Abstract
Human clearance prediction for small- and macro-molecule drugs was evaluated and compared using various scaling methods and statistical analysis.
Human clearance is generally well predicted using single or multiple species simple allometry for macro- and small-molecule drugs excreted renally.
The prediction error is higher for hepatically eliminated small-molecules using single or multiple species simple allometry scaling, and it appears that the prediction error is mainly associated with drugs with low hepatic extraction ratio (Eh). The error in human clearance prediction for hepatically eliminated small-molecules was reduced using scaling methods with a correction of maximum life span (MLP) or brain weight (BRW).
Human clearance of both small- and macro-molecule drugs is well predicted using the monkey liver blood flow method. Predictions using liver blood flow from other species did not work as well, especially for the small-molecule drugs.
Keywords: Interspecies scaling, human clearance prediction, small-molecule, macro-molecule, liver blood flow
Introduction
Allometric scaling is an empirical approach developed based on cross species similarities in anatomy, physiology, and biochemistry with a power function correlating physiological parameters with body size (Y = aWb, where Y is the parameter of interest, W is the body weight, and a and b are the coefficient and exponent of the allometric equation, respectively). This method has been applied to the projection of human pharmacokinetics for small-molecule drugs as well as therapeutic proteins and is widely used in the pharmaceutical industry for early decision making at several stages in drug discovery and development (e.g., lead compound selection and optimization, first dose in human, etc).
It is known that allometric projections generally work well for drugs mainly renally eliminated. However, for some small-molecule drugs with high cross-species variability in hepatic metabolism, this method may not work well in the extrapolation of hepatic metabolic CL from laboratory animals to humans. To improve the predictability of metabolic CL in humans, several modified scaling methods have been suggested and examined. Because longevity is frequently inversely correlated with hepatic cytochrome P450 drug oxidation rates, maximum life-span potential (MLP) and brain weight (BRW) were proposed as correction factors in allometric scaling by Boxenbaum (Boxenbaum, 1982). His work was later supported by other scientists, and their work also demonstrated that MLP and BRW corrections improved the accuracy of human CL prediction when the allometry power exponent b was higher than 0.80–0.90 (Feng et al., 2000, Mahmood and Balian, 1996). Recently, Nagilla and Ward suggested using liver blood flow (LBF) as a correction factor for the scaling of small-molecule drugs (Nagilla and Ward, 2004). Based on their analysis of 103 compounds comparing simple allometry with LBF or MLP/BRW correction, they concluded that scaling with monkey liver blood flow was the best approach among the methods tested (68% success rate). These modified approaches have improved the accuracy of prediction to some extent.
While many studies have demonstrated the use of allometric scaling in the prediction of human CL for small-molecule drugs, only a few articles reported the application of this method to macro-molecule drugs. Currently, the market of biotherapeutics, including peptide, protein and oligonucleotide drugs have been growing rapidly. The annual growth rate of biotherapeutics sales was approximately 20% from 2001 and 2006, which is much higher compared to a growth rate of only 6–8% for small-molecule drugs (Aggarwal, 2007). Although the general principles of pharmacokinetics and pharmacodynamics are applicable to biotherapeutics, their disposition in the body is known to be unique and different from conventional small-molecules (Tang et al., 2004, Lin, 2009). The binding process of biotherapeutics with receptors or other targets in the body may be species- specific and saturable exhibiting non-linear kinetics. In addition, protein drugs derived from human sources may be recognized as a foreign compound in animal species and thereby induce immune system mediated reaction, known as immunogenicity. Therefore, differences are expected in interspecies scaling from animals to humans when comparing small- versus macro-molecule drugs. Since clearance is an important pharmacokinetic parameter critical for the design of first-time-in-human study and the selection of dose regimen, it is important to understand differences and the mechanism associated with the human clearance prediction between small and macro-molecule drugs.
Positive results from human clearance prediction of macro-molecule drugs using allometric scaling have already been reported by several groups (Mordenti et al., 1991, Mahmood, 2004, Mahmood, 2009b, Ling et al., 2009, Wang and Prueksaritanont, 2010). Mordenti et al demonstrated reasonable accuracy in predicting human clearance and volume of distribution using interspecies scaling for five protein drugs with molecular weights ranging from 6 to 98 kDa (Mordenti et al., 1991). Mahmood expanded the data set to 15 therapeutic proteins and reported a low prediction error of human clearance (Mahmood, 2004, Mahmood, 2009b). He also suggested the use of at least three animal species for interspecies scaling. However, acceptable prediction of human clearance using single animal species for macro-molecule drugs has also been reported later by Ling and Wang (Ling et al., 2009, Wang and Prueksaritanont, 2010). Ling et al suggested using a fixed exponent of ‘0.85’ or ‘0.90’ for human CL prediction of monoclonal antibody drugs, and ‘0.80’ was suggested by Wang and Prueksaritanont not only for monoclonal antibodies, but also for other protein drugs.
In this study, literature data for small- and macro-molecule drugs were collected and analyzed by various allometry methods using single or multiple species scaling, and the accuracy of human clearance prediction was compared. For macro-molecule drugs, almost all the peptide and protein drugs previously reported in the literature with molecular weights ranging from 1 to 340 kDa were included in our data set, along with several oligonucleotide drugs. As a result, this study provides very useful information of the potential application of allometric scaling in human clearance prediction for both small- and macro-molecule drugs.
Methods
Data collection
Clearance data of 675 small-molecule drugs and 80 macro-molecule drugs following intravenous administration were obtained from the literature. The criteria that divide the drugs into small versus macro-molecule is 1000 Da. Drugs having molecular weights greater than 1000 Da are regarded as macro-molecule and the others as small-molecule. Based on these criteria, all biotherapeutics including protein, peptide and oligonucleotide drugs were classified as macro-molecule drugs. The clearance of 81 of the small-molecule drugs in animals and humans were collected and used for the analysis of interspecies scaling and compared with 53 of macro-molecule drugs in human clearance prediction (Tables 1 and 2).
Table 1.
Compound list for all macro-molecule drugs.
| Compounds | MW (kDa) | Observed CL (Mean (CV%), ml/hr/kg) in human | a | b | Reference | Group | |
|---|---|---|---|---|---|---|---|
| 1 | Abciximab | 47.6 | 288 (59) | (Abernethy et al., 2002) | 1 | ||
| 2 | Alefacept | 51.8 | 0.25 | (Tang et al., 2004) | |||
| 3 | Alteplasea | 67.5 | 894 (14) | 34.8 | 0.863 | (Martin et al., 1991, Martin et al., 1992) | |
| 4 | ANFa | 67.5 | 2229 (11) | 112 | 0.847 | (Yandle et al., 1986, Cernacek et al., 1988, Krieter and Trapani, 1989, Marleau et al., 1989) | |
| 5 | BM06.022a | 39.0 | 239 (13) | 6.98 | 0.893 | (Martin et al., 1991) | |
| 6 | Cetrorelix | 1.49 | 72 | (Obach et al., 2008) | |||
| 7 | Cyclosporina | 1.20 | 321 (38) | 5.51 | 1.15 | (Sangalli et al., 1988) | |
| 8 | Dalbavancin | 1.82 | 0.61 (3.6) | (Obach et al., 2008) | |||
| 9 | Daptomycin | 1.62 | 9.0 (10) | (Obach et al., 2008) | |||
| 10 | Darbepoetin alfa | 37.1 | 2.3 (35) | (Egrie Jc Fau - Dwyer et al., 2003) | |||
| 11 | Denileukin diftitox | 57.6 | 105 (20) | (Tang et al., 2004) | |||
| 12 | Desmopressin | 1.07 | 143 (34) | (Agerso H Fau - Seiding Larsen et al.) | |||
| 13 | Digoxin-Faba | 45.5 | 19 (32) | 1.017 | 0.667 | (Grene-Lerouge Na Fau - Bazin-Redureau et al., 1996, Ujhelyi and Robert, 1995) | |
| 14 | Drotrecogin-α | 55.0 | 314 (31) | (Olsen and Martin, 2002) | |||
| 15 | EPO-α | 30.4 | 8.1 (12) | (Halstenson et al., 1991) | |||
| 16 | EPO-βa | 36.0 | 7.9 (15) | 0.234 | 0.660 | (Bleuel et al., 1996, Halstenson et al., 1991) | |
| 17 | Factor IXa | 56.5 | 9.1 (16) | 0.288 | 0.715 | (Keith et al., 1995, McCarthy et al., 2002, White et al., 1998) | |
| 18 | Filgrastim | 18.8 | 36 (24) | (Tang et al., 2004) | |||
| 19 | GLQ223b | 27.0 | 130 (54) | (Gatti et al., 1991) | |||
| 20 | Glucagon | 3.50 | 810 | (Tang et al., 2004) | |||
| 21 | IFNβ1aa | 22.5 | 573 (47) | 4.98 | 0.907 | (Kagan et al., 2010) | |
| 22 | IFNβ1bb | 18.5 | 760 (37) | (Kagan et al., 2010) | |||
| 23 | ISIS 2503a | NA | 144 | 2.41 | 0.879 | (Geary et al., 2001) | |
| 24 | ISIS 3521a | 6.72 | 67 (2.7) | 2.12 | 0.599 | (Geary et al., 2001) | |
| 25 | ISIS 5132a | 6.63 | 76 (32) | 3.23 | 0.873 | (Geary et al., 2001) | |
| 26 | ISIS 104838a | 7.41 | 41 (12) | 155 | 0.709 | (Sewell et al., 2002, Geary et al., 2003) | |
| 27 | ISIS 301012b | 7.39 | 41 (12) | 96.8 | 0.487 | (Yu et al., 2007) | |
| 28 | Leuprolide | 1.21 | 114 (7.4) | (Obach et al., 2008) | |||
| 29 | Nartograstima | 19 | 29 (11) | 49.2 | 0.655 | (Kuwabara et al., 1994, Ohdo et al., 1998) | |
| 30 | Octreotide | 1.00 | 121 (25) | (Tang et al., 2004) | |||
| 31 | Pamiteplasea | 37.0 | 130 (7.7) | 2.06 | 0.913 | (Oikawa et al., 2000, Oikawa et al., 2001) | |
| 32 | PEG-EPOa | 60.0 | 0.50 (6.0) | 1.95 | 0.674 | (Macdougall et al., 2006) | |
| 33 | PEG-IL2a | 22.0 | 6.0 | 0.179 | 0.750 | (Braeckman, 2000) | |
| 34 | rCD4a | 50.0 | 49 (43) | 3.36 | 0.577 | (Mordenti et al., 1991) | |
| 35 | Relaxina | 6.00 | 139 (37) | 5.83 | 0.776 | (Mordenti et al., 1991) | |
| 36 | rEPOa | 36.0 | 10.1 (35) | 0.221 | 0.921 | (Flaharty et al., 1990, Woo and Jusko, 2007) | |
| 37 | Reteplase | 39.6 | 300 (40) | (Tang et al., 2004) | |||
| 38 | rhGHa | 20.0 | 124 | 6.47 | 0.688 | (Mordenti et al., 1991) | |
| 39 | rhIL-2a | 15.0 | 165 (54) | 4.79 | 0.650 | (Braeckman, 2000) | |
| 40 | rHirudina | 6.91 | 168 | 8.48 | 1.01 | (Nowak, 1991) | |
| 41 | rHuIFN-αAa | 19.0 | 169 (38) | 3.68 | 0.710 | (Lave et al., 1995) | |
| 42 | rHuIL-10 | 18.0 | 65 (11) | (Radwanski et al., 1998) | |||
| 43 | rt-PAa | 63.0 | 490 | 16.2 | 0.862 | (Mordenti et al., 1991, Collen et al., 1991) | |
| 44 | SK&F 107647 | 1.17 | 65 (28) | (Brocks et al., 1996) | |||
| 45 | SR 90107Aa | 1.11 | 6.3 (16) | 41.2 | 0.506 | (Herault et al., 1997) | |
| 46 | Teicoplanin A2-1 | 1.88 | 12 (5.0) | (Obach et al., 2008) | |||
| 47 | Telavancin | 1.76 | 12 (12) | (Obach et al., 2008) | |||
| 48 | Tenecteplasea | 59.0 | 83 (14) | 3.35 | 0.903 | (Tanswell et al., 2002) | |
| 49 | Valspodar | 1.22 | 156 | (Obach et al., 2008) | |||
| 50 | Vancomycin | 1.45 | 112 (5.4) | (Obach et al., 2008) | |||
| 51 | Abatacepta | 92.0 | 0.23 | 0.563 | 0.647 | (Srinivas et al., 1997, Srinivas et al., 1996a, Srinivas et al., 1996b) | 2 |
| 52 | Adalimumabb | 148 | 0.13 (31) | (Lobo et al., 2004, Weisman et al., 2003) | |||
| 53 | Basiliximab | 144 | 0.59 (46) | (Tang et al., 2004) | |||
| 54 | Bevacizumaba | 149 | 0.12 (42) | 0.0046 | 0.684 | (Lin et al., 1999, Gordon et al., 2001) | |
| 55 | CD4-IgGa | 98.0 | 1.9 | 0.102 | 0.740 | (Mordenti et al., 1991) | |
| 56 | Cetuximabb | 146 | 0.50 (37) | (Lobo et al., 2004) | |||
| 57 | CNTO136b | NA | 0.19 (32) | (Ling et al., 2009) | |||
| 58 | CNTO328b | 145 | 0.47 (34) | (Ling et al., 2009, Puchalski et al.) | |||
| 59 | CNTO95b | 180 | 0.28 (13) | (Ling et al., 2009, Mullamitha et al., 2007) | |||
| 60 | Daclizumabb | 143 | 0.19 | (Tang et al., 2004, Ling et al., 2009) | |||
| 61 | Eculizumab | 148 | 0.29 (12) | # | |||
| 62 | Efalizumabb | 150 | 0.65 (32) | (Ling et al., 2009, Bauer et al., 1999) | |||
| 63 | EGFr3a | 150 | 0.98 (18) | 0.0865 | 1.02 | (Crombet et al., 2001) | |
| 64 | Factor VIIIa | 340 | 3.0 | 0.182 | 0.744 | (Mordenti et al., 1996, Stokol et al., 1997) | |
| 65 | Gemtuzumab | 152 | 3.3 (85) | (Dowell et al., 2001) | |||
| 66 | Golimumabb | 147 | 0.28 (79) | (Ling et al., 2009) | |||
| 67 | Horse F(ab′)2a | 100 | 0.93 (84) | 0.0328 | 0.534 | (Bazin-Redureau et al., 1998, Ho et al., 1990) | |
| 68 | Infliximaba | 144 | 0.14 (20) | 0.0075 | 0.540 | (Tang et al., 2004, Palframan R Fau - Airey et al., 2009, Rojas et al., 2005) | |
| 69 | Laronidase | 83.0 | 132 (32) | (Tang et al., 2004) | |||
| 70 | Lenercepta | 120 | 0.30 | 0.008 | 1.06 | (Richter Wf Fau - Gallati et al., 1999) | |
| 71 | Natalizumabb | 149 | 0.27 (1.5) | (Ling et al., 2009, Sheremata et al., 1999) | |||
| 72 | Panitumumabb | 147 | 0.20 (10) | (Ling et al., 2009, Cohenuram and Saif, 2007) | |||
| 73 | Pertuzumaba | 150 | 0.14 (36) | 0.259 | 0.926 | (Agus et al., 2005, Adams et al., 2006) | |
| 74 | Rituximab | 145 | 0.13 | (Cartron et al., 2007) | |||
| 75 | RSHZ19a | 146 | 0.122 (15) | 0.0035 | 0.731 | (Davis et al., 1995, Everitt et al., 1996) | |
| 76 | Tanezumabb | 145 | 0.11 | # | |||
| 77 | Tositumomab | 144 | 0.97 | (Lobo et al., 2004) | |||
| 78 | Trastuzumabb | 146 | 0.41 (61) | (Tang et al., 2004, Ling et al., 2009) | |||
| 79 | Ustekinumabb | 146 | 0.093 (28) | (Ling et al., 2009) | |||
| 80 | Veltuzumab | 145 | 0.093 (39) | (Morschhauser et al., 2009, Goldenberg et al., 2009) |
PK data available in ≥ 3 animal species and in human.
