Abstract
We performed a systematic literature search regarding maternal diabetes before and during pregnancy and the risk of autism spectrum disorders (ASD) in the offspring. Of the 178 potentially relevant articles, 12 articles including three cohort studies and nine case–control studies were included in the meta-analysis. Both the meta-analyses of cohort studies and case–control studies showed significant associations. The pooled relative risk and 95 % confidence interval (CI) among cohort studies was 1.48 (1.25–1.75, p < 0.001). For case–control studies, the pooled odds ratio and 95 % CI was 1.72 (1.24–2.41, p = 0.001). No indication of significant heterogeneity across studies or publication bias was observed. In conclusion, maternal diabetes was significantly associated with a greater risk of ASD in the offspring.
Keywords: Autism spectrum disorders, Diabetes, Pregnancy
Introduction
Autism spectrum disorders (ASD) are a group of developmental disabilities characterized by deficits in socialization, communication, and repetitive or unusual behaviors (Levy et al. 2009; Rapin 2002). A substantial upward trend for ASD prevalence has been reported since the 1960s, with a recent estimate of about 113 cases per 10,000 (one in 88) children in the United States [Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators and Centers for Disease Control and Prevention (2012)]. Although the underlying mechanisms still remain to be elucidated, ASD is regarded as multifactorial, with genetic and non-genetic risk factors acting together to produce the phenotype (Bailey et al. 1995; Levy et al. 2009; London 2000). Inspired by the conceptual framework of the developmental origins of health and disease (DOHaD) hypothesis (Van den Bergh 2011) and exciting findings on the effects of early intrauterine environment insults on brain development (Gillberg 1999; Rodier et al. 1996), increasing research initiatives have concentrated on the identification of early life determinants for ASD risk (Gardener et al. 2009; Gardener et al. 2011; Guinchat et al. 2012; Kolevzon et al. 2007; Newschaffer et al. 2007).
Diabetes affects up to 15 % of pregnant women worldwide (International Diabetes Federation 2013). Approximately 87.5 % of maternal diabetes are due to gestational diabetes (GDM), defined as glucose intolerance with onset or first recognition during pregnancy (American Diabetes Association 2004), 7.5 % to pre-existing type 1 diabetes (T1DM), and 5 % to pre-existing type 2 diabetes (T2DM) (National Institute for Health and Care Excellence 2009). The prevalence of maternal diabetes is increasing (Albrecht et al. 2010; Bardenheier et al. 2013; Bell et al. 2008; Ferrara 2007; Hayes et al. 2011). Maternal diabetes has been associated with an increased risk of miscarriage, macrosomia and other adverse fetal outcomes (Herrera and Ortega-Senovilla 2010), as well as poor neurodevelopmental outcomes in the offspring (Anderson et al. 2005; Georgieff 2006; Ornoy et al. 2001; Rizzo et al. 1995). Furthermore, a positive association between maternal diabetes and ASD risk in the offspring was recently reported in a case–control study (Leonard et al. 2006) and two subsequent cohort studies (Burstyn et al. 2010; Lyall et al. 2012). However, the results from other studies have been conflicting and inconclusive (Buchmayer et al. 2009; Croen et al. 2005; Dodds et al. 2011; Elhameed et al. 2011; Hultman et al. 2002; Krakowiak et al. 2012).
In this study, our objective was to systematically review the evidence regarding the association between maternal diabetes and risk of ASD in the offspring, and to quantitatively summarize the data in a meta-analysis.
Methods
We followed the guidelines in the meta-analysis of observational studies in epidemiology (MOOSE) statement (Stroup et al. 2000) when conducting this study. The study protocol was prospectively registered in an international prospective register of systematic reviews (PROSPERO, http://www.crd.york.ac.uk/PROSPERO) as CRD42012003373.
Literature Search and Study Selection
A systematic literature search was performed in the MEDLINE/PubMed, EMBASE and PsycINFO databases through February 3rd, 2013, using a combination of free text and subheadings terms. Details of the search terms were shown in the Supplementary Text. In addition, the references listed in any relevant articles were screened. No language restriction was applied for searching or study inclusion.
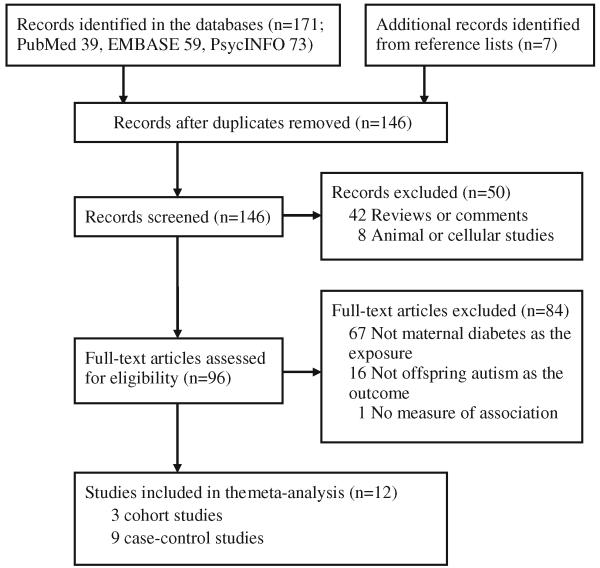
A published article was included if it had a case–control or cohort study design, evaluated the association between maternal diabetes and the risk of offspring ASD, and reported the risk estimates [relative risk (RR), or odds ratio (OR)] and corresponding 95 % confidence intervals (CIs) or standard errors, or provided sufficient data to calculate the risk estimates. Maternal diabetes herein includes GDM, pre-existing T1DM, and pre-existing T2DM. During the screening steps, several types of articles were excluded: review articles, editorials, or comments; studies on animals or cell lines; studies that did not evaluate maternal diabetes as exposure; and studies that did not include offspring ASD as the outcome. In addition, studies that did not report risk estimates or 95 % CIs for the relationships between maternal diabetes and risk of offspring ASD were excluded. The process of study selection is depicted in Fig. 1.
