Abstract
In the last decade clear evidence has accumulated that elephants are capable of vocal production learning. Examples of vocal imitation are documented in African (Loxodonta africana) and Asian (Elephas maximus) elephants, but little is known about the function of vocal learning within the natural communication systems of either species. We are also just starting to identify the neural basis of elephant vocalizations. The African elephant diencephalon and brainstem possess specializations related to aspects of neural information processing in the motor system (affecting the timing and learning of trunk movements) and the auditory and vocalization system. Comparative interdisciplinary (from behavioral to neuroanatomical) studies are strongly warranted to increase our understanding of both vocal learning and vocal behavior in elephants.
Current Opinion in Neurobiology 2014, 28:101–107
This review comes from a themed issue on Communication and language
Edited by Michael Brainard and Tecumseh Fitch
For a complete overview see the Issue and the Editorial
Available online 23rd July 2014
http://dx.doi.org/10.1016/j.conb.2014.07.001
0959-4388/© 2014 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/)
Introduction
The first evidence of vocal imitation in African elephants (Loxodonta africana) was published in 2005 [1•]. No follow-up research on elephant vocal learning was conducted until a report about a speech-imitating Asian elephant (Elephas maximus) in 2012 [2•], expanding our knowledge of vocal learning ability to both living genera of Elephantidae. Both papers document vocal imitation produced by captive elephants, some kept in socially abnormal conditions. To date, no information is available on vocal learning or the relevance of vocal learning within the natural communication system of either species. Elephants, like humans, are terrestrial, long-lived and social mammals. Accordingly, the adaptive function of their vocal learning can help reveal the original selective advantage of vocal learning in our human ancestors (because the adaptive function in modern human language, e.g. creating an extensive vocabulary, might not necessarily conform to the original function [3]).
Until recently, our knowledge of the elephant brain was limited [4]. Central to the current review are recent observations related to infrasound production and reception, as well as the control of the musculature involved. In the context of vocal learning, the most pressing question is whether elephants possess direct connections between telencephalic neurons and the primary vocal motor neurons in the brainstem controlling the vocal apparatus (following the Kuypers/Jürgens hypothesis) [5–9]. The required tract tracer studies to address this issue have not been conducted in elephants.
This review provides a synthesis of previous studies on elephant vocal learning and associated work from 1982 to 2014, along with new results and future perspectives.
Vocal flexibility and sound invention
African and Asian elephants use vocalizations with fundamental frequencies in the infrasonic range (‘rumbles’) for short-distance and long-distance communication [10,11]. The most remarkable species-specific difference in the vocal repertoire is the high-pitched, repetitive vocalizations (chirps/squeaks and squeals) of Asian elephants, which are typically absent in the African species [12]. The vocal repertoire of African and Asian elephants, with about 8–10 distinct call types [13–17] is not particularly large, but exhibits an interesting vocal plasticity (grading between call types, call type combinations [15,17], and sophisticated, context-dependent within-call type flexibility affecting all parameters including formant frequencies [18–22]). Sound visualization experiments revealed that elephants can control the vocal path from oral to nasal rumble production [12,23]. The nasal vocal tract is strongly elongated (un-extended trunk length of adult females: 1.7–1.8 m [24]). By using the nasal path during rumbling, an elephant lowers its formants by about threefold [23].
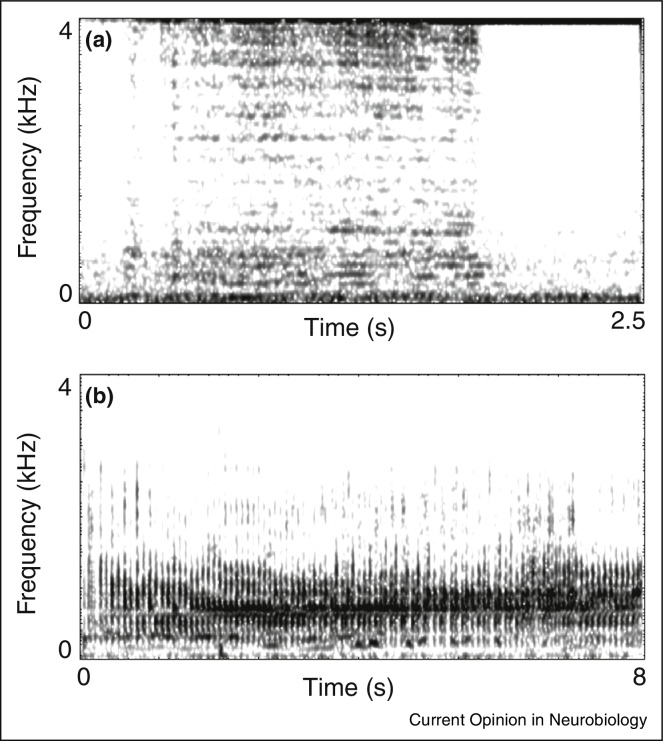
In addition, previously undocumented vocalizations sometimes emerge — mostly documented in captive elephants. These sounds appear to be vocally inventive because they are structurally unique and not socially relevant: they include trunk squelching sounds (Figure 1a, Audio Supplemental 1), croaking, creaking, and humming sounds (Figure 1b, Audio Supplemental 2) [14,17], or whistling sounds produced by, for example, pressing the trunk against the lower lip [25•,26].
