Abstract
Background
Although a rapid and efficient psychiatric treatment, electroconvulsive therapy (ECT) induces memory impairment. Modified ECT requires anesthesia for safety purposes. Although traditionally found to exert amnesic effects in general anesthesia, which is an inherent part of modified ECT, some anesthetics have been found to protect against ECT-induced cognitive impairment. However, the mechanisms remain unclear. We investigated the effects of propofol (2,6-diisopropylphenol) on memory in depressed rats undergoing electroconvulsive shock (ECS), the analog of ECT in animals, under anesthesia as well as its mechanisms.
Methods
Chronic unpredictable mild stresses were adopted to reproduce depression in a rodent model. Rats underwent ECS (or sham ECS) with anesthesia with propofol or normal saline. Behavior was assessed in sucrose preference, open field and Morris water maze tests. Hippocampal long-term potentiation (LTP) was measured using electrophysiological techniques. PSD-95, CREB, and p-CREB protein expression was assayed with Western blotting.
Results
Depression induced memory damage, and downregulated LTP, PSD-95, CREB, and p-CREB; these effects were exacerbated in depressed rats by ECS; propofol did not reverse the depression-induced changes, but when administered in modified ECS, propofol improved memory and reversed the downregulation of LTP and the proteins.
Conclusion
These findings suggest that propofol prevents ECS-induced memory impairment, and modified ECS under anesthesia with propofol improves memory in depressed rats, possibly by reversing the excessive changes in hippocampal synaptic plasticity. These observations provide a novel insight into potential targets for optimizing the clinical use of ECT for psychiatric disorders.
Keyword: electroconvulsive therapy, long-term potentiation, PSD-95, CREB
Introduction
Electroconvulsive therapy (ECT) is a commonly used treatment for some psychiatric disorders, including depression, mania, and schizophrenia.1 Compared with pharmacotherapy, ECT is more efficient and rapid, especially in patients with drug-resistant affective disorders.2 However, the development and spread of ECT have been impeded mainly because of its complications, especially amnesia. Although alternative therapies have been developed during recent years, such as vagus nerve stimulation, repetitive transcranial magnetic stimulation, and deep brain stimulation, the use of ECT has not yet been superseded.3 Encouragingly, more and more methods have been explored to alleviate ECT-induced memory deficits and to improve the final cognitive outcomes of psychiatric patients after ECT, including ECT parameter setting, electrode placement, and drug assistance.4,5 Anesthesia is required for modern ECT (modified ECT [MECT]) to enhance its safety by preventing its complications, such as fracture, asphyxia, and cardiovascular instability.6 Interestingly, although traditionally found to exert amnesic effects in general anesthesia, some anesthetics have been found to protect against ECT-induced cognitive impairment.7,8 Anesthetics are an inherent part of MECT; therefore, the cognitive benefits and underlying mechanisms of anesthetics in ECT remain to be elucidated in studies, which may offer novel insights into improvements for safer ECT performance in affective disorders.
Propofol (2,6-diisopropylphenol) is a popular intravenous anesthetic, which is well known for its rapid induction of and recovery from anesthesia, thus being a suitable and commonly-used anesthetic for MECT. Propofol was found to alleviate the memory impairment induced by ECT in previous studies.7,9 The basic synaptic mechanism of memory involves long-term potentiation (LTP), an electrophysiological model of synaptic plasticity. The mechanism of the amnesic effects of ECT is closely related to “saturation of LTP.”10,11 Propofol itself has depressive effects on LTP.12 In our previous studies, electroconvulsive shock (ECS), the analog of ECT in animals, under anesthesia with propofol was found to ameliorate LTP impairment caused by chronic unpredictable mild stress (CUMS), an animal model of depression.13 Furthermore, propofol enhanced CaMKIIα activation in the hippocampus in depressed rats undergoing ECS.14 However, to our knowledge, other evidence of the effects of propofol on LTP and the downstream mechanism underlying the alleviation of ECT-induced memory impairment is rare. In the present study, we extended our previous studies by first testing the hypothesis that the representative anesthetic propofol exerted its antiamnesic effects in ECS by regulating synaptic plasticity in the hippocampus, including LTP and its downstream effects, in a rat model of depression.
Materials and methods
Animals
Healthy adult male Wistar rats, weighing 200–240 g, from the Laboratory Animal Center of Chongqing Medical University (Chongqing, People’s Republic of China) were maintained in a standardized environment for a 1-week acclimatization period before experiments. All of the experimental procedures were approved by the Ethical Committee of Chongqing Medical University and carried out in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental groups and treatments
Rats were randomly divided into five groups: one control group of healthy rats without any treatment (group C) and four groups treated with CUMS to reproduce the rodent model of depression (groups D, P, E, and M). The rats in groups D, P, E, and M were subjected to the CUMS procedure for 28 days. On the day following completion of the CUMS procedure (ie, day 29 after the start of the experiment), the baseline measurements of behavioral tests were conducted in all the rats: sucrose preference test (SPT) on day 29, open field test (OFT) on day 30, and Morris water maze test (MWM) on days 31–36. From days 37 to 43, rats in group M received ECS with propofol pretreatment (9 mL/kg, intraperitoneal [ip], 10 g/L) (catalog number Fx061; AstraZeneca plc, London, UK); rats in group E received ECS with normal saline pretreatment (0.9% NaCl solution, 9 mL/kg, ip); rats in group P received sham ECS with propofol pretreatment (9 mL/kg, ip); and rats in group D received sham ECS with normal saline pretreatment (9 mL/kg, ip). Subsequently, behavioral tests were repeated on all rats (SPT on day 44; OFT on day 45, and MWM from days 46–51). On day 52, rats were sacrificed by decapitation, and brains were removed for preparation of hippocampal slices for electrophysiological measurements or biological assays. A timeline of the study is shown in Figure 1.
Figure 1.
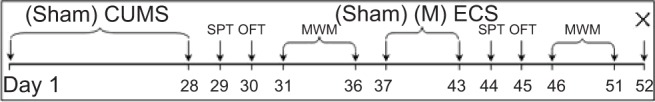
Experimental timeline of this study.
Notes: Sham CUMS, without any treatment (for group C); Sham ECS (for group D), Sham MECS (for group P).
Abbreviations: CUMS, chronic unpredictable mild stresses (for groups D, P, E, and M); ECS, electroconvulsive shock (for group E); MECS, modified ECS (for group M); MWM, Morris water maze test; OFT, open field test; SPT, sucrose preference test; ×, sacrifice.
Chronic unpredictable mild stress procedure
The CUMS procedure was adopted from a previous study with minor modifications.15 One randomly selected stressor stimulus among the panel used in this study was applied once daily to the rats in the CUMS-treated groups. The panel of stressor stimuli consisted of 1) swimming in cold water (4°C) for 5 minutes; 2) tail pinching for 1 minute; 3) food deprivation for 24 hours; 4) water deprivation for 24 hours; 5) social crowding (24 rats per cage), with cage being tilted to 30° from the horizontal plane for 24 hours; 6) shaking for 20 minutes (one shake per second); 7) 24 hours of continuous lighting; 8) housing in a soiled cage for 24 hours; 9) heat stress (45°C) for 5 minutes; 10) undesirable confinement for 2 hours. Stressor stimuli were administered three times within the 4 weeks, except for stressors 1 and 2 (two times each).
