Abstract
Background
Human metapneumovirus (HMPV) is an important cause of acute respiratory infections (ARI), but little is known about how it compares with respiratory syncytial virus (RSV) in Central America.
Objectives
In this study, we describe hospitalized cases of HMPV- and RSV-ARI in Guatemala.
Methods
We conducted surveillance at three hospitals (November 2007–December 2012) and tested nasopharyngeal and oropharyngeal swab specimens for HMPV and RSV using real-time reverse transcription-polymerase chain reaction. We calculated incidence rates, and compared the epidemiology and outcomes of HMPV-positive versus RSV-positive and RSV-HMPV-negative cases.
Results
We enrolled and tested specimens from 6288 ARI cases; 596 (9%) were HMPV-positive and 1485 (24%) were RSV-positive. We observed a seasonal pattern of RSV but not HMPV. The proportion HMPV-positive was low (3%) and RSV-positive high (41%) for age <1 month, whereas these proportions were similar (∼20%) by age 2 years. The annual incidence of hospitalized HMPV-ARI was 102/100 000 children aged <5 years [95% confidence interval (CI): 75–178], 2·6/100 000 persons aged 5–17 years (95%CI: 1·2–5·0), and 2·6/100 000 persons aged ≥18 years (95%CI: 1·5–4·9). Among children aged <5 years, HMPV-positive cases were less severe than HMPV-RSV-negative cases after adjustment for confounders [odds ratio (OR) for intensive care = 0·63, 95% CI 0·47–0·84]; OR for death = 0·46, 95% CI 0·23–0·92).
Conclusions
Human metapneumovirus is a substantial contributor to ARI hospitalization in Guatemala, but HMPV hospitalizations are less frequent than RSV and, in young children, less severe than other etiologies. Preventive interventions should take into account the wide variation in incidence by age and unpredictable timing of incidence peaks.
Keywords: Acute respiratory infection, human metapneumovirus, pneumonia, respiratory syncytial virus, surveillance
Background
Human metapneumovirus (HMPV), a virus with genetic, epidemiological, and clinical similarities to respiratory syncytial virus (RSV), was first identified in 2001 among children with acute respiratory infections (ARI) in the Netherlands.1 The prevalence of HMPV infection among ARI cases has been described in North America,2 South America,3 Asia,4 the Middle East,5 Africa,6 and Oceania.7 The first report of HMPV infections in Central America was of a pediatric case series (n = 9) in Costa Rica during 2008.8 A study among both outpatients (n = 1573) and inpatients (n = 183) with influenza-like illness in El Salvador, Honduras and Nicaragua from August 2006 to April 2009 detected only three HMPV infections using immunofluorescence.9 Schlaudecker et al.10 used a multiplex polymerase chain reaction test and found 28 (8%) cases positive for HMPV out of 345 ARI cases in children presenting to outpatient clinics in Honduras from February 2010 to June 2011.
Evidence from previous studies has been inconsistent regarding whether ARI cases that are HMPV-positive tend to be more or less severe than HMPV-negative cases. Studies have shown greater likelihood of a clinical pneumonia diagnosis and need for mechanical ventilation associated with HMPV compared with HMPV-negative cases,11,12 but another study showed that children with HMPV were less likely to require admission to an intensive care unit (ICU) than those with RSV or influenza A.2 Other studies comparing HMPV and RSV infections did not find any evidence of a difference in the probability of requiring mechanical ventilation, having longer hospital stay,12,13 or being admitted to the hospital or ICU.14 However, as these studies did not adjust for age, it is unclear to what extent these comparisons are biased by differences in the age distributions of the children infected with each virus.15
In this manuscript, we present estimates from Guatemala of population-based incidence rates of hospitalized cases of ARI that have HMPV infection (HMPV-ARI), and we use comparisons with cases RSV-positive and those negative for both HMPV and RSV (HMPV-RSV-negative) to highlight important features of the epidemiology of HMPV infection, particularly, seasonality, age distribution and disease severity.
Methods
Data collection
We have conducted ARI surveillance in hospitals in the Departments of Santa Rosa (National Hospital of Cuilapa) since November 2007, Quetzaltenango (Western Regional Hospital) since February 2009, and Guatemala (Guatemalan Social Security Institute, IGSS) from November 2009 to April 2011. We previously described the surveillance sites in more detail.16,17 This analysis focuses on cases as the start of surveillance until December 31, 2012.
Surveillance nurses searched the emergency departments and hospital wards daily for patients with respiratory admission diagnoses or chief complaints and screened them for eligibility. Eligibility criteria were as follows: (i) hospitalization; (ii) at least one sign of acute infection [temperature ≥38°C, temperature <35·5°C, abnormal white blood cell count (age <5 years and count <5550 or >15 000 cells/μl; ≥5 years and count <3000 or >11 000 cells/μl), or abnormal white blood cell differential]; and (iii) at least one of the following respiratory signs or symptoms: cough, tachypnea (age <2 months and ≥60 breaths/min; age 2–11 months and ≥50 breaths/min; age 1–4 years and ≥40 breaths/min; age ≥5 years and ≥20 breaths/min), sputum production, pleuritic chest pain, hemoptysis, difficulty breathing, shortness of breath, sore throat, or among children aged <2 years, pausing repeatedly while breastfeeding or drinking, nasal flaring, or noisy breathing. Since February 2011, we have also included all children aged <5 years meeting the World Health Organization pneumonia case definition: cough or difficulty breathing with tachypnea, chest indrawing, stridor, or a danger sign (inability to feed, loss of consciousness, lethargy, and convulsions).
