Abstract
Atrazine, one of the most commonly used herbicides worldwide, acts as an endocrine disruptor, but the mechanism of its action has not been characterized. In this study, we show that atrazine rapidly increases cAMP levels in cultured rat pituitary and testicular Leydig cells in a concentration-dependent manner, but less effectively than 3-isobutyl-1-methylxanthine, a competitive non-specific inhibitor of phosphodiesterases (PDEs). In forskolin (an activator of adenylyl cyclase)- and probenecid (an inhibitor of cyclic nucleotide transporters)-treated cells, but not in 3-isobutyl-1-methylxanthine-treated cells, atrazine further increased cAMP levels, indicating that inhibition of PDEs accounts for accumulation of cAMP. In contrast to cAMP, atrazine did not alter cGMP levels, further indicating that it inhibits cAMP-specific PDEs. Atrazine-induced changes in cAMP levels were sufficient to stimulate prolactin release in pituitary cells and androgen production in Leydig cells, indicating that it acts as an endocrine disrupter both in cells that secrete by exocytosis of prestored hormones and in cells that secrete by de novo hormone synthesis. Rolipram abolished the stimulatory effect of atrazine on cAMP release in both cell types, suggesting that it acts as an inhibitor of PDE4s, isoforms whose mRNA transcripts dominate in pituitary and Leydig cells together with mRNA for PDE8A. In contrast, immortalized lacto-somatotrophs showed low expression of these mRNA transcripts and several fold higher cAMP levels compared to normal pituitary cells, and atrazine was unable to further increase cAMP levels. These results indicate that atrazine acts as a general endocrine disrupter by inhibiting cAMP-specific PDE4s.
Keywords: endocrine disrupters, atrazine, pituitary cells, Leydig cells, cAMP-specific phosphodiesterases
Introduction
Atrazine (2-chloro-4-ethylamino-6-isopropyl-amino-s-triazine), one of the most commonly used herbicides worlwide (for reference see Pathak and Dikshit, 2012), affects reproductive functions in different species, including adult and larval stage amphibians (Hyaes et al., 2003, 2010), young fish (Spano et al., 2004), developing alligators (Crain et al., 1997), and peripubertal male and female rats (Stoker et al., 2000; Trentacoste et al., 2001; Friedmann, 2002, Cooper et al., 2007; Fraites et al., 2009). At the present time, very little is known about the mechanism by which atrazine exhibits its endocrine-disrupting effects. It has been reported that atrazine exerts an estrogen-like activity in ovarian cancer cells through G protein-coupled receptor 30, and this process requires transactivation of the epidermal growth factor receptor transduction pathway and the involvement of estrogen receptor alpha (Albanito et al. 2008). It has also been suggested that atrazine elicits estrogen action by up-regulating aromatase activity in human adrenocortical carcinoma H295R cells (Fan et al., 2007a, 2007b; Heneweer et al., 2004; Sanderson et al., 2000, 2001). Analogous induction profiles were observed in the human placental carcinoma cell line JEG-3 (Sanderson et al., 2002). It appears that atrazine induces human aromatase gene expression via promoter II in a steroidogenic factor 1-dependent manner, presumably acting as an artificial ligand for this factor (Fan et al., 2007a, 2007b). However, atrazine was unable to propagate similar increases in the human breast cancer cell line MCF-7 (Sanderson et al., 2002), the rat Leydig cell carcinoma R2C (Heneweer et al., 2004) or the human ovarian granulose-like tumor KGN cell line (Morinaga et al., 2004).
In rats, atrazine decreased serum and testicular testosterone levels when administered at doses of 100–200 mg/kg body weight (Stoker et al., 2000). It also inhibited luteinizing hormone (LH) and testosterone production when applied at concentrations at or above 100 mg/kg per day (Trentacoste et al., 2001). When applied by gavage in the dose of 50 mg/kg-bw/day, atrazine also reduced serum and intratesticular testosterone levels (Friedmann, 2002). Consistent with these studies, we have shown previously that prolonged in vivo exposure to atrazine down-regulated Leydig cell steroidogenesis via suppression of LH receptor gene expression, leading to inhibition of cAMP production and a severe decline in mRNA transcripts of several genes responsible for steroidogenesis (Pogrmic et al., 2009). However, short (1–3 days) in vivo treatment with atrazine stimulated ex vivo cyclic 3’, 5’-adenosine monophosphate (cAMP) and androgen production. The same stimulatory effect has been seen in in vitro conditions, when purified rat Leydig cells were treated with atrazine for 24 h, indicating that it also affects androgenesis independently of the status of LH receptors (Pogrmic-Majkic et al., 2010).
Atrazine was also reported to act as a competitive inhibitor of cyclic nucleotide phosphodiesterases (PDEs) derived from bovine hearts, leading to diminished conversion of cAMP to 5’-AMP in vitro (Roberge et al., 2004). This inhibition appeared to be tissue specific, as PDEs from liver and kidney tissues were not affected by atrazine, and in PDEs from brain homogenates, atrazine acted as a mixed inhibitor (Roberge et al., 2006). Consistent with these reports, we showed that cAMP, acting through its kinase (PKA), is involved in an acute stimulatory and sustained inhibitory effect of atrazine on testicular steroidogenesis (Pogrmic-Majkic et al., 2010).
In the present study, we focused on the role of PDEs and adenylyl cyclases in acute endocrine-disrupting effects of atrazine using rat anterior pituitary and Leydig cells in vitro. Cyclic nucleotides play important roles in endocrine functions of pituitary cells (Stojilkovic et al., 2012), which secrete pre-stored hormones by an exocytotic pathway. Only limited and contradictory information exists for the direct effects of atrazine on pituitary hormone release (Fakhouri et al., 2010; Cooper et al., 2000). We also used Leydig cells as a cell model for endocrine cells responding to agonist stimulation by secreting de novo produced hormones by a non-exocytotic pathway. Experiments were performed with intact cells during 1–6 h incubation with atrazine as well as by perifused pituitary cells with collection of samples every minute during a 30–60 min application of atrazine. These experiments revealed that atrazine acts as an inhibitor of cAMP-specific PDE4, causing facilitation of a cAMP-dependent signaling pathway.
Materials and methods
Chemicals
Atrazine, medium 199 containing Earle’s salt and L-glutamine (M199), Hanks' M199 containing 25 mM HEPES, Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 Ham with L-glutamine, 15 mM HEPES (DMEM/F12), HEPES, percoll, bovine serum albumin (BSA) fraction V, collagenase type IA, testosterone, trypan blue, DMSO, and IBMX (3-isobutyl-1-methylxanthine) were obtained from Sigma-Aldrich (St. Louis, MO); hCG (Pregnyl, 3000 IU/mg) was obtained from Organon (West Orange, NJ, USA).
Cell Cultures
Experiments were performed on anterior pituitary cells from 50 day old Sprague-Dawley rats obtained from Taconic Farm (Germantown, MD) and on Leydig cells from 51 days old Wistar rats raised in animal facility at the Department of Biology and Ecology (University of Novi Sad) under controlled environmental conditions (temperature 22 ± 2 °C and 14 h light/10 h dark) with food and water ad libitum. Experiments were approved by the NICHD Animal Care and Use Committee, and by the Ethics committee for protection and welfare of experimental animals at University of Novi Sad, respectively. Euthanasia of female rats was performed by asphyxiation with CO2 and the anterior pituitary glands were removed after decapitation. Pituitary cells were dispersed and cultured as mixed cells in M199 containing Earle’s salts, sodium bicarbonate, 10% heat-inactivated horse serum, penicillin (100 units/ml) and streptomycin (100 µg/ml). Experiments were performed 24–48 h after dispersion. Isolation and purification of Leydig cells was performed as previously described (Andric et al., 2008, Pogrmic-Majkic et al., 2010), and experiments were performed 3–4 h after dispersion.