Electroconvulsive shock
After pretreatment with propofol or normal saline according to the group assignments, ECS was delivered via ear clip electrodes using a Niviqure ECT system (Niviqure Meditech, Bangalore, India), with bidirectional square wave pulses, 0.8 A in amplitude, 1.5 ms in width, 125 pulses per second, duration of 0.8 seconds, generating stimulus intensity of 120 mC, once daily for 7 days.16 Oxygen was given, and the saturation of blood oxygen was monitored and maintained to prevent hypoxia. Sham ECS was performed using an identical procedure without the application of current.
Behavioral tests
Sucrose preference test
After a 23-hour period of food and water deprivation, each rat was given free access to two preweighed bottles for 1 hour (one filled with a 1% [w/v] sucrose solution and the other with water). Each bottle was weighed both before and after the 1-hour period of access for determination of the sucrose preference of each rat according to the following equation: sucrose preference percentage (SPP) = sucrose solution consumption/(water consumption + sucrose solution consumption) ×100.15
Open field test
The apparatus consisted of a black-painted circular arena, 150 cm in diameter with 60 cm walls, placed in a room with dim illumination.17 Each rat was placed at the center of the arena, and its activities were observed for 5 minutes. Indexes assessed were horizontal ambulation (the total distance traveled, indicating general locomotor activity); the number of rearing events (when a rat stood completely erect on its hind legs, indicating exploratory behavior); and time in the central zone. Data were recorded and analyzed with the SLY-WMS 2.0 system (Beijing Sunny Instruments, Beijing, People’s Republic of China).
Morris water maze
Each rat was submitted to four trials per day for 5 days from each of four quadrants in a pool (150 cm in diameter, 50 cm in height, and colored with black ink). Animals were trained to find a hidden circular platform (10 cm in diameter, 2 cm beneath the water in the middle of the southwestern quadrant) by allowing each rat a maximum of 60 seconds to reach the platform; otherwise, it was guided toward the platform and left there for 15 seconds. The time to find the platform for the first time on day 5 of the trials (ie, evasive latency [EL]) was recorded to evaluate the learning ability of the rats. On day 6, every rat was subjected to a probe trial for 60 seconds in the absence of the platform in the pool from the northeastern quadrant. The activity of rats in the 60-second period probe trial was recorded, and the percentage dwell-time spent in the southwestern quadrant (ie, time percentage in the platform quarter [TPPQ]) was positively correlated with the rats’ spatial memory. The time spent in other quarters was also recorded for each group. TPPQ = swimming time in platform quarter (seconds)/total swimming time (seconds) ×100. The rat activity data were recorded and analyzed with SLY-WMS 2.0 software (Beijing Sunny Instruments).
Electrophysiological measurements
The procedures were adopted from previous study with minor modifications.18 Hippocampal slices (400 μm thickness) were prepared in 0°C–4°C oxygenated cutting solution (2.8 mM KCl, 8 mM NaH2PO4, 31 mM NaHCO3, 0.4 mM vitamin C, 2 mM sodium pyruvate, 2 mM sodium lactate, 10 mM glucose, 183 mM sucrose, 0.5 mM CaCl2, 8 mM MgSO4, 1 mM kynurenic acid) using a vibratome. Slices were transferred to artificial cerebrospinal fluid (124 mM NaCl, 2.8 mM KCl, 1.25 mM NaH2PO4, 26 mM NaHCO3, 0.4 mM vitamin C, 10 mM glucose, 2 mM CaCl2, 2 mM MgSO4) saturated with 95% O2 and 5% CO2 at 35°C for 45 minutes and kept at room temperature (24°C) to recover for at least 1 hour before recording. Each hippocampal slice was transferred into a recording chamber and superfused continuously with artificial cerebrospinal fluid saturated with 95% O2 and 5% CO2 at a velocity of 2 mL/minute. A bipolar tungsten stimulating electrode was placed in the Schaffer collateral pathway in the CA3 region with stimulus intensities of 0.1–0.25 mA, applied to evoke a field excitatory postsynaptic potential (fEPSP), which was recorded in the stratum radiatum in the CA1 region using a glass micropipette (with resistance of 3–4 MΩ) containing 2,000 mM NaCl, 10 mM HEPES (2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid), 10 mM ethylene glycol tetraacetic acid (EGTA). fEPSPs were evoked at different intensities to determine maximal stimulation. The input/output curves were generated by evoking fEPSPs at different intensities until maximal stimulation was reached, and fEPSP slopes were plotted against incremental stimulus intensities. For LTP studies, the test stimulus intensity was set to evoke 50% of the fEPSP slope at the maximum stimulation. The baseline fEPSP slopes were stably recorded for 15 minutes, and then a high frequency stimulation (HFS) (two streams of 100 pulses at 100 Hz, at 30-second intervals) was administered to induce LTP. fEPSP continued to be recorded for at least 45 minutes after HFS was administered. Data were recorded and analyzed using the Axon Instruments system (Multiclamp700B amplifier, Digidata 1,200 transverter, pCLAMP 9.2 software; Molecular Devices, Sunnyvale, CA, USA). The relative slope of fEPSP is equal to the ratio of the slope after LTP induction and the baseline slope.
Western blotting
Western blotting was performed using antibodies specific for postsynaptic density-95 (PSD-95) (catalog number 3409s), cyclic adenosine monophosphate (cAMP)-response element binding protein (CREB) (catalog number 9197), and phospho-CREB (p-CREB) (catalog number 9198s), all obtained from Cell Signaling Technology (Danvers, MA, USA), and β-actin (catalog number AICP001; Sizhengbo, Beijing, People’s Republic of China). Hippocampal tissues were homogenized, centrifuged (12,000 rpm, 4°C, 15 minutes), and the supernatants were collected. Proteins were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transblotted onto polyvinylidene difluoride membranes. The membranes were blocked and incubated with the relevant primary antibody (1:1,000, 4°C, overnight). Membranes were washed and incubated with horseradish peroxidase-conjugated secondary antibodies (24°C, 1 hour). Immunoreactive proteins were visualized by a chemiluminescence reaction, and data were analyzed using Quantity One software (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Statistical analysis
Data were expressed as the mean ± standard error. Statistical analysis was performed using SPSS software program (version 13; SPSS, Chicago, IL, USA). The results were compared using an analysis of variance (ANOVA), followed by Fisher’s least significant difference tests. P-values <0.05 were considered significant.
Results
Behavioral tests
Sucrose preference test
Before ECS treatment, the SPP of all groups was lower than that of group C (all groups P<0.001). After ECS (or sham ECS) treatment, there were significant differences between the groups [F(4, 45)=30.701, P<0.001]. The SPP of all groups was lower than that of group C (group D, P, and E: P<0.001; group M: P=0.001). The SPP of groups E and M were higher than that of group D (both P<0.001). The SPP of groups E and M were higher than that of group P (both P<0.001). No differences were found in comparisons between all other groups (Figure 2).
Figure 2.
