Abstract
Buprenorphine and carprofen, 2 of the most commonly used analgesics in mice, must be administered every 8 to 12 h to provide sustained analgesia. Sustained-release (SR) formulations of analgesics maintain plasma levels that should be sufficient to provide sustained analgesia yet require less frequent dosing and thus less handling of and stress to the animals. The pharmacokinetics of SR formulations of buprenorphine (Bup-SR), butorphanol (Butp-SR), fentanyl (Fent-SR), carprofen (Carp-SR), and meloxicam (Melox-SR) were evaluated in mice over 72 h and compared with those of traditional, nonSR formulations. Bup-SR provided plasma drug levels greater than the therapeutic level for the first 24 to 48 h after administration, but plasma levels of Bup-HCl fell below the therapeutic level by 4 h. Fent-SR maintained plasma levels greater than reported therapeutic levels for 12 h. Therapeutic levels of the remaining drugs are unknown, but Carp-SR provided plasma drug levels similar to those of Carp for the first 24 h after administration, whereas Melox-SR had greater plasma levels than did Melox for the first 8 h. Butp-SR provided detectable plasma drug levels for the first 24 h, with a dramatic decrease over the first 4 h. These results indicate that Bup-SR provides a stable plasma drug level adequate for analgesia for 24 to 48 h after administration, whereas Carp-SR, Melox-SR, Fent-SR, and Butp-SR would require additional doses to provide analgesic plasma levels beyond 24 h in mice.
Abbreviations: Bup-HCl, buprenorphine hydrochloride; Butp, butorphanol; Carp, carprofen; Fent, fentanyl; Melox, meloxicam; SR, sustained release
Postoperative or postprocedural analgesia is imperative to eliminate undue pain or distress in murine models. The 2 classes of analgesics typically used to treat postoperative pain in laboratory mice are opioids and NSAID. Each of these has very different mechanisms of actions and side effects. Opioid analgesics bind receptors, of which µ and κ are the most frequently targeted for analgesic activity, are classified as either agonists or partial agonist-antagonists. Fentanyl is a µ receptor agonists, whereas butorphanol has primary affinity to κ receptors and buprenorphine is a partial μ agonist. Whereas µ receptors are primarily located in the cerebral cortex, κ receptors are primarily located in the spinal cord.9,16 Fentanyl and butorphanol are not routinely used in the laboratory animal setting because of their short half-lives. Effective plasma concentrations of fentanyl persist less than an hour,21 and the antinociceptive activity of butorphanol lasts only 1 to 2 h in mice,11 requiring very frequent dosing to provide prolonged analgesia. In comparison, buprenorphine offers the advantage of having a longer duration of effect. Its duration of analgesic efficacy was determined to be 3 to 5 h by using a hot plate and tail flick assay,11 whereas satisfactory analgesia was achieved with 0.1 mg/kg twice daily in a partial hepatectomy model.36 NSAID offer an alternative or adjunct to opioid analgesics. Tissue damage results in the production of prostaglandins, which increase the sensitivity of nociceptors. Prostaglandin synthesis is mediated by the cyclooxygenase enzymes COX1 and COX2. Both are constitutively expressed in tissues, with COX2 induced during the inflammatory process. NSAID inhibit the prostaglandin pathway, thereby providing analgesia. NSAID usage has some unwanted side effects including gastrointestinal toxicity, altered platelet function, and renal toxicity.4,16
Analgesia treatments for rodents continue to be developed and modified to provide optimal analgesia after surgical procedures. One of the most common analgesics used in mice is buprenorphine, which has been shown to be effective in a variety of pain models,3,5,8 exhibits a wide safety margin as a partial µ agonist,43 and has few side effects when administered appropriately.14 Depending on the severity of the procedure, many institutions, including ours, requires the provision of analgesics for the first 72 h after the procedure,6,27,37,40 thus requiring buprenorphine to be dosed every 8 to 12 h.8,11 This frequency of drug administration requires repeated handling, which can stress mice,1 and can cause waxing and waning of plasma concentrations, which may result in subtherapeutic concentrations. Sustained-release (SR) formulations that deliver a constant amount of drug over time have been developed for use in rodent models and yield adequate plasma concentrations, achieve satisfactory analgesia, and reduce the amount of handling. The efficacy of a SR formulation of buprenorphine (Bup-SR)10 was evaluated in a rat model and found to provide satisfactory analgesia for 72 h after administration. More recently, Bup-SR was evaluated in mice by using a thermal nociception model, with promising results.2
Other analgesics such as NSAID may be more appropriate for pain management depending on the nature of the procedure; NSAID can also be used in combination with opioids. Carprofen and meloxicam are 2 commonly used NSAID that provide analgesia by inhibiting the COX2 enzyme, with some activity on COX1.4 Commonly used dosing regimens for carprofen and meloxicam in mice are 5 mg/kg every 12 to 24 h and 1 to 2 mg/kg every 12 h, respectively.7,8 In addition, an SR release formulation of an NSAID would be beneficial for the management of pain in mice.
This study sought to determine the pharmacokinetics of SR formulations of buprenorphine (Bup-SR), butorphanol (Butp-SR), fentanyl (Fent-SR), carprofen (Carp-SR), and meloxicam (Melox-SR) compared with those of the nonSR formulations of buprenorphine (Bup-HCl), carprofen (Carp) and meloxicam (Melox). Our findings indicated that Bup-SR maintained plasma drug levels above therapeutic levels for the first 24 to 48 h. Fent-SR maintained plasma levels above therapeutic levels for the first 12 h. Therapeutic levels for the remaining drugs are not known in mice; however, Carp-SR provided plasma drug levels similar to those of Carp for the first 24 h, Melox-SR concentrations were greater than those of Melox for the first 8 h after administration, and Butp-SR provided measurable plasma drug levels for the first 24 h.
Materials and Methods
Mice.