Prolactin, androgens and cyclic nucleotide measurements
Pituitary cells (1 million per well) were plated in 24-well plates in serum-containing M199 and incubated overnight at 37 °C under 5% CO2-air and saturated humidity. Prior to experiments, cells were washed with serum-free medium and stimulated at 37 °C under 5% CO2-air and saturated humidity for 120 min if not otherwise stated. cAMP and hormone secretion was also monitored using cell column perifusion experiments. Briefly, 1.5 × 107 cells were incubated with preswollen cytodex-1 beads in 60-mm petri dishes for 18 h. The beads were then transferred to 0.5 ml chambers and perifused with Hanks' M199 containing 25 mM HEPES, 0.1% BSA, and penicillin (100 units/ml)/streptomycin (100 µg/ml) for 2.5 h at a flow rate of 0.5 ml/min and at 37 °C to establish stable basal secretion. Perifusion and static culture experiments were conducted with medium with or without 1 mM IBMX and different concentrations of atrazine dissolved in dimethylsulfoxide (final concentration did not exceed 0.1% in the culture medium). Static culture experiments were also conducted using medium with or without forskolin (0.1 and 1 µM) and probenecid (500 µM). Fractions were collected at 1-min intervals and later assayed for prolactin (PRL), cAMP and cyclic 3’, 5’-guanosine monophosphat (cGMP) contents using radioimmunoassay. Primary antibody and standard for PRL assay were purchased from the National Pituitary Agency and Dr. AF Parlow (Harbor-UCLA Medical Center, Torrance, CA). cAMP and cGMP were determined using specific antiserum provided by Albert Baukal (NICHD, Bethesda, MD). 125I-PRL, 125I-cAMP, and 125I-cGMP were purchased from Perkin Elmer Life Sciences (Boston, MA).
Leydig cells obtained individually from 4–5 rats were pooled and cultured in 4–8 replicates per experiments. Procedure of isolation and purification of Leydig cells is published previously (Andric et al., 2008; Pogrmic et al., 2009). Cells were allowed 3 h to attach to 24-well plates (2.5×105 cells/0.5 ml culture medium/well) and then cultured in M199–0.1% BSA with a low subsaturated dose of hCG (0.25 ng/ml) for 2 h in the absence or presence of different concentrations of atrazine, 1 mM IBMX, and/or forskolin in 0.1 or 1 µM concentration. Treatment of Leydig cells with different concentrations of atrazine in the presence or absence of IBMX or forskolin was also performed in hCG-free medium, i.e., in basal conditions and in the presence of a saturated hCG concentration (10 ng/ml). Androgen levels in the incubation medium were estimated by radioimmunoassay using [1,2,6,73H(N)]-labeled testosterone from the New England Nuclear (Brussels, Belgium) and anti-testosterone serum no. 250 provided by G.D. Niswender (Colorado State University), which has high cross-reactivity with dihydrotestosterone. The amounts of cAMP accumulated in the culture medium were measured by the cAMP EIA kit, which typically displays an IC50 value of approximately 0.5 pmol/ml and a detection limit of 0.1 pmol/ml (at 80% B/B0) for acetylated cAMP samples.
qRT-PCR analysis
Total RNA from the primary pituitary, immortalized GH3 and immortalized lacto-somatotrophs was extracted using the RNeasy Mini Kit (Qiagen, Valenica, CA). Subsequently, 1 µg of total RNA was treated with DNAse I (Invitrogen, Carlsbad, CA) and reverse transcribed with SuperScript III First Strand Synthesis SuperMix for qRT-PCR (Invitrogen). Quantitative RT-PCR was performed using pre-designed Taq-Man Gene Expression Assays for rat and mouse (Applied Biosystems, Foster City, CA) using the LightCycler® TaqMan® Master Mix and the LightCycler 2.0 Real-time PCR system (Roche, Indianopolis, IN). Total RNA from Leydig cells was extracted using RNAqueous 4PCR kit, followed by DNAse I treatment (Applied Biosystem). Subsequently, 2 µg of total RNA was reverse transcribed into cDNA in a 20 µl reaction mixture using High Capacity cDNA Reverse Transcription Kit (Applied Biosystem). Quantitative RT-PCR was performed to determine PDE gene expression in Leydig cells obtained from non-treated 51 day-old rats by using TaqMan Low Density Arrays for PDEs (TaqMan® Array Rat Phosphodiesterase, Applied Biosystems) and an ABI Prism 7900HT Sequence Detection System. The procedure was performed according to the TaqMan Low Density Array Protocol (Applied Biosystems). Each sample was run in duplicate for each of the four independent experiments. For both cell types, the relative expression of target genes in comparison to GAPDH as a reference gene was determined by the formula 2^(Ctgapdh – Cttarget gene) × 100 %.
Calculations
Cyclic nucleotide and PRL release by perifused pituitary cells are shown as representative traces. The static culture results for pituitary and Leydig cells are shown as the mean ± SEM of sextuplicate incubations in one of at least three similar experiments, each giving the same statistical conclusions. The data were analyzed by one-way ANOVA, followed by Duncan’s multiple-range post hoc test and t-test; p< 0.05 was considered as a significant difference. EC50 values and linear correlation were performed using KaleidaGraph program (Reading, PA).
Results
Effects of atrazine on cAMP levels in pituitary cells in static culture
Intracellular cyclic nucleotide levels in cultured cells with not activated Gs and Gi/o signaling pathways reflect the balance between basal production of these messengers by adenylyl and guanylyl cyclases, the activity of PDEs (a family of enzymes responsible for the hydrolysis of cAMP and cGMP), and the activity of specific efflux pumps that transport cyclic nucleotides from the cytosol to the extracellular space (Stojilkovic et al., 2012).
