Abstract
Venous thrombosis (VT) is a significant cause of morbidity and mortality in humans. Surgical animal models are crucial in studies investigating the pathogenesis of this disease and evaluating VT therapies. Because inflammation is critical to both the development and resolution of VT, analgesic medications have the potential to adversely affect multiple parameters of interest in VT research. The objective of this study was to determine how several common analgesics affect key variables in a murine ligation model of deep vein thrombosis. Male C57BL/6 mice were randomly assigned to receive either local (bupivacaine) or systemic parenteral analgesia (buprenorphine, tramadol, or carprofen) or 0.9% NaCl (control). All mice underwent laparotomy and ligation of the inferior vena cava, and treatment was continued until euthanasia at 6 or 48 h after surgery. Analysis of harvested tissues and blood included: hematology, thrombus weight, serum and vein-wall cytokines (IL1β, IL6, IL10, TNFα), soluble P-selectin, and vein-wall leukocyte infiltration. Compared with 0.9% NaCl, all of the analgesics affected multiple parameters important to VT research. Carprofen and tramadol affected the most parameters and should not be used in murine models of VT. Although they affected fewer parameters, a single dose of bupivacaine increased thrombus weight at 6 h, and buprenorphine was associated with reduced vein wall macrophages at 48 h. Although we cannot recommend the use of any of the evaluated analgesic dosages in this mouse model of VT, buprenorphine merits additional investigation to ensure the highest level of laboratory animal care and welfare.
Abbreviations: hpf, high-power field; IVC, inferior vena cava; IQR, interquartile range; sP-sel, soluble P-selectin; TW, thrombus weight; VT, venous thrombosis
Venous thrombosis (VT) is among the most common vascular diseases and is a significant cause of morbidity and mortality in humans. Numerous risk factors have been identified including advanced age,42 neoplasia,50 and obesity,1 and approximately 275,000 new cases are diagnosed annually in the United States alone, with a recurrence rate of approximately 30%.20 Historically, the primary contributors toward the development of VT have been summarized as the Virchow Triad and include hypercoagulability, endothelial injury, and vascular stasis.45 During the last 4 decades, additional attention has been paid to the role that inflammation plays in the initiation, progression, and eventual resolution of VT, emphasizing endothelial cellular and molecular interactions.47 It is now understood that the endothelium plays a key role in maintaining an antithrombotic, vasodilatory state, in part through the production of nitric oxide and IL10, an antiinflammatory cytokine.5 After endothelial dysfunction, induced by either direct physical trauma or secondary to systemic proinflammatory conditions, a cascade of changes occurs that fosters a prothrombotic, vasoconstrictive environment. In particular, for VT, the upregulation of cell adhesion molecules like P-selectin on the endothelium lead to the adhesion of activated platelets and leukocytes, contributing to thrombus formation and inflammation. Leukocyte migration across the vascular wall, particularly by polymorphonuclear cells, further contributes to early endothelial injury. Over time, these same cells, acting in concert with monocytes, are important for effective postthrombosis remodeling and eventual resolution.33,40,47
As the underlying mechanisms of thrombus formation have been elucidated and novel antithrombotic therapies have been developed in response, animal models have played an ever increasing role in both basic and applied VT research.27 Although no single species perfectly recapitulates the human disease, and both rodent and nonrodent large animal models are important to the study of VT, murine models serve a crucial role in understanding the mechanisms of VT and evaluating novel therapies headed for clinical trials.34,44 Characterizing the effects of medications, such as analgesics, on these models is an important aspect of model refinement, particularly when the model has a surgical component.
Because of the difficulties in initiating thrombus formation, most models currently used in VT research involve some degree of surgical invasiveness to access the vessel endothelium or to induce partial stasis. Because of the limited utility of minimally invasive methods due to their small body size, the absence of spontaneously occurring disease, and the need to induce thrombus formation in larger vessels, rodents generally require more invasive surgical manipulation than do some large animal models. Although all rodent models used to study VT involve some degree of surgical intervention, only 2 techniques have been shown to produce consistent, reproducible thrombus formation with minimal postoperative mortality, the electrolytic injury model12 and ligation of the inferior vena cava49 (IVC), both of which require laparotomy. Consistent with the Guide for the Care and Use of Laboratory Animals,24 PHS policy,35 and the Animal Welfare Act,3 these surgical models warrant the use of analgesia to minimize postoperative discomfort and distress unless there is compelling scientific justification for the omission of pain-relieving medication. Historically, the use of analgesics has been questioned in several laparotomy models of diseases in which inflammation plays a significant role, including sepsis and VT. The justification for withholding analgesics in these scenarios may reflect concerns that these compounds alter inflammation, immune function, and coagulation and thereby introduce a confounding experimental variable.
Although these are valid and important considerations in preserving the integrity of study results, generalizations about an individual drug may ignore the variability seen among compounds within the same analgesic class.39 The routine clinical use of analgesics in human patients afflicted with these conditions further complicates the ethical dilemma when weighing the evidence against analgesic administration in laboratory species. Despite the widespread use of animal surgical models in VT research, very few species-specific data regarding the effects of analgesia on inflammation and thrombus formation are currently available in the literature, particularly in regard to the most commonly used VT surgical models. This type of work, as it relates to analgesic use in various species and specific models with a high potential for pain and distress, is crucial if IACUC and investigators are to ensure ethical care without compromise of study data. The current study characterized the effects of 4 analgesics with varied mechanisms of action on specific endpoints of the commonly used mouse IVC ligation model of deep vein thrombosis and compared these findings with data from other species and models in the published literature.
Materials and Methods
Animals.
This study used 10- to 12-wk-old male C57BL/6 mice (n = 150) with a mean body weight of 24.6 g (Charles River Laboratories, Portage, MI). Our laboratory has historically used male mice with the ligation model of VT, because we previously have shown that male mice produce larger thrombi and female mice are unsuitable due to the vascular anatomy of the uterus and ovaries.2 We use C57BL/6 mice in light of our extensive historical data and their use as the background strain for all of the gene-targeted models that we use in our laboratory.
Five mice were housed per ventilated cage (Allentown Caging, Allentown, NJ) on a 12:12-h light:dark cycle in a temperature- and humidity-controlled room in compliance with the recommendations of the Guide.24 Mice were fed a commercial rodent diet ad libitum (irradiated Labdiet 5LOD, PMI Nutrition International, Brentwood, MO), and had continuous access to reverse-osmosis–purified water via a rack-mounted continuous watering system. The health of the colony was evaluated quarterly by using dirty-bedding transfer to 3 male CD1 sentinel mice per 70 cages. All sentinels were negative for the following pathogens for the study duration: mouse hepatitis virus, mouse parvovirus, minute virus of mice, epizootic diarrhea of infant mice, ectromelia virus, Sendai virus, pneumonia virus of mice, Theiler murine encephalomyelitis virus, reovirus type 3, lymphocytic choriomeningitis virus, mouse adenovirus, polyoma virus, Mycoplasma pulmonis, cilia-associated respiratory bacillus, and murine pinworms (Aspiculuris and Syphacia). All animal procedures were approved by the University Committee on the Use and Care of Animals at the University of Michigan in compliance with the Guide.24
Experimental design.