Surveillance nurses recorded information from medical charts, interviewed patients or caregivers, and took nasopharyngeal (NP) and oropharyngeal (OP) swabs. The nurses measured blood oxygen saturation by pulse oximetry, and we defined hypoxemia as saturation <90% in Santa Rosa (elevation approximately 900 meters), <89% in Guatemala City (elevation approximately 1500 meters), and <88% in Quetzaltenango (elevation approximately 2300 meters). Digital photographs of chest radiographs, if ordered by attending physician, were sent to a panel of three radiologists trained to interpret images according to World Health Organization guidelines.18
Nasopharyngeal and OP swabs were combined into one tube with viral transport media and sent to the Universidad del Valle de Guatemala, where they were tested by real-time reverse transcription-polymerase chain reaction (rRT-PCR) assays for HMPV, RSV, influenza A and B viruses, parainfluenza virus types 1, 2, and 3, and adenovirus,19 using primer/probe reagents and protocols provided by the US CDC. Briefly, rRT-PCR assays were performed on an ABI7500 using the AgPath-ID One-Step kit (Applied Biosystems, Foster City, CA, USA). Each reaction mixture contained 1× RT-PCR buffer, 1× RT-PCR enzyme, 1× primer and probe mixture and 5 μl of nucleic acid extract in a total reaction volume of 25 μl. Thermocycling conditions were as follows: 45°C for 10 min, 95°C for 10 min, and 45 cycles of 95°C for 15 sec, and 55°C for 1 min. For all of the viruses tested, specimens with cycle threshold values <40 were considered positive.
Data analysis
We classified the cases into three etiological groups for comparisons: HMPV-positive, RSV-positive, and HMPV-RSV-negative. HMPV-RSV co-infections were included in both HMPV-positive and RSV-positive groups for descriptive analyses. We compared demographic characteristics, clinical presentation, and severity of disease between these three groups of patients. We plotted the weekly counts of HMPV-positive and RSV-positive cases. To evaluate the shape of the associations between the probabilities of each viral infection and the age of the patients, allowing for nonlinear associations, we fit generalized additive models (GAMs) for the log odds of HMPV and RSV infections with penalized spline terms for age, using generalized cross-validation to determine the degrees of freedom and running separate models by virus and age group (<5 years and ≥5 years).20 To assess the fit of these models, we divided the children aged <5 years into age twentiles and persons aged ≥5 years into age deciles, calculated the observed proportions of ARI cases positive for each virus, and overlaid these points at the median age for the corresponding age twentile or decile on the penalized spine plots.
For incidence rate calculations, we included cases only from predefined catchment areas in the departments of Santa Rosa21 and Quetzaltenango (municipalities of Almolonga, Cantel, Concepción Chiquirichapa, La Esperanza, Olintepeque, San Juan Ostuncalco, Quetzaltenango, Salcajá, San Mateo, Zunil). Incidence was calculated for Santa Rosa for years 2008 to 2012 and Quetzaltenango for years 2009 to 2012. Population denominators were obtained from the 2002 census data and official projections [National Statistics Institute (INE), Guatemala]. We adjusted rates upwards to account for: (i) the proportion of eligible cases by age group and surveillance site for which specimens were not tested for HMPV; and (ii) the proportion of the target populations with hospitalized ARI who sought care at the surveillance hospitals, as assessed by healthcare utilization surveys (HUS). In the HUS, among those who reported seeking care at a hospital in the previous 12 months for cough and difficulty breathing for ≥2 days, or clinician-diagnosed pneumonia, 53% in Santa Rosa21 and 60% in Quetzaltenango22 sought care for their illness at surveillance hospitals. We estimated the uncertainty in the adjusted incidence rates by repeating these calculations 1999 times, each time: (i) sampling from the binomial distribution [∼Bin(n = number missing, P = probability of HMPV among those tested)] to impute the number of HMPV infections among the eligible cases not tested for HMPV; (ii) bootstrap sampling from the HUS dataset the proportion of ARI cases for which care was sought at surveillance hospitals; and (iii) sampling from the Poisson distribution based on adjusted counts. We used the bias corrected method to calculate 95% confidence intervals.23
We defined severe cases as those requiring intensive care, mechanical ventilation, or hospitalization >1 week, and those that resulted in death. We used logistic regression to test for association between etiological group (excluding HMPV-RSV co-infections) and the odds of these indicators of severity. Separate models were fit by age group (<5 and ≥5 years), and we present crude and adjusted associations, conditioning on age, sex, and surveillance area. As age ≥5 years is a broad age range across which to assume associations with disease severity are constant, we tested for interaction between etiological group and age (5–17, 18–49, and ≥50 years).