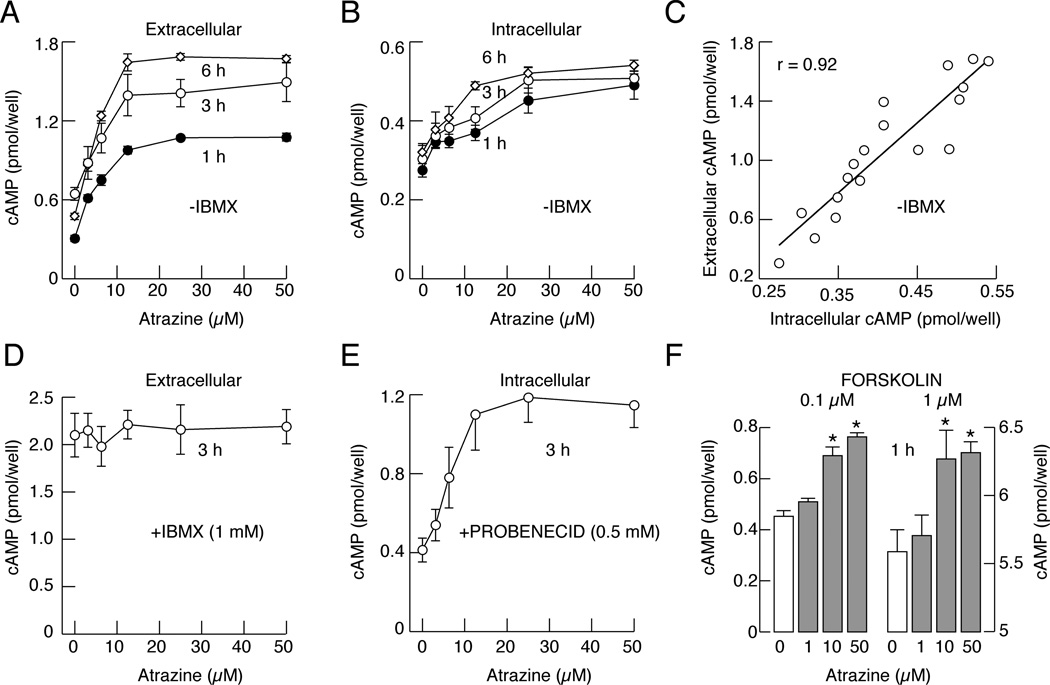
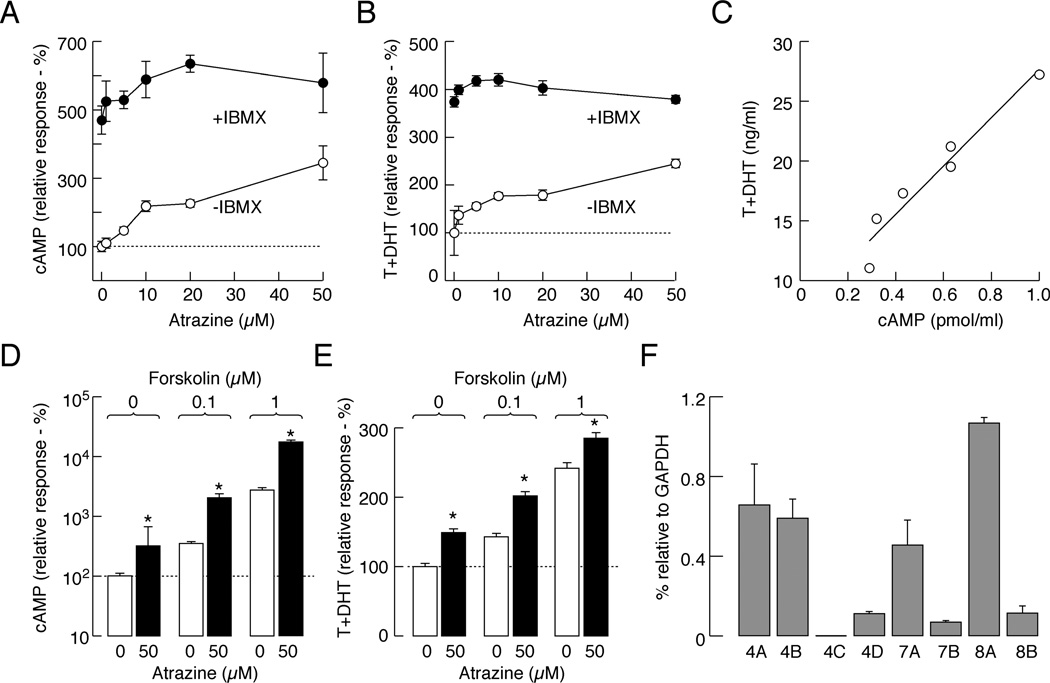
Measurements of cAMP content in medium and cell cytosol in static culture with operative and inhibited cyclic nucleotide transport provides the possibility to examine whether the activity of PDEs and/or efflux pumps are affected by atrazine. Figure 1A shows a concentration-dependent effect of atrazine on extracellular cAMP accumulation in cells bathed in IBMX-free medium for 1, 3 and 6 h, with the estimated EC50 values ranging between 5 and 6 µM at different time points. Measurements of cAMP in cell extracts also revealed a concentration-dependent increase in intracellular accumulation of this messenger (Fig. 1B), with EC50 values of 6 to 10 µM. Furthermore, there was a linear correlation between intracellular and extracellular cAMP in atrazine-treated cells (Fig. 1C). This is consistent with our earlier work showing a strong correlation between cellular content and released cAMP and cGMP in pituitary cells under other experimental conditions, indicating that the measurements of extracellular cyclic nucleotides accurately reflect the intracellular status of these messengers (Gozalez-Iglesias et al., 2006).
Fig. 1.
Atrazine inhibits PDEs in pituitary cells in static cultures. A and B, Concentration dependence of atrazine on extracellular (A) and intracellular (B) cAMP accumulation in cells bathed in medium without IBMX. C, Correlation between intracellular and extracellular cAMP levels in atrazine-treated cells. D, The lack of effects of atrazine on extracellular cAMP accumulation in cells bathed in medium containing 1 mM IBMX. E, Concentration-dependent effect of atrazine on intracellular cAMP accumulation in cells with blocked cyclic nucleotide pump by probenecid. F, Atrazine-induced increase in extracellular cAMP accumulation in cells with forskolin-stimulated adenylyl cyclase. Data are shown as the mean ± SEM from six replicates in one of three similar experiments. * p<0.01 vs. control.
In cells bathed in 1 mM IBMX-containing medium, a concentration that inhibits a majority of PDE enzymes, including the low sensitive PDE8s (IC50 about 0.6 mM; Wang et al., 2008), there was a several fold increase in extracellular cAMP levels, but atrazine was ineffective (Fig. 1D). In contrast, in cells with inhibited efflux pump by probenicide, atrazine induced a concentration-dependent increase in intracellular cAMP levels (Fig. 1E). Finally, in cells with activated adenylyl cyclase by forskolin, atrazine induced a further increase in intracellular cAMP accumulation (Fig. 1F). These data indicate that atrazine does not affect the activities of adenylyl cyclases or the cyclic nucleotide efflux pump, but it inhibits PDEs. The results further indicate that measurement of extracellular cAMP content accurately reflects the status of PDE activity in cells.