Fig. 1.
Flow chart for study selection (through February 3rd, 2013)
Data Extraction and Quality Assessment
Two investigators independently evaluated methodological quality in each study and extracted all relevant data using a standardized data extraction form. Any discrepancies were resolved by consensus.
Methodological quality of the included studies was assessed according to the Newcastle-Ottawa Scale (Wells et al. 2013), which was developed for assessing the quality of non-randomized studies in meta-analyses. A maximum of nine stars were given to each study based on the following perspectives: the selection of the study groups (four stars); the comparability of the groups (two stars); and the ascertainment of the outcome of interest in cohort study or ascertainment of the exposure of interest in case–control study (three stars). For each study, we assigned scores of 0–3, 4–6, and 7–9 for low, moderate, and high quality, respectively.
The following data were extracted from each included article: basic information (title, author, publication year), study characteristics (study design, location), participant characteristics (sample size, number of ASD cases, age, race/ethnicity), diagnostic criteria of maternal diabetes and ASD, statistical methods used for the analysis, risk estimates and 95 % CIs before and after adjustment for covariates if they were reported, and any covariates that were matched or adjusted for in the multivariate model. For studies which reported the risk estimates from several adjustment models, we extracted those that reflected the maximum extent of adjustment for potentially confounding variables. Also, we separately extracted risk estimates for the association of maternal diabetes before and during pregnancy with ASD risk if they were separately reported in the original articles.
Statistical Analysis
The risk estimates and corresponding 95 % CIs reported in the individual studies were pooled using the random-effects model (DerSimonian–Laird method), which incorporates between-study heterogeneity in addition to sampling variation (DerSimonian and Laird 1986). Data from cohort and case–control studies were analyzed separately, with pooled RR and OR as the measures of association, respectively. For cohort studies, the OR was reported in one study; however, because the incidence of ASD was sufficiently low for the rare disease assumption (<10 %) to apply, the OR was assumed to approximate the RR.
Potential sources of heterogeneity across studies were assessed using the χ2 based Cochran’s Q statistic (p < 0.10 was considered to be significant heterogeneity), and the I2 metric (I2 values of 25, 50, and 75 % were considered as low, medium, and high heterogeneity, respectively) (Higgins and Thompson 2002). Funnel plots were used to assess small-study effects. The possibility of publication bias was assessed using the Egger regression asymmetry test (Egger et al. 1997). For sensitivity analysis, we also used the fixed-effect model for all the above analyses. Additional sensitivity analyses were performed by omitting one study at a time and calculating a pooled estimate for the remainder of the studies to evaluate whether the results were affected markedly by a single study.
All statistical analyses were performed using Stata software (version 11.2; Stata Corp, College Station, TX, USA). All p values presented are two-tailed with a significance level of 0.05, except for the Cochran’s Q statistic in the heterogeneity test, in which the significance level was 0.10 (Higgins and Thompson 2002).
Results
Characteristics of Studies Included in the Meta-Analysis
We identified 178 potentially relevant articles from the literature search and reference list, of which 12 studies (Brimacombe et al. 2007; Buchmayer et al. 2009; Burstyn et al. 2010; Croen et al. 2005; Dodds et al. 2011; Elhameed et al. 2011; Hultman et al. 2002; Juul-Dam et al. 2001; Krakowiak et al. 2012; Leonard et al. 2006; Lyall et al. 2012; Piven et al. 1993) met our criteria for inclusion. The characteristics of the included studies are shown in Table 1. Of the included studies, nine were case–control studies, while the other three were cohort studies. Six studies were conducted in the USA, two in Canada, two in Sweden, one in Australia and one in Egypt. Six studies were assigned to high quality (Buchmayer et al. 2009; Burstyn et al. 2010; Croen et al. 2005; Hultman et al. 2002; Krakowiak et al. 2012; Leonard et al. 2006), five to moderate quality (Dodds et al. 2011; Elhameed et al. 2011; Juul-Dam et al. 2001; Lyall et al. 2012; Piven et al. 1993), and one to low quality (Brimacombe et al. 2007), according to the Newcastle-Ottawa Scale (Supplementary Table 1 & Supplementary Table 2).
Table 1.
