Abstract
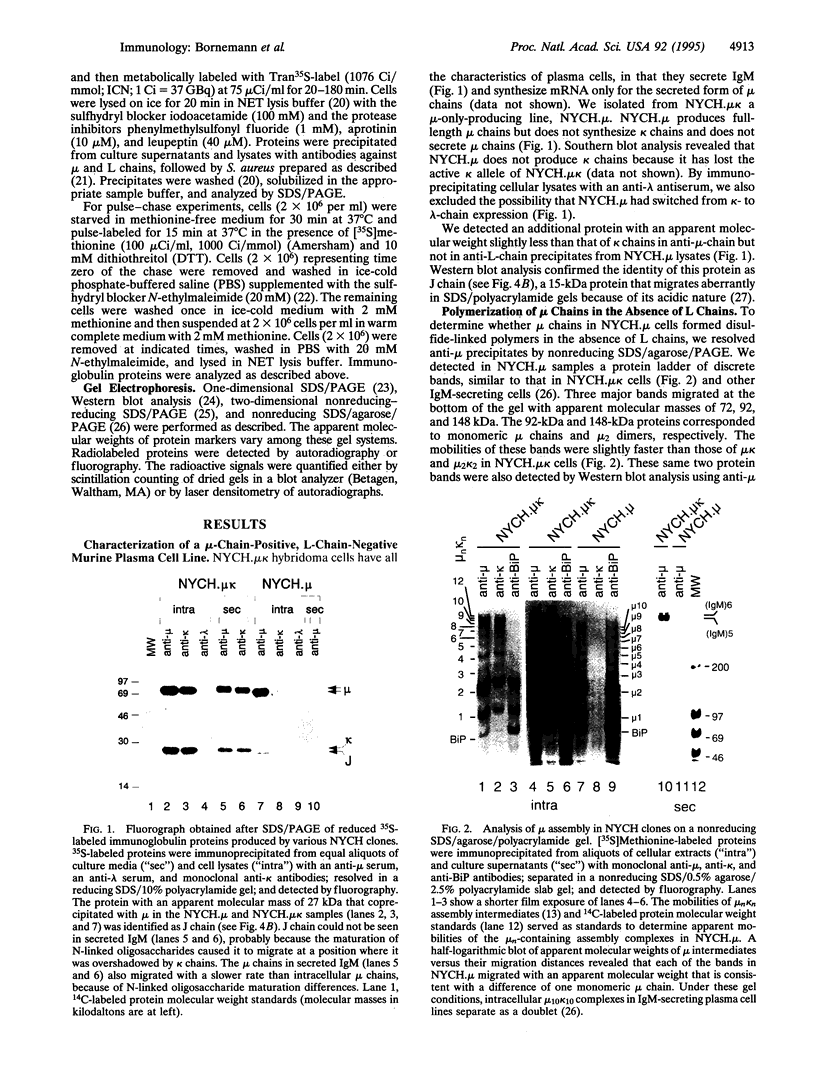
IgM antibodies are secreted as multisubunit polymers that consist of as many as three discrete polypeptides: mu heavy chains, light (L) chains, and joining (J) chains. We wished to determine whether L chains that are required to confer secretory competence on immunoglobulin molecules must be present for IgM to polymerize--that is, for intersubunit disulfide bonds to form between mu chains. Using a L-chain-loss variant of an IgM-secreting hybridoma, we demonstrated that mu chains were efficiently polymerized independent of L chains, in a manner similar to that observed for conventional microL complexes, and that the mu polymers incorporated J chain. These mu polymers were not secreted but remained associated with the endoplasmic reticulum-resident chaperone BiP (GRP78). This finding is consistent with the endoplasmic reticulum being the subcellular site of IgM polymerization. We conclude that mu chain alone has the potential to direct the polymerization of secreted IgM, a process necessary but not sufficient for IgM to attain secretory competence.
