Summary
Background
We previously detected functional brain imaging abnormalities in young adults at genetic risk for late-onset Alzheimer’s disease (AD). Here, we sought to characterize structural and functional magnetic resonance imaging (MRI), cerebrospinal fluid (CSF), and plasma biomarker abnormalities in young adults at risk for autosomal dominant early-onset AD. Biomarker measurements were characterized and compared in presenilin 1 (PSEN1) E280A mutation carriers and non-carriers from the world’s largest known autosomal dominant early-onset AD kindred, more than two decades before the carriers’ estimated median age of 44 at the onset of mild cognitive impairment (MCI) and before their estimated age of 28 at the onset of amyloid-β (Aβ) plaque deposition.
Methods
Biomarker data for this cross-sectional study were acquired in Antioquia, Colombia between July and August, 2010. Forty-four participants from the Colombian Alzheimer’s Prevention Initiative (API) Registry had structural MRIs, functional MRIs during associative memory encoding/novel viewing and control tasks, and cognitive assessments. They included 20 mutation carriers and 24 non-carriers, who were cognitively normal, 18-26 years old and matched for their gender, age, and educational level. Twenty of the participants, including 10 mutation carriers and 10 non-carriers, had lumbar punctures and venipunctures. Primary outcome measures included task-dependent hippocampal/parahippocampal activations and precuneus/posterior cingulate deactivations, regional gray matter reductions, CSF Aβ1-42, total tau and phospho-tau181 levels, and plasma Aβ1-42 levels and Aβ1-42/Aβ1-40 ratios. Structural and functional MRI data were compared using automated brain mapping algorithms and AD-related search regions. Cognitive and fluid biomarkers were compared using Mann-Whitney tests.
Findings
The mutation carrier and non-carrier groups did not differ significantly in their dementia ratings, neuropsychological test scores, or proportion of apolipoprotein E (APOE) ε4 carriers. Compared to the non-carriers, carriers had higher CSF Aβ1-42 levels (p=0·008), plasma Aβ1-42 levels (p=0·01), and plasma Aβ1-42/Aβ1-40 ratios (p=0·001), consistent with Aβ1-42 overproduction. They also had greater hippocampal/parahippocampal activations (as low as p=0·008, after correction for multiple comparisons), less precuneus/posterior cingulate deactivations (as low as p=0·001, after correction), less gray matter in several regions (p-values <0·005, uncorrected, and corrected p=0·008 in the parietal search region), similar to findings in the later preclinical and clinical stages of autosomal dominant and late-onset AD.
Interpretation
Young adults at genetic risk for autosomal dominant AD have functional and structural MRI abnormalities, along with CSF and plasma biomarker findings consistent with Aβ1-42 over-production. While the extent to which the underlying brain changes are progressive or developmental remain to be determined, this study demonstrates the earliest known biomarker changes in cognitively normal people at genetic risk for autosomal dominant AD.
Funding
Banner Alzheimer’s Foundation, Nomis Foundation, Anonymous Foundation, Forget Me Not Initiative, Boston University Department of Psychology, Colciencias (1115-408-20512, 1115-545-31651), National Institute on Aging (R01 AG031581, P30 AG19610, UO1 AG024904, RO1 AG025526, RF1AG041705), National Institute of Neurological Disorders and Stroke (F31-NS078786) and state of Arizona.
Keywords: Alzheimer’s disease, biomarkers, preclinical, early-onset, dominantly inherited, MRI, functional MRI, cerebrospinal fluid, plasma, presenilin E280A mutation, amyloid, tau, genetics, prevention
Introduction
What are the earliest brain changes associated with the predisposition to Alzheimer’s disease (AD)? According to the amyloid hypothesis, the pathogenic cascade begins with accumulation of the amyloid-β1-42 (Aβ1-42) peptide (the major constituent of neuritic plaques) into oligomeric and fibrillar assemblies, leading to neuroinflammatory changes, synaptic dysfunction and loss, accumulation and phosphorylation of the microtubule-associated protein tau (the main constituent of neurofibrillary tangles), and neuronal degeneration.1 According to the prevailing biomarker model, the corresponding sequence of brain imaging and cerebrospinal fluid (CSF) changes begins about 10-15 years prior to clinical onset with biomarker evidence of amyloid plaque deposition (reduced CSF Aβ1-42 levels and increased fibrillar Aβ positron emission tomography (PET) measurements). It is followed by biomarker evidence of neuronal dysfunction and synaptic loss (e.g., regional reductions in cerebral glucose metabolism using PET and altered patterns of functional connectivity, alterations in regional brain activity during memory encoding/novel viewing tasks, and reductions in gray matter and cortical thickness using magnetic resonance imaging [MRI]). It is also followed by biomarker evidence of neurofibrillary tangles, neuronal degeneration, and neuronal loss (e.g., elevated CSF total-tau and phosphorylated tau levels and MRI measurements of hippocampal atrophy).2, 3
We previously characterized functional brain abnormalities in young adult carriers of the apolipoprotein E (APOE) ε4 allele, the major susceptibility gene for late-onset AD (LOAD),4 even before neurochemical and histopathological evidence of Aβ accumulation.5 The functional brain abnormalities were apparent almost five decades before their estimated average at clinical onset, but did not appear to progress until older ages.4 Other studies have found that young ε4 carriers have reduced regional gray matter6 and altered functional connectivity.7
