We may know just enough about cytokines in autoinflammatory diseases to be dangerous. Insights from animal models and cell lines, observations from patient samples, and outcomes of directed cytokine modulating treatments constantly refine this understanding, while also unearthing deeper layers of complexity. The recent advances in our understanding of systemic autoinflammatory disorders have brought the interaction of basic cytokine biology and patient care to the fore.
The term “autoinflammatory” originated in the late 1990’s in an attempt to categorize the hereditary periodic fever syndromes, whose genetic roots suggested a pathogenesis distinct from autoimmune disorders. While the family of hereditary periodic fever syndromes has multiplied (1), more common rheumatic diseases with similar clinical or immunologic features, such as sarcoidosis and ankylosing spondylitis, have migrated into this fold. Classically, autoinflammatory disorders have been associated with exuberant production of cytokines such as interleukin (IL)-1β, Tumor Necrosis Factor (TNF)α, and IL-6 by cells of the innate immune system such as monocytes, macrophages, and granulocytes. However, recent advances have expanded the range of defects associated with autoinflammatory disease to include type I Interferons (IFNα/β), IL-10, IL-36, keratinocytes, and beyond (1). Throughout this turbulence in the autoinflammatory paradigm, two related entities serve as standard examples of enigmatic autoinflammatory disease: systemic Juvenile Idiopathic Arthritis (sJIA) and Adult-Onset Still’s Disease (AOSD).
The diagnostic criteria for both sJIA and AOSD are similar and include rash, prolonged quotidian fever, and arthritis. Nonetheless, diagnosing either sJIA or AOSD can be a vexing process of elimination, and fairly non-specific signs of systemic inflammation still dominate the criteria. Musculoskeletal organs are clearly not the sole sites of inflammation. While arthritis is not an absolute requirement of the various AOSD criteria, its presence may be more diagnostically specific (2). Arthritis is necessary for the diagnosis of sJIA, but it is often absent until later in the disease course, and new treatment modalities may prevent the arthritis characteristic of “burnt-out” systemics. A comparison of patients with sJIA and AOSD showed only minor clinical differences between the two entities (3). Thus, despite the imprecise nature of diagnosis, it appears reasonable to lump sJIA and AOSD for the purposes of pathogenic investigation.
In both sJIA and AOSD, recent efforts to understand pathogenesis have resulted in stratification of patients into distinct subgroups: those presenting with prominent arthritis and those with features of Macrophage Activation Syndrome (MAS). MAS is a cytokine storm syndrome characterized by acute inflammation, peripheral cytopenias, organomegaly, hyperferritinemia, hepatitis, and hemophagocytosis. MAS complicates at least 10% of sJIA, but a much higher proportion of patients show signs of subclinical MAS (4). Fall et al. identified two subgroups of sJIA patients from analysis of peripheral blood transcription, and high serum ferritin correlated with the group showing hematopoiesis and innate immune regulation signatures (5). Subsequently, Gattorno et al. looked for differences in sJIA patients grouped by IL-1 receptor antagonist (anakinra) response. Non-responders were more likely to have significant arthritis, although other parameters like serum ferritin were comparable (6). Serum analyses of IL-6 and IL-18 in sJIA resulted in correlation of arthritis with the former and risk of developing MAS in the latter (7). Finally, Ichida et al. found that serum ferritin and IL-18 were lower in AOSD patients who met criteria for rheumatoid arthritis than for those who did not (8). Given our bias that AOSD and sJIA are cytokine-driven diseases, it is not surprising that the data on subclasses runs along a cytokine divide. How these subclasses differ pathogenically and how they respond to specific anti-cytokine treatment remain open questions. The short-term evidence suggests that both IL-1 and IL-6 blocking strategies are effective regardless of arthritis.
Recently, several murine models of sJIA have been developed that may help illuminate the apparent separation of arthritis from MAS. These models variously employ Toll-Like Receptor (TLR) stimulation, inflammatory cytokine overproduction, and anti-inflammatory cytokine blockade. In this issue, a model described by Avau and colleagues adds another dimension. In this model, they administer the decades-old inflammatory trigger, complete Freund’s adjuvant (CFA), to wild-type (WT) mice and observe only a subtle inflammatory effect. However, this effect becomes more dramatic in mice unable to produce the canonically pro-inflammatory cytokine IFN-γ, and the animals develop symptoms suggestive of sJIA-like disease (arthritis, rash, anemia). Thus, in contrast to the primary role played by IFN-γ in driving MAS-like immunopathology in other mouse models, this CFA-based model suggests that IFN-γ may be a critical source of protection.