Human subjects
The surveillance protocol was approved by the ethics review committee from the Universidad del Valle de Guatemala (Guatemala City, Guatemala) and the US Centers for Disease Control and Prevention (Atlanta, GA, USA), and by the MSPAS (Guatemala City, Guatemala). Eligible patients who provided written, informed consent were enrolled in the study. For patients aged <18 years, parents or guardians served as proxies, and children aged 7–17 years were asked to provide written, informed assent.
Results
We identified 7289 eligible ARI cases, 6597 (91%) were enrolled, and 6288 (95%) of those enrolled had a specimen tested. Of these, rRT-PCR assays detected HMPV in 596 (9%) and RSV in 1485 (24%) cases; 43 (0·7%) HMPV-RSV co-infections were identified. Of the HMPV-positive cases, 87% occurred in children aged <5 years, similar to proportion of the RSV-positive cases (91%), whereas only about half of the HMPV-RSV-negative cases occurred in this age group (Table1).
Table 1.
Demographic characteristics, clinical presentation, and hospital course of patients by etiological classification and age group at three Guatemalan hospitals, November 2007–August 2012
| HMPV-positive (n = 596)* | RSV-positive (n = 1485)* | HMPV-RSV-negative cases (n = 4248) | |
|---|---|---|---|
| Age < 5 years | 520 (87) | 1356 (91) | 2295 (54) |
| Age, year (med [IQR]) | 1·0 [0·5, 1·7] | 0·5 [0·2, 1·0] | 0·8 [0·3, 1·7] |
| Sex (female) | 222 (43) | 591 (44) | 960 (42) |
| Cough | 513 (99) | 1330 (98) | 2081 (92) |
| Temperature (med [IQR]) | 38·0 [37·4, 38·5] | 38·0 [37·0, 38·5] | 37·8 [37·0, 38·5] |
| Difficulty breathing | 440 (85) | 1162 (86) | 1814 (80) |
| Tachypnea | 238 (46) | 556 (41) | 880 (38) |
| Chest indrawing | 176/472 (37) | 690/1294 (53) | 843/1978 (43) |
| Stridor | 24/472 (5) | 71/1295 (5) | 144/1982 (7) |
| Hypoxemia | 141/447 (32) | 440/1245 (35) | 497/1891 (26) |
| Radiological pneumonia | 84/293 (29) | 228/707 (32) | 325/1190 (27) |
| Hyperaeration | 147/304 (48) | 410/740 (55) | 526/1250 (42) |
| Discharge Dx pneumonia | 402 (79) | 1042 (77) | 1550 (69) |
| Discharge Dx bronchitis | 136 (27) | 300 (23) | 424 (19) |
| Intensive care admission | 67 (13) | 252 (19) | 489 (21) |
| Mechanical ventilation | 16 (3) | 87 (6) | 198 (9) |
| Hospitalized >1 week | 133/508 (26) | 427/1353 (32) | 787/2262 (35) |
| Death | 9/508 (2) | 35/1353 (3) | 101/2263 (4) |
| Age ≥ 5 years | 76 (13) | 129 (9) | 1953 (46) |
| Age, year (med [IQR]) | 47 [10,66] | 50 [20, 68] | 50 [26, 69] |
| Sex (female) | 42 (55) | 69 (53) | 1013 (52) |
| Cough | 69 (91) | 111 (89) | 1693 (91) |
| Temperature (med [IQR]) | 38·0 [37·0, 38·4] | 37·5 [37·0, 38·0] | 37·5 [37·0, 38·0] |
| Difficulty breathing | 65 (86) | 95 (76) | 1508 (81) |
| Hypoxemia | 31/66 (47) | 49/110 (45) | 718/1641 (44) |
| Radiological pneumonia | 25/48 (52) | 34/71 (48) | 507/991 (51) |
| Hyperaeration | 21/48 (44) | 36/76 (47) | 471/1038 (45) |
| Discharge Dx pneumonia | 27 (36) | 45 (35) | 705 (37) |
| Discharge Dx bronchitis | 1 (1) | 0 | 9 (0) |
| Intensive care admission | 8 (11) | 14 (11) | 224 (11) |
| Mechanical ventilation | 4 (5) | 13 (10) | 158 (8) |
| Hospitalized >1 week | 34/74 (46) | 64/127 (50) | 896/1919 (47) |
| Death | 8/74 (11) | 16/127 (13) | 204/1925 (11) |
Includes 43 cases with HMPV-RSV co-infections.
Of the 2295 HMPV-RSV-negative cases in children aged <5 years, we detected the following viruses: 180 (8%) influenza A, 43 (2%) influenza B, 99 (5%) parainfluenza virus type 1, 28 (1%) parainfluenza virus type 2, 251 (12%) parainfluenza virus type 3, and 293 (14%) adenovirus. Of the 1953 HMPV-RSV-negative cases in persons aged ≥5 years, we detected the following viruses: 153 (8%) influenza A, 30 (2%) influenza B, 28 (1%) parainfluenza virus 1, 10 (1%) parainfluenza virus 2, 62 (3%) parainfluenza virus 3, and 118 (6%) adenovirus. At least one other virus was also detected in 127 (24%) of the HMPV-positive cases in children aged <5 years and in 13 (17%) of the HMPV-positive cases among persons aged ≥5 years. At least one other virus was also detected in 271 (20%) of the RSV-positive cases in children aged <5 years and in 33 (26%) of the RSV-positive cases among persons aged ≥5 years.
