Abstract
The goal of the current study was to compare the efficacy, adverse effects, and plasma buprenorphine concentrations of sustained-release buprenorphine (SRB) and buprenorphine after subcutaneous administration in dogs undergoing ovariohysterectomy. In a prospective, randomized, blinded design, 20 healthy adult female Beagle dogs underwent routine ovariohysterectomy and received multimodal analgesia consisting of meloxicam and one of two buprenorphine formulations. Dogs were randomly assigned to receive either SRB (0.2 mg/kg SC, once) or buprenorphine (0.02 mg/kg SC every 12 h for 3 d). Blinded observers assessed all dogs by using sedation scores, pain scores, temperature, HR, RR, and general wellbeing. Dogs were provided rescue analgesia with 0.02 mg/kg buprenorphine SC if the postoperative pain score exceeded a predetermined threshold. Blood samples were collected, and mass spectrometry was used to determine plasma buprenorphine concentrations. Data were analyzed with a linear mixed model and Tukey–Kramer multiple comparison. Age, body weight, anesthetic duration, surgical duration, sevoflurane concentration, and cardiorespiratory variables did not differ significantly between groups. Dogs in both formulation groups had comparable postoperative sedation and pain scores. One dog from each formulation group had breakthrough pain requiring rescue analgesia. Plasma buprenorphine concentrations remained above a hypothesized therapeutic concentration of 0.6 ng/mL for 136.0 ± 11.3 and 10.67 ± 0.84 h for SRB and buprenorphine, respectively. Based on the results of this study, multimodal analgesic regimens consisting of meloxicam and either buprenorphine or SRB are equally efficacious in managing pain associated with an ovariohysterectomy and show comparable side effects.
Abbreviations: HR, heart rate; RR, respiratory rate; SRB, sustained-released buprenorphine
Buprenorphine, a semisynthetic opioid analgesic, is a common component of veterinary multimodal pain management. It has a strong affinity for the μ opioid receptor and slow dissociation kinetics, resulting in a longer duration of action than that of other opioid analgesics. The combination of the long duration of action, low risk of respiratory depression,22 and negligible cardiovascular effects8,23 in healthy dogs make buprenorphine an advantageous opioid analgesic agent for use with procedures associated with mild to moderate pain, including ovariohysterectomy surgery in companion animals. Numerous administration routes, including intravenous,25 intramuscular,21,29,30 subcutaneous,24 oral transmucosal,19 and transdermal24 have been reported in dogs with a high level of success in managing postoperative pain associated with ovariohysterectomy. Due to buprenorphine's slow onset of peak effect (45 to 60 min2,27), it is generally given preoperatively to provide sufficient time for onset of action. Buprenorphine may have a ceiling effect, and one study demonstrated that increasing the dose from 0.02 to 0.04 mg/kg in dogs undergoing ovariohysterectomy did not increase the analgesic effect.30
Recently, a new sterile, compounded sustained-release formulation of buprenorphine became available, and the compounder suggests that a single dose of the formulation can provide as long as 72 h of analgesia in dogs, on the basis of unpublished plasma buprenorphine concentrations. To date, clinical efficacy has been demonstrated for a maximum of 72 h in cats undergoing ovariohysterectomy and in rats undergoing surgical production of a tibial defect10 and 12 h in a hot-plate assay in male mice.6 Pharmacokinetic studies, performed in rats10 and macaques,26 have confirmed sustained, high plasma buprenorphine concentrations in those species. Although the efficacy of this buprenorphine formulation is largely untested, it has potential to decrease the number of postoperative analgesic injections and improve animal welfare by further minimizing pain and distress.
Previous studies have demonstrated the importance of multimodal analgesia28,29 in postoperative dogs, given that breakthrough pain can occur when buprenorphine19,29 or a NSAID20,29 is administered alone. As a result, multimodal analgesia has become common clinical practice. A downside of multimodal analgesia is the potential number of medications that are required and their respective dosing frequencies. Analgesic plans have become increasingly complex, leading to potential misdosing (incorrect or missed dose) in animals in the postprocedural period. To address this problem, there is a need to identify effective multimodal analgesia strategies that are easy for staff to follow and thus minimize misdosing, provide adequate analgesia, and promote animal welfare.
The objective of the current study was to evaluate the clinical efficacy and pharmacokinetics of a simplified multimodal analgesic regimen for healthy adult dogs undergoing routine ovariohysterectomy. The clinical efficacy and pharmacokinetics of sustained-release buprenorphine (SRB) and buprenorphine were directly compared in dogs undergoing ovariohysterectomy and receiving meloxicam. Dogs received 0.2 mg/kg meloxicam IV and 0.2 mg/kg SRB SC or 0.02 mg/kg buprenorphine SC prior to surgery. Postoperatively, all dogs received 0.1 mg/kg meloxicam PO once daily for 4 d. Dogs that received buprenorphine were dosed at 0.02 mg/kg SC every 12 h for 3 d, and dogs that received SRB were dosed with saline subcutaneously every 12 h for 3 d at volumes comparable to that of the buprenorphine dose. Clinical efficacy was assessed by using sedation scoring, behavioral pain scoring, temperature, heart rate (HR), respiratory rate (RR), gastrointestinal side effects, injection site reactions and the need for rescue analgesia during the postoperative monitoring period. In addition, blood was collected at regular intervals over the 7-d postoperative period, for pharmacokinetic purposes and assessment of the therapeutic plasma buprenorphine concentration.
Materials and Methods
Animals.
Female purpose-bred beagles (n = 20; age, 0.9 to 4.7 y; weight, 5.7 to 9.0 kg) were obtained from a single dealer (Marshall Bioresources, North Rose, NY) and housed in accordance with guidelines18 and regulations1 in an AAALAC-accredited program. They were maintained on a 12:12-h light:dark cycle and were fed commercial dog chow (Teklad diet 2025, Harlan-Teklad, Madison, WI) and provided ad libitum access to water. Food was withheld for 12 h prior to surgery. All dogs were deemed healthy based on a preoperative physical exam and hematologic and biochemical analyses. They were cohoused in groups of 2 to 3 animals except during the 7-d, immediate postoperative period, when they were housed individually to minimize activity. All procedures were reviewed and approved by Abbvie's IACUC.
