Abstract
Autoimmune uveitis is an organ-specific disorder characterized by irreversible lesions to the eye that predominantly affect people in their most productive years and is among the leading causes of visual deficit and blindness. Currently available therapies are effective in the treatment of a wide spectrum of uveitis, but are often associated with severe side effects. Here, we review ongoing research with promising immunomodulatory therapeutic strategies, describing their specific features, interactions and the responses triggered by the targeted immune molecules that aim to minimize clinical complications and the likelihood of disease relapse. We first review the main features of the disease, diagnostic tools, and traditional forms of therapy, as well as the animal models predominantly used to understand the pathogenesis and test the novel intervention approaches aiming to control the acute immune and inflammatory responses and to dampen chronic responses. Both exploratory research and clinical trials have targeted either the blockade of effector pathways or of their companion co-stimulatory molecules. Examples of targets are T cell receptors (CD3), their co-stimulatory receptors (CD28, CTLA-4) and corresponding ligands (B7-1 and B7-2, also known as CD80 and CD86), and cytokines like IL-2 and their receptors. Here, we summarize the available evidence on effectiveness of these treatments in human and experimental uveitis and highlight a novel CD28 antagonist monovalent Fab′ antibody, FR104, which has shown preclinical efficacy suppressing effector T cells while enhancing regulatory T cell function and immune tolerance in a humanized graft-versus-host disease (GVHD) mice model and is currently being tested in a mouse autoimmune uveitis model with encouraging results.
Keywords: Autoimmune uveitis, Experimental autoimmune uveitis, Costimulation blockade, Immune modulation, CD28 antagonists
1. Introduction
Autoimmune disorders encompass a wide range of chronic inflammatory conditions and are among the leading causes of disability in the world (report from The Autoimmune Diseases Coordinating Committee of the National Institutes of Health, 2005 at http://www.niaid.nih.gov/topics/autoimmune/research/Pages/coordComm.aspx, updated April 30, 2012). These diseases are characterized by an abnormal response of the immune system, which failing to distinguish self from non-self-molecules reacts against organs, tissues and cells of the individual. Although the specific mechanisms triggering each type of autoimmune disease are not completely understood, it is well established that the development and progression of autoimmune disorders is strongly associated with both genetic and environmental factors, which predispose the individual to the disease.
Although each specific autoimmune condition has usually a low incidence, collectively autoimmune disorders affect 14.7 to 23.5 million people in the United States (http://www.niaid.nih.gov/topics/autoimmune, updated August 1, 2013) alone. The high burden of autoimmune diseases is exacerbated by the chronic character of these conditions, the impairment in the quality of life of patients and the cost of the available treatments. More effective, safer and affordable therapies targeted at specific molecules and conditions would be ideal characteristics of a good treatment, several of which are under development, but are currently still not available.
Much clinical and experimental research on autoimmune diseases is directed towards the investigation of novel therapies or the improvement of currently available treatments. Owing to the great variability in the mechanisms underlying disease development and progression, current studies focus on the understanding of specific features and molecular pathways associated with each disorder. In particular, the use of therapies targeted at rare or distinctive autoimmune diseases currently represents promising areas of study. One such disease is autoimmune uveitis, an organ-specific disorder characterized by irreversible lesions to the eye. Autoimmune uveitis (AIU) predominantly affects people in their most productive years (from 20 to 50 years of age), and is among the leading causes (approximately 10% of all cases) of visual deficit and blindness. AIU has an incidence of 52.4 per 100,000 and a prevalence of 115.3 per 100,000 habitants in the United States [1], and is also considered a global socioeconomic problem (The International Uveitis Study Group — http://www.iusg.net, updated November 16, 2013).
The heterogeneity of underlying factors and the variable disease severity of AIU patients reinforce the need for an accurate diagnosis. Although currently available therapies are effective in the treatment of a wide spectrum of uveitis, most are associated with important and severe side effects. In this sense, treatments based on novel molecules or employing well-known molecules, tuning their use to the various hierarchical levels of the immune response, represent promising avenues of research. Moreover, the investigation of alternative or combined treatments focusing on groups of patients with a similar disorder may be also fruitful.
Here, we review ongoing research to treat AIU with promising immunomodulatory therapeutic strategies, highlighting their specific features, interactions and the responses triggered by the targeted immune molecules that aim to minimize clinical complications and the likelihood of disease relapse. For improved comprehension we first review the main features of AIU, diagnostic tools and traditional forms of therapy, as well the animal models predominantly used to understand its pathogenesis and test novel intervention approaches.
2. Autoimmune uveitis
Uveitis is an inflammation of the uvea, a layer located between the sclera and the retina of the eye that includes the iris, ciliary body, and choroid, but can also extend to adjacent tissues, such as retina, optic nerve, and vitreous humor [2]. The disease is broadly classified into infectious and non-infectious (which includes AIU) uveitis and in humans, can affect the anterior, intermediate, and posterior portions of the eye [3]. Furthermore, a series of autoimmune diseases may additionally present uveitis, with overlapping AIU features but also unique characteristics. That is the case for Behçet's disease [4], reactive arthritis [5], sarcoidosis [6], and Vogt–Koyanagi–Harada syndrome [7,8]. In contrast, AIU is confined to the eye.
In AIU, ocular antigens such as arrestin also known as S-antigen [9], the interphotoreceptor retinoid binding protein IRBP, and/or recoverin [10] are exposed and an immune response against these molecules is initiated [11]. Among the events involved in this response, blood–retinal barrier disruption and retinal antigen recognition by auto-reactive T cells are critical elements. In addition, predisposing MHC class II alleles have been identified in some populations, namely, HLA-DR4, HLA-DR3 and some HLA-DQ alleles [12]. Under normal conditions, special microanatomical features that characterize the so-called immune privilege of the eye [13] ensure that retinal molecules are protected from autoimmune responses and that tissue integrity is maintained [14,15]. These features include the blood–retinal barrier, the lack of MHC Class II positive professional antigen-presenting cells (APCs) [16,17], the absence of a lymphatic drainage, the presence of soluble immunosuppressive factors secreted by ocular cells, such as TGF-β, FasL [18], PDL-1 [19], CTLA-4 [20], CTLA-2 [21], CD200, CD55, CD46, and decay-accelerating factor (DAF) [22,23], the constitutive expression of several of these regulatory molecules, the generation of regulatory T cells [24–27], and finally, eye antigen expression in the thymus that counteracts the escape of autoreactive T cells into the peripheral blood [28].
Although many features of uveitis have been extensively studied, therapeutic interventions that work in animal models have frequently failed due to the heterogeneity of this disorder. Additionally, many of the approaches are not disease-specific leading to enhancement of local and systemic side-effects. Therefore, a precise diagnosis of the type of uveitis is often needed to guarantee the best therapeutic control of the progression of eye lesions. Diagnosis of AIU requires considering multiple factors in the patient's history like age and the exclusion of injuries resulting from trauma, infections, or tumors, as well as a detailed laboratory investigation, which should include complete blood count, erythrocyte sedimentation rate, angiotensin converting enzyme and lysozyme levels, serology for syphilis, HLA, and of course, ocular imaging including fundoscopy [29]. Eligible treatments should also be addressed aiming to minimize ocular complications that lead to decrease in visual acuity and ultimately may cause irreversible blindness. Aggravating features include cystoid macular edema, cataract, glaucoma, retinal vascular abnormalities, macular lesions, retinal detachment, corneal opacities, optic-nerve atrophy, and phthisis that have also been frequently reported [30].
Current treatments focus on immunosuppressive therapies to control acute inflammation and to ensure the maintenance of long-term remission. Corticosteroids are usually among the first chosen due to their effectiveness at controlling inflammation both in the short term and in the long term. However, a myriad of possible side effects (e.g. weight gain, gastric ulceration, osteoporosis, fluid retention, hypertension, diabetes mellitus, and changes in mental status) as well as ocular sequelae (e.g. acceleration of cataract formation and glaucoma) may be observed. More specific therapies have been associated with more positive effects [31]. Such therapies include the prescription of antimetabolite drugs (including Methotrexate, Azathioprine, Mycophenolate mofetil), T cell and calcineurin inhibitors (cyclosporine, FK506/Tacrolimus), alkylating/cytotoxic agents (cyclophosphamide, chlorambucil), intravenous immunoglobulin and modern immunobiologicals. The latter group includes several agents, such as Infliximab (a TNF-alpha antagonist mouse–human chimeric antibody), Adalimumab (a human antibody developed against TNF-alpha), Etanercept (another TNF-alpha antagonist, but less efficient than Infliximab or Adalimumab), interleukin-2 receptor antagonists such as Daclizumab, as well as interferon-alpha based therapies [32–34].