Individual cages were assigned randomly to 1 of 5 experimental groups (n = 30 mice per treatment group) prior to undergoing surgery with a model of IVC ligation as described previously.49 All of the mice within a single cage received the same treatment and were harvested at the same study time point. Surgeries were performed on one cage of animals each day at approximately the same time in the morning, and cage order was randomized so that all treatment groups were equally represented temporally over the course of the study. The same person performed all of the surgeries, treatment administrations, and tissue collections to ensure consistency. Mice were allowed to recover, and tissues were collected by terminal harvest at either 6 or 48 h postoperatively (15 mice per time point for each treatment group). Mice were anesthetized by using isoflurane, the same inhalant anesthetic administered during surgery, for cardiocentesis and tissue collection.
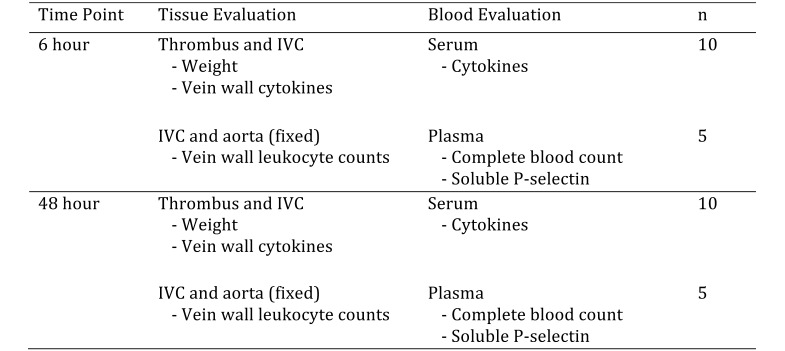
At each time point, blood was collected from 10 of the 15 mice from each group for serum cytokine analysis, and the IVC and thrombus were removed, weighed, and frozen individually for further analysis. For the remaining 5 mice in each treatment group for a given time point, blood was collected in EDTA tubes for hematology and soluble P-selectin analysis, and the IVC, thrombus, aorta, and surrounding tissues were removed for histologic evaluation (Figure 1).
Figure 1.
Allocation of mice to experimental groups. For each group, 30 mice were divided equally between 2 time points and then further assigned according to the type of tissue and blood sample that was collected for analysis.
Analgesic administration.
Mice were assigned randomly to 1 of 5 experimental groups and received either 1 of 3 parenteral analgesics or a local anesthetic or preservative-free 0.9% NaCl (no analgesia). The parenteral analgesia groups received buprenorphine (0.1 mg/kg; 0.3 mg/mL, Reckitt Benckiser Pharmaceuticals, Berkshire, UK), tramadol (20 mg/kg; 10 mg/mL, Wedgewood Pharmacy, Swedesboro, NJ), or carprofen (5 mg/kg; 50 mg/mL, Pfizer, New York, NY). All parenteral analgesics were administered subcutaneously beginning 30 min prior to surgery and continued twice daily for as long as 48 h. The local-anesthetic group received bupivacaine (5 mg/kg; 0.5%, Hospira, Lake Forest, IL) as a single subcutaneous injection on either side of the abdominal skin incision 3 to 5 min prior to initiation of surgery. All drugs were diluted with preservative-free 0.9% NaCl to produce a final volume of 0.2 mL per injection. So that the surgeon was blinded regarding treatment group, all mice received injections at the same sites and frequency. Preservative-free 0.9% NaCl was used for subsequent twice-daily injections in the local analgesia group. Animals in the no-analgesia group received preservative-free 0.9% NaCl for all injections during the course of the study.
Vein wall morphometrics.
The IVC and surrounding tissues were fixed in 10% formalin, paraffin-embedded and processed into slides, and stained with hematoxylin and eosin. A board-certified veterinary pathologist, blinded to the treatment groups, evaluated the slides and determined the number of leukocytes infiltrating the vein wall in 5 high-powered fields (hpf) on each slide. The leukocytes of interest included neutrophils, macrophages, and lymphocytes.
Vein wall and serum cytokine analysis.
The vein wall was separated from the thrombus, snap frozen in liquid nitrogen, and stored at −70 °C for later analysis. Blood (500 to 800 μL) was collected for serum cytokine analysis and held at room temperature in untreated microcentrifuge tubes (ThermoFisher Scientific, Waltham, MA) for at least 30 min until clot formation occurred. Samples then were spun at 2000 × g for 20 min. The serum was removed and stored at −70 °C until analysis. Vein wall samples were placed into 400 μL Complete Lysis Buffer (Roche, Indianapolis, IN), homogenized for 60 s, sonicated for 15 s, and then spun at 19,474 × g for 3 min. Vein wall homogenates and serum were assayed separately for IL1β, IL6, IL10, and TNFα by using a multiplex bead assay (EMD Millipore, Billerica, MA), and the protein concentration for each sample was determined by using the standard BCA protein assay (Pierce, Rockford, IL). Cytokine levels below the detection threshold were assigned a value at the lower detection limit. All cytokine results were normalized to the protein content of each sample.
Hematology.
Blood (500 μL) was collected via cardiocentesis and placed into microtainer EDTA tubes (Becton Dickson, Franklyn Lakes, NJ). A CBC was performed on each sample by using a HemaVet HV950 Multispecies Hematology Analyzer (Drew Scientific, Oxford, CT).
Mouse soluble P-selectin (sP-sel).
After a CBC, the EDTA samples were processed according to the manufacturer's recommendations by using a soluble P-selectin ELISA kit (R and D Systems, Minneapolis, MN). The protein in each sample was determined by using the standard BCA protein assay (Pierce) for normalization of the sP-sel levels.
Thrombus weight (TW).
At the time of tissue harvest, overlying connective tissue was carefully dissected from the IVC and removed with its associated thrombus from the level of the left renal vein to the bifurcation of the vena cava. The total weight of the IVC and thrombus was recorded in grams and served as a reference of thrombosis.
Statistical methods.
Statistical analysis was performed by using GraphPad Prism version 6.0 (GraphPad Software, La Jolla, CA). Data were analyzed for normality by using the D'Agostino and Pearson omnibus tests. The statistical significance of differences between the operated nonanalgesic group and each of the analgesic treatment groups was assessed for each variable at each time point by either parametric (unpaired t test with Welch's correction) or nonparametric (Mann–Whitney test) methods. Significant outliers were identified in continuous sets of data using the extreme studentized deviate test (Grubbs test, α = 0.05) and were not included in the statistical analysis. A P value of 0.05 or less was considered significant for all statistical comparisons. All parametric and nonparametric data are reported as mean ± SEM or the median and the interquartile range (IQR), respectively.