More than 200 mutations of the presenilin 1 (PSEN1), presenilin 2 (PSEN2), and amyloid precursor protein (APP) genes are known to cause autosomal dominant AD with virtually certain clinical onset before the age of 65 (www.molgen.ua.ac.be/admutations/). In contrast to LOAD, autosomal dominant AD has been associated with increased Aβ1-42 production, as reflected by increases in plasma Aβ1-42 levels, and/or an increase in Aβ1-42/Aβ1-40 ratios.8-10 Still, the study of mutation carriers provides a special opportunity to characterize the preclinical biomarker changes associated with the predisposition to AD.11 We have begun to use brain imaging and CSF biomarkers to detect and track brain changes in presymptomatic PSEN1 E280A mutation carriers from the world’s largest known autosomal dominant AD kindred. Residing in Antioquia, Colombia, this kindred is estimated to have approximately 5,000 living relatives, including about 1,500 mutation carriers.12 Carriers from this kindred have estimated median ages of 44 years at the onset of mild cognitive impairment (MCI) and 49 years at the onset of dementia.13
We previously found that cognitively normal PSEN1 E280A mutation carriers have functional14 and structural brain changes15 in the later preclinical stages of AD similar to those found by others in the later preclinical stages of autosomal dominant AD16, 17 and LOAD.18, 19 Here, we compare functional and structural MRI, CSF, and plasma measurements in 18-26 year-old PSEN1 E280A mutation carriers and non-carriers. We provide imaging evidence of structural and functional brain abnormalities and fluid biomarker evidence of Aβ1-42 over-production more than two decades before their estimated average age at clinical onset. After this study was completed, we characterized the age-related trajectory of Aβ plaque deposition in a cross-sectional florbetapir PET study of 18-60 year-old mutation carriers and non-carriers from the same kindred, and we estimated the carriers’ onset Aβ plaque deposition at age 28.20 Based upon the findings from these separate studies, we suggest that cognitively normal persons at risk for autosomal dominant AD have brain imaging and fluid biomarker changes more than two decades before their estimated age at clinical onset--and before CSF or PET biomarker evidence of Aβ plaque deposition.
Methods
Study Design and Setting
The cross-sectional study described in this report was designed to characterize some of the earliest biomarker changes associated with the predisposition to autosomal dominant AD. Between January and August 2010, clinical and cognitive assessments were performed at the University of Antioquia in 20 PSEN1 E280A mutation carriers and 24 non-carriers, who were cognitively normal, 18-26 years of age, demographically matched, descended from a common ancestor, and subsequently recruited to participate in the cross-sectional biomarker study. Between July and August 2010, structural and functional MRIs were acquired in each of the participants at the Hospital Pablo Tobón Uribe, and CSF and plasma samples were acquired in 20 of the participants at the University of Antioquia. Data were acquired blind to the participants’ genetic test results. Fluid biomarker study participants were selected based on their informed consent to participant in this optional sub-study, blind to clinical or cognitive findings and before the analysis of any brain images. Findings from this study were presented at the Alzheimer’s Association International Conference in July 2011.
Participants
Clinical ratings, neuropsychological assessments, family histories, neurological exams, APOE genotypes, functional MRIs during face-name associative memory encoding/novel viewing and control tasks, and structural MRIs were performed in forty-four 18-26 year-old mutation carriers from the PSEN1 E280A kindred. They included 20 mutation carriers and 24 non-carriers who were cognitively normal and matched for their gender, age, and educational level (Table 1). Participants were recruited from the Colombian Alzheimer’s Prevention Initiative (API) Registry, which currently includes more than 1,500 living members from this kindred, 30% of whom carry the mutation, and who have had comprehensive clinical, cognitive, and genetic assessments. Invitations to participate were based in part of the participants’ proximity to the University of Antioquia and Hospital Pablo Tobon Uribe. Thus, participants came came primarily from the Medellin area, and had a higher educational level than kindred members in the rural areas
Table 1.
PSEN1 E280A Mutation Carrier and Non-Carrier Characteristics, Clinical Ratings, and Neuropsychological Test Scores*#
|
brain imaging participants
|
fluid biomarker participants
¶
|
|||||
|
non-carriers
(n=24) |
carriers
(n=20) |
P-value † |
non-carriers
(n=10) |
carriers
(n=10) |
P-value † | |
| gender (female/male) | 12 / 12 | 11 / 9 | 0.74 | 5 / 5 | 8 / 2 | 0.16 |
| age (years) | 22 ± 2 (18-26) | 22 ± 3 (18-26) | 0.63 | 24 ± 2 | 23 ± 2 | 0.33 |
| educational level (years) | 11 ± 2 (5-15) | 11 ± 3 (5-15) | 0.71 | 12 ± 2 | 12 ± 31 | 0.71 |
| APOE ε4 carriers / non-carriers | 5 / 19 | 4 / 16 | 0.95 | 0 / 10 | 2 / 8 | 0.14 |
| MMSE Score / 30 | 29.3 ± 0.8 | 29.8 ± 0.6 | 0.06 | 29.3 ± 0.9 | 29.7 ± 0.7 | 0.55 |
| verbal fluency* | 19.9 ± 4.6 | 19.6 ± 5.5 | 0.78 | 19.8 ± 3.6 | 19.1 ± 6.3 | 0.29 |
| naming/15* | 13.4 ± 1.0 | 13.3 ± 2.0 | 0.55 | 13.5 ± 0.9 | 13.8 ± 1.0 | 0.94 |
| word memory* | ||||||
| total correct / 30* | 18.6 ± 3.8 | 20.4 ± 3.4 | 0.12 | 18.8 ± 3.7 | 20.8 ± 3.2 | 0.15 |
| total intrusions* | 0.3 ± 0.5 | 0.6 ± 0.9 | 0.25 | 0.1 ± 0.4 | 0.5 ± 1.1 | 0.66 |
| total recall* | 7.3 ± 1.3 | 7.0 ± 1.9 | 0.76 | 6.8 ± 1.8 | 7.6 ± 2.0 | 0.60 |
| recall intrusions* | 0.4 ± 0.2 | 0.2 ± 0.5 | 0.43 | 0.0 ± 0.0 | 0.1 ± 0.4 | 0.71 |
| word recognition* | ||||||
| correct “yes” / 10* | 10.0 ± 0.0 | 10.0 ± 0.2 | 0.47 | 10 ± 0.0 | 9.9 ± 0.4 | 0.71 |
| correct “no” / 10* | 9.9 ± 0.3 | 9.7 ± 0.7 | 0.27 | 10 ± 0.0 | 10.0 ± 0.0 | 1.00 |
Neuropsychological tests from the Consortium to Establish a Registry for AD (CERAD) Neuropsychological Battery
Plus-minus values are means ± SD; parentheses include range.