Buprenorphine formulation groups.
To assess differences in the efficacy of the opioid component of a multimodal analgesic plan, 20 dogs were assigned randomly to one of 2 buprenorphine formulation groups (n = 10 dogs per group), SRB (Buprenorphine SR 3 mg/mL, ZooPharm, Laramie, WY) and buprenorphine hydrochloride (Buprenex Injectable, 0.3 mg/mL, Rickett Benckiser Pharmaceuticals, Richmond, VA). Group assignment was known by only a single study technician, and all other study participants were blinded to the treatment. Dogs in the buprenorphine groups received 0.02 mg/kg buprenorphine SC every 12 h for a total of 6 injections. Dogs in the SRB group received a single injection of 0.2 mg/kg SRB SC followed by sterile saline every 12 h subcutaneously for a total of 5 saline injections at comparable volumes to buprenorphine. This dosing strategy ensured that dogs received an equal number of injections and that study participants remained blinded to the treatment groups.
Anesthesia and surgery.
All dogs were premedicated with 0.05 mg/kg acepromazine maleate (Boehringer Ingelheim, St Joseph, MO) and 0.04 mg/kg atropine sulfate (Med-Pharmex, Pomona, CA) administered intramuscularly. Immediately prior to induction, an intravenous catheter was placed, and the dogs were given an initial preoperative injection of 0.2 mg/kg IV meloxicam (Wedgewood Pharmacy, Swedesboro, NJ) and 25 mg/kg IV cefazolin (West-ward Pharmaceutical, Eatontown, NJ). The dogs were induced with propofol (Propoflo 28, Abbott Animal Health, Abbott Park, IL) intravenously until endotracheal intubation could be performed safely. All dogs were maintained at a surgical plane of anesthesia with sevoflurane (SevoFlo, Abbott Animal Health) in oxygen for the duration of the surgical procedure. Dogs were monitored continuously and RR, body temperature, HR, end-tidal carbon dioxide concentration, saturated hemoglobin oxygen concentration, and sevoflurane concentration were recorded every 10 min by an anesthesia technician for the duration of anesthesia.
Once the dogs were intubated, the injection site (dorsal cervical neck) was shaved, and the dogs received the assigned buprenorphine formulation. The injection time was recorded as time 0 for calculation of subsequent plasma collection time points. The dogs were shaved and aseptically prepared for surgery. They were positioned in dorsal recumbency with heat support, followed by final preparation and 4-corner draping. Dogs were maintained on lactated Ringers solution (Hospira, Lake Forest, IL) at a flow rate of 10 mL/kg/h.
Surgery was performed over 2 d by 4 surgeons, who followed the same procedure. All of the surgeons had extensive prior experience performing ovariohysterectomy, and each surgeon performed 3 to 8 surgeries as part of the current study. Ovariohysterectomy was accomplished via a ventral abdominal midline incision. Both ovaries and the uterus were identified visually and clamped. The ovarian vessels, ligament, and uterus were ligated with suture (Vicryl, 2-0, Ethicon, Somerville, NJ), excised, and removed. The abdomen was closed in 3 layers by using suture (Biosyn, 2-0 and 3-0, Covidien, Mansfield, MA) and staples (Appose ULC 35W staples, Tyco Healthcare Group, Norwalk, CT).
Dogs recovered from surgery in heated cages and were monitored continuously through extubation and return to sternal recumbency. Once the dogs were ambulatory, they were returned to their home cages and monitored at prescribed intervals as described below. All dogs received subsequent injections of buprenorphine or saline every 12 h as described previously and 0.1 mg/kg meloxicam (Metacam, Boehringer Ingelheim) PO once daily for 4 d after surgery.
Monitoring criteria.
The dogs were assessed by trained blinded observers for evidence of pain, distress, and sedation after surgery for 3 d at the designated time points of baseline (prior to drug administration, anesthesia, and surgery) and 1, 2, 3, 4, 5, 6, 8, 12, 14, 16, 18, 20, 24, 48, and 72 h after initial injection of SRB or buprenorphine. Assessments included sedation and pain scores (described later) as well as physiologic parameters (temperature, HR, and RR). Dogs were evaluated at every time point by 2 trained observers, and a total of 10 observers assessed all 20 dogs over the course of the study. One of the authors (EAN) trained all of the observers in the use of both the sedation and pain evaluation methods. Each observer independently observed and scored dogs for both sedation and pain at each time point. Observers did not discuss their scores at that time point, or any other time point, unless the animal was given a score that indicated a need for rescue analgesia (described later). If a dog was anesthetized or asleep at any time point, the sedation assessment was not performed.
The sedation level of the dogs was scored as previously described.19 Briefly, the dog's posture and response to noise were each given a score of 0 to 3. Posture scores were: 0, normal and standing dog; 1, mild sedation but standing; 2, laterally recumbent but able to attain sternal recumbency; and 3, laterally recumbent and unable to attain sternal recumbency. Responses to noise were: 0, normal and quick response to noise; 1, weak reaction; 2, delayed, weak reaction; and 3, no reaction.
For the purposes of the study, specific pain evaluations were performed by using a modified Colorado State University Canine Acute Pain Scale.13 This scoring system uses both visual and interactive patient assessments to evaluate the animal's body posture, body tension, behavior and mental state, and response to palpation to generate a numeric score from 0 to 4 in 0.25 increments. Three separate pain scores were collected by each observer at every time point: score A, dogs were visually assessed initially undisturbed from outside the cage, and the abdomen was not palpated; score B, the dogs were assessed as they were approached, spoken to, and gently encouraged to walk and move, but the abdomen was not palpated; and score C, the dogs were assessed as the incision and surrounding area of the abdomen were palpated by using 2 to 3 fingers.
In addition, the dogs were monitored daily for evidence of side effects for 10 postoperative days. Appetite and fecal consistency were used to evaluate the dogs for gastrointestinal side effects. The SRB and buprenorphine injection sites were observed daily for swelling, erythema, crusting, discharge, and pain on palpation.
Rescue analgesia.