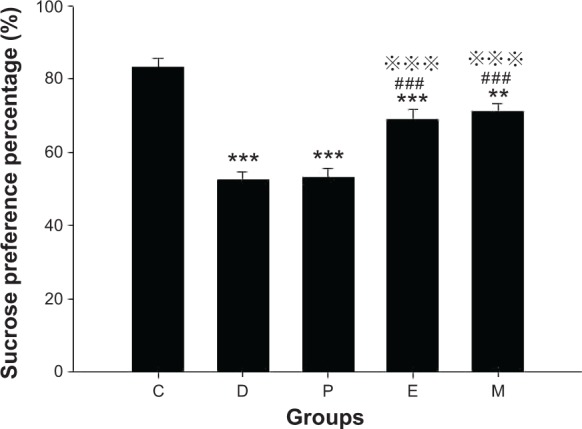
Sucrose preference in rats after ECS (or MECS) treatment.
Notes: Data are presented as the mean ± SE; **P<0.01 and ***P<0.001 versus group C; ###P<0.001 versus group D; P<0.001 versus group P. C represents control rats; D represents CUMS-pretreated rats that received sham ECS with normal saline pretreatment; P represents CUMS-pretreated rats that received sham ECS with propofol pretreatment; E represents CUMS-pretreated rats that received ECS with normal saline pretreatment; M represents CUMS-pretreated rats that received ECS with propofol pretreatment; n=10 in each group.
Abbreviations: CUMS, chronic unpredictable mild stresses; ECS, electroconvulsive shock; MECS, modified ECS; SE, standard error.
Open field test
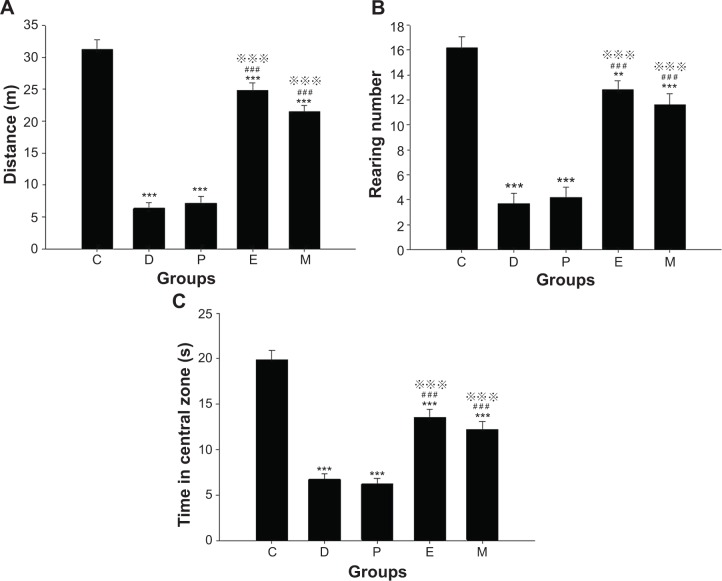
Before ECS treatment, the horizontal ambulation distances of all groups in the OFT were less than that of group C (all groups P<0.001). After ECS (or sham ECS) treatment, there were significant differences between groups [F(4, 45)=84.403, P<0.001]. The horizontal ambulation distance of all groups was less than that of group C (all groups P<0.001). The horizontal ambulation distances of groups E and M were greater than those of groups D and P (both P<0.001). No differences were found in comparisons between all other groups (Figure 3A).
Figure 3.
Indexes in open field test in rats after ECS (or MECS) treatment.
Notes: Distance (A); rearing number (B); time in the central zone (C). Data are presented as the mean ± SE. **P<0.01 and ***P<0.001 versus group C; ###P<0.001 versus group D; P<0.001 versus group P. C represents control rats; D represents CUMS-pretreated rats that received sham ECS with normal saline pretreatment; P represents CUMS-pretreated rats that received sham ECS with propofol pretreatment; E represents CUMS-pretreated rats that received ECS with normal saline pretreatment; M represents CUMS-pretreated rats that received ECS with propofol pretreatment; n=10 in each group.
Abbreviations: CUMS, chronic unpredictable mild stresses; ECS, electroconvulsive shock; MECS, modified ECS; s, seconds; SE, standard error.
Before ECS treatment, the number of rearing events in the OFT in all groups was lower than that of group C (all groups P<0.001). After ECS (or sham ECS) treatment, there were significant differences between groups [F(4, 45)=44.853, P<0.001]. The number of rearing events in all groups was lower than that of group C (groups D, P, and M: P<0.001; group E: P=0.005). The number of rearing events in groups E and M was higher than that in group D (both P<0.001). The number of rearing events in groups E and M was higher than that in group P (both P<0.001). No differences were found in comparisons between all other groups (Figure 3B).
Before ECS treatment, time in the central zone in the OFT of all groups was less than that of group C (all groups P<0.001). After ECS (or sham ECS) treatment, there were significant differences between groups [F(4, 45)=46.908, P<0.001]. The time in the central zone of all groups was less than that of group C (all groups P<0.001). Groups E and M spent more time in the central zone than group D (both P<0.001). Groups E and M spent more time in the central zone than group P (both P<0.001). No differences were found in comparisons between all other groups (Figure 3C).
Morris water maze
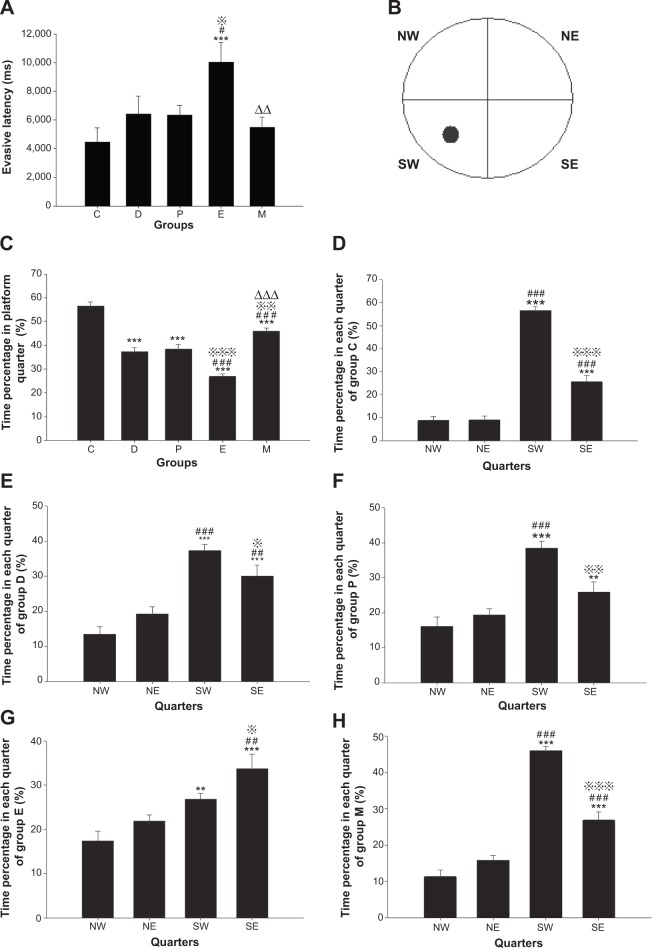
Before ECS treatment, the EL of all groups in the MWM test was longer than that of group C (all groups P<0.001). After ECS (or sham ECS) treatment, there were significant differences between groups [F(4, 45)=4.199, P=0.006]. The EL of group E was longer than those of groups C, D, and P (P<0.001; P=0.016; and P=0.014, respectively). The EL of group M was shorter than that of group E (P=0.003). No differences were found in comparisons between all other groups (Figure 4A).