Sixteen of participants who provided biological fluid samples were included in the MRI study; the other four participants were excluded from MRI studies due to dental braces.
P-values were calculated using Mann-Whitney tests to compare subject groups for age, educational level, Mini-Mental State Examination score and CERAD test scores, and with chi-square tests to compare the groups for gender and APOE ε4 carrier status.
Participants from the API Registry were selected for the cross-sectional biomarker study based on the following criteria: our pre-specified 18-26 year-old age range; presence or absence of the PSEN1 E280A mutation, and matching for the demographic features noted above; absence of significant impairment in any cognitive domain, based on a detailed clinical assessment, performance on a Colombian version of the Consortium to Establish a Registry for AD (CERAD) neuropsychological test battery, Mini-Mental State Examination (MMSE) scores, and activities of daily living; absence of memory complaints, based on self-report and a family questionnaire; a negative history of neurological or psychiatric disorders; potential suitability for MRI; and consent to participate in relevant aspects of the study.
Lumbar punctures, venipunctures, and CSF and plasma assays were performed in 20 of the relatives, including 10 mutation carriers and 10 non-carriers (Table 1). Sixteen of participants who provided biological fluid samples were included in the MRI study; the other four participants were excluded from MRI studies due to dental braces. Participants understood that they would not receive information about their PSEN1 genotype, provided their informed consent and were studied under guidelines approved by Institutional Review Boards at the University of Antioquia and Banner Health.
Between September and December 2011, florbetapir PET scans were performed in fifty 20-56 year-old PSEN E280A mutation carriers and non-carriers as part of a separate study to characterize the age-related trajectory of biomarker changes associated with autosomal dominant AD.20 The participants included 4 carriers and 4 non-carriers from the young adult study, who were selected blind to their earlier biomarker findings. The PSEN1 E280A mutation carriers had a cerebral pattern of fibrillar Aβ deposition similar to that observed in LOAD, beginning at the approximate age of 28—older than the age of the young adults in the present study.
Procedures
Clinical Ratings, Neuropsychological Tests, and Genetic Tests
Clinical ratings and neuropsychological assessments were performed using a Spanish version of the Consortium to Establish a Registry for AD (CERAD) neuropsychological battery that was adapted for Colombia as previously described.21 It includes the Mini-Mental State Examination (MMSE) and separate assessments of memory, language, praxis and orientation.
Presence or absence of the PSEN1 E280A and APOE genotypes were characterized as previously described.12, 13 The rare Christchurch APOE variant was found and confirmed using Sanger sequencing in two PSEN1 E280A mutation carriers who were homozygous for the APOE ε3 allele. Investigators were blind to the genetic status of the participants during data collection and analysis.
CSF and Plasma Assays
Lumbar punctures and venipunctures were performed in the morning at the University of Antioquia following an overnight fast. CSF and plasma samples were processed, stored in polypropylene tubes, frozen at -80°C, shipped, and assayed in the same batch following a single thaw. CSF Aβ1-42, total tau (t-tau), and phospho-tau181 (p-tau181) and plasma Aβ1-40 and Aβ1-42 levels were quantified by Luminex xMAP bead-based methods (INNO-BIA AlzBio3™, for research-use reagents, and INNO-BIA Plasma Aβ Forms Multiplex Assay, respectively, Innogenetics Ghent, Belgium) by the Knight AD Research Center Biomarker Core at Washington University in St. Louis.
Functional and Structural MRI
Functional and structural MRI pulse sequences were performed on a 1.5T Siemens Avanto scanner. Functional MRI data were acquired using a T2*-weighted gradient echoplanar blood-oxygen-level-dependent (BOLD) pulse sequence during a face-name associative memory encoding task involving the viewing of novel face-name pairs, a control task involving repeated face-name pairs, and visual fixation as previously described.14 Participants were instructed to indicate whether the name “fit” with the face and to remember the face-name pairs for later testing. Following the scanning session, recognition memory performance was assessed in response to previously viewed and new face-name pairs using a discrimination index (correctly recognized – falsely recognized pairs) and the median reaction time to correctly recognized pairs. Structural data were acquired using a T1-weighted volumetric pulse sequence.
Statistical Analyses
Mutation carriers and non-carriers in the MRI study were matched for gender, age and educational level. Carriers and non-carriers in the MRI study, and in the fluid biomarker sub-study, were compared in terms of their age, educational level, clinical ratings, neuropsychological test scores, using non-parametric Mann-Whitney tests; and their gender and APOE ε4 carrier proportions were compared using chi-square tests as shown in Table 1. CSF Aβ1-42, t-tau and p-tau181 levels, and plasma Aβ1-42 levels and Aβ1-42/Aβ1-40 ratios were compared using Mann-Whitney tests as shown in Supplementary Table 1.