Figure 1.
Spectrograms exemplifying idiosyncratic elephant vocalizations. A trunk-squelching sound produced by a five-year-old male African elephant at Vienna Zoo (a). During sound production, the elephant twined the trunk while pressing air through the squeezed trunk tips. A pulsated creak-like sound (b) produced by a captive, 18-year-old female elephant in South Africa.
Supplementary Audios 1 and 2 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.conb.2014.07.001.
‘Trunk squelching’. This audio file presents a trunk squelching sound produced by a juvenile male African elephant.
‘Creaking’. This audio recording presents a creaking sound produced by an adult female African elephant.
We know very little about how elephants generate their distinct vocalizations [12,27], but recent experiments on low-frequency rumbles suggest flow-induced vocal fold vibration (similar to human speech) [28,29].
Likewise, elephant sound perception has received little research attention, apart from early behavioral experiments revealing good low-frequency hearing [30]. Behavioral experiments further suggest that elephants may sense ground vibrations, including the seismic components of their rumbling vocalizations [31,32].
Examples of vocal imitation
Poole et al. [1•] documented two cases of vocal imitation in African savannah elephants: a 10-year-old female imitated the sounds of trucks, and a 23-year-old male named Calimero, who had spent a long time as the only African elephant in a zoo among Asian elephants, imitated the high-pitched chirping sounds typically produced by Asian elephants, but not by African elephants.
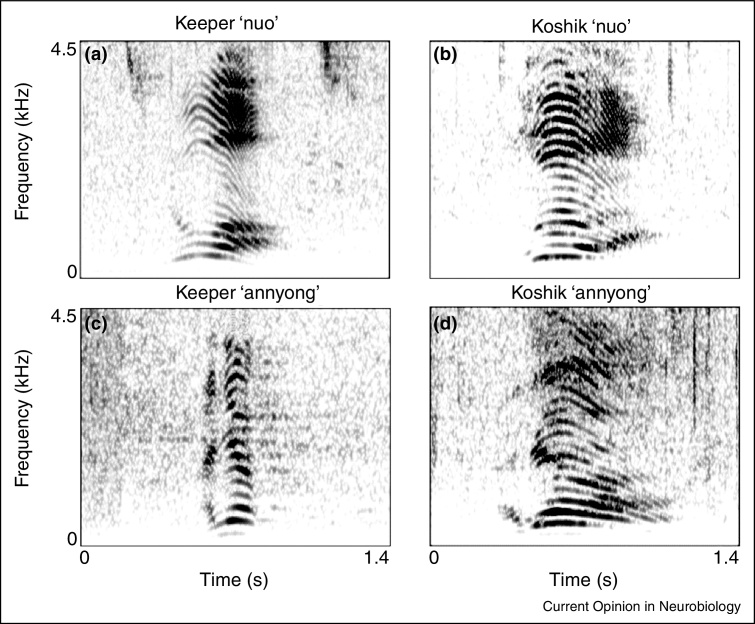
Stoeger et al. [2•] documented a male Asian elephant (Koshik) who imitates five human words in Korean with such precision that native Korean speakers could readily understand and transcribe his imitations. Koshik accurately imitates speech formant frequencies by placing his trunk inside his mouth, modulating the shape of the vocal tract during controlled phonation (Figures 2 and 3, Audio Supplemental 3 and 4).
Figure 2.
Spectral comparison of Koshik's speech imitations in Korean compared to those of a keeper. Spectrograms (a,b) show the utterance ‘nuo’ (‘lie down’), and (c,d) show ‘annyong’ (‘hello’) by a keeper and Koshik, respectively. In (d), Koshik protracts the ‘-nyong’ part of ‘annyong’ compared to this human example.
Figure 3.
Koshik's posture during speech imitation. Koshik accurately imitates human formant frequencies by placing his trunk tip into his mouth (always from the right side) at the onset of phonation. During phonation, he raises the lower jaw while keeping the trunk inside the mouth, thus modulating the shape of his vocal tract.
Supplementary Audios 3 and 4 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.conb.2014.07.001.
Sound imitation ‘Nuo’. This audio file presents the utterance ‘nuo’ (lie down) uttered first by the keeper and then by Koshik, the elephant.
Sound imitation ‘Annyong’. This audio file presents the utterance ‘annyong’ (hello) articulated by the keeper and subsequently by Koshik, the elephant.
Koshik was captive-born and, as a juvenile, the only elephant for five years at the Everland Zoo in South Korea. This suggests that his speech imitation may have been driven by social deprivation from conspecifics during an important period of bonding and development, when humans were his only social contact.
