Abstract
Background
Recent studies have raised the possibility of adverse effects of low sodium, particularly less than 2300 mg/24hr, on cardiovascular disease (CVD). However, these paradoxical findings might have resulted from suboptimal measurement of sodium and potential biases related to indication or reverse causation.
Methods and Results
Phases I and II of the Trials of Hypertension Prevention (TOHP) collected multiple 24-hour urine specimens among pre-hypertensive individuals. During extended post-trial surveillance, 193 cardiovascular events or CVD deaths occurred among 2275 participants not in a sodium reduction intervention with 10 (TOHP II) or 15 (TOHP I) years of post-trial follow-up. Median sodium excretion was 3630 mg/24hr, with 1.4% of the participants having intake <1500 mg/24hr and 10% <2300 mg/24hr, consistent with national levels. Compared to those with sodium excretion of 3600 to <4800 mg/24hr, risk for those with sodium <2300 mg/24hr was 32% lower after multivariable adjustment (HR=0.68, 95%CI = 0.34–1.37, p for trend = 0.13). There was a linear 17% increase in risk per 1000 mg/24hr (p=0.05). Spline curves supported a linear association of sodium with cardiovascular events, continuing to descend from 3600 to 2300 and 1500 mg/24hr, although the data were sparse at the lowest levels. Controlling for creatinine levels had little effect on these results.
Conclusions
Results from the TOHP studies, which overcome the major methodological challenges of prior studies, are consistent with overall health benefits of reducing sodium intake to the 1500 to 2300 mg/day range in the majority of the population, in agreement with current dietary guidelines.
Keywords: sodium, salt intake, cardiovascular disease prevention, nutrition, diet
The Institute of Medicine (IOM) recently convened a committee to review the effects of sodium intake on health outcomes other than blood pressure, focusing on intake from 1500 to 2300 mg/24hr.1 While the final report supported population-wide efforts to lower sodium, it concluded that there were insufficient data to support a lowering of sodium to <1500 mg/24hr, as recommended by the American Heart Association,2,3 or to <2000 mg/24hr, as recommended by the World Health Organization.4 The 2010 Dietary Guidelines for Americans5 recommend lowering sodium to <1500 mg/24hr for a majority of adults and to <2300 mg/24hr for all others. Several observational studies6 as well as randomized trials7,8 have examined the associations of sodium and subsequent morbidity and mortality, and generally suggest a lowering of risk with lower sodium. However, few studies have examined absolute levels of sodium intake down to those considered in the IOM report.
Some recent studies have raised the possibility of adverse effects of very low sodium intake on cardiovascular disease (CVD). Data from the FLEMENGHO and EPOGH cohorts found a higher level of CVD mortality among those in the lowest tertile of sodium.9 In addition, data from patients with CVD or diabetes suggested a J-shaped curve, with an increase in risk of CVD and of mortality at both the upper and lower levels of sodium intake.10,11 The IOM concluded, however, that these data were limited and their quality was insufficient to support the assertion of an adverse effect on CVD at very low levels of sodium intake. All of the studies reporting a paradoxical inverse or J-shaped association between sodium intake and CVD were based on secondary analyses of studies that were not designed to assess this relationship. Major concerns included suboptimal measurement of sodium and the potential for bias due to indication or reverse causality. Most studies with urinary sodium excretion collected only a spot or single 24-hour urine specimen or included a high percent of participants with a pre-existing illness.
Phases I and II of the Trials of Hypertension Prevention (TOHP) collected multiple 24-hour urine specimens over periods of either 18 months12 or 3–4 years.13 To represent long-term usual intake of sodium, these measures were averaged among those not on an active sodium intervention. In extended follow-up, a linear association of the sodium-potassium ratio with CVD was identified.14 Sodium also showed a linear association, of borderline significance, with CVD. The current report provides more detail on the relationship of sodium to CVD, particularly at the low absolute sodium intake levels considered by the IOM. It also provides more detail regarding the quality of the 24-hour urine collections, particularly the relationship of sodium and creatinine, and the impact of the latter on the association of sodium with CVD.
Methods
TOHP Trials
The TOHP Follow-up Study was an observational follow-up of TOHP, Phases I and II, and has been described previously.14 TOHP I took place from September 1987 to January 1990, and evaluated the effects of four supplement and three lifestyle interventions, including weight loss and sodium reduction interventions, on blood pressure in 2,182 men and women aged 30–54 years with high normal blood pressure.12 Those in the active sodium reduction intervention were excluded from the current analyses, leaving 1855 eligible TOHP I participants. In TOHP II, which took place from December 1990 to March 1995, the effects of sodium reduction and weight loss on blood pressure were tested over a longer 3–4 year follow-up period in a two-by-two factorial design among 2,382 pre-hypertensive men and women age 30–54.13 Eligible participants had a body mass index (BMI [calculated as weight in kilograms divided by height in meters squared]) representing 110% to 165% of desirable body weight. Those in the active sodium reduction group were excluded from the current analyses, leaving 1191 eligible TOHP II participants.
During the trial periods, three to seven 24-hour urine collections were scheduled over 18 months in TOHP I and over three to four years in TOHP II. Usual intake of sodium or potassium or their ratio was calculated as the mean of available urinary excretion measures at 5 (lifestyle interventions) or 7 (nutritional supplement interventions) scheduled collections during 18 months in TOHP I and at 3 or up to 5 scheduled collections during 3 years in TOHP II. Mean sodium and potassium excretions, representing usual intake, were computed over all collections among pre-hypertensive individuals not in the sodium reduction intervention. All of the urinary sodium and potassium measures were expressed as mg/24hr. To explore whether the relationships could be affected by under-collection, measures of creatinine were computed over the same collections, along with the creatinine-to-weight ratio (Cr/Wt), the sodium-to-creatinine ratio (Na/Cr), the potassium/creatinine ratio (K/Cr), and the sodium/potassium ratio (Na/K).