An automated brain mapping algorithm (Statistical Parametric Mapping Version 8 [SPM8], Wellcome Trust Centre for Neuroimaging) was applied to BOLD images acquired during the novel and repeated face-name association tasks to align sequential images in each participant, deform them into the coordinates of a standard brain atlas, smooth images using a 6mm full-width-at-half-maximum (FWHM) Gaussian filter, characterize and compare regional activations and deactivations associated with memory encoding/viewing of novel face-name pair associations in the mutation carrier and non-carrier groups.14 The novel and repeated conditions were modeled using a boxcar function with a length of 40 sec convolved with the canonical hemodynamic response function. The movement parameters from the realignment procedure were added as covariates to account for residual movement-related spurious activation. Hypothesis testing was restricted to hippocampal/parahippocampal and precuneus/posterior cingulate search regions14 and family-wise error corrections were used to correct for multiple comparisons in these regions (p<0·05), as shown in Table 2.
Table 2.
Location and magnitude of most significant functional and structural brain abnormalities in young adult PSEN1 E280A mutation carriers
| Atlas Coordinates † | P-Value # * | ||||
| X | Y | Z | |||
|
hippocampal/parahippocampal locations with significantly greater activation
during associative memory encoding/viewing of novel face-name pairs | |||||
| hippocampus++ | Right | 33 | −8 | −19 | <0.0001 (0.001)* |
| hippocampus++ | Left | −30 | −16 | −13 | 0.001 |
| parahippocampal gyrus++ | Right | 33 | −33 | 3 | 0.001 (0.014)* |
|
precuneus/posterior cingulate locations with significantly less deactivation
during associative memory encoding/viewing of novel face-name pairs | |||||
| precuneus++ | Right | 18 | −57 | 42 | 0.001 (0.008)* |
| precuneus++ | Left | −12 | −66 | 54 | 0.002 (0.010)* |
| posterior cingulate++ | Right | 21 | −50 | 17 | 0.002 (0.009)* |
| locations with significantly less gray matter | |||||
| parietal+ | Right | 55 | −18 | 38 | 0.0004 (0.009)* |
| parietal++ | Left | −38 | −75 | 48 | 0.0005 |
| temporal++ | Right | 51 | −27 | 5 | 0.001 |
| fusiform gyrus++ | Right | 38 | −61 | −7 | 0.0008 |
| fusiform gyrus++ | Left | −36 | −45 | −8 | 0.0008 |
| parahippocampal gyrus++ | Right | 28 | −24 | −22 | 0.002 |
| frontal | Right | 44 | 14 | 18 | 0.0006 |
| occipital | Left | −26 | −61 | 21 | 0.002 |
| cerebellum | Left | −6 | −55 | −11 | 0.002 |
Coordinates from Talairach’s brain atlas34, such that x is the distance in millimeters to the right (+) or left (−) of midline, y is the distance anterior (+) or posterior (−) to the anterior commissure, and z is the distance above (+) or below (−) a horizontal plane through the anterior and posterior commissures.
Located in an AD-affected search region.
P-values, uncorrected for multiple comparions.
Findings that remained significant after correcting for multiple regional in the postulated search regions are indicated by the corrected p-values shown in parentheses.
SPM8 was used with the Voxel-Based Morphometry Version 8 (VBM8), the DARTEL toolbox, Jacobian modulation, and an 8mm FWHM Gaussian filter to generate segmented gray matter images in each participant, deform them into brain atlas coordinates, and compare regional gray matter in the mutation carrier and non-carrier groups (unpaired t-tests, p<0·005, uncorrected for multiple comparisons). Hypothesis testing was restricted to search regions independently found to be associated with less gray matter in probable AD dementia patients, and family-wise error corrections were used to correct for multiple comparisons in these regions (p<0·05), as shown in Table 2. AD-affected search regions were characterized for this purpose using MRIs from 145 probable LOAD dementia patients and 159 cognitively normal older adults in the AD NeuroImaging Initiative (ADNI) and the same image-analysis algorithm (see Supplementary Figure 1).
Results
Participant characteristics, clinical ratings and neuropsychological test scores are shown in Table 1. The demographically matched young adult PSEN1 E280A mutation carriers and non-carriers did not differ significantly in their proportion of APOE ε4 carriers or in their clinical ratings or neuropsychological test scores.
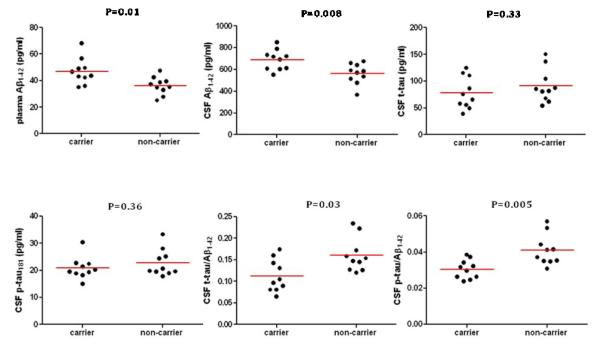
Plasma measurements and p-values are shown in Figure 1 and Supplementary Table 1. In comparison with the non-carriers, mutation carriers had significantly higher plasma Aβ1-42 levels and plasma Aβ1-42/Aβ1-40 ratios. There were no significant differences in the groups’ plasma Aβ1-40 levels. These findings are consistent with previously published findings and with overproduction of the Aβ1-42 peptide in autosomal dominant AD.8, 11
Figure 1. Plasma and cerebrospinal fluid Alzheimer’s disease biomarkers in young adult PSEN1 E280A mutation carriers and non-carriers.