PK data available in monkey and human.
Table 2.
Compound list of small-molecule drugs used for interspecies scaling.
| Compounds | MW | Observed CL (Mean (CV%), ml/min/kg) in human | Character | Elimination | Fu% | a | b | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Acivicin | 178.6 | 0.78 (27) | Acid | Mixed | 100 | 4.10 | 0.558 | (Mahmood and Balian, 1996) |
| 2 | Actisomide | 369.6 | 6.8 (16) | Base | Mixed | NA | 10.9 | 1.04 | (Mahmood and Balian, 1996) |
| 3 | Amethopterin | 454.4 | 3.5 | Acid | Renal | 50 | 10.4 | 0.724 | (Dedrick et al., 1970) |
| 4 | Amiodarone | 645.3 | 1.9 | Base | Hepatic | 4.4 | 13.5 | 0.979 | (Evans et al., 2006) |
| 5 | Amphotericin B | 924.1 | 0.34 (75) | Zwitt | Hepatic | 5.2 | 0.967 | 0.847 | (Hutchaleelaha et al., 1997, Robbie and Chiou, 1998) |
| 6 | Antipyrine | 188.2 | 0.78 (21) | Base | Hepatic | 100 | 11.7 | 0.843 | (Lave et al., 1997) |
| 7 | Ara-C | 243.2 | 1.3 | Base | Hepatic | 98 | 4.21 | 0.830 | (Dedrick et al., 1973) |
| 8 | Betamipron | 193.2 | 6.6 (12) | Acid | Renal | 48 | 13.7 | 0.782 | (Mahmood, 1998) |
| 9 | Biperiden | 311.5 | 15 | Base | Hepatic | 40 | 44.6 | 0.719 | (Evans et al., 2006) |
| 10 | Bosentan | 569.6 | 1.9 (26) | Acid | Hepatic | 2 | 24.9 | 0.562 | (Lave et al., 1997) |
| 11 | Caffeine | 194.2 | 2.0 (11) | Neutral | Hepatic | 96 | 8.07 | 0.584 | (Lave et al., 1997) |
| 12 | Cefmetazole | 471.5 | 1.7 (26) | Base | Renal | 15 | 12.8 | 0.633 | (Feng et al., 2000) |
| 13 | Cefotetan | 619.6 | 0.60 (15) | Acid | Renal | 12 | 7.15 | 0.641 | (Mahmood and Balian, 1996) |
| 14 | Cefazolin | 454.5 | 0.88 | Base | Renal | 13 | 4.79 | 0.733 | (Mahmood and Balian, 1996) |
| 15 | Cefodizime | 584.7 | 0.74 (24) | Acid | Renal | 12 | 1.51 | 1.00 | (Matsushita et al., 1990) |
| 16 | Cefoperazone | 645.7 | 1.4 (14) | Base | Hepatic | 18 | 6.75 | 0.578 | (Lave et al., 1997) |
| 17 | Cefpiramide | 612.6 | 0.48 (42) | Base | Renal | 3.7 | 4.27 | 0.443 | (Nakagawa et al., 1984) |
| 18 | Ceftizoxime | 405.4 | 2.4 (21) | Base | Renal | 72 | 10.3 | 0.544 | (Mahmood and Balian, 1996) |
| 19 | Chlorpromazine | 318.9 | 4.3 | Base | Hepatic | 10 | 47.1 | 0.889 | (Evans et al., 2006) |
| 20 | CI-1007 | 329.5 | 21 | Base | Hepatic | 2 | 35.0 | 0.905 | (Feng et al., 1998) |
| 21 | Ciprofloxacin | 331.4 | 6.0 | Acid | Renal | 60 | 14.5 | 0.939 | (Siefert et al., 1986, Nouws et al., 1988) |
| 22 | Coumarin | 206.2 | 16 (24) | Acid | Hepatic | 14 | 21.2 | 1.10 | (Ritschel et al., 1991) |
| 23 | Cytoxan | 279.1 | 2.25 (79) | Acid | Hepatic | 100 | 21.0 | 0.843 | (Mellett, 1969) |
| 24 | DA-1131 | 445.6 | 5.0 | Acid | Mixed | 90 | 11.6 | 0.825 | (Kim et al., 1998) |
| 25 | Diazepam | 284.8 | 0.32 (31) | Neutral | Hepatic | 3.2 | 37.4 | 0.735 | (Mahmood and Balian, 1996) |
| 26 | Diltiazem | 414.5 | 16 (35) | Base | Hepatic | 25 | 62.8 | 0.888 | (Evans et al., 2006) |
| 27 | Doxorubicin | 543.5 | 13 | Base | Hepatic | 25 | 40.8 | 0.887 | (Harris and Gross, 1975, Tranum et al., 1975) |
| 28 | Enoxacin | 320.3 | 9.3 (14) | Acid | Mixed | 69 | 0.353 | 1.51 | (Nakamura et al., 1983, Chang et al., 1988) |
| 29 | Enprofylline | 194.2 | 3.6 (24) | Base | Renal | 49 | 6.34 | 0.526 | (Tsunekawa et al., 1992) |
| 30 | Epiroprim | 353.4 | 3.5 (17) | Acid | Mixed | 12.5 | 34.4 | 0.609 | (Evans et al., 2006) |
| 31 | Felodipine | 384.3 | 11 (19) | Neutral | Hepatic | 1 | 43.8 | 0.575 | (Evans et al., 2006) |
| 32 | Fentanyl | 336.5 | 11 (33) | Base | Hepatic | 17 | 15.6 | 0.805 | (Bjorkman and Redke, 2000, Valverde et al., 2000) |
| 33 | Flindokalner | 359.7 | 7.6 | Acid | Hepatic | 0.38 | 21.9 | 0.629 | (Evans et al., 2006) |
| 34 | Furosemide | 330.7 | 2.3 (47) | Acid | Renal | 5 | 3.78 | 1.11 | (Doyle et al., 1982, Prandota and Pruitt, 1991, Hirai et al., 1992) |
| 35 | Garenoxacin | 426.4 | 1.2 | Acid | Mixed | 13 | 5.78 | 0.605 | (Evans et al., 2006) |
| 36 | GV150526 | 360.2 | 0.086 (24) | Acid | Hepatic | 0.002 | 1.99 | 1.20 | (Iavarone et al., 1999) |
| 37 | Haloperidol | 375.9 | 12 | Base | Hepatic | 10 | 46.1 | 0.573 | (Evans et al., 2006) |
| 38 | Imatinib | 493.6 | 3.2 (34) | Base | Hepatic | 5.9 | 29.6 | 0.866 | (Neville et al., 2004, Peng et al., 2004, Ishizuka et al., 2007, Oostendorp et al., 2009) |
| 39 | Inogatran | 439.0 | 5.8 (8.6) | Base | Renal | 0.2 | 24.7 | 0.748 | (Eriksson et al., 1998, Hauptmann, 2002) |
| 40 | ITF296 | 238.2 | 30 (20) | Acid | Hepatic | NA | 40.8 | 1.05 | (Monzani et al., 1995, Sardina et al., 1995, Giachetti et al., 1998, Monzani et al., 1999) |
| 41 | Ketoprofen | 254.3 | 1.2 (68) | Acid | Hepatic | 1 | 0.930 | 1.31 | (Lepist and Jusko, 2004) |
| 42 | Ketorolac | 376.4 | 0.80 (11) | Acid | Mixed | 0.8 | 1.28 | 0.989 | (Mroszczak et al., 1987) |
| 43 | Lamifiban | 468.5 | 1.9 | Zwitt | Renal | 94 | 6.14 | 0.884 | (Lave et al., 1996) |
| 44 | Meropenem | 383.5 | 3.2 (16) | Acid | Mixed | 98 | 17.7 | 0.418 | (Harrison et al., 1989) |
| 45 | Methadone | 429.6 | 1.4 | Base | Mixed | 20 | 43.5 | 0.775 | (Evans et al., 2006) |
| 46 | Metoprolol | 267.4 | 16 (53) | Base | Hepatic | 88 | 137 | 0.426 | (Rane et al., 1984, Belpaire et al., 1990, Bortolotti et al., 1989, Murthy et al., 1991) |
| 47 | Mibefradil | 495.6 | 7.0 | Acid | Hepatic | 1 | 66.9 | 0.804 | (Lave et al., 1997) |
| 48 | Midazolam | 309.5 | 7.6 (5.9) | Neutral | Hepatic | 4.0 | 42.4 | 0.737 | (Evans et al., 2006) |
| 49 | Mifepristone | 325.8 | 0.33 | Base | Hepatic | 2 | 34.7 | 0.720 | (Evans et al., 2006, Kenny and Grime, 2006) |
| 50 | Mofarotene | 433.6 | 11 | Base | Hepatic | 0.1 | 11.5 | 0.733 | (Mahmood and Balian, 1996) |
| 51 | Moxalactam | 520.5 | 1.6 (26) | Acid | Renal | 40 | 4.97 | 0.651 | (Mahmood and Balian, 1996) |
| 52 | Nicardipine | 479.5 | 7.0 | Base | Hepatic | 1.5 | 63.1 | 0.727 | (Higuchi and Shiobara, 1980) |
| 53 | Nifedipine | 346.3 | 7.0 | Neutral | Hepatic | 5 | 6.12 | 1.24 | (Evans et al., 2006) |
| 54 | Ofloxacin | 361.4 | 4.5 (15) | Zwitt | Renal | 75 | 9.44 | 0.569 | (Evans et al., 2006) |
| 55 | Panipenem | 339.4 | 3.1 (12) | Acid | Mixed | 92.6 | 14.8 | 0.492 | (Kurihara et al., 1992) |
| 56 | PD1 | 364.8 | 0.0043 | Base | Hepatic | 49 | 0.0276 | 0.966 | (Feng et al., 2000) |
| 57 | PD8 | 245.3 | 1.6 | Base | Renal | 16 | 5.06 | 0.629 | (Feng et al., 2000) |
| 58 | Phencyclidine | 243.4 | 5.1 (22) | Base | Hepatic | 35 | 76.3 | 0.763 | (Bachmann, 1989) |
| 59 | PNU-96391 | 281.4 | 7.2 (38) | Base | Hepatic | 0.73 | 41.7 | 0.923 | (Evans et al., 2006) |
| 60 | PNU-288034 | 403.4 | 7.3 | Base | Renal | NA | 10.7 | 0.699 | (Lai et al.) |
| 61 | Propafenone | 341.5 | 18 (19) | Base | Hepatic | 3 | 50.9 | 0.841 | (Evans et al., 2006) |
| 62 | Propranolol | 259.3 | 12 (7.7) | Base | Hepatic | 8.4 | 47.7 | 0.652 | (Mahmood and Balian, 1996) |
| 63 | Quinidine | 324.4 | 3.8 (47) | Base | Hepatic | 17.5 | 30.0 | 0.539 | (Ueda et al., 1977, Guentert et al., 1982, Iven, 1977) |
| 64 | Remoxipride | 371.3 | 1.7 (29) | Base | Hepatic | 6.5 | 91.2 | 0.506 | (Evans et al., 2006) |
| 65 | RO 24–6173 | 349.3 | 12 | NA | Hepatic | 10 | 68.8 | 0.716 | (Lave et al., 1997) |
| 66 | RO 25–6833 | 560.5 | 0.39 (29) | Acid | Mixed | 4.3 | 1.06 | 1.18 | (Richter et al., 1998) |
| 67 | Salicylic acid | 138.1 | 0.56 | Acid | Hepatic | 15 | 0.0554 | 1.63 | (Davis and Westfall, 1972) |
| 68 | Sematilide | 313.4 | 4.1 (14) | Base | Renal | 96 | 19.7 | 0.727 | (Hinderling et al., 1993) |
| 69 | Semaxanib | 238.3 | 14 (50) | Base | Mixed | 0.8 | 48.8 | 0.857 | (Evans et al., 2006) |
| 70 | Stavudine | 242.2 | 9.8 (39) | Base | Renal | 100 | 20.8 | 0.909 | (Kaul et al., 1999) |
| 71 | Susalimod | 408.0 | 0.090 | Acid | Hepatic | 23.5 | 9.32 | 0.957 | (Feng et al., 2000) |
| 72 | Tamsulosin | 408.5 | 0.69 | Base | Hepatic | 1.0 | 61.1 | 0.594 | (Bolton, 1997) |
| 73 | Tebufelone | 300.4 | 9.9 | Acid | Hepatic | 0.001 | 31.1 | 0.878 | (Cruze et al., 1995) |
| 74 | Theophylline | 180.2 | 0.86 (28) | Acid | Hepatic | 58 | 2.68 | 0.906 | (Mahmood and Balian, 1996) |