Overall, though considerable success in stemming the clinical progression of uveitis has been achieved, the search for safe and effective alternative therapies and disease-specific interventions are still going on [31].
3. Animal models of autoimmune uveitis
Owing to their ability to reproduce specific features of human diseases at diverse levels, from molecules to tissues and organs, animal models have been increasingly used to gain understanding of the pathogenesis of several autoimmune diseases. However, despite the similarities in molecular, morphological, and physiological aspects, a single animal model will often lack the ability to adequately mimic the complexity of mechanisms underlying a human disease. As a result, a number of models are usually combined to explain the many facets of autoimmune disorders.
To this date, several animal models have been used to study AIU (reviewed in [35,36]). In the next sections we review the most frequently used models to study the immunopathogenesis as well as some promising systems for evaluation of novel therapies.
3.1. Experimental autoimmune uveitis (EAU)
EAU is the most frequently used animal model of uveitis. This T-cell-mediated intraocular inflammatory disease is predominantly induced by immunization with the retinal antigens S-ag and IRBP coupled to Complete Freund's Adjuvant (CFA) and a Bordetella pertussis toxin (PTX) boost [37], with a 2-week time of onset. In mice, the resulting disease is mainly confined to the posterior part of the eye, with focal lesions affecting the retina and choroid. Vasculitis and the presence of granulomas in the posterior layers of the eye are often seen and are accompanied by serous detachment of the retina and disorganization of the photoreceptor layer. Severity of EAU is scored on a scale of 0 {no disease} to 4 {maximum disease} in half-point increments, according to a semi quantitative system described previously [37], according to lesion type, size, and number by histopathology examination of the eyes. Briefly, the minimal criteria for scoring an eye as positive for uveitis is presence of inflammatory cell in the ciliary body, choroids, or retina (EAU grade 0.5); progressive higher grades present discrete lesions in the tissue such as vasculitis, granuloma formation, retinal folding and/or detachment and photoreceptor damage [37].
Compared to other rodent models [38], mouse EAU is of longer duration and presents with recurrences, hence facilitating therapeutic handling of the disease [37].
The genetic predisposition for the development of eye autoimmunity, where only some mice lineages are susceptible to the induction of disease is quite clear in this model. Susceptibility is linked with specific H-2 MHC haplotypes, like H-2b found in C57BL/6 and C57BL/10 mice, H-2k found in B10.BR mice, and H-2r found in B10.RIII mice, with H-2r being the most susceptible, followed by H-2k and H-2b [35]. EAU susceptibility is also dependent on the pattern of immune response. For example, strains prone to a more exacerbated TH1 response are more susceptible than those with predominantly low TH1 responses [39].
As to the involvement of T-cell mediated inflammation cellular features of EAU resemble those of the human disease. T cells are mainly CD4+ exhibiting a TH1 phenotype in vivo [40], but are not required for antigen priming and retinal damage. This is suggested by the observation that IFN-γ knockout mice mount a deviant immune response against eye tissues when immunized with IRBP [41]. Although expected, TH2 lymphocytes do not confer resistance to uveitis. Nevertheless, a bias in the immune response towards the TH2 response profile has been shown to reduce pathogenic TH1 responses [39]. Recently, many groups have shown the importance of TH17 cells in the pathogenesis of AIU [42–44]. However it is difficult to determine their real role in the establishment and maintenance of disease, as it seems that both TH1 and TH17 cells can independently promote the disease onset [42].
The importance of cytokines both in EAU and in human uveitis is incontestable, but the understanding of their precise effects is a matter of great complexity. For example IFN-γ, the major cytokine of TH1 profile, is not required for onset and maintenance of EAU [41]. In addition, lack of IL-17, the main product of TH17 lymphocytes, does not abrogate EAU susceptibility either [44]. Therefore, it seems that the type of immune response will be driven not by the presence or absence of a particular cytokine, but by the balance of the cytokine milieu in the eye, which comprises IL-1, IL-12, IL-23, IL-4, IL-10 and several others (reviewed in [45]).
Altogether, these features make EAU an interesting animal model to acquire further understanding on aspects of the immune regulation taking place in the diseased eye.
3.2. Transgenic IRBP-specific T cell model of spontaneous EAU
Recently, a novel EAU model was developed to study basic autoimmune responses occurring in an immunoprivileged site such as the eye [46,47]. This spontaneous model uses an IRBP161-180 peptide-specific transgenic T cell receptor mouse model on a B10.RIII background, and shows the hallmarks of the human autoimmune uveitis as judged by histology and fundoscopic analysis. The CD4+ T cells infiltrating the uveitic eyes were of an effector/memory phenotype, but the infiltrate also included Th17 inflammatory and extrathymically-derived regulatory T cells. According to the level of transgenes present in the T cell population, disease severity varied. Additionally, in a classic adoptive transfer experiment IRBP-peptide activated Th1 cells could implement the disease in wild type naive recipients [46], according to the level of transgene in each of the lineages. On the other hand, the time course of the spontaneous disease in this model and also in Aire knockout B10.RIII mice was longer, with an onset of about 6 weeks instead of the usual 2 weeks in the original model [47]. Thus, even when using the same retinal antigen, time courses, severity of the pathology, and T cell profiles can be quite different, highlighting the variability of the disease.
3.3. Humanized model of EAU
Despite the usefulness of animal models in the understanding of mechanisms underlying the pathogenesis of autoimmune diseases, there are limitations regarding the specificity of effector cells or the genetic background underlying disease development. This is particularly important considering that AIU is more likely to be a group rather than a single disease as disease course and clinical manifestations can be highly heterogeneous. Genetic heterogeneity may underlie much of this variation associated with the clinical disease. For instance, previous evidence has shown a substantial degree of heterogeneity in the HLA loci from patients diagnosed with the classical (not linked to Behçet's, ankylosing spondylitis, or rheumatoid arthritis) autoimmune uveitis [48]. HLA-DR4, HLA-DR3 and even some HLA-DQ alleles have been linked with the pathogenesis of the disease in humans [49,50].
Humanized models have therefore been established in the last decade as important tools for the study of eye immunology. For example, although T cells from many patients diagnosed with autoimmune uveitis respond to immunization with S-ag [51], there is currently no mouse model exhibiting a similar response. Conversely, humanized HLA-DR3, HLA-DR4, and HLA-DQ8 transgenic mice can develop uveitis after immunization with retinal antigens [12]. For example, HLA-DR3 + mice develop the disease following immunization with S-ag. Similarly, HLA-A29 transgenic mice have been also shown to spontaneously develop a retinopathy resembling human birdshot chorioretinopathy [52].
Taken together, these models were important in the characterization of MHC-class II antigen presentation in AIU and represent the bottom line for studies aiming to identify relevant auto-antigenic epitopes and the establishment of antigen-specific immunotherapies.
3.4. Endotoxin-induced uveitis
In this animal model, uveitis is not induced by immunization with any retinal antigen to trigger autoimmunity, suggesting that the observed inflammatory response may be due to other mechanisms. In Lewis rats, the induction is achieved by immunization with lipopolysaccharide (LPS) [53] and in mice, immunization is carried out with a Salmonella typhimurium endotoxin, indicating an important role for the innate arm of immune response. In both rodent models the resulting disease is an anterior uveitis of short duration, which does not resemble any specific human uveitis. Although this model does not duplicate all the features of the human anterior uveitis, pathways activated during the innate response can be elicited by its use. Hence, its importance during disease onset, not only in the anterior uveitis but also in other types of uveitis might as well be studied using this model.