Characteristics of the included studies (n = 12) regarding the association between maternal diabetes and autism spectrum disorders in the offspring
| Author, year | Country | Ethnic origin |
Sample sized | Maternal diabetes criteria |
Autism criteria | Age of autism children |
Crude risk estimates (95 % CI) |
Adjusted risk estimates (95 % CI) |
Matched or adjusted covariates |
|---|---|---|---|---|---|---|---|---|---|
| Case–control studies | |||||||||
| Piven et al. (1993) | USA | N/A | 39/39 | N/A | ADI and ADOS (autism) |
Mean 14.5 year | N/A | 3.08 (0.12–77.91) |
Birth ordera |
| Juul-Dam et al. (2001) | USA | Mixed | 74/? | N/A | DSM-IV, ADI-R, CARS, and ADOS (autism and PDD- NOS) |
Range 2.5–4 year |
3.28 (1.32–8.17) | N/A | Noneb |
| Hultman et al. (2002) | Sweden | Caucasians | 408/ 2,040 |
ICD-8 and ICD- 9 (pregestational and gestational diabetes) |
ICD-9 (autism) | Mean 4.4 (boy)/ 4.6 (girl) |
1.80 (0.60–5.70) | 1.20 (0.30–5.70) | Sex, year, hospital of birth, maternal smoking, pregnancy- induced hypertensive diseases, and other maternal factors (maternal age and parity, and mother’s country of birth) |
| Croen et al. (2005) | USA | Mixed | 407/ 2,095 |
ICD-9-CM (type 1 diabetes during pregnancy) |
ICD-9-CM (ASD) | Range 3–7 year | 2.90(1.00–8.80) | 2.60 (0.80–7.90) | Sex, birth year, hospital of birth, maternal age, maternal education, maternal race/ ethnicity, and plurality |
| Leonard et al. (2006) | Australia | N/A | 191/ 236,964 |
ICD-9 (pregestational and gestational diabetes) |
DSM-IV-R (ASD with intellectual disability) |
Range 0–16 year |
2.89(1.28–6.51) | 2.95 (1.30–6.73) | Infant sex, birth order, maternal ethnicity, age group, marital status, height, country of birth, health insurance status, and paternal occupation, and the accessibility/remoteness index of Australia |
| Brimacombe et al. (2007) | USA | Mixed | 164/ 115,542 |
N/A | DSM-IV, CARS, and ADOS (ASD) |
Mean 6.8 year | 1.61 (0.82–3.17) | N/A | Nonec |
| Buchmayer et al. (2009) | Sweden | Caucasians | 1,216/ 6,080 |
ICD-9 and ICD- 10 (pregestational and gestational diabetes) |
ICD-9 and ICD-10 (autistic disorder) |
<10 year | 1.13 (0.67–1.89) | 0.90 (0.49–1.67) | Age, gender, birth year, birth hospital, maternal age, smoking, maternal country of birth, whether the mother lived with the father, and maternal schizophrenia |
| Elhameed et al. (2011) | Egypt | N/A | 14/28 | N/A | CARS (autism) | Mean 10.9 year | 6.33 (0.24–165.88) |
N/A | Nonea |
| Krakowiak et al. (2012) | USA | Mixed | 517/315 | N/A | ADI-R and ADOS (ASD) |
Mean 3.65 year | 1.51 (0.86–2.74) | 1.52 (0.82–2.83) | Age, gender, and regional center catchment area, mother’s age at delivery, race/ethnicity, education level, delivery payer, calendar time, child’s age at enrollment and gender, and catchment area |
| Cohort studies | |||||||||
| Burstyn et al. (2010) | Canada | Mixed | 1,138/ 218,890 |
ICD-9 (pregestational and gestational diabetes) |
ICD-9 (ASD) | 1,998 birth cohort: 69 mo (boy)/74 mo (girl); 2,002 birth cohort: 45 mo(boy )/40 mo (girl) |
1.87(1.14–3.06) for pregestational diabetes; 1.41 (1.07–1.85) for gestational diabetes |
1.65 (1.01–2.71) for pregestational diabetes; 1.24 (0.94–1.65) for gestational diabetes |
Maternal age, maternal weight, maternal height, bleeding, cigarette smoking, poor weight gain, parity, socioeconomic status of mother, presentation, type of labour, delivery by Caesarian section, gestational age, birth weight, Apgar at 1 min and 5 min, female child, and birth year |
| Dodds et al. (2011) | Canada | N/A | 924/ 129,733 |
ICD-9 and ICD- 10 (pregestational and gestational diabetes) |
ICD-9 (autism, other specified PDD), and ICD-10 (autism, atypical autism, Asperger syndrome, and unspecified PDD) |
Range 0–17 year |
1.98 (0.94–4.16) for pregestational diabetes; 1.29 (0.90–1.83) for gestational diabetes |
N/A | None |
| Lyall et al. (2012) | USA | Mixed | 793/ 66,445 |
N/A | ADI-R (ASD) | N/A | 2.34 (1.80–3.04) | 1.76 (1.34–2.32) | Race, marital status, income, and spouse education, age, age-at- first-birth, and parity, twin births, pregnancy-related high blood pressure, toxemia, induced abortions, and miscarriages |
ADI-R autism diagnostic interview-revised, ADOS autism diagnostic observation schedule, CARS childhood autism rating scale, DSM-IV-R diagnostic and statistical manual of mental disorders, fourth edition, recommendations, ICD international classification of diseases, PDD pervasive developmental disorders
Controls were siblings of the cases
Control group was the entire US population
Control group was the New Jersey population
Sample sizes were incident cases/participants for cohort studies, and cases/controls for case–control studies
For the maternal diabetes case definition, all the three cohort studies included data of maternal diabetes developed during pregnancy. In addition, Burstyn et al. (2010) and Dodds et al. (2011) also reported the associations of maternal diabetes before pregnancy with ASD risk in the offspring, thus we included these results separately with the data with maternal diabetes during pregnancy in the meta-analysis. Among the case–control studies, five studies included data of maternal diabetes during pregnancy, and the other four studies included mixed data of maternal diabetes before and during pregnancy. The diagnosis of maternal diabetes was made based on the International Classification of Diseases (ICD) in six studies, and in the remaining studies the diagnostic criteria were not specified.
