Abstract
Persistent and vexing health disadvantages accrue to African Americans despite decades of work to erase the effects of race discrimination in this country. Participating in these efforts, psychologists and other social scientists have hypothesized that African Americans’ continuing experiences with racism and discrimination may lie at the root of the many well-documented race-based physical health disparities that affect this population. With newly emerging methodologies in both measurement of contextual factors and functional neuroscience, an opportunity now exists to cleave together a comprehensive understanding of the ways in which discrimination has harmful effects on health. In this article, we review emerging work that locates the cause of race-based health disparities in the external effects of the contextual social space on the internal world of brain functioning and physiologic response. These approaches reflect the growing interdisciplinary nature of psychology in general, and the field of race relations in particular.
Keywords: racism, Blacks, allostatic load, social exclusion, brain, residential segregation, social cognition, cognitive appraisal, self-regulation
The ways in which race, racial prejudice, and race discrimination shape the human experience have long been of interest in psychology and the other social sciences. The purpose of this review is threefold. First, we briefly examine the disconcerting evidence for increasing Black/White disparities in health despite the radical changes over the past 50 years in race-based civil rights in the United States (Walker et al. 2004). Next, we explore the notion that African Americans’ continuing experiences with racism, discrimination, and possibly social exclusion may account for some proportion of these health disparities (Clark & Adams 2004; Everson-Rose & Lewis 2005; Guyll et al. 2001; Harrell et al. 2003; Massey 2004; Walker et al. 2004; Williams et al. 1997, 2003). Finally, we focus on three emerging perspectives that locate health disparities in the external influences of social space and the internal effects of body and brain functioning. These latter approaches reflect the growing interdisciplinary nature of research models that attempt to explain the continuing legacy of physical health disparities that harmfully affect African Americans. Our aim is to raise several important questions about the ways in which psychology can engage in a plan of research to address health disparities from race-based discrimination and also take a leadership role in informing the development of social policies that will help American society to accelerate its pace of changing negative race-based attitudes and associated social policies.
HEALTH DISPARITIES—THE BLACK/WHITE DIVIDE
In 1985, with the release of the Heckler report, America was put on notice that the health status of African Americans was significantly worse than that of their White counterparts (Heckler 1985). Unfortunately, since then, racial disparities in health have worsened in many ways. In 1990, for example, McCord and Freeman shocked the world by reporting that a Black male in Harlem had less of a chance of reaching the age of 65 than did the average male resident of Bangladesh—one of the poorest countries in the world. At the time of McCord & Freeman’s study, African American men fell behind men from Bangladesh in survival rates starting at age 40 (McCord & Freeman 1990, Sen 1993). In the United States, life expectancy for African American males experienced an unprecedented drop every year from 1984 to 1989, while all other combinations of Black/White male/female comparisons either remained the same or increased (NVSS 2004).
Today, African Americans still bear a disproportionate burden in disease morbidity, mortality, disability, and injury (MMWR 2005, Williams 1995). This continuing health disadvantage is seen particularly in the age-adjusted mortality rates: African Americans remain significantly and consistently more at risk for early death than do similar White Americans (Geronimus et al. 1996, Kochanek et al. 2004, Levine et al. 2001, MMWR 2005, Smith et al. 1998, Williams & Jackson 2005). Indeed, the overall death rate of African Americans in the United States today is equivalent to that of Whites in America 30 years ago (Levine et al. 2001, Williams & Jackson 2005).
These premature deaths arise from a broad spectrum of disorders. Diabetes, cardiovascular heart disease, hypertension, and obesity disproportionately affect African Americans (Davis et al. 2003; Krieger 1990; Mensah et al. 2005; USDHHS 1990, 2000, 2005). For example, in deaths due to heart disease, the rate per 100,000 persons for African Americans (321.3) is higher than for any other racial/ethnic group, including Asian/Pacific Islanders (137.4), American Indian/Alaska Natives (178.9), Hispanics (188.4), and Whites (245.6) (NCCDPHP 2004). This same pattern for African Americans in comparison with Asian/Pacific Islanders, American Indians/Alaska Natives, Hispanics, and Whites is repeated in deaths due to diabetes (49.9 versus 16.9, 45.3, 36.3, and 22.1, respectively) and strokes (80.0 versus 51.2, 46.1, 44.0, and 55.9, respectively). Even prevalence of hypertension per 100,000 is far greater among African Americans (34.2) than among the other major racial/ethnic groups (16.2, 25.8, 18.9, 25.8, respectively) (NCCDPHP 2004).
Furthermore, these health disadvantages occur in the context of increasing disparities in rates of disease. For example, Williams & Jackson (2005) examined Black/White health disparities using data from the National Center for Health Statistics for the years 1950 to 2000, and found that although rates of heart disease were similar for Blacks and Whites in 1950, by the year 2000, African Americans had a rate of heart disease 30% higher than that of Whites. Similarly, in 1950, African Americans had a lower cancer rate than Whites, but by the year 2000, their rate was 30% higher.
Poverty alone cannot fully explain these differences; even when socioeconomic status (SES) is controlled for, there is still an excess of 38,000 deaths per year or 1.1 million years of life lost among African Americans in the United States (Franks et al. 2005). Simple differences in skin color that might be the basis for the occurrence of discrimination also appear to be an inadequate explanation. For example, in the recent National Survey of American Life (Jackson et al. 2004), comparisons of 6000 Americans who reported being either Black of Caribbean ancestry, African American, or White revealed that of the three groups, African Americans evidenced the worst self-reported physical health status, including higher rates of hypertension, diabetes, and stroke).
DISCRIMINATION AND HEALTH
The continuing legacy of poor health in African Americans, despite the overall improved conditions of their lives, is one compelling reason to take a closer look at the role discrimination may play. The health disparities that affect African Americans in this country arise from many sources, including cultural differences in lifestyle patterns, inherited health risks, and social inequalities that are reflected in discrepancies in access to health care, variations in health providers’ behaviors, differences in socioeconomic position (Fiscella & Williams 2004; Krieger 1991, 1999; Krieger & Moss 1996; Krieger et al. 1997; Subramanian et al. 2005), and residential segregation (Massey 2004, Schulz et al. 2000). The extent to which these health disparities are also shaped by the pernicious effects of race-based discrimination is of growing interest (Clark 2003, Clark et al. 1999, Cochran & Mays 1994, Everson-Rose & Lewis 2005, Geronimus et al. 1996, Guyll et al. 2001, Harrell et al. 2003, Hertzman 2000, Krieger & Sidney 1996, Massey 2004, Mays 1995, Mays & Cochran 1998, Mays et al. 1996, McEwen 2000, Mechanic 2005, Morenoff & Lynch 2004, Walker et al. 2004, Williams et al. 2003).
From the perspective of discrimination models, the causal mechanism linking racial/ethnic minority status and health disadvantage is thought to lie in the harmful effects of chronic experiences with race-based discrimination, both actual and perceived. These experiences are thought to set into motion a process of physiological responses (e.g., elevated blood pressure and heart rate, production of biochemical reactions, hypervigilance) that eventually result in disease and mortality.
In attempting to elucidate the negative health outcome mechanisms of race-based discrimination, the effects of both overt and anticipated or perceived experiences of race-based discrimination have been examined. Studies of overt or manifest discrimination typically measure events occurring at the individual level by asking respondents if they have been “treated badly or unfairly,” “differently,” or are somehow “disadvantaged” relative to others based on their racial or ethnic background (Krieger et al. 2005). The foundation of this work came from the earlier stress research paradigm, where individual differences in vulnerability to stress were seen as key to the development of mental health morbidity (Kessler et al. 1999). Factors that were thought to predispose individuals to negative mental health outcomes include unfair treatment and social disadvantage as well as other social stressors, such as inadequate levels of social support, neuroticism, the occurrence of life events, and chronic role strain (Adler et al. 1994, Brown & Harris 1989, Henderson et al. 1981, Kanner et al. 1991, Lazarus 1993, Pearlin et al. 1981, Thoits 1983). Later studies examining the possible consequences of perceived discrimination began to document that simply the anticipation of being treated badly or unfairly had as powerful an impact on individuals as objectively measured experiences (Kessler et al. 1999). Both of these developments helped move the field toward hypothesizing that chronic experiences with perceived discrimination can have wide-ranging effects on individuals.
Several studies have now documented health effects of discrimination. In one study, experiences of perceived race-based discrimination were positively associated with raised blood pressure and poorer self-rated health (Krieger & Sidney 1996). Perceived race-based discrimination was also found to be the best predictor of smoking among African American adults in two studies (Landrine & Klonoff 2000). Moreover, smokers, as compared with nonsmokers, reported finding the experience of discrimination as subjectively more stressful. In fact, this appraisal of discrimination as stressful was a better predictor of smoking than was the measured status variables of education, gender, income, and age. Landrine & Klonoff (2000) have suggested that perceived race discrimination and the appraisal process may be key factors in explaining the Black-White differential in smoking prevalence, where smoking possibly acts as a means of coping with stress. The issue gains even greater relevance when one considers that the Black-White differential exists not only in smoking prevalence, but also in smoking-related morbidity, mortality (MMWR 1996, Rivo et al. 1989), and death from respiratory cancers (CDC 1994, USDHHS 1998). Similar findings in research on alcohol consumption among African Americans indicate that internalized racism (i.e., a belief that African Americans are inferior) is positively associated with alcohol use as well as psychological distress (Taylor & Williams 2003).
In the 1990s, the perspective in this field shifted somewhat to emphasize the importance of chronicity of discrimination exposure in negative mental health outcomes (Kessler et al. 1999). At the same time, interest in the effects of discrimination on health outcomes strengthened as the federal government released the Healthy People 2000 and Healthy People 2010 objectives, the yearly National Health Care Disparities Report (USDHHS 2005), and reports from the Institute of Medicine on Unequal Burden (Haynes & Smedley 1999) and Unequal Treatment (Smedley et al. 2003). Experts in health, social cognition, epidemiology, biology, neuroscience, and clinical psychology began to use new methodologies to study prejudice, discrimination, and racism (Everson-Rose & Lewis 2005; Karlamangala et al. 2005; Krieger 1990; Krieger & Sidney 1996; McEwen 1998, 2005; Morenoff & Lynch 2004). These studies focused on the perspective of the person being targeted (Eberhardt 2005, Everson-Rose & Lewis 2005, Golby et al. 2001, Harrell et al. 2003, Mays & Cochran 1998, Meyer 2003) as well as on the characteristics of persons who target others (Eberhardt et al. 2003, 2004; Phelps et al. 2000, 2003).