TOHP Follow-up
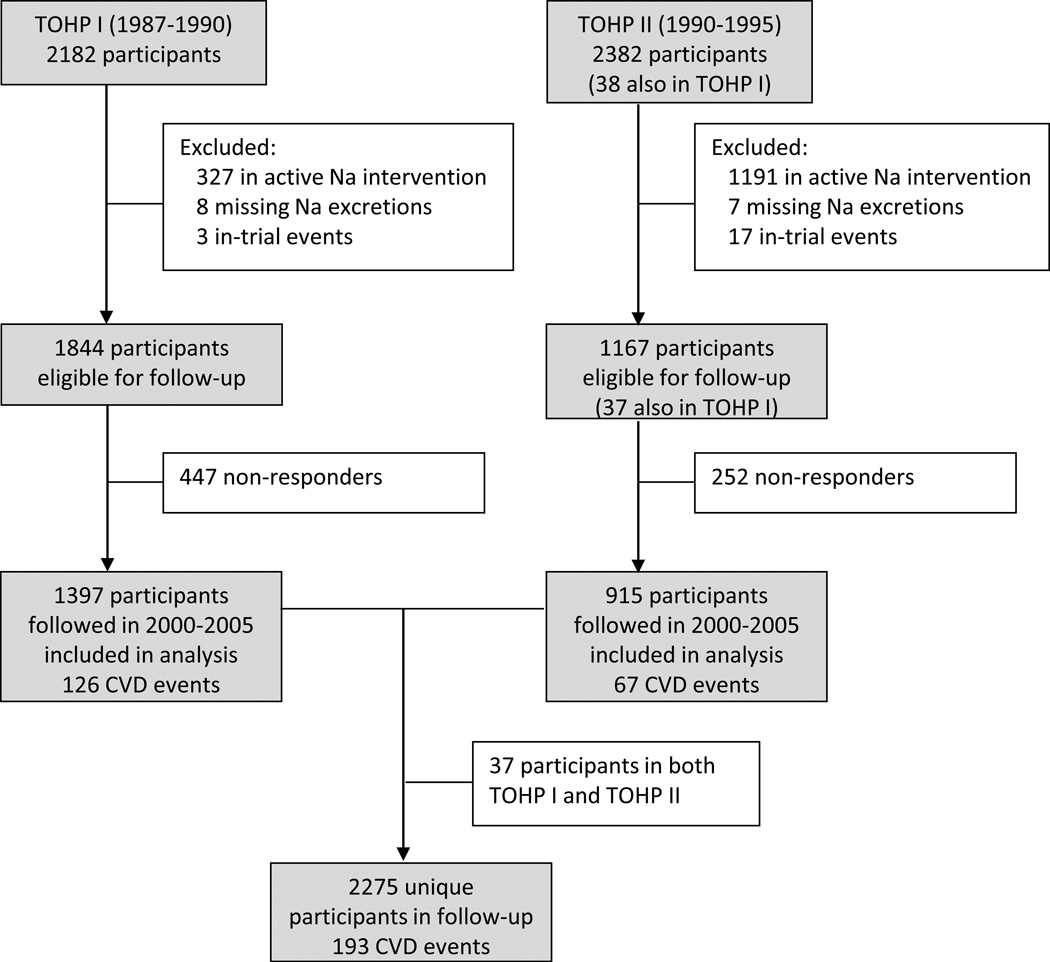
The observational follow-up for CVD began in 2000, approximately 10 years after the end of TOHP I and 5 years after the end of TOHP II, and included a total of 4,526 TOHP I and II participants (with 38 participating in both TOHP I and TOHP II) (Figure 1). Of these, 2,974 were not in a sodium reduction intervention, had available sodium excretions, and remained alive and CVD-free at the end of the trial periods. The follow-up was conducted centrally by mail and phone from the TOHP coordinating center at the Brigham and Women’s Hospital, Boston, Massachusetts, and was approved by institutional review boards there and at the participating clinic centers. Follow-up questionnaires focused on collection of CVD endpoints that had occurred following the conclusion of the trials. Additional questionnaires were sent at two-year intervals, up through early 2005, with interim annual postcards to collect information on address changes and new study endpoints.
Figure 1.
Flow diagram for TOHP follow-up.
The primary endpoint for the follow-up study was CVD or CVD death, including myocardial infarction (MI), stroke, coronary artery bypass graft (CABG), percutaneous transluminal coronary angioplasty (PTCA), or death from cardiovascular disease. Upon notification of occurrence of a primary nonfatal endpoint, consent was sought to obtain medical records. These were reviewed by a study physician, blinded to the participant’s intervention assignment, to confirm the reported events. Disconfirmed endpoints were excluded from the subsequent analyses. An NDI search was performed in the fall of 2001 and summer of 2005 to ascertain deaths from trial termination through December 2003 among non-respondents to the questionnaires. End point information was obtained for 2275 participants (2312 including duplicates in both trial phases), for an overall response rate of 76.5%. Response was slightly higher among TOHP II participants (78.4%) compared to their counterparts in TOHP I (75.8%), but did not differ by sodium quartile either crudely (p=0.39), or in fully adjusted logistic regression analyses (p=0.96) (Supplemental Table 1).
Statistical analysis
As described above, sodium levels were averaged across all collections during the course of the trial periods in those not in an active sodium intervention who remained alive and CVD-free at the end of the trial periods. Absolute levels were grouped into categories defined as <2300, 2300 to <3600, 3600 to <4800, and 4800 mg/24hr or higher, where 2300 represents current guideline recommendations for adults,5 and 3600 represents the median sodium intake in the US population aged 31–50.15 Baseline characteristics were expressed as percents or means, and were tested for trend over sodium categories using chi-square statistics or regression analysis.
Cox regression models estimated the hazard ratio (HR) for the linear effect of sodium and for the defined above. The baseline hazard was stratified by trial and those who were in both phases contributed time to the end of TOHP I until entry into TOHP II as well as any follow-up from the end of TOHP II. Models were adjusted for clinic, age, sex, race/ethnicity, and other treatment assignments (model 1), additionally for education, baseline weight, alcohol use, smoking, exercise, potassium excretion, and family history of cardiovascular disease (model 2), and also for changes in weight, smoking, and exercise during the trial periods (model 3), as pre-specified.14 Penalized splines with four degrees of freedom were fit using SPlus to examine linearity of effect;16 introducing more flexibility did not alter the relation in the lower range of sodium.
We also conducted several sensitivity analyses controlling for Cr/Wt in the multivariable models to adjust for potential inadequacy of the urine collections. First, we estimated the within-person variability of measures of creatinine (mg/24hr) divided by weight in kilograms (Cr/Wt) using mixed effects models. The coefficient of variation (CV) was computed for each participant. We then conducted analyses that excluded those with CV’s of 20% or higher and of 30% or higher. In addition, we ran models controlling for Cr/Wt or the CV of Cr/Wt in the model.17,18 Finally, we ran models including sodium, potassium, and Cr/Wt in the same models, as well as Na/Cr, K/Cr, and Cr/Wt. These were expressed per standard deviation (SD) for easier comparison of effect sizes. All analyses were conducted in SAS version 9.2, except as noted.