3.5. Experimental melanin protein-induced uveitis
In this rat model of uveitis, the disease induction is achieved by immunization with melanin protein in CFA or Hunter's Adjuvant and a PTX boost [54–56]. The resulting disease is characterized by infiltration and damage of the anterior portion of the eye by CD4+ and CD8+ T cells, macrophages and neutrophils. Clinical manifestations start approximately 14 days after disease induction and are very similar to those found in human anterior uveitis, including iritis and iridocyclitis, but with no retinal commitment.
This model comprises important innate immune response features and has been useful to clarify the role of nitric oxide (NO) in eye inflammation [56], to evaluate specific therapies aiming at the NO pathway, and even to achieve a better understanding of the differences between anterior and posterior uveitis.
3.6. TAM-receptor knockout mice
The TAM family of receptor tyrosine kinases is involved in the control of dendritic cell cytokine signaling [57] and the knockout of three receptors of this family (TAM TKO) has been shown to lead to multi-organ autoimmune disease in mice [58]. Recently, Ye and colleagues demonstrated that TAM TKO mice usually develop eye inflammation and are more susceptible to immunization with IRBP peptides. These mice show postnatal degeneration of the ocular photoreceptor layer and cellular infiltration by T lymphocytes and macrophages. Moreover, IRBP-specific T lymphocytes are found in these mice, explaining their high susceptibility to the development of uveitis even when induced by low doses of IRBP [59].
As in this model disease develops spontaneously it may provide an important tool to understand the role of the environment in overall vulnerability for autoimmunity in the eye. It may also help unravel the contribution of a deregulated population of APCs to the onset and maintenance of disease.
4. Immunomodulation strategies for non-infectious uveitis
Strategies focusing on diverse immunomodulatory targets for treatment of autoimmune diseases have been reported in humans and in experimental models. The main goal of these approaches is to control the acute immune and inflammatory responses and to dampen chronic responses. These avenues have been explored also in inflammatory ocular disorders [60]. Furthermore, exploratory research and clinical trials have targeted either the blockade of effector pathways or of their companion co-stimulatory molecules at different checkpoints of the immune response. Examples of targets evaluated are T cell receptors (CD3), their costimulatory receptors (CD28, CTLA-4) and corresponding ligands (B7-1 and B7-2, also known as CD80 and CD86), and cytokines like IL-2 and their receptors. Here, we summarize the available evidence on effectiveness of these treatments in human and experimental uveitis.
4.1. The IL-2/IL-2R pathway
IL-2 is a 15 kDa α-helical cytokine produced predominantly by activated T lymphocytes. It binds to the IL-2 receptor (IL-2R), which is formed by three subunits: α (CD25), β (CD122), and the common γ (CD132) chains (reviewed in [61]).
IL-2 has several functions in the development and maintenance of the immune system. Initially, this cytokine was known for its ability to promote the growth and clonal expansion of all T cells. However, many years of research have shown that IL-2 is also essential for the maintenance of regulatory T cells in the periphery and for the control of TH17 and follicular helper T cells (reviewed in [62]).
Because of such a central role in the control of the immune response, many efforts have targeted the IL-2 pathway as a means to control disease progression. By the time the first studies using IL-2 blockade or chimeric IL-2 toxins were conducted using experimental models of autoimmune disease, it was already known that a large proportion of eye-infiltrating cells in an EAU model bore high amounts of IL-2R [63]. These facts led Roberge and colleagues to inhibit EAU with an IL-2 chimeric toxin [64]. If treatment was initiated 7 or 10 days after induction of disease this therapy resulted in lesser incidence and disease severity in a rat EAU model. The efficacy of this approach was then confirmed with monoclonal antibodies directed against IL-2R [65,66].
In humans, treatment became available with a humanized antibody directed against the α chain of the IL-2R (Daclizumab). At first, a phase I/II clinical trial was conducted, which showed that following 12 months of Daclizumab, 8 of 10 patients diagnosed with chronic severe sight-threatening intermediate and posterior uveitis had their disease under control and immunosuppressive agent dosage could be abated with no major side effects. In some cases of anterior uveitis, however, patients responded to cyclosporin but not to Daclizumab, suggesting that different mechanisms are involved in this subtype of uveitis [67]. Subsequent clinical trials have achieved similar results [68–70], supporting the use of Daclizumab as a valid immunotherapy for some, but not all AIU patients.
4.2. Anti-CD3 therapy
Based on a similar rationale, namely aiming at a widespread downregulation of effector T cell responses, antibodies targeting CD3 molecules have been the focus of research on treatment of autoimmune disorders since the first antibody specific for humans was produced in 1979, by Kung and Goldstein [71]. Anti-CD3-induced tolerance has long been investigated (reviewed in [72]). Continuous research efforts directed towards the production of safer anti-CD3 antibodies, such as the humanized antibodies with mutated Fc regions, as well as extensive work on tolerance induction, led anti-CD3 antibodies to form a new category of immunotherapeutic agents used to treat autoimmune diseases as well as to ensure long-term survival of organ allografts [72].
In animal models, the use of oral anti-CD3 was able to suppress experimental autoimmune encephalomyelitis in a mouse model that could be explained by the induction of CD4+CD25−LAP+ regulatory T cells in a TGF-β-dependent fashion [73]. Additionally, the use of nasal anti-CD3 ameliorated systemic lupus erythematosus in two different mice models by inducing IL-10. In this case IL-10 was secreted by CD4+CD25−LAP+ regulatory cells, which downregulated follicular helper T cells and probably led to the observed tolerance [74]. Success using anti-CD3 was also obtained in animal models of diabetes [75,76].
In humans, clinical improvement of type 1 diabetic patients was observed after treatment with an anti-CD3 monoclonal antibody (hOKT3γ1(Ala-Ala); Teplizumab) and did not require use of any other immunosuppressive treatment during a 2-year follow-up [77]. Likewise, psoriatic arthritis patients treated with a similar anti-CD3 antibody showed better clinical outcome after a two-week treatment [78].
Despite the widespread use of anti-CD3 to treat autoimmunity, there is only one study testing its effectiveness in uveitis. Using the EAU model in B10.RIII mice, Ke and colleagues [79] demonstrated that anti-CD3 was able to ameliorate disease. The anti-CD3 used was directed against the invariant CD3ε chain and was unable to bind Fc receptors. The antibody given intraperitoneally for 6 or 10 days after disease induction was able to suppress EAU development in both treatment schemes, indicating its effectiveness during disease onset and upon priming of T cells. Additionally, long-term tolerance in the treated mice was achieved.
Although further research is needed to confirm these findings for other treatment schemes (such as different administration routes or under the association with other immunomodulatory drugs), the results indicate that anti-CD3 might be a promising immunotherapy for the treatment of uveitis. An important issue remaining concerns the adverse effects caused by CD3 treatment, the development of anti-drug antibodies, and the contrasting results obtained in preclinical and clinical trials [80,81].
4.3. The CD28, CTLA4, and B7 costimulation trinity
CD28, a membrane protein belonging to the well-known family of co-stimulatory molecules, transduces signals, which act synergistically as a second signal with the T cell receptor complex to activate naïve T cells. CD28 is constitutively expressed in most of CD4+ and in 50% of CD8+ T cells in humans; additionally, it is found in all mouse naïve T cells [82]. The CD28 co-stimulator receptor is a homodimer structured by disulfide bonds on the T cell surface that binds to the B7-1 (CD80), B7-2 (CD86), and ICOS-L (B7RP1) molecules expressed in activated APCs [83,84]. B7-1 and B7-2 receptors are up-regulated by inflammatory as well as antigen-specific signals and act as ligands for both CD28 and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) expressed on T cells [83].
CD28 enhances distinct T cell responses, which include survival and clonal expansion, IL-2 cytokine production, and naïve T cell differentiation into effector and memory phenotypes. In fact, there have been many reports on successful immunosuppression achieved by the blockade of the CD28/B7 pathway during treatment of autoimmune diseases, rejection of grafted organs, and in graft versus host disease [85]. However, the importance of pre-clinical and clinical studies even with promising therapeutic agents are highlighted by the extremely severe events that occurred during the phase I study of the superagonistic anti-CD28 antibody TGN1412 in six healthy human volunteers. The treatment caused massive trapping of T cells in spleen and lymph nodes in both rats and men, but the human volunteers, differently from the non-human primates tested suffered a massive cytokine storm and multiorgan failure which counteracted the possible benefits derived from the expected induction of regulatory T cells [86,87].