The result has been a great melding of disciplines, tools, and perspectives to identify the important components of the pathways linking race-based discrimination and negative health outcomes. For example, human brain imaging is now used to observe cognitive processing of experiences of social exclusion (Eisenberger & Lieberman 2004). Biological measures of race-based stress (allostatic load) reveal intricate relationships among the brain, immune system, autonomic nervous system, and the hypothalamic-pituitary-adrenal (HPA) axis (McEwen & Seeman 1999), as well as the ways in which unhealthy environmental stimuli can “get under the skin” of individuals to cause negative health outcomes (Massey 1985, Taylor et al. 1997). Political scientists interested in racial inequalities of criminal behavior and in a number of other areas are looking at the interaction between environmental exposures and brain chemistry (Masters 2001).
NEW APPROACHESTO OLD ISSUES
Across the disparate fields of psychology, sociology, and neuroscience, work is converging on “candidate” variables (e.g., social processes, functional neuroscience, contextual effects) that might be essential to understanding underlying processes associated with racial discrimination and negative health outcomes in African Americans. The challenge, at this point, is to cleave together the literatures examining the upstream side of discrimination and health with its focus on behavioral, social, and psychological factors to those studying the downstream biological pathways and molecular events that are proximal causes of the high rates of disease and disability (Kaplan 1999, Schillinger et al. 2005). In the past decade, particularly, the number of models proposed to account for the relation-ship between race-based experiences and poor physical and mental health have exploded (Clark 2003, Clark et al. 1999, Everson-Rose & Lewis 2005, Hertzman 2000, House 2002, House & Williams 2000, Kuh & Ben-Sholmo 1997, Massey 2004, McEwen 2000, Morenoff & Lynch 2004, Smedley et al. 2003). Several of the race-discrimination-health pathway models posit connections among environmental stimuli including conditions of violence, poor education, and negative social connectedness or early childhood exposure to these conditions, and resulting changes in brain functioning and bodily psychophysiological responses (Clark 2003, Clark et al. 1999, Everson-Rose & Lewis 2005, Hertzman 2000, House 2002, House & Williams 2000, Kuh & Ben-Sholmo 1997, Massey 2004, McEwen 2000, Morenoff & Lynch 2004, Smedley et al. 2003). Across these many models, three elements consistently emerge: (a) an emphasis on the importance of unhealthy social spaces in which racial stratification (particularly in the form of residential segregation) serves as a structural lattice for maintaining discrimination; (b) intergenerational and life-span effects of race discrimination that result in pernicious effects on health despite increasingly better opportunities and better environments; and (c) chronicity and magnitude of race-based discrimination (e.g., major life events, everyday hassles, and reduced opportunities) as an allostatic load factor in negative health outcomes. We discuss below three emerging areas where contributions to elucidating the candidate variables in the race-discrimination-health pathway arise in the context of new methodologies.
Social Place, Unhealthy Environments, Racial Stratification, and Health Outcomes
In recent years, the concept of place, particularly social place (e.g., geographic location, local context, neighborhood), has emerged as an important construct in understanding the contributions of discrimination in fostering ill health and health risks (Diez Roux 2002, Ellen et al. 2001, Morenoff & Lynch 2004). Traditionally, research on the health of African Americans focused on individual-level risk factors, with ownership for change residing in individual-level strategies. But the newer work argues for casting a broader net that will capture more complex and multilevel factors in the environment. These are hypothesized to play a significant role in the health status and health outcomes of individuals (Acevedo-Garcia et al. 2003; Diez Roux 2001, 2002, 2003; Diez Roux et al. 1997; Krieger 1999; O’Campo et al. 1997; Pickett & Pearl 2001; Williams & Harris-Reid 1999). For example, a number of sociologists and epidemiologists have made the case that neighborhood is a critical variable in mediating access to economic opportunities, social connections, and social capital (Diez Roux 2003, Massey 2000, O’Campo et al. 1997, Oliver & Shapiro 1995, Wilson 1987), all of which are components that mediate health status. Integrating neighborhood level effects into models of individual risk represents an intriguing new methodology for psychologists.
When neighborhoods work well, they are a place where individuals derive many social benefits. However, when neighborhoods are characterized by persistently low SES and residential segregation, often linked to ethnic/racial minority population concentrations (Acevedo-Garcia 2000, 2001; Lester 2000; O’Campo et al. 1997; Peterson & Krivo 1999), then African Americans living in those neighborhoods have higher rates of morbidity and mortality. Residential segregation that creates concentrated neighborhoods where residents are predominantly poor, racial/ethnic minority, or of immigrant status are social spaces with concentrated social problems. This increases the chances that residents, whatever their individual backgrounds, will experience greater exposure to stressful environments while also having fewer resources with which to cope with these exposures (Boardman 2004; Roberts 1997, 1999; Macintyre et al. 2002).
Roberts (1997, 1999) presents three different pathways by which poor and often racial/ethnic minority–inhabited neighborhoods can have an effect on individual health. First, poorer communities are less likely to have adequate health and social services, creating a problem of access and timely use. Also, the physical environments are more likely to expose the residents to health hazards (e.g., air pollution, lead, dust, dirt, smog, and other hazardous conditions). Finally, the concentration of poverty and its related characteristics (e.g., exposure to drugs, crime, gangs, and violence; unemployment, stress, and anxiety; substandard housing and schools; and lack of green space or fresh fruits and vegetables) often creates social environments that lessen social connectedness and provide fewer social benefits for residents.
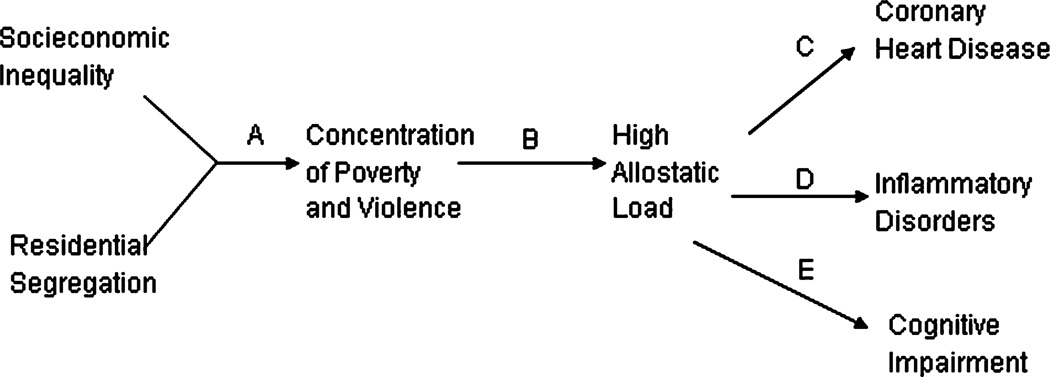
Although the perspective that some neighborhoods are less fostering of health than others is not new, researchers who are linking this idea to biological responses that might arise from chronic neighborhood stressors are gaining new insights. In Figure 1, we depict sociologist Douglas Massey’s (2004) model of the upstream/downstream process of risk for specific disease states for African Americans. The pathway begins with two correlated factors: residential segregation and social economic inequality. These factors work to concentrate social stressors, which in turn set into motion high allostatic loads that are associated with increased risk for coronary heart disease, chronic inflammation, and cognitive impairment. In racially segregated, poor neighborhoods, both chronic and acute daily stressors (e.g., violence, unemployment, personal safety concerns) repeatedly invoke a biological challenge similar to the flight/fight response. According to Massey, an African American living in this unhealthy environment responds at a biological level with persistently elevated levels of cortisol and other glucocorticoid hormones. The effect of the chronic stress response is a premature wearing down of the body and a greater tendency to develop specific disease processes.
Figure 1.
Massey’s (2004) biosocial model of racial stratification. Reprinted from Massey 2004.
One of the issues in current work in the area is identifying the core elements in an unhealthy environment that activate a sense of danger or, conversely, protect against harmful effects of chronic neighborhood stressors. Some of the pertinent work has examined the consequences of negative social interactions and has discovered positive associations between perceptions of being treated badly, lacking social support, and an absence of emotional warmth and closeness and patterns of physiological arousal associated with cardiovascular, sympathetic nervous system reactivity (Repetti et al. 2002; Seeman et al. 1993, 1995; Ursa et al. 2003). For example, Kiecolt-Glaser and colleagues found that 30 minutes of conflict between a married couple was associated with changes in norepinephrine, cortisol, and adrenocorticotropic (ACTH) levels, with both husbands and wives showing decreased immunologic responsiveness during the conflictual communication (Kiecolt-Glaser et al. 1997, Robles & Kiecolt-Glaser 2003).
Intergenerational and Developmental Perspectives
Researchers are also making a strong empirical case for the importance of positive emotions and positive relationships as critical ingredients in healthy children and healthy adults. Seeman and her colleagues investigated the relationship between social environments and the activation of biological responses (Repetti et al. 2002, Seeman et al. 1993). Their findings show that when children are exposed to environments characterized by conflict and low levels of nurturance, they are more likely to present dysregulated cortisol activity and show greater cardiovascular and sympathetic nervous system reactivity in the face of stress-related challenges. Similarly, Taylor and colleagues, working with Seeman in reviewing the literature in this area, identified three characteristics of children and young adults’ family social environments that contribute to negative mental and physical health in later adult years (Taylor et al. 1997). These are (a) social environments that are conflictual, angry, violent, or abusive; (b) parenting styles that are highly domineering or controlling; and (c) parent-child relationships that are unresponsive and lack the characteristics of warmth, social cohesiveness, and emotional support. Furthermore, a growing number of studies indicate that positive social interactions, positive expectations in the form of optimism, positive illusions, and hopeful outlooks are associated with physiological arousal patterns and biological responses (e.g., lower ambulatory blood pressures) to stress challenges that are consistent with long-term positive physical and mental health outcomes (Fredrickson 2000; Ryff & Singer 2000, 2001; Seeman & McEwen 1996; Taylor & Brown 1994; Taylor et al. 1997).