| 75 | Tolcapone | 273.2 | 1.5 (22) | Base | Hepatic | 0.1 | 12.4 | 0.654 | (Lave et al., 1997) |
| 76 | Trimethadione | 143.1 | 0.70 (14) | Neutral | Hepatic | 0.1 | 3.78 | 0.743 | (Tanaka et al., 1999) |
| 77 | Troglitazone | 441.5 | 2.5 (20) | Acid | Hepatic | 1 | 12.5 | 0.795 | (Izumi et al., 1996) |
| 78 | Valproate | 144.2 | 0.11 (18) | Acid | Hepatic | 5.2 | 3.61 | 0.947 | (Bolton, 1997) |
| 79 | Verapamil | 454.6 | 13 (31) | Base | Hepatic | 10 | 40.7 | 1.08 | (Evans et al., 2006) |
| 80 | Vinorelbine | 778.9 | 19 (19) | Acid | Hepatic | 9–21 | 25.8 | 0.915 | (Evans et al., 2006) |
| 81 | Warfarin | 308.3 | 0.058 (19) | Acid | Hepatic | 1 | 0.382 | 1.20 | (Bachmann, 1989) |
Zwitt, zwitterion; NA, information not avaliable.
Allometric scaling using single species
Human clearance was predicted using single species with the allometry exponent fixed at 0.60, 0.65, 0.70, 0.75, 0.80, 0.85, or 0.90. The following equation was used to calculate human clearance:
| (1) |
where BW is the body weight and b is the allometry exponent. Based on the availability of literature data, 36, 78, 78, and 63 small-, and 25, 40, 19, and 43 macro-molecule drugs were used in single species scaling for mouse, rat, dog, and monkey, respectively.
Single species scaling using liver blood flow
Human clearance was estimated using liver blood flow (LBF) with the following equation as proposed by Ward and Smith (Ward and Smith, 2004):
| (2) |
LBF values used for mouse, rat, dog, monkey and human were 90.0, 55.2, 30.9, 43.6 and 20.7 mL/min/kg, respectively (Davies and Morris, 1993). To be used in this equation, each LBF value was multiplied by the corresponding body weight. For example, for a mouse weighting 0.02 kg, the LBFmouse became 1.8 mL/min.
Allometric scaling using multiple species
Several methods (i.e. simple allometry, exponent rule-corrected allometry, multiexponential allometry and exponent rule-corrected multiexponential allometry) were evaluated. At least three animal species were used for the scaling and the prediction of human clearance for each compound. Based on the availability of literature data, 81 small- and 36 macro-molecule drugs were used in multiple species scaling. Small-molecule drugs were divided by 3 groups based on elimination mechanism: (1) “hepatic”– if the drugs are mainly eliminated via metabolism or biliary excretion (n = 50), (2) “renal”– if most of drug molecules are eliminated renally as unchanged (n = 19), (3) “mixed” - if both renal and hepatic routes contribute to the elimination (n = 12).
Simple allometry (SA)
Human clearance was predicted with the following allometric equation as previously described (Boxenbaum and DiLea, 1995):
| (3) |
where a is the coefficient and b is the allometry exponent.
Exponent rule-corrected allometry (ROE)
As mentioned previously, Boxenbaum (1982) proposed using maximum life-span potential (MLP) and brain weight (BRW) as correction factors in allometric scaling since longevity is frequently inversely correlated with hepatic cytochrome P450 drug oxidation rates. The application of MLP and BRW were also assessed and supported by other scientists including Feng (Feng et al., 2000), and Mahmood and Balian (Mahmood and Balian, 1996). In this study, the exponent rule-corrected method previously suggested by Mahmood and Balian was adopted: If b < 0.71 in simple allometry, no correction factor was applied; if 0.71 ≤ b < 1, MLP was used as a correction factor; if 1 ≤ b, BRW was used as an correction factor. Human clearance was predicted using the following equations:
| (4) |
| (5) |
BRW values were 1.65, 0.57, 0.78, 1.56, and 2% of body weight, and the MLP values 2.7, 4.7, 22.0, 20.0, and 93.4 for mouse, rat, dog, monkey, and human, respectively (Brown et al., 1997, Sacher, 2008).
Multiexponential allometry (MA)
Multiexponential allometry method was used to predict human clearance as suggested by Goteti et al using the following equation (Goteti et al., 2008):
| (6) |
where a and b are the coefficient and the allometry exponent determined from the simple allometry analysis.
Exponent rule-corrected multiexponential allometry (SA+MA)
Human clearance was predicted using simple allometry method if the exponent b is < 0.71 with no correction factor applied. If the exponent b is ≥ 0.71, the multiexponential allometry equation listed above was used for human clearance prediction.
Relationship between CL and molecular size
Literature data of 675 small- and 80 macro-molecule drugs were collected and the correlation between total clearance and molecular weight (MW) was assessed.
Statistical analysis
Average-fold error (AFE) for human CL prediction was calculated based on equation 7 (Bolton, 1997) and used to compare the various prediction methods,
| (7) |
By using this equation, under-estimations can have the same magnitude of error as over-estimations. For example, a 2-fold over-prediction and under-prediction would have the same value of 2 for AFE.
Student’s t-test was used to determine the statistical differences in AFE values between two groups and p < 0.05 was considered statistically significant. For multiple-group comparison in AFE values, analysis of variance (ANOVA) was performed followed by Tukey or Student’s t-test.
Results
Scaling using single species
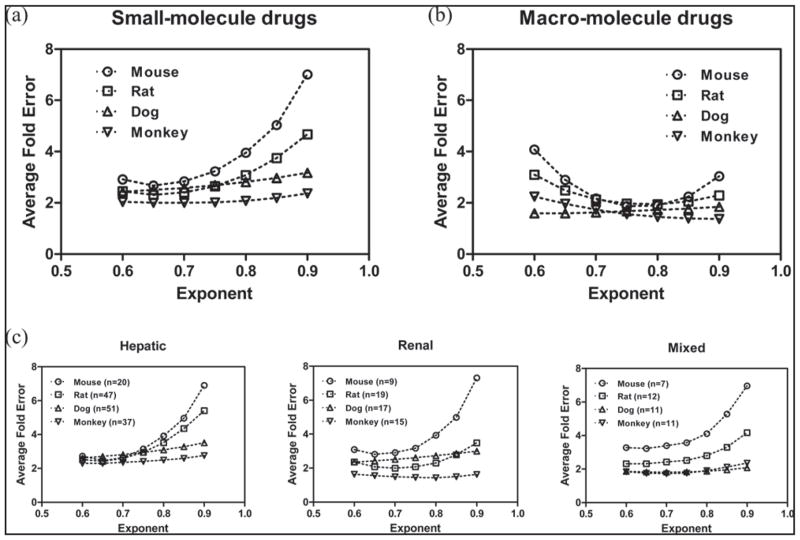
The results from single species scaling with a fixed allometry exponent are summarized in Figures 1–3. For macro-molecule drugs, human clearance is generally well predicted with average-fold error < 2 using a fixed allometry exponent of 0.75–0.80. Increase or decrease of the exponent “b” only results in a small fluctuation of the AFE (Figure 1b). It appears that human clearance of macro- molecules is best predicted using monkey as a single species with the AFE value of 1.45, which is statistically lower (p < 0.05) than the AFEs of 1.89, 1.94, 1.72 using mouse, rat or dog for single species scaling with optimal allometry exponent fixed at 0.80 (Figure 3). For the small-molecule drugs, human clearance is best predicted using a fixed allometry exponent of 0.65–0.70. The AFEs for small-molecule drugs increased significantly when the exponent “b” value was higher than 0.80 (Figure 1a). The AFEs are 2.67, 2.31, 2.50, and 2.00, respectively, using mouse, rat, dog, or monkey as a single species with an optimal allometry exponent fixed at 0.65. Those AFE values are not statistically different across the species, although human clearance appears better predicted using monkey data. As shown in Figure 1c, when the small-molecule drugs are divided by 3 groups based on elimination mechanism: “hepatic”, “renal” and “mixed”, similar trend is observed with human clearance best predicted using a fixed allometry exponent of 0.65–0.70 and the AFEs increased significantly when the exponent “b” value was higher than 0.80. The correlation between the prediction accuracy [ratio of predicted/observed (Pred/Obs)] and the actual value of human clearance for small-molecule drugs using single species allometric scaling with fixed exponent of 0.65 is presented in Figure 2 and the plots suggest the prediction error is mainly associated with drugs with low extraction ratio.
Figure 1.
Average fold-error (AFE) for human clearance predictions using single animal species with allometry exponent fixed in the range of 0.6–0.9.
Figure 3.
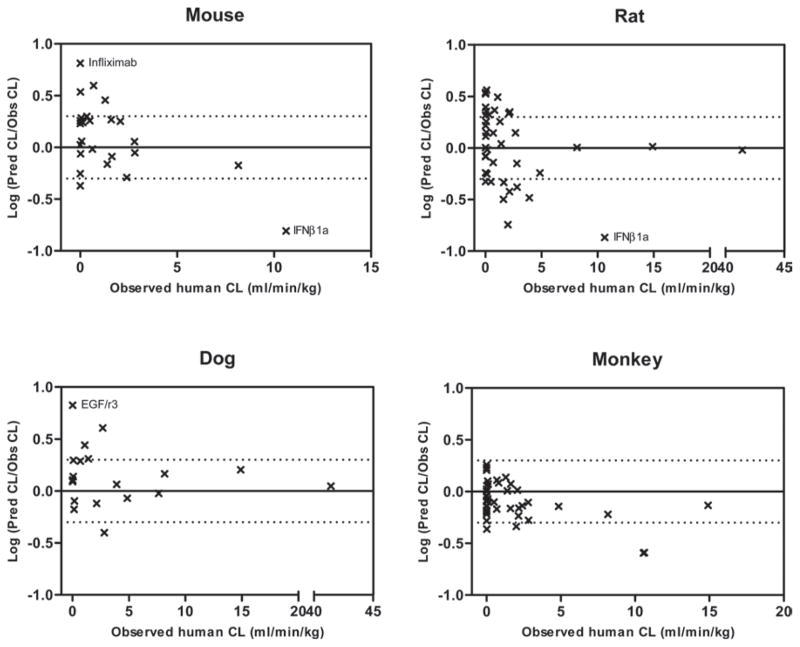
Correlation between the prediction accuracy [ratio of predicted/observed (Pred/Obs)] and the observed value of human clearance (CL) for macro-molecule drugs using single species allometric scaling with fixed exponent of 0.80 (the average optimal value from Figure 1). The solid horizontal line represents the identity with Pred/Obs ratio = 1 and the upper and lower dotted horizontal lines represent 2-fold above and 2-fold below the identity, respectively.