Blinded observers did not discuss their findings with each other unless there was concern that the animal was in pain. Any dog that scored a 2 or greater for any pain score by either observer was considered for rescue analgesia. If both observers agreed that the animal had a score of 2 or greater for any of the pain scores, the dog received rescue analgesia. An injection of 0.02 mg/kg buprenorphine SC was administered to control the breakthrough pain. Rescue analgesia was given in addition to the predetermined twice-daily dosing schedule, and dogs received their subsequent doses of buprenorphine or saline at the originally scheduled time.
Blood collection.
To assess plasma buprenorphine concentrations, 2 mL blood was collected into EDTA tubes at baseline, 0.25, 0.5, 1, 3, 6, 8, 12, 18, 24, 25, 48, 49, 72, 120, and 168 h after injection. Blood was collected from the jugular vein, and replacement fluids were not administered. The blood collection time points at 24, 25, 48, and 49 h were used to identify the peak (25 and 49 h) and trough (24 and 48 h) plasma buprenorphine concentrations. The same time points for blood collection were used for both formulation groups to maintain treatment blinding of the staff and to minimize any effects due to the blood collection itself.
During the sample collection process, tubes were placed immediately on ice following blood collection. The tubes were centrifuged at 1000 × g for 10 min at 4 °C within 15 min of collection. The plasma was collected and stored at −20 °C until they were analyzed.
Sample analysis.
All plasma samples were analyzed by using an adapted liquid chromatography–electrospray ionization–tandem mass spectrometry method.17 This method allowed for simultaneous detection of buprenorphine and its primary metabolite norbuprenorphine in the plasma sample. Briefly, 400-μL aliquots of plasma were placed in the wells of a 2-mL, 96-well plate and extracted with 1 mL of ethyl acetate:hexane at a ratio of 9:1 by using an automated liquid-handling workstation (Hamilton MICROLAB Star Hamilton Robotics, Reno, NV). The samples were removed from the workstation, vortexed for 5 min, centrifuged for 10 min at 410 × g, and then returned to the workstation. Then, 800 μL the supernatant was transferred to a clean, 96-well plate and evaporated to dryness under a gentle stream of nitrogen at room temperature. The samples were reconstituted in 30 μL acetonitrile and vortexed briefly, and then 70 μL 0.1% formic acid containing 0.025% trifluoroacetic acid was added, followed by additional vortexing. The HPLC system (Eksigent 200, AB Sciex, Framingham, MA) injected the sample onto a 50 × 0.5 mm 3- μm column (Chrom XP C18-EP-120, Eksigent, Dublin, CA) for mass spectrometric analysis (AB SCIEX Triple Quad 5500, Eksigent 200, AB Sciex). Calibration curves were used to determine the concentration of all analytes in each sample. The lower limit of quantitation of the assay for all analytes was 0.1 ng/mL for a 400-μL sample of plasma.
Pharmacokinetics.
Pharmacokinetic analysis was performed on plasma buprenorphine concentration–time data obtained after subcutaneous drug administration. Peak plasma buprenorphine concentrations (Cmax) and the times of Cmax (Tmax) were determined directly from the observed concentration–time data. Pharmacokinetic parameters (the elimination rate constant and elimination half-life t1/2) were derived by using a model-independent approach (noncompartmental analysis) according to a uniform weighting scheme. Half-life was calculated by using the harmonic mean and the AUC0–168 was calculated by using the linear trapezoidal rule. Dogs that required rescue analgesia or were misdosed were not included in the pharmacokinetic analysis. All pharmacokinetic calculations were performed by using appropriate software (version 5.2, WinNonlin, Pharsight, Cary, NC).
Statistical analysis.
The current study consisted of longitudinal observations and measurements of 2 different formulations of buprenorphine. To address the repeated-measures nature of the study and the use of multiple observers, the results (temperature, HR, RR, sedation scores, and the 3 pain scores) were analyzed by using a linear mixed-effect regression model with transformations of the outcomes, thus allowing for correction for both error and total observer (interobserver and intraobserver) variability. The covariates included time point, drug formulation, anesthesia, surgery, extubation, and recovery times, surgeon, and observer. In addition, Tukey–Kramer multiple comparison was used to compare results between groups at different time points. Direct comparison of group ages, body weight, anesthetic duration, surgical duration, extubation time, intraoperative HR, RR, temperature, maintenance end-tidal sevoflurane concentration, and hemoglobin saturation for oxygen were evaluated similarly. Statistical software (JMP 9, SAS Institute, Cary, NC; R, GNU Operating System, http://www.gnu.org/gnu/) was used for all analyses, data were reported as mean ± SEM, and significance was set at a P value of less than 0.05.
Results
Intraoperative findings.
Buprenorphine formulation groups did not differ with regard to animal age, body weight, surgical duration, anesthesia duration, or extubation time (Table 1). There were no significant differences between formulation groups in the average intraoperative RR, HR (Table 1), hemoglobin saturation for oxygen (SRB, 96.7% ± 0.5%; buprenorphine, 95.5% ± 0.7%), rectal temperature (SRB, 36.4 ± 0.2 °C; buprenorphine, 35.8 ± 0.3 °C), or sevoflurane concentration (SRB, 3.4 ± 0.1%/mL; buprenorphine 3.2% ± 0.1%/mL).
Table 1.
Data (Mean ± SEM) regarding dogs (n= 10 per group) that received SRB (0.2 mg/kg SC) or buprenorphine (0.02 mg/kg SC) prior to ovariohysterectomy
| SRB | Buprenorphine | P | |
| Body weight (kg) | 7.5 ± 0.3 | 7.4 ± 0.3 | 0.902 |
| Age (y) | 1.9 ± 0.2 | 1.7 ± 0.5 | 0.072 |
| Surgery duration (min) | 35 ± 3 | 33 ± 2 | 0.499 |
| Anesthesia duration (min) | 56 ± 3 | 59 ± 7 | 0.704 |
| Extubation time (min) | 65 ± 4 | 74 ± 8 | 0.446 |
Postoperative monitoring.
Sedation scores (posture and response to noise) were affected by anesthetic duration, extubation time, and buprenorphine formulation. Average sedation scores were greater than 0 for 16 h after drug administration in both groups. Posture and response to noise scores were slightly lower in the SRB group for the first 4 h after drug administration, but this difference was significant (P < 0.05) only at the 3- and 4-h time points for posture (Table 2). Anesthetic duration and extubation time both had significant (P < 0.05) main effects on posture scores, and there was a significant (P < 0.001) interaction effect between time after injection and drug formulation. Anesthetic duration and extubation time both had a significant (P < 0.05) main effect on response to noise scores.