Identification of candidate psychosocial variables that strongly influence health disparities in this country offers the possibility of developing more highly tailored and efficacious interventions, particularly if these interventions began in childhood. In the United States, one in five children grow up in neighborhoods characterized as poor, and for racial/ethnic minorities, particularly African Americans, the rates are even higher (Mather & Rivers 2006). Children who grow up in these poor neighborhoods are at higher risk than their counterparts in more affluent neighborhoods for a number of health challenges, including teen pregnancy, substance abuse, obesity, smoking, limited exercise, and poor dietary habits, as well as early departure from formal education activities, all of which are risk factors for premature mortality, morbidity, or disability (Mather & River 2006, Messer et al. 2005). Unfortunately, there is also strong evidence that individuals who live in poor neighborhoods as children are more likely to end up as adults living in poor neighborhoods with extended families that also live in similar neighborhoods (Mather & River 2006). Concentrations of families within higher-risk neighborhoods increase individual burden, especially when there is a local catastrophe, as occurred during Hurricane Katrina.
Efforts such as those of Seeman, Repetti, Taylor, Ryff, and others move beyond descriptive epidemiology into the realm of elucidating possible social psychological processes that mediate connections between the conditions in poor neighborhoods and the experience of the individual. This work is important to identifying the ways in which social context influences health disparities in African Americans. Nevertheless, little of the literature cited above examined the specific experiences of African Americans. Some studies have shown a negative health impact of repeated experiences with race discrimination in African Americans, particularly when the response is one of a passive coping style (Krieger 1990, Krieger & Sidney 1996). These results suggest that repeated experiences with race-based discrimination are associated with higher resting systolic blood pressure levels (Armstead et al. 1989, Clark 1992, Clark et al. 1999, Harrell et al. 2003, James et al. 1984, McNeilly et al. 1996) and more frequent reports of being diagnosed with hypertension (Krieger 1990, Krieger & Sidney 1996). Drawing from a larger body of work in this area, one can hypothesize that living in neighborhoods characterized by concentrations of poverty and violence may have harmful effects on both the immune system and neuroendocrine responses. But further study is needed to quantify the nature of these effects.
Allostatic Load: A Physiological Approach to Understanding the Effects of Race-Based Discrimination
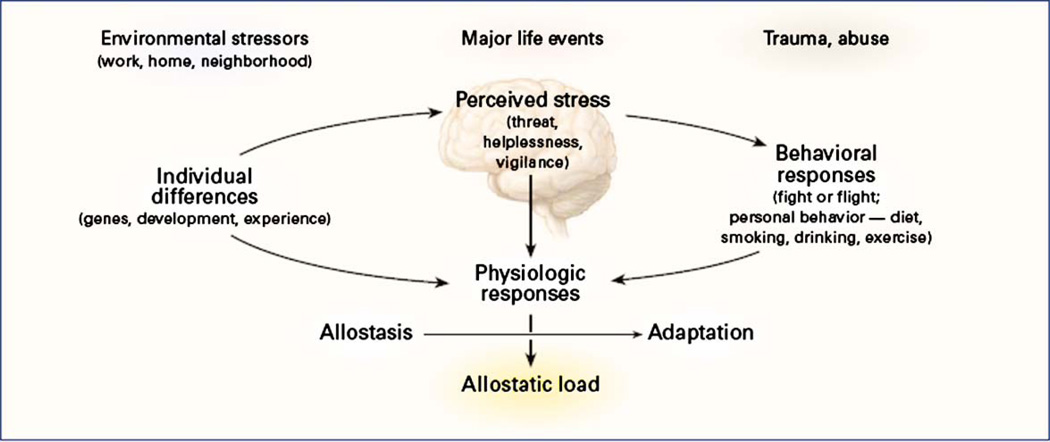
Another model developed by neuroscientist Bruce McEwen to explain the effects of stress on the human body is allostatic load. This model can also be used to conceptualize the possible deleterious effects of race-based discrimination. In McEwen’s model, the emphasis is on the interaction between cognitive processes and physiologic response (see Figure 2). In this model, race-based discrimination could be viewed as creating a chronic biological challenge to the human regulatory systems. According to this model, homeostasis is the internal processes of the body that regulate its response to challenges and demands. Allostasis, on the other hand, is the process of the body’s response to those challenges (McEwen 1998, 2004, 2005; McEwen & Stellar 1993). The simplest example of the allostasis process is the flight/fight response that occurs when one experiences a challenge, demand, or perceived danger. This response operates through the engagement of the sympathetic nervous system, the HPA axis, and the immune system. McEwen argues that when chronic and/or excessive demands are placed on the body’s regulatory systems, these systems over time will exhibit “wear and tear,” losing their ability to efficiently and effectively respond to demands (McEwen 2004, 2005). One possible consequence is overt disease pathology. Invoking McEwen’s model extends the scope of stressors (challenges) in Massey’s model to include environmental stressors of work, home, social relationships, major life events, traumas, and abuses (McEwen 2000). McEwen’s model could include racially discriminatory behavior in social interactions as stressors that send the body into allostasis (Brondolo et al. 2005, Mays & Cochran 1998, Mays et al. 1996).
Figure 2.
McEwen’s (1998) model of stress response and development of allostatic load. Reprinted from McEwen (1998).
Although increased physiological and psychological arousal during an acute stress response is temporarily and evolutionarily advantageous, continuous bouts of stress such as those daily hassles of race-based discrimination along with frequency of exposure to stressful life events (Jackson 2004, Kessler et al. 1999) could significantly alter physiological responses of African Americans (Benschop & Schedlowski 1999.) Indeed, McEwen finds that moderate challenges to the cardiovascular system actually mobilize energy through the activation of the sympathetic nervous system and enhance immune response. This can be seen in the positive role of exercise in maintaining health. However, when the stress challenge to the cardiovascular system is prolonged and excessive to the point of allostasis, immunity is suppressed, blood pressure increases, and, over time, atherosclerosis can develop (McEwen 2002), resulting in coronary vascular disease.
Although McEwen’s model is not specific to African Americans, like Massey’s model (2004), it identifies a number of downstream health effects that are expected to result from race-based discriminatory stress challenges that Massey has identified as more prevalent among African Americans. Together, these two models offer perspectives on the ways in which chronic experiences with racial discrimination might exert harmful effects on African Americans’ health. Recent studies have shown, for example, that the experience of stressful racial discrimination places African Americans at an increased risk of developing hypertension (Din-Dzietham et al. 2004) and carotid plaque (Troxel et al. 2003), both of which are related to the development of atherosclerosis and other cardiovascular diseases
These models might also facilitate an understanding of one of the important health paradoxes among African Americans: African American women, regardless of socioeconomic status, consistently exhibit the highest rates of preterm birth, and their offspring have the lowest birth weights of any group of American women (Dole et al. 2004; MMWR 1999, 2002). Furthermore, African American women as compared with White women have a threefold higher rate of very-low-birth-weight babies, secondary to preterm births (Carmichael & Iyasu 1998). The extent to which experiences with discrimination underlie this health disparity is not fully known, but in one study, African American mothers who scored high on a measure of perceived racial discrimination were twice as likely to deliver low-birth-weight infants (Ellen 2000). In a second longitudinal study of 6000 pregnant women, blood samples were taken through the first and second trimesters of pregnancy. Results suggest a correlation between high placental levels of the stress hormone corticotrophin-releasing hormone and preterm delivery (Rich-Edwards et al. 2001). Finally, in a third study, abnormally high levels of corticotrophin-releasing hormone were shown to have a strong correlation with long-term stress (Pike 2005). Although the evidence is still being accumulated, it points to a plausible set of links in a pathway model connecting race-based discrimination, stress, and negative preterm birth outcomes in African American women. In his model of racism and negative health effects, Hertzman (2000) makes the further distinction that the negative health outcomes occur not only at the time of preterm birth, but also in later life because preterm birth increases the risk of eventual coronary heart disease, high blood pressure, diabetes and other chronic diseases in offspring.
Problems with preterm and low-birth-weight babies do not disappear with improving socioeconomic conditions. College-educated African American women as compared with college-educated White American women still are more likely to deliver infants with low birth weight (Schoendorf et al. 1992). Indeed, in comparison with White American women, second-generation high-SES African American women continue to be at higher risk for low-birth-weight deliveries (Foster et al. 2000). A study of second-generation high-SES African American female college graduates and their mothers was compared with a similar cohort at Yale on rates of low birth weight and preterm delivery. Results from this study found that despite two generations of increasing SES in the African American college students, they still had the higher rates of low-birth-weight and preterm delivery, with only modest improvement over the generations (Foster et al. 2000). Furthermore, African American women born in the United States and Caribbean-born women in the United States differ in their risk for low-birth-weight deliveries, with African American women being more likely to give birth to lower-birth-weight babies (Cabral et al. 1990, Fuentes-Afflick et al. 1998, Guendelman & English 1996, Hummer et al. 1999, Pallotto et al. 2000).
EXAMINING REAL-TIME RACE-BASED PROCESSES OF DISCRIMINATION IN THE BRAIN
In the past, studying the brain directly required waiting until death to perform an autopsy or using methods too indirect to capture the subtleties of brain function. Now, with the use of methods to map the human brain, we can map hallucinations as they occur or elucidate the complex circuits and structures in the brain associated with emotions such as sadness, joy, anger, pain, or even the brain’s role in helping us to read human emotions in facial expressions (Panksepp & Gordon 2003). The tools of functional magnetic resonance imaging (fMRI), positron emission tomography, dense-array electroencephalograph technology, and the measurement of evoked potentials combined with the methods of psychoneuroimmunology offer exciting opportunities to link the occurrence of everyday social inequalities to brain function and physiologic reactions.
Exciting research that melds the study of social cognition with the techniques of brain mapping have begun to allow direct examination of the functional effects of social inequalities based on social exclusion (Eisenberger et al. 2003) interracial Black/White race contact responses (Baron & Banaji 2006; Cunningham et al. 2004; Eberhardt 2005; Golby et al. 2001; Lieberman et al. 2005; Phelps et al. 2000, 2004), and social pain (Brown et al. 2003, Macdonald & Leary 2005) in brain function. These recent studies have expanded the opportunity to study the relationship between external events and internal brain processes as well as to elucidate the factors that make up the pathways of downstream health effects. In their examinations of how the brain processes social information during functional scans, researchers have shown that neural activity occurs in the brain structures that are directly responsible for activation of stress and the allostatic load responses. In particular, two brain structures, the amygdala and anterior cingulate cortex (ACC), show activation in response to social stresses (Baron & Banaji 2006; Lieberman et al. 2005; Macdonald & Leary 2005; Phelps et al. 2000, 2004). In addition to examining the role of these brain structures in response to social stresses, it is also useful to consider what contributions the processes of cognitive appraisal, self regulation, and social exclusion may play in the effects of race-based discrimination on the health of African Americans.