Among children aged <5 years with ARI, the median age was older for those HMPV-positive (1 year) compared with those RSV-positive (6 months). There was no major difference in the respiratory signs and symptoms, presence of fever, or radiological diagnoses between the three etiological groups presented in Table1. Among children aged <5 years, hospital course (intensive care, mechanical ventilation, length of stay) and mortality proportions suggest that severity is lowest for HMPV-positive, intermediate for RSV-positive, and greatest for HMPV-RSV-negative. Among those aged ≥5 years, there was no consistent association between these indicators of severity and etiological group.
In Santa Rosa during the years 2008, 2009, 2010, 2011, and 2012, we detected HMPV in 42 (14%), 5 (1%), 75 (15%), 16 (3%), and 43 (10%) of ARI cases and RSV in 33 (11%), 242 (38%), 81 (17%), 161 (33%), and 9 (2%) of ARI cases, respectively. In Quetzaltenango during the years 2009, 2010, 2011, and 2012, we detected HMPV infection in 21 (3%), 111 (13%), 31 (7%), and 37 (8%) of ARI cases and RSV in 223 (34%), 195 (22%), 118 (26%), and 69 (15%) of ARI cases, respectively. In Guatemala City during 2010, we detected HMPV infection in 195 (16%) of ARI cases and RSV in 330 (27%). RSV infections mostly occurred from August to October, during the second half of the rainy season, with the exception of 2012, when there were few RSV cases and most occurred during November–December. In contrast, HMPV peaked at different times each year (Figure1).
Figure 1.
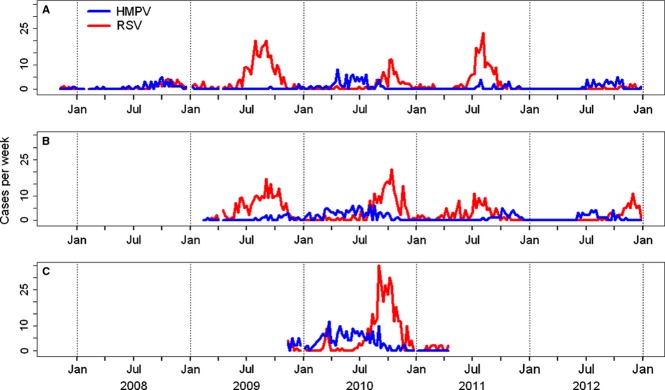
A–C. Weekly numbers of human metapneumovirus (HMPV) and respiratory syncytial virus (RSV) infections among persons hospitalized with acute respiratory infections in Guatemala: (A) Santa Rosa, (B) Quetzaltenango, and (C) Guatemala City.
Among children aged <5 years, statistically significant nonlinear relationships with age were found for both HMPV [degrees of freedom (d.f.) = 5·2, P-value <0·001) and RSV (d.f. = 2·1, P-value <0·001). The probability of HMPV was low during the first month of life and increased with age, peaking at 20% around age 16 months. The predicted probability of RSV was approximately 41% during the first month of life, after which it declined rapidly, becoming similar to the probability of HMPV by around age 2 years (Figure2). Among persons aged ≥5 years, the probability of HMPV declined from 8% at age 5 years to around 2·5% at age 20 years (d.f. = 3·6, P-value = 0·005). In contrast, there was no statistically significant evidence of an association between age and the probability of RSV in this age group (P-value = 0.402).
Figure 2.
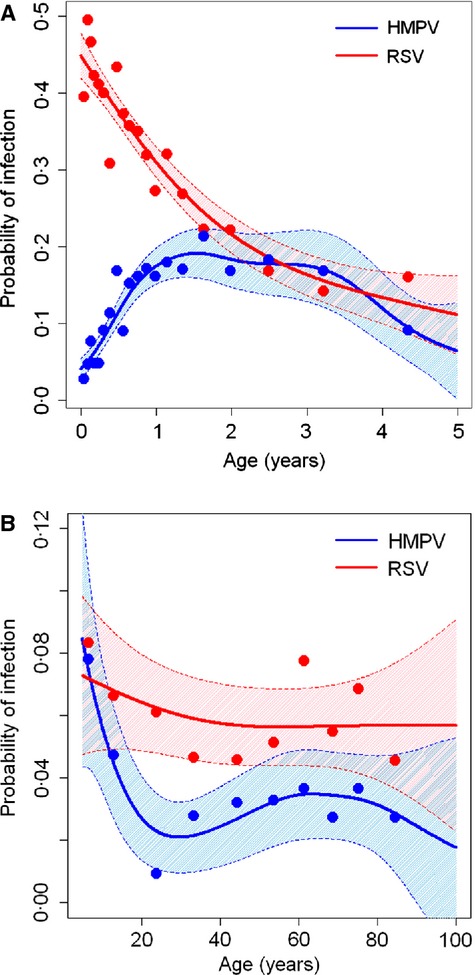
A–B. Relationships between age and probabilities of HMPV and RSV among persons aged (A) <5 years and (B) ≥5 years hospitalized with acute respiratory infection; line estimated using penalized spines in generalized additive models, shaded areas indicate 95% confidence intervals, and points indicate observed proportions and median age within twentiles of age for children aged <5 years (A) and deciles of age for persons aged ≥5 years (B).