Effects of atrazine on cyclic nucleotide and PRL release in perifused pituitary cells
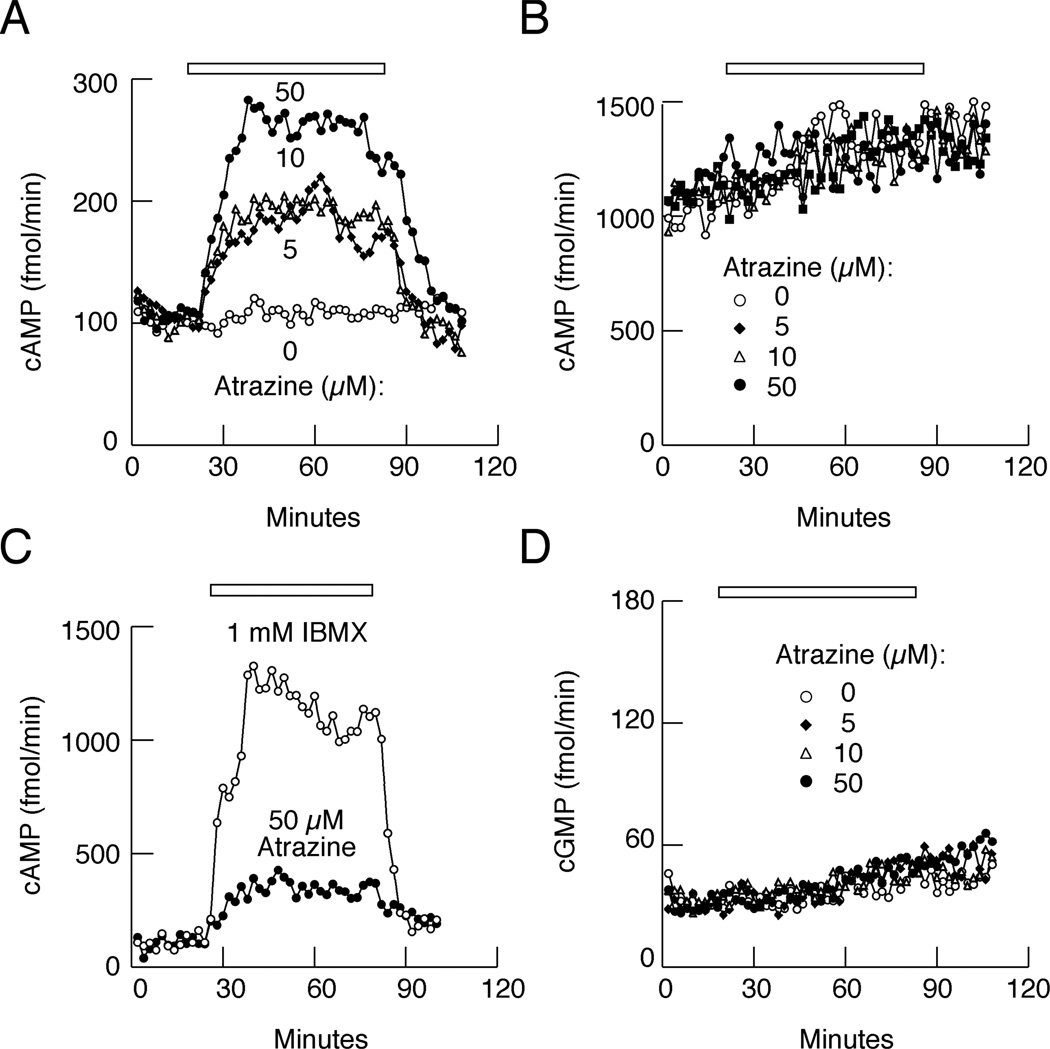
To evaluate the kinetics and concentration dependence of atrazine on cAMP release, pituitary cells were cultured on beads as described in the Materials and Methods section, loaded in perifusion chambers and washed for 2 h at a flow rate of approximately 0.5 ml per min with M199 medium with and without 1 mM IBMX to establish baseline secretion. At the end of the washing period, effluent samples were collected every minute and used to determine cAMP content. In the absence of IBMX, atrazine induced a rapid (6–8 min) and concentration-dependent elevation in cAMP release (Fig. 2A). As expected, IBMX induced a several fold increase in basal cAMP release, but the stimulatory effect of atrazine was lost in such treated cells (Fig. 2B). The time-course study further indicated that the atrazine-induced rise in cAMP release occurred at the same rate as IBMX-facilitated cAMP release, but that the amplitude of response was smaller (Fig. 2C).
Fig. 2.
Atrazine inhibits cAMP-specific PDEs in perifused pituitary cells. A, Concentration-dependent effects of atrazine on cAMP release in cells perifused with IBMX-free medium. B, The lack of effects of atrazine on cAMP release in cells bathed in 1 mM IBMX-containing medium. Notice a 10-fold increase in cAMP release compared to A. C, Comparison of effects of atrazine and IBMX on cAMP release. D, The lack of effects of atrazine on cGMP release in cells bathed in IBMX-free medium. In A, B, and D, horizontal bars indicate duration of atrazine application, and in C duration of atrazine or IBMX application. The data shown are representative of 3–4 similar experiments.
PDEs are a family of enzymes that are cGMP-specific, cAMP-specific, or degrade both messengers. To clarify whether atrazine affected cAMP alone or both messengers, we measured extracellular cGMP content from the same samples where we observed stimulatory effects of atrazine on cAMP release. Figure 2D shows the lack of effects of atrazine on cGMP release in cells perifused with IBMX-free medium. These results support the hypothesis that atrazine (between 1 and 50 µM) is less effective than IBMX because it only inhibits the activity of cAMP-specific PDEs.
Effects of atrazine on PRL release
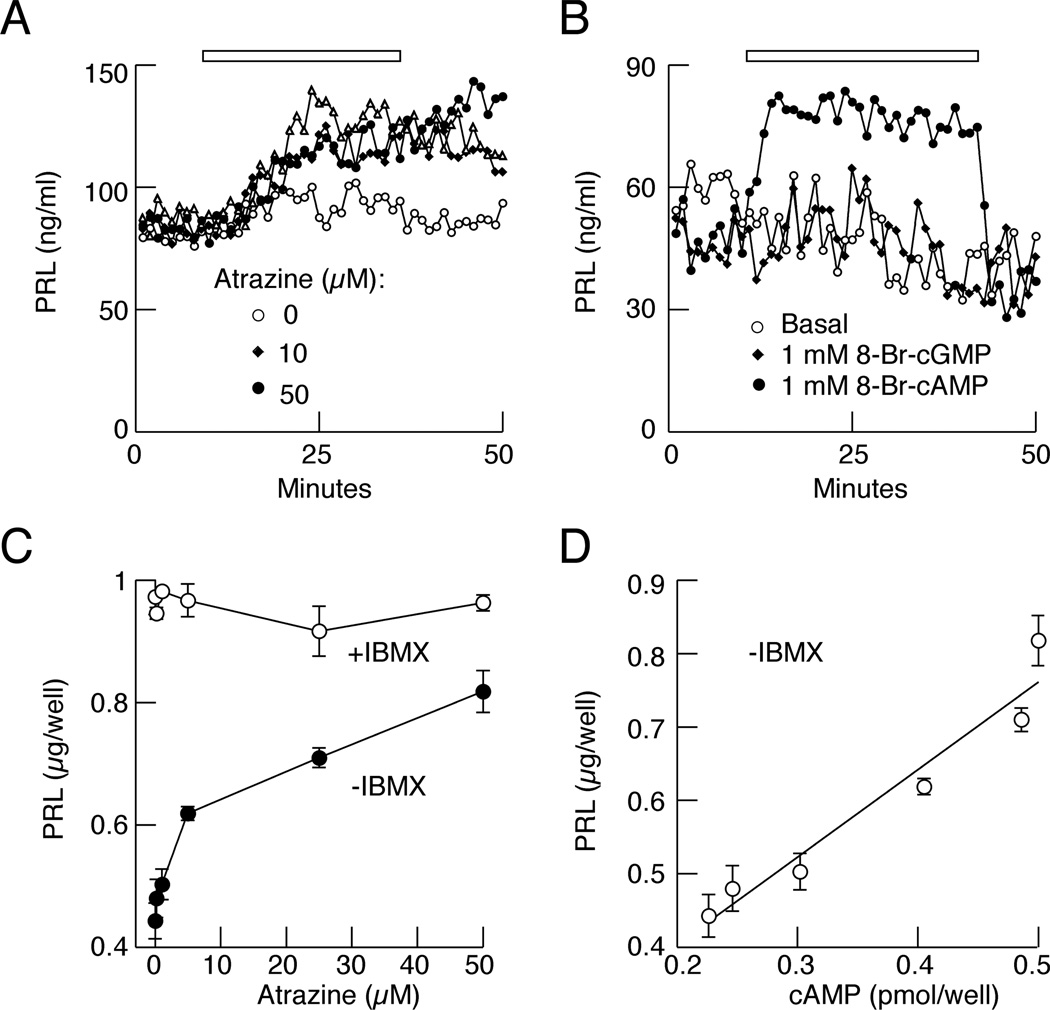
To study whether inhibition of PDEs by atrazine affects pituitary cell function, we measured basal PRL release by perifused pituitary cells in the presence and absence of atrazine. Figure 3A shows elevated basal PRL release in cells treated with 10 and 50 µM atrazine. Consistent with the hypothesis that an elevation in cAMP content accounts for stimulation of PRL release, the application of 8-Br-cAMP, a cell permeable cAMP analog, also simulated basal PRL release, whereas 8-Br-cGMP was ineffective (Fig. 3B).