The decision to perform individual t tests, rather than use a statistical method that would correct for multiple comparisons (that is, ANOVA or Kruskal–Wallis), increases the risk of type 1 errors but minimizes the likelihood of missing true significant differences associated with analgesic treatment, thereby increasing the overall power of the study.18
Results
Vein wall morphometrics.
Thrombus formation resulted in a progressive inflammatory process in the vein wall as evidenced by local leukocyte infiltration. Comparing analgesic treatment groups to the operated mice without pain management at both time points revealed 5 differences in vein wall cell counts. Vein wall neutrophil counts were significantly reduced at both 6 h (median, 4.5; IQR, 1.0 to 8.5; compared with median, 12.0; IQR, 5.5 to 24.5 cells/hpf; P = 0.0035) and 48 h (median, 7.0; IQR, 3.0 to 11.5; compared with median, 14.5; IQR, 8.0 to 17.5 cells/hpf; P = 0.0216), in mice receiving carprofen compared with the nonanalgesic groups. Vein wall macrophage numbers at 48 h were significantly reduced in the buprenorphine (median, 2.0; IQR, 1.0 to 2.5 cells/hpf; P = 0.0049), tramadol (median, 2.0; IQR, 1.0 to 3.0 cells/hpf; P = 0.0325), and carprofen (median, 1.5; IQR, 1.0 to 3.0 cells/hpf; P = 0.0167) groups compared with the nonanalgesic group (median, 3.0; IQR, 2.0 to 4.0 cells/hpf; Table 1).
Table 1.
Effects of analgesics on parameters related to thrombosis
| No analgesia | Bupivacaine | Buprenorphine | Tramadol | Carprofen | |
| Vein wall leukocytes (no. of cells/high-power field) | |||||
| 6 h | |||||
| Neutrophils | 12.0; 5.5–24.5 | ↓ 8.0; 2.0–15.8 | ↑ 8.0; 1.5–31.5 | ↓ 5.5; 1.0–11.5 | ↓ 4.5; 1.0–8.5 |
| Macrophages | 1.0; 0.0–1.0 | ↑ 1.0; 0.0–2.0 | 1.0; 0.0–1.0 | ↓ 0.0; 0.0–1.0 | ↑ 0.0; 0.0–2.0 |
| Lymphocytes | 1.0; 0.0–2.0 | 1.0; 0.0–2.0 | ↑ 1.0; 0.0–2.5 | ↑ 1.0; 0.5–3.0 | ↑ 1.0; 0.0–3.0 |
| 48 h | |||||
| Neutrophils | 14.5; 8.0–17.5 | ↑ 14.0; 4.3–21.8 | ↓ 11.0; 7.0–19.3 | ↑ 18.0; 9.5–44.0 | ↓ 7.0; 3.0–11.5 |
| Macrophages | 3.0; 2.0–4.0 | ↓ 2.0; 1.0–3.0 | ↓ 2.0; 1.0–2.5 | ↓ 2.0; 1.0–3.0 | ↓ 1.5; 1.0–3.0 |
| Lymphocytes | 3.0; 1.0–4.0 | ↓ 2.0; 1.0–3.5 | ↓ 2.0; 2.0–3.0 | ↓ 2.0; 1.0–3.0 | ↓ 3.0; 2.0–4.0 |
| Vein wall cytokines (pg/μg protein) | |||||
| 6 h | |||||
| IL1β | 18.9 ± 3.4 | ↑ 18.9 ± 4.3 | ↑ 20.1 ± 4.8 | ↑ 20.0 ± 5.8 | ↑ 22.4 ± 7.2 |
| IL6 | 4059.5 ± 545.9 | ↑ 4106.6 ± 850.7 | ↑ 5446.5 ± 1061.8 | ↑ 10066.9 ± 3144.7 | ↓ 3395.2 ± 682.2 |
| IL10 | 32.8 ± 7.0 | ↓ 17.2 ± 2.4 | ↓ 30.9 ± 3.7 | ↑ 62.4 ± 19.9 | ↓ 31.4 ± 5.6 |
| 48 h | |||||
| IL1β | 14.0 ± 3.4 | ↑ 16.3 ± 3.9 | ↑ 18.4 ± 2.8 | ↓ 11.5 ± 2.2 | ↑ 20.7 ± 5.1 |
| IL6 | 596.5 ± 79.6 | ↓ 330.6 ± 45.3 | ↓ 449.6 ± 100.4 | ↓ 444.0 ± 53.9 | ↓ 249.1 ± 64.0 |
| IL10 | 20.4; 14.1–31.6 | ↓ 13.1; 10.1–20.1 | ↑ 17.7; 7.1–49.8 | ↓ 10.9; 9.2–26.0 | ↓ 13.0; 9.3–19.5 |
| Circulating IL6 (pg/μg protein) | |||||
| 6 h | 21.6 ± 4.6 | ↓ 17.2 ± 2.4 | ↓ 11.5 ± 1.6 | ↓ 11.7 ± 2.4 | ↓ 9.7 ± 1.7 |
| 48 h | 1.2; 1.0–1.4 | ↑ 2.1; 1.1–2.9 | ↑ 1.6; 1.2–3.8 | ↑ 2.6; 1.4–4.2 | ↑ 1.1; 0.7–1.9 |
| CBC (× 103 cells/μL) | |||||
| 6 h | |||||
| WBC | 4.42; 3.22–4.83 | ↓ 4.02; 3.23–4.69 | ↑ 4.75; 3.51–6.33 | ↓ 3.82; 2.46–4.31 | ↑ 5.22; 4.79–5.41 |
| Neutrophils | 3.04; 2.40–3.58 | ↑ 2.82; 2.09–3.44 | ↑ 3.45; 2.68–4.65 | ↑ 2.03; 1.82–3.15 | ↑ 3.72; 3.58–4.01 |
| Lymphocytes | 0.92; 0.67–1.20 | ↑ 0.88; 0.68–1.26 | ↑ 1.13; 0.71–1.43 | ↓ 1.03; 0.53–1.22 | ↑ 1.22; 0.96–1.70 |
| Monocytes | 0.11; 0.10–0.20 | ↑ 0.17; 0.11–0.32 | ↑ 0.12; 0.05–0.19 | ↓ 0.07; 0.06–0.08 | ↓ 0.17; 0.11–0.29 |
| Platelets | 626; 576–659 | ↑ 683; 604–835 | ↑ 620; 572–722 | ↓ 478; 347–730 | ↑ 671; 635–708 |
| 48 h | |||||
| WBC | 4.16; 2.45–4.80 | ↑ 6.34; 3.88–6.74 | ↓ 2.82; 2.06–4.21 | ↓ 3.24; 2.21–3.95 | ↓ 3.12; 2.50–4.80 |
| Neutrophils | 1.76; 1.25–1.86 | ↑ 3.09; 1.70–3.64 | ↓ 1.54; 1.07–2.05 | ↓ 1.14; 0.90–1.51 | ↓ 1.03; 0.76–1.55 |
| Lymphocytes | 2.20; 1.15–2.89 | ↑ 2.60; 2.09–3.21 | ↓ 1.25; 0.93–2.04 | ↓ 1.82; 1.12–2.07 | ↑ 1.89; 1.56–2.95 |
| Monocytes | 0.08; 0.05–0.10 | ↓ 0.07; 0.06–0.09 | ↓ 0.05; 0.04–0.11 | ↑ 0.28; 0.12–0.37 | ↑ 0.23; 0.13–0.27 |
| Platelets | 359; 281–485 | ↑ 430; 367–549 | ↑ 484; 411–626 | ↓ 324; 277–364 | ↓ 307; 295–337 |
| Hct (%) | |||||
| 6 h | 38.4; 38.2–38.8 | ↑ 39.6; 38.6–41.5 | 38.8; 37.9–40.8 | ↓ 38.7; 35.2–40.9 | ↑ 42.1; 38.5–44.3 |
| 48 h | 36.2; 35.3–39.4 | ↓ 34.1; 31.6–35.8 | ↓ 31.6; 31.0–33.6 | ↓ 31.0; 27.4–34.2 | ↓ 36.9; 35.0–37.1 |
| Soluble P-selectin (pg/μg protein) | |||||