Results
Baseline levels
Sodium and other excretion data were averaged across all measures during the trial periods, with a median of 5 measures (range, 1–7). Median sodium excretion among the participants with follow-up data was 3630 mg/24hr, with 1.4% of the participants <1500 and 10% <2300 mg/24hr, consistent with national levels.15 Levels were higher in men, with medians of 3934 mg/24hr in men and of 3078 mg/24hr in women. Those in TOHP II, all with a BMI exceeding ideal body weight, had higher levels than participants in TOHP I, with medians of 3666 and 4292 mg/24hr in TOHP I and II, respectively, in men and of 2869 and 3340 mg/24hr, respectively, in women.
Characteristics of participants by absolute level of sodium excretion are provided in Table 1. Those with the lowest sodium levels were more likely to be college educated, more likely to drink alcohol and to exercise at least once/week, although these relationships were less consistent in women. Weight was directly correlated with sodium intake, though change in weight during the trial periods was not. Blood pressure levels, which were restricted during enrollment due to eligibility criteria, were not correlated with sodium levels.
Table 1.
Baseline characteristics among participants with follow-up information in the Trials of Hypertension Prevention by categories of urinary sodium excretion (mg/24hr).
| TOHP I (N=1397) | TOHP II (N=915) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sodium Excretion (mg/24hr) | P for Trend |
Sodium Excretion (mg/24hr) | P for Trend |
|||||||
| <2300 | 2300– <3600 |
3600– <4800 |
≥ 4800 | <2300 | 2300– <3600 |
3600– <4800 |
≥ 4800 | |||
| MEN | ||||||||||
| N | 88 | 382 | 354 | 168 | 18 | 142 | 252 | 202 | ||
| Age (yrs) | 42.1 | 42.8 | 43.3 | 42.7 | 0.42 | 42.7 | 43.6 | 43.6 | 42.5 | 0.17 |
| Black (%) | 14.8 | 6.5 | 8.8 | 7.7 | 0.44 | 11.1 | 10.6 | 9.5 | 7.9 | 0.38 |
| College degree (%) | 84.1 | 71.2 | 68.8 | 66.7 | 0.010 | 100.0 | 72.5 | 67.1 | 62.9 | 0.003 |
| Current smoker (%) | 10.2 | 10.7 | 10.5 | 10.1 | 0.90 | 22.2 | 7.0 | 8.3 | 8.9 | 0.72 |
| Past smoker (%) | 34.1 | 30.9 | 37.4 | 40.5 | 0.039 | 16.7 | 42.2 | 41.7 | 39.1 | 0.74 |
| Alcohol (% ≥1 drink/wk) | 63.6 | 55.2 | 52.5 | 42.9 | 0.001 | 55.6 | 42.2 | 44.0 | 40.1 | 0.36 |
| Exercise (% ≥once/wk) | 82.6 | 73.3 | 70.4 | 65.9 | 0.005 | 88.9 | 77.5 | 71.4 | 68.3 | 0.019 |
| Weight (lbs) | 175.8 | 185.4 | 198.1 | 211.4 | <0.0001 | 202.4 | 207.5 | 213.8 | 229.2 | <0.0001 |
| Change in wt (lbs)* | −1.6 | −1.4 | −2.2 | −3.3 | 0.083 | −6.1 | 1.9 | 1.3 | 1.8 | 0.35 |
| SBP | 124.3 | 124.2 | 125.2 | 125.3 | 0.080 | 127.7 | 127.5 | 126.7 | 127.6 | 0.88 |
| Change in SBP* | −3.7 | −4.3 | −4.5 | −4.7 | 0.31 | −4.2 | −0.4 | −0.9 | −1.1 | 0.99 |
| DBP | 84.0 | 83.7 | 84.0 | 83.7 | 0.94 | 85.8 | 86.1 | 85.9 | 86.2 | 0.52 |
| Change in DBP* | −4.3 | −4.3 | −4.5 | −4.8 | 0.35 | −7.2 | −2.9 | −3.3 | −2.9 | 0.26 |
| WOMEN | ||||||||||
| N | 101 | 208 | 73 | 23 | 29 | 161 | 89 | 22 | ||
| Age (yrs) | 44.6 | 44.7 | 43.0 | 42.7 | 0.036 | 44.4 | 43.8 | 43.1 | 44.0 | 0.45 |
| Black (%) | 19.8 | 22.6 | 28.8 | 30.4 | 0.12 | 34.5 | 30.4 | 28.1 | 18.2 | 0.21 |
| College degree (%) | 46.5 | 46.2 | 42.5 | 39.1 | 0.45 | 62.1 | 50.3 | 44.9 | 45.4 | 0.15 |
| Current smoker (%) | 14.8 | 10.6 | 11.0 | 21.7 | 0.88 | 10.3 | 8.7 | 13.5 | 9.1 | 0.56 |
| Past smoker (%) | 27.7 | 25.5 | 21.9 | 13.0 | 0.14 | 34.5 | 28.6 | 25.8 | 36.4 | 0.86 |
| Alcohol (% ≥1 drink/wk) | 41.6 | 28.8 | 23.3 | 39.1 | 0.094 | 13.8 | 19.9 | 14.6 | 27.3 | 0.69 |
| Exercise (% ≥once/wk) | 62.6 | 60.8 | 60.3 | 56.5 | 0.60 | 58.6 | 59.6 | 53.9 | 63.6 | 0.82 |
| Weight (lbs) | 151.3 | 162.4 | 174.1 | 183.7 | <0.0001 | 172.7 | 181.4 | 192.0 | 203.1 | <0.0001 |
| Change in wt (lbs)* | 0.06 | 0.12 | −0.8 | 0.6 | 0.86 | 3.0 | 1.9 | 4.3 | 3.9 | 0.32 |
| SBP | 125.2 | 125.7 | 125.3 | 123.9 | 0.70 | 128.2 | 128.4 | 128.2 | 130.0 | 0.48 |
| Change in SBP* | −3.7 | −3.5 | −2.7 | −6.4 | 0.73 | 0.9 | 0.5 | 2.6 | 0.2 | 0.47 |
| DBP | 83.7 | 83.7 | 84.5 | 83.6 | 0.26 | 85.7 | 85.7 | 85.8 | 86.2 | 0.44 |
| Change in DBP* | −4.7 | −4.4 | −4.7 | −5.9 | 0.54 | −2.4 | −2.4 | −2.0 | −3.2 | 0.97 |
Data represent means or percentages. SBP indicates systolic blood pressure; DBP, diastolic blood pressure.