Several assessment and/or diagnostic tools were used for the ascertainment of ASD cases. The ICD-9 or ICD-10 criteria were used in five studies, both the autism diagnostic interview-revised (ADI-R) and the autism diagnostic observation schedule (ADOS) in four studies, and both the diagnostic and statistical manual of mental disorders, fourth edition (DSM-IV-R) and the Childhood Autism Rating Scale (CARS) in three studies.
Maternal Diabetes and the Risk of Offspring ASD
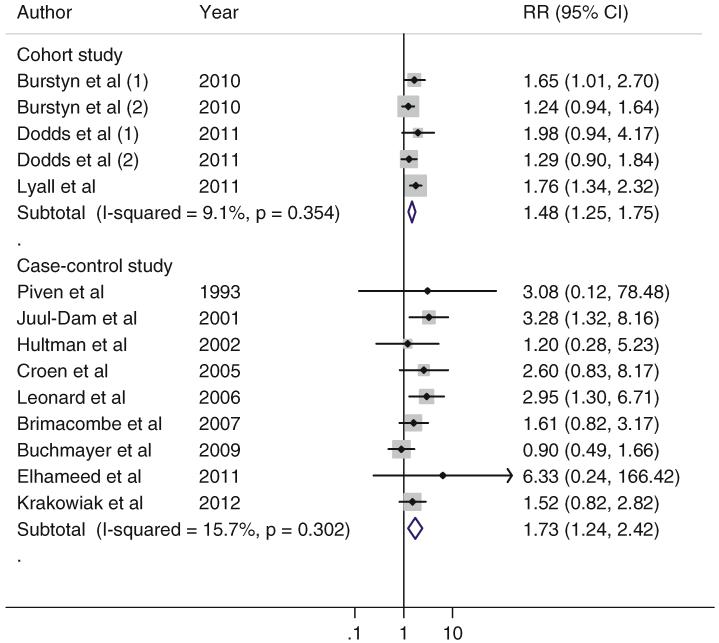
Both the meta-analyses of cohort studies and case–control studies showed significant associations between maternal diabetes and ASD risk in the offspring (Fig. 2). For cohort studies, the pooled RR (95 % CI) for ASD in mothers with maternal diabetes compared those without was 1.48 (1.25–1.75), without significant heterogeneity (I2 = 9.1, p = 0.35); for case–control studies, the pooled OR (95 % CI) for ASD was 1.73 (1.24–2.42), without significant heterogeneity (I2 = 15.7, p = 0.30). There was no evidence of publication bias as indicated by the Egger asymmetry test (p = 0.59 for cohort studies; p = 0.21 for case–control studies) (Supplementary Figure 1). Sensitivity analyses using fixed-effect model yielded similar results.
Fig. 2.
Association of maternal diabetes and ASD in the offspring in the included studies, according to study design
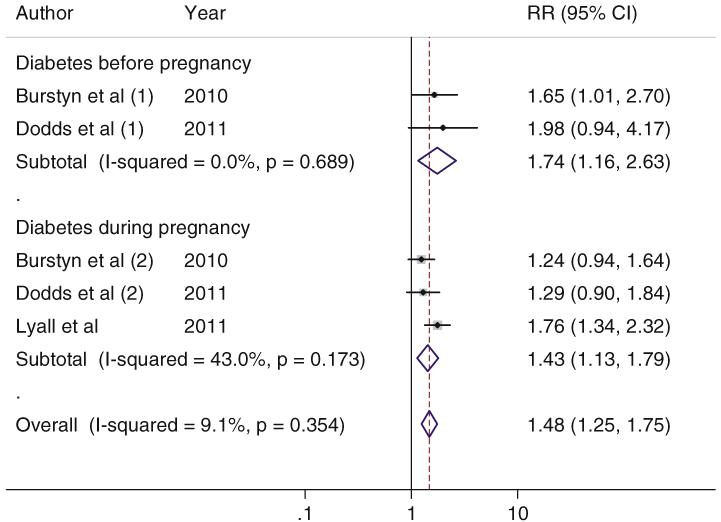
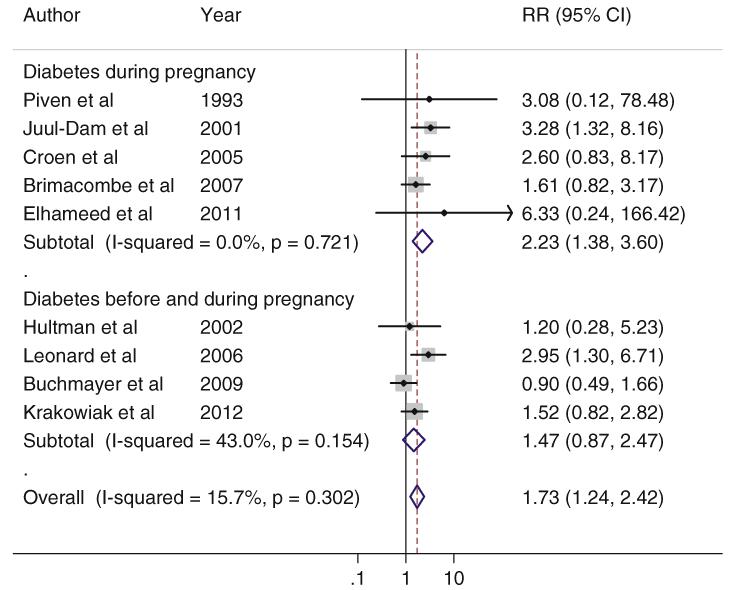
We further explored whether the associations differed by maternal diabetes developed before and during pregnancy. In the cohort studies, the pooled RRs (95 % CIs) for ASD were 1.75 (1.16–2.63) for maternal diabetes before pregnancy and 1.43 (1.13–1.79) for maternal diabetes during pregnancy. For the case–control studies, the pooled ORs (95 % CIs) were 2.23 (1.38–3.60) for maternal diabetes during pregnancy and 1.47 (0.87–2.47) for the mixed cases of maternal diabetes before and during pregnancy (Figs. 3, 4).
Fig. 3.
Association of maternal diabetes and ASD in the offspring in the included cohort studies, according to the maternal diabetes case definition
Fig. 4.
Association of maternal diabetes and ASD in the offspring in the included case–control studies, according to the maternal diabetes case definition
In the sensitivity analysis by omitting one study at a time, there was no substantial change in the pooled risk estimates among the remainder of studies (Supplementary Figure 2); the pooled RRs (95 % CIs) for ASD ranged from 1.35 (1.12–1.64) to 1.60 (1.32–1.94) in the remaining cohort studies, and the pooled ORs (95 % CIs) for ASD ranged from 1.55 (1.14–2.12) to 2.02 (1.44–2.82) in the remaining case–control studies. For case–control studies, we also performed a sensitivity analysis that only included the high quality rated studies (Buchmayer et al. 2009; Croen et al. 2005; Hultman et al. 2002; Krakowiak et al. 2012; Leonard et al. 2006); the pooled RR (95 % CI) for ASD was 1.50 (1.05–2.14).