Fig. 3.
Atrazine stimulates basal PRL release. A, Stimulatory effects of 10 and 50 µM atrazine on prolactin (PRL) release in perifused pituitary cells. B, Stimulatory effects of cell permeable 8-Br-cAMP but not 8-Br-cGMP on PRL release. Experiments were performed on cells bathed in IBMX-free medium and data shown are representative of three similar experiments. C, Concentration dependence of atrazine on PRL release in cells in static culture bathed in medium without IBMX and the lack of effects in cells bathed in medium with IBMX. D, Correlation between cAMP and PRL levels in cells in static cultures. Data are shown as the mean ± SEM from six replicates in one of three similar experiments.
In cells in static cultures bathed in medium without IBMX, atrazine also stimulated PRL release in a concentration-dependent manner (Fig. 3C). Measurements of cAMP and PRL levels from the same samples revealed a linear correlation between these two parameters (Fig. 3D). Furthermore, basal PRL release was elevated in IBMX-treated cells, but the stimulatory effect of atrazine was lost (Fig. 3C). Thus, atrazine is also a disrupter of pituitary endocrine function, reflecting effects of this compound on hydrolysis of cAMP by PDEs.
Pituitary cell type specificity of atrazine effects on cAMP levels
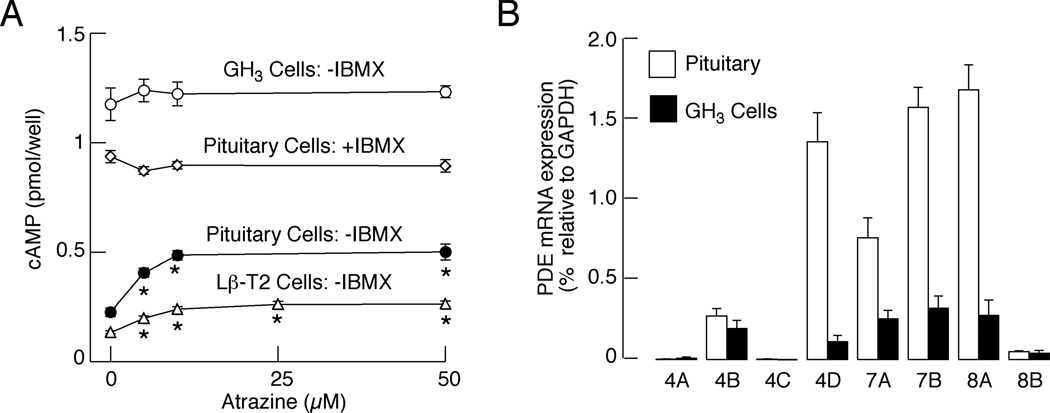
In addition to normal pituitary cells, we also used two pituitary cell lines, mouse Lβ-T2 gonadotrophs and rat GH3 lacto-somatotrophs, for studies on effects of atrazine on cAMP release. In LβT2 cells, atrazine stimulated cAMP release in a concentration-dependent manner comparable to that observed in normal pituitary cells (Fig. 4A). Surprisingly, we found that basal cAMP release is elevated in GH3 cells bathed in IBMX-free medium and higher than that observed in normal pituitary cells treated with IBMX. Furthermore, there was no further increase in cAMP release when atrazine was applied at 3 to 50 µM. We speculated that both elevated basal cAMP release and the lack of atrazine effects could reflect low expression of cAMP-specific PDEs in immortalized GH3 cells.
Fig. 4.
Expression and functional significance of cAMP-dependent PDEs in normal and immortalized pituitary cells. A, Stimulatory effect of atrazine on cAMP release by perifused LβT2 gonadotrophs and anterior pituitary cells, but not in GH3 lacto-somatotrophs, when bathed in medium without IBMX in static culture. Data are shown as the mean ± SEM from six replicates in one of three similar experiments. * p<0.01 vs. control. B, qRT-PCR analysis of expression of mRNAs for cAMP-specific PDEs in pituitary and GH3 cells. Data are shown as the mean ± SEM from five experiments.
To test this hypothesis, we estimated the expression of mRNA transcripts for cAMP-specific PDE4, PDE7, and PDE8 isoenzymes by quantitative RT-PCR. Figure 4B shows robust expression of mRNA transcripts for PDE4D, PDE7A, PDE7B, and PDE8A mRNA in normal pituitary cells. These transcripts were also found in RNAs extracted from GH3 cells, but their expression was significantly lower than in normal pituitary cells (in %: 71, 8, 32, 20 and 16 for 4B, 4D, 7A, 7B, and 8A, respectively). The mRNA for PDE4B was also present at low levels in both cell types, whereas mRNAs for PDE4A, PDE4C, and PDE8B were practically absent. These findings are consistent with the hypothesis that the lack of effects of atrazine on cAMP release in GH3 cells reflects low expression of cAMP-specific PDEs in these cells.
Atrazine inhibits PDE4 isoforms
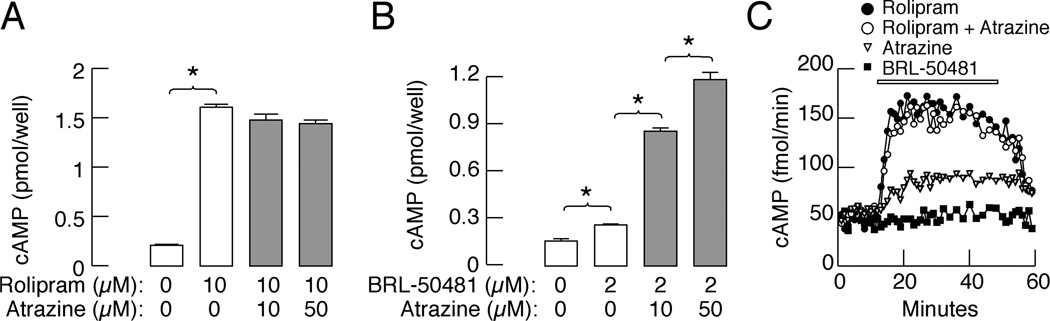
To specify which isoform of cAMP-specific PDE is a target for atrazine, pituitary cells were treated with rolipram, a PDE4-specific inhibitor, and BRL-50481, a PDE7-specific inhibitor. In cells in static culture bathed in IBMX-free medium supplemented with 10 µM rolipram, there was a significant increase in cAMP release (7.6 fold), indicating that this group of isoenzymes plays a critical role in the control of basal (in the absence of an agonist) intracellular cAMP levels (Fig. 5A). The stimulatory effect of atrazine (10 and 50 µM) on cAMP release was abolished in the presence of rolipram, further indicating that rolipram and atrazine affect PDE4 activity. In contrast to rolipram, BRL-50481 treatment increased cAMP release by 1.4-fold, and in the presence of this inhibitor, atrazine further increased cAMP release in a concentration-dependent manner (Fig. 5B). The time course study using perifused pituitary cells revealed a rapid increase in cAMP release during rolipram application. Atrazine alone also elevated cAMP release but less potently than rolipram, and in the presence of rolipram, atrazine did not further elevate cAMP release. In contrast, only a minor increase in cAMP release was observed in BRL-50481-treated cells (Fig. 5C), indicating that the contribution of PDE7s to the control of basal intracellular cAMP levels is minor.