CFA is made up of heat-killed mycobacteria in a lipid emulsion. It is used as an adjuvant in several of the most classic models of autoimmunity, including collagen-induced arthritis, as well as experimental autoimmune encephalitis, uveitis, neuritis, and orchitis. Its modes of action are protean and involve triggering of TLRs and other pathogen-recognition receptors, and prolonged delivery of coadministered autoantigens. The effects of CFA are generally dependent on MyD88, an adaptor molecule critical for IL-1, IL-18, and most TLR signaling. Many of the CFA-related models described above are primarily dependent on IL-17 and related cytokines, and are known to be more severe in the absence of IFN-γ. IFN-γ mediates its protective effects in these models by several mechanisms: inhibition of myelopoiesis, inhibition of IL-17 producing cell differentiation, and stimulation of a variety of regulatory T -cells (9). Thus, in IL-17 dominant inflammation, IFN-γ is known to be a critical anti-inflammatory cytokine.
Avau et al. intended to model a prolonged innate immune stimulus. Mice in their model developed cachexia, peripheral adenopathy, splenomegaly, neutrophilia, and thrombocytosis. These symptoms were more severe in IFN-γ deficient animals, some of whom additionally developed erosive arthritis, rash, consumptive anemia, erythro- and myelopoiesis, and hemophagocytosis. Immunologically, the phenotype of these mice was subtler than their physical manifestations might have suggested. Of the cytokines evaluated (TNFα, IL-1β, IL-18, IL-6, and IL-17), only IL-6 was elevated at the serum level and only in IFN-γ deficient mice. To probe the helper T-cell status in this model, lymph node cells were stimulated in vitro through the T-cell receptor, and found to predominantly make IL-17 over other signature T-helper cytokines. Importantly, antibodies directed against IL-17 or the p40 subunit common to both IL-12 and IL-23 rescued mice from the majority of inflammatory symptoms. Further analyses showed that CFA induced an impressive increase in the population of γδ T-cells able to make IL-17 (the CD27 negative subset), and that induction of FoxP3+ regulatory T-cells by CFA relied in part on the effects of IFN-γ. Mice in this model did not develop hepatitis or elevated ferritin. Thus, Avau et al. have described a model of prolonged immune stimulation mediated by IL-17 with some striking pathogenic similarities to the arthritis-prominent subset of sJIA.
It remains to be seen to what extent the CFA model relies on IL-17 production by “innate” lymphoid cells such as γδ T-cells versus Th17 cells. It is interesting to note that CFA-induced inflammation, in contrast to that induced by other TLR-agonists, was not immediate. Even in the absence of IFN-γ, mice did not develop overt signs of inflammation until 11 to 39 days after injection. This kinetic could suggest an additive effect of the persistent stimulation offered by CFA, and/or a substantial contribution from an adaptive response. Additionally, the contribution of IL-17 and IL-23 from T-cells may not be antigen specific. This would highlight the innate responses of adaptive immune cells, and further muddy the divide between autoimmune and autoinflammatory disorders.
How does this model fit within the context of existing models of sJIA/AOSD-like disease? The most comparable model was recently established by Strippoli et al., who administered various TLR stimuli to mice carrying a human IL6 transgene (10). They found severe inflammatory disease in this model associated with increased monocyte cytokine production and TLR signaling. Some features of disease in this model were reminiscent of MAS, including hyperferritinemia and mild thrombocytopenia. IL-6 is a complex cytokine with important functions in monocyte/macrophage activation and stimulation of IL-17 production among many others. Many questions in this model remain, including the critical cell(s) producing and responding to IL-6, as well as the response to cytokine-directed therapy. One striking finding of this model is the extent to which TLR stimulation of wild-type mice resulted in immunopathology similar in quality but of less severity than in IL6 transgenic mice.
Along these lines, Behrens et al. established that repeated stimulation of WT mice through TLR9 resulted in MAS-like disease with temperature instability, hepatitis, splenomegaly, microvascular thrombi, pancytopenia, and hyperferritinemia but no rash or arthritis (11). Importantly, disease in this model occurred even in the absence of B- and T-cells, suggesting a truly innate immune mechanism. In contrast to the CFA-induced model, IFN-γ appeared to be an important disease driver in the TLR9 model, as disease was largely abrogated in the absence of IFN-γ. Interestingly, blockade of the IL-10 receptor dramatically worsened the TLR9 model and resulted in pervasive hemophagocytosis. To further complicate matters, the pathogenic effects of IFN-γ were minimal in the absence of IL-10. This suggests that the role of IL-10 in this model is to inhibit other cytokines that, in the absence of IL-10, contribute to fulminant disease (12). Finally, this model and subsequent work (13) have shown that TLR9 stimulation appears to be uniquely suited to the development of MAS-like disease in mice.
