Abstract
Development of subunit vaccines for malaria that elicit a strong, long-term memory response is an intensive area of research, with the focus on improving the immunogenicity of a circumsporozoite (CS) protein-based vaccine. In this study, we found that a chimeric protein, formed by fusing vaccinia virus protein 14K (A27) to the CS of Plasmodium yoelii, induces strong effector memory CD8+ T cell responses in addition to high-affinity Abs when used as a priming agent in the absence of any adjuvant, followed by an attenuated vaccinia virus boost expressing CS in murine models. Moreover, priming with the chimeric protein improved the magnitude and polyfunctionality of cytokine-secreting CD8+ T cells. This fusion protein formed oligomers/aggregates that led to activation of STAT-1 and IFN regulatory factor-3 in human macrophages, indicating a type I IFN response, resulting in NO, IL-12, and IL-6 induction. Furthermore, this vaccination regimen inhibited the liver stage development of the parasite, resulting in sterile protection. In summary, we propose a novel approach in designing CS based pre-erythrocytic vaccines against Plasmodium using the adjuvant-like effect of the immunogenic vaccinia virus protein 14K.
Malaria continues to present a major public health challenge and burden on economic development in many countries. Plasmodium falciparum, the main causative agent of malaria in humans, is known to cause ~225 million cases and ~781,000 deaths annually (1). Development of a cost-effective vaccine continues to be a daunting task because of the confounding ability of the parasite to manipulate the host immune system. The most advanced malaria vaccines have focused on the use of protein-in-adjuvant formulations, and a phase III clinical trial with RTS,S/AS01E (a liposome-based adjuvant system) is under way (2). First results of the phase II trial of RTS, S/AS01E in Africa revealed vaccine-reduced clinical episodes of malaria and severe malaria by about half during 12 mo after vaccination in children 5–7 mo age (3). The rationale behind the development of RTS,S is its ability to form virus-like particles, which enhances the immunogenicity of monomeric circumsporozoite (CS) protein. The ability of protein aggregates to stimulate strong immune responses compared with their monomeric counterparts has been studied for many decades and is supported by a number of recent studies (4–6).
CS protein is a monomeric protein of ~40–60 kDa covering the surface of infective sporozoites (7). However, the native CS from sporozoites is also known to form some aggregates that may facilitate the binding of CS to hepatocytes (8). The CS protein is GPI-anchored and consists of a central portion of immunodominant repeat regions of B and T cell epitopes (9). Reports of successful vaccination against malaria in humans have been attributed to the development of CS-specific humoral and cell-mediated immune responses (10). Therefore, the strong immunogenic nature of the CS protein and its presence during pre-erythrocytic and liver stages (11) make it a promising candidate for the development of a malaria vaccine.
Control of infection for most pathogens requires different strategies of intervention, including humoral or cell-mediated immune responses at different times in their life cycle. Therefore, an effective vaccination regimen should encompass these aspects to develop sterile immunity. Viral vaccine vectors expressing CS protein and other Plasmodium Ags as promising vaccine candidates have been previously described (12, 13). Many of these studies have focused on using DNA as priming agent, which met with success rates as high as 70–100% in mouse models, even though it was short-lived (14, 15). However, DNA-based vaccines have recently come under renewed scrutiny for a number safety factors such as integration of the DNA into host gene (16) and also by their induction of tolerance against the Ag (17). Another disadvantage is the reduced capacity of DNA vaccines to induce a strong Ab response. The quality of immune responses generated by DNA vaccines administered alone is comparatively weaker to the traditional vaccines such as subunit vaccines or attenuated organisms (18). Currently, most licensed vaccines are based on attenuated whole pathogens or subunit particles. Therefore, as an alternative to DNA vectors, the use of proteins as priming agents is gaining acceptance due to their better efficacy and safety.
In this study, we describe the development of a novel CS-based chimeric protein, which when combined in a heterologus prime–boost vaccine regimen with an attenuated vaccinia virus vector-induced enhanced immune responses. Additionally, this vaccine regimen gave sterile protection in mice when challenged with sporozoites. Our chimeric CS protein (CS14K) makes use of the oligomerization domain of the A27L gene of vaccinia virus. Our previous studies helped in the understanding of the structural organization of the 14-kDa protein encoded by the A27L gene (19). Considering the drawbacks of using a DNA priming strategy and CS protein encoding its C-terminal GPI sequence, we sought to determine the possible differences in the quality and quantity of humoral and T cell responses induced by different vaccine constructs and define how this might influence protection.
We also show how the differences in the induction of CD8+ T cells and Abs could influence the protective capacity of malaria vaccines. In agreement with previous studies with fusion protein (4, 20), we found that priming with CS14K resulted in the development of high avidity and balanced IgG Ab production. An important aspect of this work is the extensive analysis of quantity and quality of CD8+ T cells during primary and memory stages. Comparing our vaccine regimen to a protective vaccination regimen based on DNA priming followed by poxvirus boost, we showed that priming with CS14K followed by a modified vaccinia virus Ankara (MVA)-CS boost triggered strong memory poly-functional effector T cell responses, an important component for providing long-term protection (21).
These results demonstrate that by fusing the vaccinia virus 14K (A27 protein) to CS we achieved a significant improvement in the quality and quantity of the humoral and T cell responses against CS in addition to protection of mice against liver stage development of malaria parasites. This study demonstrates that fusing an immunogenic protein from vaccinia virus improved the overall immunogenicity of the Plasmodium CS Ag in the absence of adjuvants.
Materials and Methods
Cells and viruses
Construction of recombinant MVA virus expressing circumsporozoite protein of Plasmodium yoelii 17XNL strain (MVA-CS) has been describe previously (13). The virus was grown and titrated in primary chicken embryo fibroblastcells and in DF-1 cells (22). Murine J774 and human THP-1 macrophages used for the in vitro cell culture studies were maintained at appropriate conditions as specified by the American Type Culture Collection.
Plasmid construction
pCI-Neo-CS
The PyCSP gene was amplified MVA-CS using the primers CS-XhoI-F (5′-ACTTACTCGAGATGTGTTACAATGAAGAAAATG-3′) and CS-NotI-R (5′-ATTGCGGCCGCTTTAAAATATACTTGAAC-3′) to yield a 972-bp fragment lacking the N-terminal signal sequence and C-terminal GPI sequence. The gene was inserted into a mammalian expression vector, pCI-Neo, that had been previously digested with XhoI and NotI followed by shrimp alkaline phosphatase treatment. The CS gene in both the virus and plasmid were sequenced (Secugen, Madrid, Spain). The plasmid was purified using a Qiagen Mega Prep kit according to the manufacturer’s protocol. Expression of CS from pCI-Neo-CS was confirmed by transfecting DF-1 cells followed by Western blot analysis with CS specific Abs.
pGEX-CS/pGEX-CS14K
The CS gene from pCI-Neo-CS plasmid was amplified using the primers CS-EcoRI-F (5′-ACTTAGAATTCATGTGT-TACAATGAAGAAAATG-3′), CS-Notl-R (5′-ATTGCGGCCGCTTTAA-AATATACTTGAAC-3′) for pGEX-CS, and with primers CS-EcorI-F and CS-14K-NotI-R (5′-ATTGCGGCCGCTATTAAATATACTTGAAC-3′) for pGEX-CS-14K. The A27L open reading frame from vaccinia strain WR (accession no. YP_233032; www.ncbi.nlm.nih.gov/genbank/) was amplified with the primers A27L-NotI-F (5′-GCTGCTAGCGGCCGCGAGG-CTAAACGCGAAG-3′) and A27L-XhoI-R (5′-CCCTCGAGTGGGTTA-CTCATATGGACG-3′) to generate a 276-bp fragment that lacks the first 28 aa from the original sequence. The chimeric gene fragment was generated by digesting with NotI followed by ligation. The fusion gene fragment was then inserted into pGEX-6p-1 plasmid to produce pGEX-CS14K plasmid.
Recombinant protein construct and protein isolation
The recombinant proteins were purified from Escherichia coli strain DH5-α. The starter culture was diluted 1:100 in fresh Luria-Bertani media and allowed to grow at 37°C until the OD600 reached 0.7, following which isopropyl β-D-thiogalactoside was added to a final concentration of 1 mM. The culture was then incubated in an orbital shaker at 18°C and 200 rpm for 24 h. After the incubation, the cells were harvested and the pellet was suspended in extraction buffer (50 mM Tris-HCl [pH 7.5], 250 mM NaCl, 1 mM EDTA and protease inhibitor tablets; Roche). The cells were then lysed with lysozyme, added to a final concentration of 1 mg/ml, and incubated on ice for 20 min. Following lysozyme treatment, 1% Sarkosyl detergent and 1% Triton X-100 was added and incubated at 37°C for 10 min. Clarified supernatant from the lysis was incubated with glutathione-Sepharose 4B beads at 4°C overnight. The beads were then washed using three washes with wash buffer I (extraction buffer with 0.5% Triton X-114) and three with wash buffer II (extraction buffer with 0.1% Triton X-114) followed by two washes with extraction buffer. The purified protein was then eluted with 20 mM reduced glutathione. The protein was desalted using an Amicon centrifugal concentrator and the protein concentration was determined using Bradford reagent. The GST tag from the protein was cleaved using PreScission protease (GE Healthcare) according to the manufacturer’s protocol. The proteins were tested for LPS contamination using a chromogenic Limulus amebocyte lysate kit (QCL-1000; Lonza), which was maintained at <2 endotoxin units per microgram of protein.
Neutralization assay
Sera from immunized rabbit were inactivated at 56°C for 30 min, following which 2-fold serial dilutions of the serum were made and incubated with 200 PFU MVA at 37°C for 1 h. Afterwards, confluent DF-1 cells were infected in triplicate and were visualized by immunostaining with anti-WR serum after 48 h. As a control, nonimmune serum from nonimmunized animals was used. The number of plaques obtained from each serum dilution was normalized to this control value.
Nitrite measurement
NO synthesis was measured by estimating the levels of nitrite present in the supernatant. Briefly, J774 cells were stimulated with proteins. For in vivo nitrite analysis, 106 splenocytes from vaccinated animals, sacrificed 53 d after boost, were treated with 5 µg/ml CS protein. Nitrite accumulation was measured by treating 50 µl supernatant with 50 µ1 Griess reagent I (1% sulfanilamide solution in 2.4 N HCl) for 10 min in dark followed by the addition of 50 µl Griess reagent II (0.1% naphthylethlyenediamine in 2.4 N HCl) for 10 min. The assay was read by a spectrophotometer at 540 nm.
RNA extraction and RT-PCR
Total RNA was extracted from cells treated with respective proteins for 24 h, using a RNeasy Mini kit according to the manufacturer’s instructions. Analysis of RNA was carried out using RT-PCR as described in the kit for reverse transcriptase (Invitrogen). Briefly, 1 µg RNA was reverse transcribed into cDNA using oligo(dT) primers (Invitrogen). For relative quantitative PCR, 2 µl cDNA was used as a template with primers specific for inducible NO synthase, IL-12p40, and GAPDH (23). All the experiments were done in triplicates and the bands from gel electrophoresis were quantified using Adobe Photoshop CS4.