Female CD1 mice (weight, 20 to 27 g; age, 8 to 10 wk) were obtained from Charles River Laboratories (Wilmington, MA). Mice were SPF for Sendai virus, mouse hepatitis virus, minute mouse virus, mouse parvovirus, mouse norovirus, Theiler murine encephalitis virus, rotavirus, Mycoplasma pulmonis, pinworms, and ectoparasites. Mice were group-housed at 5 or 6 per cage with ad libitum access to Teklad Irradiated Diet 2918 (Harlan Laboratories, Madison, WI) and filter sterilized water and allowed to acclimate for 3 to 7 d prior to initiation of the studies. Mice were maintained on a 14:10-h light:dark cycle at a temperature of 21 to 24 °C. All experimental procedures were approved by the IACUC.
Analgesics.
The 8 treatment groups and the dosages administered are presented in Table 1. The SR formulations of analgesics were developed and provided by Zoopharm (Windsor, CO). Commercially available preparations of Carp (Rimadyl injectable, Zoetis, Kalamazoo, MI), Melox (Boehringer Ingelheim, Ridgefield, CT), and Bup-HCl (Rickett Benckiser Healthcare, London, England) were diluted in sterile saline prior to administration. All SR drugs were administered once, whereas the nonSR analgesics were redosed accordingly as described following.
Table 1.
Analgesic treatment groups and dosages
| Treatment | Dose (mg/kg) | Concentration (mg/mL) |
| Bup-SR | 0.6 | 0.5 |
| Melox-SR | 6 | 2 |
| Butp-SR | 18 | 10 |
| Fent-SR | 3.5 | 2 |
| Carp-SR | 15 | 10 |
| Carp | 5 | 50 |
| Melox | 1 | 5 |
| Bup-HCl | 0.1 | 0.3 |
Pharmacokinetic study.
To assess pharmacokinetics, 168 female CD1 mice allocated into 8 treatment groups of 21 mice, with 5 or 6 mice per cage. All mice in each treatment group (n = 3 per group per time point) were manually restrained and injected subcutaneously in the interscapular region with the analgesic as outlined in Table 1. Mice in the nonSR formulations groups received subsequent dosing every 12 h for Bup-HCl and Melox, and every 24 h for Carp. Three mice from each cage were selected for analysis at each time point. If only 2 mice remained, then the additional mouse needed was selected from the next cage in the group. These studies were performed in parallel and initiated in the morning. Mice were euthanized by using carbon dioxide, and blood was immediately collected via cardiocentesis at 2, 4, 8, 12, 24, 48, and 72 h after treatment. Mice that received nonSR formulations were sampled 12 h after administration, such that the values represented the waning plasma drug levels. Blood samples were placed in heparinized microcentrifuge tubes (Becton Dickinson, Franklin Lakes, NJ), centrifuged at 10,000 × g for 15 min, and plasma collected and stored at −80 °C until analyzed. Pharmacokinetic analysis was performed by using Phoenix WinNonlin software (Pharsight, Cary, NC).
A second cohort of 9 mice was used to repeat the 48- and 72-h time points for the Bup-SR group by using a more sensitive method of detection. The group for the 48-h time point contained 4 mice, and that for the 72-h time point contained 5 mice. Mice were dosed with the same formulation and time as used in the first cohort, albeit on a different day.
Liquid chromatography–tandem mass spectrometry of analgesics.
Standard dilutions of analgesics were prepared in acetonitrile. For the analysis of each analgesic in plasma, standards (0.025 to 1000 ng/mL) were added to control plasma. Samples were prepared by using 50 µL plasma for the analysis of Bup, Butp, Fent and Melox and 100 µL plasma for the analysis of Carp. Each sample was spiked with 5 µL acetonitrile or 5 µL of the appropriate analgesic standard (10 µL of either for Carp analysis) and 5 µL of 10 µg/mL naringenin as an internal standard, samples were vortexed briefly, and then 100 µL acetonitrile was added for protein precipitation. Samples were vortexed continuously for 10 min followed by centrifugation for 10 min at 20,800 × g at 4 °C; 150 µL of each supernatant was collected and transferred to HPLC vials with inserts for analysis.
To increase sensitivity for the analysis of Bup, a liquid–liquid extraction was used (method 2). For this method, after the addition of appropriate standards and the internal standard, 1 mL methyl tert-butyl ether was added to each tube, and samples were vortexed continuously for 10 min followed by centrifugation for 10 min at 20,800 × g at 4 °C. After samples had been stored at −80 °C for 30 min, 950 µL of the organic layer was removed, placed in a fresh tube, and vacuum-dried (Automatic Environmental SpeedVac AES 1000, Savant, Farmingdale, NY) for approximately 45 min. Samples were reconstituted in 100 µL of 50 acetonitrile:50 Milli-Q–purified water and placed in HPLC vials with inserts.
Positive-ion electrospray ionization mass spectra were obtained by using a triple quadrupole mass spectrometer (MDS Sciex 3200 Q-TRAP, Applied Biosystems, Foster City, CA) with a turbo ionspray source interfaced with an HPLC system (model LC-20AD. Shimadzu, Kyoto, Japan). All samples were chromatographed by using a 2.5 µm, 4.6 × 50 mm column (XBridge Phenyl, Waters, Milford, MA) protected by a C18 guard cartridge (4.0 × 2.0 mm, Phenomenex, Torrance, CA). Gradient elution was used for all compounds. For Bup, mobile phase A consisted of 10 mM ammonium acetate in Milli-Q–purified water, and mobile phase B consisted of 100% acetonitrile. For Butp, Fent, Melox, and Carp, mobile phase A consisted of 0.1% formic acid in in Milli-Q–purified water, and mobile phase B consisted of 100% acetonitrile. For Bup, chromatographic resolution was achieved by linearly holding the B solvent at 25% for 1 min. The solvent mixture then was altered by increasing mobile phase B linearly from 25% to 98% between 1 and 2 min, maintaining at 98% between 2 and 4.5 min, and then decreasing linearly from 98% to 25% between 4.5 and 4.75 min, followed by reequilibration of the column at 25% mobile phase B from 4.75 to 6 min. The gradient conditions for Melox and Carp was achieved by linearly holding the B solvent at 25% for 0.5 min. The solvent mixture then was altered by increasing mobile phase B linearly from 25% to 98% between 0.5 and 2 min, maintaining at 98% between 2 and 4 min, and then decreasing linearly from 98% to 25% between 4 and 4.5 min, followed by reequilibration of the column at 25% mobile phase B from 4.5 to 5.5 min. The mobile phase B percentage for Fent was 25% for 1 min, then linearly increased to 98% between 1 min and 3 min, held at 98% for 1 min, linearly decreased from 98% to 25% between 4 and 4.5 min, and then equilibrated at 25% for an additional minute. For Butp, solvent B was held at 25% for 1.5 min. The solvent mixture then was altered by increasing mobile phase B linearly from 25% to 98% between 1.5 and 2.5 min, maintaining at 98% between 2 and 4 min, and then decreasing linearly from 98% to 25% between 4 and 4.75 min, followed by reequilibration of the column at 25% mobile phase B from 4.75 to 6 min. The flow rate was 750 µL/min for Butp, Melox, and Carp and 1000 µL/min for Bup and Fent. The sample injection volumes were 10 µL for Butp, Fent, Melox, and Carp, and the injection volume was 40 µL for analysis of Bup method 1 and 60 µL for Bup method 2.