Figure 2.
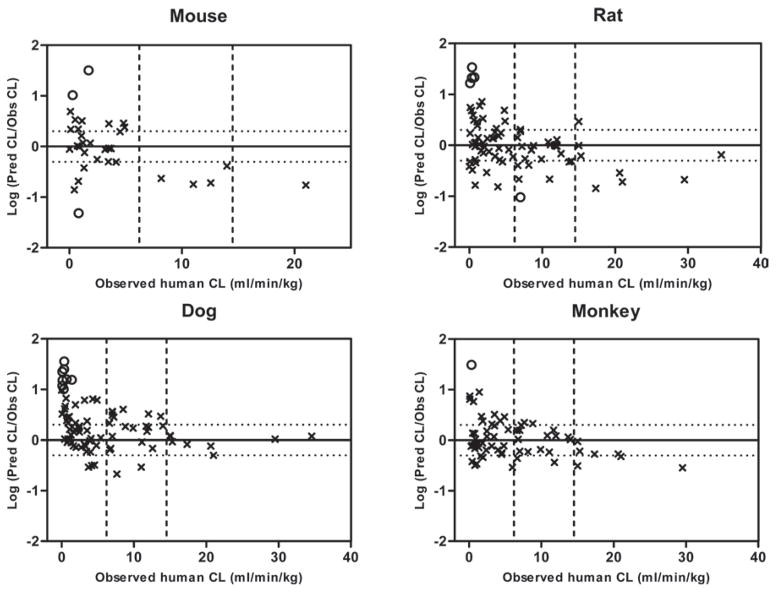
Correlation between the prediction accuracy [ratio of predicted/observed (Pred/Obs)] and the observed value of human clearance (CL) for small-molecule drugs using single species allometric scaling with fixed exponent of 0.65 (the average optimal value from Figure 1). The solid horizontal line represents the identity with Pred/Obs ratio = 1 and the upper and lower dotted horizontal lines represent 2-fold above and 2-fold below the identity, respectively. Several outliers with prediction error of greater than 10 were denoted as open circles. The two dotted vertical lines represent the criteria dividing small-molecules with low (hepatic extraction ratio < 0.3), medium, and high (hepatic extraction ratio > 0.7) clearance drugs.
Prediction of human clearance using single species liver blood flow is also examined in this study for macro- and hepatically eliminated small-molecule drugs, and the results suggest that human clearance is well predicted with AFE ≤ 2.0 for all drugs using monkey liver blood flow (Table 3). However, the liver blood flow method did not work as well using other species especially for the small-molecule drugs with high AFE values of 4.04, 3.47, and 2.83 in mouse, rat, and dog, respectively.
Table 3.
Average-fold error (AFE) of human CL prediction using liver blood flow method.
| Drugs | Mouse
|
Rat
|
Dog
|
Monkey
|
||||
|---|---|---|---|---|---|---|---|---|
| Small | Macro | Small | Macro | Small | Macro | Small | Macro | |
| # of drugs | 36 | 25 | 78 | 40 | 78 | 19 | 63 | 43 |
| AFE | 4.04 | 2.38 | 3.47 | 1.98 | 2.83 | 2.06 | 2.02 | 1.63 |
Scaling using multiple species
The multiple species scaling methods, SA, ROE, MA and exponent rule-corrected MA (SA+MA), are evaluated in this study. Although several studies have reported the scaling of 2 animal species for the prediction of human clearance, we used three or more species in our analysis and the results are summarized in Table 4a and 4b. The results indicated that the SA method delivered a high accuracy in human clearance prediction for macro-molecule drugs with an AFE of 1.67, and no additional correction using MLP or BRW seems needed. This may be explained by their elimination mechanism. The therapeutic proteins are mainly eliminated by non-specific proteolysis that is very different compared to the complicated oxidative metabolic pathways for small-molecule drugs. Therefore, the use of ROE, MA, and SA+MA did not improve the prediction of human clearance for macromolecule drugs and resulted in a higher AFE of 2.06, 1.87, and 1.95, respectively. As mentioned previously, the small-molecule drugs were grouped by mechanism of elimination to assess if the elimination mechanism may affect the prediction accuracy of human clearance. As listed in Table 4a, for drugs in the “renal” group, the human clearance is generally well predicted using simple allometry with AFE of 1.84 and it appears that no MLP or BRW correction is needed for those drugs. The AFEs from ROE, MA, and SA+MA methods are 1.95, 1.73, 1.66, respectively and not statistically different from the AFE of the SA method. The AFE of human clearance prediction is 3.14 using the SA method for drugs in the “hepatic” group, which is statistically significantly higher than the values in other groups (p < 0.05). Results from additional analysis presented in Table 4b indicated that the prediction error for hepatically eliminated small-molecules is mainly associated with drugs with low hepatic extraction ratio (Eh) with AFE of 4.51. The AFE is 2.46 and 1.35 for drugs with medium and high Eh, respectively, and the AFE in the high Eh group is significantly lower (p < 0.05) than the corresponding values in the other two groups, which is consistent with the outcome from single species scaling (Figure 2) and the literature information as the elimination process of drugs with high Eh is mainly controlled by liver blood flow, a physiological parameter extrapolated very well from animals to humans (Feng et al 1998a and 1998b). Correction with MLP or BRW did help to reduce the prediction error for hepatically eliminated drugs with low and medium Eh. The AFE is 2.25 for small-molecule drugs in the mixed group and the correction with MLP or BRW also reduced the error of prediction (Table 4a).
Table 4a.
Average-fold error (AFE) of human CL predictions using multiple species scaling: comparison of small- versus macro-molecule drugs.
| SA | ROE | MA | SA+MA | ||
|---|---|---|---|---|---|
| Small-molecule | Hepatic (n = 50) | 3.14 | 2.18 | 2.37 | 2.16 |
| Renal (n = 19) | 1.84 | 1.95 | 1.73 | 1.66 | |
| Mixed (n = 12) | 2.25 | 1.99 | 2.09 | 1.97 | |
| All (n = 81) | 2.64 | 2.10 | 2.16 | 2.00 | |
| Macro-molecule | (n = 36) | 1.67 | 2.06 | 1.87 | 1.95 |
SA, simple allometry; ROE, exponent rule-corrected SA (exponent≥1, corrected by BRW; 0.71≤exponent<1, corrected by MLP; exponent<0.71, SA); MA, multiexponential allometry; SA+MA, exponent rule-corrected MA (exponent≥0.71, MA; exponent<0.71, SA). Hepatic: drug molecules are mainly eliminated hepatically; Renal: drug molecules are mainly eliminated renally; Mixed: drug molecules are eliminated by both hepatic and renal routes.
Table 4b.
Average-fold error (AFE) of human clearance predictions using multiple species scaling for small-molecule drugs mainly hepatically eliminated with low (< 0.3), medium (0.3–0.7), and high (> 0.7) extraction ratio.
| SA | ROE | MA | SA+MA | ||
|---|---|---|---|---|---|
| Small-molecule | Low (n = 27) | 4.51 | 2.81 | 3.31 | 2.79 |
| Medium (n = 16) | 2.46 | 1.59 | 1.47 | 1.55 | |
| High (n = 7) | 1.35 | 1.71 | 1.99 | 1.74 | |
| All (n = 50) | 3.14 | 2.18 | 2.37 | 2.16 |
SA, simple allometry; ROE, exponent rule-corrected SA (exponent≥1, corrected by BRW; 0.71≤exponent<1, corrected by MLP; exponent<0.71, SA); MA, multiexponential allometry; SA+MA, exponent rule-corrected MA (exponent≥0.71, MA; exponent<0.71, SA).
Relationship between CL and molecular size
The relationship between human clearance and molecular weight is presented in Figure 4. The small-molecule drugs were divided into 3 groups with MW < 300 Da in group 1 (n = 233), 300 ≤ MW < 400 Da in group 2 (n = 221); and 400 ≤ MW < 500 Da in group 3 (n = 221). Based on one-way ANOVA analysis, the clearance values in the 3 groups are not statistically different (p > 0.05). The macro-molecule drugs were also divided into 2 groups with MW < 69 kDa in group 1 (n = 49) and MW ≥ 69 kDa in group 2 (n = 29). The clearance values in group 2 are significantly lower and statistically different compared to those in group 1 based on t-test (p < 0.01).
Figure 4.
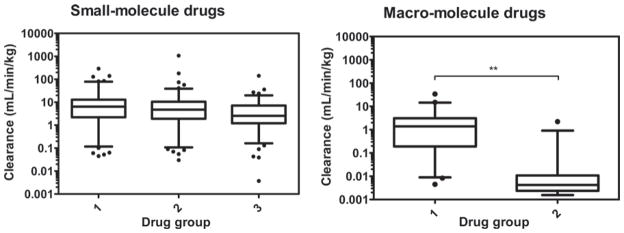
Relationship between human clearance and molecular weight for small- and macro-molecule drugs. Whiskers of box and whiskers plots represent the 5–95th percentile of data. For small-molecules: group1, MW < 300 Da (n = 233); group 2, 300 ≤ MW < 400 Da (n = 221); group 3, 400 ≤ MW (n = 221). For macro-molecules: group 1, MW < 69 kDa (n = 49) and group 2 (n = 29), MW ≥ 69 kDa (**p < 0.01 as determined by t-test).
Discussion
Scaling using single species
Single species scaling with a fixed allometry exponent or using LBF was evaluated in the current study. Although there are different opinions regarding the use of single species scaling (Mahmood, 2009a, Mahmood, 2005), the method does have advantages in the respect of cost-effectiveness. Finding an optimal value for the allometry exponent in single species scaling is always a challenge. Although previous studies have suggested there could be a universal exponent value across animal species, the reported results are still controversial (Hu and Chiu, 2009, Hu and Hayton, 2001). In our study, optimal allometry exponent values of ‘0.65–0.70’ with AFE of 2.40–2.49 were identified that generally worked best for human clearance predictions for small-molecules, while for the macro-molecules, optimal exponent value of ‘0.75–0.80’ with AFE of 1.75–1.77 were selected. These values are close to the historically recommended standard exponent value of ‘0.75’ in interspecies scaling derived from the observation that basal metabolic rates across species could be scaled by body weight with an exponent of ‘0.75’ (Kleiber, 1947, Feldman and McMahon, 1983). For the macro-molecules, the prediction error from single species scaling is generally within 2-fold for majority of the drugs in our analysis (Figure 3), which is consistent with other results in the literature (Ling et al., 2009, Wang and Prueksaritanont, 2010). Most macro-molecule drugs are peptides or proteins that are eliminated from the body via non-specific proteolysis, a process very different from the complicated oxidative metabolic pathways for small-molecule drugs. It is known that ubiquitously expressed proteolytic enzymes responsible for the elimination of macro-molecules are universal across animal species, which could help to explain the successful extrapolation from animals to humans for macro-molecule drugs. As mentioned previously, several antisense oligonucleotide drugs (e.g., ISIS compounds) were included in the macro-molecule category. Oligonucleotide drugs are mainly eliminated from the body by metabolic pathways via nucleases (Levin, 1999, Levin et al., 2001). Nucleases are also ubiquitously expressed throughout the body and could be scaled across animal species. Interestingly, it has been shown that the plasma clearance values of ISIS compounds are quite similar in rat, rabbit, dog and monkey ranging from 1 to 3 mL/min/kg (Geary et al., 2001). Several outliers (infliximab, IFNβ and EGFr3) with relatively high prediction errors (6- or 7-fold) were, however, observed in our analysis of macro-molecules (Figure 3), which may be the result of species-specific differences in binding activity and non-linear pharmacokinetics. While human IFNs bind to receptors in monkey, they lack binding activity in rodents (Kagan et al., 2010). This may help to explain why the prediction error of IFNβ was high when mouse or rat was used in single species scaling. Non-linear pharmacokinetics can be a potential explanation for the high prediction error of EGFr3 in dog, since a relatively low dose was used for the pharmacokinetic study of EGFr3 in dogs compared to other preclinical studies as previous study has shown (Wang and Prueksaritanont, 2010). For small-molecule drugs, the plots in Figure 1 and 2 suggest that human clearance is better predicted using monkey data although the cross-species comparison of AFEs does not indicate statistical differences.