The crude annual incidence rates of hospitalized HMPV-ARI (Table3) were 56 cases per 100 000 children aged <5 years (95% CI: 49, 64), 1·4 cases per 100 000 children aged 5–17 years (95% CI: 0·8, 2·4), and 1·5 cases per 100 000 persons aged ≥18 years (95% CI: 0·9, 2·2). The estimated annual incidence rates adjusted for the proportion of eligible cases tested for HMPV and for proportions of the populations seeking health care at the surveillance hospitals were 102 cases per 100 000 children aged <5 years (95% CI: 75, 178), 2·6 cases per 100 000 children aged 5–17 years (95% CI: 1·2, 5·0), and 2·6 cases per 100 000 persons aged ≥18 years (95% CI: 1·5, 4·9).
Table 3.
Odds ratios (95% confidence intervals) for indicators of disease severity associated with HMPV among patients hospitalized with acute respiratory infection
| Outcome | HMPV-positive versus RSV-positive* | HMPV-positive versus HMPV-RSV-negative* | ||
|---|---|---|---|---|
| Crude | Adjusted** | Crude | Adjusted** | |
| Age < 5 years | (n = 1804)*** | (n = 2815)*** | ||
| Intensive care | 0·66 (0·49, 0·89) | 1·08 (0·78, 1·50) | 0·55 (0·41, 0·72) | 0·63 (0·47, 0·84) |
| Mechanically ventilated | 0·46 (0·26, 0·80) | 0·70 (0·38, 1·27) | 0·34 (0·20, 0·56) | 0·38 (0·23, 0·65) |
| Hospitalized > 1 week | 0·78 (0·62, 0·99) | 1·24 (0·96, 1·61) | 0·66 (0·54, 0·82) | 0·75 (0·60, 0·93) |
| Death | 0·65 (0·30, 1·41) | 0·75 (0·33, 1·71) | 0·39 (0·19, 0·77) | 0·46 (0·23, 0·92) |
| Age ≥5 years | (n = 191)*** | (n = 2029)*** | ||
| Intensive care | 1·01 (0·40, 2·55) | 0·94 (0·34, 2·64) | 0·91 (0·43, 1·91) | 0·78 (0·37, 1·67) |
| Mechanically ventilated | 0·52 (0·16, 1·65) | 0·71 (0·20, 2·47) | 0·63 (0·23, 1·75) | 0·79 (0·28, 2·22) |
| Hospitalized > 1 week | 0·86 (0·47, 1·56) | 1·06 (0·55, 2·04) | 0·97 (0·61, 1·55) | 1·14 (0·70, 1·85) |
| Death | 0·88 (0·36, 2·18) | 1·04 (0·38, 2·86) | 1·02 (0·48, 2·16) | 1·31 (0·61, 2·84) |
Cases with HMPV-RSV co-infection (n = 43) excluded from these models.
OR estimates adjusted for age (cubic spline with 9 degrees of freedom), sex, and surveillance area (Santa Rosa, Quetzaltenango, Guatemala).
Sample sizes for some models were lower by about 1–2% due to missing data for duration of hospitalization and death.
In unadjusted logistic regression models (Table3), among children aged <5 years, having HMPV infection compared with RSV infection was associated with significantly lower odds of being admitted to the ICU, needing mechanical ventilation and being hospitalized ≥1 week. After adjusting these models for age, sex and surveillance site, there was no statistically significant association. Among children aged <5 years, after adjustment for potential confounders, we found that HMPV-positive cases versus HMPV-RSV-negative cases were associated with lower odds of ICU admission (P-value = 0·002), mechanical ventilation (P-value<0·001), hospital stay >1 week (P-value = 0·011), and death (P-value = 0·026). Among persons aged ≥5 years, there was no evidence of a difference in severity associated with etiological group, and we did not find any evidence of effect modification by age (Table3).
Table 2.