The Amygdala and Race Imaging
The amygdala plays a key role in the processing of emotions, including anger and fear. It is also the neural structure of the brain that is involved in forming and storing memories for emotional events. Historically, the amygdala was studied in relation to fear conditioning primarily in experiments that used rats. Recently, fMRI studies have demonstrated real-time effects in the amygdala when humans view fearful and threatening imagery (Delgado et al. 2006, Phelps 2006, Phelps et al. 2004). These studies show that as the amygdala responds to a fearful image, it initiates the stress response, in which the body recruits energy while it decides whether to fight or to take flight. Thus, the amygdala is key to understanding the process of allostatic load, or overactivation of the stress response.
In a series of recent studies, all from different labs, results indicate that for both White and Black participants, the viewing of Black faces, as compared with White faces, results in higher measurable levels of implicit brain activity in the amygdala (Cunningham et al. 2004, Lieberman et al. 2005, Phelps et al. 2003). This amygdala activation associated with race-related processing has been interpreted as representing fear conditioning to “culturally learned negative associations regarding African Americans” (Lieberman et al. 2005). Not so surprisingly, it also suggests that implicitly learned negative racial stereotypes about African Americans can be learned and encoded as a response by African Americans as well as White Americans (Olsson et al. 2005). Viewed from Massey’s model of racial stratification and poor health outcomes in African Americans, these findings suggest that implicitly learned fearful racial stereotypes may function to make the experience of living in race-segregated and poor neighborhoods a continuing source of chronic stress. This is not to say that racially/ethnically concentrated neighborhoods may not also be a source of providing social support, social connectedness, and a sense of belonging among African Americans (Cutrona et al. 2000). Both sets of conditions can occur simultaneously. But these laboratory findings hint that highly segregated, highly dense neighborhoods, plagued by high rates of crime and poverty, may create a social context in which chronic activation of fear responses leads to greater occurrence of the experience of allostatic load.
The Anterior Cingulate Cortex and Race
Another structure of the brain that is important to consider in examining real-time processes in race-based discrimination is the ACC. Recent studies have described the ACC as a discrepancy detector that monitors and regulates brain processes directed toward achieving goals. It is believed that the ACC functions at the subconscious level by being vigilant to conflicts to our goal attainments. In the case of conflict, the ACC engages conscious cognitive processes of the prefrontal cortex (PFC) to assist in either accommodating to or reducing the conflict (Eisenberger et al. 2003). Cognitive processes used by the ACC in its discrepancy-detector role include reasoning, decision making, motivation, and emotional regulation (Botvinick et al. 2004). These processes can facilitate an adaptive change to bodily states, such as altering heart rate, through mediation by the sympathetic nervous system (Critchley 2005; Critchley et al. 2000, 2001, 2003). The PFC also has the ability to reduce activation of the ACC (thus reducing discrepancies in goals) as well as to increase activation of the ACC, resulting in hypervigilance. Put succinctly, the actual “function of the ACC is to integrate motivationally important information, with appropriate bodily responses” (Critchley et al. 2001). In this context, the ACC emerges as a brain structure with the ability to not only recruit cognitive function to reason and to assess race-based discrimination, but also to play a role in the mediation of aspects of the physiological downstream reactions to race-based discrimination.
Those who have studied discrepancy detection view the ACC as a critical monitoring system (Botvinick et al. 2001, 2004; Eisenberger et al. 2005). When the ACC experiences any inconsistencies between desired goals and impediments or conflicts, its activation acts as a neural alarm to the body that something has gone wrong (Botvinick et al. 2001, 2004; Braver et al. 2001; Carter et al. 1998; Eisenberger et al. 2005; Weissman et al. 2003). The result is a greater demand on conscious cognitive processes with the goal to minimize the discrepancies in current actions so actions can once again lead to desired goals (Carver & Scheier 1990). As discrepancies resulting from goal conflicts are reduced, so too is activation in the ACC, quieting the alarm and returning the body to homeostasis.
In addition to activation issues with the ACC, Thayer & Friedman (2004) suggest that in a situation where a threat is ever-present, as is the case for possible experiences with race-based discrimination, inhibition of the limbic system by the prefrontal cortex PFC is also partially released. The result is a state of compromise by the brain that allows hypervigilance and perseverative thought to decrease heart rate variability and increase the allostatic load indicators of blood pressure and cortisol through the amygdala and HPA axis activation (Winters et al. 2000). Expectations of race-based discrimination might result in hypervigilance, which would then result in a greater tendency to perceive conflict discrepancies, in spite of behavior that to some may appear not to be overtly discriminatory.
A key topic in understanding the possible role of the ACC, and collaterally the PFC, in linking African Americans’ experiences with race-based discrimination and downstream health effects may be that of social exclusion. Social exclusion is a general term used by social policy makers and social scientists to refer both to the consequence of being excluded or marginalized from desired social groups as well as the processes by which this occurs (Macdonald & Leary 2005). Social exclusion may be a consequence of discrimination and prejudice, as well as a mechanism by which discrimination and prejudice can be enacted. Social exclusion is actually best thought of as a dynamic concept in which it is related to social processes that can lead to social isolation of specific groups and individuals when they are marginalized by organizations, groups, or institutions within society. For those who are socially excluded, there is the psychological experience of loss in both a sense of belonging to a desired group and denial of opportunity to participate in certain social, political, cultural, educational, or economical opportunities and rights (Tezanos 2001). For many, this description of social exclusion parallels that of discrimination, or social rejection. Underwood et al. (2004) find not only that social exclusion and social rejection are similar, but also that both create a common pathway in relationship to pain (Baumeister & Leary 1995). Indeed, it has been argued that “being excluded from social groups ranks among the most aversive of human experiences” (Labonte 2004).
In fMRI neuroimaging studies of social exclusion, Eisenberger et al. (2003) found that the anterior cingulate cortex acts as a “neural alarm system or conflict monitor” that is sensitive to the experiences of social pain when social exclusion occurs (Eisenberger & Lieberman 2004, Macdonald & Leary 2005). Social pain is described as a unique form of aversive distress that is felt specifically when rejection or social exclusion occurs (Eisenberger & Lieberman 2004, Macdonald & Leary 2005).
To the extent that experiences of race-based discrimination are perceived similarly to experiences of social exclusion, perceived discrimination too might share—at the level of brain functioning—properties similar to those of social exclusion. This may explain a second paradox in African American health: rates of psychological distress are typically higher as compared with rates for White Americans, but rates of many common, stress-sensitive major mental disorders such as major depression and most anxiety disorders are lower (MMWR 2004, USDHHS 1999). Higher distress levels may reflect chronic activation of unpleasant feelings of anger, hyper-vigilance, or being on edge. Perceived racial discrimination, with its implied obstruction in access to both belonging to a group and access to social resources, may result in social pain. This may draw the body away from homeostasis and possibly result in the activation of stress-related allostatic responses.
Self-Regulation, Social Acceptance, and Cognitive Appraisal in Race-Based Discrimination
The brain is not a passive recipient of stimulation. Self-regulation and cognitive appraisal are processes that allow individuals to achieve and maintain personal and social goals, including social relationships (Lieberman 2007). Self-regulation has been proposed as indispensable for the maintenance of social acceptance (Baumeister et al. 2005, Carver & Scheier 1981). Self-regulation refers to such functions as executive and cognitive control, emotion and affect regulation, and maintenance of motivational drive (Baumeister & Vohs 2004, Beauregard et al. 2001, Levesque et al. 2004, Muraven et al. 1998, Ochsner & Gross 2005, Ownsworth et al. 2002, Posner & Rothbart1998, Ylvisaker & Feeney 2002). Self-regulation of emotion maps onto the structures of the amygdala, ACC, and PFC, and thus may be related to the processes of race-based discrimination. In response to social stressors, individuals self-regulate in complex ways that might strengthen social bonds (Higgins 1996, Leary et al. 2006, Williams et al. 2000, Williams & Sommer 1997) or lead instead to increased levels of aggression, depression, and unhealthy behaviors such as tobacco smoking and alcohol and drug use or abuse. An examination of the role that cognitive appraisal and self-regulation play in race-based discrimination and health outcomes in African Americans may provide some useful insights into the pathways of the upstream/downstream discrimination and health relationship.
Self-regulation has been shown to increase the retention of social information about events surrounding an individual’s experience of social rejection (Gardner et al. 2000). For example, in an experiment examining the selective retention of socially relevant events, subjects experiencing social rejection had a greater recall of the events than did subjects who experienced social acceptance (Gardner et al. 2000). In addition, a main effect for negative-event recall was greater than for positive events. In particular, the negative aspects of social rejection are consolidated to memory for the individual as a source of potential future harm and avoidance. These findings raise interesting issues to consider in the dynamics of the cognitive appraisal process in race-based discrimination. For example, individuals who have been rejected as the result of perceived racial discrimination may be more likely to have a heightened surveillance for negative social cues that resemble race-based discrimination and social rejection in comparison with individuals who are commonly accepted.
The ability of the appraisal system to self-regulate emotional control can be achieved through the lateral PFC. But, Wheeler & Fiske (2005) have also found that the emotional response to race-related imagery can be altered at the level of the amygdala. For example, studies (Cunningham et al. 2004, Lieberman et al. 2005, Phelps et al. 2003) have demonstrated that presentation of Black faces as compared with White faces results in greater activation of the amygdala. This activation was thought to lie in a process in which simply viewing Black faces stimulated negative stereotypes and prejudicial amygdala activation to outgroup members. However, Wheeler & Fiske (2005) demonstrated that a differential response of the amygdala to White and Black faces could not be achieved by visual inspection alone. Instead, a face must be cognitively processed to a level at which it achieves some social relevance before differential amygdala activation occurs. This suggests that cognitive appraisal of race-based discrimination occurs at multiple levels in the brain. Indeed, the ACC in many ways acts as a social monitor by responding to stressors of social rejection (Eisenberger et al. 2003). If the processes that occur in the brain for race-based discrimination are the same or very similar to those documented in ACC activation in the study of Eisenberger et al. (2003), we would then speculate that the ACC plays an intermediary role in the process of cognitive appraisal as it directs the recruitment of higher cognitive and emotional processing in the actual appraisal process.