The mass spectrometer settings were optimized as follows: turbo ionspray temperature, 550 °C for Bup, Fent, and Melox and 575 °C for Butp and Carp; ion spray voltage, 4500 V for Butp and 5500 V for the other compounds; curtain gas, N2: 10 units for Butp, Carp, and Melox, 20 units for Fent, and 30 units for Bup; collision gas, N2: 3 units for Carp and Fent, 5 units for Bup and Butp, and 6 units for Melox; nebulizer gas, N2: 40 units for Fent, 50 units for Melox, and 60 units for the other 3 compounds; and auxiliary gas, N2: 45 units for Fent, 50 for Melox, and 60 units for the other 3 compounds. The compound-specific parameters for each compound are shown in Table 2. The predominant product ions were m/z 396.4 and 414.4 for Bup, m/z 131.1 and 157.2 for Butp, m/z 193.2 and 228.2 for Carp, m/z 105.2 and 188.3 for Fent, and m/z 115.1 and 141.2 for Melox. Samples were quantified in the multiple-reaction monitoring mode by monitoring the relevant ion transitions and then summing the counts for each transition. The dwell time for each ion transition was 100 ms for Bup, Butp, Carp, and Melox and 500 ms for Fent. Q1 and Q3 were both operated in unit resolution mode. All compounds eluted between 1.0 and 4.0 min. No interfering peaks were detected at the monitored ion transitions in the extracted matrix. Chromatographic conditions were optimized for peak shape. Quantitation of each compounds was based on linear standard curves in spiked matrix, with 1/x2 weighting of linear regression. The lower limit of detection was 0.5 ng/mL for Bup method 1 and 25 pg/mL for Bup method 2, 250 pg/mL for Fent, 1 ng/mL for Butp and Melox, and 25 ng/mL for Carp.
Table 2.
Compound-specific parameters for analysis of analgesics in mouse plasma
| Transition (m/z) | Units |
|||||
| Declustering potential | Entrance potential | Collision cell entrance potential | Collision energy | Collision cell exit potential | ||
| Bup | 468.3 → 396.4 | 80.1 | 7.2 | 46.5 | 49.4 | 3.5 |
| 468.3 → 414.4 | 71.3 | 10.7 | 68.1 | 48.9 | 4.1 | |
| Butp | 328.3 → 131.1 | 50.7 | 5.1 | 170 | 71.1 | 2.2 |
| 328.3 → 157.2 | 53.0 | 4.9 | 60.0 | 62.4 | 1.5 | |
| Carp | 274.2 → 193.2 | 22.3 | 4.6 | 21.5 | 38.2 | 2.6 |
| 274.2 → 228.2 | 24.4 | 4.8 | 25.6 | 20.5 | 3.5 | |
| Fent | 337.3 → 105.2 | 47.3 | 4.4 | 82.0 | 51.2 | 1.9 |
| 337.3 → 188.3 | 38.3 | 4.9 | 32.3 | 33.1 | 3.4 | |
| Melox | 352.2 → 115.1 | 21.0 | 5.2 | 34.9 | 24.7 | 1.8 |
| 352.2 → 141.2 | 36.6 | 4.6 | 43.1 | 28.6 | 2.2. | |
| Naringenin | 273.1 → 153 | 31.7 | 4.6 | 27.1 | 33.3 | 2.0 |
Naringenin was used as an internal standard.
Statistical analysis.
Statistical analysis was done by using SAS version 9.3 (SAS Institute, Cary, NC). Prior to analysis, any values below the limit of detection of the assay were replaced with half of the lower limit of detection, which was equal to 0.25 ng/mL for the Bup assay. There were no samples below the limit of detection for Carp and Melox. The Proc Mixed algorithm was used to fit a 2-way ANOVA model with interaction separately for each drug (Bup, Carp, Melox). The factors included time (2, 4, 8, 12, and 24 h) and treatment (SR formulation or nonSR formulation). The statistical analysis was performed prior to the redosing schedule, thus the first 12 h were compared for the Bup and Melox, whereas the first 24 h were compared for Carp. Contrasts were used to test SR formulations compared with nonSR formulations at each time point. A P value less than 0.05 was considered to be statistically significant.
Results
Pharmacokinetics.