Vocal learning and rhythmical entrainment
The vocal learning and rhythmical entrainment hypothesis raised by Patel [33•] suggests that a direct connection between the auditory centers and the motor planning regions (typical for vocal learners) is a prerequisite for the ability to synchronize with an auditory beat. This implies that only vocal learning species should be capable of rhythmical entrainment [33•,34,35]. However, recent research revealed that even some non-vocal learners show evidence for beat synchronization or natural percussive behavior [36•,37–40].
Elephants might also be capable of rhythmical entrainment [34]. An Asian elephant has been observed drumming a stable beat [41], and elephants in Asian tourist shows move rhythmically to music. Nonetheless, it remains unclear how much training was involved, whether the elephants spontaneously match their movements to the beat, or whether the accompanying music was chosen advantageously.
Untangling the evolutionarily relationship between vocal learning and rhythmical entrainment requires testing multiple species including non-vocal learners, alongside investigating whether all vocal learners indeed possess the ability for rhythmical entrainment. This should be systematically tested in both elephant species.
Elephant neuroanatomy
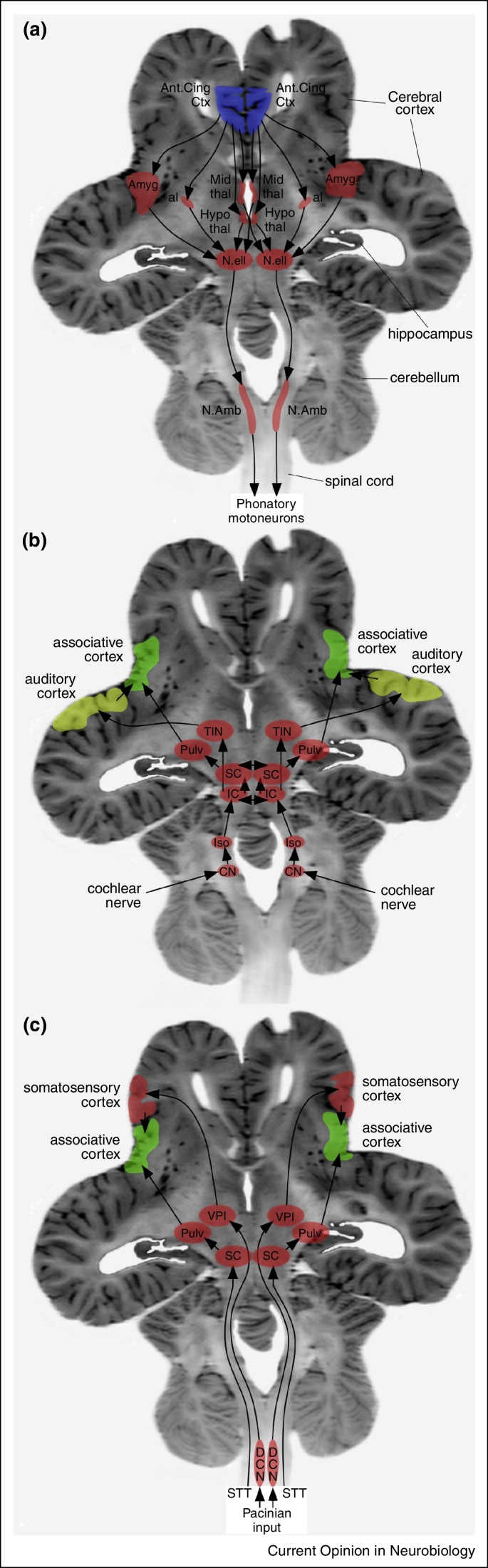
In recent years, an increasing number of studies have begun to unravel the structure of the elephant brain, the largest of any terrestrial mammal [42–55]. Regarding vocalizations, there are two important aspects: vocal production, including vocal pattern generation and muscular control, and vocal reception, including detection and extraction of information that appear to have distinct neural correlates (Figure 4). For infrasound production, the large nucleus ellipticus of the midbrain, found only in elephants, elephant seals and cetaceans, may act as a specialized vocal pattern generator [51•]. In terms of muscular control, several factors suggest that the timing and learning of movements related to trunk control are very important for elephant vocalizations. These include a relatively and absolutely large cerebellum [48] containing larger and more complex neurons than other mammals [49,50], along with a specialized substantia nigra pars compacta, facial nerve motor nucleus and inferior olivary complex [51•], with a human-sized hippocampal formation (in both absolute and relative terms) [52,53], all suggesting that the timing and learning of movements related to control of the trunk are very important for elephant vocalizations.
Figure 4.