CTLA-4 (CD152) is structurally very similar to CD28, but differs in the binding affinity and temporal expression (reviewed by [85]). It is constitutively expressed on the surface of regulatory T cells and is induced upon activation of CD4+ and CD8+ T lymphocytes [88,89] and is responsible for inhibiting the CD28-induced signals to competitively downregulate and ultimately block the T cell response by specific binding to B7. Accordingly, CTLA-4 knockout mice present enhanced T cell activation and proliferation and an increased incidence of systemic autoimmunity [90].
Due to the fact that both CTLA-4 and CD28 recognize and act through the same B7 molecules expressed on the surface of APCs, the opposite signals generated by these costimulatory molecules have been the focus of many studies. CTLA-4 blockade with monoclonal antibodies such as Ipilimumab and Tremelimumab leads to increased effector T cell activity and this approach is being used in several types of malignancies (reviewed in [91,92]), in both pre-clinical and clinical studies. In other words, the contrasting effects induced by blockade of CD28 and CTLA-4 confirm that, in the latter case, the approach does not apply to treatment of autoimmune diseases. Indeed, colitis was one of the more common adverse effects associated with this treatment [91].
Inhibition of immune responses via blockade of the CD28 receptor B7 (CD80 and CD86), however, remains a valid option for autoimmune diseases, and is being explored through the use of Abatacept (CTLA-4-Ig), a second generation recombinant fusion protein consisting of CTLA-4 and a modified Fc fragment of human IgG1 [93,94] that binds to both CD80 and CD86. Abatacept is approved by the Food and Drug Administration (FDA) for treatment of rheumatoid arthritis and juvenile idiopathic arthritis [94,95] and shows promising results in the case of systemic lupus erythematosus (SLE) patients [93].
It has become apparent however, that due to its capacity to bind both receptors, Abatacept blocks not only the CD28-mediated activation of effector cells, but also an important subset of regulatory T cells which function through CTLA4–CD80 signal transduction (reviewed in [96]). A newer generation of CTLA-4Ig antibodies addresses the issue of the double CD28 and CTLA-4 blockade aiming to enhance preferential binding of the therapeutic antibody to CD86 over CD80 [97]. This version (LEA29Y) of the molecule carries two amino acid changes and has been developed trying to increase the binding avidity for CD86 [98,99]. This change may be important because CD86 appears to be the dominant costimulatory ligand in a number of experimental models and, in treating mouse models of autoimmune disease, inhibition via CD86 was more effective than inhibition via CD80.
There are only few reports on the role of the CD28/B7 pathway in ocular autoimmune conditions that suggest CD28 may be a promising molecule for the treatment of AIU [97]. One of the trials [100] showed a percentual increase in co-stimulatory molecules, that is, of CD28, B7-1, and B7-2 positive cells in the eye biopsies of patients with ocular cicatricial pemphigoid (OCP), a chronic autoimmune disease affecting mucosal areas, including ocular (which represent about 70% of cases) and skin manifestations [101]. Authors suggested that these molecules contribute to the sustained immune activation in the OCP conjunctiva.
Intervention with anti-B7 antibodies was tested in EAU B10.A mice and led to disease remission [102]. Data were confirmed in an elegant study showing the efficacy of a combined anti-B7-1 and B7-2 treatment. Authors complemented these experiments evaluating the protection against EAU in B10.A mice in vivo with anti-CD28 antibodies. However, although these approaches were able to control effector immune responses they failed to reverse disease when mice were challenged with IRBP [103]. In another study, CTLA-4-Ig significantly decreased the average score when compared to saline-treated animals in an EAU model [104]. Additionally, Abatacept was shown to abrogate eye inflammation in cases of JIA-associated uveitis [105,106]. Taken together, these findings support promising results with CTLA-4Ig in the treatment of ocular autoimmune disorders.
4.4. Blockade of other co-stimulatory pathways
Blockade with other co-stimulatory molecules such as ICOS and PD-1 have also been essayed, with variable results.
PD-1 is a receptor broadly expressed in T and B cells and is involved in limiting T cell responses, and therefore may have a role in autoimmunity scenarios [91,107]. As occurs with CTLA-4:B7 the blockade of PD-1:PD-L1 leads to an enhancement of T cell activity, and is being explored in cancer therapy [108]; the first trials in humans with the BMS-936558 antibody were recently concluded [91,108]. In contrast, the use of surrogate PD1 ligands was shown to induce immunosuppression in multiple sclerosis [109], autoimmune glomerulonephritis [110], and SLE mouse models [111]. However, no studies on ocular autoimmunity have been carried out up to this date.
ICOS is also a co-stimulatory molecule from the CD28 family, but does not bind strongly to either B7-1 or B7-2 [84]. Instead, the major ICOS ligand is B7RP-1 and its engagement enhances cell activation and stimulates the production of inflammatory cytokines [112]. Accordingly, ICOS:B7RP-1 blockade dampened autoimmunity in experimental autoimmune encephalitis [113], in a (NZBxNZW)F1 mouse model of SLE [114], and in EAU [115]. The current understanding of overall T cell-driven immune responses in the autoimmune setting, however, points to a minor role of these pathways, and CD28 blockade remains a major therapeutic target.
4.5. Blockade with anti-CD28 Fab′ fragment
A recurrent issue in all these studies is the overlapping profile of therapeutic antibodies like Abatacept that bind to both CD80 and CD86, interrupting the function not only of the pro-inflammatory pathogenic T cells, but also of the protective regulatory T cells. Furthermore, as CTLA-4 is crucial for downregulation of the same T cells activated via CD28 costimulation, the outcome of trials with these antibodies can be quite disappointing. Ideally, a molecule should be effective in blocking CD28-based signal transduction while bypassing CTLA-4.
Therefore, exploratory research on the CD28 molecule has attempted to generate more specific effects, avoiding interference with the closely related CTLA-4. As CTLA-4 is a major player in the downregulation of the immune response mediated by the subpopulation of regulatory T cells, a directed inactivation via CD28 of effector T cells could result in a concomitant enhancement of the Treg population. The increase in molecule specificity might also favor long-term effects and increased intervals between intakes of the therapeutic products leading to safer immunobiological compounds to intervene in chronic degenerative processes [116].
Effimune, a French Biotech Company is developing a novel molecule that targets CD28 [117–119]. FR104, a CD28 antagonist monovalent Fab′ antibody, has shown preclinical efficacy suppressing effector T cells while enhancing regulatory T cell function and immune tolerance in a humanized GVHD mice model [120]. There is evidence that FR104 does not act upon the inhibitory CTLA-4 and PDL-1 pathways, as evidenced by the increased frequency of CD4highCD127low FoxP3, i.e. regulatory T cells in the peripheral blood [120]. This novel humanized pegylated Fab′ antibody fragment presented a long half-life in monkeys and was proven immunologically safe. All these features make FR104 a good candidate for further preclinical and clinical investigations on different autoimmune conditions.
FR104 is currently under study by an European Community-sponsored multicentric group of researchers (project TRIAD — for more information see https://www.triad-cd28.eu/effimune.php, updated 12/11/2013), in which our team participates studying the effect on the mouse model of EAU on the B10.RIII background as described below.
B10.RIII mice were immunized subcutaneously with the IRBP 161-180 peptide emulsified in Complete Freund's Adjuvant, plus a B. pertussis toxin boost and were then treated with the murine FR104 analog mPEG-PV1Fab′ (PV1) after the onset of disease. Histopathology analysis showed that the general disease score was significantly lower in the PV1-treated group when compared with its untreated counterpart and an impact upon incidence of the disease was also observed in the PV1-treated group. This effect seemed to correlate with higher frequencies of naïve T lymphocytes infiltrating the eyes of the mice and lower frequencies of activated T cells (results not shown). Thus, treatment with PV1 might be modulating T cell activation and memory generation (Fig. 1). These preliminary results are highly encouraging and indicate that blockade of CD28 signaling in autoimmune uveitis with this novel immunobiological may become an additional therapeutical option.
Fig. 1.