Table 2.
Postoperative sedation scores (Mean ± SEM; n= 10 per group) in dogs that received SRB or buprenorphine prior to ovariohysterectomy
| Time (h) after buprenorphine administration |
|||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 8 | 12 | ||
| Posture | SRB | 2.2 ± 0.4 | 1.5 ± 0.3 | 1.1 ± 0.3* | 0.7 ± 0.3* | 0.4 ± 0.1 | 0.6 ± 0.2 | 0.3 ± 0.2 | 0.2 ± 0.1 |
| Buprenorphine | 2.4 ± 0.3 | 1.8 ± 0.3 | 1.7 ± 0.3 | 1.4 ± 0.4 | 0.5 ± 0.2 | 0.6 ± 0.3 | 0.3 ± 0.2 | 0.2 ± 0.1 | |
| Response to noise | SRB | 2.1 ± 0.4 | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.7 ± 0.2 | 0.3 ± 0.2 | 0.7 ± 0.3 | 0.3 ± 0.2 | 0.2 ± 0.1 |
| Buprenorphine | 2.2 ± 0.4 | 1.5 ± 0.3 | 1.4 ± 0.3 | 1.0 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.1 ± 0.1 | |
, Value significantly (P < 0.05) different from that for buprenorphine.
Pain scores were useful for monitoring animal comfort in the postoperative period but required correction for interobserver variability. Different observers had significantly different (P < 0.0001) results for all 3 pain scores. When the average pain scores were corrected for interobserver variability by using a linear mixed-effect regression model, there were no significant differences between formulation groups for pain scores A or B. Corrected averages pain scores A and B peaked at 1.3 at 2 h after injection and were less than 0.25 at 24 h for both formulations. There was a small but significant (P < 0.05) difference between SRB and buprenorphine in the corrected average pain score C for the entire 72-h assessment period. Dogs that received SRB had a 0.2 lower corrected average pain score C for all time points. Corrected averages for pain score C peaked at 1.3 and 1.5 at 2 h for SRB and buprenorphine, respectively. At the 24-, 48-, and 72-h time points, the average values for pain score C were 0.6 and 0.8 for SRB and buprenorphine, respectively. There were no differences between surgeons for any of the pain scores.
One dog from each formulation group had breakthrough pain. These dogs had scores of 2 or greater for all 3 pain scores, A through C. One dog in the SRB group required rescue analgesia at the 14-h time point, and one dog in the buprenorphine group recovered poorly and required rescue analgesia between the 1- and 2-h time points. Both dogs exhibited decreased pain scores after the administration of rescue analgesia.
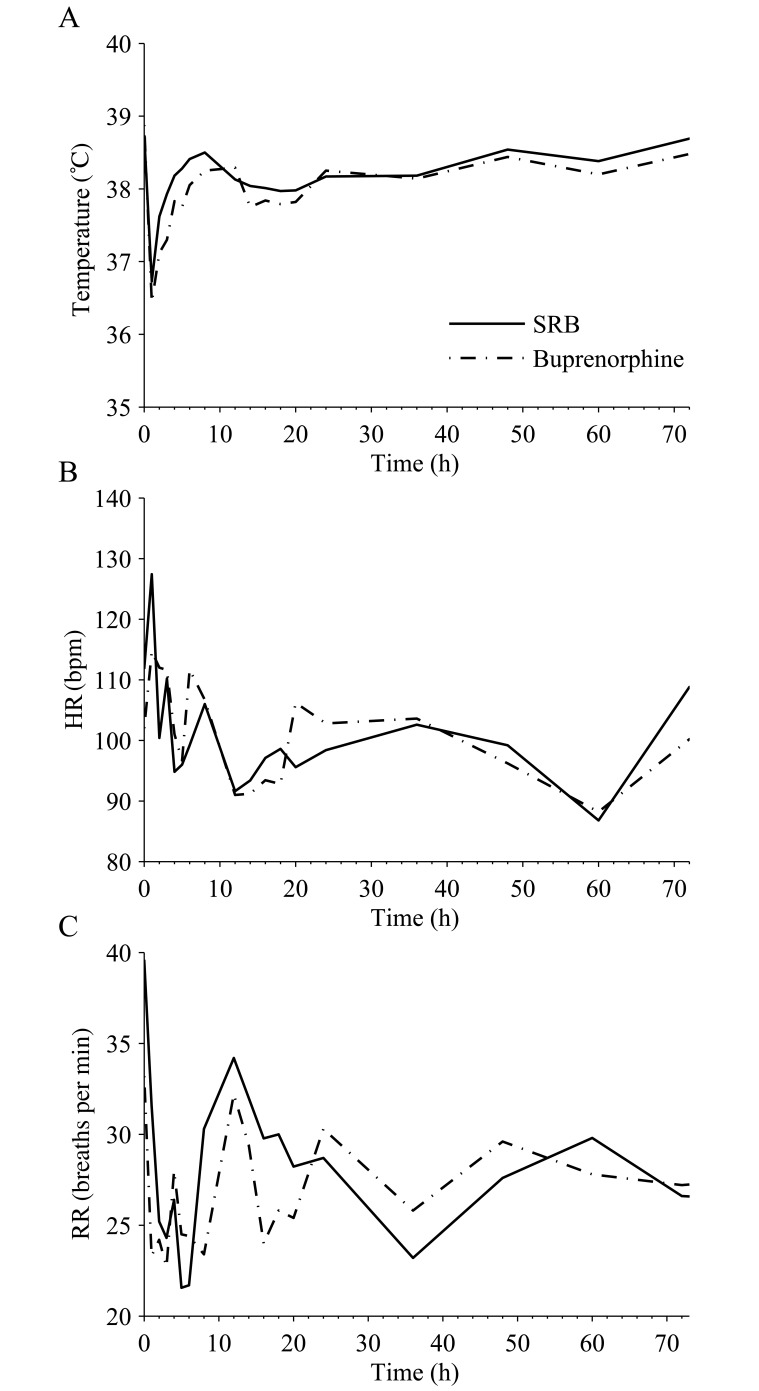
There were no significant differences in temperature, HR, or RR between the formulation groups (Figure 1). Body temperatures decreased intraoperatively but returned to 38 °C within 4 h of the initial injection (Figure 1 A). Extubation time had a significant (P < 0.05) effect on body temperature.
