Abstract
Fetal exposure to caffeine is associated with adverse pregnancy outcomes. Animal and human studies suggest that caffeine may have effects on the developing reproductive system. Here we report on mothers' smoking, coffee and alcohol use, recorded during pregnancy, and semen quality in sons in the age group of 38–47 years. Subjects were a subset of the Child Health and Development Studies, a pregnancy cohort enrolled between 1959 and 1967 in the Kaiser Foundation Health Plan near Oakland, California. In 2005, adult sons participated in a follow-up study (n = 338) and semen samples were donated by 196 participants. Samples were analyzed for sperm concentration, motility and morphology according to the National Cooperative Reproductive Medicine Network (Fertile Male Study) Protocol. Mean sperm concentration was reduced by approximately 16 million sperms for sons with high prenatal exposure (5 cups of maternal coffee use per day) compared with unexposed sons (P-value for decreasing trend = 0.09), which translates to a proportionate reduction of 25%. Mean percent motile sperm decreased by approximately 7 points (P-value = 0.04), a proportionate decline of 13%, and mean percent sperm with normal morphology decreased by approximately 2 points (P-value = 0.01), a proportionate decline of 25%. Maternal cigarette and alcohol use were not associated with son's semen quality. Adjusting for son's contemporary coffee, alcohol and cigarette use did not explain the maternal associations. Findings for son's coffee intake and father's prenatal coffee, cigarette and alcohol use were non-significant and inconclusive. These results contribute to the evidence that maternal coffee use during pregnancy may impair the reproductive development of the male fetus.
Keywords: coffee, prenatal exposure, sperm concentration, sperm motility, sperm morphology
Introduction
It has been suggested that testicular dysfunction has its origins in utero, and more specifically that the determinants of the birth defects, cryptorchidism and hypospadias share common origins with testicular cancer and low sperm counts, and the conglomeration of these has been deemed testicular dysgenesis.1 Recent study, however, has questioned whether these disorders are on the same continuum,2 stimulating new research and a further refinement of the hypothesis.3 Impaired semen quality is now considered to be heterogeneous, and both prenatal and postnatal exposures are possibly associated.3 Further evidence points to critical times of exposures relating to impaired semen quality.4,5 Studies such as the Danish Pregnancy cohort,6–9 which can combine analysis of prenatal and contemporary exposures in a single cohort, are a priority, as they minimize recall bias and errors in exposure classification. Until now, the Danish studies are the only prospective studies of semen quality. In this report, we add a second prospective study based on the Child Health and Development Studies (CHDS) pregnancy cohort.
Three prevalent behaviors are suspected to affect male reproduction, namely alcohol consumption, cigarette smoking and coffee drinking. Findings from both human and laboratory studies have recently been reviewed by Sadeu et al.5 Briefly, this review concludes that adverse effects of high levels of contemporary alcohol consumption negatively impact sperm concentration, volume, motility and morphology in humans, consistent with experimental evidence using animal models. Evidence from animal studies also suggests that alcohol exposure in the pre-pubertal period may be associated with reproductive dysfunction. Data from both human and animal studies suggest that contemporary cigarette smoking is associated with adverse effects on semen quality.10,11 One prospective study on fecundability reported that in utero exposure to tobacco smoke was associated with reduced fecundability in male offspring, and that contemporary smoking in these offspring was not associated, suggesting a critical exposure window for reproductive effects.12 The evidence for caffeine effects on male fertility or semen quality is sparse. However, one prospective study reported adverse effects of contemporary caffeine consumption on fecundability in male non-smokers, but not in male smokers, possibly because of accelerated metabolism and lower internal dose in smokers.13
The Danish prospective studies were conducted as a follow-up to pregnancies occurring between 1984 and 1987; semen studies and blood sampling on sons were performed at ages 18–21 years.6–9 Data collection in sons was stratified according to maternal smoking habits, as the study was designed to investigate the effects of prenatal smoking on adult semen quality and reproductive hormones.6 Thus, these studies specifically addressed prenatal exposures.6–9 They reported associations between prenatal maternal alcohol use and sperm concentration, but not motility or morphology;9 between prenatal maternal cigarette use and sperm count, but not motility or morphology;8 and between prenatal maternal coffee consumption and levels of inhibin B and testosterone, but not sperm concentration, motility or morphology.6 Neither son's contemporary exposures to these substances nor father's prenatal exposures confounded effects reported for maternal prenatal exposures.