Neural pathways involved with vocalizations in the elephant. Images depicting the possible pathways involved in producing vocalizations (a), the reception of the aerial component of infrasonic vocalizations (b) and the reception of the seismic component of infrasonic vocalizations (c), superimposed on a horizontal magnetic resonance image of the elephant brain. In each of these three aspects of vocalization processing, specific specializations within the brain have been observed (see text). al — ansa lenticularis; Amyg — amygdaloid body; Ant. Cing Ctx — anterior cingulate cortex; CN — cochlear nuclear complex; DCN — dorsal column nuclei; Hypothal — hypothalamus; IC — inferior colliculus; lso — lateral superior olivary nucleus; Mid thal — midline nuclei of dorsal thalamus; N. amb — nucleus ambiguus; N.ell — nucleus ellipticus; Pulv — pulvinar nucleus of dorsal thalamus; SC — superior colliculus; STT — spinothalamic tract; TIN — transverse infrageniculate nucleus; VPI — ventral posterior inferior nucleus of dorsal thalamus.
In terms of infrasound reception, specializations of the medial geniculate body of the dorsal thalamus and the lateral superior olivary nucleus within the pons appear to be related to the aerial aspect of infrasound [51•]. The specialized transverse infrageniculate nucleus within the medial geniculate body of the dorsal thalamus, which appears to have no homologue in other mammals, is likely to interact with the cerebral cortex to extract semantic information from the infrasonic vocalizations. The enlarged lateral superior olivary nucleus appears to be involved in sound source localization using interaural intensity differences. Specializations of the ventrolateral nuclei of the dorsal thalamus and the dorsal column nuclei of the medulla appear to be related to the seismic component of infrasound [51•]. The seismic component of the infrasonic vocalizations appears to be detected through Pacinian corpuscles in the feet and trunk [55], which then pass through enlarged dorsal column nuclei in the medulla and onto the ventral posterior inferior nucleus of the dorsal thalamus, a nucleus specialized for the processing of vibratory tactile stimuli that is not present in all mammals.
Elephants: terrestrial, long-lived and social vocal learners
Like humans, elephants are long-lived, terrestrial mammals with a complex social system, where individuals possess different levels of association [56,57]. Female African elephants use acoustic signals to maintain individual-specific bonds and have an extensive network of vocal recognition, that is distinguishing the calls of family and bond group members from those of outsiders [58,59]. Forming and recalling social memories of their life history is a key cognitive challenge helping ensure survival [60–62]. Vocal learning in elephants might be used to facilitate vocal recognition within their fission-fusion society by increasing the similarity between related or socially affiliated individuals (males also use vocalizations to negotiate within the social network). Call convergence in socially bonded animals also occurs in other vocal learning species (e.g. [63–66]), and regional dialects have been detected in the songs of male rock hyraxes (Procavia capensis) [67] (suggesting vocal learning in another Afrotherian mammal).
Moreover, the mechanisms that lead to the development of novel sounds in captive elephants should be verified, since vocal invention might be a form of vocal learning used to develop signals that differ from others (e.g. to increase vocal individuality) rather than converging to a model [68–71]. Identifying the function or functions of vocal learning within the natural communication system of elephants clearly provides an exciting avenue for future research.
Conclusions
This review summarized the accumulating evidence for vocal learning in elephants.
A fascinating aspect of elephant vocalizations — most striking in Koshik, the speech-imitating elephant — is that elephants use their trunk, and evolutionary highly specialized appendage, to modulate sound production in a very sophisticated manner. Notwithstanding, this review reveals that research on elephant vocal learning is still in a fledgling stage regarding context, function and neural information processing. By combining behavioral, anatomical and neuroanatomical studies, a clearer picture of elephant vocal capacities and how they relate to the life history of these iconic species should emerge.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
Acknowledgments
We acknowledge Dr. Michael Stachowitsch, Mag. Anton Baotic, Dr. Benjamin Charlton for their constructive comments on the manuscript.
We would like to thank Dr. Hilary Madzikanda of the Zimbabwe Parks and Wildlife Management Authority, and Dr. Bruce Fivaz and the team at the Malilangwe Trust, Zimbabwe. Angela Stoeger was supported by the FWF Austrian Science Fund [P23099] and [P26448], and the L’Oreal Scholarship from the Austrian Academy of Sciences. The research of Paul Manger was supported by a grant from the South African National Research Foundation to PRM [FA2005033100004].
References
- 1•.Poole J.H., Tyack P.L., Stoeger-Horwath A.S., Watwood S. Elephants are capable of vocal learning. Nature. 2005;434:455–456. doi: 10.1038/434455a. [DOI] [PubMed] [Google Scholar]; The paper describes examples of vocal imitation by African savannah elephants: a ten-year old female imitating the sounds of trucks, and a male African elephant imitating the chirping sounds of his Asian companions in captivity. The possible functional relevance of vocal learning for long-lived social species is discussed.
- 2•.Stoeger A.S., Mietchen D., Sukhun O., de Silva S., Herbst C.T., Kwon S., Fitch W.T. An Asian elephant imitates human speech. Curr Biol. 2012;22:1–5. doi: 10.1016/j.cub.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]; The paper documents speech imitation by an Asian elephant in Korean, describing in detail the matching of acoustic features and how the elephant uses the trunk in order to modulate the shape of the vocal tract during phonation. The social circumstances under which the elephant developed the speech imitations, and the potential adaptive function of vocal learning within the natural communication system of elephants are further discussed.