Figure 1.
Mean postoperative (A) temperature, (B) HR, and (C) RR over time after administration of 0.2 mg/kg SRB SC and 0.02 mg/kg buprenorphine SC twice daily. Time 0 corresponds to the time of drug administration. Values did not differ significantly between treatment groups.
Buprenorphine pharmacokinetics.
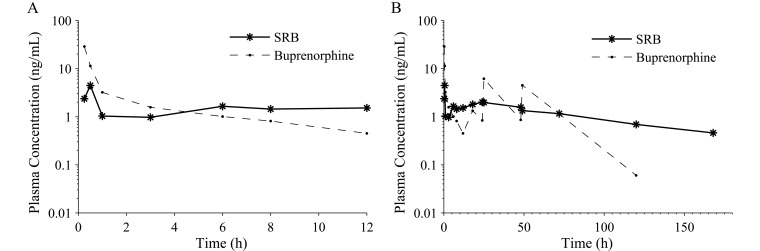
There were differences between formulations in their pharmacokinetics. The plasma buprenorphine concentration for SRB peaked at 5.6 ± 3.0 ng/mL in 13.8 ± 3.8 h and was less than 1 ng/mL within 96.3 ± 16.4 h and less than 0.6 ng/mL in 136.0 ± 11.3 h in all 9 of the dogs for which data were available (Table 3and Figure 2). Nine of the 10 dogs that received SRB still had detectable plasma buprenorphine concentrations at the last time point, 168 h (7 d). The plasma buprenorphine concentration for buprenorphine peaked at 19.6 ± 4.9 ng/mL in 0.28 ± 0.03 h after administration and then decreased over the 12-h interdose interval (Table 3, Figure 2 A). Plasma buprenorphine concentrations were less than 1 ng/mL within 7.1 ± 0.9 h in all 8 dogs and less than 0.6 ng/mL within 10.7 ± 0.8 h in 6 of the 8 dogs given buprenorphine and for which data were available. One dog in the buprenorphine group did not have quantifiable plasma buprenorphine concentrations until the 18-h time point and was excluded from all pharmacokinetic calculations. Plasma buprenorphine concentrations were detectable after 120 h in 4 of 8 dogs and after 168 h in 0 of 8 dogs in the buprenorphine group. The plasma buprenorphine concentrations at the time of breakthrough pain for the dogs receiving SRB and buprenorphine were 2.26 and 2.08 ng/mL, respectively.
Table 3.
Pharmacokinetic parameters (mean ± SEM) for plasma buprenorphine concentrations after a single dose of 0.2 mg/kg SC SRB (n= 9) and 6 doses of 0.02 mg/kg SC buprenorphine administered every 12 h (n= 8)
| SRB | Buprenorphine | |
| Cmax (ng/mL) | 5.6 ± 3.0 | 19.6 ± 4.9 |
| Tmax (h) | 13.8 ± 3.8 | 0.28 ± 0.03 |
| AUC0-168 (ng × h/mL) | 188.9 ± 15.5 | 236.3 ± 15.9 |
| AUC/D (ng × h/mL per mg/kg) | 944.7 ± 77.5 | 1969.3 ± 115.7 |
| Cmax/D (ng/mL per mg/kg) | 27.8 ± 15.2 | 163.1 ± 40.7 |
| t1/2 (h) | 64.5 | NR |
Cmax, maximal plasma concentration; D, total dose received; NR, not reported; Tmax, time when Cmax is reached; t1/2, elimination half-life for plasma (harmonic mean).
Figure 2.
Plasma buprenorphine concentrations. (A) Plasma concentrations over 12 h after a single injection of 0.2 mg/kg SRB SC or 0.02 mg/kg buprenorphine SC. (B) Plasma concentrations over 168 h for a single injection of 0.2 mg/kg SRB SC or 0.02 mg/kg buprenorphine SC every 12 h for 3 d. Plasma buprenorphine concentrations are reported as the mean ± SEM at each time point (SRB, n = 9; buprenorphine, n = 8).
Plasma buprenorphine concentrations were higher for buprenorphine than SRB for the first 4 h after administration but then were less than those for SRB for the remaining 8 h before redosing (Figure 2). Plasma buprenorphine concentrations peaked after each injection of buprenorphine and then decreased over time, but the peaks after the 12, 36, and 60 h injections were not captured because blood was not collected. Buprenorphine had higher drug exposure, reported as the AUC0–168 and dose-normalized AUC (Table 3), than did SRB. Although the elimination half-life was observed to be much shorter for buprenorphine than SRB, it could not be accurately calculated for buprenorphine due to redosing the dogs and therefore was not reported.
The plasma concentration of norbuprenorphine, the primary buprenorphine metabolite, was extremely low throughout the study. Most samples had concentrations below the limit of quantitation (< 0.1 ng/mL) and were not reported.
Adverse effects and complications.
The dogs in the current study had side effects associated with the experimental procedures. Dogs in both groups exhibited signs of soft stool and diarrhea: 8 of 10 in the SRB group and 9 of 10 dogs given buprenorphine. Dogs from both groups also exhibited evidence of appetite suppression. Decreased appetite was noted in 4 dogs in the SRB group on day 0, in 3 dogs on days 1 and 2, and 1 dog on Day 4. In the buprenorphine group, 2 dogs showed decreased appetite on days 0 through 3.
Injection-site reactions occurred only in dogs that received SRB. Seven of the 10 dogs had small (0.5–1.5 cm × 0.3 cm), nonpainful subcutaneous nodules at the site of drug administration in the dorsal cervical region. The nodules were not visually obvious and were only detectable by deliberate palpation of the injection site. The reactions were not detected until postoperative day 10, and they spontaneously resolved within 1 mo of the initial injection in 5 of the 7 dogs. One dog that had a 0.2-cm nodule was adopted out of the facility after the 1-mo period and was lost to follow-up. The nodule from another dog was removed 3 wk after the initial injection for histopathology. The lesion was described as a pyogranuloma with central clear vacuoles containing sparse gray material, consistent with an injection site reaction.