The animal models of hemophagocytic lymphohistiocytosis (HLH) offer another relevant perspective. HLH is a term used to encompass an MAS-like clinical scenario in the context of a variety of infectious, malignant, or genetic conditions. The familial/genetic form of HLH occurs when patients with defects in the machinery necessary for cytotoxicity contract an (usually viral) infection. Models of this severe form of HLH are uniformly mediated by very high levels of IFN-γ (14), and an IFN-γ blocking antibody is currently in clinical trials. Interestingly, an HLH-like phenotype has been observed in WT animals receiving a continuous infusion of either IFN-γ or the Th2 cytokine IL-4. Disease in mice receiving IFN-γ infusion is nearly absent when macrophages lack the ability to respond to it (15), suggesting that even in HLH the disease is caused by an innate response to adaptive hyperstimulation. In light of proposals that sJIA and MAS may exist on a continuum of severity, the prominent role of IFN-γ in driving models of MAS/HLH may be pathogenically significant.
Common to all of these models is the use of TLR stimulation as an inflammatory trigger, suggesting stimulation by pathogen and/or damage associated molecular patterns. Even in models of familial HLH (16), MyD88 has either directly or implicitly been shown to be necessary for disease. A TLR/MyD88 pathway may represent a critical point of convergence and potentially a therapeutic target with broad utility. Nonetheless, the effects of specific TLR ligand, dose, kinetic, and route of exposure are likely to have profound interactions with host factors. Also common to all of these models are fever, weight loss, splenomegaly, anemia, and hemophagocytosis. An increasing appreciation for systemic inflammation and expanded evaluation for hemophagocytes has underscored that these findings may be of limited diagnostic utility.
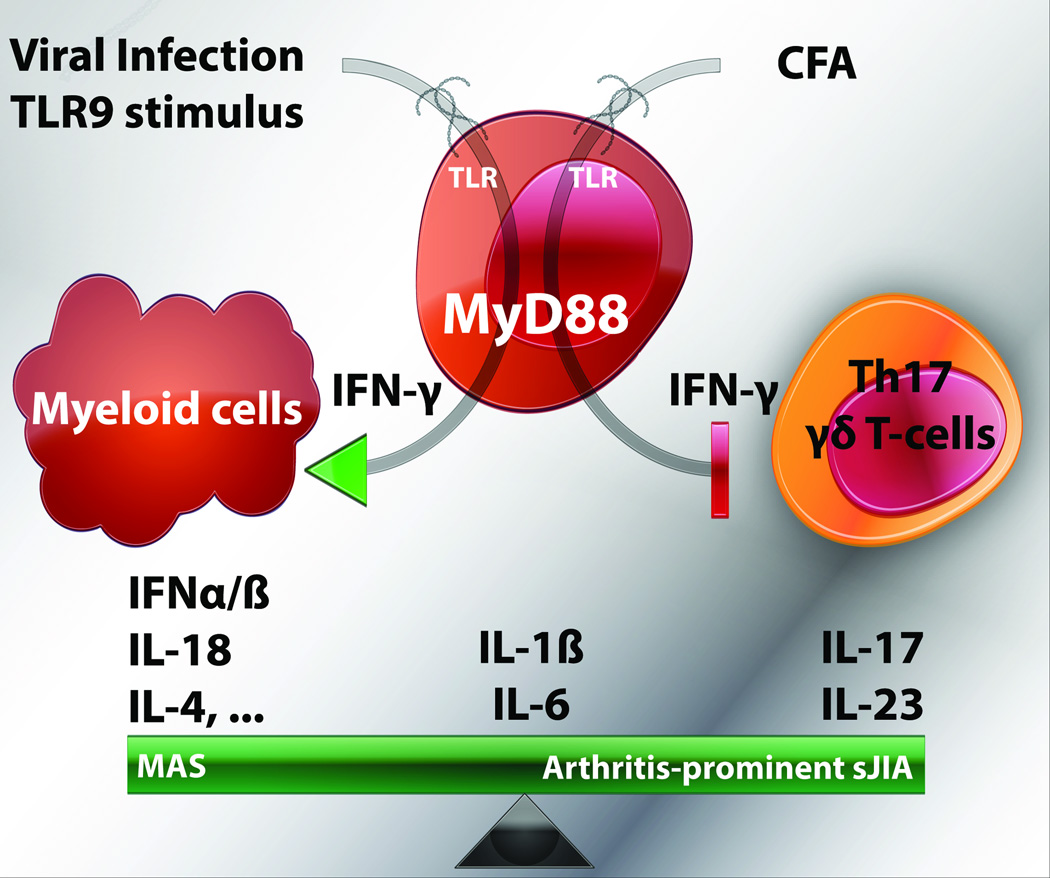
However, the differences between these models may be most instructive (schematized in Figure 1). As stated above, patients with sJIA/AOSD seem to fall into two categories: arthritis-prominent and MAS-prominent. Specific clinical features such as arthritis and thrombocytosis seem to correlate with the former, while hepatitis, thrombocytopenia, and hyperferritinemia with the latter. Correspondingly, a variety of observations from patients or animal models have associated IFN-γ, IL-10, IL-18, and IL-4 with MAS/HLH-like disease, while Avau et al. now implicate IL-17 & IL-23 as potentially important for arthritis-prominent sJIA/AOSD. Interestingly, the specific therapeutic targets approved for sJIA inhibit the actions of IL-1β and IL-6, which can participate in both IFN-γ-driven and IL-17-driven immunopathology.
