Abstract
Despite advances during the last 2 decades in every aspect of cardiovascular research (interventional cardiology, cardiopulmonary resuscitation, and so forth), Western societies still are plagued by the consequences of cardiovascular disease. Consequently the discovery of new regimens and therapeutic interventions is of utmost importance. Research using human subjects is associated with substantial methodologic and ethical considerations, and the quest for an appropriate animal model for the human cardiovascular system has led to swine. The porcine heart bears a close resemblance to the human heart in terms of its coronary circulation and hemodynamic similarities and offers ease of implementation of methods and devices from human healthcare facilities. A thorough comprehension of the anatomy and physiology of the porcine cardiovascular system should focus on differences between swine and humans as well as similarities. Understanding these differences and similarities is essential to extrapolating data appropriately and to addressing the social demand for the ethical use of animals in biomedical research.
Death due to cardiac etiology is currently one of the major fields of scientific research. It may be due to a variety of causes, and cardiac etiology represents one of the most significant causes of noninjury deaths in adults in industrialized countries.11
Coronary artery disease is one of the most common causes of cardiac arrest, and death may be the first and only manifestation of the disease. Coronary artery occlusion leads to regional ischemia, anaerobic glucolysis, intra- and extracellular acidosis, and dysfunction of membrane permeability of cardiomyocytes—all of which may trigger ectopic myocardial electrical activities and subsequent generation of arrhythmias.31,57 Other pathologic entities that may lead to fatal arrhythmias are cardiomyopathies, and for cases of unknown etiology, channelopathies may be the underlying pathology.37,46
Successful management of cardiac arrest should aim at the return of spontaneous circulation as well as cerebral resuscitation. Currently, the only internationally accepted interventions are chest compressions, defibrillation, and epinephrine administration. In particular, epinephrine has been implicated in causing severe adverse effects on the myocardium.11,24
The limited available interventions for cardiac arrest in addition to its acute nature and high social–economic impact prompt rigorous research on new pharmaceutical modalities and therapeutic techniques. Implementing these novel interventions in human patients directly is unethical and may be unproductive due to genetic heterogeneity between subjects, different life styles, need for populous statistical groups, and so forth. These gaps are accommodated through the use of animal models.
Many animal species have served as models in preclinical trials. The husbandry advantages of rodents over other animals has made them some of the most favorable species in biomedical research. Although rodents have yielded a great amount of information regarding the molecular and cellular bases of cardiovascular biology, intrinsic differences between rodents and humans in terms of heart rate, oxygen consumption, adrenergic receptor ratios, response to loss of regulatory proteins, duration of the cardiac action potential, ionic currents contributing to action potential, absence of the plateau phase, and contractile protein expression critical to the excitation–contraction coupling process as well as spontaneous reversion of experimentally induced ventricular fibrillation into normal sinus rhythm renders problematic the use of rodents and the extrapolation of data derived from these models.15,16,17,56
The need for a species that accurately approximates the human cardiovascular anatomy and physiology is imperative. Many previous advances that now constitute the basis of reperfusion treatment were achieved through studies on canine models.36,37 However during past couple of decades, pigs have gained favor over dogs, in part because of marked differences in coronary anatomy between dogs and humans and in part due to societal reluctance and concerns regarding their use in biomedical research. The initial enthusiasm over the realization that the porcine heart is almost identical to the human heart has abated somewhat, mainly through studies investigating swine as a donor for heart xenotransplantation.8 A deeper understanding of the anatomy and physiology of the porcine heart in comparison with those of other animal species and humans will further facilitate the extrapolation and interpretation of data derived from experimentation on this species.
Swine in Cardiovascular Research