One dog in the buprenorphine group, the same dog that did not have a detectable plasma buprenorphine concentration until 18 h, had minor dehiscence at the cranial aspect of the laparotomy site at the 72-h observation. A 2-cm section of omentum was removed, and the abdomen was reclosed. The dog recovered uneventfully and had no other complications for the remainder of the 10-d monitoring period.
Discussion
Postoperative analgesia is critical for animal welfare. Simplifying how that analgesia is provided improves compliance and directly affects the success of the analgesic plan. The results of this study support the clinical efficacy of SRB combined with meloxicam for providing analgesia in dogs undergoing ovariohysterectomy. When used in combination with a once-daily dosing regimen of meloxicam, a single preoperative injection of 0.2 mg/kg SC SRB provides comparable analgesia to a twice-daily dosing regimens with 0.02 mg/kg SC buprenorphine. Furthermore, in light of the single preoperative injection and stable plasma buprenorphine concentrations, using SRB may be preferred for some cases. The ability to provide postoperative analgesia with a single preoperative injection of a sustained-release opioid simplifies postoperative management, which ultimately improves compliance, provides adequate analgesia, and promotes animal welfare.
It is important to evaluate an individual animal's comfort level after surgery to ensure that the analgesic regimen is adequate. Analgesic efficacy can be assessed in multiple ways, including behavioral pain scoring9,13-16 and video monitoring to calculate useful metrics from the animals’ movement around the cage;11,12 however, all monitoring tools have their limitations. Due to ease of use, behavioral pain scoring is the most common method, and multiple scales have been developed to compare analgesic efficacy and evaluate postoperative comfort.7,9,13-16,19-21,29,30 The current study used an adapted version of the Colorado State University Canine Acute Pain Scale, because of our familiarity with that system. Although this scale is easy to use, it had not been validated for repeatability between independent observers. To address this issue, interobserver variability was evaluated, and the pain scores were corrected before statistical comparison of the 2 formulation groups. After this correction, there was a small, but statistically significant, difference between formulations for pain score C, and no difference for A and B. The minimal difference in scores between groups may have been because the dogs were either equally managed with both multimodal analgesic regimens, the effect differences were too small to detect statistically with this group size, or laboratory beagles hide their pain and behavioral manifestations were not easily observable. All of these explanations likely play a role. Furthermore, it is not possible to assess the relative contributions made by each of the analgesics individually in these multimodal regimens. Perhaps the analgesia provided by the meloxicam was sufficient to obscure differences in the analgesia provided by the different formulations of buprenorphine.
Pain sensitivity is unique to the individual animal, making it difficult to identify the therapeutic plasma concentration of an analgesic agent. In a previous study evaluating the efficacy of buprenorphine in managing pain for ovariohysterectomy, 7 of 9 dogs appeared comfortable at plasma buprenorphine concentrations equal to or less than 0.6 ng/mL, but 2 animals had breakthrough pain at plasma buprenorphine concentrations of 1.46 and 5.41 ng/mL.19 These 2 dogs were potentially more sensitive to the pain associated with ovariohysterectomy. Similarly, in the current study, 2 of 20 dogs demonstrated evidence of breakthrough pain at plasma buprenorphine concentrations of 2.26 and 2.08 ng/mL. This highlights the importance of monitoring individual animals for evidence of postoperative pain and of not relying on plasma drug concentrations. Interestingly, in both the current and previous study,19 some dogs did not demonstrate obvious signs of pain despite having plasma buprenorphine concentrations less than 0.1 ng/mL. In the current study, this may have been due to the additional use of meloxicam, thus providing the animals with multimodal analgesia and decreasing the potential for breakthrough pain. Regardless of the analgesic regimen used, careful observation is required to identify those animals that may be more sensitive and have breakthrough pain.
The pharmacokinetic parameters for buprenorphine and SRB supported the dosing strategy. Quantifiable and therapeutic plasma buprenorphine concentrations were reached within 15 min, and both formulations remained in the hypothesized therapeutic concentration range over the 72-h postoperative period. Buprenorphine achieved a higher maximum plasma concentration within a shorter timeframe than did SRB, as was expected in light of previous studies comparing the 2 formulations in macaques.26 The pharmacokinetic parameters for buprenorphine and SRB in dogs were comparable to those in other species.26,31 Despite its lower total dose, buprenorphine was associated with a higher exposure over the 168 h than was SRB, even when dose normalized (Table 3). This effect was most likely due to the high plasma buprenorphine concentrations achieved each time the buprenorphine was dosed and the gradual accumulation of buprenorphine in the body that led to detectable plasma buprenorphine concentrations in 4 of 8 dogs at the 120-h time point.
Sustained plasma buprenorphine concentrations in dogs could lead to reduced drug efficacy due to adaptive changes, such as receptor desensitization or endocytosis. This has been previously reported for the opiates morphine and heroin in cultured cells.5,32,33 In rodent studies, high doses of buprenorphine administered to pregnant females resulted in downregulation of μ opioid receptors in both adult and neonatal rat brains,3 with a greater downregulation occurring in male pups.4 The use of SRB may have had an effect on receptor expression due to the prolonged half-life of the formulation, but the anticipated associated clinical effects were not noted in the current study. Furthermore, the dose-normalized drug exposure levels were lower for SRB than buprenorphine, indicating that repeated injections of buprenorphine would be more apt to result in receptor desensitization or downregulation. Although the clinical relevance of the µ-receptor downregulation in veterinary medicine is unknown, it does warrant additional investigation, especially in the context of chronic pain management.
One dog in the buprenorphine group was misdosed initially and did not have detectable plasma buprenorphine concentrations for the first 12 h. The misdose most likely is due to technical error during the administration of buprenorphine resulting in the dog not being dosed initially. This dog did not have high pain scores during the first 12 h, with maximum pain scores of less than 1.75 at the 2-h time point for pain scores A through C. This dog also exhibited a rapid decrease in sedation scores, presumably due to that lack of detectable buprenorphine in her plasma. The lack of high pain scores in the first 12 h potentially is due to the meloxicam that she received preoperatively. NSAID alone have previously been shown to be efficacious in managing postoperative pain in dogs after ovariohysterectomy.20,29 In addition, this dog may have had either had a higher pain tolerance than did the other dogs, or perhaps observers were unable to recognize pain this dog.