Confocal microscopy
Immunostaining was carried out as described previously (24). Briefly, after fixation (30 min; 4% formaldehyde in PBS; 37°C), permeabilization in 0.1% Triton X-100 (Sigma-Aldrich), and blocking with 10% FCS in PBS, cells were incubated with primary Ab (anti-CS Ab, 1:500; C3-anti-14K, 1:400; NF-κBp65, 1:500) along with DNA staining dye, DAPI (1:200), for 1 h at room temperature. Following extensive washing with PBS secondary Abs (Alexa 546 goat anti-mouse and Alexa 488 goat anti-rabbit, 1:500) were applied for 1 h at room temperature. The slides were washed three times with PBS and mounted in ProLong antifade medium and analyzed with a Bio-Rad Radiance 2100 confocal laser microscope.
Animals and immunizations
All animal procedures were approved by the Ethical Committee of Animal Experimentation of Centro Nacional de Biotecnologia (Consejo Superior de Investigaciones Cientificas). Female BALB/C mice (H-2d), 6–8 wk old, were obtained from Harlan U.K. A standard immunization protocol based on a heterologus prime–boost approach designed in the laboratory was followed (25). In short, animals were primed with DNA (100 µg; DNA-CS or empty DNA-ϕ) or protein (20 µg; CS or CS-14K) via intradermal route and were boosted after 2 wk with 2×107PFU respective sucrose-purified viruses (MVA or MVA-CS) through i.p. injection. All the preparations were made in endotoxin-free PBS.
P. yoelii sporozoite challenge study
Challenge experiments were performed as previously described (26). Briefly, sporozoites were obtained from the salivary glands of Anopheles stephensi mosquitoes. Two weeks after boost, mice were challenged with 2×104 sporozoites via i.v. route through the tail vein. After 42 h, animals were sacrificed and levels of P. yoelii 18S rRNA levels were assessed by quantitative RT-PCR. To determine sterile protection 2 wk after immunization, mice were challenged i.v. with 300 P. yoelii sporozoites. Parasitemia was monitored by performing daily blood smears from days 3 to 21.
ELISA and Ab avidity
Abs present in the serum of immunized animals were determined using ELISA as previously described (25). Purified CS protein was coated to the 96-well Nunc MaxiSorp plates at a concentration of 2 µg/ml in coating buffer (NaHCO3/Na2CO3) at 4°C overnight. Bound Abs were detected using 1:2000 dilution of alkaline phosphate-conjugated goat anti-mouse Ab total IgG or IgG1 or IgG2a (SouthernBiotech, Birmingham, AL). Plates were developed by adding TMB substrate (Sigma-Aldrich) and stopping the reaction with 1 M H2SO4. OD was read at 450 nm. Endpoint titer values were determined as the last positive dilution of serum giving an absorbance value 3-fold higher than naive serum. For analyzing the avidity of Abs, an initial serum dilution that gave an absorbance of 2.7 ELISA units was selected. Following incubation, with serum, the Ag/Ab interaction was disrupted using a range of dilutions from 0 to 5 M urea (Invitrogen) in Tris-HCl (pH 8.0) for 15 min before the addition of secondary Ab. EC50 was then calculated by linear regression (R2 = 0.99) between 1 and 5 M urea concentration (log of 50% reduction, 1.699).
IFN-γ ELISPOT analysis
Fresh IFN-γ ELISPOT analysis was carried out as previously described (27).
Multiparameter flow cytometry
Flow cytometry and intracellular cytokine staining analysis were performed to detect the different phenotype of lymphocytes secreting cytokines, as reported previously (25). Briefly, 4 × 106 splenocytes were stimulated with 1 µg/ml CD8+ peptide, PyCS280–288 (SYVPSAEQI), with GolgiPlug (BD Biosciences) for 6 h in a 96-well plate. The cells were then washed and Fc receptors were blocked using anti-CD16/CD32, following which the cells were stained with surface-specific mouse Abs, namely CD4-Alexa 700, CD3-FITC, and CD8-PerCP for adaptive response studies, or CD8-FITC, CD44-PeCy5, and CD62L-PE for memory studies. Cells were permeabilized using the BD Cytofix/Cytoperm kit (BD Biosciences) and were stained for intracellular cytokines IFN-γ/allophycocyanin, IL-2/PE, and TNF-α/PeCy7 for adaptive response or with IFN-γ/PeCy7, IL-2/allo-phycocyanin, and TNF-α/PE for memory response. A million cells were then passed through an LSRII flow cytometer (BD Biosciences) and the data were analyzed with FlowJo (Tree Star) and Spice (version 5.0). Appropriate controls were used and the values from unstimulated samples were subtracted.
Statistical Analysis
Statistical analysis was performed using Minitab for Windows. For ELISA, to determine the differences between groups we performed a linear regression of the logarithmic absorbance versus the logarithmic dilution, removing those samples clearly not following a linear trend (R2 > 0.98). We then computed the logarithmic dilution at which this regression line was crossing the threshold given by twice the absorbance of the control values for that mouse. Doing this for the four mice we have four estimates of the logarithmic dilution beyond which the absorbance is smaller than twice the absorbance given by the control. We will refer to this value as the critical logarithmic dilution. Finally, we compared with a Student t test the hypothesis that the critical logarithmic dilution between the groups. For intracellular cytokine staining and ELISPOT, statistical analysis was done based on a previously described method (25). Briefly, we developed a novel method for analysis by correcting the control values (RPMI 1640) for determining standard deviations and p values for the samples.
Results
14K fusion aids CS protein aggregation
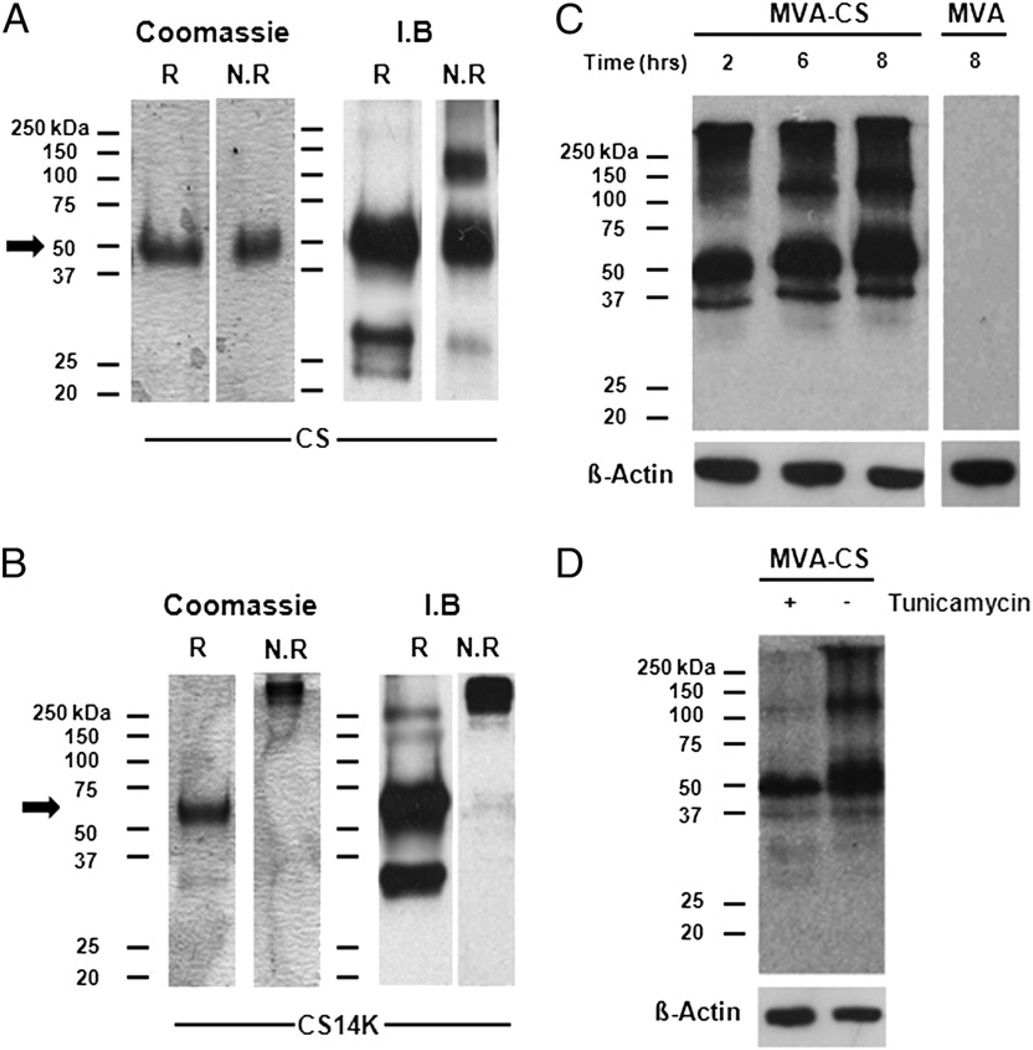
To investigate the effects of 14K conjugation with CS, we determined the structural organization of CS protein and its reactivity against Abs generated from P. yoelii sporozoites. With the aim of improving the immune response against CS, we removed the GPI motif (28) and signal sequence in the CS and fused it with the 14K protein after deleting the first 28 aa from A27, which is responsible for producing neutralizing Abs against vaccinia virus (Supplemental Fig. 1A). Abs generated against CS14K did not neutralize the infectivity of MVA as seen by neutralization assay (30–36 spots/well) (Supplemental Fig. 1B). Using SDS-PAGE analysis under reducing and nonreducing conditions, we evaluated the oligomerization/aggregation status of recombinant proteins and their reactivity with a CS-specific mAb obtained after immunization with sporozoites (Fig. 1A, 1B). Under reducing conditions CS14K has a molecular mass of ~60 kDa, compared with ~50 kDa for CS, whereas under nonreducing conditions CS14K has a size apparently >250 kDa, in contrast to 50 kDa for CS. Additional experiments by analytical ultracentrifugation revealed that CS14K protein has a main sedimenting species with a standard s value of 4.3 ± 0.1, which is compatible with a moderately elongated protein dimer (calculated frictional ratio f/f0 = 1.8). The CS14K has high tendency to form oligomers/aggregates (data not shown). To determine the kinetics of CS expression by the vector MVA-CS, we performed time-course analysis of cells infected with the MVA recombinant expressing CS (Fig. 1C). We observed that after infection with MVA-CS, CS was detectable 2 h postinfection, and these expression levels remained elevated up to 6 h postinfection. Next, to determine whether CS expressed by MVA undergoes posttranslational modifications, we incubated infected cells in the presence of tunicamycin and observed that most of the CS expressed in the virus-infected cells was glycosylated, as indicated by the reduced intensity of the protein bands (Fig. 1D). We also investigated whether there was any difference in the localization of proteins in macrophages expressed by virus or upon transient transfection. To study the subcellular localization of full-length CS during infection with MVA-CS, J774 cells were infected at a multiplicity of infection of 5 PFU/cell for 18 h. We observed that MVA-CS expresses CS as punctuated spots with complete cytoplasmic spread (Supplemental Fig. 1C). In contrast, when macrophages were transfected with DNA encoding CS and CS14K, we observed that CS and CS14K were strongly localized with the nuclear envelope of the cell.