Annual crude and adjusted incidence rates (95% confidence intervals) of hospitalized acute respiratory infections with HMPV by age group—Santa Rosa, 2008–2012 and Quetzaltenango, 2009–2012
| Rates per 100 000 persons per year | |||||
|---|---|---|---|---|---|
| HMPV cases | Person-years | Crude | Adjustment for enrollment and RSV testing1 | Plus adjustment for healthcare seeking1,2 | |
| All ages | 265 | 27 749 038 | 9·5 (8·4, 10·8) | 9·9 (8·3, 10·9) | 17·4 (11·9, 25·0) |
| <5 years | 231 | 412 560 | 56·0 (49·0, 63·7) | 57·7 (49·9, 64·5) | 101·6 (75·0, 177·6) |
| <6 months | 48 | 42 456 | 113·1 (83·4, 149·9) | 114·8 (82·4, 148·4) | 205·2 (133·5, 385·1) |
| 6–23 months | 148 | 125 880 | 117·6 (99·4, 138·1) | 123·1 (103·3, 143·0) | 215·3 (155·3, 386·6) |
| 2–4 years | 35 | 244 224 | 14·3 (10·0, 19·9) | 14·5 (9·8, 19·7) | 25·3 (15·7, 45·5) |
| 5–17 years | 13 | 921 286 | 1·4 (0·8, 2·4) | 1·5 (0·8, 2·4) | 2·6 (1·2, 5·0) |
| ≥18 years | 21 | 1 441 092 | 1·5 (0·9, 2·2) | 1·5 (0·9, 2·2) | 2·6 (1·5, 4·9) |
| 18–49 years | 5 | 1 089 578 | 0·5 (0·1, 1·1) | 0·5 (0·2, 1·0) | 0·8 (0·2, 2·0) |
| ≥50 years | 16 | 351 514 | 4·6 (2·6, 7·4) | 4·7 (2·6, 7·1) | 8·4 (4·4, 16·7) |
| 50–64 years | 6 | 212 973 | 2·8 (1·0, 6·1) | 3·0 (0·9, 6·1) | 5·4 (1·8, 12·7) |
| ≥65 years | 10 | 138 541 | 7·2 (3·5, 13·3) | 7·3 (3·6, 13·0) | 13·0 (5·4, 28·0) |
Adjusted for proportion of eligible cases enrolled and tested for HMPV by age group and site, which ranged from 90% to 100%.
Adjusted for the proportion in health utilization surveys stating that they were hospitalized at the surveillance hospital among those hospitalized for pneumonia from Santa Rosa (53%) and from Quetzaltenango (60%).
Discussion
To our knowledge, this is the first report from Central America of HMPV infection in the context of systematic hospital-based surveillance for ARI, and these data from three sites in Guatemala covering a span of 5 years demonstrate that HMPV is a substantial cause of ARI hospitalization in Guatemala. Among children aged <5 years, we found population incidence rates of HMPV-positive ARI similar to a previous study among US children.24 In contrast, the incidence rate we found for adults was many times lower than that reported in Tennessee, USA.25 However, lower access to and utilization of healthcare services may result in underestimation of the burden in Guatemala, despite our attempt to correct for healthcare seeking behavior. The proportion of ARI cases HMPV-positive among children aged <5 years in our study (13%) was higher than other previous studies from USA (New York and Tennessee), Italy, France and Korea,26–29 but similar to studies in Alaska and Brazil.12,30 The proportion of hospitalized ARI cases that were HMPV-positive among adults ≥50 years (3%) was similar to that reported in TN, USA (4·5%).25
There was no clear seasonality of HMPV infection that we observed. In contrast, it has been reported that peak incidence of HMPV coincides with the latter half of RSV seasonal peaks in both the USA and Brazil.31,32 The broad distribution of HMPV cases over time, in contrast to the sharper peak of RSV, is, however, consistent with previous studies in both temperate and tropical environments.31–33 The wide variation we found between years in the numbers and proportions of ARI cases attributable to HMPV infection is also consistent with findings from prospective ARI surveillance in other countries.31,32
Our study is consistent with previous findings that HMPV and RSV are major causes of ARI during early childhood and fairly important during later childhood and adulthood, and that HMPV-ARI is less frequent than RSV-ARI.34 Hall et al. 35found that incidence of RSV hospitalization in the United States was highest for infants 1 month of age, which is similar to our findings for proportion of ARI cases RSV-positive. Our detailed analysis of the association between age and the probability of detecting each virus among ARI cases, however, provides new information about the relative burden of disease attributable to each virus at specific ages. For instance, during the neonatal period, HMPV was detected in only about 3% of ARI cases, whereas RSV was detected in 41%. Most population-based studies that have reported the proportions of ARI positive for HMPV and RSV have not provided this level of detail because they have grouped children aged <6 months or <2 years.27,36,37 The decline in RSV and increase in HMPV over the first year of life suggest that the timing of vaccination would have important implications for the relative burden of disease that would be prevented by potential future HMPV and RSV vaccines. The small number of HMPV cases in the first months of life suggests that an effective early childhood vaccine could prevent the majority of its disease burden, in contrast to RSV, which may require maternal immunization to prevent the high burden during the first few months of life.
Among children aged <5 years, after adjusting for potential confounders, ARI cases with HMPV were no less severe than those with RSV but were less severe than those negative for both viruses, which included 1512 (66%) negative for the eight viruses tested (data not shown). Among persons aged ≥5 years, there was no evidence of a difference in severity when comparing HMPV-positive to either RSV-positive or HMPV-RSV-negative cases, which included 1575 (81%) negative for the eight viruses tested. Our crude results are consistent with evidence that HMPV hospitalizations are less severe and of shorter duration compared with RSV hospitalizations.32,38 However, it is also informative to know, as the adjusted models indicate, that children aged <5 years of similar age and same sex admitted to the same hospital would be expected to have similar hospital course and outcomes, regardless of whether the infection is HMPV or RSV, but that HMPV-positive cases can be expected to be less severe than HMPV-RSV-negative cases.