Figure 4.
Learning and memory in Morris water maze test in rats after ECS (or MECS) treatment.
Notes: Evasive latency (A); division of different quarters in the test (B); time percentage in the platform quarter of each group (C); time percentage in each quarter in group C (D); time percentage in each quarter in group D (E); time percentage in each quarter in group P (F); time percentage in each quarter in group E (G); time percentage in each quarter in group M (H). Data are presented as the mean ± standard error; **P<0.01, ***P<0.001 versus group C or quarter NW; #P<0.05, ##P<0.01, and ###P<0.001 versus group D or quarter NE; P<0.05, P<0.01, and P<0.001 versus group P or quarter SW; ΔΔP<0.01, ΔΔΔP<0.001 versus group E. C represents control rats; D represents CUMS-pretreated rats that received sham ECS with normal saline pretreatment; P represents CUMS-pretreated rats that received sham ECS with propofol pretreatment; E represents CUMS-pretreated rats that received ECS with normal saline pretreatment; M represents CUMS-pretreated rats that received ECS with propofol pretreatment. • Represents platform; n=10 in each group.
Abbreviations: CUMS, chronic unpredictable mild stresses; ECS, electroconvulsive shock; MECS, modified ECS; NE, northeastern quarter; NW, northwestern quarter; SE, southeastern quarter; SW, southwestern quarter (ie, the platform quarter).
Before ECS treatment, the TPPQ of all groups was lower than that of group C (all groups P<0.001). After ECS (or sham ECS) treatment, there were significant differences between groups [F(4, 45)=49.073, P<0.001]. The TPPQ of all groups was lower than that of group C (all groups P<0.001). The TPPQ of group E was lower than that of group D (P<0.001), while that of group M was higher (P<0.001). The TPPQ of group E was lower than that of group P (P<0.001), while that of group M was higher (P=0.002). The TPPQ of group M was higher than that of group E (P<0.001). No differences were found in comparisons between all other groups (Figure 4C).
In group C, there were significant differences between the times spent in each quarter [F(3, 36)=121.359, P<0.001]; compared with the northwestern quarter (NW), the percentage of time spent in the southwestern quarter (SW) (ie, the platform quarter) or the southeastern quarter (SE) was greater (both P<0.001); compared with the northeastern quarter (NE), the percentage of time spent in SW or SE was greater (both P<0.001); compared with SW, the percentage of time spent in SE was less (P<0.001) (Figure 4D).
In group D, there were significant differences between the times spent in each quarter [F(3, 36)=20.725, P<0.001]; compared with NW, the percentage of time spent in SW or SE was greater (both P<0.001); compared with NE, the percentage of time spent in SW or SE was greater (P<0.001 versus SW and P=0.002 versus SE); compared with SW, the percentage of time spent in SE was less (P=0.035) (Figure 4E).
In group P, there were significant differences between the time spent in each quarter [F(3, 36)=17.650, P<0.001]; compared with NW, the percentage of time spent in SW or SE was greater (P<0.001 versus SW and P=0.006 versus SE); compared with NE, the percentage of time spent in SW was greater (P<0.001); compared with SW, the percentage of time spent in SE was less (P=0.001) (Figure 4F).
In group E, there were significant differences between the times spent in each quarter [F(3, 36)=10.154, P<0.001]; compared with NW, the percentage of time spent in SW or SE was greater (P=0.004 versus SW and P<0.001 versus SE); compared with NE, the percentage of time spent in SE was larger (P=0.001); compared with SW, the percentage of time spent in SE was greater (P=0.034) (Figure 4G).
In group M, there were significant differences between the time spent in each quarter [F(3, 36)=84.585, P<0.001]; compared with NW, the percentage of time spent in SW or SE was greater (both P<0.001); compared with NE, the percentage of time spent in SW or SE was greater (both P<0.001); compared with SW, the percentage of time spent in SE was less (P<0.001) (Figure 4H).
Electrophysiological measurements
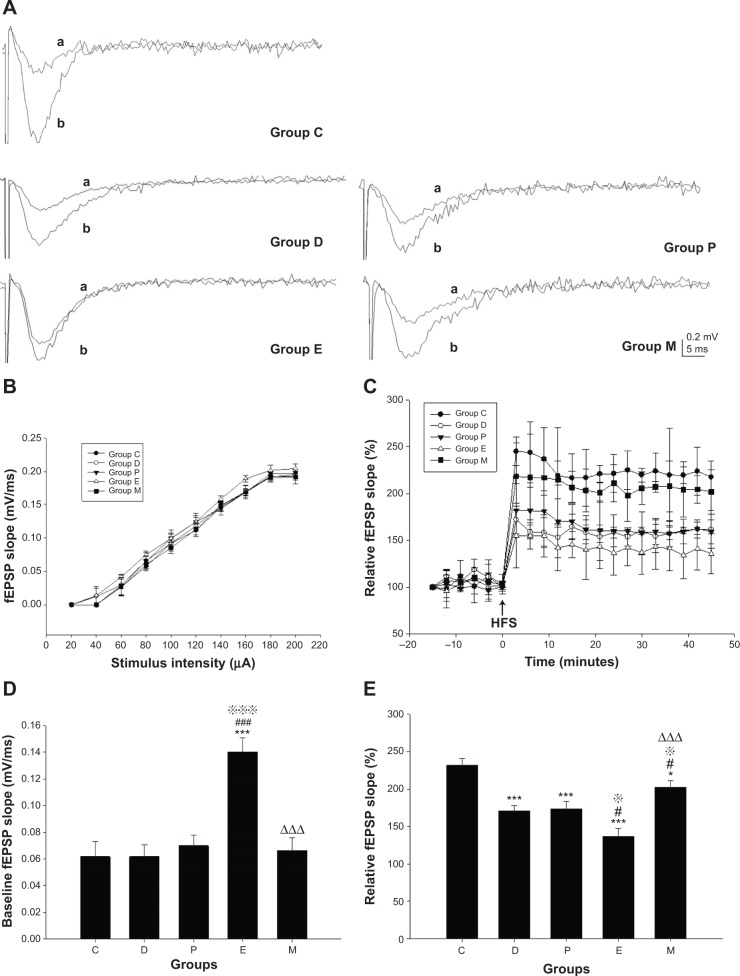
There were significant differences in the baseline fEPSP slopes between groups [F(4, 20)=12.628, P<0.001]. Compared with groups C, D, and P, the baseline fEPSP slope of group E was steeper (all P<0.001). However, compared with group E, the baseline fEPSP slope of group M was less steep (P<0.001).
There were significant differences in the relative fEPSP slopes between groups [F(4, 20)=14.971, P<0.001]. Compared with group C, the relative fEPSP slopes of groups D, P, E, and M were less steep (all P<0.001 versus groups D, P, and E; P=0.037 versus group M). Compared with group D, the relative fEPSP slope of group E was less steep (P=0.016), while that of group M was steeper (P=0.026). Compared with group P, the relative fEPSP slopes of group E were less steep (P=0.011), while that of group M was steeper (P=0.040). Compared with group E, the relative fEPSP slope of group M was steeper (P<0.001) (Figure 5).