Change during the trial periods.
Baseline urinary excretion levels are shown in Table 2 by phase and gender. Median potassium excretion was 2327 mg/24hr, and was higher in men (2502 mg/24hr) than women (1952 mg/24hr), but similar by phase. Median creatinine was 1569 mg/24hr, again higher in men (1726 mg/24hr) than women (1155 mg/24hr). The median creatinine/weight ratio was 19.3 in men and 15.0 in women. Both potassium and creatinine, as well as Cr/Wt, Na/K, and Na/Cr, were positively associated with average sodium excretion.
Table 2.
Baseline urinary excretion variables among participants with follow-up information in the Trials of Hypertension Prevention by categories of urinary sodium excretion (mg/24hr).
| TOHP I | TOHP II | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sodium Excretion (mg/24hr) | P for Trend |
Sodium Excretion (mg/24hr) | P for Trend |
|||||||
| <2300 | 2300– <3600 |
3600– <4800 |
≥ 4800 | <2300 | 2300– <3600 |
3600– <4800 |
≥ 4800 | |||
| MEN | ||||||||||
| N | 88 | 382 | 354 | 168 | 18 | 142 | 252 | 202 | ||
| Sodium (mg/24hr) | 1928.2 | 3054.5 | 4145.8 | 5923.0 | 1995.2 | 3119.5 | 4200.5 | 5728.8 | ||
| Potassium (mg/24hr) | 1986.1 | 2357.8 | 2665.2 | 3058.0 | <0.0001 | 2176.8 | 2341.4 | 2694.4 | 2919.9 | <0.0001 |
| Sodium/Potassium | 1.13 | 1.47 | 1.71 | 2.11 | <0.0001 | 1.08 | 1.48 | 1.72 | 2.14 | <0.0001 |
| Creatinine (mg/24hr) | 1270.2 | 1508.6 | 1705.8 | 1993.2 | <0.0001 | 1483.3 | 1703.6 | 1936.0 | 2175.4 | <0.0001 |
| Cr/weight (mg/kg) | 16.2 | 18.2 | 19.3 | 21.1 | <0.0001 | 16.5 | 18.3 | 20.1 | 21.1 | <0.0001 |
| Sodium/Cr | 1.66 | 2.12 | 2.50 | 3.04 | <0.0001 | 1.42 | 1.91 | 2.24 | 2.70 | <0.0001 |
| CV Cr/wt (%)(median)* | 23.6 | 19.7 | 16.4 | 17.7 | 0.0007 | 17.6 | 19.4 | 14.6 | 13.1 | <0.0001 |
| % CV Cr/wt >= 20%* | 61.2 | 48.8 | 34.0 | 41.6 | <0.0001 | 43.8 | 47.0 | 34.4 | 22.8 | <0.0001 |
| % CV Cr/wt >= 30%* | 30.6 | 25.7 | 16.3 | 18.6 | 0.0013 | 25.0 | 18.2 | 15.1 | 9.8 | 0.013 |
| WOMEN | ||||||||||
| N | 101 | 208 | 73 | 23 | 29 | 161 | 89 | 22 | ||
| Sodium (mg/24hr) | 1847.6 | 2908.1 | 4088.6 | 5941.2 | 1965.0 | 3032.8 | 4034.6 | 5596.7 | ||
| Potassium (mg/24hr) | 1653.2 | 1920.0 | 2243.3 | 2622.4 | <0.0001 | 1522.6 | 1962.2 | 2168.4 | 2637.4 | <0.0001 |
| Sodium/Potassium | 1.27 | 1.67 | 1.96 | 2.38 | <0.0001 | 1.51 | 1.76 | 2.06 | 2.34 | <0.0001 |
| Creatinine (mg/24hr) | 894.3 | 1097.6 | 1277.2 | 1546.4 | <0.0001 | 1017.4 | 1239.2 | 1417.2 | 1522.1 | <0.0001 |
| Cr/weight (mg/kg) | 13.3 | 15.1 | 16.4 | 18.9 | <0.0001 | 13.1 | 15.1 | 16.4 | 16.6 | <0.0001 |
| Sodium/Cr | 2.21 | 2.74 | 3.26 | 3.95 | <0.0001 | 2.11 | 2.54 | 3.00 | 3.99 | <0.0001 |
| CV Cr/wt (%)(median)* | 21.0 | 19.8 | 20.7 | 21.9 | 0.92 | 22.1 | 17.5 | 17.4 | 16.1 | 0.39 |
| % CV Cr/wt >= 20%* | 52.0 | 47.6 | 52.9 | 60.0 | 0.61 | 65.4 | 38.2 | 32.5 | 31.8 | 0.016 |
| % CV Cr/wt >= 30%* | 31.6 | 22.1 | 25.7 | 25.0 | 0.38 | 26.9 | 16.4 | 13.8 | 18.2 | 0.35 |
Data represent means unless otherwise indicated. Cr indicates creatinine; Cr/wt, creatinine/weight ratio; CV, coefficient of variation.
In those with at least 2 urine excretions.
We computed the coefficients of variation for the Cr/Wt data, using the within-person standard deviation. These were slightly higher in women and in TOHP II, with medians of 18% and 20% for men and women, resp., in TOHP I, and of 15% and 18% for men and women, resp., in TOHP II. The CVs declined across absolute levels of sodium in men, but were more stable in women, as were the percents with CVs above 20% or 30%.
Association with CVD
During the post-trial follow-up, 193 cardiovascular events or CVD deaths occurred. First events included 68 MIs, 77 coronary revascularizations, 22 strokes (1 participant reported both MI and stroke), and 27 CVD deaths. Crude proportions developing CVD were lowest in the two lower sodium excretion groups in each phase (Table 3). In models adjusted for clinic, treatment assignment and demographic variables (model 1), for these plus baseline covariables (model 2), and additionally for changes in weight, smoking and exercise over the trial periods (model 3), there was a non-significant trend of increasing risk with increasing sodium level. In the fully adjusted model, compared to those with sodium 3600 to <4800 mg/24hr, risk for those with sodium <2300 mg/24hr was 32% lower after multivariable adjustment (p for trend = 0.13). When sodium was considered as a continuous term, risk increased linearly, with a 17% increase in risk per 1000 mg/24hr (p=0.054) in the fully adjusted model.
