), azithromycin (15 µg), nalidixic acid (30 µg), ciprofloxacin (5 µg). The MICs of tetracycline, chloramphenicol, streptomycin and trimethoprim/sulfamethoxazole were also determined by using E-test Strips (BioMerieux). The MIC of florfenicol (Sigma) was addressed by using the agar dilution method [23] on Mueller-Hinton plates containing varying concentrations of this antibiotic ranging from 4 mg/L to 32 mg/L. In this test, the inoculum was brought to an optical density of 1.0 at a wavelength of 610 nm (∼1×108 bacterial cells/ml) according to the McFarland scale. When no interpretive criteria for V. cholerae were available based on CLSI guidelines, breakpoints for enterobacteriaceae were applied.
The ICEs gene content and insertion site on the chromosome were determined based on the whole-genome sequencing of VC833 (Nigeria, 2009) and VC504 (Nepal, 1994) strains. Single-end 454 pyrosequencing (GS-Junior from Roche Diagnostics, USA) from the high-throughput platform of Oswaldo Cruz Institute/FIOCRUZ. The reads were assembled by using Newbler (Roche Diagnostics) and UGENE v1.9.8 [24] software. Custom primer walking by using Sanger sequencing closed the gaps of the ICE region. The recovered ICEs were annotated by RAST [14] and PGAAP software [15]. The nucleotide sequences of the identified ICEs were deposited in GenBank under accession numbers KC886258 and KC886257.
Complete ICE sequences were retrieved from the ICEberg database (db-mml.sjtu.edu.cn/ICEberg/), a web-based resource for integrative and conjugative elements found in bacteria. In the ICEberg site, there are 58 V. cholerae ICEs described, but only eight of these have entire ICE sequences. So, these eight ICE sequences were used for comparison with the ICEVchNig1 and ICEVchNep1. The ICE distribution was addressed using HS4, HS5 and int SXT as queries in BLASTN searches against V. cholerae genomes from Genbank, Sequence Reads Archive (SRA) [20] and European Nucleotide Archive (ENA) [7]. Moreover, we performed searches against CAMERA metagenome database (camera.calit2.net/) with a similarity threshold based on an e-value below to 1e-20 (April 2014).
Supporting Information
V. cholerae O1 strains from the Bacteria Culture Collection of Environment and Health at the Oswaldo Cruz Foundation, FIOCRUZ screened in this study.
(DOC)
V. cholerae O1 genomes harboring the group 1 ICE.
(DOC)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Instituto Oswaldo Cruz grant (PROEP), FAPERJ, CAPES, CAPES-PNPD, CNPq and FIOCRUZ fellowships. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kitaoka M, Miyata ST, Unterweger D, Pukatzki S (2011) Antibiotic resistance mechanisms of Vibrio cholerae. J Med Microbiol 60: 397–407 Available: http://www.ncbi.nlm.nih.gov/pubmed/21252269. Accessed 4 July 2013. [DOI] [PubMed] [Google Scholar]
- 2. Burrus V, Waldor MK (2004) Shaping bacterial genomes with integrative and conjugative elements. Res Microbiol 155: 376–386 Available: http://www.ncbi.nlm.nih.gov/pubmed/15207870. Accessed 4 July 2013. [DOI] [PubMed] [Google Scholar]
- 3. Beaber JW, Burrus V, Hochhut B, Waldor MK (2002) Comparison of SXT and R391, two conjugative integrating elements: definition of a genetic backbone for the mobilization of resistance determinants. Cell Mol Life Sci 59: 2065–2070 Available: http://www.ncbi.nlm.nih.gov/pubmed/12568332. Accessed 4 July 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burrus V, Marrero J, Waldor MK (2006) The current ICE age: biology and evolution of SXT-related integrating conjugative elements. Plasmid 55: 173–183 Available: http://www.ncbi.nlm.nih.gov/pubmed/16530834. Accessed 12 March 2013. [DOI] [PubMed] [Google Scholar]
- 5. Wozniak RAF, Fouts DE, Spagnoletti M, Colombo MM, Ceccarelli D, et al. (2009) Comparative ICE genomics: insights into the evolution of the SXT/R391 family of ICEs. PLoS Genet 5: e1000786 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2791158&tool=pmcentrez&rendertype=abstract. Accessed 2 September 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Balado M, Lemos ML, Osorio CR (2013) Integrating conjugative elements of the SXT/R391 family from fish-isolated Vibrios encode restriction-modification systems that confer resistance to bacteriophages. FEMS Microbiol Ecol 83: 457–467 Available: http://www.ncbi.nlm.nih.gov/pubmed/22974320. Accessed 4 July 2013. [DOI] [PubMed] [Google Scholar]
- 7. Mutreja A, Kim DW, Thomson NR, Connor TR, Lee JH, et al. (2011) Evidence for several waves of global transmission in the seventh cholera pandemic. Nature 477: 462–465 Available: http://www.nature.com/doifinder/10.1038/nature10392. Accessed 18 February 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sjölund-Karlsson M, Reimer A, Folster JP, Walker M, Dahourou GA, et al. (2011) Drug-resistance mechanisms in Vibrio cholerae O1 outbreak strain, Haiti, 2010. Emerg Infect Dis 17: 2151–2154 Available: http://www.ncbi.nlm.nih.gov/pubmed/22141136. Accessed 11 July 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ceccarelli D, Spagnoletti M, Bacciu D, Danin-Poleg Y, Mendiratta DK, et al. (2011) ICEVchInd5 is prevalent in epidemic Vibrio cholerae O1 El Tor strains isolated in India. Int J Med Microbiol IJMM 301: 318–324 Available: http://www.ncbi.nlm.nih.gov/pubmed/21276749. Accessed 7 May 2013. [DOI] [PubMed] [Google Scholar]