The LBF method appears useful in human clearance prediction for both small and macro-molecule drugs when monkey data are available. If data from other animal species were used, the prediction accuracy may be acceptable for most of the macro-molecule drugs (within 2-fold), but the error appears high for small-molecule drugs.
Scaling using multiple species
Interspecies scaling using 3 or more animal species was also evaluated in this study. The results suggest that the SA method delivered a high accuracy prediction of human clearance for macro-molecule drugs (AFE = 1.67, Table 4a), which is consistent with the phenomena that the clearance mechanism of macro-molecule drugs is evolutionally well conserved compared to the species-specific metabolism of small-molecule drugs. Only EGFr3 is a notable outlier in the SA method and it may be associated with nonlinear pharmacokinetics as previously discussed.
In general, the SA method also worked well with AFEs < 2.0 for small-molecule drugs excreted renally (Table 4a). The AFE from the SA method is higher for drugs mainly eliminated hepatically, and a correction with MLP or BRW (using the ROE, MA, or SA+MA methods) helped to reduce the prediction error for those drugs (Table 4a and 4b). The correction with MLP was proposed in 1982 by Boxenbaum based on the observation that longevity was frequently inversely correlated with hepatic cytochrome P450 drug oxidation rates (Boxenbaum, 1982, Boxenbaum and Ronfeld, 1983). The MLP Clearance product represents the volume from which drug would be cleared per person’s maximum life-span potential assuming constant drug exposure. Using antipyrine as an example, Boxenbaum demonstrated that the regression of volume cleared per MLP is approximately proportional to body weight. With respect to humans, it would appear that the relatively low intrinsic unbound clearance (with respect to liver weight) is synchronized to his (or hers) longevity; i.e. the low activity is conserved over the relatively longer chronological MLP. Boxenbaum also explored the possibility of using brain weight (BRW) as a correction factor since MLP is closely correlated to BRW MLP=185.4 (BRW0.636) (W(− 0.225)). Boxenbaum’s work was later supported by the results of other scientists. Results from our previous work also demonstrated that the MLP and BRW correction improved the accuracy of human clearance predictions when the allometry power exponent b was higher than 0.80–0.90 (Feng et al., 1998a, Feng et al., 1998b, Feng et al., 2000, Mahmood and Balian, 1996). The results from our current study indicated that multiple species scaling generally worked better than single species allometry for small-molecule drugs, and the ROE, MA and SA+MA methods using a correction with MLP or BRW could help to increase the accuracy of prediction for small-molecule drugs mainly hepatically eliminated. However, there were a few notable outliers with high prediction error even after using MLP or BRW as a correction factor. All these drugs are mainly hepatically eliminated either by metabolism or by biliary excretion and have relatively low clearance.
Relationship between CL and molecular size
The results of this study also indicated that the pharmacokinetic properties of small- and macro-molecule drugs are quite different when the relationship between clearance and molecular weight was examined. A trend for macro-molecules is observed (Figure 4), in which the CL values of those drugs in group 2 (MW ≥ 69 kDa) are significantly lower than those in group 1 (MW < 69 kDa). It is generally known that a molecule with MW < 10 kDa is readily excreted by glomerular filtration in the kidney, while a molecule with MW ≥ 69 kDa (MW of albumin ≈ 69 kDa) is highly restricted at the glomerulus (Braeckman, 2000, Lin, 2009). Therefore, glomerular filtration could limit the renal excretion of group 2 drugs, and this may help us to understand the differences in clearance between group 1 and 2. However, one needs to be cautious in directly relating clearance with molecular weight, since MW does not necessarily represent the effective molecular size and the effective molecular radius may be a better way to determine the degree of glomerular filtration. Drug molecules in group 2 may be eliminated mainly by non-specific proteolysis or receptor mediated degradation. Other physicochemical properties such as lipophilicity, charge and functional groups may further influence the distribution and elimination mechanisms (Braeckman, 2000).
Conclusion
In summary, human clearance of macro-molecule drugs may be predicted using single-species allometric scaling with an optimal component value of “0.80” or using multiple-species simple allometry scaling. No correction by MLP or BRW seems needed for the scaling of macro-molecules probably due to their elimination mechanism. The therapeutic proteins are mainly eliminated by non-specific proteolysis that is very different compared to the complicated oxidative metabolic pathways of the small-molecules. In general, human clearance of small-molecule drugs may be predicted (AFE value of 2.0) using monkey body weight scaling and an optimal allometry exponent value of “0.65”. However, the prediction appears less accurate when mouse, rat or dog data are used for single species allometric scaling. Human clearance is also well predicted using SA method with AFE < 2.0 for small-molecules renally excreted. The prediction error is higher for small-molecules hepatically excreted, and correction using MLP or BRW (ROE, MA and SA+MA methods) could help to reduce the prediction error. Human clearance of both small- and macro-molecule drugs could also be predicted using the monkey liver blood flow method, but the prediction using liver blood flow from other species did not work as well especially for the small molecules.
For small molecule drugs with complicated oxidative metabolic pathway and significant cross-species differences in hepatic metabolism, the simple allometry power equation may not work well for the estimation of human clearance, and in addition to MLP and BRW, other correction factors (e.g. liver blood blow, in vitro metabolic clearance, free fraction in blood, binding affinity to receptors or subcellular components, etc.) have been proposed to enhance the accuracy of prediction. Each of these techniques mentioned above has its own merits and drawbacks, and some of them have had only partial success in predicting human clearance. Research work using in vitro, in vivo, and in silico models are still ongoing to further improve the estimation accuracy of human pharmacokinetic profiles to help reducing the risk and the tremendous financial costs associated with failed clinical trials.
References
- Abernethy DR, Pezzullo J, Mascelli MA, Frederick B, Kleiman NS, Freedman J. Pharmacodynamics of abciximab during angioplasty: comparison to healthy subjects. Clin Pharmacol Ther. 2002;71:186–195. doi: 10.1067/mcp.2002.121775. [DOI] [PubMed] [Google Scholar]
- Adams CW, Allison DE, Flagella K, Presta L, Clarke J, Dybdal N, McKeever K, Sliwkowski MX. Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol Immunother. 2006;55:717–727. doi: 10.1007/s00262-005-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agerso H, Seiding Larsen L, Riis a, Lovgren U, Karlsson MO, Senderovitz T. Pharmacokinetics and renal excretion of desmopressin after intravenous administration to healthy subjects and renally impaired patients. Br J Clin Pharmacol. 2004;58:352–358. doi: 10.1111/j.1365-2125.2004.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S. What’s fueling the biotech engine? Nat Biotechnol. 2007;25:1097–1104. doi: 10.1038/nbt1007-1097. [DOI] [PubMed] [Google Scholar]
- Agus DB, Gordon MS, Taylor C, Natale RB, Karlan B, Mendelson DS, Press MF, Allison DE, Sliwkowski MX, Lieberman G, Kelsey SM, Fyfe G. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol. 2005;23:2534–2543. doi: 10.1200/JCO.2005.03.184. [DOI] [PubMed] [Google Scholar]
- Bachmann K. Predicting toxicokinetic parameters in humans from toxicokinetic data acquired from three small mammalian species. J Appl Toxicol. 1989;9:331–338. doi: 10.1002/jat.2550090509. [DOI] [PubMed] [Google Scholar]
- Bauer RJ, Dedrick RL, White ML, Murray MJ, Garovoy MR. Population pharmacokinetics and pharmacodynamics of the anti-CD11a antibody hu1124 in human subjects with psoriasis. J Pharmacokinet Biopharm. 1999;27:397–420. doi: 10.1023/a:1020917122093. [DOI] [PubMed] [Google Scholar]
- Bazin-Redureau M, Pepin S, Hong G, Debray M, Scherrmann JM. Interspecies scaling of clearance and volume of distribution for horse antivenom F(ab′)2. Toxicol Appl Pharmacol. 1998;150:295–300. doi: 10.1006/taap.1997.8363. [DOI] [PubMed] [Google Scholar]
- Belpaire FM, de Smet F, Vynckier LJ, Vermeulen AM, Rosseel MT, Bogaert MG, Chauvelot-Moachon L. Effect of aging on the pharmcokinetics of atenolol, metoprolol and propranolol in the rat. J Pharmacol Exp Ther. 1990;254:116–122. [PubMed] [Google Scholar]
- Björkman S, Redke F. Clearance of fentanyl, alfentanil, methohexitone, thiopentone and ketamine in relation to estimated hepatic blood flow in several animal species: application to prediction of clearance in man. J Pharm Pharmacol. 2000;52:1065–1074. doi: 10.1211/0022357001774985. [DOI] [PubMed] [Google Scholar]
- Bleuel H, Hoffmann R, Kaufmann B, Neubert P, Ochlich PP, Schaumann W. Kinetics of subcutaneous versus intravenous epoetin-beta in dogs, rats and mice. Pharmacology. 1996;52:329–338. doi: 10.1159/000139398. [DOI] [PubMed] [Google Scholar]
- Bolton S. Pharmaceutical statistics: practical and clinical applications. New York: Marcel Dekker Inc; 1997. [Google Scholar]
- Bortolotti A, Castelli D, Verotta D, Bonati M. Pharmacokinetic and pharmacodynamic modelling of metoprolol in rabbits with liver failure. Eur J Drug Metab Pharmacokinet. 1989;14:145–151. doi: 10.1007/BF03190855. [DOI] [PubMed] [Google Scholar]
- Boxenbaum H. Interspecies scaling, allometry, physiological time, and the ground plan of pharmacokinetics. J Pharmacokinet Biopharm. 1982;10:201–227. doi: 10.1007/BF01062336. [DOI] [PubMed] [Google Scholar]
- Boxenbaum H, DiLea C. First-time-in-human dose selection: allometric thoughts and perspectives. J Clin Pharmacol. 1995;35:957–966. doi: 10.1002/j.1552-4604.1995.tb04011.x. [DOI] [PubMed] [Google Scholar]
- Braeckman R. Pharmacokinetics and pharmacodynamics of protein therapeutics. CRC Press; 2000. [Google Scholar]
- Brocks DR, Freed MI, Martin DE, Sellers TS, Mehdi N, Citerone DR, Boppana V, Levitt B, Davies BE, Nemunaitis J, Jorkasky DK. Interspecies pharmacokinetics of a novel hematoregulatory peptide (SK&F 107647) in rats, dogs, and oncologic patients. Pharm Res. 1996;13:794–797. doi: 10.1023/a:1016020221300. [DOI] [PubMed] [Google Scholar]
- Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health. 1997;13:407–484. doi: 10.1177/074823379701300401. [DOI] [PubMed] [Google Scholar]