Table 3.
Cardiovascular events after the Trials of Hypertension Prevention by categories of urinary sodium excretion (mg/24hr).
| Sodium Excretion (mg/24hr) | P Value for Trend |
HR per 1000 mg/d |
P Value |
||||
|---|---|---|---|---|---|---|---|
| <2300 | 2300– <3600 |
3600– <4800 |
≥ 4800 | ||||
| TOHP I | |||||||
| CVD events/ Total (%) | 15/189 (7.9) | 48/590 (8.1) | 40/427 (9.4) | 23/191 (12.0) | |||
| TOHP II | |||||||
| CVD events/ Total (%) | 2/47 (4.3) | 13/303 (4.3) | 34/341 (10.0) | 18/224 (8.0) | |||
| Model 1 | |||||||
| HR | 0.92 | 0.80 | 1.00 | 1.11 | 0.18 | 1.13 | 0.044 |
| 95%CI | 0.53–1.60 | 0.56–1.13 | (Reference) | 0.75–1.64 | 1.00–1.27 | ||
| Model 2 | |||||||
| HR | 0.87 | 0.78 | 1.00 | 1.12 | 0.19 | 1.18 | 0.025 |
| 95%CI | 0.48–1.58 | 0.54–1.13 | (Reference) | 0.74–1.67 | 1.02–1.36 | ||
| Model 3 | |||||||
| HR | 0.68 | 0.75 | 1.00 | 1.05 | 0.13 | 1.17 | 0.054 |
| 95%CI | 0.34–1.37 | 0.50–1.11 | (Reference) | 0.68–1.62 | 1.00–1.36 | ||
From Cox proportional hazards regression models stratified by trial phase and adjusted as follows: Model 1 (age, sex, race/ethnicity, clinic, and treatment assignment), Model 2 (Model 1 variables plus education status, baseline weight, alcohol use, smoking, exercise, potassium excretion, and family history of cardiovascular disease), and Model 3 (Model 2 variables plus changes in weight, smoking, and exercise during the trial periods). CVD indicates cardiovascular disease; HR, hazard ratio; CI, confidence interval.
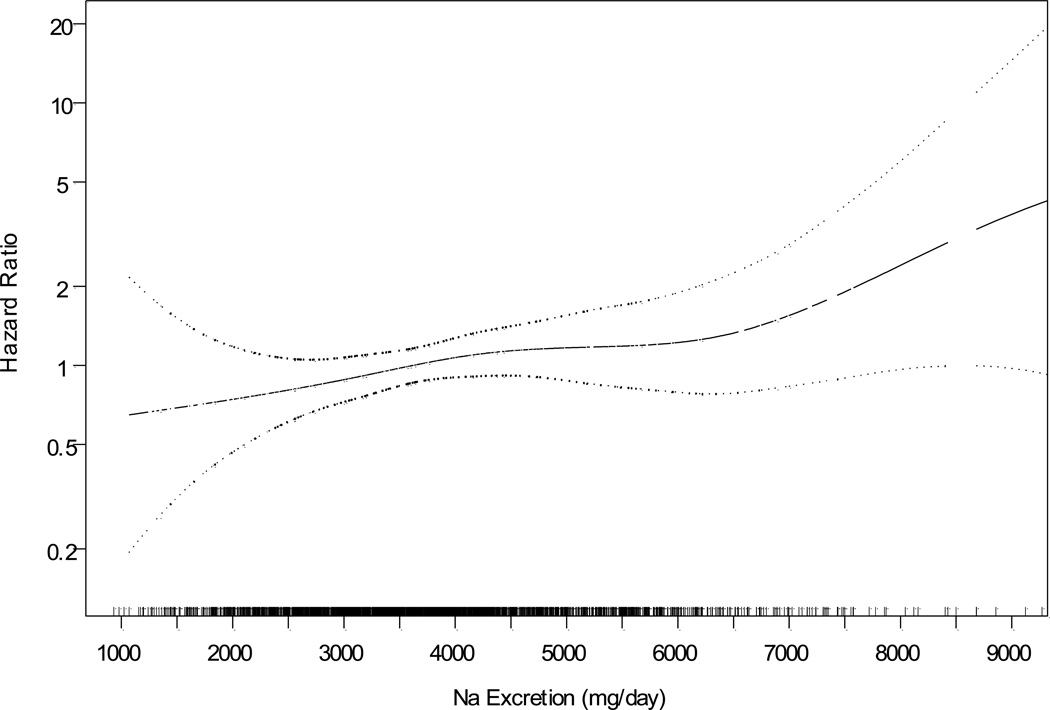
The spline plot (Figure 2) supports a linear association of sodium with CVD (P=0.044), with a p value for nonlinearity = 0.76. In particular, the curve continues to descend from 3600 to 2300 and 1500 mg/24hr, although the data are sparse at the lowest levels. In this nonlinear curve, compared to those consuming 3600 mg/24hr, the estimated HRs for those consuming 2300 and 1500 mg/24hr were 0.78 and 0.69, respectively.
Figure 2.
Spline plot of the hazard ratio for cardiovascular disease by mean sodium excretion expressed as mg/24hr, adjusted for covariables. P value for linearity = 0.044; p value for nonlinearity = 0.76. Rug plot indicates distribution of sodium excretion.
To determine the impact of differences in creatinine levels on the association of sodium and CVD, we conducted several sensitivity analyses (Table 4). We first excluded all those with a CV for Cr/Wt of 20% or greater, leaving a sample size of 1298 participants. The association of sodium with CVD was slightly stronger, with hazard ratios that were 33% lower in those <2300 mg/24hr and 35% higher in those ≥ 4800 mg/24hr, but the trend was not statistically significant. The linear trend also increased to a 20% increase in risk per 1000 mg/24hr (p=0.09). When excluding those with a CV for Cr/Wt of 30% or greater, or when controlling for Cr/Wt or the CV of Cr/Wt in the model, there was little change in the estimated coefficients, though the effect of sodium became stronger with control for the CV of Cr/Wt.
Table 4.