A puzzling dilemma is posed to African Americans with regard to self-regulation. The authors of self-regulation and social rejection studies have pointed to a role of self-regulation to achieve social goals and acceptance. But the challenge may be for African Americans to quiet a regulatory system that is stimulated toward hypervigilance. Race-based discrimination may exact a chronic toll on the self-regulatory system and shape cognitive appraisals in ways that have yet to be studied.
NEXT STEPS IN A RESEARCH, INTERVENTION, AND PREVENTION AGENDA
Although there is growing support for the importance of a relationship between experiences with race-based discrimination and various physical health outcomes in African Americans, full understanding of the phenomenon is still a long way off. Indeed, in the next couple of decades, work from other fields, such as the genetic bases of mental and physical health morbidities, promises to complicate attempts to fully capture the complex causal pathways that link the effects of discrimination in the social environment to health at an individual level. But the emerging methodological tools and the increasingly interdisciplinary nature of health-related research offer the promise of articulating how social unfairness works to harm individual health. The work that we have reviewed here merely highlights new directions in the research in this area.
If these methodologies succeed in reliably identifying insidious neighborhood effects on health or specific areas of the brain that are damaged by discrimination, psychologists will face new challenges. What are our social responsibilities to change or alter the conditions that harm health? How do we develop interventions to alter neighborhood structure? If the effects of discrimination are such that early experiences with being treated badly or unfairly alter brain function toward greater sensitivity, quicker reactivity, and greater vulnerability to the impact of later experiences, would we then assign less blame or responsibility to either the behavior of individuals who experience discrimination or the behavior of perpetrators of discrimination on individuals who themselves have had early exposures to discrimination? What will be our societal response if science establishes that discrimination is a brain assault with tangible harmful effects on both quality of life and physical health?
A number of important areas could be more fully explored to help us gain insights into the pathways that may account for the relationship of experiences of race-based discrimination to negative health status in African Americans. Because of page constraints in this review, we could not fully address all of the areas, and for some areas that we discussed above, there was a need for brevity. However, we have presented a number of concepts, many of which are still only possibilities that remain in need of further study to explain upstream/downstream health effects in African Americans.
Psychology is unique as a discipline in being located with one foot solidly in the science of upstream effects and the other in the direct examination of downstream effects. That places us in an enviable position to study the effects of racial discrimination and negative health outcomes in African Americans in a comprehensive manner. In doing so, we honor our long tradition of efforts to use the scientific method to pursue social justice and fairness (Deutsch 1975, Lewin 1997). Much of America’s ability to dialogue dispassionately about race had its birth in the early research done by social psychologists on racial attitudes and symbolic racism (Bobo 1983; Bobo & Fox 2003; Sears 1998; Sears & Henry 2003, 2005). This work has also informed the justice system. With these emerging methods, approaches, and collaborative relationships, psychology as a discipline has even greater opportunities to shape our understanding of the ways in which race, racism, and race-based discrimination affect the health of African Americans.
ACKNOWLEDGMENTS
Work on this review was supported by funding from the National Institute of Drug Abuse (DA 15539) and an EXPORT grant from the National Center for Minority Health and Health Disparities (MD P60–000508). We thank our colleagues who read various parts of this manuscript or discussed ideas with us, particularly Teresa Seeman and Jason Woods, though we take full responsibility for the ideas presented.
Contributor Information
Vickie M. Mays, Email: mays@ucla.edu.
Susan D. Cochran, Email: cochran@ucla.edu.
Namdi W. Barnes, Email: nbarnesn@ucla.edu.
LITERATURE CITED
- Acevedo-Garcia D. Residential segregation and the epidemiology of infectious diseases. Soc. Sci. Med. 2000;51:1143–1161. doi: 10.1016/s0277-9536(00)00016-2. [DOI] [PubMed] [Google Scholar]
- Acevedo-Garcia D. Zip code level risk factors for tuberculosis: neighborhood environment and residential segregation, New Jersey, 1985–1992. Am. J. Public Health. 2001;91:734–741. doi: 10.2105/ajph.91.5.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo-Garcia D, Lochner KA, Ospuyk TL, Subramanian SV. Future directions in residential segregation and health research: a multilevel approach. Am. J. Public Health. 2003;93:215–221. doi: 10.2105/ajph.93.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler N, Boyce T, Chesney M, Cohen S, Folkman S, et al. Socioeconomic status and health: the challenge of the gradient. Am. Psychol. 1994;9:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Armstead C, Lawler K, Gordon G, Cross J, Gibbons J. The relationship of racial stressors to blood pressure responses and anger expressions in black college students. Health Psychol. 1989;8:541–556. doi: 10.1037//0278-6133.8.5.541. [DOI] [PubMed] [Google Scholar]
- Baron AS, Banaji MR. The development of implicit attitudes. Evidence of race evaluations from ages 6 and 10 and adulthood. Psychol. Sci. 2006;17:53–58. doi: 10.1111/j.1467-9280.2005.01664.x. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, DeWall CN, Ciarocco NJ, Twenge JM. Social exclusion impairs self-regulation. J. Personal. Soc. Psychol. 2005;88:589–604. doi: 10.1037/0022-3514.88.4.589. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychol. Bull. 1995;117:497–529. [PubMed] [Google Scholar]
- Baumeister RF, Vohs KD. Handbook of Self-Regulation: Research, Theory, and Applications. New York: Guilford; 2004. [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J. Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benschop RJ, Schedlowski M. Acute psychological stress. In: Schedlowski M, Tuwes U, editors. Psychoneuroimmunology: An Interdisciplinary Introduction. New York: Kluwer Acad./Plenum; 1999. pp. 293–306. [Google Scholar]
- Boardman JD. Stress and physical health: the role of neighborhoods as mediating and moderating mechanisms. Soc. Sci. Med. 2004;58:2473–2483. doi: 10.1016/j.socscimed.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Bobo L. Whites’ opposition to busing: symbolic racism or realistic group conflict? J. Personal. Soc. Psychol. 1983;45:1196–1210. [Google Scholar]
- Bobo L, Fox C. Race, racism and discrimination: bridging problems, methods, and theory in social psychological research. Soc. Psychol. Q. 2003;66:319–332. [Google Scholar]
- Botvinick MM, Carter CS, Braver TS, Barch DM, Cohen Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn. Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb. Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Brondolo E, Thompson S, Brady N, Appel R, Cassells A, et al. The relationship of racism to appraisals and coping in a community sample. Ethnicity Dis. 2005;15:S5–S10. [PubMed] [Google Scholar]
- Brown GW, Harris TO. Life Events and Illness. New York: Guilford; 1989. [Google Scholar]
- Brown JL, Sheffield D, Leary MR, Robinson ME. Social support and experimental pain. Psychosom. Med. 2003;65:276–283. doi: 10.1097/01.psy.0000030388.62434.46. [DOI] [PubMed] [Google Scholar]
- Cabral H, Fried LE, Levenson S, Amaro H, Zuckerman B. Foreign-born and US-born black women: differences in health behaviors and birth outcomes. Am. J. Public Health. 1990;80:70–72. doi: 10.2105/ajph.80.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SL, Iyasu S. Changes in the black-white infant mortality gap from 1983 to 1991 in the United States. Am. J. Prevent. Med. 1998;15:220–227. doi: 10.1016/s0749-3797(98)00052-x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF. The self-attention-induced feedback loop and social facilitation. J. Exp. Soc. Psychol. 1981;17:545–568. [Google Scholar]
- Carver CS, Scheier MF. Origins and functions of positive and negative affect: a control-process view. Psychol. Rev. 1990;97:19–35. [Google Scholar]
- Centers for Disease Control (CDC) Advance Report of Final Mortality Statistics, 1992. Hyattsville, MD: US Dep. Health Human Serv. Public Health; 1994. [Google Scholar]
- Clark R. Self-reported racism and social support predict blood pressure reactivity in blacks. Ann. Behav. Med. 2003;25:127–136. doi: 10.1207/S15324796ABM2502_09. [DOI] [PubMed] [Google Scholar]
- Clark R, Adams JH. Moderating effects of perceived racism on John Henryism and blood pressure reactivity in black female college students. Ann. Behav. Med. 2004;28:126–131. doi: 10.1207/s15324796abm2802_8. [DOI] [PubMed] [Google Scholar]
- Clark R, Anderson NB, Clark VR, William DR. Racism as a stressor for African Americans: a biopsychosocial model. Am. Psychol. 1999;54:805–816. doi: 10.1037//0003-066x.54.10.805. [DOI] [PubMed] [Google Scholar]
- Clark WA. Residential preferences and residential choices in a multiethnic context. Demography. 1992;29:451–466. [PubMed] [Google Scholar]
- Cochran SD, Mays VM. Depressive distress among homosexually active African American men and women. Am. J. Psychiatry. 1994;151:524–529. doi: 10.1176/ajp.151.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J. Comp. Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigations in humans. J. Physiol. 2000;523:250–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29:537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, et al. Human cingu-late cortex & autonomic control: converging neuroimaging & clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Johnson MK, Rave CL, Gatenby J, Gore JC, Banaji MR. Separable neural components in the processing of black and white faces. Psychol. Sci. 2004;12:806–813. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- Cutrona CE, Russell DW, Hessling RM, Brown PA, Murry V. Direct and moderating effect of community context on the psychological well-being of African-American women. J. Personal. Soc. Psychol. 2000;79:1088–1101. doi: 10.1037//0022-3514.79.6.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SK, Liu Y, Gibbons GH. Disparities in trends of hospitalization for potentially preventable chronic conditions among African Americans during the 1990s: implications and benchmarks. Am. J. Public Health. 2003;93:447–455. doi: 10.2105/ajph.93.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol. Psychol. 2006 doi: 10.1016/j.biopsycho.2006.01.006. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Deutsch M. Equity, equality and need: what determines which value will be used as a basis for distributive justice. J. Soc. Issues. 1975;31:137–150. [Google Scholar]