- Cartron G, Blasco H, Paintaud G, Watier H, Le Guellec C. Pharmacokinetics of rituximab and its clinical use: thought for the best use? Crit Rev Oncol Hematol. 2007;62:43–52. doi: 10.1016/j.critrevonc.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Cernacek P, Maher E, Crawhall JC, Levy M. Renal dose response and pharmacokinetics of atrial natriuretic factor in dogs. Am J Physiol. 1988;255:R929–R935. doi: 10.1152/ajpregu.1988.255.6.R929. [DOI] [PubMed] [Google Scholar]
- Chang T, Black A, Dunky A, Wolf R, Sedman A, Latts J, Welling PG. Pharmacokinetics of intravenous and oral enoxacin in healthy volunteers. J Antimicrob Chemother. 1988;21(Suppl B):49–56. doi: 10.1093/jac/21.suppl_b.49. [DOI] [PubMed] [Google Scholar]
- Cohenuram M, Saif MW. Panitumumab the first fully human monoclonal antibody: from the bench to the clinic. Anticancer Drugs. 2007;18:7–15. doi: 10.1097/CAD.0b013e32800feecb. [DOI] [PubMed] [Google Scholar]
- Collen D, Lu HR, Lijnen HR, Nelles L, Stassen JM. Thrombolytic and pharmacokinetic properties of chimeric tissue-type and urokinase-type plasminogen activators. Circulation. 1991;84:1216–1234. doi: 10.1161/01.cir.84.3.1216. [DOI] [PubMed] [Google Scholar]
- Crombet T, Torres O, Neninger E, Catalá M, Rodríguez N, Ramos M, Fernández E, Iznaga N, Pérez R, Lage A. Phase I clinical evaluation of a neutralizing monoclonal antibody against epidermal growth factor receptor. Cancer Biother Radiopharm. 2001;16:93–102. doi: 10.1089/108497801750096122. [DOI] [PubMed] [Google Scholar]
- Cruze CA, Kelm GR, Meredith MP. Interspecies scaling of tebufelone pharmacokinetic data and application to preclinical toxicology. Pharm Res. 1995;12:895–901. doi: 10.1023/a:1016273306956. [DOI] [PubMed] [Google Scholar]
- Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- Davis CB, Hepburn TW, Urbanski JJ, Kwok DC, Hart TK, Herzyk DJ, Demuth SG, Leland M, Rhodes GR. Preclinical pharmacokinetic evaluation of the respiratory syncytial virus-specific reshaped human monoclonal antibody RSHZ19. Drug Metab Dispos. 1995;23:1028–1036. [PubMed] [Google Scholar]
- Davis LE, Westfall BA. Species differences in biotransformation and excretion of salicylate. Am J Vet Res. 1972;33:1253–1262. [PubMed] [Google Scholar]
- Dedrick R, Bischoff KB, Zaharko DS. Interspecies correlation of plasma concentration history of methotrexate (NSC-740) Cancer Chemother Rep. 1970;54:95–101. [PubMed] [Google Scholar]
- Dedrick RL, Forrester DD, Cannon JN, el-Dareer SM, Mellett LB. Pharmacokinetics of 1-beta-D-arabinofuranosylcytosine (ARA-C) deamination in several species. Biochem Pharmacol. 1973;22:2405–2417. doi: 10.1016/0006-2952(73)90342-0. [DOI] [PubMed] [Google Scholar]
- Dowell JA, Korth-Bradley J, Liu H, King SP, Berger MS. Pharmacokinetics of gemtuzumab ozogamicin, an antibody-targeted chemotherapy agent for the treatment of patients with acute myeloid leukemia in first relapse. J Clin Pharmacol. 2001;41:1206–1214. doi: 10.1177/00912700122012751. [DOI] [PubMed] [Google Scholar]
- Doyle E, Chasseaud LF, Miller JN. Comparative pharmacokinetics of frusemide in female rhesus monkeys, cynomolgus monkeys and baboons. Comp Biochem Physiol C, Comp Pharmacol. 1982;71C:89–93. doi: 10.1016/0306-4492(82)90015-6. [DOI] [PubMed] [Google Scholar]
- Egrie JC, Dwyer E, Browne JK, Hitz A, Lykos MA. Darbepoetin alfa has a longer circulating half-life and greater in vivo potency than recombinant human erythropoietin. 2003 doi: 10.1016/s0301-472x(03)00006-7. [DOI] [PubMed]
- Eriksson UG, Renberg L, Bredberg U, Teger-Nilsson AC, Regårdh CG. Animal pharmacokinetics of inogatran, a low-molecular-weight thrombin inhibitor with potential use as an antithrombotic drug. Biopharm Drug Dispos. 1998;19:55–64. doi: 10.1002/(sici)1099-081x(199801)19:1<55::aid-bdd74>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Evans CA, Jolivette LJ, Nagilla R, Ward KW. Extrapolation of preclinical pharmacokinetics and molecular feature analysis of “discovery-like” molecules to predict human pharmacokinetics. Drug Metab Dispos. 2006;34:1255–1265. doi: 10.1124/dmd.105.006619. [DOI] [PubMed] [Google Scholar]
- Everitt DE, Davis CB, Thompson K, DiCicco R, Ilson B, Demuth SG, Herzyk DJ, Jorkasky DK. The pharmacokinetics, antigenicity, and fusion-inhibition activity of RSHZ19, a humanized monoclonal antibody to respiratory syncytial virus, in healthy volunteers. J Infect Dis. 1996;174:463–469. doi: 10.1093/infdis/174.3.463. [DOI] [PubMed] [Google Scholar]
- Feldman HA, McMahon TA. The ¾ mass exponent for energy metabolism is not a statistical artifact. Respir Physiol. 1983;52:149–163. doi: 10.1016/0034-5687(83)90002-6. [DOI] [PubMed] [Google Scholar]
- Feng MR, Loo J, Wright J. Disposition of the antipsychotic agent CI-1007 in rats, monkeys, dogs, and human cytochrome P450 2D6 extensive metabolizers. Species comparison and allometric scaling. Drug Metab Dispos. 1998;26:982–988. [PubMed] [Google Scholar]
- Feng MR, Lou X, Brown RR, Hutchaleelaha A. Allometric pharmacokinetic scaling: towards the prediction of human oral pharmacokinetics. Pharm Res. 2000;17:410–418. doi: 10.1023/a:1007520818956. [DOI] [PubMed] [Google Scholar]
- Flaharty KK, Caro J, Erslev A, Whalen JJ, Morris EM, Bjornsson TD, Vlasses PH. Pharmacokinetics and erythropoietic response to human recombinant erythropoietin in healthy men. Clin Pharmacol Ther. 1990;47:557–564. doi: 10.1038/clpt.1990.76. [DOI] [PubMed] [Google Scholar]
- Gatti G, Kahn JO, Lifson J, Williams R, Turin L, Volberding PA, Gambertoglio JG. Pharmacokinetics of GLQ223 in rats, monkeys, and patients with AIDS or AIDS-related complex. Antimicrob Agents Chemother. 1991;35:2531–2537. doi: 10.1128/aac.35.12.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary RS, Yu RZ, Levin AA. Pharmacokinetics of phosphorothioate antisense oligodeoxynucleotides. Curr Opin Investig Drugs. 2001;2:562–573. [PubMed] [Google Scholar]
- Geary RS, Yu RZ, Watanabe T, Henry SP, Hardee GE, Chappell A, Matson J, Sasmor H, Cummins L, Levin AA. Pharmacokinetics of a tumor necrosis factor-alpha phosphorothioate 2′-O-(2-methoxyethyl) modified antisense oligonucleotide: comparison across species. Drug Metab Dispos. 2003;31:1419–1428. doi: 10.1124/dmd.31.11.1419. [DOI] [PubMed] [Google Scholar]
- Giachetti C, Bertolino M, Canali S, Lombardini E, Monzani MV, Sala A, Zanolo G. Pharmacokinetic study in dogs and monkeys after single intravenous and oral administrations of [14C]-ITF-296. Eur J Drug Metab Pharmacokinet. 1998;23:239–250. doi: 10.1007/BF03189346. [DOI] [PubMed] [Google Scholar]
- Goldenberg DM, Rossi EA, Stein R, Cardillo TM, Czuczman MS, Hernandez-Ilizaliturri FJ, Hansen HJ, Chang CH. Properties and structure-function relationships of veltuzumab (hA20), a humanized anti-CD20 monoclonal antibody. Blood. 2009;113:1062–1070. doi: 10.1182/blood-2008-07-168146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MS, Margolin K, Talpaz M, Sledge GW, Jr, Holmgren E, Benjamin R, Stalter S, Shak S, Adelman D. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19:843–850. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]
- Goteti K, Brassil PJ, Good SS, Garner CE. Estimation of human drug clearance using multiexponential techniques. J Clin Pharmacol. 2008;48:1226–1236. doi: 10.1177/0091270008320369. [DOI] [PubMed] [Google Scholar]
- Grene-Lerouge NA, Bazin-Redureau MI, Debray M, Scherrmann JM. Interspecies scaling of clearance and volume of distribution for digoxin-specific Fab. Toxicol Appl Pharmacol. 1996;138:84–89. doi: 10.1006/taap.1996.0101. [DOI] [PubMed] [Google Scholar]
- Guentert TW, Huang JD, Oie S. Disposition of quinidine in the rabbit. J Pharm Sci. 1982;71:812–815. doi: 10.1002/jps.2600710723. [DOI] [PubMed] [Google Scholar]
- Halstenson CE, Macres M, Katz SA, Schnieders JR, Watanabe M, Sobota JT, Abraham PA. Comparative pharmacokinetics and pharmacodynamics of epoetin alfa and epoetin beta. Clin Pharmacol Ther. 1991;50:702–712. doi: 10.1038/clpt.1991.210. [DOI] [PubMed] [Google Scholar]
- Harris PA, Gross JF. Preliminary pharmacokinetic model for adriamycin (NSC-123127) Cancer Chemother Rep. 1975;59:819–825. [PubMed] [Google Scholar]
- Harrison MP, Moss SR, Featherstone A, Fowkes AG, Sanders AM, Case DE. The disposition and metabolism of meropenem in laboratory animals and man. J Antimicrob Chemother. 1989;24(Suppl A):265–277. doi: 10.1093/jac/24.suppl_a.265. [DOI] [PubMed] [Google Scholar]
- Hauptmann J. Pharmacokinetics of an emerging new class of anticoagulant/antithrombotic drugs. A review of small-molecule thrombin inhibitors. Eur J Clin Pharmacol. 2002;57:751–758. doi: 10.1007/s00228-001-0392-7. [DOI] [PubMed] [Google Scholar]
- Hérault JP, Donat F, Bàrzu T, Crépon B, Bernat A, Lormeau JC, Herbert JM. Pharmacokinetic study of three synthetic AT-binding pentasaccharides in various animal species-extrapolation to humans. Blood Coagul Fibrinolysis. 1997;8:161–167. doi: 10.1097/00001721-199704000-00002. [DOI] [PubMed] [Google Scholar]
- Higuchi S, Shiobara Y. Comparative pharmacokinetics of nicardipine hydrochloride, a new vasodilator, in various species. Xenobiotica. 1980;10:447–454. doi: 10.3109/00498258009033779. [DOI] [PubMed] [Google Scholar]
- Hinderling PH, Dilea C, Koziol T, Millington G. Comparative kinetics of sematilide in four species. Drug Metab Dispos. 1993;21:662–669. [PubMed] [Google Scholar]
- Hirai J, Miyazaki H, Taneike T. The pharmacokinetics and pharmacodynamics of furosemide in the anaesthetized dog. J Vet Pharmacol Ther. 1992;15:231–239. doi: 10.1111/j.1365-2885.1992.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Ho M, Silamut K, White NJ, Karbwang J, Looareesuwan S, Phillips RE, Warrell DA. Pharmacokinetics of three commercial antivenoms in patients envenomed by the Malayan pit viper, Calloselasma rhodostoma, in Thailand. Am J Trop Med Hyg. 1990;42:260–266. doi: 10.4269/ajtmh.1990.42.260. [DOI] [PubMed] [Google Scholar]
- Hu T-M, Chiu S-J. Prediction of human drug clearance using a single-species, fixed-exponent allometric approach. Journal of Medical Sciences. 2009;29:331–339. [Google Scholar]
- Hu TM, Hayton WL. Allometric scaling of xenobiotic clearance: uncertainty versus universality. AAPS PharmSci. 2001;3:E29. doi: 10.1208/ps030429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchaleelaha A, Chow HH, Mayersohn M. Comparative pharmacokinetics and interspecies scaling of amphotericin B in several mammalian species. J Pharm Pharmacol. 1997;49:178–183. doi: 10.1111/j.2042-7158.1997.tb06775.x. [DOI] [PubMed] [Google Scholar]
- Iavarone L, Hoke JF, Bottacini M, Barnaby R, Preston GC. First time in human for GV196771: interspecies scaling applied on dose selection. J Clin Pharmacol. 1999;39:560–566. doi: 10.1177/00912709922008164. [DOI] [PubMed] [Google Scholar]
- Ishizuka M, Nagai S, Sakamoto KQ, Fujita S. Plasma pharmacokinetics and CYP3A12-dependent metabolism of c-kit inhibitor imatinib in dogs. Xenobiotica. 2007;37:503–513. doi: 10.1080/00498250600962849. [DOI] [PubMed] [Google Scholar]
- Iven H. The pharmacokinetics and organ distribution of ajmaline and quindine in the mouse. Naunyn Schmiedebergs Arch Pharmacol. 1977;298:43–50. doi: 10.1007/BF00510985. [DOI] [PubMed] [Google Scholar]
- Izumi T, Enomoto S, Hosiyama K, Sasahara K, Shibukawa A, Nakagawa T, Sugiyama Y. Prediction of the human pharmacokinetics of troglitazone, a new and extensively metabolized antidiabetic agent, after oral administration, with an animal scale-up approach. J Pharmacol Exp Ther. 1996;277:1630–1641. [PubMed] [Google Scholar]