Full text
PDF
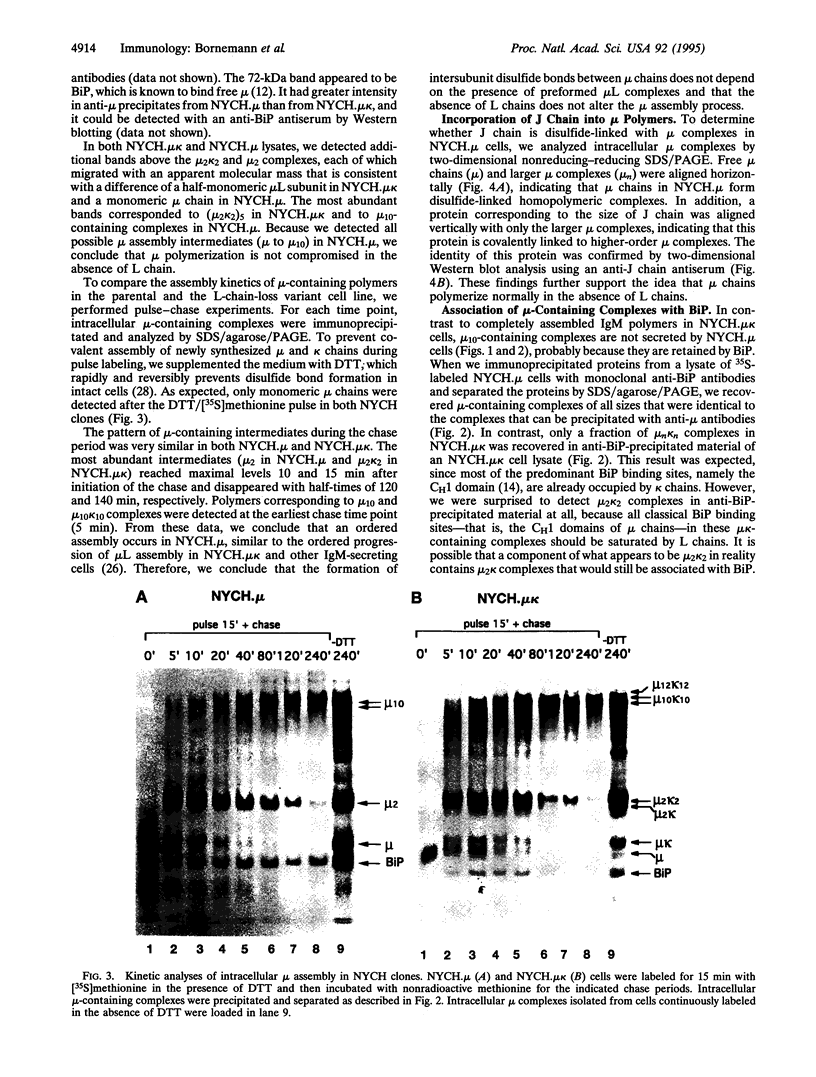
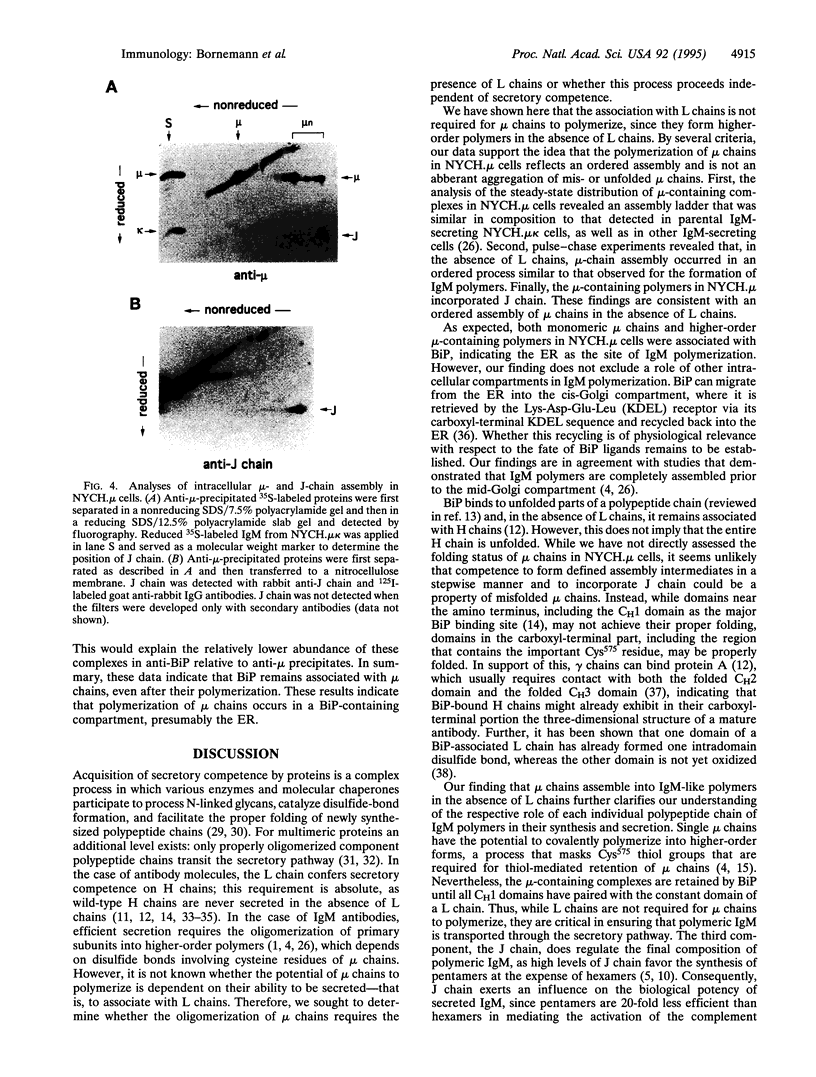

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck-Engeser G., Jäck H. M., Wabl M. Allelic inclusion in a pre-B-cell line that generates immunoglobulin heavy chain genes in vitro. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1060–1064. doi: 10.1073/pnas.84.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J., Lötscher E., Steimer K. S., Capon D. J., Baenziger J., Jäck H. M., Wabl M. Bispecific antibodies that mediate killing of cells infected with human immunodeficiency virus of any strain. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4723–4727. doi: 10.1073/pnas.88.11.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I., Helenius J., Helenius A. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 1992 May;11(5):1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer J. W., Randall T. D., Parkhouse R. M., Corley R. B. IgM hexamers? Immunol Today. 1994 Apr;15(4):165–168. doi: 10.1016/0167-5699(94)90313-1. [DOI] [PubMed] [Google Scholar]
- Brewer J. W., Randall T. D., Parkhouse R. M., Corley R. B. Mechanism and subcellular localization of secretory IgM polymer assembly. J Biol Chem. 1994 Jun 24;269(25):17338–17348. [PubMed] [Google Scholar]
- Burrows P. D., Beck G. B., Wabl M. R. Expression of mu and gamma immunoglobulin heavy chains in different cells of a cloned mouse lymphoid line. Proc Natl Acad Sci U S A. 1981 Jan;78(1):564–568. doi: 10.1073/pnas.78.1.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A., Neuberger M. S. Polymeric immunoglobulin M is secreted by transfectants of non-lymphoid cells in the absence of immunoglobulin J chain. EMBO J. 1987 Sep;6(9):2753–2758. doi: 10.1002/j.1460-2075.1987.tb02569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis R. M., Koshland M. E. Mechanism of IgM polymerization. Proc Natl Acad Sci U S A. 1974 Mar;71(3):657–661. doi: 10.1073/pnas.71.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E. Experimental studies of protein folding and unfolding. Prog Biophys Mol Biol. 1978;33(3):231–297. doi: 10.1016/0079-6107(79)90030-0. [DOI] [PubMed] [Google Scholar]
- Davis A. C., Roux K. H., Shulman M. J. On the structure of polymeric IgM. Eur J Immunol. 1988 Jul;18(7):1001–1008. doi: 10.1002/eji.1830180705. [DOI] [PubMed] [Google Scholar]