Fig. 5.
B, Atrazine selectively inhibits PDE4 in pituitary cells. A and B, Concentration dependence of atrazine on cAMP release in the presence of BRL-50481, a PDE7 inhibitor (B), but not in the presence of rolipram, a PDE4 inhibitor (A). Data are shown as the mean ± SEM from six replicates in one of three similar experiments. C, Time course of rolipram and atrazine-induced cAMP release. Notice the lack of effects of atrazine on cAMP release in the presence of rolipram and of BRL-50481 alone. Data are shown as the mean ± SEM from six replicates in one of three similar experiments. * p<0.01 vs. control.
Effects of atrazine on hCG-stimulated cAMP and androgen production by Leydig cells
In further experiments, we studied whether the endocrine-disrupting action of atrazine is specific for cells that secrete by releasing pre-stored hormones, i.e., the pituitary lactotrophs that secrete PRL by the exocytotic pathway, or if this action also occurs in endocrine cells that secrete by de novo synthesis of hormones, i.e., rat testicular Leydig cells which express the Gs-coupled LH receptors and secrete androgens. To activate these receptors, we stimulated Leydig cells with a low concentration of hCG (0.25 ng/ml) for 2 h in the absence or presence of IBMX. In the absence of IBMX, atrazine stimulated cAMP accumulation and androgen production in a concentration-dependent manner (Fig. 6A and B) and there was a linear correlation between cAMP and androgen levels (Fig. 6C). As in pituitary cells, cAMP and androgen levels were significantly increased in the presence of 1 mM IBMX (cAMP: 4.7 times; T+DHT: 3.7 times), but the stimulatory action of atrazine was practically lost. Moreover, in Leydig cells with forskolin-activated adenylyl cyclase, atrazine induced further increases in extracellular cAMP and androgen accumulation (Fig. 6D and E). The effects of atrazine in IBMX- and forskolin-treated Leydig cells bathed in hCG-free medium (basal conditions) were similar to those obtained in the presence of low-dose hCG. However, in the presence of a saturated concentration of hCG, atrazine did not stimulate androgen production (data not shown).
Fig. 6.
Atrazine stimulates hCG-supported cAMP and androgen production in Leydig cells. A and B, Concentration-dependent effects of atrazine on cAMP (A) and androgen (B) accumulation in the incubation medium of Leydig cells. Notice the lack of effects of atrazine on cAMP and androgen release in the presence of 1 mM IBMX. C, Correlation between cAMP and androgen levels in the absence of IBMX. D and E, Atrazine-induced increase in cAMP accumulation (D) and androgen production (E) in forskolin-stimulated Leydig cells. Data are shown as the mean ± SEM from six replicates in one of three similar experiments * p<0.05 vs. control. F, qRT-PCR analysis of expression of mRNAs for cAMP-specific PDEs in Leydig cells. Data are shown as the mean ± SEM from four experiments.
As in pituitary cells, mRNA transcripts for cAMP-specific PDEs were also present in Leydig cells, including the rolipram-sensitive PDE4s and the BRL-50481-sensitive PDE7 isoenzymes (Fig. 6F). This provides a rationale for a concentration dependent effect of atrazine on cAMP levels in agonist stimulated Leydig cells. The relative expression of PDE7A and PDE8A was comparable in both cells types, whereas PDE4A and PDE4B dominate in Leydig cells vs. PDE4D in pituitary cells. Pituitary cells, but not Leydig cells, also highly express mRNA transcripts for PDE7B (Fig. 4B vs. 6F). Like in pituitary cells, the stimulatory effect of 50 µM atrazine on hCG-stimulated cAMP accumulation was abolished in the presence of 10 µM rolipram (data not shown). Atrazine-simulated T+DHT production was also abolished in Leydig cells in the presence of rolipram (Table 1). These findings suggest that atrazine inhibits different subtypes of PDE4 isoenzymes.
Table 1.
The lack of effect of atrazine on hCG-stimulated T+DHT production in Leydig cells in the presence of 10 µM rolipram. Data shown are the mean ± SEM.
| Atrazine (µM) | hCG-stimulated T+DHT production (ng/ml) | |
|---|---|---|
| − rolipram | + rolipram | |
| 0 | 74±10 | 110±5 |
| 50 | 128±11 | 104+4 |
Discussion
Intracellular cyclic nucleotide concentrations reflect the balance between de novo production of these messengers by adenylyl and guanylyl cyclases and activities of cyclic nucleotide PDEs. PDEs are a large family of enzymes responsible for the hydrolysis of cAMP and cGMP, and cyclic nucleotide efflux pumps that transport cyclic nucleotides from cytosol to the extracellular fluid (Stojilkovic et al., 2012). Here we show that atrazine, an established endocrine disrupter, elevates cAMP levels in anterior pituitary cells and Leydig cells in vitro in a concentration-dependent manner. This response was lost in IBMX-treated cells, but not in cells with either inhibited cyclic nucleotide efflux pumps by probenicide or stimulated adenylyl cyclases by forskolin. Thus, it is reasonable to conclude that atrazine inhibits PDEs, not only in tissue extracts, as has been previously shown (Roberge et al., 2004, 2006), but also in intact cells.
Mammalian cells contain more than 50 cyclic nucleotide PDEs that are classified into 11 families based on their amino acid sequences, substrate specificities, allosteric regulatory characteristics, and pharmacological properties. These diverse PDEs share a modular architecture, with a conserved central catalytic domain, N- and C-terminal regulatory domains, and an N-terminal targeting domain. The substrate specificities of the PDEs families include cAMP-specific (PDE4, PDE7, and PDE8), cGMP-specific (PDE5, PDE6, and PDE9), and mixed specificity enzymes (PDE1, PDE2, PDE3, PDE10, and PDE11) (Bender and Beavo, 2006).
In our experiments, the time course of atrazine-induced cAMP release was comparable to that induced by IBMX, a nonselective blocker of PDEs in concentrations used (Beavo, 1995), but the peak amplitude of response was smaller. Because atrazine was unable to facilitate cGMP release, in contrast to IBMX, which also inhibits mixed specificity enzymes PDE1, PDE2, PDE3, PDE10, and PDE11, it was reasonable to conclude that atrazine affects cAMP-specific PDEs. Consistent with this, our qRT-PCR analysis indicated the expression of cAMP-specific enzymes in order: PDE8A ≥ PDE7B ≥ PDE4D > PDE7A >> PDE4B in anterior pituitary cells and PDE8A > PDE4A ≥ PDE4B ≥ PDE7A in Leydig cells.
In general, the contribution of cAMP-specific PDEs could be analyzed by siRNA technology, but the efficacy of transient transfection of primary pituitary cells was very low. For that reason, we used immortalized |GH3 pituitary cells, which are easily transfectable. Fortunately, these cells poorly express cAMP-specific PDEs, indicating that they represent a natural system for testing the specificity of atrazine for cAMP-specific PDEs. In these cells, basal cAMP release was much higher than in normal pituitary cells, and atrazine was practically ineffective in elevating cAMP release, confirming the hypothesis.