- Diez Roux AV. Investigating neighborhood and area effects on health. Am. J. Public Health. 2001;91:1783–1789. doi: 10.2105/ajph.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV. Invited commentary: places, people, and health. Am. J. Epidemiol. 2002;155:516–519. doi: 10.1093/aje/155.6.516. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV. Residential environments and cardiovascular risk. J. Urban Health. 2003;90:569–589. doi: 10.1093/jurban/jtg065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV, Nieto FJ, Muntaner C, Tyroler HA, Comstock GW, et al. Neighborhood environments and coronary heart disease: a multilevel analysis. Am. J. Epidemiol. 1997;146:48–63. doi: 10.1093/oxfordjournals.aje.a009191. [DOI] [PubMed] [Google Scholar]
- Din-Dzietham R, Nembhard WN, Collins R, Davis SK. Perceived stress following race-based discrimination at work is associated with hypertension in African-Americans. The metro Atlanta heart disease study, 1999–2001. Soc. Sci. Med. 2004;58:449–461. doi: 10.1016/s0277-9536(03)00211-9. [DOI] [PubMed] [Google Scholar]
- Dole N, Savitz DA, Siega-Riz AM, Hertz-Picciotto I, McMahon MJ, Buekens P. Psy-chosocial factors and preterm birth among African American and white women in central North Carolina. Am. J. Public Health. 2004;94:1358–1365. doi: 10.2105/ajph.94.8.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan YF, Winters R, McCabe PM, Green EJ, Huang Y, Schneiderman N. Behavioral characteristics of defense and vigilance reactions elicited by electrical stimulation of the hypothalamus in rabbits. Behav. Brain Res. 1996;81:33–41. doi: 10.1016/s0166-4328(96)00042-3. [DOI] [PubMed] [Google Scholar]
- Eberhardt JL. Imaging race. Am. Psychol. 2005;60:181–190. doi: 10.1037/0003-066X.60.2.181. [DOI] [PubMed] [Google Scholar]
- Eberhardt JL, Goff PA, Purdie VJ, Davies PG. Seeing black: race, crime, and visual processing. J. Personal. Soc. Psychol. 2004;87:876–893. doi: 10.1037/0022-3514.87.6.876. [DOI] [PubMed] [Google Scholar]
- Eberhardt JL, Dasgupta N, Banaszynki TL. Believing is seeing: the effects of racial labels and implicit beliefs on face perception. Personal. Soc. Psychol. Bull. 2003;29:360–370. doi: 10.1177/0146167202250215. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn. Sci. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Satpute AB. Personality from a controlled processing perspective: an fMRI study of neuroticism, extraversion, and self-consciousness. Cogn. Affect. Behav. Neurosci. 2005;5:169–181. doi: 10.3758/cabn.5.2.169. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:237–239. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Ellen IG. Is segregation bad for your health? The case of low birth weight. In: Gale WG, Rothenberg Pack JR, editors. Brookings-Wharton Papers on Urban Affairs. Washington, DC: Brookings Inst. Press; 2000. pp. 203–229. [Google Scholar]
- Ellen IG, Mijanovich T, Dillman KN. Neighborhood effects on health: exploring the links and assessing the evidence. J. Urban Affairs. 2001;23:391–408. [Google Scholar]
- Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular disease. Annu. Rev. Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- Fiscella K, Williams DR. Health disparities based on socioeconomic inequities: implications for urban health care. Acad. Med. 2004;79:1139–1147. doi: 10.1097/00001888-200412000-00004. [DOI] [PubMed] [Google Scholar]
- Foster HW, Wu L, Bracken MB, Semenya K, Thomas J, Thomas J. Intergenerational effects of high socioeconomic status on low birthweight and preterm birth in African Americans. J. Natl. Med. Assoc. 2000;92:213–221. [PMC free article] [PubMed] [Google Scholar]
- Franks P, Muennig P, Lubetkin E, Jia H. The burden of disease associated with being African-American in the United States and the contribution of socio-economic status. Soc. Sci. Med. 2006;62(10):2469–2478. doi: 10.1016/j.socscimed.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL. Extracting meaning from past affective experiences: the importance of peaks, ends, and specific emotions. Cogn. Emot. 2000;14:577–606. [Google Scholar]
- Fuentes-Afflick E, Hessol NA, Perez-Stable EJ. Maternal birthplace, ethnicity, and low birth weigh in California. Arch. Pediatr. Adolesc. Med. 1998;152:1105–1012. doi: 10.1001/archpedi.152.11.1105. [DOI] [PubMed] [Google Scholar]
- Gardner WL, Pickett CL, Brewer MB. Social exclusion and selective memory: how the need to belong affects memory for social information. Personal. Soc. Psychol. Bull. 2000;26:486–496. [Google Scholar]
- Geronimus AT, Bound J, Wadimann TA, Hillemeier MM, Burns PB. Excess mortality among blacks and whites in the United States. N. Engl. J. Med. 1996;35:1552–1558. doi: 10.1056/NEJM199611213352102. [DOI] [PubMed] [Google Scholar]
- Golby AJ, Gabrieli JDE, Chiao JY, Eberhardt JL. Differential responses in the fusiform region to same-race and other race faces. Nat. Neurosci. 2001;4:845–850. doi: 10.1038/90565. [DOI] [PubMed] [Google Scholar]
- Guendelman S, English PB. Effect of United States residence on birth outcomes among Mexican immigrants: an exploratory study. Am. J. Epidemiol. 1995;142(9 Suppl.):S30–S38. doi: 10.1093/aje/142.supplement_9.s30. [DOI] [PubMed] [Google Scholar]
- Guyll M, Matthews KA, Bromberger JT. Discrimination and unfair treatment: relationship to cardiovascular reactivity among African American and European American women. Health Psychol. 2001;20:315–325. doi: 10.1037//0278-6133.20.5.315. [DOI] [PubMed] [Google Scholar]
- Harrell JP, Halls S, Taliaferro J. Physiological responses to racism and discrimination: an assessment of the evidence. Am. J. Public Health. 2003;93:243–248. doi: 10.2105/ajph.93.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes MA, Smedley BD. The Unequal Burden of Cancer: An Assessment of NIH Research and Programs for Ethnic Minorities and the Medically Underserved. Washington, DC: Natl. Acad. Press; 1999. [PubMed] [Google Scholar]
- Heckler MM. Report of the Secretary’s Task Force on Black and Minority Health. Washington, DC: U.S. Dep. Health Human Serv; 1985. U.S. Task Force on Black and Minority Health. [Google Scholar]
- Henderson S, Byrne DG, Duncan-Jones P. Neurosis and the Social Environment. Sydney: Academic; 1981. [Google Scholar]
- Hertzman C. The biological embedding of early experiences and its effects on health in adulthood. Ann. NY Acad. Sci. 2000;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- Higgins ET. The “self-digest”: self-knowledge serving self-regulatory functions. J. Personal. Soc. Psychol. 1996;71:1062–1083. doi: 10.1037//0022-3514.71.6.1062. [DOI] [PubMed] [Google Scholar]
- House JS. Understanding social factors and inequalities in health: twentieth century progress and twenty-first century prospects. J. Health Soc. Behav. Res. 2002;43:125–142. [PubMed] [Google Scholar]
- House JS, Williams D. Understanding and reducing socioeconomic and racial/ethnic disparities in health. In: Smedley BD, Syme SL, editors. Promoting Health: Intervention Strategies from Social and Behavioral Research. Washington, DC: Natl. Acad. Press; 2000. pp. 81–124. [Google Scholar]
- Hummer RA, Beigler M, DeTurk PB, Forbes D, Frisbie WP, et al. Race, ethnicity, nativity, and infant mortality in the United States. Soc. Forces. 1999;77:1083–1118. [Google Scholar]
- Jackson JS. The National Survey of American Life, data and tables. [Accessed Jan. 15, 2006];Prog. Res. Black Am. 2004 http://www.rcgd.isr.umich.edu/prba/rta/index.htm.
- Jackson JS, Neighbors HW, Neese RM, Trierweiler SJ, Torres M. Methodological innovations in the National Survey of American Life. Int. J. Methods Psychiatr. Res. 2004;13:288–298. doi: 10.1002/mpr.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SA, LaCroix AZ, Kleinbaum DG, Strogatz DS. John Henryism and blood pressure differences among black men. II. The role of occupational stressors. J. Behav. Med. 1984;7:259–275. doi: 10.1007/BF00845359. [DOI] [PubMed] [Google Scholar]
- Kanner AD, Feldman SS, Weinberger DA, Ford ME. Uplifts, hassles, and adaptational outcomes inearly adolescents. In: Monet A, Lazarus RS, editors. Stress and Coping: An Anthology. New York: Columbia Univ. Press; 1991. pp. 158–182. [Google Scholar]
- Kaplan GA. Part III Summary: What is the role of the social environment in understanding inequalities in health? In: Adler NE, Marmot M, McEwen B, Stewart J, editors. Socioeconomic Status and Health in Industrialized Nations. Vol. 896. New York: NY Acad. Sci; 1999. pp. 116–119. [DOI] [PubMed] [Google Scholar]
- Karlamangala AS, Singer BH, Williams DR, Schwartz JE, Matthews KA, et al. Impact of socioeconomic status on longitudinal accumulation of cardiovascular risk in young adults: the CARDIA study (USA) Soc. Sci. Med. 2005;60:999–1015. doi: 10.1016/j.socscimed.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Mickelson KD, Williams DR. The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. J. Health Soc. Behav. 1999;40:208–230. [PubMed] [Google Scholar]
- Kiecolt-Glaser J, Glaser R, Cacioppo J, MacCallum R, Snydersmith M, et al. Marital conflict in older adults: endocrinological and immunological correlates. Psychosomatic Med. 1997;59:339–349. doi: 10.1097/00006842-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Kochanek KD, Murphy SL, Anderson RN, Scott C. Deaths: final data for 2002. Natl. Vital Statis. Rep. 2004;53:1–116. [PubMed] [Google Scholar]
- Krieger N. Racial and gender discrimination: risk factors for high blood pressure? Soc. Sci. Med. 1990;30:1273–1281. doi: 10.1016/0277-9536(90)90307-e. [DOI] [PubMed] [Google Scholar]