Figure 5.
LTP in the CA1 region in hippocampus of rats after ECS (or MECS) treatment.
Notes: Original traces of fEPSP in each group (A); input/output curves in each group (B); the induction and maintenance of LTP (C); baseline fEPSP slope (D); relative fEPSP slope (E). Data are presented as the mean ± SE; *P<0.05, ***P<0.001 versus group C; #P<0.05, ###P<0.001 versus group D; P<0.05, P<0.001 versus group P; ΔΔΔP<0.001 versus group E. C represents control rats; D represents CUMS-pretreated rats that received sham ECS with normal saline pretreatment; P represents CUMS-pretreated rats that received sham ECS with propofol pretreatment; E represents CUMS-pretreated rats that received ECS with normal saline pretreatment; M represents CUMS-pretreated rats that received ECS with propofol pretreatment; n=5 in each group. aoriginal trace of baseline fEPSP; boriginal trace of fEPSP after HFS.
Abbreviations: CUMS, chronic unpredictable mild stresses; ECS, electroconvulsive shock; fEPSP, field excitatory postsynaptic potential; HFS, high frequency stimulation; LTP, long-term potentiation; MECS, modified ECS; SE, standard error.
Western blotting measurements
PSD-95
There were significant differences in PSD-95 protein expression between groups [F(4, 25)=21.591, P<0.001]. Compared with group C, PSD-95 protein expression was lower in groups D, P, E, and M (all P<0.001). Expression in group E was lower than that in groups D and P (P=0.014 versus group D and P=0.024 versus group P). In contrast, expression in group M was higher than that in group E (P=0.004). No differences were found in comparisons between all other groups (Figure 6).
Figure 6.
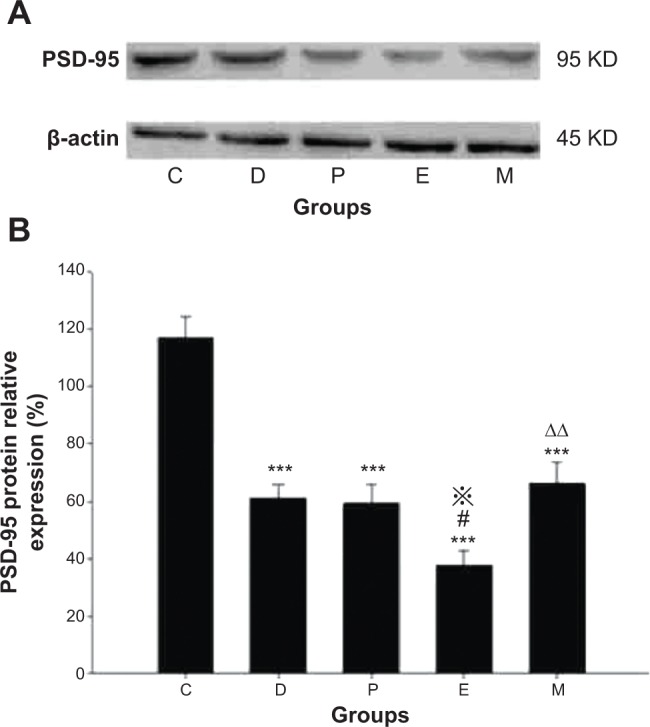
Hippocampal PSD-95 protein expression in rats after ECS (or MECS) treatment.
Notes: Western blot bands of PSD-95 and β-actin (A); relative expression of PSD-95 protein (B). Data are presented as the mean ± SE; ***P<0.001 versus group C; #P<0.05 versus group D; P<0.05 versus group P; ΔΔP<0.01 versus group E. C represents control rats; D represents CUMS-pretreated rats that received sham ECS with normal saline pretreatment; P represents CUMS-pretreated rats that received sham ECS with propofol pretreatment; E represents CUMS-pretreated rats that received ECS with normal saline pretreatment; M represents CUMS-pretreated rats that received ECS with propofol pretreatment; n=6 in each group.
Abbreviations: CUMS, chronic unpredictable mild stresses; ECS, electroconvulsive shock; MECS, modified ECS; PSD-95, postsynaptic density-95; SE, standard error.
CREB and p-CREB
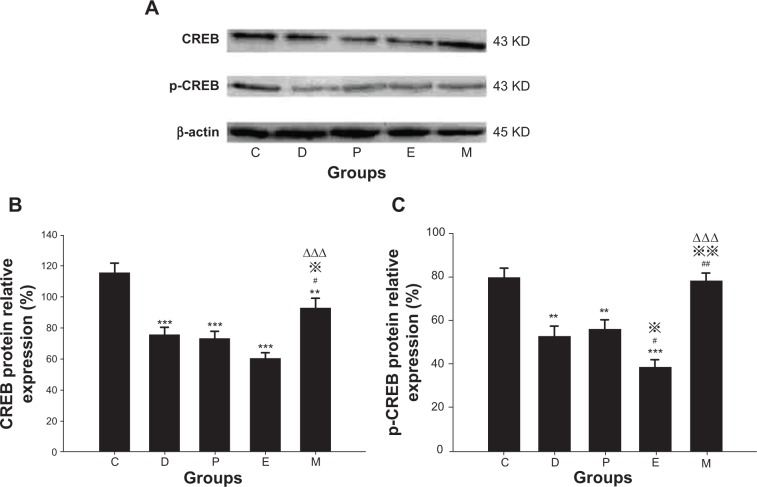
There were significant differences in CREB protein expression between groups [F(4, 24)=14.895, P<0.001]. Compared with group C, CREB protein expression was lower in groups D, P, E, and M (all P<0.001 versus groups D, P, E, and P=0.007 versus group M). Expression was higher in group M compared with that in groups D, P, and E (P=0.028 versus group D, P=0.013 versus group P, and P<0.001 versus group E). No differences were found in comparisons between all other groups.
There were significant differences in p-CREB protein expression between groups [F(4, 24)=13.764, P<0.001]. Compared with group C, p-CREB protein expression was lower in groups D, P, and E (P=0.001 versus group D, P=0.002 versus group P, and P<0.001 versus group E). Compared with group D, expression in group E was lower, while expression in group M was higher (P=0.043 versus group E, and P=0.001 versus group M). Compared with group P, expression in group E was lower, while that in group M was higher (P=0.014 versus group E and P=0.002 versus group M). Compared with group E, expression in group M was higher (P<0.001). No differences were found in comparisons between all other groups (Figure 7).
Figure 7.
Hippocampal CREB/p-CREB protein expression in rats after ECS (or MECS) treatment.
Notes: Western blot bands of CREB/p-CREB and β-actin (A); relative expression of CREB protein (B); relative expression of p-CREB protein (C). Data are presented as the mean ± SE; **P<0.01, ***P<0.001 versus group C; #P<0.05, ##P<0.01 versus group D; P<0.05, P<0.01 versus group P; ΔΔΔP<0.001 versus group E. C represents control rats; D represents CUMS-pretreated rats that received sham ECS with normal saline pretreatment; P represents CUMS-pretreated rats that received sham ECS with propofol pretreatment; E represents CUMS-pretreated rats that received ECS with normal saline pretreatment; M represents CUMS-pretreated rats that received ECS with propofol pretreatment; n=5–6 in each group.