Discussion
In this systematic review and meta-analysis, we found that maternal diabetes was associated with an increased risk of offspring ASD. Similar results were observed in the meta-analysis of cohort studies and case–control studies.
Our results support the DOHaD hypothesis which highlights the effects of early life exposure to environmental insults, in particular during a sensitive period in utero or early postnatal development, on short- and long-term health and disease risk (Van den Bergh 2011). In utero exposure to hyperglycemia, a consequence of any types of maternal diabetes, may increase the risk of offspring ASD involving several plausible biological mechanisms. First, maternal hyperglycemia can result in hypoxia in the fetus (Eidelman and Samueloff 2002), and a depleted oxygen supply to the fetus may impair neurodevelopment and thus contribute to a greater risk of ASD (Burstyn et al. 2011; Kolevzon et al. 2007). Second, maternal hyperglycemia has been associated with increased free-radical production and impaired antioxidant defense system that lead to oxidative stress in the cord blood and placental tissue (Biri et al. 2006; Chen and Scholl 2005). The positive association between oxidative stress and ASD in children was reported in several studies (Chauhan et al. 2004; Ming et al. 2005; Yao et al. 2006). Third, excessive adiposity that commonly accompanies T2DM and gestational diabetes are inducers of chronic inflammation. A number of studies have identified both neuro and systemic markers of inflammation in children with autism (Onore et al. 2012). Also, it has been hypothesized that insulin signaling may contribute to development of autism in genetically susceptible individuals via activation of PI3K/Tor pathway in neurons (Stern 2011). Fourth, T1DM is an autoimmune disorder resulting from a cellular-mediated autoimmune destruction of pancreatic beta-cells (American Diabetes Association 2013). Maternal altered autoimmunity may influence brain development in the off-spring either by creating a hostile intrauterine environment or by modifying the offspring’s autoimmunity in early development through immunoglobulin G (Keil et al. 2010). Fifth, epigenetic modification by hyperglycemia (Fernandez-Morera et al. 2010; Schanen 2006) may also be implicated in the pathogenesis of ASD, but the current evidence is still sparse.