Figure 1.
Of all these cytokines, IFN-γ appears to be the double-edged sword that creates the dichotomy. With the animal models described, the genetic backgrounds are not equivalent. This difference has relevance to their broader interpretation. The TLR9, IL6-transgenic, and HLH models were all performed on a Th1-primed background (C57Bl/6), where animals easily clear IFN-γ dependent infections but are prone to developing IFN-γ mediated pathology. The CFA-model was performed in mice intrinsically poor at mounting Th1 inflammatory responses (BALB/c), highlighting the potential immunoregulatory properties of IFN-γ. Further investigation should examine how strain differences might illuminate mechanisms of host protection/susceptibility. These seemingly subtle immunologic differences between strains of WT mice may help model the different responses to the same trigger we often observe in humans.
In summary, Avau et al. have demonstrated that prolonged CFA administration can result in a model of arthritis-predominant sJIA/AOSD, but only in the absence of IFN-γ. In concert with the other models relevant to sJIA/AOSD, we are left to conclude that either too much or not enough IFN-γ activity can predispose to a disease like sJIA/AOSD. Further work in this model may also help elucidate the different contributions of innate versus adaptive responses. The interpretation of all of these models should come with the usual caveats that murine models help illustrate how disease may develop in general, but not necessarily how it actually does develop in humans. Nonetheless, our challenges in diagnosis and categorization of sJIA/AOSD are great and our need for further basic/translational research even greater.
Acknowledgments
I would like to thank Edward Behrens and Peter Nigrovic for insightful comments.
Dr. Canna receives grant support from the Arthritis National Research Foundation.
References
- 1.Montealegre Sanchez GA, Almeida de Jesus A, Goldbach-Mansky R. Monogenic autoinflammatory diseases: disorders of amplified danger sensing and cytokine dysregulation. Rheumatic diseases clinics of North America. 2013;39(4):701–734. doi: 10.1016/j.rdc.2013.08.001. doi: 10.1016/j.rdc.2013.08.001. PubMed PMID: 24182851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crispin JC, Martinez-Banos D, Alcocer-Varela J. Adult-onset Still disease as the cause of fever of unknown origin. Medicine (Baltimore) 2005;84(6):331–337. doi: 10.1097/01.md.0000188009.47085.76. PubMed PMID: 16267408. [DOI] [PubMed] [Google Scholar]
- 3.Pay S, Turkcapar N, Kalyoncu M, Simsek I, Beyan E, Ertenli I, et al. A multicenter study of patients with adult-onset Still's disease compared with systemic juvenile idiopathic arthritis. Clinical rheumatology. 2006;25(5):639–644. doi: 10.1007/s10067-005-0138-5. doi: 10.1007/s10067-005-0138-5. PubMed PMID: 16365690. [DOI] [PubMed] [Google Scholar]
- 4.Behrens EM, Beukelman T, Paessler M, Cron RQ. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J Rheumatol. 2007;34(5):1133–1138. Epub 2007/03/09. doi: 07/13/039 [pii]. PubMed PMID: 17343315. [PubMed] [Google Scholar]
- 5.Fall N, Barnes M, Thornton S, Luyrink L, Olson J, Ilowite NT, et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum. 2007;56(11):3793–3804. doi: 10.1002/art.22981. Epub 2007/10/31. doi: 10.1002/art.22981. PubMed PMID: 17968951. [DOI] [PubMed] [Google Scholar]