- Davis A. C., Shulman M. J. IgM--molecular requirements for its assembly and function. Immunol Today. 1989 Apr;10(4):118–128. doi: 10.1016/0167-5699(89)90244-2. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Jones T. A., Huber R., Sjödahl J., Sjöquist J. Crystallization, crystal structure analysis and atomic model of the complex formed by a human Fc fragment and fragment B of protein A from Staphylococcus aureus. Hoppe Seylers Z Physiol Chem. 1978 Aug;359(8):975–985. doi: 10.1515/bchm2.1978.359.2.975. [DOI] [PubMed] [Google Scholar]
- Fra A. M., Fagioli C., Finazzi D., Sitia R., Alberini C. M. Quality control of ER synthesized proteins: an exposed thiol group as a three-way switch mediating assembly, retention and degradation. EMBO J. 1993 Dec;12(12):4755–4761. doi: 10.1002/j.1460-2075.1993.tb06164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Haas I. G., Wabl M. R. Immunoglobulin heavy chain toxicity in plasma cells is neutralized by fusion to pre-B cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7185–7188. doi: 10.1073/pnas.81.22.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas I. G., Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983 Nov 24;306(5941):387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- Hendershot L., Bole D., Köhler G., Kearney J. F. Assembly and secretion of heavy chains that do not associate posttranslationally with immunoglobulin heavy chain-binding protein. J Cell Biol. 1987 Mar;104(3):761–767. doi: 10.1083/jcb.104.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Jäck H. M., Beck-Engeser G., Lee G., Wofsy D., Wabl M. Tumorigenesis mediated by an antigen receptor. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8482–8486. doi: 10.1073/pnas.89.18.8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäck H. M., Beck-Engeser G., Sloan B., Wong M. L., Wabl M. A different sort of Mott cell. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11688–11691. doi: 10.1073/pnas.89.24.11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Knittler M. R., Dirks S., Haas I. G. Molecular chaperones involved in protein degradation in the endoplasmic reticulum: quantitative interaction of the heat shock cognate protein BiP with partially folded immunoglobulin light chains that are degraded in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1764–1768. doi: 10.1073/pnas.92.5.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland M. E. The coming of age of the immunoglobulin J chain. Annu Rev Immunol. 1985;3:425–453. doi: 10.1146/annurev.iy.03.040185.002233. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Lodish H. F. Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell. 1986 Sep 12;46(6):929–937. doi: 10.1016/0092-8674(86)90075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leptin M., Potash M. J., Grützmann R., Heusser C., Shulman M., Köhler G., Melchers F. Monoclonal antibodies specific for murine IgM I. Characterization of antigenic determinants on the four constant domains of the mu heavy chain. Eur J Immunol. 1984 Jun;14(6):534–542. doi: 10.1002/eji.1830140610. [DOI] [PubMed] [Google Scholar]