The contribution of these enzymes in control of cAMP signaling was further evaluated pharmacologically. Rolipram is a specific inhibitor of PDE4s (Shimizu-Albergine et al., 2012) that has helped to clarify the role of these enzymes in inflammatory diseases (Castro et al., 2005). Previous experiments suggested that PDE4s play important roles in the control of cAMP levels in agonist-stimulated pituitary corticotrophs (Ang and Antoni, 2002). Our results suggest that atrazine is a PDE4-specific inhibitor because in the presence of rolipram, the effect of atrazine on cAMP production was abolished. Specificity of atrazine toward PDE4 is further supported by the findings that the atrazine effect on cAMP production in pituitary cells is present in cells treated with BRL-50481, a specific inhibitor of PDE7, whose mRNA transcripts are also abundant in pituitary cells together with PDE4D and PDE8A.
Because the mRNA transcripts for PDE4A and B are also present in normal Leydig cells, and selectivity of atrazine to PDE4 is also established in these cells, we concluded that PDE4 isoforms are also a target of atrazine in Leydig cells. Both cell types also express PDE8A, which could also contribute to atrazine action. According to Shimizu-Albergine et al. (2012), PDE8s in mouse Leydig cells control steroidogenesis at the resting basal state and at low-to-moderate agonist stimulation, but fail to do so at high agonist stimulation. In this study, we did not investigate the possible effect of atrazine on PDE8A in pituitary and Leydig cells, because PF-04957325, a specific blocker of PDE8A (Shimizu-Albergine et al., 2012), is not commercially available.
There are several reports indicating than atrazine affects pituitary secretory function in vivo. It appears that LH release was inhibited by atrazine, reflecting altered GnRH secretion and steroid hormone actions in the hypothalamus (Trentacoste et al., 2001; Ashby et al., 2002; McMullin et al., 2004). It has also been suggested that atrazine exhibits toxic effects on the nervous system and on the induction of mammary tumors, reflecting altered expression of PRL (Missale et al., 1996; Sagrillo and Elmanoff, 1988; O’Connor et al., 2000). However, variable effects of atrazine on PRL release have been reported (Stoker et al., 1999; O’Conner et al., 2000; Cooper et al. 2000). In vitro, atrazine was reported to inhibit the expression of mRNA and protein transcripts for growth hormone and LH, whereas both transcripts for PRL were elevated (Fakhouri et al., 2010). Others reported no effects of 100 µM atrazine on basal and agonist-stimulated LH and PRL release (Cooper et al., 2000). Consistent with the first report, here we show that atrazine elevated basal PRL release both in perifused and pituitary cells in static cultures. We further correlate cAMP and PRL levels in atrazine treated cells.
The dual effects of atrazine, elevation in intracellular cAMP concentration and facilitation of basal PRL release, are in accordance with our knowledge about stimulus secretion coupling. Lactotrophs secrete by exocytosis, and spontaneous electrical activity and accompanied voltage-gated calcium influx are sufficient to trigger fusion of secretory vesicles and release of PRL (Stojilkovic et al., 2012). Spontaneous electrical activity, calcium signaling, and PRL release in lactotrophs are facilitated by cAMP in a PKA-dependent and -independent manner (Stojilkovic et al., 2012). Additionally, the potential role of cAMP on PRL release downstream of calcium signaling should not be excluded (Sikdar et al., 1990). In contrast to lactotrophs, Leydig cells do not pre-store hormones, but activation of LH receptors causes de novo androgen synthesis and release. The ability of atrazine to stimulate both cAMP and androgen production is consistent with the PKA dependence of androgenesis (Payne and Youngblood, 1995; Pogrmic-Majkic et al., 2010).
In summary, we show for the first time that atrazine is an inhibitor of cAMP-specific PDEs, leading to elevated intracellular and extracellular cAMP accumulation and, presumably, PKA activation. The elevation in intracellular cAMP levels was sufficient to enhance basal PRL release in pituitary cells and hCG-stimulated androgen production in Leydig cells. Thus, atrazine acts as endocrine disrupter both in cells that secrete by exocytosis of pre-stored hormones and in cells that secrete by de novo hormone synthesis. These effects are rapid, and no change in protein expression is required. Because cAMP/PKA is also involved in the control of transcriptional activity, it is also reasonable to speculate that atrazine also exhibits sustained effects on variable signaling pathways. Additionally, this may account for the rapid and sustained in vivo effects of atrazine. Finally, the finding that atrazine specifically affects cAMP-specific PDE4 isoforms in pituitary cells could provide a potential clue for next-generation PDE inhibitors targeting predominantly this subgroup of enzymes.
Acknowledgments
We are grateful to Dr. G. D. Niswender for the supply of testosterone antiserum. This work is supported by the Ministry of Science, Republic of Serbia (grant no. 173037), and a Eunce Kennedy Shiver National Institute of Health and Human Development intramural grant.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Andric N, Kostic T, Kaisarevic S, Fa S, Pogrmic K, Kovacevic R. In vivo and in vitro effects of PCB126 and PCB153 on rat testicular androgenesis. Environ. Toxicol. Pharmacol. 2008;25:222–226. doi: 10.1016/j.etap.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Ang KL, Antoni FA. Functional plasticity of cyclic AMP hydrolysis in rat adenohypophysial corticotroph cells. Cell. Signal. 2002;14:445–452. doi: 10.1016/s0898-6568(01)00267-4. [DOI] [PubMed] [Google Scholar]
- Ashby J, Tinwell H, Stevens J, Pastoor T, Breckenridge CB. The effects of atrazine on the sexual maturation of female rats. Regul. Toxicol. Pharmacol. 2002;35:468–473. doi: 10.1006/rtph.2002.1571. [DOI] [PubMed] [Google Scholar]
- Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol. Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol. Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- Castro A, Jerez MJ, Gil C, Martinez A. Cyclic nucleotide phosphodiesterases and their role in immunomodulatory responses: advances in the development of specific phosphodiesterase inhibitors. Med. Res. Rev. 2005;25:229–244. doi: 10.1002/med.20020. [DOI] [PubMed] [Google Scholar]
- Connor K, Howell J, Chen I, Liu H, Berhane K, Sciarretta C, et al. Failure of chloro-S-triazine derived compounds to induce estrogenic receptor-mediated responses in vivo and in vitro. Fundam. Appl. Toxicol. 1996;30:93–101. [PubMed] [Google Scholar]
- Cooper RL, Stoker TE, Tyrey L, Goldman JM, McElroy WK. Atrazine disrupts the hypothalamic control of pituitary-ovarian function. Toxicol. Sci. 2000;53:297–307. doi: 10.1093/toxsci/53.2.297. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Laws SC, Das PC, Norotsky MG, Goldman JM, Tyrey EL, Stoker TE. Atrazine and reproductive function: mode and mechanism of action studies. Birth Defects Res. B Dev. Reprod. Toxicol. 2007;80:98–112. doi: 10.1002/bdrb.20110. [DOI] [PubMed] [Google Scholar]