| 6 h | 6.13; 5.90–7.67 | ↓ 5.78; 5.35–6.09 | ↑ 9.95; 6.62–10.90 | ↑ 8.47; 7.80–10.31 | ↑ 7.22; 6.29–7.73 |
| 48 h | 9.98; 8.94–11.39 | ↑ 11.99; 8.84–12.23 | ↑ 9.76; 8.43–13.11 | ↑ 13.63; 10.16–13.88 | ↓ 9.73; 9.31–10.89 |
| Thrombus weight (mg) | |||||
| 6 h | 13.1 ± 0.7 | ↑ 17.6 ± 1.4 | ↑ 13.9 ± 1.5 | ↑ 15.6 ± 1.2 | ↓ 12.3 ± 4.2 |
| 48 h | 30.5 ± 1.2 | ↓ 27.6 ± 1.3 | ↓ 27.5 ± 1.3 | ↓ 26.7 ± 1.3 | ↓ 26.6 ± 1.1 |
Results reported as mean ± SEM for parametric data and median and interquartile range for nonparametric data. Bold text indicates values that are significantly (P < 0.05) different from that of the no-analgesia group.
Vein wall cytokines.
IL1β, IL6, and IL10 were present at detectable levels in the majority of the vein wall homogenate samples at both the 6- and 48-h time points, whereas TNFα was below the detection threshold in all assayed samples. In general, the cytokine results were higher for IL6 and at or near the low end of the detection range for IL1β and IL10. At 6 h, none of the treatment groups differed significantly from the no-analgesia group for any of the 3 measurable cytokines. Similarly, there were no apparent treatment effects on IL1β or IL10 at 48 h. Bupivacaine (330.6 ± 45.3 pg/μg protein, P = 0.0113) and carprofen (249.1 ± 64.0 pg/μg protein, P = 0.0037) reduced vein wall IL6 concentrations at 48 h compared with that of the no-analgesia group (596.5 ± 79.6 pg/μg protein; Table 1).
Serum cytokine analysis.
IL6 levels were present at detectable levels in most of the serum samples and showed marked decreases between the 6- and 48-h time points (Table 1). IL1β, IL10, and TNFα were consistently below the detection limits of the assay for most of the serum samples.
At 6 h after surgery, IL6 levels were significantly lower in the mice receiving carprofen (9.7 ± 1.7 pg/μg protein; P = 0.0379) compared with those with no analgesic treatment (21.6 ± 4.6 pg/μg protein). Mice in the tramadol group (median, 2.6; IQR, 1.4 to 4.2 pg/μg protein; P = 0.0056) had increased serum IL6 at 48 h after surgery, compared with those without analgesics (median, 1.2; IQR, 1.0 to 1.4 pg/μg protein; Table 1).
Hematology.
Peripheral leukocyte counts.
Among surgical groups, treatment did not significantly affect circulating numbers of neutrophils or lymphocytes. Total WBC at 6 h was higher in the carprofen group (median, 5.22; IQR, 4.79 to 5.41) compared with mice that did not receive any analgesia (median, 4.42; IQR, 3.22 to 4.83 cells × 103/μL; P = 0.0397). Depression of peripheral monocyte numbers occurred at 6 h in mice receiving tramadol (median, 0.07; IQR, 0.06 to 0.08) compared with those receiving no analgesia (median, 0.11; IQR, 0.10 to 0.20 × 103 cells/μL; P = 0.0079). Circulating monocyte numbers were significantly (P = 0.0238) increased at 48 h in the carprofen group (median, 0.23; IQR, 0.13 to 0.27) compared with no analgesia (median, 0.08; IQR, 0.05 to 0.10 cells/μL; Table 1).
Circulating erythrocytes and platelets.
Treatment had no effect on Hct at 6 h after surgery compared with the no-analgesia group. Mice receiving buprenorphine (median, 31.6; IQR, 31.0% to 33.6%; P = 0.0079) and tramadol (median, 31.0; IQR, 27.4% to 34.2%; P = 0.0079) had values that were significantly lower than did animals that did not receive analgesia (median, 36.2; IQR, 35.3% to 39.4%; Table 1).
All treatment groups demonstrated a marked reduction in platelets between 6 and 48 h as thrombus formation progressed, but there were no significant differences between the no-analgesia group and any of the treatment groups at either time point (Table 1).
Mouse soluble P-selectin.
At 6 h after surgery, sP-sel levels were significantly (P = 0.0159) higher in mice receiving tramadol (median, 8.47; IQR, 7.80 to 10.31) than in those that did not receive analgesia (median, 6.13; IQR, 5.90 to 7.67 pg/μg protein). There were no significant differences in circulating sP-sel levels between the no-analgesia group and any of the analgesic treatment groups at 48 h (Table 1).
Thrombus weight.
Mice receiving bupivacaine analgesia had significantly increased TW at 6 h after ligation than did animals receiving no analgesia (17.6 ± 1.4 compared with 13.1 ± 0.7 mg; P = 0.0116). Compared with the no-analgesia group (30.5 ± 1.2 mg) at 48 h, TW were lower in mice treated with tramadol (26.7 ± 1.3 mg; P = 0.0487) and carprofen (26.6 ± 1.1 mg; P = 0.0264). There were no other significant differences at either time point when groups that received an analgesic were compared with those that did not (Table 1).