- Krieger N. Women and social class: a methodological study comparing individual, household, and census measures of black/white differences in reproductive history. J. Epidemiol. Comm. Health. 1991;45:35–42. doi: 10.1136/jech.45.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N. Embodying inequality: a review of concepts, measures, and methods for studying health consequences of discrimination. Int. J. Health Serv. 1999;29:295–352. doi: 10.2190/M11W-VWXE-KQM9-G97Q. [DOI] [PubMed] [Google Scholar]
- Krieger N, Moss N. Accounting for the public’s health: an introduction to selected papers from a U.S. conference on “measuring social inequalities in health. Int. J. Health Serv. 1996;26:383–390. doi: 10.2190/20CQ-LUE1-MC7X-H2KQ. [DOI] [PubMed] [Google Scholar]
- Krieger N, Sidney S. Racial discrimination and blood pressure. The CARDIA study of young black and white women and men. Am. J. Public Health. 1996;6:1370–1378. doi: 10.2105/ajph.86.10.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc. Sci. Med. 2005;61:1576–1596. doi: 10.1016/j.socscimed.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Krieger N, Williams DR, Moss NE. Measuring social class in U.S. public health research: concepts, methodologies, and guidelines. Annu. Rev. Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- Kuh D, Ben-Sholmo Y. A Life Course Approach to Chronic Disease Epidemiology. Oxford, UK: Oxford Univ. Press; 1997. [Google Scholar]
- Labonte R. Social inclusion/exclusion: dancing the dialectic. Health Promot. Int. 2004;19:115–121. doi: 10.1093/heapro/dah112. [DOI] [PubMed] [Google Scholar]
- Landrine H, Klonoff EA. Racial discrimination and cigarette smoking among blacks: finding from two studies. Ethnicity Dis. 2000;10:195–202. [PubMed] [Google Scholar]
- Lazarus RS. From psychological stress to the emotions: a history of changing outlooks. Annu. Rev. Psychol. 1993;44:1–21. doi: 10.1146/annurev.ps.44.020193.000245. [DOI] [PubMed] [Google Scholar]
- Leary MR, Twenge JM, Quinlivan E. Interpersonal rejection as a determinant of anger and aggression. Personal. Soc. Psychol. Rev. 2006;10:111–132. doi: 10.1207/s15327957pspr1002_2. [DOI] [PubMed] [Google Scholar]
- Lester D. Does residential segregation in cities predict African-American suicide rates? Percept. Mot. Skills. 2000;91:870. doi: 10.2466/pms.2000.91.3.870. [DOI] [PubMed] [Google Scholar]
- Levesque J, Joanette Y, Mensour B, Beudoin G, Leroux JM, et al. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129:361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Levine RS, Foster JE, Fullilove RE, Fullilove MT, Briggs NC, et al. Black-white inequalities in mortality and life expectancy, 1933–1999: implications for Healthy People 2010. Public Health Rep. 2001;116:474–483. doi: 10.1093/phr/116.5.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin K. Resolving Social Conflicts and Field Theory in Social Science. Washington, DC: Am. Psychol. Assoc; 1997. [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annu. Rev. Psychol. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Hariri A, Jarcho JM, Eisenberger NI, Bookheimer SY. An fMRI investigation of race-related amygdala activity in African-American and Caucasian-American individuals. Nat. Neurosci. 2005;6:720–722. doi: 10.1038/nn1465. [DOI] [PubMed] [Google Scholar]
- Macdonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychol. Bull. 2005;131:202–223. doi: 10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- Macintyre S, Ellaway A, Cummins S. Place effects on health: How can we conceptualize, operationalize and measure them? Soc. Sci. Med. 2002;55:125–139. doi: 10.1016/s0277-9536(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Massey DS. Residential segregation and neighborhood conditions in U.S. metropolitan areas. In: Smesler N, Wilson WJ, Mitchell F, editors. America Becoming Racial: Trends and Their Consequences. Washington, DC: Natl. Acad. Press; 1985. pp. 391–434. [Google Scholar]
- Massey DS. Housing discrimination 101. Popul. Today. 2000;28:1–4. [PubMed] [Google Scholar]
- Massey DS. Segregation and stratification: a biosocial perspective. DuBois Rev. Soc. Sci. Rev. Race. 2004;1:7–25. [Google Scholar]
- Masters RD. Biology and politics: linking nature and nurture. Annu. Rev. Polit. Sci. 2001;4:345–369. [Google Scholar]
- Mather M, Rivers KL. City Profiles of Child Well-Being: Results from the American Community Survey. Washington, DC: Annie E. Casey Found; 2006. [Google Scholar]
- Mays VM. Black women, work stress, and perceived discrimination: the Focused Support Group Model as an intervention for stress reduction. Cult. Divers. Ment. Health. 1995;1:53–65. [PMC free article] [PubMed] [Google Scholar]
- Mays VM, Cochran SD. Proc. 27th Public Health Conf. Records Stat. Natl. Committee Vital Health Stat. 47th Annu. Symp. Washington, DC: US Dep. Health Human Serv; 1998. Racial discrimination and health outcomes in African Americans. [Google Scholar]
- Mays VM, Coleman LS, Jackson JS. Perceived race-based discrimination, employment status, and job stress in a national sample of black women: implication for health outcomes. J. Occup. Health Psychol. 1996;3:319–329. doi: 10.1037//1076-8998.1.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord C, Freeman HP. Excess mortality in Harlem. N. Engl. J. Med. 1990;322:1606–1667. doi: 10.1056/NEJM199001183220306. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: the good and bad sides of the response to stress. Metabolism. 2002;51(6 Suppl. 1):2–4. doi: 10.1053/meta.2002.33183. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress, allostasis and allo-static load overload and relevance to the pathophysiology of psychiatric disorders. Ann. NY Acad. Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stressed or stressed out: What is the difference? J. Psychiatry Neurosci. 2005;30:315–318. [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann. NY Acad. Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: mechanisms leading to disease. Arch. Intern. Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- McNeilly A, Anderson NB, Armstead CA, Clark R, Corbett MO, et al. The Perceived Racism Scale: a multidimensional assessment of the perceptions of white racism among African Americans. Ethnicity Dis. 1996;6:154–166. [PubMed] [Google Scholar]
- Mechanic D. Policy challenges in addressing racial disparities and improving population health. Health Affairs. 2005;24:335–338. doi: 10.1377/hlthaff.24.2.335. [DOI] [PubMed] [Google Scholar]
- Mensa GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- Messer LC, Kaufman JS, Dole N, Savitz DA, Laraia BA. Neighborhood crime, deprivation, and preterm birth. Ann. Epidemiol. 2005;16(6):455–462. doi: 10.1016/j.annepidem.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Meyer IH. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol. Bull. 2003;129:674–697. doi: 10.1037/0033-2909.129.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MMWR. State-specific prevalence of cigarette smoking—United States, 1995. Morb. Mortal. Weekly Rep. 1996;45:962–966. [PubMed] [Google Scholar]
- MMWR. Preterm singleton births—United States, 1989–1996. Morb. Mortal. Weekly. Rep. 1999;48:185–189. [PubMed] [Google Scholar]
- MMWR. Infant mortality and low birth weight among black and white infants—United States, 1980–2000. Morb. Mortal. Weekly Rep. 2002;51:589–592. [PubMed] [Google Scholar]
- MMWR. Self-reported frequent mental distress among adults—United States, 1993- 2001. Morb. Mortal. Weekly Rep. 2004;53:963–966. [PubMed] [Google Scholar]
- MMWR. Health disparities experienced by black or African Americans—United States. Morb. Mortal. Weekly Rep. 2005;54:1–3. [PubMed] [Google Scholar]
- Morenoff JD, Lynch JW. What makes a place healthy? Neighborhood influences on racial/ethnic disparities in health over the life course. In: Anderson NB, Rodolfo AB, Cohen B, editors. Critical Perspectives on Racial and Ethnic Difference in Health in Late Life. Washington, DC: Natl. Acad. Press; 2004. pp. 406–449. [Google Scholar]
- Muraven M, Tice DM, Baumeister RF. Self-control as limited resource: regulatory depletion patterns. J. Personal. Soc. Psychol. 1998;74:774–789. doi: 10.1037//0022-3514.74.3.774. [DOI] [PubMed] [Google Scholar]
- Natl. Cent. Chronic Dis. Prevent. Health Promot. (NCCDPHP) The Burden of Chronic Diseases and Their Risk Factors National and State Perspectives 2004. Atlanta, GA: USDHHS; 2004. http://www.cdc.gov/nccdphp/burdenbook2004/ [Google Scholar]
- Natl. Vital Stat. Rep. (NVSS) Deaths: final data for 2002. Natl. Vital Stat. Rep. 2004;53:1–116. [PubMed] [Google Scholar]
- O’Campo P, Xue X, Wang MC, Caughy M. Neighborhood risk factors for low birth-weight in Baltimore: a multilevel analysis. Am. J. Public Health. 1997;87:1113–1118. doi: 10.2105/ajph.87.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Oliver ML, Shapiro TM. Black Wealth/White Wealth: A New Perspective on Racial Inequality. New York: Routledge; 1995. [Google Scholar]
- Olsson A, Ebert JP, Banaji MR, Phelps EA. The role of social groups in the persistence of learned fear. Science. 2005;5735:785–787. doi: 10.1126/science.1113551. [DOI] [PubMed] [Google Scholar]
- Ownsworth TL, McFarland K, Young RM. The investigation of factors underlying deficits in self-awareness and self-regulation. Brain Injury. 2002;16:291–309. doi: 10.1080/02699050110103986. [DOI] [PubMed] [Google Scholar]
- Pallotto EK, Collins JWJ, David RJ. Enigma of maternal race and infant birth weight: a population-based study of US-born black and Caribbean-born black women. Am. J. Epidemiol. 2000;151:1080–1085. doi: 10.1093/oxfordjournals.aje.a010151. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Gordon N. The instinctual basis of human affect: affective imaging of laughter and crying. Conscious. Emot. 2003;4:197–205. [Google Scholar]
- Pearlin LI, Lieberman MA, Menaghan EG, Mullan JT. The stress process. J. Health Soc. Behav. 1981;22:337–356. [PubMed] [Google Scholar]
- Peterson ED, Krivo LJ. Racial segregation, the concentration of disadvantage, and black and white homicide victimization. Soc. Forum. 1999;14:465–493. [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu. Rev. Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Cannistraci CJ, Cunningham WA. Intact performance on an indirect measure of race bias following amygdala damage. Neuropsychologia. 2003;41:203–208. doi: 10.1016/s0028-3932(02)00150-1. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Cunningham WA, Funayama ES, Gatenby JC, et al. Performance on indirect measures of race evaluation predicts amygdala activation. J. Cogn. Neurosci. 2000;12:729–738. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Pearl M. Multilevel analyses of neighborhood socioeconomic context and health outcomes: a critical review. J. Epidemiol. Commun. Health. 2001;55:111–122. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike IL. Maternal stress and fetal responses: evolutionary perspectives on preterm delivery. Am. J. Hum. Biol. 2005;17:55–65. doi: 10.1002/ajhb.20093. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychol. Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- Rich-Edwards JW, Krieger N, Majzoub J, Zierler S, Lieberman E, Gillman M. Maternal experiences of racism and violence as predictors of preterm birth: rationale and study design. Paediatr. Perinat. Epidemiol. 2001;15(Suppl. 2):124–135. doi: 10.1046/j.1365-3016.2001.00013.x. [DOI] [PubMed] [Google Scholar]