The strengths of this study include comprehensive literature search, clearly defined criteria for study selection, and meticulous protocol for review. Several limitations merit discussion. First, the number of studies was limited, which may influence the statistical power of testing the small-study effects and heterogeneity. Even though the number of studies was small, little inter-study heterogeneity was detected using statistical tests (i.e., Cochran’s Q statistic and I2 metric) in this meta-analysis. Second, most of the included studies were conducted in western populations. Studies conducted in other populations, among which an increase of ASD prevalence has been documented (Wong and Hui 2008), are warranted. Third, the study quality in several included studies was low to moderate, which may also impact the overall stability of the pooled statistic. For instance, the studies by Juul-Dam et al. (2001), Brimacombe et al. (2007), Dodds et al. (2011) and Elhameed et al. (2011) did not adjust for potential confounders. The studies by Piven et al. (1993) and Elhameed et al. (2011) had small sample size and high imprecision (very wide CIs) of ORs. The studies by Juul-Dam et al. (2001) and Brimacombe et al. (2007) used suboptimal control groups. The study by Croen et al. (2005) only examined maternal T1DM in relation to ASD in children; this form of diabetes is an autoimmune condition that is rarer than the etiologically similar T2DM and GDM, and so women with the latter two forms of diabetes may have been included as controls. Fourth, misclassification bias is possible for self-reported maternal diabetes in the included studies. However, this may not be a substantial concern because individuals generally tend to report their diabetes status with moderate to high accuracy according to several validation studies (Huerta et al. 2009; Okura et al. 2004; Solomon et al. 1997). Moreover, if there was any misclassification in prospective cohort studies, which should be non-differential because of the prospective nature, the association between maternal diabetes and ASD risk in offspring may have been underestimated. Fifth, mixed definitions of maternal diabetes and different assessment criteria of ASD were used in the included studies. In the current study, the associations between maternal diabetes and ASD in the offspring were rather consistent across different definitions. However, we cannot fully exclude the possibility of potential heterogeneity due to the limited number of included studies. Clearly, when more accumulating evidence is available, the associations of different types of maternal diabetes (i.e., T1DM, T2DM, and GDM) with different phenotypes of ASD (i.e., autism, Asperger syndrome, pervasive developmental disorder not otherwise specified, etc.) merit further evaluation. Finally, we cannot rule out the possibility of publication bias. Conceptually, studies with small sample size and non-significant results are prone to be unpublished. However, this concern may not be substantial in our study because we found that a number of such studies have already been published and their results were included in our meta-analysis.
Conclusions
In summary, maternal diabetes was associated with a significantly greater risk of ASD in the offspring. Because the number of available studies is still limited, our findings should be taken with caution. More future studies, in particular population-based prospective cohort studies, are needed to confirm the association. In addition, to better understand the association, future studies exploring the underlying molecular mechanisms are warranted.
Supplementary Material
Acknowledgments
This study was funded by the National Natural Science Foundation of China (No. 81172687) and the Doctoral Fund of Ministry of Education of China (No. 20110171110053). Dr. Wei Bao was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, USA.
Abbreviations
- ADI-R
Autism diagnostic interview-revised
- ADOS
The autism diagnostic observation schedule
- ASD
Autism spectrum disorders
- CARS
The childhood autism rating scale
- CI
Confidence interval
- DSM-IV
The diagnostic and statistical manual of mental disorders, fourth edition
- ICD
International classification of diseases
- OR
Odds ratio
- RR
Relative risk
Footnotes
Registration: This review was registered at the PROSPERO as CRD42012003373.
Electronic supplementary material The online version of this article (doi:10.1007/s10803-013-1928-2) contains supplementary material, which is available to authorized users.
Contributor Information
Guifeng Xu, Department of Maternal and Child Health, School of Public Health, Sun Yat-Sen University, Guangzhou 510080, China.
Jin Jing, Department of Maternal and Child Health, School of Public Health, Sun Yat-Sen University, Guangzhou 510080, China.
Katherine Bowers, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH 45229, USA.
Buyun Liu, Department of Maternal and Child Health, School of Public Health, Sun Yat-Sen University, Guangzhou 510080, China.
Wei Bao, Epidemiology Branch, Division of Epidemiology, Statistics, and Prevention Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Rockville, MD 20852, USA.