FIGURE 1.
Biophysical and biochemical properties of recombinant proteins: Coomassie blue-stained SDS page and immunoblot with mAb against CS (NYS1; 1:1000 dilution) of 1 µg recombinant (A) CS protein or (B) CS14K protein, under reduced (R) and nonreduced (NR) conditions. Western blots of DF-1 cells infected with MVA-CS virus at 1 MOI for (C) 2, 6, and 8 h or for (D) 16 h in the presence and absence of 10 µg/ml tunicamycin and probed with NYS1 Ab.
Taken together, these data show that the 14K (A27L) protein of vaccinia virus when fused to CS protein of Plasmodium is able to form oligomers/aggregates displaying an apparent molecular mass of >250 kDa compared with its monomeric CS counterpart of 50 kDa. A moderately elongated protein forming oligomers/aggregates was observed when CS14K protein was run under nonreducing conditions by analytical ultracentrifugation. The lower band that we see in the immunoblot under reducing condition is the cleaved CS14K protein. We therefore sought to investigate a possible biological effect on the immunogenicity of CS protein when fused with A27.
CS14K protein modulates innate immune responses in macrophages
Fusion of Ags to immunomodulatory fragments such as TLRs (5) or to complement proteins (29), which is aimed at improving immunogenicity by aggregation of proteins or by exploiting the innate immune signaling of macrophages, is an intense area of research. Because fusion of 14K protein to CS facilitates its aggregation, we sought to investigate whether this could also modulate the innate immune responses in macrophages.
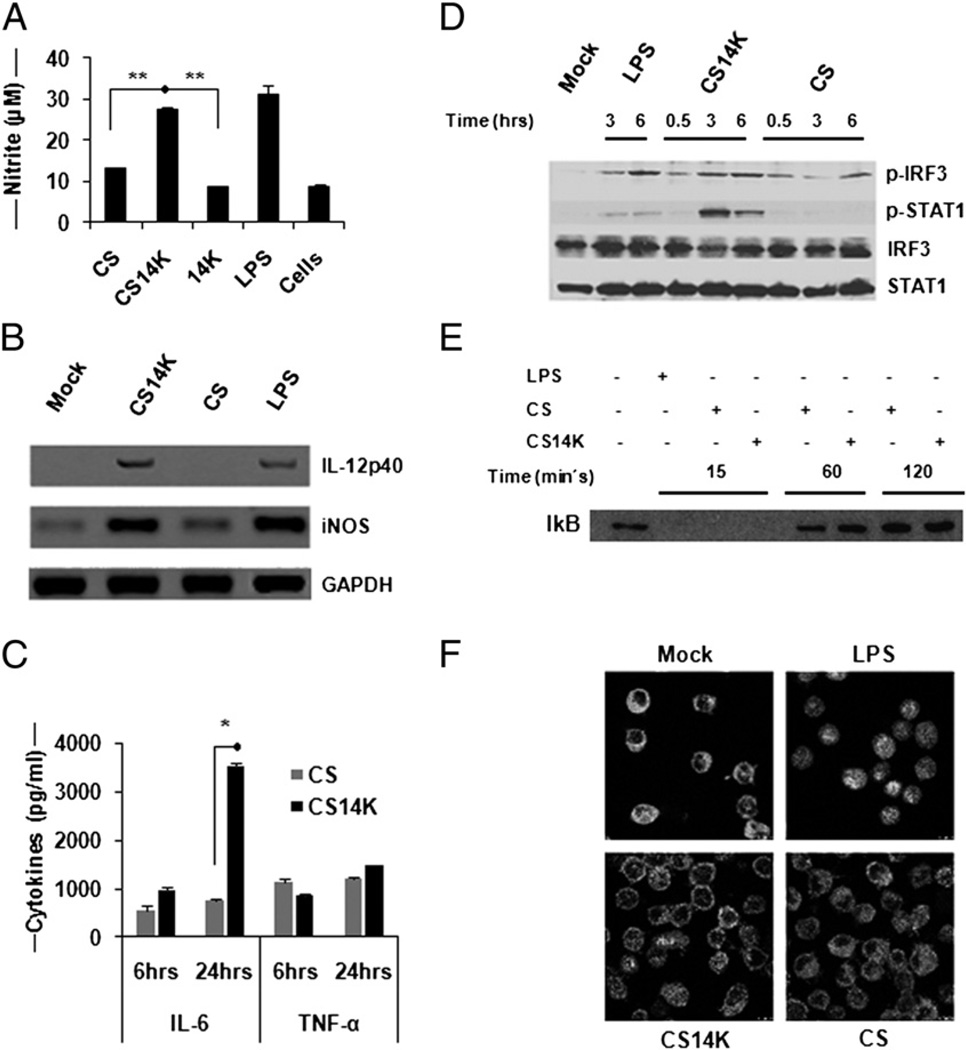
Ability of macrophages to induce NO to inhibit the growth of parasite has been well documented (30); therefore, we sought to evaluate the ability of the fusion protein to induce NO production. When comparing the NO levels in supernatant of J774 cells treated with fusion protein or normal protein, it was apparent that only CS14K resulted in NO production (Fig. 2A). However, when the cells were stimulated with proteins along with recombinant IFN-γ, elevated levels of NO were found in all samples, regardless of the presence of additional proteins (data not shown). Further analysis revealed a significant increase in NO (p = 0.044) and IL-12p40 (p = 0.05) mRNA levels in CS14K-treated macrophages compared with CS protein treatment (Fig. 2B). We next investigated the effects of proteins on IL-6 and TNF-α secretion, given their possible role in controlling pre-erythrocytic stages of malaria. Chimeric protein was able to induce a significant increase in IL-6 in macrophages (Fig. 2C). Although not statistically significant, we also observed increased TNF-α production at 24 h with CS14K treatment.
FIGURE 2.
Modulation in innate immune sensing of CS14K by macrophages: fusion protein can activate innate sensing in macrophages. THP-1 or J774 macrophage cells were stimulated with 5 µg/ml proteins or as positive control with 1 µg/ml LPS. (A) Induction of NO by proteins in macrophages. Nitrite accumulation in the supernatant was determined after 48 h protein treatment in J774. (B) Semiquantitative RT-PCR analysis of NO and IL-12p40 expression. RNA was extracted and the corresponding cDNA was obtained as mentioned in Materials and Methods. Proportionate volume of amplified DNA was run on 8% agarose gel. (C) Cytokine analysis by Luminex assay. Supernatants were collected after 6 and 24 h treatment. Cytokine levels in the supernatant were measured by Luminex. (D) CS14K induces IRF-3 and STAT-1 phosphorylation: 2 × 106 THP-1 cells were stimulated with proteins, following which the samples were collected at different time intervals and probed with phospho-Abs against IRF-3 and STAT-1. Normalization was carried out using total STAT-1 and IRF-3 Abs (1:1000). (E and F) Proteins inhibit NF-κB activation: proteins block the movement of p65 (mouse anti-p65 NF-κB Ab p65, 1:500) into the nucleus in J774 macrophages inspite of IkB degradation. Images were acquired at ×63 objective magnification. Data are expressed as mean ± s.e. of triplicate observation and are representative of two independent experiments. *p < 0.05, **p < 0.005.
Given the significance of TLR activation and the induction of type I IFN, experiments focused on the analysis of the downstream molecules involved in IFN signaling such as IFN regulatory factor (IRF)-3 and STAT-1 were performed. To determine whether there are differences between CS and CS14K, human macrophage THP-1 cells were stimulated and harvested at different time intervals and probed with phospho-Abs against STAT-1 and IRF-3 (Fig. 2D). It is notable that CS14K was particularly effective in activating STAT-1 and IRF-3. To ensure that the activation of STAT-1 and IRF-3 is not influenced by any dsRNA contamination, the cells were stimulated with proteins previously digested with RNAse III (Supplemental Fig. 2).
NF-κB, an important transcription factor responsible for activating several genes involved in innate immune signaling in macrophages, has been shown to be blocked by CS protein (11). Therefore, we analyzed whether CS14K could overcome this constraint. Although both CS and CS14K proteins led to early degradation of IkB (Fig. 2E), the movement of p65 into the nucleus was inhibited (Fig. 2F).
In summary, these data establish that the fusion of A27 protein to CS could alter the innate immune responses in macrophages by STAT-1 and IRF-3 activation without the involvement of NF-κB, which possibly resulted in increased levels of NO, IL-6, and IL-12 production, suggesting signaling via the TLR4 pathway. CS14K was also able to activate higher levels of CD54, a marker for macrophage activation (data not shown). We therefore sought to investigate whether such a response could alter the immunogenicity of the protein in vivo.
CS14K fusion protein priming arms the humoral responses in vivo
It is well established that a strong Ab response against CS correlates with higher protection in many animal and human models (31, 32). The magnitude, isotype, and systemic availability of the Ab produced are important parameters that govern the control of parasites and help to resolve disease (33, 34).