P-values were calculated using Mann-Whitney tests
CSF measurements and p-values are also shown in Figure 1 and Supplementary Table 1. In comparison with the non-carriers, the PSEN1 E280A mutation carriers had significantly higher CSF Aβ1-42 levels, consistent with Aβ1-42 overproduction and in contrast to previously published findings in the later preclinical and clinical stages of LOAD22 and autosomal dominant AD.23 The groups did not differ significantly in their CSF t-tau or p-tau181 levels. Mutation carriers had significantly lower CSF t-tau/Aβ1-42 and p-tau/Aβ1-42 ratios, in contrast to the elevated ratios reported in the clinical and later preclinical stages of LOAD22 and autosomal dominant AD23, and mostly attributable to elevated CSF Aβ1-42 levels.
The mutation carriers and non-carriers did not differ significantly in their post-scan face-name pair recognition memory performance: their respective discrimination indices were 0·44±0.17 and 0·46±0.14 (p=0·60); their respective median reaction times to respond correctly to previously viewed face-name pairs were 2,010±306 and 2,027±268 milliseconds (p=0·43). As expected, encoding/viewing of novel face-name pairs was associated in each subject group with activation in bilateral fusiform gyrus, medial temporal lobe and prefrontal regions, and deactivation in posterior parietal regions (p<0·005, uncorrected for multiple comparisons).14 Compared to non-carriers, mutation carriers showed significantly greater activation in hippocampal/parahippocampal regions and less deactivation in precuneus/posterior cingulate regions (Table 2, Figure 2, Supplementary Figure 2). Interactions remained significant in the right hippocampal/parhahipocampal and precuneus/posterior cingulate regions after correction for multiple comparisons in the postulated search regions (Table 2).
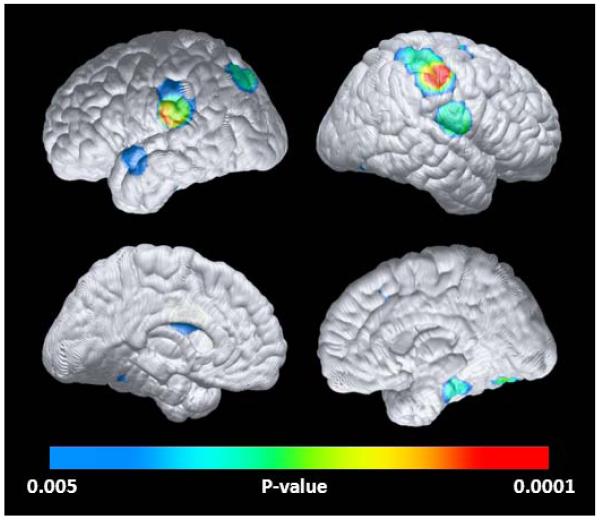
Figure 2. Task-dependent functional brain abnormalities in young adult PSEN1 E280A mutation carriers.
In comparison with non-carriers, young adult mutation carriers have a) significantly greater activation bilaterally in a hippocampal/parahippocampal search regions and b) significantly less deactivation bilaterally in precuneus and posterior cingulate search regions previously implicated in the later preclinical stages of AD14. Statistical maps are projected onto the medial and lateral surfaces of a spatially standardized brain. (The map of significantly greater activation in the bilateral hippocampal/parahippocampal regions is also displayed on coronal sections in Supplementary Figure 2.) Between-group differences in the postulated search regions correspond to the color scale. Differences in other regions are shown in light blue (P<0·005, uncorrected for multiple comparisons). Maximal differences within the search regions are listed in Table 2 and remained significant after correction for multiple comparisons.
Brain regions with abnormally reduced gray matter in the young adult mutation carriers are shown in Table 2 and Figure 3. In comparison with the non-carriers, the mutation carriers had significantly less gray matter in bilateral parietal and parietotemporal, right parahippocampal and frontal, and left cingulate and temporal regions. The reduction in right parietal lobe gray matter remained significant after correction for multiple comparisons in the AD-affected search region (Table 2). The structural MRI abnormalities are similar but not identical to those previously reported in the clinical and later preclinical stages of autosomal dominant AD,17 LOAD dementia patients19 (Supplementary Figure 1), and APOE ε4-carrying children, adolescents, and young adults.6
Figure 3. Structural brain abnormalities in young adult young adult PSEN1 E280A mutation carriers.
In comparison with non-carriers, young adult mutation carriers have significantly less gray matter in bilateral parietal, parieto-temporal and fusiform, right parahippocampal, left temporal and mid-cingulate regions (P<0 005, uncorrected for multiple comparisons). Statistical maps are projected onto the medial and lateral surfaces of a spatially standardized brain. Maximally significant gray matter reductions are listed in Table 2.
As expected from the small sample sizes and narrow 18-26 year-old age range, post-hoc analyses failed to detect significant associations between the implicated biomarkers and age in the mutation carriers or significant interactions between carrier status and age. Studies involving larger samples, a broader age range, and longitudinal follow-up will be needed to clarify the extent to which any of the observed changes are progressive or developmental.
Discussion
This report studied cognitively normal young adult PSEN1 E280A mutation carriers and non-carriers from the world’s largest known autosomal dominant kindred in order to characterize some of the earliest CSF, plasma, and brain imaging abnormalities associated with the predisposition to autosomal dominant AD.