Swine belong to the species Sus scrofa domestica, which comprises many different breeds that vary in size and appearance. The range of swine breeds can be grossly subdivided into 2 categories: farm pigs (the most common breeds being the Yorkshire, Landrace, and Duroc as well as their crosses) and minipigs (such as Yucatan, Hanford, Göttingen, and Sinclair). One of the major advantages of minipigs over farm breeds is that for the same body weight, minipigs are more mature, and their tissues are more resilient to experimental procedures.49 Given that intubation in farm pigs presents numerous challenges (deep larynx, extended soft palate, fragile trachea, predisposition to laryngospasm),16 this trait of minipigs may be important to some researchers. Another disadvantage of farm breeds is that they are susceptible to ventricular fibrillation, and some genetic lines are predisposed to malignant hyperthermia. Furthermore the high growth rate and adult size of farm pigs may present a husbandry challenge for standard laboratory facilities. For these reasons, many researchers favor the use of minipigs, especially in long-term survival studies.22
The ratio of heart weight to body weight in 20- to 30-kg pigs, which are used frequently in cardiovascular studies, is identical (5 g/kg) to that of adult humans.23 One should bear in mind that this ratio in such young animals is markedly higher than that of adult pigs (2.5 to 2.9 g/kg). This ratio is approximately 7 g/kg for adult dogs and approximately 3 g/kg for adult sheep.23
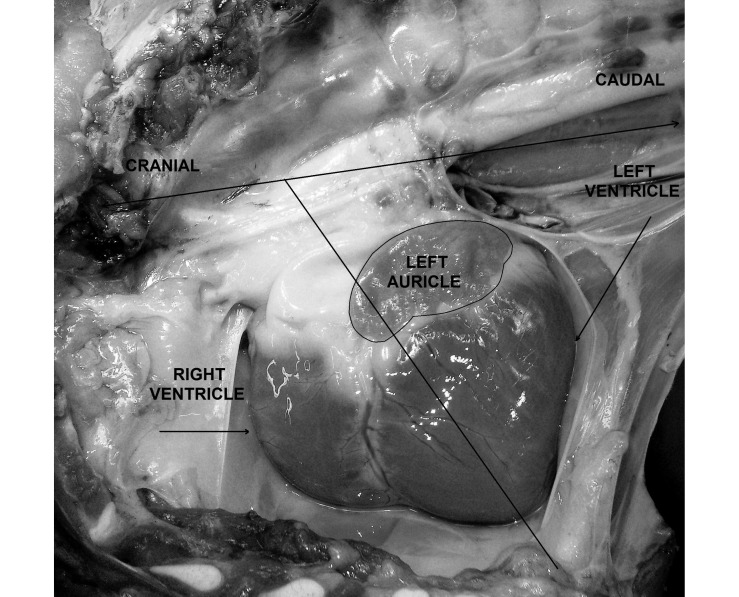
Many of the differences in cardiac anatomy between pigs and humans are the result of a quadruped compared with biped stance as well as the unusual conformation of the human thorax, which is dorsoventrally compressed, compared with the thorax of other mammals, which typically is laterally compressed. Consequently, the morphology and topography as well as the terminology of the thoracic organs differ between most mammals used in biomedical research and humans. As far as topography is concerned, the long axis of the swine heart tips forward, forming an acute angle to the vertical plane (Figure 1). Furthermore, the heart of these animals species are rotated counterclockwise compared with the human heart. As a result, the left ventricle and left atrium faces caudally, whereas the right atrium and ventricle are cranially situated (Figure 1). Due to the quadruped stance, the heart appears to be overhanging the thoracic cavity by its major vessels; therefore, venal drainage has a gravitational component. The orifices of the caval veins of pigs and other animals form an angle as they enter the right atrium, in contrast to the human caval veins, which are aligned along the same axis.8 Regarding terminology, the superior and inferior caval veins of humans are called the cranial and caudal caval veins in the majority of mammals used in biomedical research. An important difference is the presence of the left azygous vein in pigs, which drains directly into the coronary sinus; the human heart lacks this anatomic arrangement.50 In the swine heart, the right auricle has a narrow tubular appearance, compared with the triangular shape of that in humans. The number of orifices for the left atrium varies among mammals. In pigs the left atrium receives oxygenated blood from 2 pulmonary veins, in dogs from 5 or 6, and in humans from 4 or 5.25
Figure 1.
Right recumbency; left hemithorax, adjacent lung, and pericardium removed. Position of the porcine heart in the thoracic cavity. The upper line is parallel to the spine; the oblique line represents the long axis of the heart. The acute angle in this figure is overestimated due to lateral recumbency.
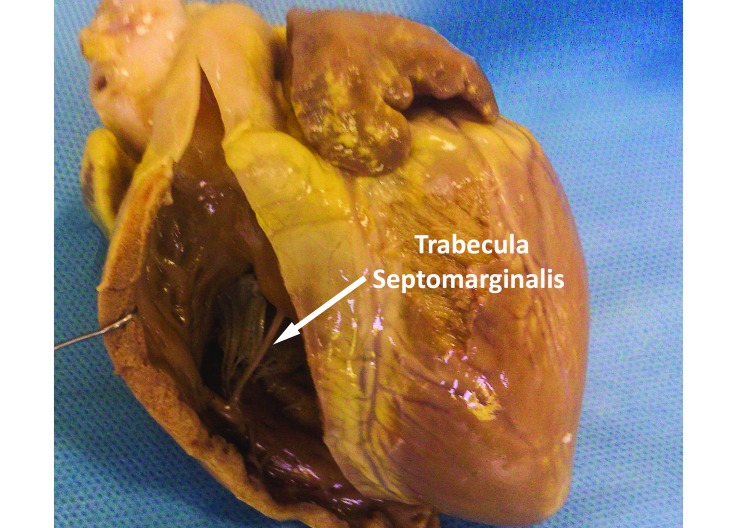
In the trabecular component of the right ventricle, the trabeculae carnae and papillary muscles of pigs are much coarser and broader than those of the human right ventricle. Similar to the arrangement in humans, the tendineae chordae of swine arise from the apices of the papillary muscles to the free border of the 3 leaflets of the tricuspid valve. One of the most prominent features in the right ventricle of the porcine heart is the trabecula septomarginalis, formerly known as moderator band. This muscular strand connects the septal wall of the right ventricle to its free wall (Figure 2) and carries Purkinje fibers from the right atrioventricular bundle across the right ventricle's lumen. Compared with that in humans, the trabecula septomarginalis of swine is more prominent and situated more proximally relative to the base of the heart.18
Figure 2.
Porcine heart, right ventricle. The arrow indicates the trabecula septomarginalis. Compared with that in humans, the porcine structure is much thicker and more proximally situated to the base of the heart.