- 10. Ceccarelli D, Spagnoletti M, Cappuccinelli P, Burrus V, Colombo MM (2011) Origin of Vibrio cholerae in Haiti. Lancet Infect Dis 11: 262 Available: http://www.ncbi.nlm.nih.gov/pubmed/21453867. Accessed 2011 Jul 18. [DOI] [PubMed] [Google Scholar]
- 11. Talkington D, Bopp C, Tarr C, Parsons MB, Dahourou G, et al. (2011) Characterization of Toxigenic Vibrio cholerae from Haiti, 2010–2011. Emerg Infect Dis 17: 2122–2129 Available: http://www.ncbi.nlm.nih.gov/pubmed/22099116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marin MA, Thompson CC, Freitas FS, Fonseca EL, Aboderin aO, et al. (2013) Cholera outbreaks in Nigeria are associated with multidrug resistant atypical El Tor and non-O1/non-O139 Vibrio cholerae. PLoS Negl Trop Dis 7: e2049 Available: http://dx.plos.org/10.1371/journal.pntd.0002049. Accessed 2013 Apr 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reimer AR, Domselaar GVan, Stroika S, Walker M, Kent H, et al. (2011) Comparative Genomics of Vibrio cholerae from Haiti, Asia, and Africa. Emerg Infect Dis 17: 2113–2121 Available: http://www.ncbi.nlm.nih.gov/pubmed/22099115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, et al. (2008) The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9: 75 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2265698&tool=pmcentrez&rendertype=abstract. Accessed 2013 Mar 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Angiuoli SV, Gussman A, Klimke W, Cochrane G, Field D, et al. (2008) Toward an online repository of Standard Operating Procedures (SOPs) for (meta)genomic annotation. OMICS 12: 137–141 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3196215&tool=pmcentrez&rendertype=abstract. Accessed 2013 Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pugliese N, Maimone F, Scrascia M, Materu SF, Pazzani C (2009) SXT-related integrating conjugative element and IncC plasmids in Vibrio cholerae O1 strains in Eastern Africa. J Antimicrob Chemother 63: 438–442 Available: http://www.ncbi.nlm.nih.gov/pubmed/19155226. Accessed 2013 Jul 4. [DOI] [PubMed] [Google Scholar]
- 17. Opintan Ja, Newman MJ, Nsiah-Poodoh OA, Okeke IN (2008) Vibrio cholerae O1 from Accra, Ghana carrying a class 2 integron and the SXT element. J Antimicrob Chemother 62: 929–933 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2566517&tool=pmcentrez&rendertype=abstract. Accessed 2012 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amita Chowdhury SR, Thungapathra M, Ramamurthy T, Nair GB, et al. (2003) Class I integrons and SXT elements in El Tor strains isolated before and after 1992 Vibrio cholerae O139 outbreak, Calcutta, India. Emerg Infect Dis 9: 500–502 Available: http://www.ncbi.nlm.nih.gov/pubmed/19763428. Accessed 24 March 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bani S, Mastromarino PN, Ceccarelli D, Le Van A, Salvia AM, et al. (2007) Molecular characterization of ICEVchVie0 and its disappearance in Vibrio cholerae O1 strains isolated in 2003 in Vietnam. FEMS Microbiol Lett 266: 42–48 Available: http://www.ncbi.nlm.nih.gov/pubmed/17233716. Accessed 2014 Mar 25. [DOI] [PubMed] [Google Scholar]
- 20. Hendriksen RS, Price LB, Schupp JM, Gillece JD, Kaas RS, et al. (2011) Population genetics of Vibrio cholerae from Nepal in 2010: evidence on the origin of the Haitian outbreak. MBio 2: e00157–11 Available: http://mbio.asm.org/content/2/4/e00157-11.short. Accessed 2013 Mar 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Valia R, Taviani E, Spagnoletti M, Ceccarelli D, Cappuccinelli P, et al. (2013) Vibrio cholerae O1 epidemic variants in Angola: a retrospective study between 1992 and 2006. Front Microbiol 4: 354 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3842873&tool=pmcentrez&rendertype=abstract. Accessed 2014 Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Piarroux R, Faucher B (2012) Cholera epidemics in 2010: respective roles of environment, strain changes, and human-driven dissemination. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 18: 231–238 Available: http://www.ncbi.nlm.nih.gov/pubmed/22288560. [DOI] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute (2012) Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Second Informational Supplement. M100–S22. CLSI, Wayne, PA. [Google Scholar]
- 24. Okonechnikov K, Golosova O, Fursov M (2012) Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics 28: 1166–1167 Available: http://www.ncbi.nlm.nih.gov/pubmed/22368248. Accessed 2012 Dec 24. [DOI] [PubMed] [Google Scholar]
- 25. Waldor MK, Tschäpe H, Mekalanos JJ (1996) A new type of conjugative transposon encodes resistance to sulfamethoxazole, trimethoprim, and streptomycin in Vibrio cholerae O139. J Bacteriol 178: 4157–4165 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=178173&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
V. cholerae O1 strains from the Bacteria Culture Collection of Environment and Health at the Oswaldo Cruz Foundation, FIOCRUZ screened in this study.
(DOC)
V. cholerae O1 genomes harboring the group 1 ICE.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.