More than two decades before the kindred’s median age of 44 at MCI onset and its median age of 49 at dementia onset, young adult PSEN1 mutation carriers have elevated CSF levels, plasma Aβ1-42 levels, and plasma Aβ1-42/Aβ1-40 ratios. These findings are consistent with cellular evidence of Aβ1-42 overproduction and increased in Aβ1-42/Aβ1-40 ratios in autosomal dominant AD8-10, including a reported association between the PSEN1 E280A mutation and enhanced production and secretion of Aβ1-42.24 They appear to precede the reduced CSF Aβ1-42 levels that have been observed in the subsequent preclinical and clinical stages of AD and which are thought to reflect deposition of diffuse and neuritic plaques in the subsequent preclinical stages of AD.25
Of particular interest, the 18-26 PSEN1 year-old mutation carriers also had functional and structural MRI abnormalities, similar to those found in the later preclinical stages of autosomal dominant AD16, 17 and LOAD.18, 19 Indeed, these brain abnormalities were observed before the ages at which mutation carriers in this kindred have florbetapir PET or CSF evidence of Aβ plaque accumulation. In the absence of post-mortem brain samples, we cannot exclude the possibility that the PET and CSF biomarkers are underestimating the accumulation of diffuse amyloid plaques in autosomal dominant AD; nor can we exclude the possibility that the elevated CSF levels in the young adult carriers have already begun to decline. Using a linear mixed model to characterize the trajectory of different biomarkers in a cross-sectional study of different autosomal AD mutation carriers and non-carriers over a larger age range, investigators from the Dominantly Inherited Alzheimer’s Network (DIAN) recently suggested that CSF Aβ1-42 levels begin to decline 25 years before their estimated age at clinical onset, though no significant differences were reported in the 13 carriers and non-carriers who were studied more than 20 years before their estimated age at clinical onset (see Research in Context Panel).11 Still, our latest findings, along with those reported previously in APOE ε4 carriers decades before their anticipated age at clinical onset and/or before evidence of elevated fibrillar or soluble Aβ in brain,4 raise new questions about the earliest brain changes associated with the predisposition to autosomal dominant AD and LOAD.
Additional studies are needed to clarify the extent to which the structural and functional abnormalities identified in young adults at genetic risk for autosomal dominant AD or LOAD precede Aβ plaque deposition, the extent to which these changes are progressive or developmental, the extent to which they provide a foothold for the subsequent Aβ or tau pathology, and the extent to which they are the consequence of soluble Aβ or other molecular species (including but not limited to elevated Aβ1-42 levels or Aβ1-42/Aβ1-40 ratios in person’s at risk for autosomal dominant AD). In previous studies, we suggested that the functional brain abnormalities we found in living young adult APOE ε4 carriers4 were apparent in brain samples from expired young adult ε4 carriers before demonstrable soluble Aβ elevations, Aβ plaques, or significant tau pathology,5 that they were not progressive between young adulthood and late middle age,26 but that they anticipated progressive brain changes and some of the earliest Aβ plaque deposition in the later preclinical stages of AD.4 Other researchers have demonstrated striking similarities between brain regions associated with the highest metabolic activity and aerobic glycolysis in young adults and those associated with Aβ plaque deposition in the later preclinical and clinical stages of AD,27 that these brain regions correspond to the “default-mode network” that is most active in a person’s resting state,28 and that synaptic activity is involved in the production, secretion, and regional deposition of Aβ.29-31
Additional studies are needed to clarify the nature of the structural and functional brain abnormalities implicated in the early predisposition to AD. We postulate that the reductions in regional gray matter are related to a very early age-related or developmental reduction in the density of terminal neuronal fields innervating the implicated regions, which could help account for the similar pattern metabolic reductions found in FDG PET studies of persons at risk for LOAD4 and autosomal dominant AD.32 Whether they begin before or after Aβ plaque deposition, increases in hippocampal activity during a memory encoding/novel viewing task could reflect the effort to compensate for neuronal or synaptic impairments or an inefficient inhibition of synaptic functions.14
This study has several strengths: It studied young adult members from an extremely large and extensively studied kindred of autosomal dominant AD mutation carriers, who in the absence of an effective prevention therapy are certain to develop AD symptoms and have well characterized trajectories of cognitive decline. The more homogeneous nature of the disease in carriers of this single mutation may afford the chance to characterize preclinical AD with improved statistical power and greater confidence in relating the brain changes to the estimated age at clinical onset. In addition, it compared several different brain imaging and fluid biomarker measurements in mutation carriers and non-carriers to characterize some of the earliest biomarker changes associated with the predisposition to autosomal dominant AD.
This study also has several limitations, including relatively small sample sizes and uncertainty in the extent to which our findings may be generalizable to other causes of autosomal dominant AD and LOAD. While there are fewer participants with CSF and plasma samples than with structural and functional MRIs, we note our sensitivity to detect abnormal increases in Aβ1-42, levels in our relatively homogeneous mutation carrier group. While the regional gray matter findings should be regarded as exploratory, the uncorrected significance levels, bilateral pattern, and resemblance to the pattern observed in the later stages of AD reduce the likelihood that they are attributable to the Type I error associated with multiple regional comparisons. While our findings are currently limited to PSEN1 E280A carriers, we have sought to harmonize our biomarker measurements and perform biological fluid assays in the same laboratory used in the study of many other autosomal dominant AD mutation carriers and non-carriers from the Dominantly Inherited Alzheimer’s Network (DIAN),8, 11 thus providing complementary data and converging evidence in the preclinical study of autosomal dominant AD.
In conclusion, young adult PSEN1 E280A mutation carriers have functional and structural MRI changes, along with CSF and plasma biomarker findings consistent with Aβ1-42 over-production. This study demonstrates some of the earliest known brain changes in autosomal dominant AD mutation carriers, it suggests that these changes may begin before biomarker evidence of Aβ plaque deposition, and it underscores the need for studies to clarify the earliest brain changes associated with the predisposition to AD. Under the auspices of the Alzheimer’s Prevention Initiative (API),33 we continue to characterize the age-related trajectory of biomarker changes associated with preclinical AD in this extremely large and well-studied kindred and to set the stage for the first clinical trial of an anti-amyloid therapy in the preclinical treatment of AD.