Cardiovascular events after the Trials of Hypertension Prevention by categories of urinary sodium excretion (mg/24hr) – Sensitivity analyses adjusting for creatinine.
| Sodium Excretion (mg/24hr) | P Value for Trend |
HR per 1000 mg/d |
P Value |
||||
|---|---|---|---|---|---|---|---|
| <2300 | 2300– <3600 |
3600– <4800 |
≥ 4800 | ||||
| Excluding those with CV Cr/Wt >= 20%* (CVD events/ Total = 104/1298) | |||||||
| HR | 0.67 | 0.83 | 1.00 | 1.35 | 0.14 | 1.20 | 0.094 |
| 95%CI | 0.23–1.91 | 0.48–1.44 | (Reference) | 0.76–2.42 | 0.97–1.50 | ||
| Excluding those with CV Cr/Wt >= 30%* (CVD events/ Total = 141/1780) | |||||||
| HR | 0.71 | 0.79 | 1.00 | 1.00 | 0.31 | 1.18 | 0.078 |
| 95%CI | 0.30–1.68 | 0.50–1.24 | (Reference) | 0.61–1.65 | 0.98–1.42 | ||
| Controlling for Cr/Wt in Model (CVD events/ Total = 193/2312) | |||||||
| HR | 0.70 | 0.76 | 1.00 | 1.03 | 0.19 | 1.16 | 0.085 |
| 95%CI | 0.34–1.45 | 0.51–1.13 | (Reference) | 0.66–1.60 | 0.98–1.37 | ||
| Controlling for CV Cr/Wt in Model* (CVD events/ Total = 180/2218) | |||||||
| HR | 0.61 | 0.72 | 1.00 | 1.10 | 0.066 | 1.18 | 0.040 |
| 95%CI | 0.29–1.28 | 0.48–1.09 | (Reference) | 0.71–1.71 | 1.01–1.39 | ||
From Cox proportional hazards regression models stratified by trial phase and adjusted for age, sex, race/ethnicity, clinic, and treatment assignment, education status, baseline weight, alcohol use, smoking, exercise, potassium excretion, and family history of cardiovascular disease, and changes in weight, smoking, and exercise during the trial periods. Cr/wt indicates creatinine/weight ratio; CV, coefficient of variation; HR, hazard ratio; CI, confidence interval.
In those with at least 2 urine excretions.
For easier comparison, we further considered the excretion variables expressed per standard deviation (Supplemental Table 2). The average creatinine/weight ratio was not a predictor of CVD by itself, with a small 4% increase in risk per SD (p=0.72). When the first baseline measure of Cr/Wt was used, rather than the average over the trial period, however, the risk increased by 20% per SD (p=0.0087) (data not shown). The risk of CVD increased by 21% per SD of average sodium excretion (p=0.05) and decreased by 15% per SD of average potassium excretion (p=0.12), with little change after adjustment for Cr/Wt. Similar results were seen using the sodium/ creatinine and potassium/creatinine ratios.
Discussion
An extensive body of information documents the presence of a direct relationship between sodium intake and blood pressure. During the past year, three new meta-analyses of randomized, controlled clinical trials were published.19–21 Although they differed in their trial inclusion and exclusion criteria, each of the meta-analyses reported a significant and clinically important decrement in blood pressure following a reduction in sodium intake. Consistent with prior knowledge, the reduction in blood pressure was greater in those with a higher starting level of blood pressure, older persons, African-Americans, and those with a more successful intervention. In the meta-analyses that are most generalizeable to clinical practice and public health,17,19 total cholesterol and catecholamine levels were essentially unchanged and changes in urinary protein excretion were consistent with a beneficial effect of sodium reduction. Blood pressure is one of the best validated surrogate markers for CVD,22–24 and has been identified as one of the leading preventable causes of death in the U.S.25
Direct observations of the relationship between sodium reduction and CVD are more limited, especially at lower absolute levels of sodium intake. A preponderance of reports based on observational epidemiology support the presence of a direct relationship between sodium intake and CVD, especially for stroke.26 While the DASH-Sodium clinical trial demonstrated an increasingly beneficial effect of reduced sodium intake on blood pressure at progressively lower levels of dietary sodium down to 1500 mg/day,27 some reports have identified null, inverse or J-shaped relationships with CVD.26
In its most recent review of dietary sodium,1 the IOM committee was charged with examining the effects of very low levels of sodium intake in the range of 1500–2300 mg/day on health outcomes, defined as clinical CVD events. This and other reviews have uniformly concluded that the underlying data showing adverse effects of sodium in this range are of insufficient quality to support a firm conclusion.1,19,26 Many suffer from some combination of potential for bias in the assessment of sodium intake, reverse causality, residual confounding and random error. Despite this, the reports of a paradoxical inverse or J-shaped association between sodium intake and CVD have received considerable media28,29 as well as scientific community attention30–32 and have been the basis for addition of sodium to food products.33
Several analyses of CVD or total mortality were based on NHANES data, which employed a single 24-hour diet recall to estimate sodium intake. This approach underestimates sodium intake by failing to measure sodium added at the table, in the kitchen, in supplements, or in drinking water. Falsely low estimation due to underreporting of food and beverage intake and portion sizes is also a concern. Finally, inaccuracies can result from changes in commercial food composition that are not reflected in the nutrient databases used to estimate sodium content. Use of food frequency questionnaires to estimate sodium intake is prone to many of the same challenges encountered with use of dietary recalls. In addition, sodium content can vary considerably across brands of processed foods, which are typically incompletely assessed in these questionnaires.
The gold standard for sodium assessment in healthy persons is 24-hour urine collection. Overnight and spot urine samples are less burdensome for study participants and research staff. However, they are affected by diurnal variation, and the methods supporting the validity of their use are inadequate. They represent a weak substitute for 24-hour urine collections, especially when they are used to estimate sodium intake and relationships in an individual.26 Despite being considered the gold standard, even 24-hour collections are subject to quality control concerns, particularly under-collection of urine specimens. Measurements based on a single 24-hour urine fail to capture day-to-day variability, making them less than optimal for study of the within-person relationship between sodium and CVD.
The TOHP Follow-up Study used an average of 3–7 carefully collected 24-hour urine collections as a measure of sodium exposure, greatly reducing bias due to measurement error and random error due to within-person variability over time. We found a continued decrease in CVD events among those with sodium levels as low as 1500 mg/day, with no evidence of a J-shape when examining spline curves. Risk reductions among those at the lowest levels of sodium excretion were substantial, with a 32% reduction among those excreting less than 2300 mg/day, although this was not statistically significant due to small numbers in this subgroup and limited power.