Abbreviations: CUMS, chronic unpredictable mild stresses; ECS, electroconvulsive shock; MECS, modified ECS; SE, standard error.
Discussion
This study performed in a rat model demonstrated that depression induced memory damage and downregulated LTP, PSD-95, CREB, and p-CREB protein expression. ECS further impaired memory, downregulated LTP by elevating the baseline fEPSP slope, and downregulated PSD-95 and p-CREB protein expression in depressed rats. Propofol alone did not reverse depression-induced changes, but when administered in modified ECS (MECS) (ie, ECS with anesthesia), it improved memory as compared with depressed rats receiving either ECS or sham ECS. Furthermore, the effects on the baseline fEPSP slope and LTP, PSD-95, CREB, and p-CREB protein expression were reversed. Moreover, propofol in MECS did not compromise the antidepressant effectiveness of ECS.
CUMS is a valid method used to reproduce depression in animal models.19,20 The SPT is used to evaluate rats’ enjoyment of food, with decreased interest in sucrose (reflected by decreased SPP) indicating the degree of anhedonia, which is a core symptom of depression. Indexes of OFT indicate spontaneous activity such as excitation and exploratory abilities in unfamiliar environments, which assist in evaluation of the depressive condition. CUMS can depress the responses of animals to their interests and novel environments. In this study, the SPP and indexes of OFT in CUMS-treated rats were lower than those in the control group, and these effects were not recovered by sham ECS treatment. These results are consistent with the behavioral traits of depressed animals, indicating the successful reproduction of a depressive animal model. There were no significant differences in SPP and OFT indexes between the ECS-treated group and the MECS-treated group, indicating that propofol does not compromise the antidepressant efficacy of ECS.
Patients or animals with depression have cognitive deficits, including memory impairment, which lead to decreased social activities and also affect final cognitive outcomes. In this study, we also found that rats displayed memory dysfunction after receiving CUMS treatment. Exerting amnesic effects, ECS has been used to reproduce an animal model of learning and memory deficits since the 1960s.21 We found that ECS exacerbated memory impairment in depressed rats. As a representative general anesthetic, propofol used at either anesthetic or nonsedative doses was found to induce amnesia in rodents.22,23 However, later studies showed that propofol anesthesia did not affect spatial memory in aged rats.24 Our results also indicate that repeated administration of propofol (once a day for 7 days) did not exacerbate the memory impairment caused by depression. When combined with other general anesthetics used in ECT (including methohexital and thiopental), propofol did not damage cognitive function and was even found to provide benefit in this regard compared with the effects of the other anesthetics used alone.7,25 In our previous studies, it was shown that propofol itself had a definite antiamnesic effect when used as the sole anesthetic in ECS.9,14,26 In the present study, we further showed that propofol combined with MECS (in group M) could reverse memory impairment to a significantly higher level than was observed in the other two depressed groups without undergoing ECS treatments (groups D and P). These results indicate that MECS not only reverses the memory impairment as compared with ECS, but also reverses the memory damage caused by depression, although not to the level observed in the control group.
As an essential mechanism of memory, hippocampal synaptic plasticity can be measured in terms of LTP, which is damaged by depression. As an etiological factor of depression, chronic stresses depress hippocampal LTP and subsequently result in memory impairment in rodents.27 Compared with control rats, the enhancement of synaptic efficacy induced by HFS in the dentate gyrus (DG) region of the hippocampus was depressed significantly in a rat model of depression.28 In this study, we also found that hippocampal LTP of CUMS-treated rats was depressed without regulating the baseline fEPSP slope, as compared with control rats. In vivo animal studies have demonstrated that a single local electroconvulsive seizure can impair LTP in the CA1 region of the hippocampus, and repeated ECS can impair LTP in the DG region in rats.29,30 However, whether repeated ECS inhibits LTP in the CA1 region in rodent hippocampal slices in vitro remains controversial.31 In this study, we found that ECS upregulated the baseline fEPSP slope in the CA1 region in CUMS-treated rats, thus further downregulating HFS-induced LTP, which is similar to the effects of ECS found in the DG region in hooded Lister rats in vivo. This evidence supports the hypothesis that repeated ECS can induce the “saturation of LTP.”32 This hypothesis presumes that the baseline fEPSP slope had been excessively raised by ECS, which could simulate the induction of LTP by HFS; therefore, the potential response of synapses to the further LTP-inducing procedures (ie, HFS) was relatively decreased. Propofol inhibits LTP in the CA1 region,12 although LTP in the DG region did not change in a rat model of cerebral ischemia following intravenous infusion of propofol for 1 hour.33 We found that although repeated administration of propofol (once a day for 7 days) did not reverse the inhibitory effects of depression on LTP, it did not further exacerbate the effects. However, LTP in depressed rats undergoing MECS following propofol pretreatment (group M) was reversed to a higher level than that of groups D, P, and E. This observation indicated that propofol did exert completely enhancing effects on LTP in MECS for rats with depression, and that MECS could improve LTP in depressed rats. The elevation of LTP by propofol is a novel discovery in this animal model of depression and ECS. It can be speculated that the mechanism of this effect of propofol differs from the depressive effects reported in previous studies in other disease models.
The formation and regulation of LTP is associated with synapse-related proteins. ECS induces decreased expression of hippocampal synaptic proteins, including N-methyl-D-aspartate receptor (NMDAR) (a glutamic acid receptor subtype), PSD-95, and CREB proteins without changing GluR1 (another glutamic acid receptor subtype), and synaptophysin in healthy rats.34 We previously found that hippocampal NMDAR expression was downregulated, reversed excessively after undergoing ECS, and that propofol inhibited this effect of ECS, leading to improved memory in depressed rats.26 Furthermore, propofol combined with MECS also reversed hippocampal synaptophysin expression in depressed rats.13
Linked with NMDARs at synaptic sites, PSD-95 plays a key role in mediating trafficking, clustering, and downstream signaling events following receptor activation in synaptic plasticity.35,36 PSD-95 was found to be decreased in the CA1 region of depressed monkeys.37 Repeated ECS downregulated PSD-95 in healthy rats.34 In accordance with these reports, in this study, PSD-95 protein expression was downregulated in depressed rats, as compared with that in the control group. Furthermore, we found that ECS further exacerbated the decrease in PSD-95 protein expression in depressed rats, while propofol combined with MECS reverses these changes, albeit only to the level of untreated depressed rats. The results of hippocampal PSD-95 expression obtained in this study are generally in accordance with those of behavior indicating memory in rats, which indicates the participation of PSD-95 in the mechanism by which propofol regulates the effects of MECS on memory.