- 3.Fitch W.T. The evolution of speech: a comparative review. Trends Cogn Sci. 2000;4:258–267. doi: 10.1016/s1364-6613(00)01494-7. [DOI] [PubMed] [Google Scholar]
- 4.Cozzi B., Spagnoli S., Bruno L. An overview of the central nervous system of the elephant through a critical appraisal of the literature published in the XIX and XX centuries. Brain Res Bull. 2001;54:219–227. doi: 10.1016/s0361-9230(00)00456-1. [DOI] [PubMed] [Google Scholar]
- 5.Kuypers H.G.J.M. Corticobular connections to the pons and lower brainstem in man: an anatomical study. Brain. 1958;81:364–388. doi: 10.1093/brain/81.3.364. [DOI] [PubMed] [Google Scholar]
- 6.Kuypers H.G.J.M. Some projections from the peri-central cortex to the pons and lower brain stem in monkey and chimpanzee. J Comp Neurol. 1958;110:221–255. doi: 10.1002/cne.901100205. [DOI] [PubMed] [Google Scholar]
- 7.Kuypers H.G.J.M. The anatomical organization of the descending pathways and their contributions to motor control especially in primates. In: Desmedt J.E., editor. New Developments in EMG and Clinical Neurophysiology. Karger; 1973. pp. 38–68. [Google Scholar]
- 8.Jürgens U., Kirzinger A., Cramon D.Y. The effects of deep-reaching lesions in the cortical face area on phonation. A combined case report and experimental monkey study. Cortex. 1982;18:125–139. doi: 10.1016/s0010-9452(82)80024-5. [DOI] [PubMed] [Google Scholar]
- 9.Fitch W.T., Huber L., Bugnyar T. Social cognition and the evolution of language: constructing cognitive phylogenies. Neuron. 2010;65:795–814. doi: 10.1016/j.neuron.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Payne K.B., Langbauer W.R., Thomas E.M. Infrasonic calls of the Asian elephant (Elephas maximus) Behav Ecol Sociobiol. 1986;18:297–301. [Google Scholar]
- 11.Poole J.H., Payne K., Langbauer W.R.J., Moss C. The social contexts of some very low frequency calls of African elephants. Behav Ecol Sociobiol. 1988;22:385–392. [Google Scholar]
- 12.Stoeger A.S., de Silva S. African and Asian elephant vocal communication: a cross species comparison. In: Witzany G., editor. Biocommunication of Animals. Springer; Netherlands: 2014. pp. 21–39. [Google Scholar]
- 13.Stoeger-Horwath A.S., Stoeger S., Schwammer H.M., Kratochvil H. Call repertoire of infant African elephants — first insights into the early vocal ontogeny. J Acoust Soc Am. 2007;121:3922–3931. doi: 10.1121/1.2722216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leong K.M., Ortolani A., Burks K.D., Mellen J.D., Savage A. Quantifying acoustic and temporal characteristics of vocalizations for a group of captive African elephants Loxodonta africana. Bioacoustics. 2003;13:213–231. [Google Scholar]
- 15.de Silva S. Acoustic communication in the Asian elephant, Elephas maximus. Behaviour. 2010;147:825–852. [Google Scholar]
- 16.Nair B.R., Balakrishnan R., Seelamantula C.S., Sukumar R. Vocalizations of wild Asian elephants (Elephas maximus): structural classification and social context. J Acoust Soc Am. 2009;126:2768–2778. doi: 10.1121/1.3224717. [DOI] [PubMed] [Google Scholar]
- 17.Poole J.H. Behavioral contexts of elephant acoustic communication. In: Moss C.J., Croze H., Lee P.C., editors. The Amboseli Elephants: A Long-Term Perspective on a Long-Lived Mammal. University of Chicago Press; 2011. pp. 125–161. [Google Scholar]
- 18.King L.E., Soltis J., Douglas-Hamilton I., Savage A., Vollrath F. Bee threat elicits alarm call in African elephants. PLoS ONE. 2010 doi: 10.1371/journal.pone.0010346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soltis J., Blowers T.E., Savage A. Measuring positive and negative affect in the voiced sounds of African elephants (Loxodonta africana) J Acoust Soc Am. 2011;129:1059–1066. doi: 10.1121/1.3531798. [DOI] [PubMed] [Google Scholar]
- 20.Stoeger A.S., Charlton B.D., Kratochvil H., Fitch W.T. Vocal cues indicate level of arousal in infant African elephant roars. J Acoust Soc Am. 2011;130:1700–1711. doi: 10.1121/1.3605538. [DOI] [PubMed] [Google Scholar]
- 21.Soltis J. Emotional communication in African elephants. In: Altenmüller E., Schmidt S., Zimmermann E., editors. The Evolution of Emotional Communication: From Sounds in Nonhuman Mammals to Speech and Music in Man. Oxford University Press; 2013. pp. 105–115. [Google Scholar]
- 22.Soltis J., King L.E., Douglas-Hamilton I., Vollrath F., Savage A. African elephant alarm calls distinguish between threats from humans and bees. PLoS ONE. 2014 doi: 10.1371/journal.pone.0089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoeger A.S., Heilmann G., Zeppelzauer M., Ganswindt A., Hensman S., Charlton B.D. Visualizing sound emission of elephant vocalizations: evidence fort two rumble production types. PLoS ONE. 2012 doi: 10.1371/journal.pone.0048907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sikes S.K., editor. The Natural History of the African Elephant. Weidenfeld and Nicolson Ltd.; 1971. [Google Scholar]
- 25•.Wemmer C.M., Mishra H.R. Observational learning by an Asiatic elephant of an unusual sound production method. Mammalia. 1982;46:556–655. [Google Scholar]; The paper, for the first time, describes an idiosyncratic method of sound production and its transmission to an unrelated elephant calf by observational learning.