To further consider the quality of urine specimens, we also adjusted for creatinine using various approaches. Since the rate of creatinine formation is fairly constant in healthy individuals, urinary creatinine serves as a measure of completeness of urine collection.17 Those with the lowest levels of urinary creatinine excretion are likely to include individuals who have collected an incomplete 24-hour specimen. They may be less likely to exhibit healthy behaviors and more likely to have poorer health outcomes. Creatinine level, however, is also related to other factors, such as age, race, and BMI.17 While average sodium levels in TOHP were positively associated with creatinine levels and the Cr/Wt ratio, these had little impact on the relationship between sodium and CVD in these analyses.
In our study, the average Cr/Wt was not a predictor of CVD. However, when using only the first baseline urinary excretion, it was a strong predictor. This suggests that using the average for many measurements over several years, as in these TOHP analyses, adjusts for variations in dilution and removes any effect of under-collection, as well as reducing within-person variability. Estimates based on a single urine collection, however, may be distorted by under-collection of the specimen,9,34 and in such a circumstance creatinine adjustment should be considered.
Reverse causation is another important source of error in observational studies of the relationship between dietary sodium and CVD. This is most likely an issue in studies that include patients with heart failure, coronary heart disease, chronic kidney disease, diabetes mellitus, or even hypertension. Such patients are likely to reduce their sodium intake either because of a poor diet or health advice. This can result in an inverse relationship between sodium intake and CVD that is a consequence of their underlying disease rather than the lower intake of dietary sodium (reverse causation). In many of these studies the situation is further compounded by the use of potent diuretics and other medications that can distort estimates of sodium intake. Often, the number of CVD events in these studies is small so that even a trivial number of events resulting from reverse causation can distort the results. Eliminating early periods of follow-up and controlling for illness in the analysis may not be a sufficient remedy. Indeed, controlling for blood pressure, hypertension, or hypertension medication in the analysis may eliminate the indirect effect of sodium through blood pressure, which may be the main biologic mechanism.35 A major strength of the TOHP follow-up analyses is that the cohorts were restricted to healthy, free living prehypertensive individuals who were not taking blood pressure medications. Thus reverse causation is very unlikely to have been an issue in the TOHP follow-up study. However, since it did not include those with high blood pressure, it could also have eliminated those who were most sensitive to the effects of sodium on blood pressure, and in whom the impact on CVD could be even stronger.
Limitations of the current analyses should also be considered. First, the response rate to the TOHP follow-up questionnaires was less than 80%. However, much of this was due to changes in address after 5–10 years of no contact; the response rate was 85% among those with a valid address. We also collected information on cardiovascular deaths for all participants through the NDI. In addition, there was no difference in response by sodium level, reducing the likelihood of bias. Second, it is unclear if sodium levels were maintained throughout follow-up. However, this concern is shared by most observational studies, and our repeat 24-hour urine collections over 1–4 years represent very accurate assessments of usual sodium intake. Third, we cannot rule out residual confounding. Those on a low sodium diet may exhibit other healthy lifestyle behaviors, including better diet quality and increased exercise. We adjusted for weight, exercise and smoking in our models, as well as changes in these during the intervention periods, but uncontrolled confounding remains a possibility.
Approximately 37% of US adults have prehypertension,36 and these data show that they would benefit from sodium reduction. Given the strong impact of sodium on blood pressure among hypertensives, the 16% of US adults with hypertension36 would also be expected to benefit. Thus, these results are consistent with overall health benefits of reducing sodium intake to the 1500 to 2300 mg/day range in the majority of the population, in agreement with current dietary guidelines.5 While there is likely a biologic benefit to lowering sodium to these levels, however, only 1% of US adults have a sodium intake as low as 1500 mg/24hr currently,15 and fewer than 10% have intakes less than 2300 mg/24hr. Reasonable questions remain about the practical implementation37 of such low sodium targets, but even a small reduction in the population’s average intake of dietary sodium could result in a major improvement in CVD health.38,39
Supplementary Material
Acknowledgments
Funding Sources: TOHP I and II were supported by cooperative agreements HL37849, HL37852, HL37853, HL37854, HL37872, HL37884, HL37899, HL37904, HL37906, HL37907, and HL37924, and the TOHP Follow- up Study was supported by grant HL57915, all from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health. The NHLBI had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Dr. Cook had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Disclosures: Dr. Appel is an investigator on a grant from the McCormick Foundation.
References
- 1.IOM (Institute of Medicine) Sodium intake in populations: Assessment of evidence. Washington, DC: The National Academies Press; 2013. [Google Scholar]
- 2.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett GK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD on behalf of the American Heart Association Strategic Planning Task Force and Statistics Committe. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s Strategic Impact Goal Through 2020 and Beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 3.Appel LJ, Frohlich ED, Hall JE, Pearson TA, Sacco RL, Seals DR, Sacks FM, Smith SC, Jr, Vafiadis DK, Van Horn LV. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: a call to action from the American Heart Association. Circulation. 2011;123:1138–1143. doi: 10.1161/CIR.0b013e31820d0793. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Guideline: Sodium intake for adults and children. Geneva: World Health Organization (WHO); 2012. [PubMed] [Google Scholar]
- 5.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. Washington, DC: U.S. Government Printing Office; 2010. Dec, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strazzulo P, D'Elia L, Kandela N-B, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang H-Y, Hu Y-W, Yue C-SJ, Wen Y-W, Yeh W-T, Hsu L-S, Tsai S-Y, Pan W-H. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr. 2006;83:1289–1296. doi: 10.1093/ajcn/83.6.1289. [DOI] [PubMed] [Google Scholar]