In the left ventricle of pigs, the apical trabeculations are coarse and not obviously different from those of the right ventricle, contrary to the finer trabeculations of the left ventricle of the human heart. These anatomic differences between the 2 species reflect the high variation of the Purkinje fiber network and probably result in differences in ventricular conductivity and contractility.26 The ratio of wall thickness between the left to right ventricle is much higher in the porcine heart than in the human heart.8
In mammals, the cardiac valves follow in general the same pattern. The atria are divided from the ventricles by the atrioventricular valves, which are connected to the papillary muscles through the tendinae chordae. The tricuspid valve of swine has 3 leaflets, whereas dogs typically have 2.10 The porcine mitral valve has similar characteristics to those of the human valve regarding size, leaflets, and tendinae chordae configuration.28 Substantial similarities between the 2 species are apparent as far as their semilunar valves are concerned. The noncoronary cusp of the porcine aortic valve has a fibrous attachment. This structure is absent in bovine and ovine aortic valves.40 Furthermore, swine and humans (although to a lesser extent in humans) display a fibrous continuity between the leaflets of the mitral and aortic valve.8 Another difference is the increased myocardial support of the porcine aortic valve when compared with that of humans.40 Differences between the 2 species exist regarding the size and geometry of the respective cusps of their aortic valve.43 In addition, differences between the 2 species have been observed at the microstructural level of their valves, in regard to metalloproteinase I expression and proteoglycan distribution.47
Despite several differences, the vast anatomic and physiologic similarities render the porcine heart a possible source for human valve bioprostheses. Compared with mechanical valves, porcine valves do not require anticoagulant regimens, but their rapid deterioration poses a significant disadvantage. At first, this degenerative process was attributed to ‘wear-and tear’ phenomena, but substantial current evidence suggests that an immune-mediated reaction contributes significantly.29 Genetically modified pigs donors could, to some extent, overcome the human host-mediated immune reaction.29 The implementation of a new prosthetic heart valve in clinical practice prerequisites an animal model that accurately predicts the efficacy and safety of the valve being tested. Even though swine closely approximate the human cardiovascular, coagulation, and inflammatory systems, intraoperative and postoperative difficulties have limited their use as an animal model of valve disease. Although the ovine model is considered to be the animal species of choice in this context,5 skepticism about its use has emerged after its failure to predict the safety of a new prosthetic valve.44 Moreover, the significant differences between the ovine and human cardiovascular systems should not be overlooked. These limitations of the ovine model led a team of researchers to propose a reproducible porcine model for the long-term evaluation of valvular prostheses.44
In addition, swine often demonstrate congenital heart anomalies and therefore have been used as a spontaneous model for the study of ventricular septal defect, atrial septal defect, patent foramen ovale, patent ductus arteriosus, and tricuspid dysplasia.50 As in humans, nonesterified fatty acids are the predominant source of energy in pigs, supplying a maximum of 80% of the energy needed; in situations where the oxidation of fatty acids decreases, energy is delivered through increased glucose extraction (Randle cycle).22
Coronary Circulation
In the past, dogs were used extensively as animal models to study myocardial ischemia. However, this species demonstrates significant variation in the coronary circulation pattern between subjects10 (mongrels or purebreds) and an extensive preexisting collateral epicardial circulation which can supply as much as 40% of the blood flow after the occlusion of a coronary artery.22 These features have prompted skepticism regarding the appropriateness of using dogs in cardiovascular research. In contrast, surface intercoronary anastomosis is rarely seen in the swine heart, and the variation in coronary pattern distribution between subjects is analogous to that in humans.19,39 After the occlusion of a branch of the left anterior descending artery, swine had the least collateral flow (expressed as a percentage of the ischemic zone) to the nonischemic zone, followed by rabbits, baboons, ferrets, rats, cats, and dogs; guinea pigs had the highest percentage of collateral circulation.19
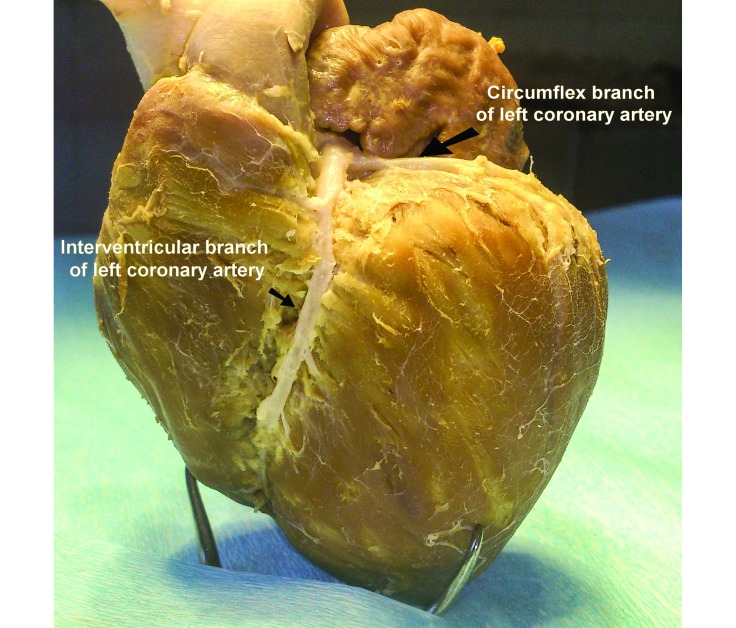
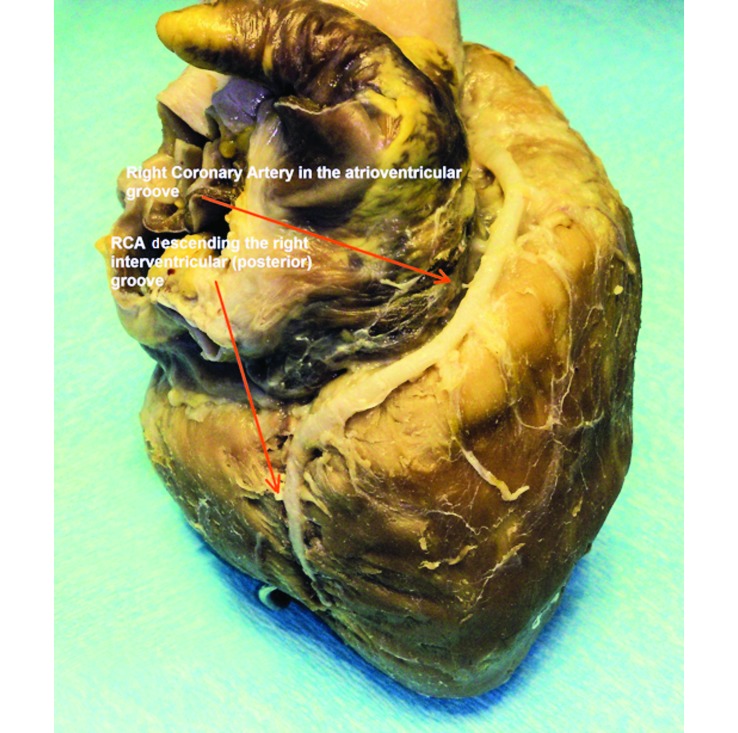
The porcine heart is characterized by right-coronary dominancy.8 Although in both humans and swine, the left coronary artery (Figure 3) supplies the majority of the myocardium, right dominance defines the origin of the artery descending into the posterior (right) interventricular sulcus. In left dominance, the artery of the posterior (right) interventricular sulcus originates from the circumflex artery (ruminants, dogs) whereas in right dominance, it originates from the right coronary artery (swine, humans). Substantial differences exist between studies about the percentage of right dominance in the porcine heart. In some studies, right dominance was an absolute phenomenon in the swine population examined,39 which agrees with our observation regarding Landrace–Large White breed hearts (Figure 4). However, in other studies, the posterior descending coronary artery was reported to originate from the left circumflex in 5% to 10% of the swine population examined.42,55 The inclusion of minipigs in the study population or variations in the coronary circulation between breeds may account for these differences. Furthermore, humans and swine are similar in regard to the circulation of the interventricular septum. Although significant interspecies variations exist, the general pattern of interventricular vascularization in both species consists of anterior and posterior septal arteries that arise from the left and right coronary arteries, respectively. In addition, the atrioventricular node and the bundle of His in both humans and swine are irrigated predominantly by the posterior septal artery.4 This anatomic and structural resemblance implies that ischemia-associated injury to the conduction system of the swine heart is analogous to that in humans, contrary to the canine model, in which the blood supply originates from the anterior septal artery.50