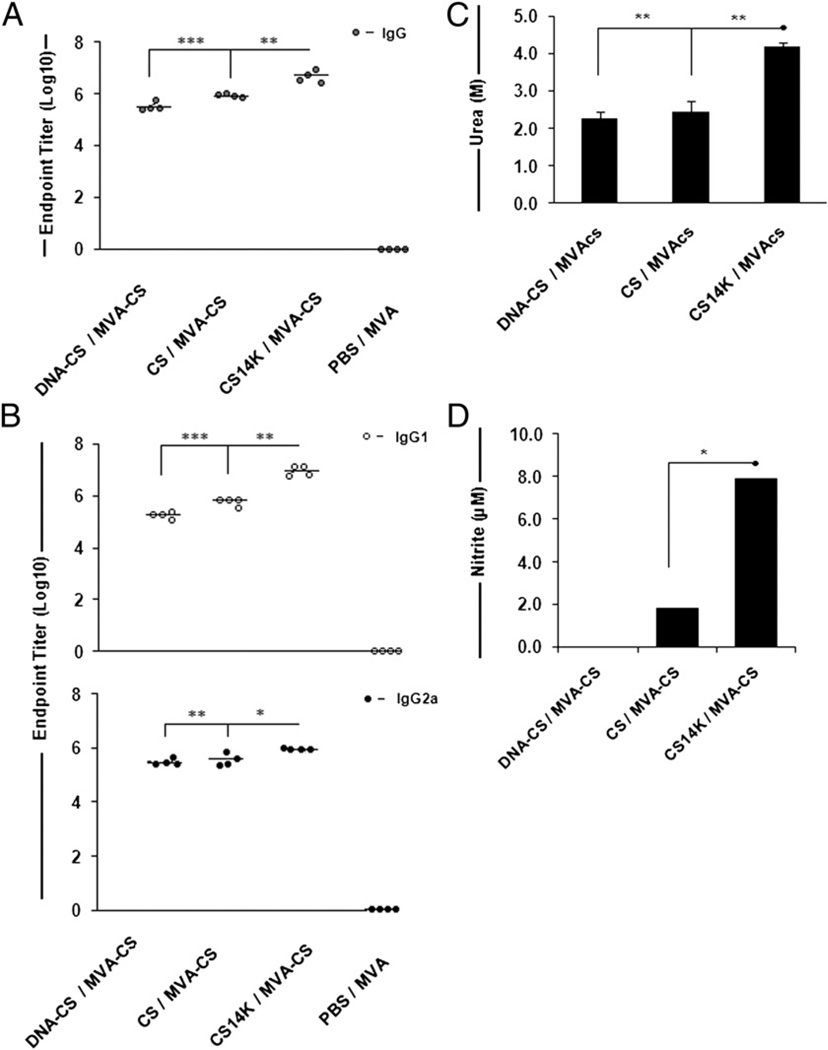
Two weeks after the final phase of the prime–boost vaccination protocol described in Materials and Methods, quantitative analysis of the Abs produced showed that priming with CS14K chimeric protein followed by MVA-CS induced significantly higher titers of Abs than mice primed with DNA-CS or CS protein alone (Fig. 3A). The Ab titers of CS14K-primed group was 2-fold greater than the CS-primed group (p = 0.008) and 8-fold more than in the DNA-CS-primed group (p = 0.002). Even 53 d after boost, the Ab levels in all of the immunized groups were maintained at similar levels except for DNA-CS, which showed a reduction by 1 log (data not shown).
FIGURE 3.
Chimeric protein generates elevated levels of high-avidity Abs in addition to NO. (A) Total IgG serum Ab responses against CS protein as measured by ELISA. (B) IgG isotypes induced by immunizations. Levels of IgG1 and IgG2a Abs generated by the vaccine regimes are shown. Control groups represent the animals receiving empty DNA or PBS for priming and MVA as boost. (C) Avidity indices of the Abs against CS protein. The avidity index was arbitrarily considered as the molarity of urea required to reduce the initial absorbance by 50% (i.e., log10 50% = 1.69). (D) Splenocytes from vaccinated groups harvested after 53 d after boost were incubated with 5 µg/ml recombinant CS protein for a period of 48 h. Amount of NO in control groups was deducted from DNA-CS- and protein-primed groups. Data are expressed as means ± SEM of triplicate observation (n = 4 mice/group) and are representative of two independent experiments. *p < 0.05, **p < 0.005, ***p < 0.0005.
Further analysis of IgG isotype switching showed that fusion protein induced a higher IgG1 response whereas the DNA-vaccinated group showed a predominance of IgG2a response. The levels of IgG1 (p = 0.012) and IgG2a (p = 0.030) induced by the fusion protein-primed group were almost double those of the CS-primed group (Fig. 3B). Finally, we sought to determine whether the vaccination with chimeric protein affected the avidity of the CS-specific Abs. The Abs from mice primed with CS14K showed higher avidity (EC50 = 4.2 M urea) whereas those primed with DNA-CS (EC50 = 2.3 M urea) or CS (EC50 = 2.5 M urea) protein had lower affinity (Fig. 3C). Taken together, these data indicate that priming with proteins, as compared with DNA alone, enhances levels of Abs against CS. Additionally, priming with CS14K not only increases the overall production of Abs but appears to induce a more balanced production of high-affinity IgG1 and IgG2a Abs.
Having established that CS14K can mediate the induction of NO in cultured macrophages, we sought to assess the ability of splenocytes from vaccinated mice to respond to purified CS protein by evaluating NO production. Consequently, splenocytes harvested from animals receiving a different prime–boost vaccine regimen 53 d after boost were stimulated with 5 µg/ml purified CS protein for 48 h. Mice that were primed with DNA-CS showed no detectable NO. Alternatively, mice that received proteins as priming agents were able to induce NO production (Fig. 3D). Whereas CS protein was able to increase NO by low levels, CS14K increased NO production by 4-fold (p = 0.001). These data indicate a reduced capability of CS-based vaccination to induce NO compared with CS14K. The unique nature of CS-based malaria vaccines to induce NO has not been reported before. Thus, the fusion of oligomerization domain of 14K protein to CS improves the humoral arm of immune responses against the Ag along with increased production of NO.
CS14K generates durable and polyfunctionel CS-specific CD8+ T cells in mice
Given the critical role of 14K fusion in aggregating CS protein and the enhancement of innate immune responses, we examined the influence of these factors may have on the development and maintenance of CD8+ T cells in vivo.
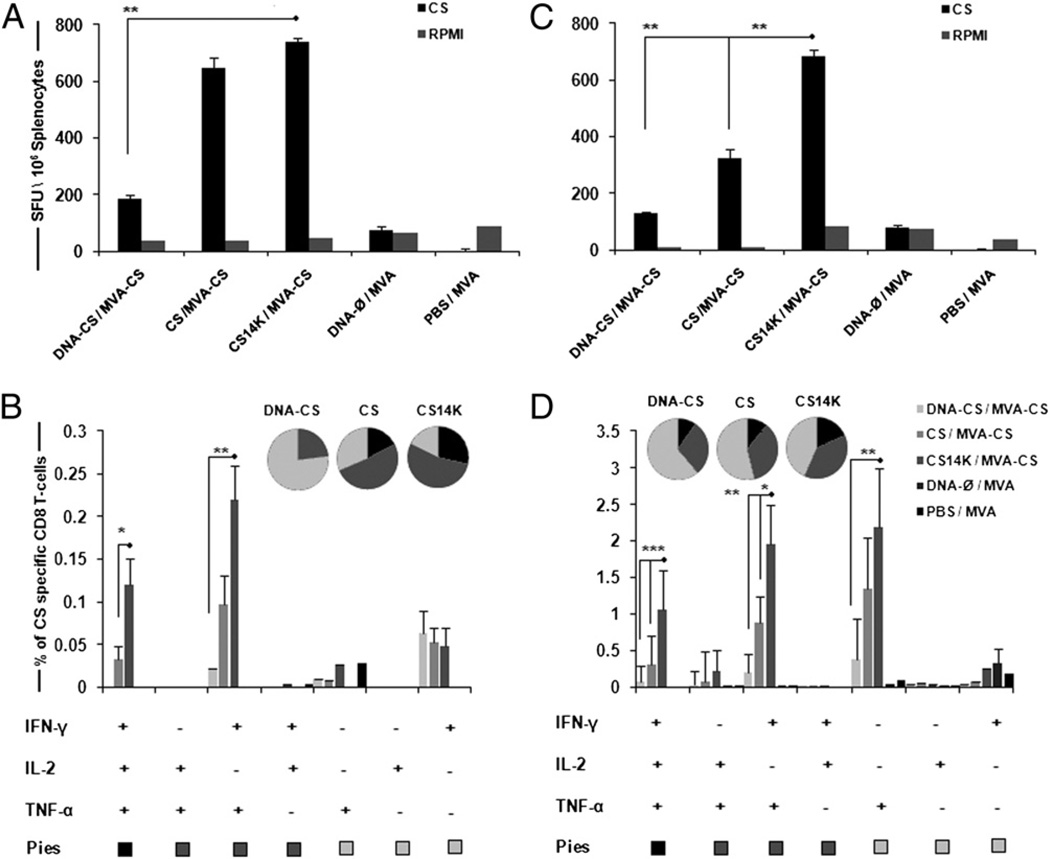
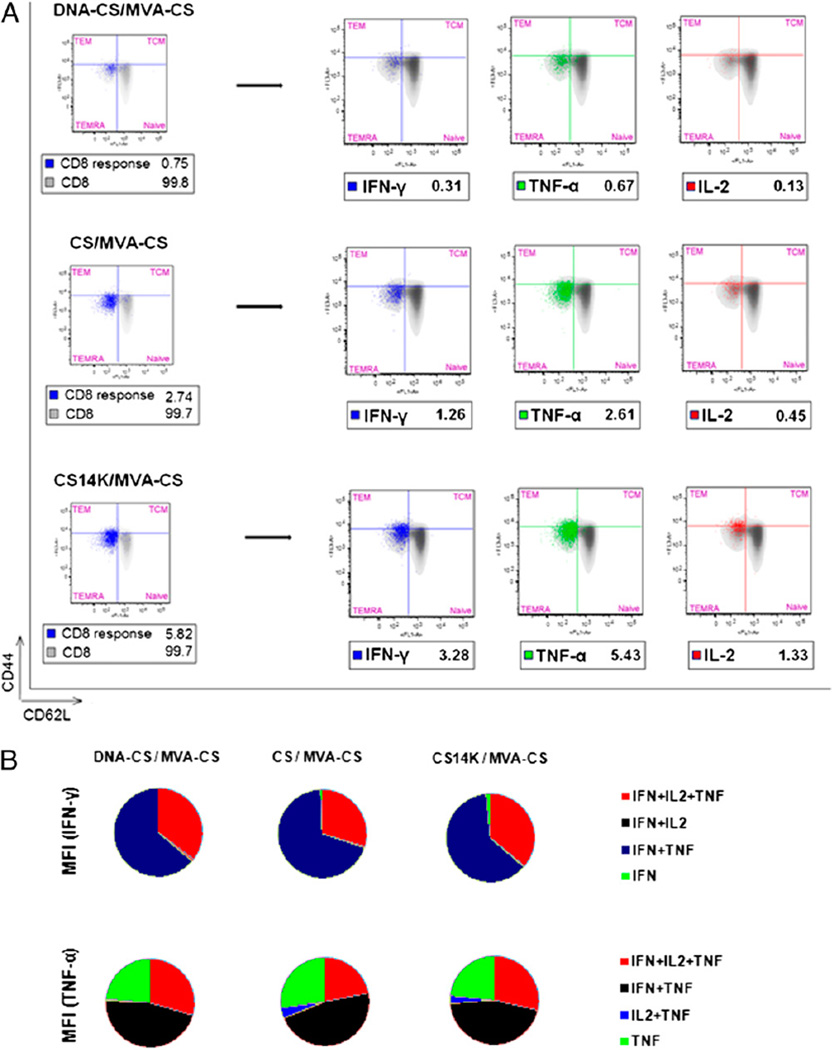
After mice were immunized, they were sacrificed on day 14 to study the adaptive immune response and on day 53 for memory analysis. Efficacy of the vaccination regimen was analyzed using an IFN-γ–based ELISPOT assay and multiparameter flow cytometry. The fusion protein significantly improved the CS-specific IFN-γ– secreting cells (p < 0.05) compared with other vaccine regimes (Fig. 4A, 4B). We characterized the immune responses in terms of polyfunctionality, CD8+ T cells specific for the peptide 280–288 of P. yoelii CS protein, secreting IFN-γ, TNF-α, IL-2, or any combination among these three cytokines. A clear dominance in CD8+ T cell-secreting cytokines was seen in CS14K-primed group of animals over CS protein (2.2-fold)- or DNA-CS (4.3-fold)-primed groups (Fig. 4B) at day 14 after boost. Clearly, cells that secreted IL-2 were associated with triple cytokine-producing cells. Vaccine regimen involving DNA-CS priming resulted in single- or double-positive cells and did not induce any triple-positive population. In contrast, animals receiving protein prime were able to induce IFN-γ+TNF-α+IL-2+-secreting cells with CS14K bringing an ~3.6-fold increase over CS. Additionally, CS14K significantly increased double-positive IFN-γ+TNF-α+ over both CS protein and DNA-CS priming.