This study adds to this literature, with a follow-up of pregnancies occurring between 1959 and 1967, with semen and blood sampling from sons at ages 38–47 years, in the CHDS cohort. This report presents findings on the relation of maternal and paternal prenatal exposures to adult son's semen parameters for behaviors that are highly correlated: cigarette smoking, coffee drinking and alcohol use. Our findings complement the work in the Danish cohort by presenting results based on maternal self-report – which occurred before recommendations – that cigarette smoking, and alcohol and coffee consumption be curtailed during pregnancy. The CHDS mothers provided information about their smoking and alcohol use largely before the broad dissemination of public health warnings against smoking (after 1964) and alcohol use (after 1973) during pregnancy, mitigating the potential for misclassification due to underreporting that might have resulted from these warnings. This study also provides a contrast of effects of prenatal exposures at older ages (39–47 years) in the CHDS to effects on young men (ages 18–21 years) in the Danish cohort.
Methods
Analysis sample
The CHDS enrolled 98% of eligible pregnant women who were members of the Kaiser Foundation Health Plan in the Oakland, California area. Its purpose was to investigate events of pregnancy, labor, delivery and subsequent childhood development.14 During the 7-year enrollment period from 1959 to 1966 (with deliveries extending into 1967), a total of 20,530 pregnancies were observed among the 15,528 women who were recruited to the study. At baseline, information was collected from face-to-face interviews with mothers. A detailed description of the CHDS can be found in the featured introduction article of this publication and online at http://www.chdstudies.org.
In 2005, a series of follow-up studies of the adult CHDS children who were in their 40s were initiated as part of an integrated effort to investigate the long-term impacts of fetal and childhood growth and development on adult health (see the ‘Introduction’ section). Among these, the Study of the Environment and Reproduction (SER) was conducted from December, 2005 through April, 2008 to examine associations between prenatal organochlorine exposures and subsequent reproduction, as measured by time to pregnancy (assessed via interview) and semen characteristics (assessed via semen examination).
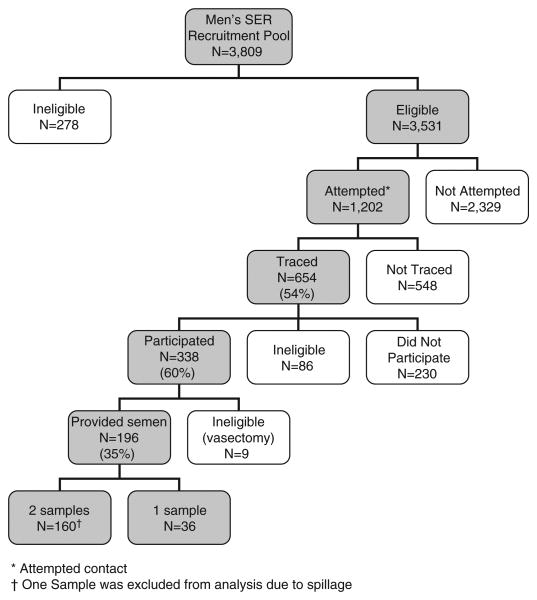
Subjects were eligible for the SER study only if they had data on birth length and weight, at least one same-day childhood height and weight measure between 6 months and 5.5 years of age, mother's interview data, sufficient second trimester and post-partum prenatal serum for serological measures (reported elsewhere) and residence within commutable distance to the Kaiser Oakland Clinic (approximately a 100-mile geographic radius in all directions from the facility). The residence criterion was applied because study participation involved an in-person clinic visit during which participants were asked to provide semen and blood samples and complete an in-person interview. These selection criteria resulted in a pool of 3809 eligible subjects among whom 654 were traced; of those, 568 remained eligible, resulting in 338 participants (60%) who provided an interview and 196 (35%) who provided at least one semen sample (Fig. 1). This study was approved by the Institutional Review Boards at Columbia University Medical Center and Kaiser Permanente.
Fig. 1.
Men's Study of the Environment and Reproduction: an adult follow-up study of sons in the Child Health and Development Studies, 1959–1967.