- 6.Gattorno M, Piccini A, Lasiglie D, Tassi S, Brisca G, Carta S, et al. The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis and rheumatism. 2008;58(5):1505–1515. doi: 10.1002/art.23437. Epub 2008/04/29. doi: 10.1002/art.23437. PubMed PMID: 18438814. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu M, Nakagishi Y, Yachie A. Distinct subsets of patients with systemic juvenile idiopathic arthritis based on their cytokine profiles. Cytokine. 2013;61(2):345–348. doi: 10.1016/j.cyto.2012.11.025. doi: 10.1016/j.cyto.2012.11.025. PubMed PMID: 23276493. [DOI] [PubMed] [Google Scholar]
- 8.Ichida H, Kawaguchi Y, Sugiura T, Takagi K, Katsumata Y, Gono T, et al. Clinical manifestations of adult-onset still's disease presenting with erosive arthritis: Association with low levels of ferritin and IL-18. Arthritis care & research. 2013 doi: 10.1002/acr.22194. doi: 10.1002/acr.22194. PubMed PMID: 24124073. [DOI] [PubMed] [Google Scholar]
- 9.Kelchtermans H, Billiau A, Matthys P. How interferon-gamma keeps autoimmune diseases in check. Trends Immunol. 2008;29(10):479–486. doi: 10.1016/j.it.2008.07.002. doi: 10.1016/j.it.2008.07.002. PubMed PMID: 18775671. [DOI] [PubMed] [Google Scholar]
- 10.Strippoli R, Carvello F, Scianaro R, De Pasquale L, Vivarelli M, Petrini S, et al. Amplification of the response to Toll-like receptor ligands by prolonged exposure to interleukin-6 in mice: implication for the pathogenesis of macrophage activation syndrome. Arthritis and rheumatism. 2012;64(5):1680–1688. doi: 10.1002/art.33496. Epub 2011/11/24. doi: 10.1002/art.33496. PubMed PMID: 22108837. [DOI] [PubMed] [Google Scholar]
- 11.Behrens EM, Canna SW, Slade K, Rao S, Kreiger PA, Paessler M, et al. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J Clin Invest. 2011;121(6):2264–2277. doi: 10.1172/JCI43157. Epub 2011/05/18. doi: 10.1172/JCI43157. PubMed PMID: 21576823; PubMed Central PMCID: PMC3104738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canna SW, Wrobel J, Chu N, Kreiger PA, Paessler M, Behrens EM. Interferon-gamma mediates anemia but is dispensable for fulminant toll-like receptor 9-induced macrophage activation syndrome and hemophagocytosis. Arthritis and rheumatism. 2013 doi: 10.1002/art.37958. Epub 2013/04/05. doi: 10.1002/art.37958. PubMed PMID: 23553372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohyagi H, Onai N, Sato T, Yotsumoto S, Liu J, Akiba H, et al. Monocyte-derived dendritic cells perform hemophagocytosis to fine-tune excessive immune responses. Immunity. 2013;39(3):584–598. doi: 10.1016/j.immuni.2013.06.019. Epub 2013/09/17. doi: 10.1016/j.immuni.2013.06.019. PubMed PMID: 24035363. [DOI] [PubMed] [Google Scholar]
- 14.Pachlopnik Schmid J, Cote M, Menager MM, Burgess A, Nehme N, Menasche G, et al. Inherited defects in lymphocyte cytotoxic activity. Immunological reviews. 2010;235(1):10–23. doi: 10.1111/j.0105-2896.2010.00890.x. Epub 2010/06/12. doi: 10.1111/j.0105-2896.2010.00890.x. PubMed PMID: 20536552. [DOI] [PubMed] [Google Scholar]
- 15.Zoller EE, Lykens JE, Terrell CE, Aliberti J, Filipovich AH, Henson PM, et al. Hemophagocytosis causes a consumptive anemia of inflammation. J Exp Med. 2011;208(6):1203–1214. doi: 10.1084/jem.20102538. Epub 2011/06/01. doi: 10.1084/jem.20102538. PubMed PMID: 21624938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krebs P, Crozat K, Popkin D, Oldstone MB, Beutler B. Disruption of MyD88 signaling suppresses hemophagocytic lymphohistiocytosis in mice. Blood. 2011;117(24):6582–6588. doi: 10.1182/blood-2011-01-329607. Epub 2011/05/10. doi: 10.1182/blood-2011-01-329607. PubMed PMID: 21551232. [DOI] [PMC free article] [PubMed] [Google Scholar]