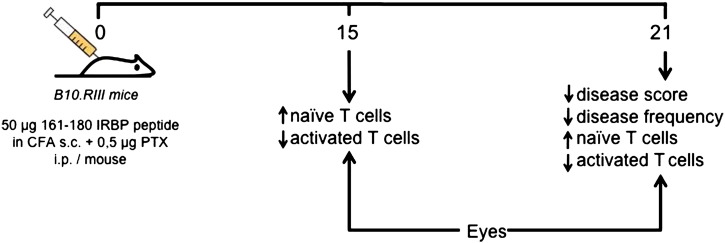
Summarizes the preliminary data from our group showing that in an autoimmune uveitis B10.RIII model, treatment with a murine analog of FR104 (Effimune) protects against severe disease when compared to untreated mice. Moreover, this effect seems to be associated with higher frequencies of naïve T cells infiltrating the eyes of the treated mice, suggesting that the CD28 blockade by this novel Fab′ ´CD28 antagonist prevents a full activation of T cells, rendering them in a naïve state. Furthermore, this effect appears to be associated with a dampened memory formation. Thus, the T cells from treated mice infiltrate the eyes but cannot induce a potent inflammatory response, restoring immune equilibrium in the eyes and protecting against disease progression.
5. Conclusion
There is great interest in the understanding of the mechanisms involved in the initiation and progression of autoimmune diseases, particularly in light of the need to uncover novel and more specific treatments targeted at the modulation of key molecules involved in disease pathogenesis. Additionally, individual approaches that take into account specific immunological profiles are ideal to improve therapeutic effectiveness and prevent systemic and non-related side effects associated with available immunosuppressive therapies.
The present review shows that current research on AIU is diversified, aiming at multiple aspects of disease pathogenesis. As T cells have a central participation in the development of uveitis, encompassing the recognition of retinal antigens to the genesis of immunological memory, intervention schemes focused on lymphocyte signaling pathways are encouraging. Among them, the more promising strategies are targeted at the IL-2 cytokine involved in disease progression and also at the CD28/B7 molecular pathway, bypassing the antagonistic CTLA-4 signal transduction.
Take-home messages
-
•
Autoimmune uveitis is a T-cell mediated disease caused by immune responses against ocular arrestin, interphotoreceptor retinoid binding protein and/or recoverin.
-
•
Experimental autoimmune uveitis in genetically susceptible mice exhibits focal lesions affecting retina and choroid, with vasculitis and granulomas, serous detachment of the retina and disorganization of the photoreceptor layer.
-
•
Research and clinical trials aim at the blockade of effector pathways or of their companion co-stimulatory molecules, such as CD3 T cell receptors and their costimulatory CD28, CTLA-4 receptors and corresponding ligands, and the IL-2 pathway.
-
•
FR104, a CD28 antagonist monovalent Fab′ antibody, has shown preclinical efficacy suppressing effector T cells while enhancing regulatory T cell function and immune tolerance in autoimmune disease models.
Acknowledgments
We are thankful for the funding support of the European project TRIAD (EU-FP7-Health program EC-GA N281493; www.triad-CD28.eu). LVR (303763/2010-8) and ACG (304900/2010-9) are recipients of a personal fellowship from CNPq.
References
- 1.Gritz D.C., Wong I.G. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111(3):491–500. doi: 10.1016/j.ophtha.2003.06.014. [discussion 500] [DOI] [PubMed] [Google Scholar]
- 2.Whitcup S.M., Nussenblatt R.B. Immunologic mechanisms of uveitis. New targets for immunomodulation. Arch Ophthalmol. 1997;115(4):520–525. doi: 10.1001/archopht.1997.01100150522013. [DOI] [PubMed] [Google Scholar]
- 3.Selmi C. Diagnosis and classification of autoimmune uveitis. Autoimmun Rev. 2014;13(4–5):591–594. doi: 10.1016/j.autrev.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Comarmond C.B., Wechsler B., Bodaghi B., Cacoub P., Saadoun D. Biotherapies in Behcet's disease. Autoimmun Rev. 2014;13(7):762–769. doi: 10.1016/j.autrev.2014.01.056. [DOI] [PubMed] [Google Scholar]
- 5.Selmi C., Gershwin M.E. Diagnosis and classification of reactive arthritis. Autoimmun Rev. 2014;13(4–5):546–549. doi: 10.1016/j.autrev.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Jamilloux Y.L., Kodjikian L., Broussolle C., Seve P. Sarcoidosis and uveitis. Autoimmun Rev. 2014;13(8):840–849. doi: 10.1016/j.autrev.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Sakata V.M., da Silva F.T., Hirata C.E., de Carvalho J.F., Yamamoto J.H. Diagnosis and classification of Vogt–Koyanagi–Harada disease. Autoimmun Rev. 2014;13(4–5):550–555. doi: 10.1016/j.autrev.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Greco A., Fusconi M., Gallo A., Turchetta R., Marinelli C., Macri G.F. Vogt–Koyanagi–Harada syndrome. Autoimmun Rev. 2013;12(11):1033–1038. doi: 10.1016/j.autrev.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Wacker W.B., Donoso L.A., Kalsow C.M., Yankeelov J.A., Jr., Organisciak D.T. Experimental allergic uveitis. Isolation, characterization, and localization of a soluble uveitopathogenic antigen from bovine retina. J Immunol. 1977;119(6):1949–1958. [PubMed] [Google Scholar]
- 10.Gery I., Wiggert B., Redmond T.M., Kuwabara T., Crawford M.A., Vistica B.P. Uveoretinitis and pinealitis induced by immunization with interphotoreceptor retinoid-binding protein. Invest Ophthalmol Vis Sci. 1986;27(8):1296–1300. [PubMed] [Google Scholar]
- 11.Roitt I.M. The role of autoantigens in the driving of autoimmune diseases. Immunol Ser. 1993;59:119–129. [PubMed] [Google Scholar]
- 12.Pennesi G., Mattapallil M.J., Sun S.H., Avichezer D., Silver P.B., Karabekian Z. A humanized model of experimental autoimmune uveitis in HLA class II transgenic mice. J Clin Invest. 2003;111(8):1171–1180. doi: 10.1172/JCI15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medawar P.B. Immunological tolerance. Nature. 1961;189:14–17. doi: 10.1038/189014a0. [DOI] [PubMed] [Google Scholar]
- 14.Caspi R.R. Ocular autoimmunity: the price of privilege? Immunol Rev. 2006;213:23–35. doi: 10.1111/j.1600-065X.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- 15.Ohta K., Wiggert B., Taylor A.W., Streilein J.W. Effects of experimental ocular inflammation on ocular immune privilege. Invest Ophthalmol Vis Sci. 1999;40(9):2010–2018. [PubMed] [Google Scholar]
- 16.Hamrah P., Dana M.R. Corneal antigen-presenting cells. Chem Immunol Allergy. 2007;92:58–70. doi: 10.1159/000099254. [DOI] [PubMed] [Google Scholar]
- 17.Forrester J.V., Xu H.., Kuffova L., Dick A.D., McMenamin P.G. Dendritic cell physiology and function in the eye. Immunol Rev. 2010;234(1):282–304. doi: 10.1111/j.0105-2896.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- 18.Griffith T.S., Ferguson T.A. The role of FasL-induced apoptosis in immune privilege. Immunol Today. 1997;18(5):240–244. doi: 10.1016/s0167-5699(97)81663-5. [DOI] [PubMed] [Google Scholar]
- 19.Usui Y., Okunuki Y., Hattori T., Kezuka T., Keino H., Ebihara N. Functional expression of B7H1 on retinal pigment epithelial cells. Exp Eye Res. 2008;86(1):52–59. doi: 10.1016/j.exer.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Sugita S., Keino H., Futagami Y., Takase H., Mochizuki M., Stein-Streilein J. B7 + iris pigment epithelial cells convert T cells into CTLA-4 +, B7-expressing CD8 + regulatory T cells. Invest Ophthalmol Vis Sci. 2006;47(12):5376–5384. doi: 10.1167/iovs.05-1354. [DOI] [PubMed] [Google Scholar]
- 21.Sugita S., Horie S., Nakamura O., Futagami Y., Takase H., Keino H. Retinal pigment epithelium-derived CTLA-2alpha induces TGFbeta-producing T regulatory cells. J Immunol. 2008;181(11):7525–7536. doi: 10.4049/jimmunol.181.11.7525. [DOI] [PubMed] [Google Scholar]