Discussion
Effects of analgesics on leukocyte infiltration into vein walls.
The role of leukocytes in thrombus formation and resolution is well documented in experimental VT. It begins with the initial local response to endothelial activation, when P-selectin expression on platelets and the endothelial surface is increased. Receptors present on the surface of both leukocytes and platelets, called P-selectin glycoprotein ligands, bind to the newly expressed P-selectin and attach to the endothelium at the site of injury as well as throughout the developing thrombus matrix.14 This process also stimulates the attached leukocytes (especially monocytes), platelets, and endothelium to produce microparticles, prothrombotic phospholipids that contribute significantly to ongoing thrombus formation.9,15 The progression of leukocyte migration to the site of thrombus formation is similar to that observed in other inflammatory processes—specifically, polymorphonuclear cells arrive at the early stages of the disease followed by large numbers of monocytes and small numbers of lymphocytes. In the early stages of thrombus resolution, polymorphonuclear cells contribute by promoting both fibrinolysis and collagenolysis, whereas at the late stages, monocytes play a crucial role in remodeling and resolution of the mature thrombus.23 Because the presence of leukocytes within the thrombus and vein wall are critical to the pathogenesis of VT, we particularly were concerned about the potential effects of various analgesics on leukocyte function and migration.
Local anesthetics, as a class, have known effects on the migration and function of leukocytes,21 although the effects are seen more frequently in association with high doses, systemic rather than local administration, or in in vitro assays. Bupivacaine seems to be less likely to affect neutrophil function than do other local anesthetics,30 and although both lidocaine and bupivacaine were found to alter local inflammatory factors, neither resulted in an impairment of tissue remodeling when evaluated in a mouse model of wound healing.46 There is evidence that subcutaneous local anesthetic administration can affect inflammation at a distant anatomic site,29 but the referenced study used lidocaine rather than bupivacaine. The results of our current work are in concordance with the majority of the evidence in the literature and confirmed no significant effect of bupivacaine on leukocyte infiltration after surgery.
Several recent reviews37,39 have addressed the antiinflammatory and immunomodulatory properties of opioids, specifically the differences between the immunosuppressive effects of those exhibiting strong activity at the µ receptor (morphine, fentanyl) and those with weaker µ affinity that appear to have little effect (buprenorphine) or even immunostimulatory effects (tramadol). Decreased numbers of vein wall monocytes at 48 h in mice that received buprenorphine and tramadol were the only opioid-associated effect that we noted in the vein wall leukocyte counts. To the best of our knowledge, there are no prior reports in the literature of buprenorphine or tramadol inhibiting monocyte migration in vivo. A study of the effects of buprenorphine on human neutrophil chemotaxis in vitro revealed a dose-dependent inhibitory effect31 and morphine suppressed the in vitro migration of macaque neutrophils and monocytes in response to IL8.32 In contrast, monocyte migration in rats was unaffected by tramadol, a weak µ agonist.6 In fact, several published reviews37,39 have suggested that buprenorphine, as a partial µ agonist, has minimal effect on immune function, including leukocyte migration. This conclusion is supported by 2 studies that used a murine cecal ligation-and-puncture model of sepsis and found no difference in monocyte numbers in peritoneal fluid after surgery with and without buprenorphine.11,22 In addition, one of these studies22 also noted a transient increase in airway macrophage numbers in mice that received either tramadol or buprenorphine. Taken together, these findings imply that the effects on chemotaxis are not only drug- and dose-specific but may also depend on the anatomic location and degree of the injury as well as the type of inflammatory cell of interest.
As a category, NSAID have been shown to suppress leukocyte function, chemotaxis, and endothelial migration in a dose-related fashion,16 and carprofen specifically reduced neutrophil and total leukocyte accumulations in experimentally induced subcutaneous airspaces in a rat model.13 Of the 4 analgesics that we selected for the current study, carprofen has the most well documented and potent antiinflammatory effects; it therefore is not surprising our study yielded similar findings. Carprofen had the greatest effect on leukocyte migration of any treatment we evaluated and reduced polymorphonuclear cellular infiltration at both time points and numbers of vein-wall monocytes at 48 h. The observation that carprofen does not affect TW at 6 h but does result in a smaller thrombus at 48 h suggests that the impairment of thrombus formation is due in part to decreased inflammation and consequently smaller thrombi.
An additional contribution to the apparent antithrombotic effect of carprofen that we noted is its potential effect on the proinflammatory cytokine concentrations in the vein wall. Evaluation of the IVC vein wall at 48 h showed a significant reduction in IL6 compared with that in the no-analgesia group. IL6 has been associated with the development of deep vein thrombosis and intimal fibrosis, and neutralization of IL6 in a mouse IVC ligation model of VT significantly reduced the weight of the resulting thrombus at 48 h after surgery.49 Attenuation of IL6 production by carprofen occurred in an equine tissue culture model of osteoarthritis4 and a rodent model of traumatic brain injury.41 The combined effects of carprofen on monocyte migration and IL6 production may contribute to the reduction in thrombosis that we found in this study. We saw similar reductions in vein wall IL6 without a concurrent decrease in TW in mice that received bupivacaine.
Carprofen's effect on IL6 does not appear to be limited to the vein wall, as significant reductions in serum IL6, relative to animals not receiving analgesia, were observed at the 6 h time point. Tramadol was the only other treatment that had any significant effect on circulating IL6 and increased serum levels at 48 h after surgery. In both of these instances, the difference we noted was inconsistent with the findings of published studies evaluating similar effects.28,36,48 Importantly, all of the previous studies lacked parity with ours with regard to the model and species investigated, thus highlighting the challenges of extrapolating data between species and confirming the need for model-specific investigations.
Although the effect of leukocyte infiltration at the site of injury has been established, the role of circulating leukocytes on the course of disease is less certain. In the current study, only tramadol and carprofen affected blood leukocyte numbers, and these effects primarily were limited to monocytes. Tramadol was associated with a reduction in monocytes at 6 h but not 48 h, in contrast to a murine CLP study,22 which found decreases in peripheral monocytes only at 48 h and at a significantly higher dose than that we used. Carprofen increased the circulating WBC count at 6 h and monocyte count at 48 h; effects that seem counterintuitive and difficult to explain for a compound with well-documented antiinflammatory properties. Although these differences achieved statistical significance between groups, the small sample size for each experimental group (n = 5) warrants cautious interpretation of these findings, especially in light of an uncertain mechanism and the lack of previously published data revealing similar effects on leukocyte numbers.