- Kagan L, Abraham AK, Harrold JM, Mager DE. Interspecies scaling of receptor-mediated pharmacokinetics and pharmacodynamics of type I interferons. Pharm Res. 2010;27:920–932. doi: 10.1007/s11095-010-0098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul S, Dandekar KA, Schilling BE, Barbhaiya RH. Toxicokinetics of 2′,3′-didehydro-3′-deoxythymidine, stavudine (D4T) Drug Metab Dispos. 1999;27:1–12. [PubMed] [Google Scholar]
- Keith JC, Jr, Ferranti TJ, Misra B, Frederick T, Rup B, McCarthy K, Faulkner R, Bush L, Schaub RG. Evaluation of recombinant human factor IX: pharmacokinetic studies in the rat and the dog. Thromb Haemost. 1995;73:101–105. [PubMed] [Google Scholar]
- Kenny JR, Grime K. Pharmacokinetic consequences of time-dependent inhibition using the isolated perfused rat liver model. Xenobiotica. 2006;36:351–365. doi: 10.1080/00498250600637946. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kim WB, Lee MG. Interspecies pharmacokinetic scaling of a new carbapenem, DA-1131, in mice, rats, rabbits and dogs, and prediction of human pharmacokinetics. Biopharm Drug Dispos. 1998;19:231–235. doi: 10.1002/(sici)1099-081x(199805)19:4<231::aid-bdd96>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Kleiber M. Body size and metabolic rate. Physiol Rev. 1947;27:511–541. doi: 10.1152/physrev.1947.27.4.511. [DOI] [PubMed] [Google Scholar]
- Krieter PA, Trapani AJ. Metabolism of atrial natriuretic peptide. Extraction by organs in the rat. Drug Metab Dispos. 1989;17:14–19. [PubMed] [Google Scholar]
- Kurihara A, Naganuma H, Hisaoka M, Tokiwa H, Kawahara Y. Prediction of human pharmacokinetics of panipenem-betamipron, a new carbapenem, from animal data. Antimicrob Agents Chemother. 1992;36:1810–1816. doi: 10.1128/aac.36.9.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara T, Kato Y, Kobayashi S, Suzuki H, Sugiyama Y. Nonlinear pharmacokinetics of a recombinant human granulocyte colony-stimulating factor derivative (nartograstim): species differences among rats, monkeys and humans. J Pharmacol Exp Ther. 1994;271:1535–1543. [PubMed] [Google Scholar]
- Lai Y, Sampson KE, Balogh LM, Brayman TG, Cox SR, Adams WJ, Kumar V, Stevens JC. Preclinical and clinical evidence for the collaborative transport and renal secretion of an oxazolidinone antibiotic by organic anion transporter 3 (OAT3/SLC22A8) and multidrug and toxin extrusion protein 1 (MATE1/SLC47A1) J Pharmacol Exp Ther. 2010;334:936–944. doi: 10.1124/jpet.110.170753. [DOI] [PubMed] [Google Scholar]
- Lave T, Dupin S, Schmitt C, Chou RC, Jaeck D, Coassolo P. Integration of in vitro data into allometric scaling to predict hepatic metabolic clearance in man: application to 10 extensively metabolized drugs. J Pharm Sci. 1997;86:584–590. doi: 10.1021/js960440h. [DOI] [PubMed] [Google Scholar]
- Lave T, Levet-Trafit B, Schmitt-Hoffmann AH, Morgenroth B, Richter W, Chou RC. Interspecies scaling of interferon disposition and comparison of allometric scaling with concentration-time transformations. J Pharm Sci. 1995;84:1285–1290. doi: 10.1002/jps.2600841106. [DOI] [PubMed] [Google Scholar]
- Lave T, Saner A, Coassolo P, Brandt R, Schmitt-Hoffmann AH, Chou RC. Animal pharmacokinetics and interspecies scaling from animals to man of lamifiban, a new platelet aggregation inhibitor. J Pharm Pharmacol. 1996;48:573–577. doi: 10.1111/j.2042-7158.1996.tb05976.x. [DOI] [PubMed] [Google Scholar]
- Lepist EI, Jusko WJ. Modeling and allometric scaling of s(+)-ketoprofen pharmacokinetics and pharmacodynamics: a retrospective analysis. J Vet Pharmacol Ther. 2004;27:211–218. doi: 10.1111/j.1365-2885.2004.00579.x. [DOI] [PubMed] [Google Scholar]
- Levin AA. A review of the issues in the pharmacokinetics and toxicology of phosphorothioate antisense oligonucleotides. Biochim Biophys Acta. 1999;1489:69–84. doi: 10.1016/s0167-4781(99)00140-2. [DOI] [PubMed] [Google Scholar]
- Levin AA, Yu RZ, Geary RS. Basic principles of the pharmacokinetics of antisense oligonucleotide drugs. CRC Press; 2001. [Google Scholar]
- Lin JH. Pharmacokinetics of Biotech Drugs: Peptides, Proteins and Monoclonal Antibodies. Curr Drug Metab. 2009;10:661–691. doi: 10.2174/138920009789895499. [DOI] [PubMed] [Google Scholar]
- Lin YS, Nguyen C, Mendoza JL, Escandon E, Fei D, Meng YG, Modi NB. Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of a humanized monoclonal antibody against vascular endothelial growth factor. J Pharmacol Exp Ther. 1999;288:371–378. [PubMed] [Google Scholar]
- Ling J, Zhou H, Jiao Q, Davis HM. Interspecies scaling of therapeutic monoclonal antibodies: initial look. J Clin Pharmacol. 2009;49:1382–1402. doi: 10.1177/0091270009337134. [DOI] [PubMed] [Google Scholar]
- Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93:2645–2668. doi: 10.1002/jps.20178. [DOI] [PubMed] [Google Scholar]
- Macdougall IC, Robson R, Opatrna S, Liogier X, Pannier A, Jordan P, Dougherty FC, Reigner B. Pharmacokinetics and pharmacodynamics of intravenous and subcutaneous continuous erythropoietin receptor activator (C.E.R.A.) in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2006;1:1211–1215. doi: 10.2215/CJN.00730306. [DOI] [PubMed] [Google Scholar]
- Mahmood I. Interspecies scaling of renally secreted drugs. Life Sci. 1998;63:2365–2371. doi: 10.1016/s0024-3205(98)00525-6. [DOI] [PubMed] [Google Scholar]
- Mahmood I. Interspecies scaling of protein drugs: prediction of clearance from animals to humans. J Pharm Sci. 2004;93:177–185. doi: 10.1002/jps.10531. [DOI] [PubMed] [Google Scholar]
- Mahmood I. Prediction of clearance using monkey liver blood flow. Rockville: Pine House Publishers; 2005. [Google Scholar]
- Mahmood I. Application of fixed exponent 0.75 to the prediction of human drug clearance: an inaccurate and misleading concept. Drug Metabol Drug Interact. 2009;24:57–81. doi: 10.1515/dmdi.2009.24.1.57. [DOI] [PubMed] [Google Scholar]
- Mahmood I. Pharmacokinetic allometric scaling of antibodies: application to the first-in-human dose estimation. J Pharm Sci. 2009;98:3850–3861. doi: 10.1002/jps.21682. [DOI] [PubMed] [Google Scholar]
- Mahmood I, Balian JD. Interspecies scaling: predicting clearance of drugs in humans. Three different approaches. Xenobiotica. 1996;26:887–895. doi: 10.3109/00498259609052491. [DOI] [PubMed] [Google Scholar]
- Marleau S, Ong H, De Léan A, du Souich P. Disposition and dynamics of atrial natriuretic factor in conscious rabbits. J Pharmacol Exp Ther. 1989;251:328–333. [PubMed] [Google Scholar]
- Martin U, Köhler J, Sponer G, Strein K. Pharmacokinetics of the novel recombinant plasminogen activator BM 06.022 in rats, dogs, and non-human primates. Fibrinolysis. 1992;6:39–43. [Google Scholar]
- Martin U, von Möllendorff E, Akpan W, Kientsch-Engel R, Kaufmann B, Neugebauer G. Pharmacokinetic and hemostatic properties of the recombinant plasminogen activator bm 06.022 in healthy volunteers. Thromb Haemost. 1991;66:569–574. [PubMed] [Google Scholar]
- Matsushita H, Suzuki H, Sugiyama Y, Sawada Y, Iga T, Hanano M, Kawaguchi Y. Prediction of the pharmacokinetics of cefodizime and cefotetan in humans from pharmacokinetic parameters in animals. J Pharmacobio-dyn. 1990;13:602–611. doi: 10.1248/bpb1978.13.602. [DOI] [PubMed] [Google Scholar]
- McCarthy K, Stewart P, Sigman J, Read M, Keith JC, Jr, Brinkhous KM, Nichols TC, Schaub RG. Pharmacokinetics of recombinant factor IX after intravenous and subcutaneous administration in dogs and cynomolgus monkeys. Thromb Haemost. 2002;87:824–830. [PubMed] [Google Scholar]
- Mellett LB. Comparative drug metabolism. Prog Drug Res. 1969;13:136–169. doi: 10.1007/978-3-0348-7068-9_3. [DOI] [PubMed] [Google Scholar]
- Monzani MV, Coltro G, Jiritano L, Sala A. Pharmacokinetic profile of ITF 296 in rats and dogs. J Cardiovasc Pharmacol. 1995;26(Suppl 4):S67–S71. [PubMed] [Google Scholar]
- Monzani MV, Coltro G, Sala A, Sardina M. Pharmacokinetics of ITF 296 (Sinitrodil) a novel organic nitrate, in healthy volunteers. Eur J Pharm Sci. 1999;7:179–184. doi: 10.1016/s0928-0987(98)00026-8. [DOI] [PubMed] [Google Scholar]
- Mordenti J, Chen SA, Moore JA, Ferraiolo BL, Green JD. Interspecies scaling of clearance and volume of distribution data for five therapeutic proteins. Pharm Res. 1991;8:1351–1359. doi: 10.1023/a:1015836720294. [DOI] [PubMed] [Google Scholar]
- Mordenti J, Osaka G, Garcia K, Thomsen K, Licko V, Meng G. Pharmacokinetics and interspecies scaling of recombinant human factor VIII. Toxicol Appl Pharmacol. 1996;136:75–78. doi: 10.1006/taap.1996.0008. [DOI] [PubMed] [Google Scholar]
- Morschhauser F, Leonard JP, Fayad L, Coiffier B, Petillon MO, Coleman M, Schuster SJ, Dyer MJ, Horne H, Teoh N, Wegener WA, Goldenberg DM. Humanized anti-CD20 antibody, veltuzumab, in refractory/recurrent non-Hodgkin’s lymphoma: phase I/II results. J Clin Oncol. 2009;27:3346–3353. doi: 10.1200/JCO.2008.19.9117. [DOI] [PubMed] [Google Scholar]
- Mroszczak EJ, Lee FW, Combs D, Sarnquist FH, Huang BL, Wu AT, Tokes LG, Maddox ML, Cho DK. Ketorolac tromethamine absorption, distribution, metabolism, excretion, and pharmacokinetics in animals and humans. Drug Metab Dispos. 1987;15:618–626. [PubMed] [Google Scholar]
- Mullamitha SA, Ton NC, Parker GJ, Jackson A, Julyan PJ, Roberts C, Buonaccorsi GA, Watson Y, Davies K, Cheung S, Hope L, Valle JW, Radford JA, Lawrance J, Saunders MP, Munteanu MC, Nakada MT, Nemeth JA, Davis HM, Jiao Q, Prabhakar U, Lang Z, Corringham RE, Beckman RA, Jayson GC. Phase I evaluation of a fully human anti-alphav integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumors. Clin Cancer Res. 2007;13:2128–2135. doi: 10.1158/1078-0432.CCR-06-2779. [DOI] [PubMed] [Google Scholar]
- Murthy SS, Nelson WL, Shen DD, Power JM, Cahill CM, McLean AJ. Pharmacokinetic interaction between verapamil and metoprolol in the dog. Stereochemical aspects. Drug Metab Dispos. 1991;19:1093–1100. [PubMed] [Google Scholar]
- Nagilla R, Ward KW. A comprehensive analysis of the role of correction factors in the allometric predictivity of clearance from rat, dog, and monkey to humans. J Pharm Sci. 2004;93:2522–2534. doi: 10.1002/jps.20169. [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Koyama M, Matsui H, Ikeda C, Yano K, Nakatsuru N, Yoshinaga K, Noguchi T. Pharmacokinetics of cefpiramide (SM-1652) in humans. Antimicrob Agents Chemother. 1984;25:221–225. doi: 10.1128/aac.25.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Kurobe N, Kashimoto S, Ohue T, Takase Y, Shimizu M. Pharmacokinetics of AT-2266 administered orally to mice, rats, dogs, and monkeys. Antimicrob Agents Chemother. 1983;24:54–60. doi: 10.1128/aac.24.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville K, Parise RA, Thompson P, Aleksic A, Egorin MJ, Balis FM, McGuffey L, McCully C, Berg SL, Blaney SM. Plasma and cerebrospinal fluid pharmacokinetics of imatinib after administration to nonhuman primates. Clin Cancer Res. 2004;10:2525–2529. doi: 10.1158/1078-0432.ccr-03-0155. [DOI] [PubMed] [Google Scholar]