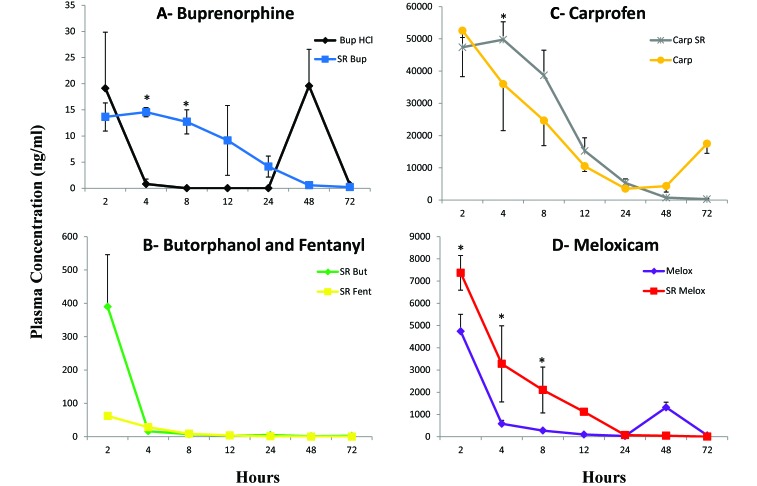
Mice were given analgesics subcutaneously at the doses specified in Table 1, and plasma concentrations were determined over a 72-h period (n = 3 per time point). SR release formulations were given once. Mice receiving Melox and Bup-HCl were dosed every 12 h, and those receiving Carp were dosed every 24 h. Plasma concentrations of the Bup-SR were highest at 4 h after administration (14.5 ng/mL) and steadily decreased over the first 24 h to 4.2 ng/mL (Figure 1 A). There was one sample at 12 h that was below the limit of detection, whereas the other samples were 10.9 and 16.3 ng/mL. Initial mice at the 48- and 72-h time point for Bup-SR had plasma concentrations that were below the limit of detection of the first Bup analysis method (0.5 ng/mL). Therefore, we repeated these time points in additional mice by using a more sensitive technique for drug level determination (method 2) that had a lower limit of detection of 25 pg/mL. The plasma concentrations of Bup at 48 h (n = 4) ranged from 0.26 to 0.59 ng/mL, with an average of 0.56 ng/mL (1 SD, 0.25 ng/mL). At 72 h (n = 5), the plasma concentration ranged from 0.03 to 0.45 ng/mL, with an average of 0.22 ng/mL (1 SD, 0.16 ng/mL). The peak concentration of Bup-HCl occurred at 2 h after administration (19.1 ng/mL). At 4 h, 1 sample was detectable (1.91 ng/mL), where the other 2 samples were below the limit of detection of the assay, and at 8 h, all samples were below the limit of detection (Figure 1 A). The levels were below the limit of detection after 12, 24, and 72 h. These values likely represent the nadir plasma levels because mice were euthanized prior to redosing. At the 48-h time point, the mice were inadvertently dosed with Bup-HCl, and the samples collected within 1 h after dosing, yielding plasma levels at 19.5 ng/mL. The other SR opioids, Fent-SR and Butp-SR (Figure 1 B), had very high plasma concentrations at 2 h after administration (62.1 ng/mL and 389.7 ng/mL, respectively). These were followed by a drop at 4 h (Fent-SR, 28.3 ng/mL; Butp-SR, 16.05 ng/mL). The plasma concentrations of Fent-SR averaged 8.8 ng/mL, 3.5 ng/mL, and 1.0 ng/mL at 8, 12, and 24 h, respectively. Plasma levels of Fent-SR were below the limit of detection at 48 and 72 h. Mice that received Fent-SR had decreased activity during the first 12 h after administration. The plasma concentration of Butp-SR was 7.1 ng/mL, 3.0 ng/mL, and 5.0 ng/mL at 8, 12, and 24 h. At 48 h, 2 Butp-SR samples were below the limit of detection; the remaining sample was 1.24 ng/mL. Similarly at 72 h, 2 samples were below the limit of detection, and the remaining sample was 2.7 ng/mL. Due to the short duration of action of Fent and Butp, only the SR formulations were assessed.
Figure 1.
Plasma concentrations of analgesics over time. Three mice were sampled at each time point for the first 72 h, except for Bup-SR (4 mice sampled at 48 h; 5 mice sampled at 72 h). NonSR formulations were analyzed at 12 h after administration, representing the nadir plasma levels. Bars represent 1 SD; *, significant (P < 0.05) difference between the SR formulation and the nonSR formulation at that given time point prior to the redosing interval. (A) Bup-SR and Bup-HCl. (B) Butp-SR and Fent-SR. (C) Carp-SR and Carp. (D) Melox-SR and Melox.
The NSAID analgesics showed similar pharmacokinetic profiles as those of the opioids. Carp-SR (Figure 1 C) had similar levels at 2 and 4 h after administration (47.4 μg/mL and 49.7 μg/mL, respectively), whereas Melox-SR (Figure 1 D) had a peak level at 2 h after administration (73.7 μg/mL). Carp and Melox had peak levels at 2 h postadministration (52.5 μg/mL and 47.4 μg/mL, respectively). Both SR formulations and nonSR formulations of Carp and Melox showed decreases in plasma concentration over the first 24 h but maintained values above the limit of detection of the assay (Carp, 25 ng/mL; Melox, 1 ng/mL) for the duration of the 72-h study. Values at 24, 48, and 72 h represent the nadir plasma levels for Carp and Melox, except at the Melox 48-h time point, when mice were dosed inadvertently, and the samples were collected within 1 h after dosing. Figure 1 shows the plasma concentrations over the entire time period tested for each drug and formulation.
There were no morbidities, including injection site reactions, or mortalities in any of the treatment groups.
The noncompartmental analysis for Bup-SR, Fent-SR, Butp-SR, Melox-SR, Melox, Carp-SR, and Carp are provided in Table 3. Noncompartmental analysis was not performed for Bup-HCl because of its short duration of action and the inability to detect plasma drug levels beyond the 8-h time point. As expected, the half-lives for all SR formulations are greater than those for nonSR formulations. The SR formulation leads to an approximate doubling of the terminal half-life for Melox and an approximate 50% increase for Carp. In addition, exposure times (apparent by AUC) are increased with the SR formulations—roughly 2.8 times for both Melox and Carp. Although apparent clearance is only slightly increased for Carp-SR compared with Carp, there is an increase in clearance for Melox-SR.
Table 3.