- Mestecky J., Schrohenloher R. E. Site of attachment of J chain to human immunoglobulin M. Nature. 1974 Jun 14;249(458):650–652. doi: 10.1038/249650a0. [DOI] [PubMed] [Google Scholar]
- Morrison S. L., Scharff M. D. Heavy chain-producing variants of a mouse myeloma cell line. J Immunol. 1975 Feb;114(2 Pt 1):655–659. [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Recycling of proteins between the endoplasmic reticulum and Golgi complex. Curr Opin Cell Biol. 1991 Aug;3(4):585–591. doi: 10.1016/0955-0674(91)90027-v. [DOI] [PubMed] [Google Scholar]
- Randall T. D., Brewer J. W., Corley R. B. Direct evidence that J chain regulates the polymeric structure of IgM in antibody-secreting B cells. J Biol Chem. 1992 Sep 5;267(25):18002–18007. [PubMed] [Google Scholar]
- Randall T. D., King L. B., Corley R. B. The biological effects of IgM hexamer formation. Eur J Immunol. 1990 Sep;20(9):1971–1979. doi: 10.1002/eji.1830200915. [DOI] [PubMed] [Google Scholar]
- Randall T. D., Parkhouse R. M., Corley R. B. J chain synthesis and secretion of hexameric IgM is differentially regulated by lipopolysaccharide and interleukin 5. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):962–966. doi: 10.1073/pnas.89.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. A., Pierce S. B. In vivo cross-linking of protein disulfide isomerase to immunoglobulins. Biochemistry. 1987 Jul 14;26(14):4179–4182. doi: 10.1021/bi00388a001. [DOI] [PubMed] [Google Scholar]
- Sitia R., Neuberger M., Alberini C., Bet P., Fra A., Valetti C., Williams G., Milstein C. Developmental regulation of IgM secretion: the role of the carboxy-terminal cysteine. Cell. 1990 Mar 9;60(5):781–790. doi: 10.1016/0092-8674(90)90092-s. [DOI] [PubMed] [Google Scholar]
- Sonenshein G. E., Siekevitz M., Siebert G. R., Gefter M. L. Control of immunoglobulin secretion in the murine plasmacytoma line MOPC 315. J Exp Med. 1978 Jul 1;148(1):301–312. doi: 10.1084/jem.148.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton D. E., Desaymard C., Scharff M. D. Use of monoclonal anti-mouse immunoglobulin to detect mouse antibodies. Hybridoma. 1981;1(1):5–11. doi: 10.1089/hyb.1.1981.1.5. [DOI] [PubMed] [Google Scholar]