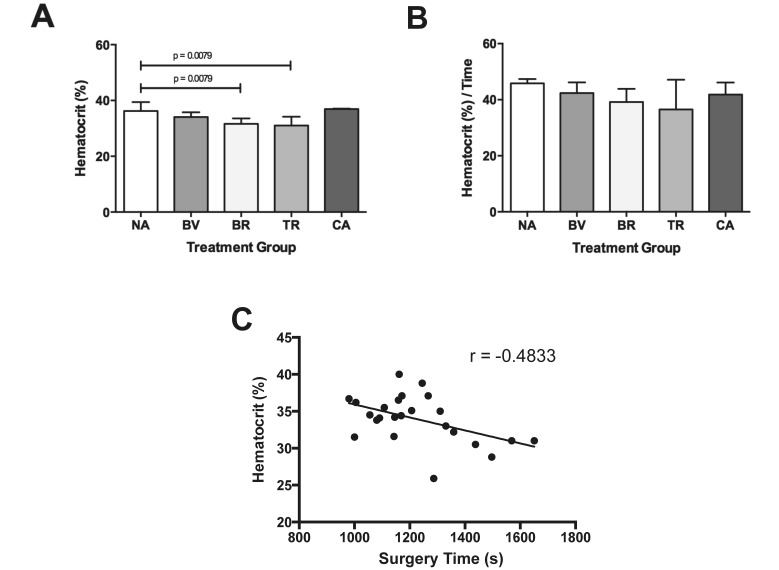
The only other significant hematologic effect of analgesia appeared to be a reduction in circulating erythrocyte mass at 48 h in both opioid groups. Because none of the animals demonstrated evidence of overt postoperative hemorrhage or hemolysis and given that neither of the implicated analgesic classes are routinely associated with bone marrow suppression, the cause of the relative reduction is difficult to explain. A variable that does show moderate correlation with the decreased Hct is the duration of surgery (Figure 2). Additional investigation is needed to better characterize this unexpected result.
Figure 2.
Significant differences in (A) Hct at 48 h are eliminated when (B) Hct is normalized to surgery time (s). (C) The Hct at 48 h shows an inverse correlation with the duration of surgery (P = 0.0167) Treatment groups are no analgesia (NA), bupivacaine (BV), buprenorphine (BR), tramadol (TR), and carprofen (CA). Results are reported as median and interquartile range.
As previously mentioned, sP-sel plays a critical role throughout the process of thrombus formation. Not only does membrane-associated sP-sel facilitate the recruitment of inflammatory cells to the site of endothelial injury, it also plays important roles in the promotion of coagulation, fibrin deposition, and eventually increased thrombus mass and stability. Studies have shown that sP-sel plays an active part in leukocyte recruitment and subsequent increases in microparticles, which contain tissue factor, an important component of the extrinsic coagulation pathway leading to thrombin production.9,38 Another consequence of increased sP-sel expression by the endothelium and activated platelets is a concurrent rise in sP-sel in the plasma that is proportional to the degree of injury as well as the thrombotic potential.9
In general, analgesic therapy did not have a marked effect on sP-sel concentrations in plasma, and the only effect that was statistically different from the value obtained from the no-analgesia group was that for the tramadol group at 6 h. Although the difference in sP-sel concentrations between tramadol and no analgesia at 48 h did not achieve statistical significance, there was a trend (P = 0.0952) toward increased levels at this time point as well.
Interestingly, the expected consequence of increased sP-sel, increased TW, was not observed in the tramadol groups. Instead, mice given tramadol had smaller thrombi at 48 h compared with those without analgesia. Tramadol's observed antiinflammatory properties6 and potential to inhibit the procoagulant actions of serotonin17,19 and norepinephrine43 could account for the reduction in TW that we noted. Although these effects may not lead to clinically relevant alterations in hemostasis,8,25 they are important considerations in the decision to use tramadol in models of thrombosis. Like tramadol, carprofen reduced TW at 48 h, and in this case is a finding that is consistent with its documented effects on inflammation and hemostasis.7
One aspect of the results that was difficult to understand was the significant increase in TW at 6 h in mice that received bupivacaine. Of all of the analgesic groups, those receiving bupivacaine seemed the least likely to show increased thrombus formation, given the local nature of administration, as well as the documented antithrombotic properties of local anesthetics both in vitro26 and after intravenous administration in human patients.10 In our mouse study, bupivacaine did not affect any of the other measured parameters in a manner consistent with increased TW at the same time point. Although the use of bupivacaine led to an increase in serum IL6 and a reduction in vein-wall IL6 at 48 h, neither change correlates temporally with the observation of increased TW at the earlier time point. Coupled with the common clinical observation that local anesthetics reduce thrombosis and inflammation, the results we obtained in this study deserve additional investigation and characterization.
The primary goal of this study was to evaluate the effects of common analgesics on inflammation and hemostasis in mice, specifically within the context of a surgical model of VT. According to our results, none of the analgesic candidates are completely devoid of effects on important variables relevant to the ligation model of VT, and their use could be problematic at the doses and intervals used here. However, rather than eliminating all of the selected analgesics from further consideration in light of the potential to affect a single variable, these results serve as an opportunity to pursue additional investigation of those medications that had fewer effects. In this study, our choices to use analgesic doses at the upper end of the recommended range, the use of 48 h of therapy, and the incorporated method of statistical analysis were intended to provide a conservative evaluation of the effects of these compounds and discern a possible response. This practice facilitates the selection of one or more analgesics for additional investigation by using a dose-response approach to determine whether a lower dose or a shorter duration of therapy would provide a measure of pain relief without compromising the scientific integrity of the model. Similarly, various analgesics can be removed from consideration when numerous or pronounced effects on research variables are noted, thereby preventing the use of excessive animal numbers and financial resources.
Carprofen was selected for evaluation despite its classification as a NSAID due to its widespread use, demonstrated efficacy, and lack of regulatory burden as a noncontrolled substance. Not unexpectedly, carprofen affected the greatest number of study parameters and should not be considered for VT studies because of its significant effects on cytokine levels, leukocyte chemotaxis, and thrombus formation. Tramadol has experienced increased use in laboratory animal medicine as a noncontrolled analgesic drug with several mechanisms of action, including activation of opioid receptors. A review of the literature suggests that tramadol has a limited effect on inflammation and immune response. However, in the current study, compared with the no-analgesia group, tramadol-treated mice showed a reduction in TW and alterations in serum IL6, sP-sel levels, and circulating monocytes, thus limiting the utility of tramadol in this particular model of VT.