- Rivo ML, Kofie V, Schwartz E, Levy ME, Tuckson RV. Comparisons of black and white smoking-attributable mortality, morbidity, and economic costs in the District of Columbia. J. Natl. Med. Assoc. 1989;81:1125–1130. [PMC free article] [PubMed] [Google Scholar]
- Roberts EM. Neighborhood social environments and the distribution of low birthweight in Chicago. Am. J. Public Health. 1997;87:597–603. doi: 10.2105/ajph.87.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SA. Socioeconomic position and health: the independent contribution of community socioeconomic context. Annu. Rev. Sociol. 1999;25:489–516. [Google Scholar]
- Robles TF, Kiecolt-Glaser JK. The physiology of marriage: pathways to health. Physiol. Behav. 2003;79:409–416. doi: 10.1016/s0031-9384(03)00160-4. [DOI] [PubMed] [Google Scholar]
- Ryff C, Singer B. Interpersonal flourishing: a positive health agenda for the new millennium. Personal. Soc. Psychol. Rev. 2000;4:30–44. [Google Scholar]
- Ryff C, Singer B. From social structure to biology: integrative science in pursuit of human health and well-being. In: Snyder CR, Lopez SJ, editors. Handbook of Positive Psychology. New York: Oxford Univ. Press; 2001. pp. 541–555. [Google Scholar]
- Schillinger M, Exner M, Mlekusch W, Sabeti S, Amighi J, et al. Inflammation and Carotid Artery Risk for Atherosclerosis Study (ICARAS) Circulation. 2005;111:2203–2209. doi: 10.1161/01.CIR.0000163569.97918.C0. [DOI] [PubMed] [Google Scholar]
- Schoendorf KC, Hogue CJ, Kleinman JC, Rowley D. Mortality among infants of black as compared with white college-educated parents. N. Engl. J. Med. 1992;326:1522–1526. doi: 10.1056/NEJM199206043262303. [DOI] [PubMed] [Google Scholar]
- Schulz A, Williams D, Israel B, Becker A, Parker E, et al. Unfair treatment, neighborhood effects, and mental health in the Detroit metropolitan area. J. Health Soc. Behav. 2000;41:314–332. [PubMed] [Google Scholar]
- Sears DO. Symbolic racism. In: Katz PA, Taylor DA, editors. Eliminating Racism: Profiles in Controversy, Perspectives in Social Psychology. New York: Plenum; 1988. pp. 53–84. [Google Scholar]
- Sears DO, Henry JP. The origins of symbolic racism. J. Personal. Soc. Psychol. 2003;85:259–275. doi: 10.1037/0022-3514.85.2.259. [DOI] [PubMed] [Google Scholar]
- Sears DO, Henry JP. Over thirty years later: a contemporary look at symbolic racism. In: Zanna MP, editor. Advances in Experimental Social Psychology. San Diego, CA: Academic; 2005. pp. 95–150. [Google Scholar]
- Seeman TE, Berkman LF, Gulanski BI, Robbins RJ, Greenspan SL, et al. Self-esteem and neuroendocrine response to challenge: MacArthur studies of successful aging. J. Psychosom. Res. 1995;39:69–84. doi: 10.1016/0022-3999(94)00076-h. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Berkman LF, Kohout F, LaCroix A, Glynn R, Blazer D. Intercommunity variations in the association between social ties and mortality in the elderly: a comparative analysis of three communities. Ann. Epidemiol. 1993;3:325–335. doi: 10.1016/1047-2797(93)90058-c. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS. Impact of social environment characteristics on neuroen-docrine regulation. Psychosom. Med. 1996;58:459–471. doi: 10.1097/00006842-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Sen A. The economics of life and death. Sci. Am. 1993;268:40–47. doi: 10.1038/scientificamerican0593-40. [DOI] [PubMed] [Google Scholar]
- Smedley BD, Smith AY, Nelson AR. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: Natl. Acad. Press; 2003. [PubMed] [Google Scholar]
- Smith GD, Neaton JD, Wentworth D, Stamler R, Stamler J. Mortality differences between black and white men in the USA: contribution of income and other risk factors among men screened for the MRFIT. Lancet. 1998;51:934–939. doi: 10.1016/s0140-6736(00)80010-0. [DOI] [PubMed] [Google Scholar]
- Subramanian SV, Chen JT, Rehkopf DH, Waterman PD, Krieger N. Racial disparities in context: a multilevel analysis of neighborhood variations in poverty and excess mortality among black populations in Massachusetts. Am. J. Public Health. 2005;95:260–265. doi: 10.2105/AJPH.2003.034132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Brown JD. Positive illusions and well-being revisited: separating fact from fiction. Psychol. Bull. 1994;116:21–27. doi: 10.1037/0033-2909.116.1.21. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Repetti RL, Seeman T. Health psychology: What is an unhealthy environment and how does it get under the skin? Annu. Rev. Psychol. 1997;48:411–447. doi: 10.1146/annurev.psych.48.1.411. [DOI] [PubMed] [Google Scholar]
- Tezanos JF. La Sociedad divida. Estructuras de clases y desigualdades en las sociedades tecnologicas. Madrid: Biblioteca Nueva; 2001. [Google Scholar]
- Thayer JF, Friedman BH. A neurovisceral integration model of health disparities in aging. In: Anderson NB, Bulatao RA, Cohen B, editors. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. Washington, DC: Natl. Acad. Press; 2004. pp. 567–603. [Google Scholar]
- Thoits PA. Dimensions of life events that influence psychological distress: an evaluation and synthesis of the literature. In: Kaplan HB, editor. Psychosocial Research: Trends in Theory and Research. New York: Academic; 1983. pp. 33–103. [Google Scholar]
- Troxel WM, Matthews KA, Bromberger JT, Sutton-Tyrrell K. Chronic stress burden, discrimination, and subclinical carotid artery disease in African American and Caucasian women. Health Psychol. 2003;22:300–309. doi: 10.1037/0278-6133.22.3.300. [DOI] [PubMed] [Google Scholar]
- Underwood MK, Scott BL, Galperin MB, Bjornstad GJ, Sexton AM. An observational study of social exclusion under varied conditions: gender and developmental differences. Child Dev. 2004;75:1538–1555. doi: 10.1111/j.1467-8624.2004.00756.x. [DOI] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychol. Sci. 2003;14:347–353. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- US Dep. Health Human Serv. Healthy People 2000: National Health Promotion and Disease Prevention. Washington, DC: USDHHS; 1990. [Google Scholar]
- US Dep. Health Human Serv. Tobacco Use Among U.S Racial/Ethnic Minority Groups— African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics a Report of the Surgeon General. Atlanta, GA: USDHHS; 1998. [PubMed] [Google Scholar]
- US Dep. Health Human Serv. Mental Health: Culture, Race and Ethnicity A Supplement to Mental Health A Report of the Surgeon General. Washington, DC: U.S. Public Health Serv; 1999. [Google Scholar]
- US Dep. Health Human Serv. Healthy People 2010: Understanding and Improving Health. 2nd ed. Washington, DC: USDHHS; 2000. [Google Scholar]
- US Dep. Health Human Serv. Health, United States, 2005, with Chartbook on Trends in the Health of Americans. Hyattsville, MD: US GPO; 2005. [Google Scholar]
- US Dep. Health Human Serv. National Health Care Disparities Report. Rockville, MD: Agency Healthc. Res. Qual.; 2005. http://www.qualitytools.ahrq.gov/disparitiesreport/ [Google Scholar]
- Walker B, Mays VM, Warren R. The changing landscape for the elimination of racial/ethnic health status disparities. J. Health Care Poor Underserved. 2004;15:506–521. doi: 10.1353/hpu.2004.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Giesbrecht B, Song AW, Mangun GR, Woldorff MG. Conflict monitoring in the human anterior cingulate cortex during selective attention to global and local object features. Neuroimage. 2003;19:1361–1368. doi: 10.1016/s1053-8119(03)00167-8. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Fiske ST. Controlling racial prejudice: social-cognitive goals affect amygdala and stereotype activation. Psychol. Sci. 2005;16:56–63. doi: 10.1111/j.0956-7976.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- Williams DR. African American mental health: persisting questions and paradoxal findings. Afr. Am. Res. Perspect. 1995;2:2–6. [Google Scholar]
- Williams DR, Harris-Reid M. Race and mental health: emerging patterns and promising approaches. In: Horwitz AV, Schneid TL, editors. A Handbook for the Study of Mental Health: Social Contexts, Theories, and Systems. New York: Cambridge Univ. Press; 1999. pp. 295–314. [Google Scholar]
- Williams DR, Jackson PB. Social sources of racial disparities in health. Health Affairs. 2005;24:325–334. doi: 10.1377/hlthaff.24.2.325. [DOI] [PubMed] [Google Scholar]
- Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: findings from community studies. Am. J. Public Health. 2003;93(2):200–208. doi: 10.2105/ajph.93.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: socioeconomic status, stress, and discrimination. J. Health Psychol. 1997;2:335–351. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- Williams KD, Cheung CK, Choi W. Cyberostracism: effects of being ignored over the Internet. J. Personal. Soc. Psychol. 2000;79:748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Williams KD, Sommer KL. Social ostracism by coworkers: Does rejection lead to loafing or compensation? Personal. Soc. Psychol. Bull. 1997;23:693–706. [Google Scholar]
- Wilson WJ. The Truly Disadvantaged: The Inner City, the Underclass, and Public Policy. Chicago: Univ. Chicago Press; 1987. [Google Scholar]
- Winters EW, McCabe PM, Green EJ, Schneiderman N. Stress responses, coping, and cardiovascular neurobiology: central nervous system circuitry underlying learned and unlearned affective responses to stressful stimuli. In: McCabe PM, Schneiderman N, Field T, Wellens AR, editors. Stress, Coping, and Cardiovascular Disease. Hillsdale, NJ: Erlbaum; 2000. pp. 1–49. [Google Scholar]
- Ylvisaker M, Feeney T. Executive functions, self-regulation, and learned optimism in pediatric rehabilitation: a review and implications for intervention. Pediatr. Rehab. 2002;5:51–70. doi: 10.1080/1363849021000041891. [DOI] [PubMed] [Google Scholar]