Noncompartmental analysis of analgesic formulations
| Tmax (h) | Cmax (ng/mL) | t1/2 (h) | AUClast | Tlast (h) | AUCinf (hr*ng/mL) | Cl/Fpred | |
| Bup-SR | 4 | 14.5 ng/mL | 10.1 | 322 h×ng/mL | 72 | 325 h×ng/mL | 1.85 L/h/kg |
| Butp-SR | 2 | 390 ng/mL | not done | 910 h×ng/mL | 24 | not done | not done |
| Fent-SR | 2 | 62.1 ng/mL | 6.28 | 279 h×ng/mL | 24 | 288 h×ng/mL | 12.2 L/h/kg |
| Melox-SR | 2 | 7370 ng/mL | 6.96 | 44,200 h×ng/mL | 72 | 44,300 h×ng/mL | 0.136 L/h/kg |
| Melox | 2 | 4740 ng/mL | 3.08 | 12,500 h×ng/mL | 12 | 12,900 h×ng/mL | 0.077 L/h/kg |
| Carp-SR | 4 | 149 µg/mL | 10.2 | 1130 h×µg/mL | 72 | 1140 h×µg/mL | 13.2 mL/h/kg |
| Carp | 2 | 52.5 µg/mL | 6.88 | 399 h×µg/mL | 24 | 434 h×µg/mL | 11.5 mL/h/kg |
Tmax, time of Cmax; Cmax, maximal concentration measured ; t1/2, terminal half-life; AUClast, AUC from time 0 to Tlast; Tlast, time point at which AUClast was calculated; AUCinf, AUC to infinity; Cl/Fpred, predicted clearance.
Discussion
According to the pharmacokinetic profiles of the various analgesic regimens used in this study, Bup-SR maintained plasma drug levels above its therapeutic level for 24 to 48 h, and Fent-SR maintained plasma drug levels above its therapeutic level for 12 h. In addition, Butp-SR, Carp-SR, and Melox-SR maintained detectable plasma drug levels for 24 h. Whether any of these concentrations exceed estimated therapeutic levels is discussed later.
The dosing regimen for Bup-HCl was 0.1 mg/kg SC every 12 h, and the Bup-SR loading dose was based on the cumulative dose of the Bup-HCl, or 0.6 mg/kg SC. The targeted therapeutic plasma level was 0.5 ng/mL, on the basis of previous studies that determined this dose provided effective analgesia in 50% of the population; however, a therapeutic plasma level of 1.0 ng/mL is reported to be effective in a majority of the population.14 The pharmacokinetic analysis demonstrated that Bup-SR maintained a plasma level concentration greater than 1.0 ng/mL for the first 24 h, with levels below that at the 48- and 72-h time points (0.58 ng/mL and 0.22 ng/mL, respectively). The half-life of the Bup-SR formulation was 10.1 h in our study, compared with 2.9 h in previous studies.44 Mice treated with Bup-SR at a dose of 1.0 mg/kg had analgesia that was effective for 12 to 24 h, but no pharmacokinetic analysis was performed.2 A pharmacokinetic study in rats treated with Bup-SR at a dose of 0.9 mg/kg maintained levels above 1.0 ng/mL for a 72-h period.10 The loading dose used and species-specific differences may account for the differences seen between the mice in the current study and the previous mouse and rat studies.
The Bup-HCl levels acquired at the end of the 72-h dosing regimen were consistently below the level of detection of the assay (0.5 ng/mL) after 8 h. The exception was at 48 h, when the sample was collected after mice inadvertently received a subsequent dose, therefore leading to the observed high plasma level (19.6 ng/mL). Our results are consistent with previous pharmacokinetic analyses. The kinetics of Bup in mice after various routes of administration demonstrated that the serum concentration of Bup rapidly declined after a subcutaneous bolus of 0.05 mg/kg and was below 1 ng/mL at 6 h after administration.20 Similarly, mice given Bup-HCl intravenously at 2.4 mg/kg demonstrated plasma levels less than 5 ng/mL at 12 h and less than 1.0 ng/mL at 15 h.44 In previous studies, rats treated with 0.1 mg/kg Bup-HCl demonstrated a peak plasma level of 3.0 ng/mL at 4 h after administration, and this level dropped to below 0.5 ng/mL at 24 h.10 It is important to note that the Bup-HCl time points likely represent the lowest plasma drug levels when mice are dosed at 12-h intervals for analgesia. Although not evaluated in the current study, it is very likely that during the first 8 h after subsequent administration of Bup-HCl, there is a peak plasma level that is comparable to the levels seen during the first 8 h of this study. This scenario was demonstrated at the 48-h time point, when the mice inadvertently were dosed prior to euthanasia and within 1 h demonstrated a rise in plasma drug level. Although we did not determine analgesic efficacy, the results of our Bup-HCl pharmacokinetic analysis suggest that plasma levels are inadequate if the drug is provided every 12 h and that more frequent dosing is required, perhaps even as frequently as every 3 to 4 h,11 to maintain plasma drug levels.
We evaluated the pharmacokinetics of SR formulations of Butp and Fent as potential replacements for Bup, which becomes unavailable occasionally. Published analgesic protocols using Butp or Fent for postoperative pain in mice are scarce, likely due to the drugs’ very short duration of action and need for frequent administration. Butp at 5 mg/kg in mice provided short-term analgesia, which declined in 2 h, in the hot-plate and tail-flick analgesiometric assays.11 Other published analgesic protocols using Butp required 1 to 2 mg/kg SC every 4 h.7 The loading dose of 18 mg/kg that we used was based on this 1-mg/kg dosing regimen. The therapeutic levels of Butp in mice are unknown. According to ranges from studies in other species (horses and cats), the therapeutic levels may be 10 to 45 ng/mL.34,41 The plasma levels of Butp remained above 10 ng/mL for the first 4 h of our study and fell below 10 ng/mL by 8 h.