- 22.Zhou R., Caspi R.R. Ocular immune privilege. F1000 Biol Rep. 2010:2. doi: 10.3410/B2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forrester J.V., Xu H., Lambe T., Cornall R. Immune privilege or privileged immunity? Mucosal Immunol. 2008;1(5):372–381. doi: 10.1038/mi.2008.27. [DOI] [PubMed] [Google Scholar]
- 24.Taylor A., Shang F., Obin M. Relationships between stress, protein damage, nutrition, and age-related eye diseases. Mol Aspects Med. 1997;18(5):305–414. doi: 10.1016/s0098-2997(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida M., Kezuka T., Streilein J.W. Participation of pigment epithelium of iris and ciliary body in ocular immune privilege. 2. Generation of TGF-beta-producing regulatory T cells. Invest Ophthalmol Vis Sci. 2000;41(12):3862–3870. [PubMed] [Google Scholar]
- 26.Zamiri P., Sugita S., Streilein J.W. Immunosuppressive properties of the pigmented epithelial cells and the subretinal space. Chem Immunol Allergy. 2007;92:86–93. doi: 10.1159/000099259. [DOI] [PubMed] [Google Scholar]
- 27.Sugita S., Horie S., Yamada Y., Keino H., Usui Y., Takeuchi M. Suppression of bystander T helper 1 cells by iris pigment epithelium-inducing regulatory T cells via negative costimulatory signals. Invest Ophthalmol Vis Sci. 2010;51(5):2529–2536. doi: 10.1167/iovs.09-4460. [DOI] [PubMed] [Google Scholar]
- 28.DeVoss J., Hou Y., Johannes K., Lu W., Liou G.I., Rinn J. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203(12):2727–2735. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biscette O.M.F., H.F., Flynn T.E. Uveitis Diagnosis. Management, and Treatment Retinal Physician. 2007 [Google Scholar]
- 30.Rothova A., Buitenhuis H.J., Meenken C., Brinkman C.J., Linssen A., Alberts C. Uveitis and systemic disease. Br J Ophthalmol. 1992;76(3):137–141. doi: 10.1136/bjo.76.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heo J., Sepah Y.J., Yohannan J., Renner M., Akhtar A., Gregory A. The role of biologic agents in the management of non-infectious uveitis. Expert Opin Biol Ther. 2012;12(8):995–1008. doi: 10.1517/14712598.2012.688021. [DOI] [PubMed] [Google Scholar]
- 32.Smith J.A., Thompson D.J., Whitcup S.M., Suhler E., Clarke G., Smith S. A randomized, placebo-controlled, double-masked clinical trial of etanercept for the treatment of uveitis associated with juvenile idiopathic arthritis. Arthritis Rheum. 2005;53(1):18–23. doi: 10.1002/art.20904. [DOI] [PubMed] [Google Scholar]
- 33.Foster C.S., Tufail F., Waheed N.K., Chu D., Miserocchi E., Baltatzis S. Efficacy of etanercept in preventing relapse of uveitis controlled by methotrexate. Arch Ophthalmol. 2003;121(4):437–440. doi: 10.1001/archopht.121.4.437. [DOI] [PubMed] [Google Scholar]
- 34.Smith J.R., Levinson R.D., Holland G.N., Jabs D.A., Robinson M.R., Whitcup S.M. Differential efficacy of tumor necrosis factor inhibition in the management of inflammatory eye disease and associated rheumatic disease. Arthritis Rheum. 2001;45(3):252–257. doi: 10.1002/1529-0131(200106)45:3<252::AID-ART257>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 35.Caspi R.R. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120(9):3073–3083. doi: 10.1172/JCI42440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gasparin F., Takahashi B.S., Scolari M.R., Gasparin F., Pedral L.S., Damico F.M. Experimental models of autoimmune inflammatory ocular diseases. Arq Bras Oftalmol. 2012;75(2):143–147. doi: 10.1590/s0004-27492012000200016. [DOI] [PubMed] [Google Scholar]
- 37.Caspi R.R., Roberge F.G., Chan C.C., Wiggert B., Chader G.J., Rozenszajn L.A. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol. 1988;140(5):1490–1495. [PubMed] [Google Scholar]
- 38.Wildner G., Kaufmann U. What causes relapses of autoimmune diseases? The etiological role of autoreactive T cells. Autoimmun Rev. 2013;12(11):1070–1075. doi: 10.1016/j.autrev.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Sun B., Rizzo L.V., Sun S.H., Chan C.C., Wiggert B., Wilder R.L. Genetic susceptibility to experimental autoimmune uveitis involves more than a predisposition to generate a T helper-1-like or a T helper-2-like response. J Immunol. 1997;159(2):1004–1011. [PubMed] [Google Scholar]
- 40.Rizzo L.V., Silver P., Wiggert B., Hakim F., Gazzinelli R.T., Chan C.C. Establishment and characterization of a murine CD4 + T cell line and clone that induce experimental autoimmune uveoretinitis in B10.A mice. J Immunol. 1996;156(4):1654–1660. [PubMed] [Google Scholar]
- 41.Jones L.S., Rizzo L.V., Agarwal R.K., Tarrant T.K., Chan C.C., Wiggert B. IFN-gamma-deficient mice develop experimental autoimmune uveitis in the context of a deviant effector response. J Immunol. 1997;158(12):5997–6005. [PubMed] [Google Scholar]
- 42.Tang J., Zhu W., Silver P.B., Su S.B., Chan C.C., Caspi R.R. Autoimmune uveitis elicited with antigen-pulsed dendritic cells has a distinct clinical signature and is driven by unique effector mechanisms: initial encounter with autoantigen defines disease phenotype. J Immunol. 2007;178(9):5578–5587. doi: 10.4049/jimmunol.178.9.5578. [DOI] [PubMed] [Google Scholar]
- 43.Amadi-Obi A., Yu C.R., Liu X., Mahdi R.M., Clarke G.L., Nussenblatt R.B. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13(6):711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 44.Luger D., Silver P.B., Tang J., Cua D., Chen Z., Iwakura Y. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205(4):799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vallochi A.L., Commodaro A.G., Schwartzman J.P., Belfort R., Jr, Rizzo L.V. The role of cytokines in the regulation of ocular autoimmune inflammation. Cytokine Growth Factor Rev. 2007;18(1–2):135–141. doi: 10.1016/j.cytogfr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 46.Horai R., Silver P.B., Chen J., Agarwal R.K., Chong W.P., Jittayasothorn Y. Breakdown of immune privilege and spontaneous autoimmunity in mice expressing a transgenic T cell receptor specific for a retinal autoantigen. J Autoimmun. 2013;44:21–33. doi: 10.1016/j.jaut.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen J., Qian H., Horai R., Chan C.C., Falick Y., Caspi R.R. Comparative analysis of induced vs. spontaneous models of autoimmune uveitis targeting the interphotoreceptor retinoid binding protein. PLoS One. 2013;8(8):e72161. doi: 10.1371/journal.pone.0072161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caspi R.R. Immunogenetic aspects of clinical and experimental uveitis. Reg Immunol. 1992;4(5):321–330. [PubMed] [Google Scholar]
- 49.Davey M.P., Rosenbaum J.T. The human leukocyte antigen complex and chronic ocular inflammatory disorders. Am J Ophthalmol. 2000;129(2):235–243. doi: 10.1016/s0002-9394(99)00433-x. [DOI] [PubMed] [Google Scholar]
- 50.Levinson R.D. Immunogenetics of ocular inflammatory disease. Tissue Antigens. 2007;69(2):105–112. doi: 10.1111/j.1399-0039.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 51.de Smet M.D., Yamamoto J.H., Mochizuki M., Gery I., Singh V.K., Shinohara T. Cellular immune responses of patients with uveitis to retinal antigens and their fragments. Am J Ophthalmol. 1990;110(2):135–142. doi: 10.1016/s0002-9394(14)76981-8. [DOI] [PubMed] [Google Scholar]
- 52.Szpak Y., Vieville J.C., Tabary T., Naud M.C., Chopin M., Edelson C. Spontaneous retinopathy in HLA-A29 transgenic mice. Proc Natl Acad Sci U S A. 2001;98(5):2572–2576. doi: 10.1073/pnas.051595998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenbaum J.T., McDevitt H.O., Guss R.B., Egbert P.R. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286(5773):611–613. doi: 10.1038/286611a0. [DOI] [PubMed] [Google Scholar]