Panel: Research in Context
Systematic Review
In preparation for our revised manuscript, PUBMED was used iteratively to search for relationships between the respective terms “preclinical familial Alzheimer’s,” “dominantly inherited,” and “early-onset” and the respective terms “cerebral spinal fluid,” “MRI,” and “PET.” As of August 20, 2012, 18 articles reported brain imaging and CSF biomarker findings in more than one autosomal dominant AD mutation carrier before the clinical onset of AD (see Supplementary Table 2 and Supplementary References). Five articles reported CSF findings, 8 reported structural MRI findings, 2 reported functional MRI findings, 4 reported FDG PET findings, and 5 reported fibrillar Aβ PET findings in healthy mutation carriers and non-carriers. Most of these studies included a preponderance of carriers who were older and closer to the estimated age at clinical onset than those in this study. To our knowledge, none of the reports included a sufficient number of mutation carriers and non-carriers to investigate or detect significant biomarker changes in mutation carriers younger than age 26 or more than two decades before the carriers’ estimated age at MCI onset.
In August 2012, DIAN investigators reported a cross-sectional brain imaging and fluid biomarker study of 43 clinically affected mutation carriers, 45 clinically unaffected mutation carriers, and 40 non-carriers from 51 PSEN1, PSEN2, and APP pedigrees over a large age range.11 Using a linear mixed model to characterize the trajectory of different biomarkers, they suggested that CSF Aβ1-42 levels begin to decline 25 years before their estimated age at clinical onset. However, the report included a total of 13 participants who were studied more than 20 years before the parent’s estimated age at clinical onset, and did not report significant differences in CSF, plasma or brain imaging biomarker data in this younger sub-set. While the DIAN report included structural MRI and FDG PET measurements in preselected regions-of-interest, it did not include functional MRI data or the voxel-based analysis of regional gray matter differences. API and DIAN will continue to play complementary and potentially converging roles in the preclinical study of autosomal dominant AD.
To our knowledge, this is the first study to report significant brain imaging and CSF differences in young-adults at genetic risk for autosomal dominant AD, more than two decades years before their estimated age at clinical onset. After this study was completed, we conducted a separate study to characterize the age-related trajectory of brain imaging and fluid biomarker changes in PSEN1 E280A mutation carriers and non-carriers 18-60 years. Florbetapir PET findings are described in a companion report. Also after this study was completed, we conducted a structural and functional MRI study in 8-17 year-old PSEN1 280A mutation carriers and non-carriers. Analyses are underway, and findings will be described in a future report.
Interpretation
This study demonstrates functional and structural brain imaging changes, along with CSF and plasma biomarker findings consistent with Aβ1-42 over-production, in young adult PSEN1 E280A mutation carriers, more than two decades before the carriers’ estimated median age at MCI onset, and before CSF or PET biomarker evidence of fibrillar Aβ deposition. It supports the possibility that brain changes begin many years before the clinical onset of AD, perhaps even before the onset of Aβ plaque deposition.
Additional research is needed to clarify a) the extent to which the observed biomarker findings are progressive or developmental, b) whether or not the elevated CSF Aβ42 levels are already in decline, c) whether or not there is any cerebral fibrillar Aβ deposition in expired young-adult mutation carriers (even in the absence of PET or CSF biomarker findings), d) the extent to which our findings are relevant to other genetic and non-genetic forms of AD, and e) the extent to which brain changes implicated in the MRI studies provide a foothold for the subsequent onset of AD neuropathology.
Supplementary Material
Acknowledgements
We thank Drs. Natalia Montes and Feliza Restrepo, Sandra Lainez, Erika Munoz and their colleagues from the Hospital Pablo Tobón Uribe and our colleagues at the University of Antioquia and Xiaofen Liu, Pradeep Thiyyagura, Hua Mo, Hillary Protas, Napatkamon Ayutyanont, Wendy Lee, Auttawut Roontiva, Stephanie Parks, Mark Nishimura, and Weihua Chen from the Banner Alzheimer’s Institute for their technical assistance. We thank Dr. Paul Thompson and his colleagues for software used to help generate the cortical surface maps in Figures 2 and 3. We thank our valued research participants and other members of the PSEN1 E280A mutation kindred for their invaluable dedication and inspiration.
Role of the Funding Source This study was supported by a grant to investigate the earliest brain changes associated with the predisposition to autosomal dominant AD from an Anonymous Foundation (EMR, FL) and by the Banner Alzheimer’s Foundation, the Nomis Foundation, and the Boston University Department of Psychology (CES. YTQ). Additional support for structural MRI analyses was provided by the National Institute on Aging (R01 AG031581 [EMR], P30 AG19610 [EMR], U01 AG024904 [Michael Weiner, PI], RO1 AG025526 [GEA], and RF1AG041705 [EMR, PNT, FL), the National Institute of Neurological Disorders and Stroke (F31-NS078786 [YTQ]), Colciencias (1115-408-20512 fl, 1115-545-31651 fl), and the state of Arizona. The study sponsors had no role in the study design, the collection, analysis, or interpretation of data, or the preparation or review of this manuscript. The authors had complete access to study data and final responsibility for the submitted manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors EMR and FL contributed to study design, data acquisition and analysis, manuscript preparation, and acquisition of funding. YTQ contributed to study design, data acquisition and analysis, and manuscript preparation. ASF contributed to study design, data analysis, and manuscript preparation. KC contributed to data analysis and manuscript preparation. CVP contributed to study design, biological sample preparation, and manuscript preparation. M.J.D-R contributed to study design, biological sample preparation, and manuscript preparation. AMF contributed to biological sample preparation and analysis and manuscript preparation. SA, AA, MG, NA, VT, CM, RAR, MJH, and KK contributed to data acquisition and manuscript preparation. RAS and BCD contributed to study design and manuscript preparation. CES contributed to study design and manuscript preparation. GEA contributed to data analysis and manuscript preparation. JBL and PNT contributed to study design, data analysis, and manuscript preparation.