- 26.Wemmer C.M., Mishra H.R., Dinerstein E. Unusual use of the trunk for sound production in a captive Asian elephant: a second case. J Bombay Nat Hist Soc. 1985;82:187. [Google Scholar]
- 27.Herler A., Stoeger A.S. Vocalizations and associated behaviour of Asian elephant (Elephas maximus) calves. Behaviour. 2012;149:575–599. [Google Scholar]
- 28.Herbst C.T., Stoeger A.S., Frey R., Lohscheller J., Titze I.R., Gumpenberger M., Fitch W.T. How low can you go? Physical production mechanism of elephant infrasound vocalizations. Science. 2012;337:595–599. doi: 10.1126/science.1219712. [DOI] [PubMed] [Google Scholar]
- 29.Herbst C.T., Svenc J., Lohscheller J., Frey R., Gumpenberger M., Stoeger A.S., Fitch W.T. Complex vibratory patterns in an elephant larynx. J Exp Biol. 2013;216:4054–4064. doi: 10.1242/jeb.091009. [DOI] [PubMed] [Google Scholar]
- 30.Heffner R.S., Heffner H.E. Hearing in the elephant (Elephas maximus) Science. 1980;208:518–529. doi: 10.1126/science.7367876. [DOI] [PubMed] [Google Scholar]
- 31.O’Connell C., Hart L.A., Arnason B.T. Comments on “Elephant hearing”. J Acoust Soc Am. 1998;105:2051–2052. doi: 10.1121/1.426748. [DOI] [PubMed] [Google Scholar]
- 32.O’Connell-Rodwell C., Arnason B.T., Hart L.A. Seismic properties of Asian elephant (Elephas maximus) vocalizations and locomotion. J Acoust Soc Am. 2000;108:3066–3072. doi: 10.1121/1.1323460. [DOI] [PubMed] [Google Scholar]
- 33•.Patel A.D. Musical rhythm, linguistic rhythm, and human evolution. Music Percept. 2006;24:99–104. [Google Scholar]; In this commentary Patel suggests breaking music cognition into its underlying components and determining whether any of these are innate, specific to music, and unique to humans. He proposes the ‘vocal learning and rhythmic synchronization hypothesis’ (implying that a neural circuitry for complex vocal learning is a prerequisite for the ability to synchronize with an auditory beat).
- 34.Schachner A., Brady T.F., Pepperberg I.M., Hauser M.D. Spontaneous motor entrainment to music in multiple vocal mimicking species. Curr Biol. 2009;19:831–836. doi: 10.1016/j.cub.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 35.Patel A.D., Iversen J.R., Bregman M.R., Schulz I. Experimental evidence for synchronization to a musical beat in a nonhuman animal. Curr Biol. 2009;19:827–830. doi: 10.1016/j.cub.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 36•.Cook P., Rouse A., Wilson M., Reichmuth C. A California sea lion (Zalophus californianus) can keep the beat: motor entrainment to rhythmic auditory stimuli in a non vocal mimic. J Comp Psychol. 2013;127:412–427. doi: 10.1037/a0032345. [DOI] [PubMed] [Google Scholar]; The paper challenges the ‘vocal learning and rhythmical entrainment’ hypothesis by presenting evidence for beat synchronization in a California sea lion (a non-vocal learning species).