- 54.Broekhuyse R.M., Kuhlmann E.D., Winkens H.J. Experimental autoimmune anterior uveitis (EAAU). II. Dose-dependent induction and adoptive transfer using a melanin-bound antigen of the retinal pigment epithelium. Exp Eye Res. 1992;55(3):401–411. doi: 10.1016/0014-4835(92)90112-6. [DOI] [PubMed] [Google Scholar]
- 55.Bora N.S., Kim M.C., Kabeer N.H., Simpson S.C., Tandhasetti M.T., Cirrito T.P. Experimental autoimmune anterior uveitis. Induction with melanin-associated antigen from the iris and ciliary body. Invest Ophthalmol Vis Sci. 1995;36(6):1056–1066. [PubMed] [Google Scholar]
- 56.Matteson D.M., Shen D.F., Chan C.C. Inhibition of experimental melanin protein-induced uveitis (EMIU) by targeting nitric oxide via phosphatidylcholine-specific phospholipase C. J Autoimmun. 1999;13(2):197–204. doi: 10.1006/jaut.1999.0319. [DOI] [PubMed] [Google Scholar]
- 57.Rothlin C.V., Ghosh S., Zuniga E.I., Oldstone M.B., Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131(6):1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 58.Lu Q., Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293(5528):306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 59.Ye F., Li Q., Ke Y., Lu Q., Han L., Kaplan H.J. TAM receptor knockout mice are susceptible to retinal autoimmune induction. Invest Ophthalmol Vis Sci. 2011;52(7):4239–4246. doi: 10.1167/iovs.10-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saadoun D., Bodaghi B., Bienvenu B., Wechsler B., Sene D., Trad S. Biotherapies in inflammatory ocular disorders: interferons, immunoglobulins, monoclonal antibodies. Autoimmun Rev. 2013;12(7):774–783. doi: 10.1016/j.autrev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Hoyer K.K., Dooms H., Barron L., Abbas A.K. Interleukin-2 in the development and control of inflammatory disease. Immunol Rev. 2008;226:19–28. doi: 10.1111/j.1600-065X.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- 62.Banchereau J., Pascual V., O'Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol. 2012;13(10):925–931. doi: 10.1038/ni.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chan C.C., Nussenblatt R.B., Wiggert B., Redmond T.M., Fujikawa L.S., Chader G.J. Immunohistochemical analysis of experimental autoimmune uveoretinitis (EAU) induced by interphotoreceptor retinoid-binding protein (IRBP) in the rat. Immunol Invest. 1987;16(1):63–74. doi: 10.3109/08820138709055713. [DOI] [PubMed] [Google Scholar]
- 64.Roberge F.G., Lorberboum-Galski H., Le Hoang P., de Smet M., Chan C.C., Fitzgerald D. Selective immunosuppression of activated T cells with the chimeric toxin IL-2-PE40. Inhibition of experimental autoimmune uveoretinitis. J Immunol. 1989;143(11):3498–3502. [PubMed] [Google Scholar]
- 65.Higuchi M., Diamantstein T., Osawa H., Caspi R.R. Combined anti-interleukin-2 receptor and low-dose cyclosporine therapy in experimental autoimmune uveoretinitis. J Autoimmun. 1991;4(1):113–124. doi: 10.1016/0896-8411(91)90011-z. [DOI] [PubMed] [Google Scholar]
- 66.Guex-Crosier Y., Raber J., Chan C.C., Kriete M.S., Benichou J., Pilson R.S. Humanized antibodies against the alpha-chain of the IL-2 receptor and against the beta-chain shared by the IL-2 and IL-15 receptors in a monkey uveitis model of autoimmune diseases. J Immunol. 1997;158(1):452–458. [PubMed] [Google Scholar]
- 67.Nussenblatt R.B., Fortin E., Schiffman R., Rizzo L., Smith J., Van Veldhuisen P. Treatment of noninfectious intermediate and posterior uveitis with the humanized anti-Tac mAb: a phase I/II clinical trial. Proc Natl Acad Sci U S A. 1999;96(13):7462–7466. doi: 10.1073/pnas.96.13.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nussenblatt R.B., Thompson D.J., Li Z., Chan C.C., Peterson J.S., Robinson R.R. Humanized anti-interleukin-2 (IL-2) receptor alpha therapy: long-term results in uveitis patients and preliminary safety and activity data for establishing parameters for subcutaneous administration. J Autoimmun. 2003;21(3):283–293. doi: 10.1016/s0896-8411(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 69.Papaliodis G.N., Chu D., Foster C.S. Treatment of ocular inflammatory disorders with daclizumab. Ophthalmology. 2003;110(4):786–789. doi: 10.1016/S0161-6420(02)01932-2. [DOI] [PubMed] [Google Scholar]
- 70.Yeh S., Wroblewski K., Buggage R., Li Z., Kurup S.K., Sen H.N. High-dose humanized anti-IL-2 receptor alpha antibody (daclizumab) for the treatment of active, non-infectious uveitis. J Autoimmun. 2008;31(2):91–97. doi: 10.1016/j.jaut.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kung P., Goldstein G., Reinherz E.L., Schlossman S.F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- 72.Chatenoud L. CD3-specific antibody-induced active tolerance: from bench to bedside. Nat Rev Immunol. 2003;3(2):123–132. doi: 10.1038/nri1000. [DOI] [PubMed] [Google Scholar]
- 73.Ochi H., Abraham M., Ishikawa H., Frenkel D., Yang K., Basso A.S. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4 + CD25 − LAP + T cells. Nat Med. 2006;12(6):627–635. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- 74.Wu H.Y., Quintana F.J., Weiner H.L. Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4 + CD25 − LAP + regulatory T cell and is associated with down-regulation of IL-17 + CD4 + ICOS + CXCR5 + follicular helper T cells. J Immunol. 2008;181(9):6038–6050. doi: 10.4049/jimmunol.181.9.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herold K.C., Bluestone J.A., Montag A.G., Parihar A., Wiegner A., Gress R.E. Prevention of autoimmune diabetes with nonactivating anti-CD3 monoclonal antibody. Diabetes. 1992;41(3):385–391. doi: 10.2337/diab.41.3.385. [DOI] [PubMed] [Google Scholar]
- 76.Chatenoud L., Thervet E., Primo J., Bach J.F. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A. 1994;91(1):123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herold K.C. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54(6):1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Utset T.O. Modified anti-CD3 therapy in psoriatic arthritis: a phase I/II clinical trial. J Rheumatol. 2002;29(9):1907–1913. [PubMed] [Google Scholar]
- 79.Ke Y. Anti-CD3 antibody ameliorates experimental autoimmune uveitis by inducing both IL-10 and TGF-beta dependent regulatory T cells. Clin Immunol. 2011;138(3):311–320. doi: 10.1016/j.clim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dean Y., Depis F., Kosco-Vilbois M. Combination therapies in the context of anti-CD3 antibodies for the treatment of autoimmune diseases. Swiss Med Wkly. 2012;142:w13711. doi: 10.4414/smw.2012.13711. [DOI] [PubMed] [Google Scholar]
- 81.Phillips B., Trucco M., Giannoukakis N. Current state of type 1 diabetes immunotherapy: incremental advances, huge leaps, or more of the same? Clin Dev Immunol. 2011;2011:432016. doi: 10.1155/2011/432016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gross J.A., Callas E., Allison J.P. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992;149(2):380–388. [PubMed] [Google Scholar]