- 8.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK for the Trials of Hypertension Collaborative Research Group. The long-term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the Trials of Hypertension Prevention. BMJ. 2007;334:885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerova J, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E, Filipovsky J, Kawecka-Jaszcz K, Nikitin Y, Staessen JA for the European Project on Genes in Hypertension (EPOGH) Investigators. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305:1777–1785. doi: 10.1001/jama.2011.574. [DOI] [PubMed] [Google Scholar]
- 10.O’Donnell MJ, Yusuf S, Mente A, Gao P, Mann JF, Teo K, McQueen M, Sleight P, Sharma AM, Dans A, Probstfield J, Schmieder RE. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306:2229–2238. doi: 10.1001/jama.2011.1729. [DOI] [PubMed] [Google Scholar]
- 11.Thomas MC, Moran J, Forsblom C, Harjutsalo V, Thorn L, Ahola A, Wadén J, Tolonen N, Saraheimo M, Gordin D, Groop PH. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diab Care. 2011;34:861–866. doi: 10.2337/dc10-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Trials of Hypertension Prevention Collaborative Research Group. The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. J Amer Med Assoc. 1992;267:1213–1220. doi: 10.1001/jama.1992.03480090061028. [DOI] [PubMed] [Google Scholar]
- 13.The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high- normal blood pressure. The Trials of Hypertension Prevention, Phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997;157:657–667. [PubMed] [Google Scholar]
- 14.Cook NR, Obarzanek E, Cutler JA, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK for the Trials of Hypertension Collaborative Research Group. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: The Trials of Hypertension Prevention (TOHP) Follow-up Study. Arch Intern Med. 2009;169:32–40. doi: 10.1001/archinternmed.2008.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cogswell ME, Zhang Z, Carriquiry AL, Gunn JP, Kuklina EV, Saydah SH, Yang Q, Moshfegh AJ. Sodium and potassium intakes among US adults: NHANES 2003–2008. Am J Clin Nutr. 2012;96:647–657. doi: 10.3945/ajcn.112.034413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thurston SW, Eisen EA, Schwartz J. Smoothing in survival models: An application to workers exposed to metalworking fluids. Epidemiol. 2002;13:685–692. doi: 10.1097/00001648-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Khaw K-T, Barrett-Connor E. The association between blood pressure, age, dietary sodium and potassium: a population study. Circ. 1988;77:53–56. doi: 10.1161/01.cir.77.1.53. [DOI] [PubMed] [Google Scholar]
- 18.Schisterman EF, Whitcomb BW, Buck Louis GM, Louis TA. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect. 2005;113:853–857. doi: 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aburto N, Ziolkovska N, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ. 2013;346:f1326. doi: 10.1136/bmj.f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graudal NA, Hubeck-Graudal T, Jürgens G. Effects of low-sodium diet vs. high-sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Cochrane review) Am J Hypertens. 2012;25:1–15. doi: 10.1038/ajh.2011.210. [DOI] [PubMed] [Google Scholar]
- 21.He FJ, Li J, MacGregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346:f1325. doi: 10.1136/bmj.f1325. [DOI] [PubMed] [Google Scholar]
- 22.Desai M, Stockbridge N, Temple R. Blood pressure as an example of a biomarker that functions as a surrogate. AAPS J. 2006;8:E146–E152. doi: 10.1208/aapsj080117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleming TR, Powers JH. Biomarkers and surrogate endpoints in clinical trials. Stat Med. 2012;31:2973–2984. doi: 10.1002/sim.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.IOM (Institute of Medicine) Evaluation of biomarkers and surrogate endpoints in chronic disease. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 25.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJL, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. Plos Med. 2009;6:1–23. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whelton PK, Appel LJ, Sacco RL, Anderson CAM, Antman EM, Campbell N, Dunbar SB, Frohlich ED, Hall JE, Jessup M, Labarthe DR, MacGregor GA, Sacks FM, Stamler J, Vafiadis DK, Van Horn LV. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation. 2012;126:2880–2889. doi: 10.1161/CIR.0b013e318279acbf. [DOI] [PubMed] [Google Scholar]
- 27.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, III, Simons-Morton DG, Karanja N, Lin P-H for the DASH-Sodium Collaborative Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 28.Kelland K. Special report: a pinch of doubt over salt. Reuters. 2011 Sep 1; [Google Scholar]
- 29.Taubes G. Salt, we misjudged you. The New York Times. 2012 Jun 2; [Google Scholar]
- 30.Alderman MH. Reducing dietary sodium: The case for caution. JAMA. 2010;303:448–449. doi: 10.1001/jama.2010.69. [DOI] [PubMed] [Google Scholar]
- 31.Furberg CD. Public health policies: no place for surrogates. Am J Hypertens. 2012;25:21. doi: 10.1038/ajh.2011.203. [DOI] [PubMed] [Google Scholar]
- 32.McCarron DA. Data rather than opinion dictates that a definitive clinical trial must determine if the US government’s sodium guideline Is safe and effective. Am J Hypertens. 2011;24:859–860. doi: 10.1038/ajh.2011.111. [DOI] [PubMed] [Google Scholar]
- 33.Arumugam N. Campbell Soup increases sodium as new studies vindicate salt. Forbes. 2011 Jul 18; [Google Scholar]
- 34.He FJ, Appel LJ, Cappuccio FP, de Wardener HE, MacGregor GA. Does reducing salt intake increase cardiovascular mortality? Kidney Int. 2011;80:696–698. doi: 10.1038/ki.2011.246. [DOI] [PubMed] [Google Scholar]
- 35.Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiol. 1992;3:143–155. doi: 10.1097/00001648-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Qureshi AQ, Suri MFK, Kirmani JF, Divani AA. Prevalence and trends of prehypertension and hypertension in United States: National Health and Nutrition Examination Surveys 1976 to 2000. Med Sci Monit. 2005;11:CR403–CR409. [PubMed] [Google Scholar]
- 37.IOM (Institute of Medicine) Strategies to reduce sodium intake in the United States. Washington, DC: The National Academies Press; 2010. [Google Scholar]
- 38.Beaglehole R, Bonita R, Horton R, Adams C, Alleyne G, Asaria P, Baugh V, Bekedam H, Billo N, Casswell S, Cecchini M, Colagiuri R, Colagiuri S, Collins T, Ebrahim S, Engelgau M, Galea G, Gaziano T, Geneau R, Haines A, Hospedales J, Jha P, Keeling A, Leeder S, Lincoln P, McKee M, Mackay J, Magnusson R, Moodie R, Mwatsama M, Nishtar S, Norrving B, Patterson D, Piot P, Ralston J, Rani M, Reddy K, Sassi F, Sheron N, Stuckler D, Suh I, Torode J, Varghese C, Watt J for the Lancet NCD Action Group and the NCD Alliance. Priority actions for the non-communicable disease crisis. Lancet. 2011;377:1438–1447. doi: 10.1016/S0140-6736(11)60393-0. [DOI] [PubMed] [Google Scholar]
- 39.Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, Goldman L. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362:590–599. doi: 10.1056/NEJMoa0907355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.