- Nouws JF, Mevius DJ, Vree TB, Baars AM, Laurensen J. Pharmacokinetics, renal clearance and metabolism of ciprofloxacin following intravenous and oral administration to calves and pigs. Vet Q. 1988;10:156–163. doi: 10.1080/01652176.1988.9694165. [DOI] [PubMed] [Google Scholar]
- Nowak G. Pharmacokinetics of hirudin. Semin Thromb Hemost. 1991;17:145–149. doi: 10.1055/s-2007-1002603. [DOI] [PubMed] [Google Scholar]
- Obach RS, Lombardo F, Waters NJ. Trend analysis of a database of intravenous pharmacokinetic parameters in humans for 670 drug compounds. Drug Metab Dispos. 2008;36:1385–1405. doi: 10.1124/dmd.108.020479. [DOI] [PubMed] [Google Scholar]
- Ohdo S, Arata N, Furukubo T, Yukawa E, Higuchi S, Nakano S, Ogawa N. Chronopharmacology of granulocyte colony-stimulating factor in mice. J Pharmacol Exp Ther. 1998;285:242–246. [PubMed] [Google Scholar]
- Oikawa K, Kamimura H, Watanabe T, Miyamoto I, Higuchi S. Pharmacokinetic properties of a novel tissue-type plasminogen activator pamiteplase after single intravenous administration to rats, dogs, and monkeys. Thromb Res. 2001;101:493–500. doi: 10.1016/s0049-3848(00)00414-x. [DOI] [PubMed] [Google Scholar]
- Oikawa K, Watanabe T, Miyamoto I, Higuchi S. Determination, pharmacokinetics and protein binding of a novel tissue-type plasminogen activator, pamiteplase in human plasma. Xenobiotica. 2000;30:993–1003. doi: 10.1080/00498250050200140. [DOI] [PubMed] [Google Scholar]
- Olsen KM, Martin SJ. Pharmacokinetics and clinical use of drotrecogin alfa (activated) in patients with severe sepsis. Pharmacotherapy. 2002;22:196S–205S. doi: 10.1592/phco.22.18.196s.33708. [DOI] [PubMed] [Google Scholar]
- Oostendorp RL, Buckle T, Beijnen JH, van Tellingen O, Schellens JH. The effect of P-gp (Mdr1a/1b), BCRP (Bcrp1) and P-gp/BCRP inhibitors on the in vivo absorption, distribution, metabolism and excretion of imatinib. Invest New Drugs. 2009;27:31–40. doi: 10.1007/s10637-008-9138-z. [DOI] [PubMed] [Google Scholar]
- Palframan R, Airey M, Moore A, Vugler A, Nesbitt A. Use of biofluorescence imaging to compare the distribution of certolizumab pegol, adalimumab, and infliximab in the inflamed paws of mice with collagen-induced arthritis. J Immunol Methods. 2009;348:36–41. doi: 10.1016/j.jim.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Peng B, Dutreix C, Mehring G, Hayes MJ, Ben-Am M, Seiberling M, Pokorny R, Capdeville R, Lloyd P. Absolute bioavailability of imatinib (Glivec) orally versus intravenous infusion. J Clin Pharmacol. 2004;44:158–162. doi: 10.1177/0091270003262101. [DOI] [PubMed] [Google Scholar]
- Prandota J, Pruitt AW. Pharmacokinetic, biliary excretion, and metabolic studies of 14C-furosemide in the rat. Xenobiotica. 1991;21:725–736. doi: 10.3109/00498259109039512. [DOI] [PubMed] [Google Scholar]
- Puchalski T, Prabhakar U, Jiao Q, Berns B, Davis HM. Pharmacokinetic and pharmacodynamic modeling of an anti-interleukin- 6 chimeric monoclonal antibody (siltuximab) in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2010;16:1652–1661. doi: 10.1158/1078-0432.CCR-09-2581. [DOI] [PubMed] [Google Scholar]
- Radwanski E, Chakraborty A, Van Wart S, Huhn RD, Cutler DL, Affrime MB, Jusko WJ. Pharmacokinetics and leukocyte responses of recombinant human interleukin-10. Pharm Res. 1998;15:1895–1901. doi: 10.1023/a:1011918425629. [DOI] [PubMed] [Google Scholar]
- Rane A, Högstedt S, Lindberg B, Regårdh CG, Jorulf H. Comparison of different clearance estimates for metoprolol in the rhesus monkey. J Pharmacol Exp Ther. 1984;228:774–778. [PubMed] [Google Scholar]
- Richter WF, Gallati H, Schiller CD. Animal pharmacokinetics of the tumor necrosis factor receptor-immunoglobulin fusion protein lenercept and their extrapolation to humans. Drug Metab Dispos. 1999;27:21–25. [PubMed] [Google Scholar]
- Richter WF, Heizmann P, Meyer J, Starke V, Lave T. Animal pharmacokinetics and interspecies scaling of Ro 25-6833 and related (lactamylvinyl)cephalosporins. J Pharm Sci. 1998;87:496–500. doi: 10.1021/js970261f. [DOI] [PubMed] [Google Scholar]
- Ritschel WA, Vachharajani NN, Johnson RD, Hussain AS. Interspecies scaling of the pharmacokinetic parameters of coumarin among six different mammalian species. Methods Find Exp Clin Pharmacol. 1991;13:697–702. [PubMed] [Google Scholar]
- Robbie G, Chiou WL. Elucidation of human amphotericin B pharmacokinetics: identification of a new potential factor affecting interspecies pharmacokinetic scaling. Pharm Res. 1998;15:1630–1636. doi: 10.1023/a:1011923704731. [DOI] [PubMed] [Google Scholar]
- Rojas JR, Taylor RP, Cunningham MR, Rutkoski TJ, Vennarini J, Jang H, Graham MA, Geboes K, Rousselle SD, Wagner CL. Formation, distribution, and elimination of infliximab and anti-infliximab immune complexes in cynomolgus monkeys. J Pharmacol Exp Ther. 2005;313:578–585. doi: 10.1124/jpet.104.079277. [DOI] [PubMed] [Google Scholar]
- Sacher GA. Relation of Lifespan to Brain Weight and Body Weight in Mammals. John Wiley & Sons, Ltd; 2008. [Google Scholar]
- Sangalli L, Bortolotti A, Jiritano L, Bonati M. Cyclosporine pharmacokinetics in rats and interspecies comparison in dogs, rabbits, rats, and humans. Drug Metab Dispos. 1988;16:749–753. [PubMed] [Google Scholar]
- Sardina M, Love R, Mizrahi J, Monzani V, Bianchini C. Safety and pharmacologic activity of a new nitrate ester, ITF 296, after intravenous administration in healthy volunteers. J Cardiovasc Pharmacol. 1995;26(Suppl 4):S72–S79. [PubMed] [Google Scholar]
- Sewell KL, Geary RS, Baker BF, Glover JM, Mant TG, Yu RZ, Tami JA, Dorr FA. Phase I trial of ISIS 104838, a 2′-methoxyethyl modified antisense oligonucleotide targeting tumor necrosis factor-alpha. J Pharmacol Exp Ther. 2002;303:1334–1343. doi: 10.1124/jpet.102.036749. [DOI] [PubMed] [Google Scholar]
- Sheremata WA, Vollmer TL, Stone LA, Willmer-Hulme AJ, Koller M. A safety and pharmacokinetic study of intravenous natalizumab in patients with MS. Neurology. 1999;52:1072–1074. doi: 10.1212/wnl.52.5.1072. [DOI] [PubMed] [Google Scholar]
- Siefert HM, Maruhn D, Scholl H. Pharmacokinetics of ciprofloxacin. 2nd communication: distribution to and elimination from tissues and organs following single or repeated administration of [14C]ciprofloxacin in albino rats. Arzneimittelforschung. 1986;36:1503–1510. [PubMed] [Google Scholar]
- Srinivas NR, Shyu WC, Weiner RS, Warner G, Comereski C, Tay LK, Greene DS, Barbhaiya RH. Assessment of dose proportionality, absolute bioavailability, and immunogenicity response of CTLA4Ig (BMS-188667), a novel immunosuppressive agent, following subcutaneous and intravenous administration to rats. Pharm Res. 1997;14:911–916. doi: 10.1023/a:1012156001831. [DOI] [PubMed] [Google Scholar]
- Srinivas NR, Weiner RS, Shyu WC, Calore JD, Tritschler D, Tay LK, Lee JS, Greene DS, Barbhaiya RH. A pharmacokinetic study of intravenous CTLA4Ig, a novel immunosuppressive agent, in mice. J Pharm Sci. 1996;85:296–298. doi: 10.1021/js950428+. [DOI] [PubMed] [Google Scholar]
- Srinivas NR, Weiner RS, Warner G, Shyu WC, Davidson T, Fadrowski CG, Tay LK, Lee JS, Greene DS, Barbhaiya RH. Pharmacokinetics and pharmacodynamics of CTLA4lg (BMS-188667), a novel immunosuppressive agent, in monkeys following multiple doses. J Pharm Sci. 1996;85:1–4. doi: 10.1021/js950347d. [DOI] [PubMed] [Google Scholar]
- Stokol T, Trepanier L, Parry BW, Finnin BC. Pharmacokinetics of von Willebrand factor and factor VIII in canine von Willebrand disease and haemophilia A. Res Vet Sci. 1997;63:23–27. doi: 10.1016/s0034-5288(97)90153-3. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Ishikawa A, Horie T. In vivo and in vitro trimethadione oxidation activity of the liver from various animal species including mouse, hamster, rat, rabbit, dog, monkey and human. Hum Exp Toxicol. 1999;18:12–16. doi: 10.1177/096032719901800102. [DOI] [PubMed] [Google Scholar]
- Tang L, Persky AM, Hochhaus G, Meibohm B. Pharmacokinetic aspects of biotechnology products. J Pharm Sci. 2004;93:2184–2204. doi: 10.1002/jps.20125. [DOI] [PubMed] [Google Scholar]
- Tanswell P, Modi N, Combs D, Danays T. Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet. 2002;41:1229–1245. doi: 10.2165/00003088-200241150-00001. [DOI] [PubMed] [Google Scholar]
- Tranum BL, Stephens RL, Lehane DE, Hoogstraten B, Lane M, Haut A. Adriamycin (NSC-123127) plus 5-fluorouracil (NSC-19893): a phase I study. Cancer Chemother Rep. 1975;59:1163–1165. [PubMed] [Google Scholar]
- Tsunekawa Y, Hasegawa T, Nadai M, Takagi K, Nabeshima T. Interspecies differences and scaling for the pharmacokinetics of xanthine derivatives. J Pharm Pharmacol. 1992;44:594–599. doi: 10.1111/j.2042-7158.1992.tb05471.x. [DOI] [PubMed] [Google Scholar]
- Ueda CT, Ballard B, Rowland M. Concentration-time effects of quinidine disposition kinetics in rhesus monkeys. J Pharmacol Exp Ther. 1977;200:459–468. [PubMed] [Google Scholar]
- Ujhelyi MR, Robert S. Pharmacokinetic aspects of digoxin-specific Fab therapy in the management of digitalis toxicity. Clin Pharmacokinet. 1995;28:483–493. doi: 10.2165/00003088-199528060-00006. [DOI] [PubMed] [Google Scholar]
- Valverde CR, Mama KR, Kollias-Baker C, Steffey EP, Baggot JD. Pharmacokinetics and cardiopulmonary effects of fentanyl in isoflurane-anesthetized rhesus monkeys (Macaca mulatta) Am J Vet Res. 2000;61:931–934. doi: 10.2460/ajvr.2000.61.931. [DOI] [PubMed] [Google Scholar]
- Wang W, Prueksaritanont T. Prediction of human clearance of therapeutic proteins: simple allometric scaling method revisited. Biopharm Drug Dispos. 2010;31:253–263. doi: 10.1002/bdd.708. [DOI] [PubMed] [Google Scholar]
- Ward KW, Smith BR. A comprehensive quantitative and qualitative evaluation of extrapolation of intravenous pharmacokinetic parameters from rat, dog, and monkey to humans. I. Clearance. Drug Metab Dispos. 2004;32:603–611. doi: 10.1124/dmd.32.6.603. [DOI] [PubMed] [Google Scholar]
- Weisman MH, Moreland LW, Furst DE, Weinblatt ME, Keystone EC, Paulus HE, Teoh LS, Velagapudi RB, Noertersheuser PA, Granneman GR, Fischkoff SA, Chartash EK. Efficacy, pharmacokinetic, and safety assessment of adalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: a pilot study. Clin Ther. 2003;25:1700–1721. doi: 10.1016/s0149-2918(03)80164-9. [DOI] [PubMed] [Google Scholar]
- White G, Shapiro A, Ragni M, Garzone P, Goodfellow J, Tubridy K, Courter S. Clinical evaluation of recombinant factor IX. Semin Hematol. 1998;35:33–38. [PubMed] [Google Scholar]
- Woo S, Jusko WJ. Interspecies comparisons of pharmacokinetics and pharmacodynamics of recombinant human erythropoietin. Drug Metab Dispos. 2007;35:1672–1678. doi: 10.1124/dmd.107.015248. [DOI] [PubMed] [Google Scholar]
- Yandle TG, Richards AM, Nicholls MG, Cuneo R, Espiner EA, Livesey JH. Metabolic clearance rate and plasma half life of alpha-human atrial natriuretic peptide in man. Life Sci. 1986;38:1827–1833. doi: 10.1016/0024-3205(86)90137-2. [DOI] [PubMed] [Google Scholar]
- Yu RZ, Kim TW, Hong A, Watanabe TA, Gaus HJ, Geary RS. Cross-species pharmacokinetic comparison from mouse to man of a second-generation antisense oligonucleotide, ISIS 301012, targeting human apolipoprotein B-100. Drug Metab Dispos. 2007;35:460–468. doi: 10.1124/dmd.106.012401. [DOI] [PubMed] [Google Scholar]