Figure 3.
Anatomy of the circumflex branch and the left (anterior) interventricular branch of the left coronary artery in the Landrace–Large White breed.
Figure 4.
Anatomy of the right coronary artery of the porcine heart. The branch descending the right (posterior) interventricular groove originates from the right coronary artery in the Landrace–Large White breed.
The porcine heart responds similarly to the human heart after infraction and presents arrhythmogenity with reperfusion, contrary to the canine heart with its multiple preexisting collateral anastomoses. For human cases where the infraction has developed gradually, allowing time for the formation of collateral circulation, dogs might serve as an appropriate animal model. Swine could mimic such a situation after gradual occlusion of a coronary artery by using balloon angioplasty and the administration of an atherogenic diet.7,13,14 When compared with sheep, swine resemble humans more closely regarding the healing characteristics of the myocardium, given that in ruminants, healing is characterized by the formation of collagenous scars.30
A feature of paramount importance, especially for interventional cardiology, is the way arteries respond to traumatic injuries. Many species have been used in vascular biology research. Restenosis studies on the arteries of mice have gained favor particularly through advances in molecular biology and genome manipulation.21,41 The main disadvantage of this model is the extremely small size of the vessels, which hampers the implementation of traditional surgical methods.52 Atherosclerosis and restenosis studies have been implemented on the carotid artery of rats. However, the failure of this model to predict responses to angiotensin-converting inhibitors analogous with those in humans led researchers to devaluate its use.3,32 The different histopathologic lesions observed in the iliac artery of the rabbit model after balloon angioplasty (the abundance of foamy cells compared with their rarity in humans) caused researchers to question the use of rabbits in interventional cardiology. In contrast to those in other animals, vascular studies in swine are implemented directly on the coronary arteries. Neointimal thickening is observed within 28 d after vessel insult induced by balloon angioplasty.48 The lesions closely resemble the restenotic neointima observed in humans, and their severity is proportional to injury,52 thus permitting the quantitative evaluation of the injury progression or the regression of the lesion through the prophylactic or therapeutic interventions of the study's protocol. In addition, the porcine model has proven to be accurately predictive of negative results in clinical trials. Although many results suggest that the porcine model performs similarly appropriately as a positive model, additional data are needed for its full characterization.52
Swine have been used as a model in atherosclerosis research. In this animal species, 60% of the circulating cholesterol is present as low-density lipoproteins, with high-density lipoproteins representing 38%. These values are almost identical to those in humans, where the percentages are 63% and 28%, respectively.27 Furthermore, aging pigs may spontaneously develop atherosclerosis.7 In biomedical research, experimentally induced lesions in the coronary arteries of minipigs are achieved through the administration of a diet containing 2% to 4% cholesterol and 40% fat. The prolonged time needed for the development of these lesions (6 mo) can be accelerated by traumatizing the endothelium with a balloon catheter. In addition, a strain of pigs with mutations in Lpb5 and Lpu1 may present extensive atherosclerotic lesions in all 3 coronary arteries after the consumption of a normal diet.33,35
Hemodynamics
The acquisition of hemodynamic parameters is fundamental in cardiovascular research. Interpretation and extrapolation of such data to humans are a key factor of any hemodynamic study. Prognostic indexes are derivatives of these data, especially in studies concerning cardiac arrest and cardiopulmonary resuscitation.58,59 Although the implementation of devices adapted to the small size of animals such as rodents is now feasible, large animals provide values more analogous to those in humans. Swine show high resemblance to humans in regard to their hemodynamic parameters. However, direct interspecies and intraspecies comparisons should be made prudently. Significant alterations in values emanate from differences in age, weight, breed, and the use of anesthesia (various regimens). When animals of different breeds are compared, they should be weight- and age-matched.45 This is not possible when comparisons are made between farm pigs and minipigs. Furthermore, when values are obtained from anesthetized animals, the pharmaceutical combination as well as the depth of anesthesia may play important roles.50,51 Table 1 presents reference values for key parameters of cardiovascular function. However these values must be taken into account cautiously, mainly due to differences in the age and weight of the pigs and the methods used for data acquisition.