FIGURE 4.
Enhanced immunogenicity of vaccine regimen based on chimeric protein. BALB/c mice were primed intradermally with DNA or proteins and boosted with i.p administration of 2 × 107 PFU MVA-CS after 2 wk. Splenocytes were stimulated with CS peptide SYVPSAEQI. The primary immune responses were analyzed on day 14 by (A) IFN-γ ELISPOT assay and (B) CD8+ cells secreting IFN-γ, TNF-α, and IL-2 by polychromatic flow cytometry. Memory responses were analyzed on day 53 after boost (C) IFN-γ ELISPOT assay and (D) memory CD8 analysis using CD44 and CD62L memory markers. Pie charts represent the polyfunctionality of CD8+ T cells secreting single, double, and triple cytokines. Data are expressed as means ± SEM of triplicate observations (n = 4 mice/group) and are representative of two independent experiments. *p < 0.05, **p < 0.005, ***p < 0.0005.
To study memory responses, the CD8+ T cells were classified into central memory (CD44highCD62Lhigh), effector cells (CD44highCD62Llow), terminally differentiated memory effector memory cells (CD44lowCD62Llow), and naive cells (CD44low CD62Lhigh) (35, 36). Upon analyzing memory responses on day 53 and also on day 120 (data not shown), we observed rapid proliferation of the peptide-specific effector CD8 T cell population. Interestingly, the frequency of cells secreting IFN-γ was maintained even during the memory stage by CS14K priming compared with other groups (Fig. 4C). In terms of poly-functionality, priming with fusion protein had a significant increase in the triple-positive (IFN-γ+TNF-α+IL-2+) and double-positive (IFN-γ+TNF-α+) population of cytokine-secreting cells over both DNA as well as CS protein (Fig. 4D). Most of the single-positive cells were dominant for TNF-α. A 3.5-fold increase in IFN-γ+TNF-α+IL-2+–secreting cells by the CS14K-primed group over the CS protein-primed group and a nearly 14-fold increase over the DNA-CS–primed groups was seen. A clear hierarchy in CS peptide-specific total CD8 cells secreting cytokines was observed when priming was carried out with CS14K (Fig. 5A). Mice immunized with proteins showed significant increase in magnitude of cytokines released over DNA-CS priming. Indeed, priming with CS14K protein rather that CS protein led to a 2-fold increase in total IFN-γ (p < 0.05) and TNF-α (p < 0.0005) in addition to the 3-fold increase in IL-2–secreting cells (p < 0.05). Interestingly, all of the positive CD8 T cells produced by the vaccine regimen had a phenotype resembling either T effector cells or terminally differentiated effector memory cells. To extend the immune analysis, we evaluated the amount of IFN-γ or TNF-α secreted by the different populations based on the mean fluorescence intensity (MFI) calculation (Fig. 5B). We observed that, irrespective of priming agent, most of the IFN-γ was produced by IFN-γ+TNF-α+IL-2+ or IFN-γ+TNF-α+ cells with a similar pattern for TNF-α. The lack of CM CD8 T cells could be explained by the fact that upon exposure to peptide stimulus most of the CM cells rapidly acquired effector memory characteristics. These data indicate that proteins are able to prime to a higher extent an effective long-term CD8 T cell response than does DNA. The enhanced CD8 T cell responses in mice immunized with CS14K rather that CS is due to the adjuvant-like effect of the unique A27 element.
FIGURE 5.
Polyfunctionality related to increased cytokine production by CS14K induced memory CD8+ T cells: characterization of memory CD8+ T cells. (A) Representation of total CS peptide specific CD8 cells were gated on CD44 and CD62L cell surface markers represented on left-hand side of the diagram. Total CD8 responses for each group are represented inside the box. Different populations of memory CD8 cells secreting different cytokines are represented on the right-hand side with their respective total responses as depicted insdie the box. (B) MFI of IFN-γ and TNF-α produced by different polyfunctional population of effector memory CD8+ T cells (n = 4 mice/group).
CS14K fusion protein abrogates the liver stage development of parasites when challenged with sporozoites
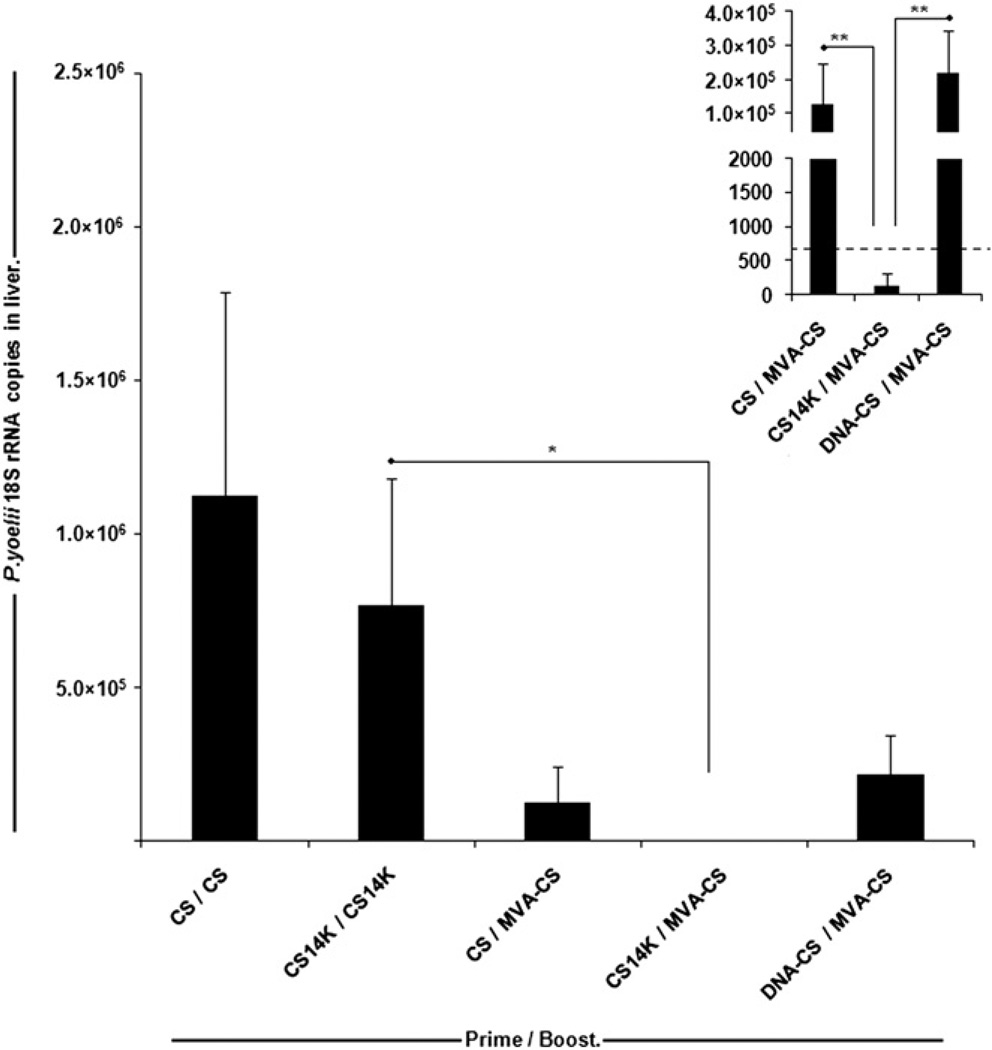
Given that polyfunctional CD8+ T cell responses in addition to high-avidity Abs are crucial in enhancing protection against parasite infection, and also that CS14K improved both adaptive and memory immune responses, compared with CS or DNA-CS priming, we sought to investigate the efficacy of immunization against sporozoite challenge. Protection was evaluated by measuring levels of P. yoelii 18S rRNA in liver and also by determining parasitemia in sporozoite-challenged animals. To investigate the protective capacity of this subunit vaccine, groups of mice were immunized in various prime–boost protocols (Fig. 6). We show that a heterologus protein prime/vaccinia virus boost regimen was found to be more effective than a homologous protein prime/protein boost regimen, and a 32% reduction in the liver stage burden of parasites was observed in mice receiving CS14K (CS14K/CS14K) compared with CS (CS/CS) protein. Furthermore, a MVA-CS boost significantly lowered the parasite levels in the liver. Moreover, the CS14K protein priming followed by MVA-CS resulted in a near complete inhibition (~99.9%; p < 0.005) of parasite development in liver compared with CS protein and DNA-CS (Fig. 6).
FIGURE 6.
Strong inhibition of liver stage parasite development by CS14K. Mice were immunized as described in Materials and Methods. Two weeks after boost animals were challenged with 2 × 10 P. yoelii sporozoites through i.v. route via tail vein. After 42 h, the amounts of parasites in the liver were estimated by measuring the number of copies of 18S rRNA by RT-PCR. The dotted line represents the minimum detectable levels by the highly sensitive quantitative RT-PCR (26). Data are expressed as means ± SEM (n = 6 mice/group). *p < 0.05, **p < 0.005.
To evaluate whether protection from liver-stage parasites induced by CS14K/MVA prime–boost can prevent the development of blood stages, mice were challenged with 300 live P. yoelii sporozoites and monitored until patency. Importantly, none of the vaccinated animals developed blood stages from days 3 to 21 of daily follow-up after challenge with sporozoites (Table I).
Table I.
CS14K priming induces sterile protection
| Priminga | Boostingb | Challengec | Protected/Challenged (% Protected) |
Prepatent Period |
|---|---|---|---|---|
| CS14K | MVA-CS | 300 sporozoites | 10/10 (100) | __ |
| PBS | PBS | 14 d after boost | 0/10 (0) | 4d |
Protein (20µg) administered intradermally.