Our study has a few important limitations. Our initial ARI case definition is likely to have missed a proportion of illnesses caused by HMPV and RSV, particularly among young infants and adults, as they are often afebrile and lack significant WBC abnormalities.39–41 However, this underestimation of burden and potential selection bias was no longer a limitation for children aged <5 years after we added the WHO pneumonia case definition in February 2011. Furthermore, the small proportion (9%) of cases after February 2011 in children aged <5 years that lacked one of these signs of infection suggests this source of bias was not substantial for this age group. Interpretation of the comparisons between HMPV and HMPV-RSV-negative cases is limited by the incomplete information on the etiologies of this heterogeneous reference group, among which we detected a viral infection in only 27% of the cases, as well as the diverse clinical and epidemiological features of these infections. Our crude population incidence rates of HMPV-ARI hospitalization are known to substantially underestimate the burden of severe disease in the community that should have led to hospitalization. On the other hand, the adjustment of incidence rates for healthcare seeking behavior was based on self-reported illness and healthcare seeking practices, which introduces substantial uncertainty due to small sample size, as reflected in the wider confidence intervals, and is also subject to reporting bias.
Our study suggests that HMPV is important though less frequent than RSV as a cause of ARI hospitalizations and mortality in Guatemala. These findings highlight the importance of considering HMPV testing within ARI surveillance platforms in Central America. Additionally, interventions to prevent ARI due to HMPV and evaluations of their impact should take into account the potentially unpredictable timing of incidence peaks, rarity of cases during the first few months of life, and lesser severity compared with HMPV-RSV-negative cases among young children.
Acknowledgments
This publication was supported by Cooperative Agreement Number UO1 GH000028-02 from the US Centers for Disease Control and Prevention (CDC). We are grateful to Dr. Juan Carlos Moir, epidemiologist from the Guatemala Ministry of Health who supported the implementation of the surveillance system, to María Reneé López for performing PCR tests and reviewing the manuscript, and to Dean Erdman for assisting with the description of laboratory methods.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boivin G, De Serres G, Cote S, et al. Human metapneumovirus infections in hospitalized children. Emerg Infect Dis. 2003;9:634–640. doi: 10.3201/eid0906.030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray GC, Capuano AW, Setterquist SF, et al. Human metapneumovirus, Peru. Emerg Infect Dis. 2006;12:347–350. doi: 10.3201/eid1202.051133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loo LH, Tan BH, Ng LM, Tee NW, Lin RT, Sugrue RJ. Human metapneumovirus in children, Singapore. Emerg Infect Dis. 2007;13:1396–1398. doi: 10.3201/eid1309.070220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali SA, Williams JV, Chen Q, et al. Human metapneumovirus in hospitalized children in Amman, Jordan. J Med Virol. 2010;82:1012–1016. doi: 10.1002/jmv.21768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ijpma FF, Beekhuis D, Cotton MF, et al. Human metapneumovirus infection in hospital referred South African children. J Med Virol. 2004;73:486–493. doi: 10.1002/jmv.20116. [DOI] [PubMed] [Google Scholar]
- 7.Sloots TP, Mackay IM, Bialasiewicz S, et al. Human metapneumovirus, Australia, 2001-2004. Emerg Infect Dis. 2006;12:1263–1266. doi: 10.3201/eid1208.051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ulloa-Gutierrez R, Vargas-Jimenez F, Mora-Chavarria A, Umana MA, Calvo-Espinoza M, Alfaro-Bourrouet W. Human metapneumovirus in Costa Rica. Pediatr Infect Dis J. 2009;28:452–453. doi: 10.1097/INF.0b013e31819d0c64. [DOI] [PubMed] [Google Scholar]
- 9.Laguna-Torres VA, Sanchez-Largaespada JF, Lorenzana I, et al. Influenza and other respiratory viruses in three Central American countries. Influenza Other Respir Viruses. 2011;5:123–134. doi: 10.1111/j.1750-2659.2010.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlaudecker EP, Heck JP, Macintyre ET, et al. Etiology and Seasonality of Viral Respiratory Infections in Rural Honduran Children. Pediatr Infect Dis J. 2012;31:1113–1118. doi: 10.1097/INF.0b013e31826052eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdullah Brooks W, Erdman D, Terebuh P, et al. Human metapneumovirus infection among children, Bangladesh. Emerg Infect Dis. 2007;13:1611–1613. doi: 10.3201/eid1310.070337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singleton RJ, Bulkow LR, Miernyk K, et al. Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol. 2010;82:1282–1290. doi: 10.1002/jmv.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caracciolo S, Minini C, Colombrita D, et al. Human metapneumovirus infection in young children hospitalized with acute respiratory tract disease: virologic and clinical features. Pediatr Infect Dis J. 2008;27:406–412. doi: 10.1097/INF.0b013e318162a164. [DOI] [PubMed] [Google Scholar]