Adult son interview
Men were asked to come into the Kaiser, Oakland facility to complete a 1-h in-person interview, during which demographic information, health histories, health behaviors and reproductive history were obtained. At their first appointment, subjects were also asked for a blood sample, which was drawn after the semen collection.
Semen evaluation
Men were asked to provide two semen specimens approximately 2 weeks apart, each after 2–5 days of sexual abstinence. All samples were obtained at the clinic visit. A container was provided, and subjects were asked to report whether the entire specimen was collected. Only completely collected samples were included in the analysis sample. If a subject reported incomplete sample, then that sample was discarded and the subject was rescheduled for another semen examination. At the time of the appointment, the subject was asked the date and time of his last ejaculation. Three andrology laboratory technicians who were trained at the University of California, Davis (UCD), performed the semen analysis according to a standard protocol also used by the National Institute of Child Health and Human Development-funded National Cooperative Reproductive Medicine Network (Fertile Male Study).15, 16 Briefly, analyses were performed within 1 h of sample collection and included measurements of ejaculate volume by weight,17 determinations of sperm concentration using a microcell chamber and assessments of the percentage of motile sperm.16 Seminal smears were prepared at the Kaiser Clinic and shipped to the Andrology Laboratory at UCD where the smears were stained using a modified Papanicolaou method. The technician who read slides for the Fertile Male Study also assessed the sperm morphology.15 Sperms were classified as having normal or abnormal morphologic features according to strict criteria.18
For concentration and motility assessments, duplicate evaluations were performed, each from a different aliquot of semen. Duplicate readings were obtained from different areas of the seminal smear for morphology evaluation. A total of 200 or 300 sperms were evaluated for motility and morphology; at least 400 were evaluated for concentration, unless the concentration was extremely low. Values obtained from duplicate assessments of concentrations and motility were compared, and where the difference exceeded 10% an additional vial/slide was scored. For morphology, an additional reading was made when the first 2% normal values differed by >4%.
Throughout the study, external quality control procedures were performed by the UCD Andrology Laboratory. These methods are described in detail in a previous publication.19 In summary, clinic technicians received blinded triplicates from several diluted semen specimens from the UCD Laboratory for assessing concentration at various points throughout the study. Videotapes were also sent out, which had several semen samples repeated randomly throughout the tape for assessing motility, again at several points throughout the study. Percent difference from the UCD standard and coefficients of variation (CV) were calculated for these samples for each technician. The goal of the quality control protocol was to maintain percent differences and CVs that were below 15%.
Variable construction
Prenatal smoking and alcohol and coffee intake were determined based on mother's interview during her pregnancy with her son. Father's prenatal use was also based on the mother's report at interview. Son's contemporary use was based on his report at his adult follow-up interview. Coffee consumption was measured continuously in cups per day. Smoking was measured as two dichotomous variables (yes v. no) representing current and past smokers, with never smokers as the reference for both. Smoking quantity was measured using the number of cigarettes per day for past and current smokers. Alcohol consumption was based on the sum of the usual quantity of beer, wine and hard liquor consumed per week and measured continuously in total drinks per week.
Sperm concentration was measured as millions of sperm per milliliter (ml) of sample. Motility and morphology were measured as the percent of sperm that were motile or which had normal morphology. Concentration was cube-root transformed to approximate a normal distribution; however, distributions for motility and morphology were best untransformed, as measured in their original units.
Potential confounder variables were classified as follows: mother's age (continuous); mother's race/ethnicity (dummy variables for African American, Hispanic, Asian and Other v. Caucasian); mother's parity (continuous); son's genital birth defects, specifically hypospadias and cryptorchidism (yes v. no); son's age (continuous years); abstinence between last ejaculation and semen examination (continuous number of days); and analysis delay or time between ejaculation at the time of examination and analysis of motility (continuous minutes).
Alcohol and smoking were identified as the primary confounders of coffee findings based on their widely known interrelationships with one another20–22 and on previous reports about their associations with semen measures.5–8 Other maternal characteristics – age, race and parity – were identified as potential confounders, as they were known to be associated with both exposure and outcome in the CHDS data. Son's age and presence of genital birth defects, hypospadias or cryptorchidism, were examined for confounding also because of known associations between these characteristics and both exposure (prenatal coffee)23 and outcome (semen quality).2 Abstinence and analysis delay (interval between ejaculation at the time of examination and semen evaluation) are thought to influence quality of semen; specifically, longer periods of abstinence are associated with increased sperm concentration, and longer analysis delay is associated with lower measures of motility.24–26
Statistical methods
Analyses are based on 355 semen samples donated by 196 participants (Fig. 1). For men who donated two semen samples, we calculated the mean of the concentration, motility and morphology measures.