Virus (2×107 PFU) administered i.p.
Challenge via i.v. route.
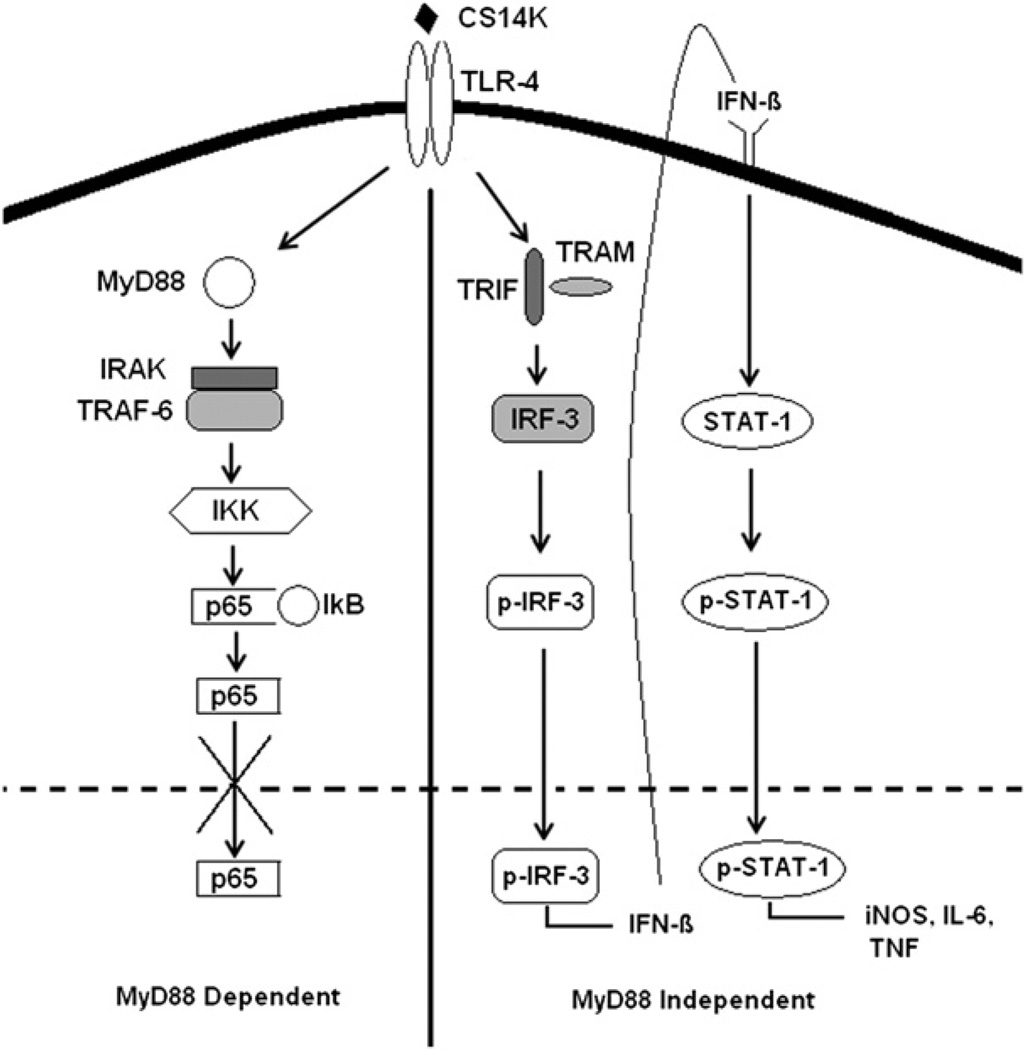
Thus, priming with CS14K protein in combination with a boost by MVA-CS is a highly effective vaccine regimen against murine malaria liver stage parasites. A proposed mechanism is shown in Fig. 7.
FIGURE 7.
Proposed TLR signaling mechanism by CS14K: a hypothetical model of TLR4 signaling, pathway activated by fusion protein, in a MyD88-independent fashion. Inhibition of p65 movement to the nucleus by CS14K prevents the action of NF-κB while activation of IRF-3 and STAT-1 leads to elevated levels of NO, IL-6, and TNF-a, which could inhibit the growth of parasites.
Discussion
In this study, we report a novel approach in designing effective protein vaccines based on the fusion of 14K (A27) protein of vaccinia virus with a model Ag, CS of P. yoelii, for developing an effective vaccination regimen based on protein prime/vaccinia virus boost against murine malaria. An obvious advantage of this vaccine strategy is the concomitant increase in both CS-specific humoral and CD8+ T cells up to 4 mo. By comparing CS14K protein with other priming agents such as CS- and DNA-CS–based vaccines, we showed the correlation between the immune responses generated and protective efficacy generated in the murine malaria model.
In this study, we showed that the fusion of 14K protein with CS results in a protein with high tendency to form oligomers/aggregates of CS14K, which, in turn, enhanced the immune response profile leading to protection against murine malaria. Previous studies have demonstrated the differences in CS–specific T cell proliferation using recombinant soluble CS protein, viable sporozoites, or heat-killed sporozoites (37). This could explain the enhancement of polyfunctional T cells and Abs when animals are primed with chimeric protein rather than CS, which contains lower amounts of CS aggregates. An obvious advantage of this chimeric protein is the incorporation of near full-length CS protein containing both B and T cell epitopes whose presentation would be enhanced as a result of aggregation. Additionally, the use of viral vaccinia vectors for boosting could further enhance the development of long-term effector CS-specific T cells. In Fig. 6, we show how the vaccine regimen based on CS14K prime/MVA-CS boost inhibits the liver stage development of the parasite in mice when challenged with a high dose of sporozoites. When we calculated the CT value of individual mice in each group we found that for the CS14K/MVA-CS group the values were well >35, the reference value in naive mice (indicated as dotted lines in Fig. 6). This suggests that the mice in this group did not have any parasite in the liver. Moreover, given that sterile protection is the target of pre-erythrocytic vaccines, we showed that CS14K/MVA-CS prime–boost prevented the development of blood stage parasites (Table I). The maintenance of both cellular and humoral responses even after 4 mo indicated the strong nature of the vaccine regimen used in this study.
Besides protein aggregation, another mechanism that could explain the enhanced immunogenicity of CS14K is its ability to activate type I IFN signaling. Given that an early activation of innate immune responses curbs the pre-eryfhrocytic development of parasites (38, 39), activation of type I IFN signaling by fusion protein further validates its use as a priming agent. Indeed, activation of type I IFN signaling by the chimeric protein did result in elevated levels of cytokines belonging to this family such as IL-6, TNF-α, and IL-12. It is thought that the parasiticidal action of TNF-α in hepatocytes is mediated through the synthesis of IL-6 (40). Furthermore, engaging TLRs is critical in developing antimalarial immunity, which could be explained by the poor immunogenicity of RTS,S without a strong adjuvant such as AS01E, a TLR4 agonist (41). A recent study also shows the importance of TLR signaling for effective development of protective Abs to Plasmodium (42). This is in agreement with our study, which shows how enhancement in STAT-1 and IRF-3 by CS14K suggested a role in TLR4 activation (Fig. 7), resulting in improved immunogenicity and inhibiting the liver stage development of the parasite (43, 44).
It is known how parasites such as Leishmania and Plasmodium downregulate synthesis of NO (45). Our study shows the inherent capacity of chimeric protein to enhance the production of NO both in vitro and in vivo. Because neither CS nor 14K protein alone could induce NO, it seems reasonable to conclude that the CS 14K protein aggregation is responsible for NO induction. The persistently elevated levels of NO at 53 d after vaccination with chimeric CS14K protein compared with priming with DNA-CS or protein alone suggest that differences exist in the processing of these Ags by APCs. Additionally, early production of NO has been proposed to be required for proliferation of CD8+ T cells against the parasite (46). In conclusion, fusion of 14K to CS leads to a more favorable immunogenic milieu to enhance the efficacy of the vaccine.
Protective immunity against malaria associates with circulating IgG Abs against CS (47). Previous studies have also shown the ability of fusion proteins to induce strong humoral responses based on their ability to form large molecular mass aggregates (20). Similar to the CS14K priming approach presented in this study, the malaria vaccine in the latest stages of clinical trials, RTS,S, is also dependent on a higher magnitude of Abs belonging to the IgG1 subclass (48). Vaccine studies based only on DNA-CS vaccination lacking the GPI anchor improve protection up to 95% based only on Ab titers (49). Also, the serum Ab titers obtained by fusion protein priming are much higher than those obtained in presence of strong adjuvants such as Freund’s adjuvant (50). However, unlike vaccination with DNA-CS or protein alone, vaccination with the CS14K fusion protein results in the concomitant induction of high levels of both IgG1 and IgG2a Abs, and this could be beneficial in providing protection against malaria. The chimeric protein proved to be superior in inducing high-avidity Abs against CS comparable to those achieved by using nanoparticle-based CS peptide vaccination (51). High-avidity Abs produced by the chimeric protein could be due to the unique folding pattern attained by CS14K protein resulting in exposure of other potent epitopes. These data have important implications in the choice of Ags designed to induce Ab responses against malaria.
CD8+ T cell responses against CS protein are known to be an important factor in the development of sterile immunity using irradiated sporozoites (52). A role of CD8+T cells in subunit vaccine-induced protection against malaria was initially established in a heterologus prime–boost approach with flu and vaccinia virus vectors expressing CS (53).Vaccine regimes based on DNA as well as protein prime followed by vaccinia boost have shown to induce CS peptide-specific CD8+ T cell responses (15, 54). The ability of the CS14K regimen to produce significantly elevated levels of IFN-γ and TNF-α single-positive– as well as double-positive–secreting CD8 T cells even after 53 d makes it a suitable vaccine candidate. We also report a shift in polyfunctionality of CD8 T cells secreting all three cytokines produced by chimeric protein, which may be effective in controlling the growth of parasites. Furthermore, the ability of CS14K vaccine regimen to induce a large amount of TNF-α has an added advantage because it is known to be an important cytokine for the maintenance of memory CD8 T cells in malaria (55). The concomitant increase in TNF-α by CS14K could also contribute to elevated NO and Ab responses (56, 57). The need for IL-12 in the development of effective adaptive responses is in agreement with our study, which shows the ability of CS14K protein to prime an enhanced T cell response (58). The data from MFI studies show that double- or triple-positive populations are better in producing elevated levels of cytokines, both of which are elevated by CS14K priming. The low levels of CD8 responses against DNA-CS suggest that the protective capability of this regimen may be based on the Abs induced. The maintenance of such durable and polyfunctional memory responses even after 4 mo of vaccination, taken together with sterile protection, justifies the potent nature of A27 protein fusion and its use for developing better malaria vaccines.