Table 1.
Hemodynamic parameters in various swine breeds
| Landrace–Large Whitea | Hanfordb | Yucatan minipigb | Yucatan micropigb | |
| Age | 10–15 wk | 4 mo | 4 mo | 4 mo |
| Heart rate (bpm) | 116.41 ± 8.11 | 105 ± 7 | 112 ± 3 | 106 ± 5 |
| Cardiac output (L/min) | 5.12 ± 0.53 | — | — | — |
| Left ventriclar systolic pressure (mm Hg) | 108.97 ± 12.06 | 116 ± 4 | 58 ± 2 | 59 ± 3 |
| Left ventricular diastolic pressure (mm Hg) | 8.88 ± 1.81 | 4 ± 1 | 3 ± 1 | 6 ± 2 |
| Right ventricular peak pressure (mm Hg) | 21.24 ± 2.16 | 30 ± 1 | 24 ± 2 | 27 ± 2 |
| Right ventricular diastolic pressure (mm Hg) | 4.20 ± 0.72 | 4 ± 1 | 2 ± 1 | 5 ± 2 |
Values are presented as mean ± 1 SD.
From reference 58.
From reference 51.
Electrophysiology
A productive and harmonic cardiac cycle is achieved through the synchronized transmission of an electrical impulse. The fibrous skeleton of the heart plays an important role in this process, mainly due to electrical insulation of the atria from the ventricles. The signal is transmitted through specialized cardiac muscle cells. Afferent and efferent neural pathways convey messages from and to the heart, according to need.12 Different parts of the system retain different intrinsic characteristics concerning time and velocity of electrical transmission. Although the basic architecture between mammals is similar, substantial differences exist.1 Regarding the conduction system, one fundamental difference is the presence of numerous nerves in swine whereas humans have few. These fibers are cholinergic and adrenergic in origin. This specific feature has led some researchers to characterize the swine conduction system as neuromyogenic in contrast to the myogenic conduction system of the human.50
In the swine heart, the sinoatrial node lies at the right side of the terminal crest, at the junction of the cranial vena cava and the right atrial appendage and is relatively lower on the septum than is the node in humans. The swine node appears to be rectangular in its longitudinal direction but has a flattened appearance perpendicularly, whereas the human node has a central broad region with tapered ends.18 The innervation of the swine atrial node has a uniform distribution, thus varying substantially from the heterogeneous distribution of the human node. This anatomic difference may account for the unifocal impulse generation in the swine in contrast to the multifocal impulse generation observed in the human and canine nodes.9,34 In swine, numerous ganglionated nerve trunks populate the node, especially in its epicardial periphery, indicating either substantial innervation of intrinsic origin or a synaptic contact with cardiac ganglia.9 Differences in the sinus node also are apparent between humans and other commonly used small-animal models. In rabbits, the sinus node is in the intercaval region and spans the full thickness of the atrial wall, whereas the human node demonstrates a wedge appearance at the cavoatrial junction.1
The swine atrioventricular node resides on the right side of the ventricular septum, an anatomic position similar to that in humans,6 but is more densely innervated. In most mammals, the bundle branches are insulated from the ventricular myocardium by a fibrous sheath. Such sheaths are not evident in the mouse heart.1 Furthermore, ganglion cell bodies are present within the compact atrioventricular node of swine9 but are absent in humans, baboons, dogs, and cats. The penetrating bundle is shorter, and the bifurcation into bundle branches occurs more proximally in swine than in humans. Furthermore the right bundle branch in the trabecula septomarginalis is situated more proximally to the base of the swine heart compared with the human heart.8 All of these anatomic features contribute to the electrocardiographic differences observed between the 2 species: the sinus rhythm is faster and the PR interval is shorter in swine. Although the sinus rhythm decreases as a pig matures, it remains faster than that of a human of equivalent maturity6 (Table 2).
Table 2.
Swine and human electrophysiologic parameters
| Swine | Human | |
| Heart rate (bpm) | 91–167 | 60–100 |
| PR interval (ms) | 50–120 | 3-5 y of age: 110–150 |
| 5-9 y of age: 120–160 | ||
| QT interval (ms) | 150–340 | Heart rate 150 bpm: 210–280 |
| Heart rate 100 bpm: 260–350 | ||
| HRA–LRA (ms) | 10 | 2–5 y of age: 6–38 |
| 6–10 y of age: 0–41 | ||
| LRA–H (ms) | 60–65 | 2–5 y of age: 45–101 |
| 6–10 y of age: 40–124 | ||
| H–V (ms) | 20–35 | 2–5 y of age: 27–59 |
| 6–10 y of age: 28–52 |
H, bundle of His; HRA, high right atrium; LRA, low right atrium; V, ventricle
Modified from reference 6.
The pigs were 10 to 15 wk old and weighted 30 to 40 kg.
The same pattern of increased innervation is also present in the Purkinje fiber network. The Purkinje system of swine is characterized by fast electrical coupling. Purkinje fibers are connected to the ventricular myocardium at Purkinje–ventricular junctions. In humans, dogs, and rabbits, these junctions have been identified only subendocardially, whereas they usually lie transmurally in sheep and pigs.2,20,38,53,54 This difference may explain why the endocardium and epicardium are activated simultaneously in the swine heart. At the microscopic level, the swine conduction system appears to possess more connective than elastic tissue, compared with that in humans.50,51
Conclusion
In Western societies, cardiovascular disease is a leading cause of mortality. Research in laboratory animals is a fundamental cornerstone of modern biomedical science and provides tools for deeper understanding of biologic processes and responses. Therefore, animals are crucial to proving critical hypotheses as far as basic or complex biologic mechanisms are concerned. Although the primary benefits of research pertain to humans, we should always remember that animals themselves benefit from medical research. Researchers must thoroughly consider various key scientific and ethical questions in every experimental protocol. We consider these 2 components to be complementary to each other, and they should be dealt equally meticulously. Our knowledge increases not only because of similarities between animal species and the direct extrapolation of scientific results but also from their differences and deeper insight into how they affect the scientific data obtained.