References
- Albrecht SS, Kuklina EV, Bansil P, Jamieson DJ, Whiteman MK, Kourtis AP, et al. Diabetes trends among delivery hospitalizations in the US, 1994-2004. Diabetes Care. 2010;33:768–773. doi: 10.2337/dc09-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association Gestational diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S88–S90. doi: 10.2337/diacare.27.2007.s88. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JL, Waller DK, Canfield MA, Shaw GM, Watkins ML, Werler MM. Maternal obesity, gestational diabetes, and central nervous system birth defects. Epidemiology. 2005;16:87–92. doi: 10.1097/01.ede.0000147122.97061.bb. [DOI] [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators. Centers for Disease Control and Prevention Prevalence of autism spectrum disorders–Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61:1–19. [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychological Medicine. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Bardenheier BH, Elixhauser A, Imperatore G, Devlin HM, Kuklina EV, Geiss LS, et al. Variation in prevalence of gestational diabetes mellitus among hospital discharges for obstetric delivery across 23 states in the United States. Diabetes Care. 2013;36:1209–1214. doi: 10.2337/dc12-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R, Bailey K, Cresswell T, Hawthorne G, Critchley J, Lewis-Barned N. Trends in prevalence and outcomes of pregnancy in women with pre-existing type I and type II diabetes. BJOG: An International Journal of Obstetrics and Gynaecology. 2008;115:445–452. doi: 10.1111/j.1471-0528.2007.01644.x. [DOI] [PubMed] [Google Scholar]
- Biri A, Onan A, Devrim E, Babacan F, Kavutcu M, Durak I. Oxidant status in maternal and cord plasma and placental tissue in gestational diabetes. Placenta. 2006;27:327–332. doi: 10.1016/j.placenta.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Brimacombe M, Ming X, Lamendola M. Prenatal and birth complications in autism. Maternal and Child Health Journal. 2007;11:73–79. doi: 10.1007/s10995-006-0142-7. [DOI] [PubMed] [Google Scholar]
- Buchmayer S, Johansson S, Johansson A, Hultman CM, Sparen P, Cnattingius S. Can association between preterm birth and autism be explained by maternal or neonatal morbidity? Pediatrics. 2009;124:e817–e825. doi: 10.1542/peds.2008-3582. [DOI] [PubMed] [Google Scholar]
- Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Diseases in Canada. 2010;30:125–134. [PubMed] [Google Scholar]
- Burstyn I, Wang X, Yasui Y, Sithole F, Zwaigenbaum L. Autism spectrum disorders and fetal hypoxia in a population-based cohort: accounting for missing exposures via Estimation-Maximization algorithm. BMC Medical Research Methodology. 2011;11:2. doi: 10.1186/1471-2288-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, Chauhan V, Brown WT, Cohen I. Oxidative stress in autism: increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin–the antioxidant proteins. Life Sciences. 2004;75:2539–2549. doi: 10.1016/j.lfs.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Chen X, Scholl TO. Oxidative stress: Changes in pregnancy and with gestational diabetes mellitus. Current Diabetes Reports. 2005;5:282–288. doi: 10.1007/s11892-005-0024-1. [DOI] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: A case–control study. Archives of Pediatrics and Adolescent Medicine. 2005;159:151–157. doi: 10.1001/archpedi.159.2.151. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S. The role of prenatal, obstetric and neonatal factors in the development of autism. Journal of Autism and Developmental Disorders. 2011;41:891–902. doi: 10.1007/s10803-010-1114-8. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelman AI, Samueloff A. The pathophysiology of the fetus of the diabetic mother. Seminars in Perinatology. 2002;26:232–236. doi: 10.1053/sper.2002.34215. [DOI] [PubMed] [Google Scholar]
- Elhameed MAA, Elbaky AEOA, Kamel EA. A controlled study of the risk factors and clinical picture of children with autism in an Egyptian sample. Egypt Journal of Neurology, Neurosurgery and Psychiatry. 2011;48:271–276. [Google Scholar]
- Fernandez-Morera JL, Rodriguez-Rodero S, Menendez-Torre E, Fraga MF. The possible role of epigenetics in gestational diabetes: Cause, consequence, or both. Obstetrics and Gynecology International. 2010;2010:605163. doi: 10.1155/2010/605163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara A. Increasing prevalence of gestational diabetes mellitus: A public health perspective. Diabetes Care. 2007;30(Suppl 2):S141–S146. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: Comprehensive meta-analysis. British Journal of Psychiatry. 2009;195:7–14. doi: 10.1192/bjp.bp.108.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: A comprehensive meta-analysis. Pediatrics. 2011;128:344–355. doi: 10.1542/peds.2010-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieff MK. The effect of maternal diabetes during pregnancy on the neurodevelopment of offspring. Minnesota Medicine. 2006;89:44–47. [PubMed] [Google Scholar]
- Gillberg C. Neurodevelopmental processes and psychological functioning in autism. Development and Psychopathology. 1999;11:567–587. doi: 10.1017/s0954579499002217. [DOI] [PubMed] [Google Scholar]
- Guinchat V, Thorsen P, Laurent C, Cans C, Bodeau N, Cohen D. Pre-, peri- and neonatal risk factors for autism. Acta Obstetricia et Gynecologica Scandinavica. 2012;91:287–300. doi: 10.1111/j.1600-0412.2011.01325.x. [DOI] [PubMed] [Google Scholar]
- Hayes DK, Fan AZ, Smith RA, Bombard JM. Trends in selected chronic conditions and behavioral risk factors among women of reproductive age, behavioral risk factor surveillance system, 2001-2009. Preventing Chronic Disease. 2011;8:A120. [PMC free article] [PubMed] [Google Scholar]
- Herrera E, Ortega-Senovilla H. Disturbances in lipid metabolism in diabetic pregnancy: Are these the cause of the problem? Best Practice and Research Clinical Endocrinology and Metabolism. 2010;24:515–525. doi: 10.1016/j.beem.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Huerta JM, Tormo MJ, Egea-Caparros JM, Ortola-Devesa JB, Navarro C. Accuracy of self-reported diabetes, hypertension and hyperlipidemia in the adult Spanish population. DINO study findings. Revista Espanola de Cardiologia. 2009;62:143–152. doi: 10.1016/s1885-5857(09)71532-4. [DOI] [PubMed] [Google Scholar]
- Hultman CM, Sparen P, Cnattingius S. Perinatal risk factors for infantile autism. Epidemiology. 2002;13:417–423. doi: 10.1097/00001648-200207000-00009. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation [Accessed July 10, 2013];IDF Policy Briefing: Diabetes in Pregnancy: Protecting Maternal Health. 2013 http://www.idf.org/sites/default/files/Policy_Briefing_DiabetesInPregancy.pdf.