In recent years, many experimental CS-based vaccines have been developed using novel adjuvants or by modifying the structure of CS protein (Supplemental Fig. 3). The assessment of protective capacity of vaccine regimen gave a good platform to study the efficacy of chimeric protein generated by 14K fusion. Chimeric protein prime–boost alone could inhibit the liver stage development better than those attained by DNA-CS prime and MVA-CS boost under a high challenge dosage. This could be explained by the potent nature of the attenuated vaccinia viral boost (59).
Developing an effective pre-erythrocytic vaccine against malaria is difficult because it requires high levels of both humoral and cell-mediated immune responses. The role of CSP Abs in providing protection is a much debated topic. However, the latest trial of RTS,S vaccine does show that the protection could be mediated by CSP Abs (60, 61). In this study, we describe an optimal prime–boost approach using modified CS protein fused to the 14K protein of vaccinia virus as priming and MVA-CS as a boost. This unique approach is able to improve the magnitude and polyfunctionality of both humoral as well as cell-mediated immune responses, resulting in complete protection of vaccinated animals compared with other experimental CS based vaccines (62–65). Additional studies in primates could be performed using the chimeric protein in prime–boost protocols. These data establish a ground for the development of vaccines based on structural modifications of Ags using the immunogenic molecules from vaccinia virus.
Supplementary Material
Acknowledgments
We thank Victoria Jimenez for expert technical assistance with the cells and viruses. We also thank Susana Garcia of the Confocal Microscopy Core Facility at Centro Nacional de Biotecnologia.
This work was supported by Spanish Grant SAF2008/02036 from the Ministry of Science and Innovation (to M.E.) and by a La-Caixa International Ph.D. fellowship (to A.V.). F.Z. is supported by National Institutes of Health Grant AI44375. This work is, in part, for the fulfillment of the Ph.D. degree by A.V. at the Universidad Autonóma de Madrid.
Abbreviations used in this article
- CS
circumsporozoite
- IRF
IFN regulatory factor
- MFI
mean fluorescence intensity
- MVA
modified vaccinia virus Ankara
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.World Health Organization. World Malaria Report 2010. Geneva, Switzerland: WHO Press; 2010. [Google Scholar]
- 2.Good MF, Doolan DL. Malaria vaccine design: immunological considerations. Immunity. 2010;33:555–566. doi: 10.1016/j.immuni.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Bejon P, Lusingu J, Olotu A, Leach A, Lievens M, Vekemans J, Mshamu S, Lang T, Gould J, Dubois MC, et al. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N. Engl. J. Med. 2008;359:2521–2532. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg AS. Effects of protein aggregates: an immunologic perspective. AAPS J. 2006;8:E501–E507. doi: 10.1208/aapsj080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kastenmüller K, Wille-Reece U, Lindsay RW, Trager LR, Darrah PA, Flynn BJ, Becker MR, Udey MC, Clausen BE, Igyarto BZ, et al. Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets. J. Clin. Invest. 2011;121:1782–1796. doi: 10.1172/JCI45416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ilyinskii PO, Thoidis G, Sherman MY, Shneider A. Adjuvant potential of aggregate-forming polyglutamine domains. Vaccine. 2008;26:3223–3226. doi: 10.1016/j.vaccine.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 7.Nussenzweig V, Nussenzweig RS. Circumsporozoite proteins of malaria parasites. Cell. 1985;42:401–403. doi: 10.1016/0092-8674(85)90093-5. [DOI] [PubMed] [Google Scholar]
- 8.Sinnis P, Clavijo P, Fenyö D, Chait BT, Cerami C, Nussenzweig V. Structural and functional properties of region II-plus of the malaria circumsporozoite protein. J. Exp. Med. 1994;180:297–306. doi: 10.1084/jem.180.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lal AA, de la Cruz VF, Welsh JA, Charoenvit Y, Maloy WL, McCutchan TF. Structure of the gene encoding the circumsporozoite protein of Plasmodium yoelii: a rodent model for examining antimalarial sporozoite vaccines. J. Biol. Chem. 1987;262:2937–2940. [PubMed] [Google Scholar]
- 10.Kumar KA, Baxter P, Tarun AS, Kappe SH, Nussenzweig V. Conserved protective mechanisms in radiation and genetically attenuated uis3(−) and uis4(−) Plasmodium sporozoites. PLoS ONE. 2009;4:e4480. doi: 10.1371/journal.pone.0004480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh AP, Buscaglia CA, Wang Q, Levay A, Nussenzweig DR, Walker JR, Winzeler EA, Fujii H, Fontoura BM, Nussenzweig V. Plasmodium circumsporozoite protein promotes the development of the liver stages of the parasite. Cell. 2007;131:492–504. doi: 10.1016/j.cell.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Dunachie SJ, Walther M, Vuola JM, Webster DP, Keating SM, Berthoud T, Andrews L, Bejon P, Poulton I, Butcher G, et al. A clinical trial of prime-boost immunisation with the candidate malaria vaccines RTS,S/ AS02A and MVA-CS. Vaccine. 2006;24:2850–2859. doi: 10.1016/j.vaccine.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 13.González-Aseguinolaza G, Nakaya Y, Molano A, Dy E, Esteban M, Rodríguez D, Rodríguez JR, Palese P, García-Sastre A, Nussenzweig RS. Induction of protective immunity against malaria by priming-boosting immunization with recombinant cold-adapted influenza and modified vaccinia Ankara viruses expressing a CD8+-T-cell epitope derived from the circumsporozoite protein of. Plasmodium yoelii. J. Virol. 2003;11:11859–11866. doi: 10.1128/JVI.77.21.11859-11866.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sedegah M, Brice GT, Rogers WO, Doolan DL, Charoenvit Y, Jones TR, Majam VF, Belmonte A, Lu M, Belmonte M, et al. Persistence of protective immunity to malaria induced by DNA priming and poxvirus boosting: characterization of effector and memory CD8+-T-cell populations. Infect. Immun. 2002;70:3493–3499. doi: 10.1128/IAI.70.7.3493-3499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sedegah M, Jones TR, Kaur M, Hedstrom R, Hobart P, Tine JA, Hoffman SL. Boosting with recombinant vaccinia increases immuno-genicity and protective efficacy of malaria DNA vaccine. Proc. Natl. Acad. Sci. USA. 1998;95:7648–7653. doi: 10.1073/pnas.95.13.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Troilo PJ, Wang X, Griffiths TG, Pacchione SJ, Barnum AB, Harper LB, Pauley CJ, Niu Z, Denisova L, et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004;11:711–721. doi: 10.1038/sj.gt.3302213. [DOI] [PubMed] [Google Scholar]
- 17.Mor G, Yamshchikov G, Sedegah M, Takeno M, Wang R, Houghten RA, Hoffman S, Klinman DM. Induction of neonatal tolerance by plasmid DNA vaccination of mice. J. Clin. Invest. 1996;98:2700–2705. doi: 10.1172/JCI119094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang Y, Calvo PA, Daly TM, Long CA. Comparison of humoral immune responses elicited by DNA and protein vaccines based on merozoite surface protein-1 from Plasmodium yoelii, a rodent malaria parasite. J. Immunol. 1998;161:4211–219. [PubMed] [Google Scholar]
- 19.Vázquez MI, Esteban M. Identification of functional domains in the 14-kilodalton envelope protein (A27L) of vaccinia virus. J. Virol. 1999;73:9098–9109. doi: 10.1128/jvi.73.11.9098-9109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu. Rev. Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- 21.Reyes-Sandoval A, Wyllie DH, Bauza K, Milicic A, Forbes EK, Rollier CS, Hill AV. CD8+ T effector memory cells protect against liver-stage malaria. J. Immunol. 2011;187:1347–1357. doi: 10.4049/jimmunol.1100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gómez CE, Nájera JL, Jiménez EP, Jimenez V, Wagner R, Graf M, Frachette MJ, Liljeström P, Pantaleo G, Esteban M. Head-to-head comparison on the immunogenicity of two HIV/AIDS vaccine candidates based on the attenuated poxvirus strains MVA and NYVAC co-expressing in a single locus the HIV-1BX08 gp120 and HIV-1IIIB Gag-Pol-Nef proteins of clade B. Vaccine. 2007;25:2863–2885. doi: 10.1016/j.vaccine.2006.09.090. [DOI] [PubMed] [Google Scholar]
- 23.Maffei CM, Mirels LF, Sobel RA, Clemons KV, Stevens DA. Cytokine and inducible nitric oxide synthase mRNA expression during experimental murine cryptococcal meningoencephalitis. Infect. Immun. 2004;72:2338–2349. doi: 10.1128/IAI.72.4.2338-2349.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerra S, López-Fernández LA, Pascual-Montano A, Muñoz M, Harshman K, Esteban M. Cellular gene expression survey of vaccinia virus infection of human HeLa cells. J. Virol. 2003;77:6493–6506. doi: 10.1128/JVI.77.11.6493-6506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García-Arriaza J, Nájera JL, Gómez CE, Sorzano CO, Esteban M. Immunogenic profiling in mice of a HIV/AIDS vaccine candidate (MVA-B) expressing four HIV-1 antigens and potentiation by specific gene deletions. PLoS ONE. 2010;5:e12395. doi: 10.1371/journal.pone.0012395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruña-Romero O, Hafalla JC, González-As eguinolaza G, Sano G, Tsuji M, Zavala F. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 2001;31:1499–1502. doi: 10.1016/s0020-7519(01)00265-x. [DOI] [PubMed] [Google Scholar]
- 27.Gómez CE, Nájera JL, Sánchez R, Jiménez V, Esteban M. Multimeric soluble CD40 ligand (sCD40L) efficiently enhances HIV specific cellular immune responses during DNA prime and boost with attenuated poxvirus vectors MVA and NYVAC expressing HIV antigens. Vaccine. 2009;27:3165–3174. doi: 10.1016/j.vaccine.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 28.Ophorst OJ, Radosević K, Ouwehand K, van Beem W, Mintardjo R, Sijtsma J, Kaspers J, Companjen A, Holterman L, Goudsmit J, Havenga MJ. Expression and immunogenicity of the Plasmodium falciparum circumsporozoite protein: the role of GPI signal sequence. Vaccine. 2007;25:1426–1436. doi: 10.1016/j.vaccine.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 29.Bergmann-Leitner ES, Duncan EH, Leitner WW, Neutzner A, Savranskaya T, Angov E, Tsokos GC. C3d–defined complement receptor-binding peptide p28 conjugated to circumsporozoite protein provides protection against Plasmodium berghei . Vaccine. 2007;25:7732–7736. doi: 10.1016/j.vaccine.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 30.Sharma A, Eapen A, Subbarao SK. Parasite killing in Plasmodium vivax malaria by nitric oxide: implication of aspartic protease inhibition. J. Biochem. 2004;136:329–334. doi: 10.1093/jb/mvh128. [DOI] [PubMed] [Google Scholar]