- 14.Martin ET, Kuypers J, Heugel J, Englund JA. Clinical disease and viral load in children infected with respiratory syncytial virus or human metapneumovirus. Diagn Microbiol Infect Dis. 2008;62:382–388. doi: 10.1016/j.diagmicrobio.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermos CR, Vargas SO, McAdam AJ. Human metapneumovirus. Clin Lab Med. 2010;30:131–148. doi: 10.1016/j.cll.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langley GF, McCracken J, Arvelo W, et al. The epidemiology and clinical characteristics of young children hospitalized with respiratory syncytial virus infections in Guatemala (2007–2010) Pediatr Infect Dis J. 2013;32:629–635. doi: 10.1097/INF.0b013e318289e3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindblade KA, Arvelo W, Gray J, et al. A comparison of the epidemiology and clinical presentation of seasonal influenza A and 2009 pandemic influenza A (H1N1) in Guatemala. PLoS One. 2010;5:e15826. doi: 10.1371/journal.pone.0015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherian T, Mulholland EK, Carlin JB, et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ. 2005;83:353–359. [PMC free article] [PubMed] [Google Scholar]
- 19.Fry AM, Chittaganpitch M, Baggett HC, et al. The burden of hospitalized lower respiratory tract infection due to respiratory syncytial virus in rural Thailand. PLoS ONE. 2010;5:e15098. doi: 10.1371/journal.pone.0015098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood SN. Generalized Additive Models: An Introduction With R. Chapman & Hall: Boca Raton, FL; 2006. [Google Scholar]
- 21.Lindblade KA, Johnson AJ, Arvelo W, et al. Low usage of government healthcare facilities for acute respiratory infections in guatemala: implications for influenza surveillance. BMC Public Health. 2011;11:885. doi: 10.1186/1471-2458-11-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan OW, Frenkel G, Zhang X, et al. Health utilization survey in Quetzaltenango, Guatemala. International Conference on Emerging Infectious Diseases: Atlanta, GA; 2010. [Google Scholar]
- 23.Efron B, Tibshirani R. An Introduction to the Bootstrap. Chapman & Hill: New York, NY; 1993. [Google Scholar]
- 24.Williams JV, Edwards KM, Weinberg GA, et al. Population-based incidence of human metapneumovirus infection among hospitalized children. J Infect Dis. 2010;201:1890–1898. doi: 10.1086/652782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widmer K, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis. 2012;206:56–62. doi: 10.1093/infdis/jis309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim CK, Choi J, Callaway Z, et al. Clinical and epidemiological comparison of human metapneumovirus and respiratory syncytial virus in seoul, Korea, 2003-2008. J Korean Med Sci. 2010;25:342–347. doi: 10.3346/jkms.2010.25.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwane MK, Edwards KM, Szilagyi PG, et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113:1758–1764. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 28.Freymuth F, Vabret A, Cuvillon-Nimal D, et al. Comparison of multiplex PCR assays and conventional techniques for the diagnostic of respiratory virus infections in children admitted to hospital with an acute respiratory illness. J Med Virol. 2006;78:1498–1504. doi: 10.1002/jmv.20725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierangeli A, Gentile M, Di Marco P, et al. Detection and typing by molecular techniques of respiratory viruses in children hospitalized for acute respiratory infection in Rome, Italy. J Med Virol. 2007;79:463–468. doi: 10.1002/jmv.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomazelli LM, Vieira S, Leal AL, et al. Surveillance of eight respiratory viruses in clinical samples of pediatric patients in southeast Brazil. J Pediatr (Rio J) 2007;83:422–428. doi: 10.2223/JPED.1694. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira DB, Durigon EL, Carvalho AC, et al. Epidemiology and genetic variability of human metapneumovirus during a 4-year-long study in Southeastern Brazil. J Med Virol. 2009;81:915–921. doi: 10.1002/jmv.21436. [DOI] [PubMed] [Google Scholar]
- 32.Williams JV, Harris PA, Tollefson SJ, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams JV, Wang CK, Yang CF, et al. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J Infect Dis. 2006;193:387–395. doi: 10.1086/499274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papenburg J, Boivin G. The distinguishing features of human metapneumovirus and respiratory syncytial virus. Rev Med Virol. 2010;20:245–260. doi: 10.1002/rmv.651. [DOI] [PubMed] [Google Scholar]
- 35.Hall CB, Weinberg GA, Blumkin AK, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132:e341–e348. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- 36.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heikkinen T, Osterback R, Peltola V, Jartti T, Vainionpaa R. Human metapneumovirus infections in children. Emerg Infect Dis. 2008;14:101–106. doi: 10.3201/eid1401.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marguet C, Lubrano M, Gueudin M, et al. In very young infants severity of acute bronchiolitis depends on carried viruses. PLoS One. 2009;4:e4596. doi: 10.1371/journal.pone.0004596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falsey AR, Walsh EE. Viral pneumonia in older adults. Clin Infect Dis. 2006;42:518–524. doi: 10.1086/499955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh EE, Peterson DR, Falsey AR. Is clinical recognition of respiratory syncytial virus infection in hospitalized elderly and high-risk adults possible? J Infect Dis. 2007;195:1046–1051. doi: 10.1086/511986. [DOI] [PubMed] [Google Scholar]
- 41.El-Radhi AS, Barry W, Patel S. Association of fever and severe clinical course in bronchiolitis. Arch Dis Child. 1999;81:231–234. doi: 10.1136/adc.81.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]