According to our current knowledge, swine appear to be the most appropriate species in cardiovascular research. Nevertheless, the use of swine is associated with several limitations, including anatomic variation in the thoracic cavity, a trait that is common to most mammals used in biomedical research, and substantial differences between the conduction systems of swine compared with humans. However, the conduction systems of other commonly used animal species also differ significantly anatomically and physiologically from that of humans.50 Swine have gained in importance as an animal model in cardiovascular research through social acceptance of their use, the similarity of their coronary circulation to that of humans, the approximation of their hemodynamic values, and their size and the relative convenience associated with implementing methods and equipment used in human medical practice.
References
- 1.Anderson RH, Yanni J, Boyett MR, Chandler NJ, Dobrzynski H. 2009. The anatomy of the cardiac conduction system. Clin Anat 22:99–113 [DOI] [PubMed] [Google Scholar]
- 2.Ansari A, Ho S, Anderson RH. 1999. Distribution of the Purkinje fibers in the sheep heart. Anat Rec 254:92–97 [DOI] [PubMed] [Google Scholar]
- 3.Berger PB, Holmes DR, Jr, Ohman EM, O'Hanesian MA, Murphy JG, Schwartz RS, Serruys PW, Faxon DP. 1996. Restenosis, reocclusion, and adverse cardiovascular events after successful balloon angioplasty of occluded versus nonoccluded coronary arteries. Results from the Multicenter American Research trial with Cilazapril after Angioplasty to Prevent Transluminal coronary Obstruction and Restenosis (MARCATOR). J Am Coll Cardiol 27:1–7 [DOI] [PubMed] [Google Scholar]
- 4.Bertho E, Gagnon G. 1964. A comparative study in 3 dimensions of the blood supply of the normal interventricular septum in human, canine, bovine, porcine, ovine, and equine heart. Dis Chest 46:251–262 [DOI] [PubMed] [Google Scholar]
- 5.Bianco RW, Callegos RP, Rivald Al, Voigt J, Dalmasso AP. 2009. Animal models for cardiac research, p 393–410. In: Laizzo PA. Handbook of cardiac anatomy, physiology, and devices, 2nd ed. Minneapolis (MN): Springer [Google Scholar]
- 6.Bharati S, Levine M, Huang SKS, Handler B, Parr GVS, Bauernfeind R, Lev M. 1991. The conduction system of the swine heart. Chest 100:207–212 [DOI] [PubMed] [Google Scholar]
- 7.Bloor CM, White FC, Roth DM. 1992. The pig as a model of myocardial ischemia and gradual coronary artery occlusion, p 163–175. In: Swindle MM. Swine as models in biomedical research. Ames (IA): Iowa State University Press [Google Scholar]
- 8.Crick S, Sheppard MN, Ho SY, Gebstein L, Anderson RH. 1998. Anatomy of the pig heart: comparisons with normal human cardiac structure. J Anat 193:105–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crick SJ, Sheppard MN, Ho SY, Anderson RH. 1999. Localisation and quantitation of autonomic innervation in the porcine heart. I: conduction system. J Anat 195:341–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans HE. 1993. The heart and arteries, p 586–681. In: Evans HE. Miller's anatomy of the dog, 3rd ed. Philadelphia (PA): Saunders [Google Scholar]
- 11.Field JM, Hazinski MF, Sayre MR, Chameides L, Schexnayder SM, Hemphill R, Samson RA, Kattwinkel J, Berg RA, Bhanji F, Cave DM, Jauch EC, Kudenchuk PJ, Neumar RW, Peberdy MA, Perlman JM, Sinz E, Travers AH, Berg MD, Billi JE, Eigel B, Hickey RW, Kleinman ME, Link MS, Morrison LJ, O'Connor RE, Shuster M, Callaway CW, Cucchiara B, Ferguson JD, Rea TD, Vanden Hoek TL. 2010. Part 1. Executive summary: American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 122:S640–S656 [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald K, Wilson RF, Laizzo PA. 2009. Autonomic nervous system, p 177–189. In: Laizzo PA. Handbook of cardiac anatomy, physiology, and devices, 2nd ed. Minneapolis (MN): Springer [Google Scholar]
- 13.Gal D, Isner JM. 1992. Atherosclerotic Yucatan microswine as a model for novel cardiovascular interventions and imaging, p 118–140. In: Swindle MM. Swine as models in biomedical research. Ames (IA): Iowa State University Press [Google Scholar]
- 14.Gardner TJ, Johnson DL. 1988. Cardiovascular system, p 74–124. In: Swindle MM, Adams RJ. Experimental surgery and physiology: induced animal models of human disease. Baltimore (MD): Williams and Wilkins [Google Scholar]
- 15.Ginis I, Luo Y, Miura T, Thies S, Brandenberger R, Gerecht-Nir S, Amit M, Hoke A, Carpenter MK, Itskovitz-Eldor J, Rao MS. 2004. Differences between human and mouse embryonic stem cells. Dev Biol 269:360–380 [DOI] [PubMed] [Google Scholar]
- 16.Greene SA, Benson GJ. 2002. Porcine anesthesia, p 273–274. In: Greene SA. Veterinary anesthesia and pain management secrets. Philadelphia (PA): Hanley and Belfus [Google Scholar]
