Abstract
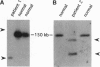
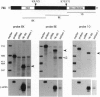
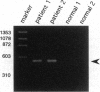
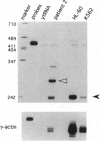
Chromosomal rearrangements involving band 12p13 are found in a wide variety of human leukemias but are particularly common in childhood acute lymphoblastic leukemia. The genes involved in these rearrangements, however, have not been identified. We now report the cloning of a t(12;21) translocation breakpoint involving 12p13 and 21q22 in two cases of childhood pre-B acute lymphoblastic leukemia, in which t(12;21) rearrangements were not initially apparent. The consequence of the translocation is fusion of the helix-loop-helix domain of TEL, an ETS-like putative transcription factor, to the DNA-binding and transactivation domains of the transcription factor AML1. These data show that TEL, previously shown to be fused to the platelet-derived growth factor receptor beta in chronic myelomonocytic leukemia, can be implicated in the pathogenesis of leukemia through its fusion to either a receptor tyrosine kinase or a transcription factor. The TEL-AML1 fusion also indicates that translocations affecting the AML1 gene can be associated with lymphoid, as well as myeloid, malignancy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birren B. W., Lai E., Hood L., Simon M. I. Pulsed field gel electrophoresis techniques for separating 1- to 50-kilobase DNA fragments. Anal Biochem. 1989 Mar;177(2):282–286. doi: 10.1016/0003-2697(89)90052-3. [DOI] [PubMed] [Google Scholar]
- Erickson P., Gao J., Chang K. S., Look T., Whisenant E., Raimondi S., Lasher R., Trujillo J., Rowley J., Drabkin H. Identification of breakpoints in t(8;21) acute myelogenous leukemia and isolation of a fusion transcript, AML1/ETO, with similarity to Drosophila segmentation gene, runt. Blood. 1992 Oct 1;80(7):1825–1831. [PubMed] [Google Scholar]
- Frohman M. A. Rapid amplification of complementary DNA ends for generation of full-length complementary DNAs: thermal RACE. Methods Enzymol. 1993;218:340–356. doi: 10.1016/0076-6879(93)18026-9. [DOI] [PubMed] [Google Scholar]
- Golub T. R., Barker G. F., Lovett M., Gilliland D. G. Fusion of PDGF receptor beta to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell. 1994 Apr 22;77(2):307–316. doi: 10.1016/0092-8674(94)90322-0. [DOI] [PubMed] [Google Scholar]
- Johansson B., Mertens F., Mitelman F. Cytogenetic deletion maps of hematologic neoplasms: circumstantial evidence for tumor suppressor loci. Genes Chromosomes Cancer. 1993 Dec;8(4):205–218. doi: 10.1002/gcc.2870080402. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Montgomery K. T., Bohlander S. K., Adra C. N., Lim B. L., Kucherlapati R. S., Donis-Keller H., Holt M. S., Le Beau M. M., Rowley J. D. Fluorescence in situ hybridization mapping of translocations and deletions involving the short arm of human chromosome 12 in malignant hematologic diseases. Blood. 1994 Nov 15;84(10):3473–3482. [PubMed] [Google Scholar]
- Kobayashi H., Rowley J. D. Identification of cytogenetically undetected 12p13 translocations and associated deletions with fluorescence in situ hybridization. Genes Chromosomes Cancer. 1995 Jan;12(1):66–69. doi: 10.1002/gcc.2870120112. [DOI] [PubMed] [Google Scholar]
- Levanon D., Negreanu V., Bernstein Y., Bar-Am I., Avivi L., Groner Y. AML1, AML2, and AML3, the human members of the runt domain gene-family: cDNA structure, expression, and chromosomal localization. Genomics. 1994 Sep 15;23(2):425–432. doi: 10.1006/geno.1994.1519. [DOI] [PubMed] [Google Scholar]
- Liu P., Tarlé S. A., Hajra A., Claxton D. F., Marlton P., Freedman M., Siciliano M. J., Collins F. S. Fusion between transcription factor CBF beta/PEBP2 beta and a myosin heavy chain in acute myeloid leukemia. Science. 1993 Aug 20;261(5124):1041–1044. doi: 10.1126/science.8351518. [DOI] [PubMed] [Google Scholar]
- Mitani K., Ogawa S., Tanaka T., Miyoshi H., Kurokawa M., Mano H., Yazaki Y., Ohki M., Hirai H. Generation of the AML1-EVI-1 fusion gene in the t(3;21)(q26;q22) causes blastic crisis in chronic myelocytic leukemia. EMBO J. 1994 Feb 1;13(3):504–510. doi: 10.1002/j.1460-2075.1994.tb06288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H., Shimizu K., Kozu T., Maseki N., Kaneko Y., Ohki M. t(8;21) breakpoints on chromosome 21 in acute myeloid leukemia are clustered within a limited region of a single gene, AML1. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10431–10434. doi: 10.1073/pnas.88.23.10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisson P. E., Watkins P. C., Sacchi N. Transcriptionally active chimeric gene derived from the fusion of the AML1 gene and a novel gene on chromosome 8 in t(8;21) leukemic cells. Cancer Genet Cytogenet. 1992 Oct 15;63(2):81–88. doi: 10.1016/0165-4608(92)90384-k. [DOI] [PubMed] [Google Scholar]
- Nucifora G., Begy C. R., Erickson P., Drabkin H. A., Rowley J. D. The 3;21 translocation in myelodysplasia results in a fusion transcript between the AML1 gene and the gene for EAP, a highly conserved protein associated with the Epstein-Barr virus small RNA EBER 1. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7784–7788. doi: 10.1073/pnas.90.16.7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora G., Begy C. R., Kobayashi H., Roulston D., Claxton D., Pedersen-Bjergaard J., Parganas E., Ihle J. N., Rowley J. D. Consistent intergenic splicing and production of multiple transcripts between AML1 at 21q22 and unrelated genes at 3q26 in (3;21)(q26;q22) translocations. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):4004–4008. doi: 10.1073/pnas.91.9.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa E., Maruyama M., Kagoshima H., Inuzuka M., Lu J., Satake M., Shigesada K., Ito Y. PEBP2/PEA2 represents a family of transcription factors homologous to the products of the Drosophila runt gene and the human AML1 gene. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6859–6863. doi: 10.1073/pnas.90.14.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi S. C. Current status of cytogenetic research in childhood acute lymphoblastic leukemia. Blood. 1993 May 1;81(9):2237–2251. [PubMed] [Google Scholar]
- Romana S. P., Le Coniat M., Berger R. t(12;21): a new recurrent translocation in acute lymphoblastic leukemia. Genes Chromosomes Cancer. 1994 Mar;9(3):186–191. doi: 10.1002/gcc.2870090307. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Hahn S. L., Giovane A. The Ets family of transcription factors. Eur J Biochem. 1993 Jan 15;211(1-2):7–18. doi: 10.1007/978-3-642-78757-7_2. [DOI] [PubMed] [Google Scholar]