- Juul-Dam N, Townsend J, Courchesne E. Prenatal, perinatal, and neonatal factors in autism, pervasive developmental disorder-not otherwise specified, and the general population. Pediatrics. 2001;107:E63. doi: 10.1542/peds.107.4.e63. [DOI] [PubMed] [Google Scholar]
- Keil A, Daniels JL, Forssen U, Hultman C, Cnattingius S, Soderberg KC, et al. Parental autoimmune diseases associated with autism spectrum disorders in offspring. Epidemiology. 2010;21:805–808. doi: 10.1097/EDE.0b013e3181f26e3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: a review and integration of findings. Archives of Pediatrics and Adolescent Medicine. 2007;161:326–333. doi: 10.1001/archpedi.161.4.326. [DOI] [PubMed] [Google Scholar]
- Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129:e1121–e1128. doi: 10.1542/peds.2011-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard H, de Klerk N, Bourke J, Bower C. Maternal health in pregnancy and intellectual disability in the offspring: A population-based study. Annals of Epidemiology. 2006;16:448–454. doi: 10.1016/j.annepidem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Levy SE, Mandell DS, Schultz RT. Autism. Lancet. 2009;374:1627–1638. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London EA. The environment as an etiologic factor in autism: A new direction for research. Environmental Health Perspectives. 2000;108(Suppl 3):401–404. doi: 10.1289/ehp.00108s3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Pauls DL, Spiegelman D, Ascherio A, Santangelo SL. Pregnancy complications and obstetric suboptimality in association with autism spectrum disorders in children of the Nurses’ Health Study II. Autism Research. 2012;5:21–30. doi: 10.1002/aur.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Stein TP, Brimacombe M, Johnson WG, Lambert GH, Wagner GC. Increased excretion of a lipid peroxidation biomarker in autism. Prostaglandins Leukotrienes and Essential Fatty Acids. 2005;73:379–384. doi: 10.1016/j.plefa.2005.06.002. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence [Accessed July 10, 2013];NICE clinical guidelines 63: Diabetes in pregnancy: Management of diabetes and its complications from pre-conception to the postnatal period. 2009 http://guidance.nice.org.uk/CG63.
- Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, et al. The epidemiology of autism spectrum disorders. Annual Review of Public Health. 2007;28:235–258. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. Journal of Clinical Epidemiology. 2004;57:1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain, Behavior, and Immunity. 2012;26:383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornoy A, Ratzon N, Greenbaum C, Wolf A, Dulitzky M. School-age children born to diabetic mothers and to mothers with gestational diabetes exhibit a high rate of inattention and fine and gross motor impairment. Journal of Pediatric Endocrinology and Metabolism. 2001;14(Suppl 1):681–689. doi: 10.1515/jpem.2001.14.s1.681. [DOI] [PubMed] [Google Scholar]
- Piven J, Simon J, Chase GA, Wzorek M, Landa R, Gayle J, et al. The etiology of autism: Pre-, peri- and neonatal factors. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32:1256–1263. doi: 10.1097/00004583-199311000-00021. [DOI] [PubMed] [Google Scholar]
- Rapin I. The autistic-spectrum disorders. New England Journal of Medicine. 2002;347:302–303. doi: 10.1056/NEJMp020062. [DOI] [PubMed] [Google Scholar]
- Rizzo TA, Dooley SL, Metzger BE, Cho NH, Ogata ES, Silverman BL. Prenatal and perinatal influences on long-term psychomotor development in offspring of diabetic mothers. American Journal of Obstetrics and Gynecology. 1995;173:1753–1758. doi: 10.1016/0002-9378(95)90422-0. [DOI] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. Journal of Comparative Neurology. 1996;370:247–261. doi: 10.1002/(SICI)1096-9861(19960624)370:2<247::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Schanen NC. Epigenetics of autism spectrum disorders. Hum Mol Genet. 2006;15(2):R138–R150. doi: 10.1093/hmg/ddl213. [DOI] [PubMed] [Google Scholar]
- Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. The Journal of the American Medical Association. 1997;278:1078–1083. [PubMed] [Google Scholar]
- Stern M. Insulin signaling and autism. Frontiers in Endocrinology. 2011;2:54. doi: 10.3389/fendo.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group Meta-analysis of observational studies in epidemiology: a proposal for reporting. The Journal of the American Medical Association. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR. Developmental programming of early brain and behaviour development and mental health: a conceptual framework. Developmental Medicine and Child Neurology. 2011;53(Suppl 4):19–23. doi: 10.1111/j.1469-8749.2011.04057.x. [DOI] [PubMed] [Google Scholar]
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. [Accessed January 30, 2013];The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- Wong VC, Hui SL. Epidemiological study of autism spectrum disorder in China. Journal of Child Neurology. 2008;23:67–72. doi: 10.1177/0883073807308702. [DOI] [PubMed] [Google Scholar]
- Yao Y, Walsh WJ, McGinnis WR, Pratico D. Altered vascular phenotype in autism: correlation with oxidative stress. Archives of Neurology. 2006;63:1161–1164. doi: 10.1001/archneur.63.8.1161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.