Conflicts of Interest The authors declare no relevant conflicts of interest.
Reference List
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Jack CR, Jr., Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valla J, Yaari R, Wolf AB, et al. Reduced posterior cingulate mitochondrial activity in expired young adult carriers of the APOE epsilon4 allele, the major late-onset Alzheimer’s susceptibility gene. J Alzheimers Dis. 2010;22(1):307–313. doi: 10.3233/JAD-2010-100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw P, Lerch JP, Pruessner JC, et al. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 2007;6(6):494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- 7.Filippini N, MacIntosh BJ, Hough MG, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bateman RJ, Aisen PS, De Strooper B, et al. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res Ther. 2011;2(6):35. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ringman JM, Younkin SG, Pratico D, et al. Biochemical markers in persons with preclinical familial Alzheimer disease. Neurology. 2008;71(2):85–92. doi: 10.1212/01.wnl.0000303973.71803.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chavez-Gutierrez L, Bammens L, Benilova I, et al. The mechanism of gamma-Secretase dysfunction in familial Alzheimer disease. EMBO J. 2012;31(10):2261–2274. doi: 10.1038/emboj.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. N Engl J Med. 2012 doi: 10.1056/NEJMoa1202753. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopera F, Ardilla A, Martinez A, et al. Clinical features of early-onset Alzheimer disease in a large kindred with an E280A presenilin-1 mutation. JAMA. 1997;277(10):793–799. [PubMed] [Google Scholar]
- 13.Acosta-Baena N, Sepulveda-Falla D, Lopera-Gomez CM, et al. Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer’s disease: a retrospective cohort study. Lancet Neurol. 2011;10(3):213–220. doi: 10.1016/S1474-4422(10)70323-9. [DOI] [PubMed] [Google Scholar]
- 14.Quiroz YT, Budson AE, Celone K, et al. Hippocampal hyperactivation in presymptomatic familial Alzheimer’s disease. Ann Neurol. 2010;68(6):865–875. doi: 10.1002/ana.22105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quiroz Y, Stern C, Reiman EM, et al. Cortical signature of Alzheimer’s disease-related thinning in presymptomatic presenilin-1 mutation carriers. Alzheimers Dement. 2011;7(4):S220. [Google Scholar]
- 16.Mondadori CR, Buchmann A, Mustovic H, et al. Enhanced brain activity may precede the diagnosis of Alzheimer’s disease by 30 years. Brain. 2006;129(Pt 11):2908–2922. doi: 10.1093/brain/awl266. [DOI] [PubMed] [Google Scholar]
- 17.Knight WD, Kim LG, Douiri A, Frost C, Rossor MN, Fox NC. Acceleration of cortical thinning in familial Alzheimer’s disease. Neurobiol Aging. 2011;32(10):1765–1773. doi: 10.1016/j.neurobiolaging.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Celone KA, Calhoun VD, Dickerson BC, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. J Neurosci. 2006;26(40):10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickerson BC, Bakkour A, Salat DH, et al. The Cortical Signature of Alzheimer’s Disease: Regionally Specific Cortical Thinning Relates to Symptom Severity in Very Mild to Mild AD Dementia and is Detectable in Asymptomatic Amyloid-Positive Individuals. Cerebral Cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleisher AS, Chen K, Quiroz YT, et al. Age-associated fibrillar amyloid-β deposition in individuals from the presenilin 1 E280A autosomal-dominant Alzheimer’s disease kindred. Lancet Neurol. doi: 10.1016/S1474-4422(12)70227-2. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aguirre-Acevedo DC, Gomez RD, Moreno S, et al. Validity and reliability of the CERAD-Col neuropsychological battery. Rev Neurol. 2007;45(11):655–660. [PubMed] [Google Scholar]
- 22.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/β- amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64(3):343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 23.Ringman JM, Coppola G, Elashoff D, et al. Cerebrospinal fluid biomarkers and proximity to diagnosis in preclinical familial Alzheimer’s disease. Dement Geriatr Cogn Disord. 2012;33(1):1–5. doi: 10.1159/000335729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikeuchi T, Dolios G, Kim SH, Wang R, Sisodia SS. Familial Alzheimer disease-linked presenilin 1 variants enhance production of both Abeta 1-40 and Abeta 1-42 peptides that are only partially sensitive to a potent aspartyl protease transition state inhibitor of “gamma-secretase”. J Biol Chem. 2003;278(9):7010–7018. doi: 10.1074/jbc.M209252200. [DOI] [PubMed] [Google Scholar]
- 25.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol. 2006;59(3):512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 26.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the ε4 allele for apolipoprotein E. N Engl J Med. 1996;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 27.Vlassenko AG, Vaishnavi SN, Couture L, et al. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta ) deposition. Proc Natl Acad Sci U S A. 2010;107(41):17763–17767. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei W, Nguyen LN, Kessels HW, Hagiwara H, Sisodia S, Malinow R. Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci. 2010;13(2):190–196. doi: 10.1038/nn.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bero AW, Yan P, Roh JH, et al. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci. 2011;14(6):750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cirrito JR, Yamada KA, Finn MB, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48(6):913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 32.Mosconi L, Sorbi S, de Leon MJ, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer’s disease. J Nucl Med. 2006;47(11):1778–1786. [PubMed] [Google Scholar]
- 33.Reiman EM, Langbaum JBS, Tariot PN. Alzheimer’s Prevention Initiative: a proposal to evaluate presymptomatic treatments as quickly as possible. Biomarkers in Medicine. 2010;4(1):3–14. doi: 10.2217/bmm.09.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.