Buprenorphine use has been associated with common opioid side effects, including respiratory depression, cardiovascular depression, decreased body temperature, sedation, nausea, vomiting, and diarrhea. The observed decreases in body temperature, HR, and RR (Figure 1) were consistent with dogs given buprenorphine8,19 but also are common in dogs undergoing general anesthesia. Although opioids can cause cardiovascular depression, these effects are not of clinical importance in healthy dogs,23 and there were no significant differences in HR between formulation groups. Buprenorphine can cause sedation, especially in combination with general anesthesia, and dogs in the current study demonstrated signs of sedation for a maximum of 16 h. The higher sedation scores observed during the first 4 h in dogs that received buprenorphine were most likely due to the higher plasma buprenorphine concentrations measured in dogs given buprenorphine instead of SRB (Figure 2 A). This result was consistent with a previous study evaluating sedation scores between 2 different doses of oral transmucosal buprenorphine.19
Although the number of studies evaluating SRB is limited, injection site reactions of varying severity have been reported in every species.6,7,10,26 In rodents and cats, the injection reaction tends to be acute in nature, causing a transient ulceration that scabs and heals.6,7,10 In macaques26 and dogs, the process is slower and is consistent with a subcutaneous foreign-body reaction to either the dl-lactide and ϵ-caprolactone copolymer or the organic solvent. In both macaques and dogs, this process appears to be self-limiting, and the mild subcutaneous nodules resolve without intervention. Neither reaction type appears to be associated with pain in the affected animals,6,7,10,26 and these events would not prevent the drug from being used for analgesia in these species.
A major advantage to using SRB is the convenient dosing strategy that provides stable plasma buprenorphine concentrations. A single injection provides as long as 5 d of analgesia when used in combination with meloxicam. This feature eliminates the need to provide owners with a controlled substance that they may not correctly handle or administer. This characteristic also decreases the stress of repeated opioid administration in animals that are fractious or difficult to restrain for injections. Furthermore, there is no need for the constant animal monitoring associated with either a patch or continuous-rate infusion to achieve stable plasma drug concentrations. This situation eliminates concerns about opioid ingestion toxicity, intravenous line patency, and potential opioid over- or underdosing.
In conclusion, when either SRB or buprenorphine was used as part of a multimodal analgesic regimen with meloxicam for an ovariohysterectomy, equally effective analgesia was provided with minimal intraoperative and postoperative adverse effects. A single injection of 0.2 mg/kg SC SRB provided a prolonged, stable plasma buprenorphine concentration that exceeded the therapeutic plasma concentration threshold. Collectively, the results described herein indicate that SRB can be used with meloxicam to provide analgesia in healthy dogs undergoing routine ovariohysterectomy. The use of SRB simplifies the postoperative multimodal analgesic regimen by decreasing the total number and frequency of medications administered postoperatively. This benefit makes it easier for staff to dose animals correctly, to provide analgesia to postoperative animals, and to promote animal welfare.
Acknowledgments
We thank Tricia Galassi, Tricia Rinaldo, Marissa Erickson, Julie Carriker, Jodi Ternes, Michael McNally, Lisa Charwicz, Kelsey Feiza, Dawn Oldenburg, Dianna Laurent, and Drs Lisa Rehm, Matthew Rieser, and Wayne Buck for technical, resource, and analytical assistance.
EAN was an employee of University of Illinois at Chicago and contracted to AbbVie during this study. DFS, JM, ASW, GJJ, and CLM were employees of AbbVie during this study. The design, study conduct, and financial support for these studies were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the manuscript.
References
- 1.Animal Welfare Regulations . 2009. 9 CFR §2.30–2.38, 3.1–3.19. [Google Scholar]
- 2.Andaluz A, Moll X, Abellan R, Ventura R, Carbo M, Fresno L, Garcia F. 2009. Pharmacokinetics of buprenorphine after intravenous administration of clinical doses to dogs. Vet J 181:299–304 [DOI] [PubMed] [Google Scholar]
- 3.Belcheva MM, Barg J, McHale RJ, Dawn S, Ho MT, Ignatova E, Coscia CJ. 1993. Differential down- and up-regulation of rat brain opioid receptor types and subtypes by buprenorphine. Mol Pharmacol 44:173–179 [PMC free article] [PubMed] [Google Scholar]
- 4.Belcheva MM, Bohn LM, Ho MT, Johnson FE, Yanai J, Barron S, Coscia CJ. 1998. Brain opioid receptor adaptation and expression after prenatal exposure to buprenorphine. Brain Res Dev Brain Res 111:35–42 [DOI] [PubMed] [Google Scholar]
- 5.Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. 2003. Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of µ-opioid receptors. J Biol Chem 278:18776–18784 [DOI] [PubMed] [Google Scholar]
- 6.Carbone ET, Lindstrom KE, Diep S, Carbone L. 2012. Duration of action of sustained-release buprenorphine in 2 strains of mice. J Am Assoc Lab Anim Sci 51:815–819 [PMC free article] [PubMed] [Google Scholar]
- 7.Catbagan DL, Quimby JM, Mama KR, Rychel JK, Mich PM. 2011. Comparison of the efficacy and adverse effects of sustained-release buprenorphine hydrochloride following subcutaneous administration and buprenorphine hydrochloride following oral transmucosal administration in cats undergoing ovariohysterectomy. Am J Vet Res 72:461–466 [DOI] [PubMed] [Google Scholar]