We expected that bupivacaine, as a local anesthetic, would prove to be an effective analgesic without significant effects on our model. Few published in vivo studies have examined the effects of bupivacaine on hemostasis and inflammation, and we noted few differences on these parameters. However, the increased TW and effects on IL6 coupled with bupivicaine's relatively short duration of action reduce the promise of this modality in the current murine laparotomy plus IVC ligation model. Additional research to elucidate the mechanisms underlying our findings is warranted for this class of drugs and specifically for bupivacaine. At our institution, due to its efficacy, safety margin, and favorable dosing interval, buprenorphine is the most commonly used opioid in rodent species. Reports in both the human and veterinary literature have characterized buprenorphine as having minimal effects on the immune system and inflammation, although there are no published data regarding its effects on hemostasis. In our study, the reduction in vein wall macrophage infiltration represents a significant component of thrombosis pathophysiology that could affect investigators’ results at the dose and interval evaluated here.
In light of our study's results, we do not recommend the routine use of any of the evaluated analgesics at the doses used in the murine IVC ligation model. In addition, due to the variety and number of the parameters they affected in this study and considering their potential antiinflammatory action, neither carprofen nor tramadol should be used in mouse models of thrombosis. Buprenorphine, although not appropriate for use in a murine IVC ligation model at the dose and interval that we evaluated, may be a reasonable candidate for future studies using a different doses or administration interval. We invite investigative groups to continue to evaluate the suitability of selected analgesics for their own models, especially when model-specific scientific justification for withholding analgesics is not well documented. Historically, our laboratory has not used analgesics in VT models because of potential confounding effects on research outcomes like those shown in the present study. Yet we believe that model-specific studies like the current one are necessary for challenging current analgesic dogma while ensuring the highest level of laboratory animal care and welfare.
Acknowledgments
Funding for this project was provided by the Unit for Laboratory Animal Medicine at the University of Michigan and by the Conrad Jobst Foundation. Gerry Hish's participation in the comparative medicine training program was sponsored by the Unit for Laboratory Animal Medicine. Special thanks go to Joel Whitfield at the University of Michigan Immunology core for his extensive guidance and advice related to the performance of the multiplex bead assays and to Shirley Wrobleski for providing surgical training and participating in the preparation of this manuscript.
References
- 1.Allman-Farinelli MA. 2011. Obesity and venous thrombosis: a review. Semin Thromb Hemost 37:903–907 [DOI] [PubMed] [Google Scholar]
- 2.Alvarado CM, Diaz JA, Hawley AE, Wrobleski SK, Sigler RE, Myers DD., Jr 2011. Male mice have increased thrombotic potential: sex differences in a mouse model of venous thrombosis. Thromb Res 127:478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Animal Welfare Act as Amended 2008. USC §2131–2159. [Google Scholar]
- 4.Armstrong S, Lees P. 2002. Effects of carprofen (R and S enantiomers and racemate) on the production of IL1, IL6, and TNFα by equine chondrocytes and synovicytes. J Vet Pharmacol Ther 25:145–153 [DOI] [PubMed] [Google Scholar]
- 5.Becker BF, Heindl B, Kupatt C, Zahler S. 2000. Endothelial function and hemostasis. Z Kardiol 89:160–167 [DOI] [PubMed] [Google Scholar]
- 6.Bianchi M, Rossoni G, Sacerdote P, Panerai AE. 1999. Effects of tramadol on experimental inflammation. Fundam Clin Pharmacol 13:220–225 [DOI] [PubMed] [Google Scholar]
- 7.Brainard BM, Meredith CP, Callan MB, Budsberg SC, Schofer FS, Driessen B, Otto CM. 2007. Changes in platelet function, hemostasis, and prostaglandin expression after treatment with nonsteroidal antiinflammatory drugs with various cyclooxygenase selectivities in dogs. Am J Vet Res 68:251–257 [DOI] [PubMed] [Google Scholar]
- 8.Brondani JT, Luna SPL, Marcello GCG, Padovani CR. 2009. Perioperative administration of vedaprofen, tramadol, or their combination does not interfere with platelet aggregation, bleeding time and biochemical variables in cats. J Feline Med Surg 11:503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cambien B, Wagner DD. 2004. A new role in hemostasis for the adhesion receptor P-selectin. Trends Mol Med 10:179–186 [DOI] [PubMed] [Google Scholar]
- 10.Cooke ED, Lloyd MJ, Bowcock SA, Pilcher MF. 1977. Intravenous lignocaine in prevention of deep venous thrombosis after elective hip surgery. Lancet 310:797–799 [DOI] [PubMed] [Google Scholar]
- 11.Cotroneo TM, Hugunin KMS, Shuster KA, Hwang HJ, Kakaraparthi BN, Nemzek-Hamlin JA. 2012. Effects of buprenorphine on a cecal ligation-and-puncture model in C57BL/6 mice. J Am Assoc Lab Anim Sci 51:357–365 [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz JA, Hawley AE, Alvarado CM, Berguer AM, Baker NK, Wrobleski SK, Wakefield TW, Lucchesi BR, Myers DD., Jr 2010. Thrombogenesis with continuous blood flow in the inferior vena cava: a novel mouse model. Thromb Haemost 104:366–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Essien BE, Kotiw M. 2012. Anti-inflammatory activity of hyperimmune plasma in a lipopolysaccharide-mediated rat air pouch model of inflammation. Inflammation 35:58–64 [DOI] [PubMed] [Google Scholar]
- 14.Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, Celi A, Croce K, Furie BC, Furie B. 2003. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med 197:1585–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furie B, Furie BC. 2004. Role of platelet P-selectin and microparticle PSGL1 in thrombus formation. Trends Mol Med 10:171–178 [DOI] [PubMed] [Google Scholar]
- 16.Furst SM, Khan KN, Komocsar WJ, Fan L, Mennear J. 2005. Screening new drugs for immunotoxic potential: II. Assessment of the effects of selective and nonselective COX2 inhibitors on complement activation, superoxide anion production and leukocyte chemotaxis and migration through endothelial cells. J Immunotoxicol 2:85–96 [DOI] [PubMed] [Google Scholar]
- 17.Galan AM, Lopez-Vilchez I, Diaz-Ricart M, Navalon F, Gomez E, Gasto C, Escolar G. 2009. Serotonergic mechanisms enhance platelet-mediated thrombogenicity. Thromb Haemost 102:511–519 [DOI] [PubMed] [Google Scholar]