- 37.Bregman M.R., Iversen J.R., Lichman D., Reinhart M., Patel A.D. A method for testing synchronization to a musical beat in domestic horses (Equus ferus caballus) Empir Musicol Rev. 2012;7:3–4. [Google Scholar]
- 38.Ravignani A., Gingras B., Asano R., Sonnweber R., Matellan V., Fitch W.T. The evolution of rhythmic cognition: new perspectives and technologies in comparative research. Proc Cog Sci. 2013:1199–1204. [Google Scholar]
- 39.Schachner A. If horses entrain, don’t entirely reject vocal learning: an experience-based vocal learning hypothesis. Empir Musicol Rev. 2012;7:3–4. [Google Scholar]
- 40.Hattori Y., Tomonaga M., Matsuzawa T. Spontaneous synchronized tapping to an auditory rhythm in a chimpanzee. Sci Rep. 2013;3:1566. doi: 10.1038/srep01566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel A., Iversen J. A non-human animal can drum a steady beat on a musical instrument. In: Baroni M., Addessi A.R., Caterina R., Costa M., editors. Proceedings of the 9th International Conference on Music, Perception & Cognition (ICMPC9); Italy; 2006. p. 447. [Google Scholar]
- 42.Hakeem A.Y., Hof P.R., Sherwood C.C., Switzer R.C., Rasmussen L.R.L., Allman J.M. Brain of the African elephant (Loxodonta africana): neuroanatomy form magnetic resonance images. Anat Rec. 2005;287A::1117–1127. doi: 10.1002/ar.a.20255. [DOI] [PubMed] [Google Scholar]
- 43.Hakeem A.Y., Sherwood C.C., Bonar C.J., Butti C., Hof P.R., Allman J.M. Von Economo neurons in the elephant brain. Anat Rec. 2009;292:242–248. doi: 10.1002/ar.20829. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs B., Lubs J., Hannan M., Anderson Km Butti C., Sherwood C.C., Hof P.R., Manger P.R. Neuronal morphology in the African elephant (Loxodonta africana) neocortex. Brain Struct Funct. 2011;215:273–298. doi: 10.1007/s00429-010-0288-3. [DOI] [PubMed] [Google Scholar]
- 45.Manger P.R., Pillay P., Maseko B.C., Bhagwandin A., Gravett N., Moon D., Jillani N.E., Hemingway J. Acquisition of the brain of the Africa elephant (Loxodonta africana): perfusion-fixation and dissection. J Neurosci Methods. 2009;179:16–21. doi: 10.1016/j.jneumeth.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Manger P.R., Prowse M., Haagensen M., Hemingway J. Quantitative analysis of neocortical gyrencephaly in African elephants (Loxodonta africana) and six species of cetaceans: comparison with other mammals. J Comp Neurol. 2012;520:2430–2439. doi: 10.1002/cne.23046. [DOI] [PubMed] [Google Scholar]
- 47.Maseko B.C., Spocter M.A., Haagensen M., Manger P.R. Volumetric analysis of the African elephant ventricular system. Anat Rec. 2011;294:1412–1417. doi: 10.1002/ar.21431. [DOI] [PubMed] [Google Scholar]
- 48.Maseko B.C., Spocter M.A., Haagensen M., Manger P.R. Elephants have relatively the largest cerebellum size of mammals. Anat Rec. 2012;295:661–672. doi: 10.1002/ar.22425. [DOI] [PubMed] [Google Scholar]
- 49.Maseko B.C., Jacobs B., Spocter M.A., Sherwood C.C., Hof P.R., Manger P.R. Qualitative and quantitative aspects of the microanatomy of the African elephant cerebellar cortex. Brain Behav Evol. 2013;81:40–55. doi: 10.1159/000345565. [DOI] [PubMed] [Google Scholar]
- 50.Herculano-Houzel S., Avelino-de-Souza K., Neves K., Porfírio J., Messeder D., Mattos Feijó L., Maldonado J., Manger P.R. The elephant brain in numbers. Front Neuroanat. 2014 doi: 10.3389/fnana.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51•.Maseko B.C., Patzke N., Fuxe K., Manger P.R. Architectural organization of the African elephant diencephalon and brainstem. Brain Behav Evol. 2013;82:83–128. doi: 10.1159/000352004. [DOI] [PubMed] [Google Scholar]; The paper describes the elephant diencephalon and brainstem, emphasising subtle but important differences and variations related to firstly, the motor system, secondly, the auditory and vocalization system, thirdly the orex-inergic satiety/wakefulness centre of the hypothalamus and the locus coeruleus, and finally the potential neurogenic lining of the brainstem. The authors further outline how these adaptations may relate to the life history and observed behaviors of the African elephant.