- 31.Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, Wellde BT, Garçon N, Krzych U, Marchand M. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria: RTS,S Malaria Vaccine Evaluation Group. N. Engl. J. Me. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 32.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat. Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 33.Charoenvit Y, Mellouk S, Cole C, Bechara R, Leef MF, Sedegah M, Yuan LF, Robey FA, Beaudoin RL, Hoffman SL. Monoclonal, but not polyclonal, antibodies protect against Plasmodium yoelii sporozoites. J. Immunol. 1991;146:1020–1025. [PubMed] [Google Scholar]
- 34.Kester KE, McKinney DA, Tornieporth N, Ockenhouse CF, Heppner DG, Hall T, Krzych U, Delchambre M, Voss G, Dowler MG, et al. RTS,S Malaria Vaccine Evaluation Group. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J. Infect Dis. 2001;183:640–647. doi: 10.1086/318534. [DOI] [PubMed] [Google Scholar]
- 35.Gerberick GF, Cruse LW, Miller CM, Sikorski EE, Ridder GM. Selective modulation of T cell memory markers CD62L and CD44 on murine draining lymph node cells following allergen and irritant treatment. Toxicol. Appl. Pharmacol. 1997;146:1–10. doi: 10.1006/taap.1997.8218. [DOI] [PubMed] [Google Scholar]
- 36.Waters WR, Rahner TE, Palmer MV, Cheng D, Nonnecke BJ, Whipple DL. Expression of L-Selectin (CD62L), CD44, and CD25 on activated bovine T cells. Infect. Immun. 2003;71:317–326. doi: 10.1128/IAI.71.1.317-326.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krzych U, Jareed T, Link HT, Loomis LD, Ballou WR. Distinct T cell specificities are induced with the authentic versus recombinant Plasmodium berghei circumsporozoite protein. J. Immunol. 1992;148:2530–2538. [PubMed] [Google Scholar]
- 38.Shio MT, Kassa FA, Bellemare MJ, Olivier M. Innate inflammatory response to the malarial pigment hemozoin. Microbes Infect. 2010;12:889–899. doi: 10.1016/j.micinf.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Arnold L, Tyagi RK, Mejia P, Van Rooijen N, Pèrignon JL, Druilhe P. Analysis of innate defences against Plasmodium falciparum in immuno-deficient mice. Malar. J. 2010;9:197. doi: 10.1186/1475-2875-9-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nussler A, Pied S, Goma J, Rènia L, Miltgen F, Grau GE, Mazier D. TNF inhibits malaria hepatic stages in vitro via synthesis of IL-6. Int. Immunol. 1991;3:317–321. doi: 10.1093/intimm/3.4.317. [DOI] [PubMed] [Google Scholar]
- 41.Mettens P, Dubois PM, Demoitiè MA, Bayat B, Donner MN, Bourguignon P, Stewart VA, Heppner DG, Jr, Garçon N, Cohen J. Improved T cell responses to Plasmodium falciparum circumsporozoite protein in mice and monkeys induced by a novel formulation of RTS,S vaccine antigen. Vaccine. 2008;26:1072–1082. doi: 10.1016/j.vaccine.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 42.Wiley SR, Raman VS, Desbien A, Bailor HR, Bhardwaj R, Shakri AR, Reed SG, Chitnis CE, Carter D. Targeting TLRs expands the antibody repertoire in response to a malaria vaccine. Sci. Transl. Med. 2011;3:93ra69. doi: 10.1126/scitranslmed.3002135. [DOI] [PubMed] [Google Scholar]
- 43.Imada K, Leonard WJ. The Jak-STAT pathway. Mol. Immunol. 2000;37:1–11. doi: 10.1016/s0161-5890(00)00018-3. [DOI] [PubMed] [Google Scholar]
- 44.Späth GF, Schlesinger P, Schreiber R, Beverley SM. A novel role for Stat1 in phagosome acidification and natural host resistance to intracellular infection by Leishmania major . PLoS Pathog. 2009;5:e1000381. doi: 10.1371/journal.ppat.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liew FY. Regulation of nitric oxide synthesis in infectious and autoimmune diseases. Immunol. Lett. 1994;43:95–98. doi: 10.1016/0165-2478(94)00157-x. [DOI] [PubMed] [Google Scholar]
- 46.Scheller LF, Green SJ, Azad AF. Inhibition of nitric oxide interrupts the accumulation of CD8+ T cells surrounding Plasmodium berghei-infected hepatocytes. Infect. Immun. 1997;65:3882–3888. doi: 10.1128/iai.65.9.3882-3888.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White MT, Griffin JT, Riley EM, Drakeley CJ, Moorman AM, Sumba PO, Kazura JW, Ghani AC, John CC. Efficacy model for antibody-mediated pre-erythrocytic malaria vaccines. Proc. Biol. Sci. 278:1298–1305. doi: 10.1098/rspb.2010.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliveira GA, Wetzel K, Calvo-Calle JM, Nussenzweig R, Schmidt A, Birkett A, Dubovsky F, Tierney E, Gleiter CH, Boehmer G, et al. Safety and enhanced immunogenicity of a hepatitis B core particle Plasmodium falciparum malaria vaccine formulated in adjuvant Montanide ISA 720 in a phase I trial. Infect. Immun. 2005;73:3587–3597. doi: 10.1128/IAI.73.6.3587-3597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheiblhofer S, Chen D, Weiss R, Khan F, Mostböck S, Fegeding K, Leitner WW, Thalhamer J, Lyon JA. Removal of the circumsporozoite protein (CSP) glycosylphosphatidylinositol signal sequence from a CSP DNA vaccine enhances induction of CSP-specific Th2 type immune responses and improvesprotection against malaria infection. Eur. J. Immunol. 2001;31:692–698. doi: 10.1002/1521-4141(200103)31:3<692::aid-immu692>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 50.Wang R, Charoenvit Y, Corradin G, Porrozzi R, Hunter RL, Glenn G, Alving CR, Church P, Hoffman SL. Induction of protective polyclonal antibodies by immunization with Plasmodium yoelii circumsporozoite protein multiple antigen peptide vaccine. J. Immunol. 1995;155:1637. [PubMed] [Google Scholar]
- 51.Kaba SA, Brando C, Guo Q, Mittelholzer C, Raman S, Tropel D, Aebi U, Burkhard P, Lanar DE. A nonadjuvanted polypeptide nanoparticle vaccine confers long-lasting protection against rodent malaria. J. Immunol. 2009;183:7268–7277. doi: 10.4049/jimmunol.0901957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Overstreet MG, Cockburn IA, Chen YC, Zavala F. Protective CD8 T cells against Plasmodium liver stages: immunobiology of an “unnatural” immune response. Immunol. Rev. 2008;225:272–283. doi: 10.1111/j.1600-065X.2008.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li S, Rodrigues M, Rodriguez D, Rodriguez JR, Esteban M, Palese P, Nussenzweig RS, Zavala F. Priming with recombinant influenza virus followed by administration of recombinant vaccinia virus induces CD8+ T-cell-mediated protective immunity against malaria. Proc. Natl. Acad. Sci. USA. 1993;90:5214–5218. doi: 10.1073/pnas.90.11.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stewart VA, McGrath SM, Dubois PM, Pau MG, Mettens P, Shott J, Cobb M, Burge JR, Larson D, Ware LA, et al. Priming with an adenovirus 35-circumsporozoite protein (CS) vaccine followed by RTS,S/ AS01B boosting significantly improves immunogenicity to Plasmodium falciparum CS compared to that with either malaria vaccine alone. Infect. Immun. 2007;75:2283–2290. doi: 10.1128/IAI.01879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Butler NS, Schmidt NW, Harty JT. Differential effector pathways regulate memory CD8 T cell immunity against Plasmodium berghei versus P. yoelii sporozoites. J. Immunol. 2010;184:2528–2538. doi: 10.4049/jimmunol.0903529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li C, Langhorne J. Tumor necrosis factor a p55 receptor is important for development of memory responses to blood-stage malaria infection. Infect. Immun. 2000;68:5724–5730. doi: 10.1128/iai.68.10.5724-5730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pombo DJ, Lawrence G, Hirunpetcharat C, Rzepczyk C, Bryden M, Cloonan N, Anderson K, Mahakunkijcharoen Y, Martin LB, Wilson D, et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum . Lancet. 2002;360:610–617. doi: 10.1016/S0140-6736(02)09784-2. [DOI] [PubMed] [Google Scholar]
- 58.Hoffman SL, Crutcher JM, Puri SK, Ansari AA, Villinger F, Franke ED, Singh PP, Finkelman F, Gately MK, Dutta GP, Sedegah M. Sterile protection of monkeys against malaria after administration of interleukin-12. Nat. Med. 1997;3:80–83. doi: 10.1038/nm0197-80. [DOI] [PubMed] [Google Scholar]
- 59.Delaloye J, Roger T, Steiner-Tardivel QG, Le Roy D, Knaup Reymond M, Akira S, Petrilli V, Gomez CE, Perdiguero B, Tschopp J, et al. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 2009;5:e1000480. doi: 10.1371/journal.ppat.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Olotu A, Lusingu J, Leach A, Lievens M, Vekemans J, Msham S, Lang T, Gould J, Dubois MC, Jongert E, et al. Efficacy of RTS,S/AS01E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5–17 months in Kenya and Tanzania: a randomised controlled trial. Lancet Infect Dis. 2011;11:102–109. doi: 10.1016/S1473-3099(10)70262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olotu A, Moris P, Mwacharo J, Vekemans J, Kimani D, Janssens M, Kai O, Jongert E, Lievens M, Leach A, et al. Circumsporozoite-specific T cell responses in children vaccinated with RTS,S/AS01E and protection against P falciparum clinical malaria. PLoS ONE. 2011;6:e25786. doi: 10.1371/journal.pone.0025786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schneider J, Gilbert SC, Blanchard TJ, Hanke T, Robson KJ, Hannan CM, Becker M, Sinden R, Smith GL, Hill AV. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 63.Yoshida S, Kondoh D, Arai E, Matsuoka H, Seki C, Tanaka T, Okada M, Ishii A. Baculovirus virions displaying Plasmodium berghei circumsporozoite protein protect mice against malaria sporozoite infection. Virology. 2003;316:161–170. doi: 10.1016/j.virol.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 64.Tartz S, Rüssmann H, Kamanova J, Sebo P, Sturm A, Heussler V, Fleischer B, Jacobs T. Complete protection against P. berghei malaria upon heterologous prime/boost immunization against circumsporozoite protein employing Salmonella type III secretion system and Bordetella adenylate cyclase toxoid. Vaccine. 2008;26:5935–5943. doi: 10.1016/j.vaccine.2008.08.057. [DOI] [PubMed] [Google Scholar]
- 65.Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, Wilson JM, Schmieg J, Kronenberg M, Nakayama T, Taniguchi M, Koezuka Y, Tsuji M. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J. Exp. Med. 2002;195:617–624. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.