- 17.Haghighi K, Kolokathis F, Pater L, Lynch RA, Asahi M, Gramolini AO, Fan GC, Tsiapras D, Hahn HS, Adamopoulos S, Liggett SB, Dorn GW, 2nd, MacLennan DH, Kremastinos DT, Kranias EG. 2003. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest 111:869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill AJ, Laizzo PA. 2009. Comparative cardiac anatomy, p 87–108. In: Laizzo PA. Handbook of cardiac anatomy, physiology, and devices, 2nd ed. Minneapolis (MN): Springer [Google Scholar]
- 19.Hearse DJ. 2000. The elusive coypu: the importance of collateral flow and the search for an alternative to the dog. Cardiovasc Res 45:215–219 [Google Scholar]
- 20.Holland RP, Brooks H. 1976. The QRS complex during myocardial ischemia. An experimental analysis in the porcine heart. J Clin Invest 57:541–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horiba M, Kadomatsu K, Nakamura E, Muramatsu H, Ikematsu S, Sakuma S, Hayashi K, Yuzawa Y, Matsuo S, Kuzuya M, Kaname T, Hirai M, Saito H, Muramatsu T. 2000. Neointima formation in a restenosis model is suppressed in midkine-deficient mice. J Clin Invest 105:489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes GC, Post MJ, Simons M, Annex BH. 2003. Translational physiology: porcine models of human coronary artery disease. Implications for preclinical trials of therapeutic angiogenesis. J Appl Physiol (1985) 94:1689–1701 [DOI] [PubMed] [Google Scholar]
- 23.Hughes HC. 1986. Swine in cardiovascular research. Lab Anim Sci 36:348–350 [PubMed] [Google Scholar]
- 24.Ibrahim WH. 2007. Recent advances and controversies in adult cardiopulmonary resuscitation. Postgrad Med J 83:649–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.König HE, Ruberte J, Liebich J. 2004. Organs of the cardiovascular system (systema cardiovasculare), p 415–450. In: König HE, Liebich J. Veterinary anatomy of domestic mammals. New York (NY): Schattauer [Google Scholar]
- 26.Laske TG, Shrivastav M, Laizzo PA. 2009. The cardiac conduction system, p 159–175. In: Laizzo PA. Handbook of cardiac anatomy, physiology, and devices, 2nd ed. Minneapolis (MN): Springer [Google Scholar]
- 27.Liedtke AJ, Hughes HC, Neely JR. 1975. An experimental model for studying myocardial ischemia. Correlation of hemodynamic performance and metabolism in the working swine heart. J Thorac Cardiovasc Surg 69:203–211 [PubMed] [Google Scholar]
- 28.Lomholt M, Nielsen SL, Hansen SB, Andersen NT, Hasenkam JM. 2002. Differential tension between secondary and primary mitral chordae in an acute in-vivo porcine model. J Heart Valve Dis 11:337–345 [PubMed] [Google Scholar]
- 29.Manji RA, Menkis AH, Ekser B, Cooper DKC. 2012. Porcine bioprosthetic heart valves: the next generation. Am Heart J 164:177–185 [DOI] [PubMed] [Google Scholar]
- 30.Mehran RJ, Ricci MA, Graham AM, Carter K, Smyes JF. 1991. Porcine model for vascular graft studies. J Invest Surg 4:37–44 [DOI] [PubMed] [Google Scholar]
- 31.Packer M. 1985. Sudden unexpected death in patients with congestive heart failure: a second frontier. Circulation 72:681–685 [DOI] [PubMed] [Google Scholar]
- 32.Powell JS, Clozel JP, Müller RK, Kuhn H, Hefti F, Hosang M, Baumgartner HR. 1989. Inhibitors of angiotensisn converting enzyme prevent myointimal proliferation after vascular injury. Science 245:186–188 [DOI] [PubMed] [Google Scholar]
- 33.Prescott MF, McBride CH, Hasler-Rapacz J, Von Linden J, Rapacz J. 1991. Development of complex atherosclerotic lesions in pigs with inherited hyperLDL cholesterolemia bearing mutant alleles for apolipoprotein B. Am J Pathol 139:139–147 [PMC free article] [PubMed] [Google Scholar]
- 34.Priola DV. 1980. Intrinsic innervation of the canine heart. Effects on conduction in the atrium, atrioventricular node, and proximal bundle branch. Circ Res 47:74–79 [DOI] [PubMed] [Google Scholar]
- 35.Rapacz J, Hasler-Rapacz J, Taylor KM, Checovich WJ, Attie AD. 1986. Lipoprotein mutations in pigs are associated with elevated plasma cholesterol and atherosclerosis. Science 234:1573–1577 [DOI] [PubMed] [Google Scholar]
- 36.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. 1977. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation 56:786–794 [DOI] [PubMed] [Google Scholar]
- 37.Reimer KA, Jennings RB. 1979. The ‘wavefront phenomenon’ of myocardial ischemic cell death, II Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest 40:633–644 [PubMed] [Google Scholar]
- 38.Ryu S, Yamamoto S, Andersen CR, Nakazawa K, Miyake F, James TN. 2009. Intramural Purkinje cell network of sheep ventricles as the terminal pathway of conduction system. Anat Rec (Hoboken) 292:12–22 [DOI] [PubMed] [Google Scholar]
- 39.Sahni D, Kaur GD, Jit H, Jit I. 2008. Anatomy and distribution of coronary arteries in pig in comparison with human. Indian J Med Res 127:564–570 [PubMed] [Google Scholar]