Fent is a common postoperative analgesic used in large animal species that is frequently delivered via a transdermal patch. The use of Fent in mice postoperatively has rarely been reported. In a chronic (7 d) Fent infusion study in mice, 2 mg/kg/d was found to be well tolerated in mice with adequate µ-receptor inhibition concentrations.35 However, in early preliminary studies with Fent-SR, heavy sedation and labored breathing occurred with this bolus formulation. Fent at a dose of 0.2 mg/kg as a single bolus provided analgesia in an experimental model of femoral bone cancer with varying success.31 Fent administered at a dose of 7.5 μg/h (180 μg/d) by using an osmotic pump, thereby mimicking the dosage of transdermal patches used in humans, had analgesic properties in a hot-plate assay.28 A dose of 0.9 mg/kg demonstrated a 1-ng/mL serum concentration for 60 min and led to analgesic effects and therapeutic antinociception at 3 to 10 ng/mL.21 We chose to use a loading dose of 3.5 mg/kg for Fent, which would be the total dose if dosed daily at 1.2 mg/kg. The half-life of Fent-SR was 6.3 h compared with a previously published half-life of less than 10 min,21 and the plasma levels remained above 3 ng/mL until the 12-h evaluation and were below the limit of detection at 48 and 72 h. However, mice experienced profound sedation for the first 12 h after administration, as evidenced by their decreased activity level. Given the high loading dose of Fent-SR and the initial burst of drug released from the matrix, the initial plasma levels likely exceed the effective analgesic dose and approach anesthetic doses.
Given the inability to maintain an adequate plasma level past 12 h, additional evaluations of Butp-SR and Fent-SR are needed for them to be useful analgesics in mice. Specifically, additional pharmacokinetic and efficacy studies could be performed on nonSR formulations of these drugs to determine therapeutic levels. Because of cost constraints and their short duration of action, we did not perform these pharmacokinetic studies. Other formulations could be evaluated to minimize the initial burst effect, particularly of Fent-SR, which likely led to the profound sedation we noted during the first few hours after treatment. Although Fent-SR does not provide analgesia for 72 h, the formulations could be manipulated to provide Fent for a shorter duration (12 to 24 h) without the sedative effect that we noted.
Although primarily used for treating low to moderate pain intensity, both Carp and Melox have been demonstrated to provide analgesia with longer half-lives than those of opioids.9,16,26 Our loading dose was based on the published doses of 5 mg/kg Carp every 24 h9,12,30 and 1 to 2 mg/kg Melox every 12 h,7,12,15,33,36 both of which are commonly used dosages, according to recommendations from multiple animal resource websites.19,38,39 The therapeutic levels of Carp and Melox are unknown in mice. In other species, therapeutic levels range from 20 to 24 μg/mL for Carp25 and 390 to 911 ng/mL for Melox,13,18,24 according to in vitro assays evaluating inhibition of cyclooxygenase. From the pharmacokinetic profiles, both Carp-SR and Melox-SR provided sustained plasma drug levels for at least the first 24 h postadministration. Carp-SR demonstrated levels comparable to those of Carp given every 24 h, albeit with higher levels noted 4 to 8 h after administration. However, Carp-SR maintained levels higher than the estimated therapeutic level of 24 μg /mL for the first 12 h, as did Carp. Given the similarity in pharmacokinetic profiles, the benefit of using Carp-SR over Carp once daily may be minimal, and twice-daily dosing of each formulation likely is needed to maintain therapeutic levels.
Similar to the Carp analysis, Melox plasma levels were higher for the first 12 h after treatment with Melox-SR compared with Melox, with similar profiles from 24 to 72 h. The exception was an increased plasma level of Melox at 48 h due to inadvertently dosing the mice prior to sample collection, so the value does not represent the nadir of Melox, as at other points. Melox-SR maintained plasma levels greater than the estimated therapeutic level of 911 ng/mL for the first 12 h, whereas Melox concentrations fell below that level after 4 h. Although we were able to demonstrate that SR formulations are capable of sustaining plasma drug levels above therapeutic levels for 12 h, we did not assess analgesic efficacy in this study. Several recent publications suggest that the analgesic doses of NSAID in mice are much greater than originally proposed. Much of the Carp use in mice is extrapolated from efficacy studies from rats;9,32 however, a significantly higher dosage of Carp, 20 to 25 mg/kg, may be needed to reduce postoperative pain when using the mouse grimace scale as a means of assessment.29 Early studies demonstrated antinociceptive effects of Melox in several models of chemical and thermal nociception in mice at doses ranging from 0.98 to 2.6 mg/kg,33 but dose ranges of 2 to 20 mg/kg typically are used for the treatment of partial hepatectomies,36 vasectomies,23,42 cerebral ischemia,17 and immunization with Freund adjuvant.22 The evaluation of Carp and Melox in the cited studies17,23,33,36,42 were based on behavioral criteria and did not include correlation with the plasma drug levels. Nonetheless the SR formulations provided sustained plasma drug levels for 12 h above estimated therapeutic levels in our current study. Future studies with modifications to the matrix and dosage of Carp and Melox are needed to determine whether therapeutic levels are maintained beyond 12 h.
In this study, we sought to determine the plasma drug levels of SR formulations of commonly used analgesics in laboratory mice and to compare these data with those of nonSR formulations at various time points over 72 h. We sampled mice initially to determine the profile for each formulation immediately after administration for the first 12 h. Samples then were collected every 12 h after administration to determine whether plasma drug concentrations for SR formulations were comparable or greater than those of nonSR formulations. There were no morbidities, including injection site reactions, or mortalities in any of the treatment groups, suggesting the SR formulations were safe. We found that Bup-SR provided plasma levels greater than therapeutic levels for 24 to 48 h after administration, suggesting that this drug can be used for postoperative analgesia in mice. However, additional studies evaluating the clinical efficacy of Bup-SR are needed to determine its suitability to relieve postoperative pain in mice. The other SR formulations yielded concentrations that were below reported therapeutic levels for other species at 8 to 12 h but provided prolonged plasma drug levels that remained detectable for 12 to 24 h. These formulations require additional evaluation before assessing their efficacy to provide postoperative analgesia. For example, an appropriate loading dose and schedule to minimize the heavy sedative effect of the Fent-SR requires additional investigation. The Melox-SR and Carp-SR formulations require modifications to provide a prolonged plasma drug level and need to be assessed in efficacy studies to determine an optimal analgesic dose.