- 83.Greenwald R.J. CTLA-4 regulates cell cycle progression during a primary immune response. Eur J Immunol. 2002;32(2):366–373. doi: 10.1002/1521-4141(200202)32:2<366::AID-IMMU366>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 84.Yao S. B7-h2 is a costimulatory ligand for CD28 in human. Immunity. 2011;34(5):729–740. doi: 10.1016/j.immuni.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Greenfield E.A., Nguyen K.A., Kuchroo V.K. CD28/B7 costimulation: a review. Crit Rev Immunol. 1998;18(5):389–418. doi: 10.1615/critrevimmunol.v18.i5.10. [DOI] [PubMed] [Google Scholar]
- 86.Stebbings R. “Cytokine storm” in the phase I trial of monoclonal antibody TGN1412: better understanding the causes to improve preclinical testing of immunotherapeutics. J Immunol. 2007;179(5):3325–3331. doi: 10.4049/jimmunol.179.5.3325. [DOI] [PubMed] [Google Scholar]
- 87.St Clair E.W. The calm after the cytokine storm: lessons from the TGN1412 trial. J Clin Invest. 2008;118(4):1344–1347. doi: 10.1172/JCI35382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guinan E.C. Pivotal role of the B7:CD28 pathway in transplantation tolerance and tumor immunity. Blood. 1994;84(10):3261–3282. [PubMed] [Google Scholar]
- 89.Takahashi T. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192(2):303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stohl W. Global T cell dysregulation in non-autoimmune-prone mice promotes rapid development of BAFF-independent, systemic lupus erythematosus-like autoimmunity. J Immunol. 2008;181(1):833–841. doi: 10.4049/jimmunol.181.1.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramsay A.G. Immune checkpoint blockade immunotherapy to activate anti-tumour T-cell immunity. Br J Haematol. 2013;162(3):313–325. doi: 10.1111/bjh.12380. [DOI] [PubMed] [Google Scholar]
- 93.Romo-Tena J., Gomez-Martin D., Alcocer-Varela J. CTLA-4 and autoimmunity: new insights into the dual regulator of tolerance. Autoimmun Rev. 2013;12(12):1171–1176. doi: 10.1016/j.autrev.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 94.Kremer J.M. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349(20):1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 95.Rosman Z., Shoenfeld Y., Zandman-Goddard G. Biologic therapy for autoimmune diseases: an update. BMC Med. 2013;11:88. doi: 10.1186/1741-7015-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bluestone J.A., St Clair E.W., Turka L.A. CTLA4Ig: bridging the basic immunology with clinical application. Immunity. 2006;24(3):233–238. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 97.Salomon B., Bluestone J.A. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 98.Moreland L.W. Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose-finding, double-blind, placebo-controlled clinical trial evaluating CTLA-4Ig and LEA29Y eighty-five days after the first infusion. Arthritis Rheum. 2002;46(6):1470–1479. doi: 10.1002/art.10294. [DOI] [PubMed] [Google Scholar]
- 99.Larsen C.P. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5(3):443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 100.Tesavibul N. Costimulatory molecules in ocular cicatricial pemphigoid. Invest Ophthalmol Vis Sci. 1998;39(6):982–988. [PubMed] [Google Scholar]
- 101.Hardy K.M. Benign mucous membrane pemphigoid. Arch Dermatol. 1971;104(5):467–475. [PubMed] [Google Scholar]
- 102.Fukai T. The role of costimulatory molecules B7-1 and B7-2 in mice with experimental autoimmune uveoretinitis. Graefes Arch Clin Exp Ophthalmol. 1999;237(11):928–933. doi: 10.1007/s004170050388. [DOI] [PubMed] [Google Scholar]
- 103.Silver P.B. Blockade of costimulation through B7/CD28 inhibits experimental autoimmune uveoretinitis, but does not induce long-term tolerance. J Immunol. 2000;165(9):5041–5047. doi: 10.4049/jimmunol.165.9.5041. [DOI] [PubMed] [Google Scholar]
- 104.Verwaerde C. Ocular transfer of retinal glial cells transduced ex vivo with adenovirus expressing viral IL-10 or CTLA4-Ig inhibits experimental autoimmune uveoretinitis. Gene Ther. 2003;10(23):1970–1981. doi: 10.1038/sj.gt.3302101. [DOI] [PubMed] [Google Scholar]
- 105.Zulian F. Abatacept for severe anti-tumor necrosis factor alpha refractory juvenile idiopathic arthritis-related uveitis. Arthritis Care Res (Hoboken) 2010;62(6):821–825. doi: 10.1002/acr.20115. [DOI] [PubMed] [Google Scholar]
- 106.Kenawy N. Abatacept: a potential therapy in refractory cases of juvenile idiopathic arthritis-associated uveitis. Graefes Arch Clin Exp Ophthalmol. 2011;249(2):297–300. doi: 10.1007/s00417-010-1523-6. [DOI] [PubMed] [Google Scholar]
- 107.Wu Y.L. Immunotherapies: the blockade of inhibitory signals. Int J Biol Sci. 2012;8(10):1420–1430. doi: 10.7150/ijbs.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lipson E.J. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19(2):462–468. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ortler S. B7-H1 restricts neuroantigen-specific T cell responses and confines inflammatory CNS damage: implications for the lesion pathogenesis of multiple sclerosis. Eur J Immunol. 2008;38(6):1734–1744. doi: 10.1002/eji.200738071. [DOI] [PubMed] [Google Scholar]
- 110.Reynolds J. Stimulation of the PD-1/PDL-1 T-cell co-inhibitory pathway is effective in treatment of experimental autoimmune glomerulonephritis. Nephrol Dial Transplant. 2012;27(4):1343–1350. doi: 10.1093/ndt/gfr529. [DOI] [PubMed] [Google Scholar]
- 111.Wong M., La Cava A., Hahn B.H. Blockade of programmed death-1 in young (New Zealand Black × New Zealand White)F1 mice promotes the suppressive capacity of CD4 + regulatory T cells protecting from lupus-like disease. J Immunol. 2013;190(11):5402–5410. doi: 10.4049/jimmunol.1202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sporici R.A., Perrin P.J. Costimulation of memory T-cells by ICOS: a potential therapeutic target for autoimmunity? Clin Immunol. 2001;100(3):263–269. doi: 10.1006/clim.2001.5093. [DOI] [PubMed] [Google Scholar]
- 113.Rottman J.B. The costimulatory molecule ICOS plays an important role in the immunopathogenesis of EAE. Nat Immunol. 2001;2(7):605–611. doi: 10.1038/89750. [DOI] [PubMed] [Google Scholar]
- 114.Hu Y.L. B7RP-1 blockade ameliorates autoimmunity through regulation of follicular helper T cells. J Immunol. 2009;182(3):1421–1428. doi: 10.4049/jimmunol.182.3.1421. [DOI] [PubMed] [Google Scholar]
- 115.Usui Y. The role of the ICOS/B7RP-1 T cell costimulatory pathway in murine experimental autoimmune uveoretinitis. Eur J Immunol. 2006;36(11):3071–3081. doi: 10.1002/eji.200636138. [DOI] [PubMed] [Google Scholar]
- 116.Hunig T. Manipulation of regulatory T-cell number and function with CD28-specific monoclonal antibodies. Adv Immunol. 2007;95:111–148. doi: 10.1016/S0065-2776(07)95004-X. [DOI] [PubMed] [Google Scholar]
- 117.Poirier N. Inducing CTLA-4-dependent immune regulation by selective CD28 blockade promotes regulatory T cells in organ transplantation. Sci Transl Med. 2010;2(17):17ra10. doi: 10.1126/scitranslmed.3000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Poirier N. Preclinical efficacy and immunological safety of FR104, an antagonist anti-CD28 monovalent Fab′ antibody. Am J Transplant. 2012;12(10):2630–2640. doi: 10.1111/j.1600-6143.2012.04164.x. [DOI] [PubMed] [Google Scholar]
- 119.Poirier N., Blancho G., Vanhove B. A more selective costimulatory blockade of the CD28-B7 pathway. Transpl Int. 2011;24(1):2–11. doi: 10.1111/j.1432-2277.2010.01176.x. [DOI] [PubMed] [Google Scholar]
- 120.Poirier N., Blancho G., Vanhove B. CD28-specific immunomodulating antibodies: what can be learned from experimental models? Am J Transplant. 2012;12(7):1682–1690. doi: 10.1111/j.1600-6143.2012.04032.x. [DOI] [PubMed] [Google Scholar]