- 8.Cowan A, Doxey JC, Harry EJ. 1977. The animal pharmacology of buprenorphine, an oripavine analgesic agent. Br J Pharmacol 60:547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Firth AM, Haldane SL. 1999. Development of a scale to evaluate postoperative pain in dogs. J Am Vet Med Assoc 214:651–659 [PubMed] [Google Scholar]
- 10.Foley PL, Liang H, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50:198–204 [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen BD. 2003. Assessment of pain in dogs: veterinary clinical studies. ILAR J 44:197–205 [DOI] [PubMed] [Google Scholar]
- 12.Hardie EM, Hansen BD, Carroll GS. 1997. Behavior after ovariohysterectomy in the dog: what's normal? Appl Anim Behav Sci 51:111–128 [Google Scholar]
- 13.Hellyer PW. 2002. Objective, categoric methods for assessing pain and analgesia, p 82–107. In: Gaynor JS, Muir W. Handbook of veterinary pain management; St Louis (MO): Mosby [Google Scholar]
- 14.Holton L, Reid J, Scott EM, Pawson P, Nolan A. 2001. Development of a behaviour-based scale to measure acute pain in dogs. Vet Rec 148:525–531 [DOI] [PubMed] [Google Scholar]
- 15.Holton LL, Scott EM, Nolan AM, Reid J, Welsh E. 1998. Relationship between physiological factors and clinical pain in dogs scored using a numerical rating scale. J Small Anim Pract 39:469–474 [DOI] [PubMed] [Google Scholar]
- 16.Holton LL, Scott EM, Nolan AM, Reid J, Welsh E, Flaherty D. 1998. Comparison of 3 methods used for assessment of pain in dogs. J Am Vet Med Assoc 212:61–66 [PubMed] [Google Scholar]
- 17.Huang W, Moody DE, McCance-Katz EF. 2006. The in vivo glucuronidation of buprenorphine and norbuprenorphine determined by liquid chromatography–electrospray ionization–tandem mass spectrometry. Ther Drug Monit 28:245–251 [DOI] [PubMed] [Google Scholar]
- 18.Institute of Laboratory Animal Research 2011. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press [Google Scholar]
- 19.Ko JC, Freeman LJ, Barletta M, Weil AB, Payton ME, Johnson BM, Inoue T. 2011. Efficacy of oral transmucosal and intravenous administration of buprenorphine before surgery for postoperative analgesia in dogs undergoing ovariohysterectomy. J Am Vet Med Assoc 238:318–328 [DOI] [PubMed] [Google Scholar]
- 20.Lascelles BD, Cripps PJ, Jones A, Waterman-Pearson AE. 1998. Efficacy and kinetics of carprofen, administered preoperatively or postoperatively, for the prevention of pain in dogs undergoing ovariohysterectomy. Vet Surg 27:568–582 [DOI] [PubMed] [Google Scholar]
- 21.Linton DD, Wilson MG, Newbound GC, Freise KJ, Clark TP. 2012. The effectiveness of a long-acting transdermal fentanyl solution compared to buprenorphine for the control of postoperative pain in dogs in a randomized, multicentered clinical study. J Vet Pharmacol Ther 35 Suppl 2:53–64 [DOI] [PubMed] [Google Scholar]
- 22.Martin WR. 1979. History and development of mixed opioid agonists, partial agonists, and antagonists. Br J Clin Pharmacol 7 Suppl 3:273S–279S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez EA, Hartsfield SM, Melendez LD, Matthews NS, Slater MR. 1997. Cardiovascular effects of buprenorphine in anesthetized dogs. Am J Vet Res 58:1280–1284 [PubMed] [Google Scholar]
- 24.Moll X, Fresno L, Garcia F, Prandi D, Andaluz A. 2011. Comparison of subcutaneous and transdermal administration of buprenorphine for preemptive analgesia in dogs undergoing elective ovariohysterectomy. Vet J 187:124–128 [DOI] [PubMed] [Google Scholar]
- 25.Morgaz J, Navarrete R, Munoz-Rascon P, Dominguez JM, Fernandez-Sarmiento JA, Gomez-Villamandos RJ, Granados MM. 2013. Postoperative analgesic effects of dexketoprofen, buprenorphine, and tramadol in dogs undergoing ovariohysterectomy. Res Vet Sci 95:278–282 [DOI] [PubMed] [Google Scholar]
- 26.Nunamaker EA, Halliday LC, Moody DE, Fang WB, Lindeblad M, Fortman JD. 2013. Pharmacokinetics of 2 formulations of buprenorphine in macaques (Macaca mulatta and Macaca fascicularis). J Am Assoc Lab Anim Sci 52:48–56 [PMC free article] [PubMed] [Google Scholar]
- 27.Pascoe PJ. 2000. Opioid analgesics. Vet Clin North Am Small Anim Pract 30:757–772 [DOI] [PubMed] [Google Scholar]
- 28.Peeters ME, Kirpensteign J. 2011. Comparison of surgical variables and short-term postoperative complications in healthy dogs undergoing ovariohysterectomy or ovariectomy. J Am Vet Med Assoc 238:189–194 [DOI] [PubMed] [Google Scholar]
- 29.Shih AC, Robertson S, Isaza N, Pablo L, Davies W. 2008. Comparison between analgesic effects of buprenorphine, carprofen,, and buprenorphine with carprofen for canine ovariohysterectomy. Vet Anaesth Analg 35:69–79 [DOI] [PubMed] [Google Scholar]
- 30.Slingsby LS, Taylor PM, Murrell JC. 2011. A study to evaluate buprenorphine at 40 μg kg-1 compared to 20 μg kg-1 as a postoperative analgesic in the dog. Vet Anaesth Analg 38:584–593 [DOI] [PubMed] [Google Scholar]
- 31.Steagall PV, Mantovani FB, Taylor PM, Dixon MJ, Luna SP. 2009. Dose-related antinociceptive effects of intravenous buprenorphine in cats. Vet J 182:203–209 [DOI] [PubMed] [Google Scholar]
- 32.Varga EV, Rubenzik MK, Stropova D, Sugiyama M, Grife V, Hruby VJ, Rice KC, Roeske WR, Yamamura HI. 2003. Converging protein kinase pathways mediate adenylyl cyclase superactivation upon chronic δ-opioid agonist treatment. J Pharmacol Exp Ther 306:109–115 [DOI] [PubMed] [Google Scholar]
- 33.von Zastrow M, Svingos A, Haberstock-Debic H, Evans C. 2003. Regulated endocytosis of opioid receptors: cellular mechanisms and proposed roles in physiological adaptation to opiate drugs. Curr Opin Neurobiol 13:348–353 [DOI] [PubMed] [Google Scholar]