As a final downstream component of various pathways, the transcription factor CREB and its phosphorylated form are essential in the formation of hippocampal LTP, synaptic plasticity, and memory. In accordance with the results of previous studies, we found that hippocampal CREB was downregulated in depressed rats. The effects of ECS on CREB and its phosphorylation remain controversial. In early studies, it was found that ECS raised Ser-133 phosphorylation of CREB in rat hippocampus, but not in the cerebellum.38 However, others found that a single ECS procedure or repetitive treatment did not change CREB expression patterns.39 Subsequently, other studies showed that CREB was reduced in the frontal cortices of rats immediately after ECS treatment,40 and the repeated ECS downregulated CREB in the hippocampus of rats.34 Recently, it has been reported that electroconvulsive stimuli increased p-CREB levels and the ratio of p-CREB/CREB in both saline-treated and adrenocorticotropic hormone (ACTH)-treated rats.41 In the present study, ECS downregulated hippocampal p-CREB levels without changing CREB levels in depressed rats. It can be speculated that the discrepancies between the findings of these studies are due to differences in the time points at which measurements were taken or in animal models; however, this requires further investigation. It was found that propofol at clinically relevant concentrations blocked NMDAR-dependent activation of CREB,42 and subanesthetic doses of propofol induced suppression of CREB phosphorylation.43 However, others found that propofol had no effect on t-butyl hydroperoxide-induced downregulation of CREB expression and activation in cultured astrocytes,44 while propofol upregulated CREB phosphorylation in adult neural stem cells.45 In this study, propofol had no effects on the decrease in hippocampal CREB or p-CREB in depressed rats, but significantly reversed both CREB and p-CREB expression in MECS-treated depressed rats as compared with either the ECS-treated or untreated depressed rats. The differences between these reports of the effects of propofol on CREB may be caused by differences in doses or pathophysiological conditions, although this requires confirmation in further studies.
The limitation of this study is that the behavioral tests might influence the electrophysiological function and protein expression in rats. However, all the rats in this study were subjected to the same behavioral tests. Moreover, the statistically significant differences between groups in this study provide confidence in the interpretation of our results although further studies will be required to confirm the absence of influence due to the behavioral tests employed.
Conclusion
This work revealed that propofol alleviated ECS-induced memory impairment, possibly by reversing the excessive changes in hippocampal synaptic plasticity and related proteins caused by ECS, without interference with the antidepressant effects of ECS in a rodent model of depression. Furthermore, such findings are expected to provide novel insights into potential targets for optimizing the safety and efficacy of the clinical use of ECT.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (numbers 81271501 and 81201053), the Natural Science Foundation Project of Chongqing Science and Technology Commission (CQ CSTC) (number cstc2012jjA10056), the National Clinical Key Subject Construction Project of China (Caishe [2011] number 170), and the Medical Key Discipline Construction Project of Chongqing (Yuweikejiao [2007] number 2).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Goodman WK. Electroconvulsive therapy in the spotlight. N Engl J Med. 2011;364(19):1785–1787. doi: 10.1056/NEJMp1101096. [DOI] [PubMed] [Google Scholar]
- 2.Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357(19):1939–1945. doi: 10.1056/NEJMct075234. [DOI] [PubMed] [Google Scholar]
- 3.Wani A, Trevino K, Marnell P, Husain MM. Advances in brain stimulation for depression. Ann Clin Psychiatry. 2013;25(3):217–224. [PubMed] [Google Scholar]
- 4.Andrade C, Shaikh SA, Narayan L, Blasey C, Belanoff J. Administration of a selective glucocorticoid antagonist attenuates electroconvulsive shock-induced retrograde amnesia. J Neural Transm. 2012;119(3):337–344. doi: 10.1007/s00702-011-0712-8. [DOI] [PubMed] [Google Scholar]
- 5.Sackeim HA, Prudic J, Devanand DP, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med. 1993;328(12):839–846. doi: 10.1056/NEJM199303253281204. [DOI] [PubMed] [Google Scholar]
- 6.Zahavi GS, Dannon P. Comparison of anesthetics in electroconvulsive therapy: an effective treatment with the use of propofol, etomidate, and thiopental. Neuropsychiatr Dis Treat. 2014;10:383–389. doi: 10.2147/NDT.S58330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butterfield NN, Graf P, Macleod BA, Ries CR, Zis AP. Propofol reduces cognitive impairment after electroconvulsive therapy. J ECT. 2004;20(1):3–9. doi: 10.1097/00124509-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 8.McDaniel WW, Sahota AK, Vyas BV, Laguerta N, Hategan L, Oswald J. Ketamine appears associated with better word recall than etomidate after a course of 6 electroconvulsive therapies. J ECT. 2006;22(2):103–106. doi: 10.1097/00124509-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Luo J, Min S, Wei K, Li P, Dong J, Liu YF. Propofol protects against impairment of learning-memory and imbalance of hippocampal Glu/GABA induced by electroconvulsive shock in depressed rats. J Anesth. 2011;25(5):657–665. doi: 10.1007/s00540-011-1199-z. [DOI] [PubMed] [Google Scholar]
- 10.Pigot M, Andrade C, Loo C. Pharmacological attenuation of electro-convulsive therapy – induced cognitive deficits: theoretical background and clinical findings. J ECT. 2008;24(1):57–67. doi: 10.1097/YCT.0b013e3181616c14. [DOI] [PubMed] [Google Scholar]
- 11.Reid IC, Stewart CA. Seizures, memory and synaptic plasticity. Seizure. 1997;6(5):351–359. doi: 10.1016/s1059-1311(97)80034-9. [DOI] [PubMed] [Google Scholar]
- 12.Nagashima K, Zorumski CF, Izumi Y. Propofol inhibits long-term potentiation but not long-term depression in rat hippocampal slices. Anesthesiology. 2005;103(2):318–326. doi: 10.1097/00000542-200508000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Liu L, Liu YY, et al. Effects of electroconvulsive stimulation on long-term potentiation and synaptophysin in the hippocampus of rats with depressive behavior. J ECT. 2012;28(2):111–117. doi: 10.1097/YCT.0b013e31824a47ca. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Li W, Luo J, et al. Effects of propofol on the activation of hippocampal CaMKIIα in depressed rats receiving electroconvulsive therapy. J ECT. 2012;28(4):242–247. doi: 10.1097/YCT.0b013e31826140c7. [DOI] [PubMed] [Google Scholar]