- Crain DA, Guillette LJJr, Rooney AA, Pickford DB. Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ. Health Perspect. 1997;105:528–533. doi: 10.1289/ehp.97105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan WQ, Yanase T, Morinaga H, Shigeki, Gondo S, Okabe T, Nomura M, Hayes TB, Takayanagi R, Hajime, Nawata H. Herbicide atrazine activates SF-1 by direct affinity and concomitant co-activators recruitments to induce aromatase expression via promoter II. Biochem. Biophys. Res. Commun. 2007;355:1012–1018. doi: 10.1016/j.bbrc.2007.02.062. [DOI] [PubMed] [Google Scholar]
- Fakhouri WD, Nunez JL, Trail F. Atrazine binds to the growth hormone releasing hormone receptor and affects growth hormone gene expression. Environ. Health Perspect. 2010;118:1400–1405. doi: 10.1289/ehp.0900738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraites MJ, Cooper RL, Buckalew A, Jayaraman S, Mills L, Laws SC. Characterization of the hypothalamic-pituitary-adrenal axis response to atrazine and metabolites in the female rat. Toxicol. Sci. 2009;112:88–99. doi: 10.1093/toxsci/kfp194. [DOI] [PubMed] [Google Scholar]
- Friedmann AS. Atrazine inhibition of testosterone production in rat males following peripubertal exposure. Reprod. Toxicol. 2002;16:275–279. doi: 10.1016/s0890-6238(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Hayes T, Haston K, Tsui M, Hoang A, Haeffele C, Vonk A. Atrazine-induced hermaphroditism at 0.1 ppb in American leopard frogs (Rana pipiens): laboratory and field evidence. Environ. Health Perspect. 2003;111:568–575. doi: 10.1289/ehp.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes TB, Khoury V, Narayan A, Nazir M, Park A, Brown T, Adame L, Chan E, Buchholz D, Stueve T, Gallipeau S. Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis) Proc. Natl. Acad. Sci. U S A. 2010;107:4612–4617. doi: 10.1073/pnas.0909519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneweer M, Van der Berg M, Sanderson T. A comparison of human H295R and rat R2C cell lines as in vitro screening tools for effects on aromatase. Toxicol. Lett. 2004;146:183–194. doi: 10.1016/j.toxlet.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Iglesias AE, Jiang Y, Tomic M, Kretschmannova K, Andric SA, Zemkova H, Stojilkovic SS. Dependence of electrical activity and calcium influx-controlled prolactin release on adenylyl cyclase signaling pathway in pituitary lactotrophs. Mol. Endocrinol. 2006;20:2231–2246. doi: 10.1210/me.2005-0363. [DOI] [PubMed] [Google Scholar]
- McMullin TS, Andersen ME, Nagahara A, Lund TD, Pak T, Handa RJ, et al. Evidence that atrazine and diaminochlorotirazine inhibit the estrogen/progesterone induced surge of luteinizing hormone in female Sprague-Dawley rats without changing estrogen reception action. Toxicol. Sci. 2004;79:278–286. doi: 10.1093/toxsci/kfh127. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Plowchalk DR, Van Pelt CS, Davis LG, Cook JC. Role of prolactin in chloro-S-triazine rat mamm ary tumorigenesis. Drug Chem. Toxicol. 2000;23:575–601. doi: 10.1081/dct-100101972. [DOI] [PubMed] [Google Scholar]
- Pathak RK, Dikshit AK. Atrazine and its Use. Int. J. Res. Chem. Environ. 2012;2:1–6. [Google Scholar]
- Payne AH, Youngblood GL. Regulation of expression of steroidogenic enzymes in Leydig cells. Biol. Reprod. 1995;52:217–225. doi: 10.1095/biolreprod52.2.217. [DOI] [PubMed] [Google Scholar]
- Pogrmic K, Fa S, Dakic V, Kaisarevic S, Kovacevic R. Atrazine oral exposure of peripubertal male rats down-regulates steroidogenesis gene expression in Leydig Cells. Toxicol. Sci. 2009;111:189–197. doi: 10.1093/toxsci/kfp135. [DOI] [PubMed] [Google Scholar]
- Pogrmic-Majkic K, Fa S, Dakic V, Kaisarevic S, Kovacevic R. Up-regulation of peripubertal rat Leydig cell steroidogenesis following 24 hour in vitro and in vivo exposure to atrazine. Toxicol. Sci. 2010;118:52–60. doi: 10.1093/toxsci/kfq227. [DOI] [PubMed] [Google Scholar]
- Roberge M, Hakk H, Larsen G. Atrazine is a competitive inhibitor of phosphodiesterase but does not affect the estrogen receptor. Toxicol. Lett. 2004;154:61–68. doi: 10.1016/j.toxlet.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Roberge MT, Hakk H, Larsen G. Cytosolic and localized inhibition of phosphodiesterase by atrazine in swine tissue homogenates. Food Chem. Toxicol. 2006;44:885–890. doi: 10.1016/j.fct.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Sagrillo CA, Elmanoff M. Effects of prolactin on expression of the mRNAs encoding the immediate early genes zif/268 (NGF1-A), nur/77 (NGF1-B), c-fos and c-jun in the hypothalamus. Mol. Brain Res. 1998;61:62–68. doi: 10.1016/s0169-328x(98)00198-3. [DOI] [PubMed] [Google Scholar]
- Sanderson JT, Seinen W, Giesy JP, Van der Berg M. 2-Chloro-s-triazine herbicides induce aromatase (CYP19) activity in H295R human adrenocortical carcinoma cells: a novel mechanism for estrogenicity? Toxicol. Sci. 2000;54:121–127. doi: 10.1093/toxsci/54.1.121. [DOI] [PubMed] [Google Scholar]
- Sanderson TJ, Boerma J, Lansbergen GWA, Berg M. Induction and inhibition of aromatase CYP19 activity by various classes of pesticides in H295R human adrenocortical carcinoma cells. Toxicol. Appl. Pharmacol. 2002;182:44–54. doi: 10.1006/taap.2002.9420. [DOI] [PubMed] [Google Scholar]
- Sikdar SK, Zorec R, Mason WT. cAMP directly facilitates Ca-induced exocytosis in bovine lactotrophs. FEBS Lett. 1990;273:150–154. doi: 10.1016/0014-5793(90)81072-v. [DOI] [PubMed] [Google Scholar]
- Shimizu-Albergine M, Tsai LCL, Patrucco E, Beavo JA. cAMP-specific phosphodiesterases 8A and 8B, essential regulators of Leydig cell steroidogenesis. Mol. Pharmacol. 2012;81:556–566. doi: 10.1124/mol.111.076125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano L, Tyler CR, van Aerle R, Devos P, Mandiki SN. Effects of atrazine on sex steroid dynamics, plasma vitellogenin concentration and gonad development in adult goldfish (Carassius auratus) Aquat. Toxicol. 2004;66:369–379. doi: 10.1016/j.aquatox.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Robinette CL, Cooper RL. Maternal exposure to atrazine during lactation suppresses sucking-induced prolactin release and results in prostatitis in the adult offspring. Toxicol. Sci. 1999;52:68–79. doi: 10.1093/toxsci/52.1.68. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol. Endocrinol. 2005;19:2647–2659. doi: 10.1210/me.2004-0532. [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS, Kretschmannova K, Tomic M, Stratakis CA. Dependence of the excitability of pituitary cells on cyclic nucleotides. J. Neuroendocrinol. 2012;24:1–18. doi: 10.1111/j.1365-2826.2012.02335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker TE, Laws SC, Guidici DL, Cooper RL. The effects of atrazine on puberty in male Wistar rats: An evaluation in the protocol for the assessment of pubertal development and thyroid function. Toxicol. Sci. 2000;58:50–59. doi: 10.1093/toxsci/58.1.50. [DOI] [PubMed] [Google Scholar]
- Trentacoste SV, Friedmann AS, Youker RT, Breckenridge CB, Zirkin BR. Atrazine effects on testosterone levels and androgen-dependent reproductive organs in peripubertal male rats. J. Androl. 2001;22:142–148. [PubMed] [Google Scholar]
- Wang H, Yan Z, Yang S, Cai J, Robinson H, Ke H. Kinetic and structural studies of phosphodiesterase-8A and implication on the inhibitor selectivity. Biochemistry. 2008;47:12760–12768. doi: 10.1021/bi801487x. [DOI] [PMC free article] [PubMed] [Google Scholar]