- 18.Graphpad PRISM [Internet]. T tests after one-way ANOVA, without correction for multiple comparisons. [Cited 2 July 2014]. Available at http://http://www.graphpad.com/support/faq/t-tests-after-one-way-anova-without-correction-for-multiple-comparisons/
- 19.Hallback I, Hagg S, Eriksson AC, Whiss PA. 2012. In vitro effects of serotonin and norepinephrine reuptake inhibitors on human platelet adhesion and coagulation. Pharmacol Rep 64:979–983 [DOI] [PubMed] [Google Scholar]
- 20.Heit JA. 2008. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 28:370–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollomann MW, Durieux ME. 2000. Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology 93:858–875 [DOI] [PubMed] [Google Scholar]
- 22.Hugunin KM, Fry C, Shuster K, Nemzek JA. 2010. Effects of tramadol and burprenorphine on select immunologic factors in a cecal ligation and puncture model. Shock 34:250–260 [DOI] [PubMed] [Google Scholar]
- 23.Humphries J, McGuinness CL, Smith A, Waltham M, Poston R, Burnand KG. 1999. Monocyte chemotactic protein 1 (MCP1) accelerates the organization and resolution of venous thrombi. J Vasc Surg 30:894–899 [DOI] [PubMed] [Google Scholar]
- 24.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press [Google Scholar]
- 25.Isik B, Arslan M, Ozsoylar O, Akcabay M. 2009. Effects of preoperative lornoxicam versus tramadol on postoperative pain and side effects in adult tonsillectomy patients. Agri 21:113–120 [PubMed] [Google Scholar]
- 26.Kohrs R, Hoenemann CW, Feirer N, Durieux ME. 1999. Bupivacaine inhibits whole blood coagulation in vitro. Reg Anesth Pain Med 24:326–330 [DOI] [PubMed] [Google Scholar]
- 27.Levi M, Dorffler-Melly J, Johnson GJ, Drouet L, Badimon L. 2001. Usefulness and limitations of animal models of venous thrombosis. Thromb Haemost 86:1331–1333 [PubMed] [Google Scholar]
- 28.Liu YM, Zhu SM, Wang KR, Feng ZY, Chen QL. 2008. Effect of tramadol on immune responses and nociceptive thresholds in a rat model of incisional pain. J Zhejiang Univ Sci B 9:895–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCafferty DM, Sharkey KA, Wallace JL. 1994. Beneficial effects of local or systemic lidocaine in experimental colitis. Am J Physiol 266:G560–G567 [DOI] [PubMed] [Google Scholar]
- 30.Mikawa K, Akamatsu H, Nishina K, Shiga M, Obara H, Niwa Y. 2003. Effects of ropivacaine on human neutrophil function: comparison with bupivacaine and lidocaine. Eur J Anaesthesiol 20:104–110 [DOI] [PubMed] [Google Scholar]
- 31.Mikawa K, Akamatsu H, Nishina K, Uesugi T, Niwa Y. 2006. The effects of pentazocine, buprenorphine and butorphanol on human neutrophil functions. Acta Anaesthesiol Scand 50:643–644 [DOI] [PubMed] [Google Scholar]
- 32.Miyagi T, Chuang LF, Lam KM, Kung H, Wang JM, Osburn BI, Chuang RY. 2000. Opioids suppress chemokine-mediated migration of monkey neutrophils and monocytes—an instant response. Immunopharmacology 47:53–62 [DOI] [PubMed] [Google Scholar]
- 33.Modarai B, Burnand KG, Humphries J, Waltham M, Smith A. 2005. The role of neovascularisation in the resolution of venous thrombus. Thromb Haemost 93:801–809 [DOI] [PubMed] [Google Scholar]
- 34.Myers DD., Jr 2012. Nonhuman primate models of thrombosis. Thromb Res 129:S65–S69 [DOI] [PubMed] [Google Scholar]
- 35.Office of Laboratory Animal Welfare [Internet]. 2002. Public health service policy on humane care and use of laboratory animals. [Cited 2 July 2014]. Available at http://grants.nih.gov/grants/olaw/references/phspol.htm
- 36.Pang WY, Earley B, Murray M, Sweeney T, Gath V, Crowe MA. 2011. Banding or burdizzo castration and carprofen administration on peripheral leukocyte inflammatory cytokine transcripts. Res Vet Sci 90:127–132 [DOI] [PubMed] [Google Scholar]
- 37.Pergolizzi J, Aloisi AM, Dahan A, Filitz J, Langford R, Likar R, Mercadante S, Morlion B, Raffa RB, Sabatowski R, Sacerdote P, Torres LM, Weinbroum AA. 2010. Current knowledge of buprenorphine and its unique pharmacologic profile. Pain Pract 10:428–450 [DOI] [PubMed] [Google Scholar]
- 38.Polgar J, Matuskova J, Wagner DD. 2005. The P-selectin–tissue factor–coagulation triad. J Thromb Haemost 3:1590–1596 [DOI] [PubMed] [Google Scholar]
- 39.Sacerdote P. 2006. Opioids and the immune system. Palliat Med 20:s9–s15 [PubMed] [Google Scholar]
- 40.Saha P, Humphries J, Modarai B, Mattock K, Waltham M, Evans CE, Ahmad A, Patel AS, Premaratne S, Lyons OT, Smith A. 2011. Leukocytes and the natural history of deep vein thrombosis: current concepts and future directions. Arterioscler Thromb Vasc Biol 31:506–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thau-Zuchman O, Shohami E, Alexandrovich AG, Trembovler V, Leker RR. 2012. The antiinflammatory drug carprofen improves long-term outcome and induces gliogenesis after traumatic brain injury. J Neurotrauma 29:375–384 [DOI] [PubMed] [Google Scholar]
- 42.Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. 2002. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med 162:1182–1189 [DOI] [PubMed] [Google Scholar]
- 43.Tschuor C, Asmis LM, Lenzlinger PM, Tanner M, Harter L, Keel M, Stocker R, Stover JF. 2008. In vitro norepinephrine significantly activates isolated platelets form healthy volunteers and critically ill patients following severe traumatic brain injury. Crit Care 12:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vilahur G, Padro T, Badimon L. 2011. Atherosclerosis and thrombosis: insights from large animal models. J Biomed Biotechnol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Virchow R. 1998. Thrombosis and emboli (1846–1856). Canton (MA): Science History Publications [Google Scholar]
- 46.Waite A, Gilliver SC, Masterson GR, Hardman MJ, Ashcroft GS. 2010. Clinically relevant doses of lidocaine and bupivacaine do not impair cutaneous wound healing in mice. Br J Anaesth 104:768–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wakefield TW, Myers DD, Henke PK. 2008. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol 28:387–391 [DOI] [PubMed] [Google Scholar]
- 48.Wang G, Weng Y, Ishiguro Y, Sakamoto H, Morita S. 2005. The effect of tramadol on serum cytokine response in patients undergoing pulmonary lobectomy. J Clin Anesth 17:444–450 [DOI] [PubMed] [Google Scholar]
- 49.Wojcik BM, Wrobleski S, Hawley AE, Wakefield TW, Myers DD, Diaz J. 2011. Interleukin 6: a potential target for post-thrombotic syndrome. Ann Vasc Surg 25:229–239 [DOI] [PubMed] [Google Scholar]
- 50.Young A, Chapman O, Connor C, Poole C, Rose P, Kakkar AK. 2012. Thrombosis and cancer. Nat Rev Clin Oncol 9:437–449 [DOI] [PubMed] [Google Scholar]