Acknowledgments
We are very grateful to Elisa French for her technical assistance and to Ann Hess for her statistical analysis. This work was supported by Colorado State University's Office of the Vice President for Research, the American Society for Laboratory Animal Practitioners Summer Fellowship Grant, and the University of Colorado Cancer Center Shared Resources Grant (P30CA046934).
References
- 1.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51 [PubMed] [Google Scholar]
- 2.Carbone ET, Lindstromo KE, Diep S, Carbone L. 2012. Duration of action of sustained-release buprenorphine in 2 strains of mice. J Am Assoc Lab Anim Sci 51:815–819 [PMC free article] [PubMed] [Google Scholar]
- 3.Christoph T, Kogel B, Schiene K, Meen M, DeVry J, Friderichs E. 2005. Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. Eur J Pharmacol 507:87–98 [DOI] [PubMed] [Google Scholar]
- 4.Clark TP. 2006. The clinical pharmacology of cyclooxygenase-2-selective and dual inhibitors. Vet Clin North Am Small Anim Pract 36:1061–1085 [DOI] [PubMed] [Google Scholar]
- 5.Cowan A, Doxey JC, Harry EJR. 1977. The animal pharmacology of buprenorphine, an oripavine analgesic agent. Br J Pharmacol 60:547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emory University Division of Animal Resources. [Internet] 2013. Analgesic drugs- rodents and rabbits. [Cited 20 November 2013]. Available at http://www.dar.emory.edu/vetcare/analgesic_drugs.php
- 7.Flecknell PA. 2001. Analgesia of small mammals. Vet Clin North Am Exot Anim Pract 4:47–56 [DOI] [PubMed] [Google Scholar]
- 8.Flecknell PA. 2009. Laboratory animal anaesthesia, 3rd ed. London (UK): Academic Press [Google Scholar]
- 9.Flecknell PA, Orr HE, Roughan JV, Stewart R. 1999. Comparison of the effects of oral or subcutaneous carprofen or ketoprofen in rats undergoing laparotomy. Vet Rec 144:65–67 [DOI] [PubMed] [Google Scholar]
- 10.Foley PL, Liang H, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50:198–204 [PMC free article] [PubMed] [Google Scholar]
- 11.Gades NM, Danneman PJ, Wixson SK, Tolley EA. 2000. The magnitude and duration of the analgesic effect of morphine, butrophanol, and buprenorphine in rats and mice. Contemp Top Lab Anim Sci 39:8–13 [PubMed] [Google Scholar]
- 12.Gargiulo S, Greco A, Gramanzini M, Esposito S, Affuso A, Brunetti A, Vesce G. 2012. Mice anesthesia, analgesia and care, part 1: anesthetic considerations in preclinical research. ILAR J 53:E70–E81 [DOI] [PubMed] [Google Scholar]
- 13.Giraudel JM, Diquelou A, Laroute V, Lees P, Toutain PL. 2005. Pharmacokinetic–pharamcodynamic modelling of NSAIDs in a model of reversible inflammatin in the cat. Br J Pharmacol 146:642–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guarnieri M, Brayton C, DeTolla L, Forbes-McBean N, Sarabia-Estrada R, Zadnik P. 2012. Safety and efficacy of buprenorphine for analgesia in laboratory mice and rats. Lab Anim (NY) 41:337–343 [DOI] [PubMed] [Google Scholar]
- 15.Harkness JE, Turner PV, VandeWoude S, Wheler CL. 2010. Harkness and Wagner's biology and medicine of rabbits and rodents, 5th ed. Ames (IA): Wiley-Blackwell Publishing [Google Scholar]
- 16.Heavaner JE, Cooper DM. 2008. Pharmacology of analgesics, p 97–118. In Fisher RE, Brown MJ, Danneman PJ, Karas AZ. Anesthesia and analgesia in laboratory animals, 2nd ed. San Diego (CA): Academic Press [Google Scholar]
- 17.Jacobsen KR, Fauerby N, Raida Z, Kalliokoski O, Hau J, Johansen FF, Abelson KS. 2013. Effects of buprenorphine and meloxicam analgesia on induced cerebral ischemia in C57BL/6 male mice. Comp Med 63:105–113 [PMC free article] [PubMed] [Google Scholar]
- 18.Jeunesse EC, Bargues IA, Toutain CE, Lacroix MZ, Letellier M, Giraudel JM, Toutain PL. 2011. Paw inflammation model in dogs for preclinical pharmacokinetic–pharmacodyanmic investigations of nonsteroidal antiinflmmatory drugs. J Pharmacol Exp Ther 338:548–558 [DOI] [PubMed] [Google Scholar]
- 19.Johns Hopkins University Animal Care and Use Committee. [Internet] 2013. Rodent drug formulary quick reference guide. [Cited 2 December 2013]. Available at: http://web.jhu.edu/animalcare/rdf/index.html
- 20.Kalliokoski O, Jacobsen KR, Hau J, Abelson KSP. 2011. Serum concentrations of buprenorphine after oral and parenteral administration in male mice. Vet J 187:251–254 [DOI] [PubMed] [Google Scholar]
- 21.Kalvass JC, Olson ER, Cassidy MP, Selley DE, Pollack GM. 2007. Pharmacokinetics and pharmacodynamics of 7 opioids in p-glycoprotein-competent mice: assessment of unbound brain EC50,u and correlation of in vitro, preclinical, and clinical data. J Pharmacol Exp Ther 323:346–355 [DOI] [PubMed] [Google Scholar]
- 22.Kolstad AM, Rodriguiz RM, Kim CJ, Hale LP. 2012. Effect of pain management on immunization efficacy in mice. J Am Assoc Lab Anim Sci 51:448–457 [PMC free article] [PubMed] [Google Scholar]