- 52.Patzke N., Olaleye O., Haagensen M., Hof P.R., Ihunwo A.O., Manger P.R. Organization and chemical neuroanatomy of the African elephant (Loxodonta africana) hippocampus. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0587-6. [DOI] [PubMed] [Google Scholar]
- 53.Patzke N., Spocter M.A., Karlsson K.Æ., Bertelsen M.F., Haagensen M., Chawana R., Streicher S., Kaswera C., Gilissen E., Alagaili A.N., Mohammed O.B., Repp R.L., Bennett N.C., Siegel J.M., Ihunwo A.O., Manger P.R. In contrast to many other mammals, cetaceans have relatively small hippocampi that appear to lack adult neurogenesis. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shoshani J., Kupsky W.J., Marchant G.H. Elephant brain. 1. Gross morphology, functions, comparative anatomy, and evolution. Brain Res Bull. 2006;70:124–157. doi: 10.1016/j.brainresbull.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 55.Bouley D.M., Alarcon C.N., Hildebrandt T., O’Connell-Rodwell C.E. The distribution, density and three-dimensional histomorphology of Pacinian corpuscles in the foot of the Asian elephant (Elephas maximus) and their potential role in seismic communication. J Anat. 2007;211:426–435. doi: 10.1111/j.1469-7580.2007.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Silva S., Wittemyer G. A comparison of social organization in Asian elephants and African savannah elephants. Int J Primatol. 2012;33:1125–1141. [Google Scholar]
- 57.de Silva S., Ranjeewa A.D.G., Kryazhimskiy S. The dynamics of social networks among female Asian elephants. BMC Ecol. 2011 doi: 10.1186/1472-6785-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McComb K., Moss C., Sayialel S., Baker L. Unusually extensive networks of vocal recognition in African elephants. Anim Behav. 2000;59:1103–1109. doi: 10.1006/anbe.2000.1406. [DOI] [PubMed] [Google Scholar]
- 59.O’Connell-Rodwell C.E., Wood J.D., Kinzley C., Rodwell T.C., Poole J.H., Puria S. Wild African elephants (Loxodonta africana) discriminate between familiar and unfamiliar conspecific seismic alarm calls. J Acoust Soc Am. 2007;122:823–830. doi: 10.1121/1.2747161. [DOI] [PubMed] [Google Scholar]
- 60.Hart B.L., Hart L.A., Pinter-Wollman N. Large brains and cognition: where do elephants fit in? Neurosci Biobehav Rev. 2008;32:86–89. doi: 10.1016/j.neubiorev.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 61.McComb K., Shannon G., Durant S.M., Sayialel K., Slotow R., Poole J., Moss C. Leadership in elephants: the adaptive value of age. Proc Roy Soc B. 2011;278:3270–3276. doi: 10.1098/rspb.2011.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McComb K., Moss C., Durant S., Baker L., Sayialel S. Matriarchs as repositories of social knowledge in African elephants. Science. 2001;292:491–494. doi: 10.1126/science.1057895. [DOI] [PubMed] [Google Scholar]
- 63.Tyack P.L. Convergence of calls as animals form social bonds, active compensation for noisy communication channels, and the evolution of vocal learning in mammals. J Comp Psychol. 2008;122:319–331. doi: 10.1037/a0013087. [DOI] [PubMed] [Google Scholar]
- 64.Fripp D., Owen C., Quintana-Rizzo E., Shapiro A., Buckstaff K., Jankowski K., Wells R., Tyack P. Bottlenose dolphin (Tursiops truncatus) calves appear to model their signature whistles on the signature whistles of community members. Anim Cogn. 2005;8:17–26. doi: 10.1007/s10071-004-0225-z. [DOI] [PubMed] [Google Scholar]
- 65.Nousek A.E., Slater P.J., Wang C., Miller P.J.O. The influence of social affiliation on individual vocal signatures of northern resident killer whales (Orcinus orca) Biol Lett. 2006;4:481–484. doi: 10.1098/rsbl.2006.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hile A.G., Striedter G.F. Call convergence within groups of female budgerigars (Melopsitta cus undulatus) Ethology. 2000;106:1105–1114. [Google Scholar]
- 67.Kershenbaum A., Ilany A., Blaustein L., Geffen E. Syntactic structure and geographical dialects in the songs of male rock hyraxes. Proc R Soc B. 2012;279:2974–2981. doi: 10.1098/rspb.2012.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Janik V.M., Slater P.J.B. The different roles of social learning in vocal communication. Anim Behav. 2000;60:1–11. doi: 10.1006/anbe.2000.1410. [DOI] [PubMed] [Google Scholar]
- 69.Janik V.M. Cognitive skills in bottlenose dolphin communication. Trends Cogn Sci. 2013;17:157–159. doi: 10.1016/j.tics.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 70.Quick N.J., Janik V.M. Bottlenose dolphins exchange signature whistles when meeting at sea. Proc R Soc B. 2012;279:2539–2545. doi: 10.1098/rspb.2011.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schusterman R.J., Reichmuth C. Novel sound production through contingency learning in the Pacific walrus (Odobenus rosmarus divergens) Anim Cogn. 2008;11:319–327. doi: 10.1007/s10071-007-0120-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
‘Trunk squelching’. This audio file presents a trunk squelching sound produced by a juvenile male African elephant.
‘Creaking’. This audio recording presents a creaking sound produced by an adult female African elephant.
Sound imitation ‘Nuo’. This audio file presents the utterance ‘nuo’ (lie down) uttered first by the keeper and then by Koshik, the elephant.
Sound imitation ‘Annyong’. This audio file presents the utterance ‘annyong’ (hello) articulated by the keeper and subsequently by Koshik, the elephant.