- 40.Sands MP, Rittenhouse EA, Mohri H, Merendino KA. 1969. An anatomical comparison of human, pig, calf, and sheep aortic valves. Ann Thorac Surg 8:407–414 [DOI] [PubMed] [Google Scholar]
- 41.Sata M, Maejima Y, Adachi F, Fukino K, Saiura A, Sugiura S, Aoyagi T, Imai Y, Kurihara H, Kimura K, Omata M, Makuuchi M, Hirata Y, Nagai R. 2000. A mouse model of vascular injury that induces rapid onset of medial cell apoptosis followed by reproducible neointimal hyperplasia. J Mol Cell Cardiol 32:2097–2104 [DOI] [PubMed] [Google Scholar]
- 42.Schuleri KH, Boyle AJ, Centola M, Amado LC, Evers R, Zimmet JM, Evers KS, Ostbye KM, Scorpio DG, Hare JM, Lardo AC. 2008. The adult Göttingen minipig as a model for chronic heart failure after myocardial infarction: focus on cardiovascular imaging and regenerative therapies. Comp Med 58:568–579 [PMC free article] [PubMed] [Google Scholar]
- 43.Sim EKW, Muskawad S, Lim CS, Yeo HJ, Lim KH, Grignani RT, Durrani A, Lau G, Duran C. 2003. Comparison of human and porcine aortic valves. Clin Anat 16:193–196 [DOI] [PubMed] [Google Scholar]
- 44.Smerup M, Pederson TF, Nyboe C, Funder JA, Christensen TD, Nielsen SL, Hjortdal V, Hasenkam M. 2004. A long-term porcine model for evaluation of prosthetic heart valves. Heart Surg Forum 7:E259–E264 [DOI] [PubMed] [Google Scholar]
- 45.Smith AC, Ehler W, Swindle MM. 1997. Anesthesia and analgesia in swine, p 313–366. In: Kohn DH, Wixson SK, White WJ, Benson GJ. Anesthesia and analgesia in laboratory animals. New York (NY): Academic Press [Google Scholar]
- 46.Štengl M. 2010. Experimental models of spontaneous ventricular arrhythmias and of sudden cardiac death. Physiol Res 59:S25–S 31 [DOI] [PubMed] [Google Scholar]
- 47.Stephens EH, Kearney DL, Grande Allen KJ. 2012. Insight into pathologic abnormalities in congenital semilunar valve disease based on advances in understanding normal valve microstructure and extracellular matrix. Cardiovasc Pathol 21:46–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki Y, Yeung AC, Ikeno F. 2009. The preclinical animal model in the translational research of interventional cardiology. JACC Cardiovasc Interv 2:373–383 [DOI] [PubMed] [Google Scholar]
- 49.Swindle MM. 2007. Biology, handling husbandry, and anatomy, p 1–32. In: Swindle MM. Swine in the laboratory. Surgery, anesthesia, and experimental techniques, 2nd ed. Boca Raton (FL): CRC Press [Google Scholar]
- 50.Swindle MM. 2007. Cardiothoracic and vascular surgery–chronic intravascular catheterization, p 195–252. In: Swindle MM. Swine in the laboratory. Surgery, anesthesia, and experimental techniques, 2nd ed. Boca Raton (FL): CRC Press [Google Scholar]
- 51.Swindle MM. 2007. Cardiovascular catheterization, electrophysiology and imaging laboratory procedures, p 299–343. In: Swindle MM. Swine in the laboratory. Surgery, anesthesia, and experimental techniques, 2nd ed. Boca Raton (FL): CRC Press [Google Scholar]
- 52.Swartz RS, Chronos NA, Virmani R. 2004. Preclinical restenosis models amd drug-eluting stents still important, still much to learn. J Am Coll Cardiol 44:1373–1385 [DOI] [PubMed] [Google Scholar]
- 53.Tranum-Jensen J, Wlde AAM, Vermeulen JT, Janse MJ. 1991. Morphology of electrophysiologically identified junctions between Purkinje fibers and ventricular muscle in rabbit and pig hearts. Circ Res 69:429–437 [DOI] [PubMed] [Google Scholar]
- 54.Tribulova N, Novakova S, Macsaliova A, Sass S, Thomas S, Goetzfried S, Podzuweit T, Manoach M. 2002. Histochemical and ultrastructural characterization of an arrhythmogenic substrate in ischemic pig heart. Acta Histochem 104:393–397 [DOI] [PubMed] [Google Scholar]
- 55.Weaver ME, Pantely GA, Bristow JD, Ladley HD. 1986. A quantitative study of the anatomy and distribution of coronary arteries in swine in comparison with other mammals and man. Cardiovasc Res 20:907–917 [DOI] [PubMed] [Google Scholar]
- 56.Winfree AT. 1994. Electrical turbulence in 3-dimensional heart muscle. Science 266:1003–1006 [DOI] [PubMed] [Google Scholar]
- 57.Waller BF. 1985. Exercise-related sudden death in young (age less than or equal to 30 years) and old (age greater than 30 years) conditioned subjects. Cardiovasc Clin 15:9–73 [PubMed] [Google Scholar]
- 58.Xanthos T, Bassiakou E, Koudouna E, Tsirikos-Karapanos N, Lelovas P, Papadimitriou D, Dontas I, Papadimitriou L. 2007. Baseline hemodynamics in anesthetized Landrace–Large White swine: reference values for research in cardiac arrest and cardiopulmonary resuscitation models. J Am Assoc Lab Anim Sci 46:21–25 [PubMed] [Google Scholar]
- 59.Xanthos T, Lelovas P, Vlachos I, Tsirikos-Karapanos N, Kouskouni E, Perrea D, Dontas I. 2007. Cardiopulmonary arrest and resuscitation in Landrace–Large White swine: a research model. Lab Anim 41:353–362 [DOI] [PubMed] [Google Scholar]