- 23.Leach MC, Klaus K, Miller AL, di Perrotolo MS, Sotocinal SF, Flecknell PA. 2012. The assessment of postvasectomy pain in mice using behaviour and the mouse grimace scale. PLoS ONE 7:e35656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lees P, Giraudel J, Landoni MF, Toutain PL. 2004. PK–PD integration and PK–PD modelling of nonsteroidal antiinflammatory drugs: principles and application in veterinary pharmacology. J Vet Pharmacol Ther 27:491–502 [DOI] [PubMed] [Google Scholar]
- 25.Lees P, Landoni MF, Giraudel J, Toutain PL. 2004. Pharmacodynamics and pharmacokinetics of nonsteroidal antiinflammatory drugs in species of veterinary interest. J Vet Pharmacol Ther 27:479–490 [DOI] [PubMed] [Google Scholar]
- 26.Liles JH, Flecknell PA. 1994. A comparison of the effectts of buprenorphine, carprofen, and flunixin following laparotomy in rats. J Vet Pharmacol Ther 17:284–290 [DOI] [PubMed] [Google Scholar]
- 27.McGill University Comparative Medicine and Animal Resources Centre. [Internet] 2013. Rodent analgesia. [Cited 20 November 2013]. Available at: http://www.mcgill.ca/research/sites/mcgill.ca.research/files/101-rodent_analgesia_0.pdf
- 28.Martucci C, Panerai AE, Sacerdote P. 2004. Chronic fentanyl or buprenoprhine infusion in the mouse: similar analgesic profile but different effects on immune responses. Pain 110:385–392 [DOI] [PubMed] [Google Scholar]
- 29.Matsumiya LC, Sorge RE, Sotocinal SG, Tabaka JM, Wieskopf JS, Zaloum A, King OD, Mogil JS. 2012. Using the mouse grimace scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J Am Assoc Lab Anim Sci 51:42–49 [PMC free article] [PubMed] [Google Scholar]
- 30.Miller AL, Richardson CA. 2011. Rodent analgesia. Vet Clin North Am Exot Anim Pract 14:81–92 [DOI] [PubMed] [Google Scholar]
- 31.Minami K, Hasegawa M, Ito H, Nakamura A, Tomii T, Matsumoto M, Orita S, Matsushima S, Miyoshi T, Masuno K, Torii M, Koike K, Shimada S, Kanesmasa T, Kihara T, Narita M, Suzuki T, Kato A. 2009. Morphine, oxycodone, and fentanyl exhibit different analgesic profiles in mouse pain models. J Pharmacol Sci 111:60–72 [DOI] [PubMed] [Google Scholar]
- 32.Roughan JV, Flecknell PA. 2001. Behavioural effects of laparotomy and analgesic effects of ketoprofen and carprofen in rats. Pain 90:65–74 [DOI] [PubMed] [Google Scholar]
- 33.Santos AR, Vedana EM, DeFreita GA. 1998. Antinociceptive effect of meloxicam in neurogenic and inflammatory nociceptive models in mice. Inflamm Res 47:302–307 [DOI] [PubMed] [Google Scholar]
- 34.Sellon DC, Papich MG, Palm L, Remund B. 2009. Pharmacokinetics of butorphanol in horses after intramuscular injection. J Vet Pharmacol Ther 32:62–65 [DOI] [PubMed] [Google Scholar]
- 35.Sirohi S, Dighe SV, Walker EA, Yoburn BC. 2008. The analgesic efficacy of fentanyl: relationship to tolerance and µ-opioid receptor regulation. Pharmacol Biochem Behav 91:115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tubbs JT, Kissling GE, Travlos GS, Goulding DR, Clark JA, King-Herbert AP, Blankenship-Paris TL. 2011. Effects of buprenorphine, meloxicam, and flunixin meglumine as postoperative analgesia in mice. J Am Assoc Lab Anim Sci 50:185–191 [PMC free article] [PubMed] [Google Scholar]
- 37.University of Illinois at Chicago Office of Animal Care and Institutional Biosafety. [Internet] 2013. Guidelines—rodent surgical classifications and analgesic guidelines. [Cited 20 November 2013]. Available at http://www.brl.uic.edu/media/PDF/guidelines_rodent_surgery_anesthesia.pdf
- 38.University of Minnesota Research Animal Resources. [Internet] 2013. Analgesic choices for rats and mice. [Cited 2 December 2013]. Available at http://www.ahc.umn.edu/rar/umnusers/formulary.html
- 39.University of Pennsylvania Office of Regulatory Affairs. [Internet] 2013. Rodent anesthesia and analgesia formulary. [Cited 2 December 2013]. Available at hhttp://www.upenn.edu/regulatoryaffairs/Documents/Guideline-RODENT_ANESTHESIA_AND_ANALGESIA_FORMULARY.pdf
- 40.University of Texas-San Antonio Laboratory Animal Resources Center. [Internet] 2013. Guidelines for evaluating and treating surgical pain in mice and rats. [Cited 20 November 2013]. Available at http://research.utsa.edu/files/larc/TreatingPostoperativePain.pdf
- 41.Wells SM, Glerum LE, Papich MG. 2008. Pharmacokinetics of burophanol in cats after intramuscular and buccal transmucsal administration. Am J Vet Res 69:1548–1554 [DOI] [PubMed] [Google Scholar]
- 42.Wright-Williams SL, Courade J, Richardson CA, Roughan JV, Flecknell PA. 2007. Effects of vasectomy surgery and meloxciam treatment on faecal corticosterone levels and behaviour in 2 strains of laboratory mice. Pain 130:108–118 [DOI] [PubMed] [Google Scholar]
- 43.Yassen A, Olofsen E, Kan J, Dahan A, Danhof M. 2008. Pharmacokinetic–pharmacodynamic modeling of the effectiveness and safety of buprenorphine and fentanyl in rats. Pharm Res 25:183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu S, Zhang X, Sun Y, Pen Y, Johnson J, Mandrell T, Shukla AJ, Laizure SC. 2006. Pharmacokinetics of buprenorphine after intravenous administration in the mouse. J Am Assoc Lab Anim Sci 45:12–16 [PubMed] [Google Scholar]