- 15.Luo KR, Hong CJ, Liou YJ, Hou SJ, Huang YH, Tsai SJ. Differential regulation of neurotrophin S100B and BDNF in two rat models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1433–1439. doi: 10.1016/j.pnpbp.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 16.Luo J, Min S, Wei K, Zhang J, Liu Y. Propofol interacts with stimulus intensities of electroconvulsive shock to regulate behavior and hippocampal BDNF in a rat model of depression. Psychiatry Res. 2012;198(2):300–306. doi: 10.1016/j.psychres.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Qi X, Lin W, Li J, et al. Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress. Neurobiol Dis. 2008;31(2):278–285. doi: 10.1016/j.nbd.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson JL, Gorski JA, Gibson ES, et al. AKAP150-anchored calcineurin regulates synaptic plasticity by limiting synaptic incorporation of Ca2+-permeable AMPA receptors. J Neurosci. 2012;32(43):15036–15052. doi: 10.1523/JNEUROSCI.3326-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willner P, Mitchell PJ. The validity of animal models of predisposition to depression. Behav Pharmacol. 2002;13(3):169–188. doi: 10.1097/00008877-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Mohamed BM, Aboul-Fotouh S, Ibrahim EA, et al. Effects of pentoxifylline, 7-nitroindazole, and imipramine on tumor necrosis factor-α and indoleamine 2,3-dioxygenase enzyme activity in the hippocampus and frontal cortex of chronic mild-stress-exposed rats. Neuropsychiatr Dis Treat. 2013;9:697–708. doi: 10.2147/NDT.S41020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153(3742):1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- 22.O’Gorman DA, O’Connell AW, Murphy KJ, Moriarty DC, Shiotani T, Regan CM. Nefiracetam prevents propofol-induced anterograde and retrograde amnesia in the rodent without compromising quality of anesthesia. Anesthesiology. 1998;89(3):699–706. doi: 10.1097/00000542-199809000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Pain L, Angst MJ, LeGourrier L, Oberling P. Effect of a nonsedative dose of propofol on memory for aversively loaded information in rats. Anesthesiology. 2002;97(2):447–453. doi: 10.1097/00000542-200208000-00023. [DOI] [PubMed] [Google Scholar]
- 24.Lee IH, Culley DJ, Baxter MG, Xie Z, Tanzi RE, Crosby G. Spatial memory is intact in aged rats after propofol anesthesia. Anesth Analg. 2008;107(4):1211–1215. doi: 10.1213/ane.0b013e31817ee879. [DOI] [PubMed] [Google Scholar]
- 25.Geretsegger C, Nickel M, Judendorfer B, Rochowanski E, Novak E, Aichhorn W. Propofol and methohexital as anesthetic agents for electroconvulsive therapy: a randomized, double-blind comparison of electroconvulsive therapy seizure quality, therapeutic efficacy, and cognitive performance. J ECT. 2007;23(4):239–243. doi: 10.1097/0b013e31814da971. [DOI] [PubMed] [Google Scholar]
- 26.Dong J, Min S, Wei K, Li P, Cao J, Li Y. Effects of electroconvulsive therapy and propofol on spatial memory and glutamatergic system in hippocampus of depressed rats. J ECT. 2010;26(2):126–130. doi: 10.1097/yct.0b013e3181a9947a. [DOI] [PubMed] [Google Scholar]
- 27.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33(1):88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 28.Diana G, Domenici MR, Loizzo A, Scotti de Carolis A, Sagratella S. Age and strain differences in rat place learning and hippocampal dentate gyrus frequency-potentiation. Neurosci Lett. 1994;171(1–2):113–116. doi: 10.1016/0304-3940(94)90618-1. [DOI] [PubMed] [Google Scholar]
- 29.Hesse GW, Teyler TJ. Reversible loss of hippocampal long term potentiation following electronconvulsive seizures. Nature. 1976;264(5586):562–564. doi: 10.1038/264562a0. [DOI] [PubMed] [Google Scholar]
- 30.Stewart CA, Reid IC. Repeated ECS and fluoxetine administration have equivalent effects on hippocampal synaptic plasticity. Psychopharmacology (Berl) 2000;148(3):217–223. doi: 10.1007/s002130050045. [DOI] [PubMed] [Google Scholar]
- 31.Stewart CA, Davies SN. Repeated electroconvulsive stimulation impairs synaptic plasticity in the dentate gyrus in vivo but has no effect in CA1 in vitro. Neurosci Lett. 1996;213(3):177–180. doi: 10.1016/0304-3940(96)12853-6. [DOI] [PubMed] [Google Scholar]
- 32.Andrade C, Singh NM, Thyagarajan S, Nagaraja N, Sanjay Kumar Rao N, Suresh Chandra J. Possible glutamatergic and lipid signalling mechanisms in ECT-induced retrograde amnesia: experimental evidence for involvement of COX-2, and review of literature. J Psychiatr Res. 2008;42(10):837–850. doi: 10.1016/j.jpsychires.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Kakehata J, Togashi H, Yamaguchi T, Morimoto Y, Yoshioka M. Effects of propofol and halothane on long-term potentiation in the rat hippocampus after transient cerebral ischaemia. Eur J Anaesthesiol. 2007;24(12):1021–1027. doi: 10.1017/S0265021507000749. [DOI] [PubMed] [Google Scholar]
- 34.Yao Z, Guo Z, Yang C, et al. Phenylbutyric acid prevents rats from electroconvulsion-induced memory deficit with alterations of memory-related proteins and tau hyperphosphorylation. Neuroscience. 2010;168(2):405–415. doi: 10.1016/j.neuroscience.2010.03.060. [DOI] [PubMed] [Google Scholar]
- 35.Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(1):70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hashimoto K, Malchow B, Falkai P, Schmitt A. Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur Arch Psychiatry Clin Neurosci. 2013;263(5):367–377. doi: 10.1007/s00406-013-0399-y. [DOI] [PubMed] [Google Scholar]
- 37.Willard SL, Hemby SE, Register TC, McIntosh S, Shively CA. Altered expression of glial and synaptic markers in the anterior hippocampus of behaviorally depressed female monkeys. Neurosci Lett. 2014;563:1–5. doi: 10.1016/j.neulet.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeon SH, Seong YS, Juhnn YS, et al. Electroconvulsive shock increases the phosphorylation of cyclic AMP response element binding protein at Ser-133 in rat hippocampus but not in cerebellum. Neuropharmacology. 1997;36(3):411–414. doi: 10.1016/s0028-3908(97)00047-6. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh TF, Simler S, Vergnes M, et al. BDNF restores the expression of Jun and Fos inducible transcription factors in the rat brain following repetitive electroconvulsive seizures. Exp Neurol. 1998;149(1):161–174. doi: 10.1006/exnr.1997.6686. [DOI] [PubMed] [Google Scholar]
- 40.Kang UG, Jeon WJ, Kim Y, et al. Transient activation of protein phosphatase 2A induced by electroconvulsive shock in the rat frontal cortex. Neurosci Lett. 2005;390(3):171–175. doi: 10.1016/j.neulet.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 41.Kuwatsuka K, Hayashi H, Onoue Y, et al. The mechanisms of electroconvulsive stimuli in BrdU-positive cells of the dentate gyrus in ACTH-treated rats. J Pharmacol Sci. 2013;122(1):34–41. doi: 10.1254/jphs.13015fp. [DOI] [PubMed] [Google Scholar]
- 42.Kozinn J, Mao L, Arora A, Yang L, Fibuch EE, Wang JQ. Inhibition of glutamatergic activation of extracellular signal-regulated protein kinases in hippocampal neurons by the intravenous anesthetic propofol. Anesthesiology. 2006;105(6):1182–1191. doi: 10.1097/00000542-200612000-00018. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H, Zhang SB, Zhang QQ, et al. Rescue of cAMP response element-binding protein signaling reversed spatial memory retention impairments induced by subanesthetic dose of propofol. CNS Neurosci Ther. 2013;19(7):484–493. doi: 10.1111/cns.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holownia A, Mroz RM, Wielgat P, et al. Propofol protects rat astroglial cells against tert-butyl hydroperoxide-induced cytotoxicity; the effect on histone and cAMP-response-element-binding protein (CREB) signalling. J Physiol Pharmacol. 2009;60(4):63–69. [PubMed] [Google Scholar]
- 45.Tao T, Zhao Z, Hao L, Gu M, Chen L, Tang J. Propofol promotes proliferation of cultured adult rat hippocampal neural stem cells. J Neurosurg Anesthesiol. 2013;25(3):299–305. doi: 10.1097/ANA.0b013e31828baa93. [DOI] [PubMed] [Google Scholar]