Spearman correlation coefficients were used to examine the relationship among exposures between mothers, fathers and sons. Scatter plots of maternal coffee use v. sperm concentration, motility and morphology were prepared to examine the data-based, unadjusted relationship between prenatal exposure and semen quality. Adjusted and unadjusted effects of prenatal and contemporary coffee, alcohol and smoking were examined using linear regression. These analyses were performed using Proc Mixed in SAS 9.1 to account for the 14 sibships (12 pairs and 2 threesomes) among the 196 semen donors in our study. Outcomes were analyzed as cube-root-transformed sperm concentration and percent motility and percent morphology (both untransformed). Concentration was transformed to adhere to model assumptions, but it was unnecessary to transform motility and morphology.
We first analyzed each of the mother's pregnancy behaviors (maternal coffee consumption, maternal alcohol consumption and maternal cigarette smoking) separately and each of the son's contemporary behaviors (coffee, alcohol and smoking) separately for the three semen parameters (Table 3, unadjusted models 1a–f). We subsequently examined all three of the maternal pregnancy behaviors in one model (Table 3, model 2a) and then added all three of the son's behaviors to adjust the prenatal exposure for son's contemporary use (Table 3, model 2b). A parallel series of analyses was run using father's and son's behavior (data not presented).
Table 3. Associations between mothers' pregnancy behaviors in the Child Health and Development Studies and adult sons' semen measures in the Study of the Environment and Reproduction study, estimated from linear regression.
| Concentrationa | Motility (% motile) | Morphology (% normal) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Coefficientb | 95% CIc | Coefficientc | 95% CIc | Coefficientb | 95% CIc | |
| (1) Unadjusted models | ||||||
| (a) Maternal coffee consumption (5 cups per day) | −0.4* | −0.8, 0.1 | −6.6+ | −12.7, −0.4 | −2.1+ | −3.7, −0.5 |
| (b) Maternal alcohol consumption (1 drink per week) | 0.0 | −0.0, 0.1 | 0.4 | −0.5, 1.3 | 0.1 | −0.2, 0.3 |
| (c) Maternal cigarette smoking | ||||||
| Current (yes/no) | 0.0 | −0.4, 0.4 | −3.8 | −9.7, 2.0 | −0.8 | −2.3, 0.7 |
| Past (yes/no) | −0.2 | −0.6, 0.2 | −0.7 | −6.6, 5.2 | −0.0 | −1.5, 1.5 |
| (d) Son coffee consumption (1 cup per day) | 0.0 | −0.1, 0.1 | 0.5 | −0.6, 1.6 | 0.1 | −0.2, 0.4 |
| (e) Son alcohol consumption (1 drink per week) | −0.0 | −0.0, 0.0 | −0.1 | −0.3, 0.2 | −0.1 | −0.1, 0.0 |
| (f) Son cigarette smoking | ||||||
| Current (yes/no) | 0.1 | −0.4, 0.6 | −9.2+ | −16.1, −2.2 | −1.9+ | −3.7, −0.1 |
| Past (yes/no) | −0.2 | −0.7, 0.3 | −4.3 | −11.0, 2.4 | −1.8+ | −3.6, 0.0 |
| (2) Adjusted Models | ||||||
| (a) Adjusted for maternal behaviors: | ||||||
| Maternal coffee consumption (5 cups per day) | −0.5* | −1.0, 0.0 | −6.0* | −12.7, 0.7 | −2.1+ | −3.8, −0.4 |
| Maternal alcohol consumption (1 drink per week)) | 0.0 | −0.0, 0.1 | 0.7 | 20.3, 1.6 | 0.1 | 20.1, 0.4 |
| Maternal cigarette smoking | ||||||
| Current (yes/no) | 0.0 | −0.4, 0.5 | −3.9 | −10.4, 2.6 | −0.6 | −2.2, 1.1 |
| Past (yes/no) | −0.4 | −0.9, 0.1 | −1.5 | −8.2, 5.2 | −0.8 | −2.5, 0.9 |
| (b) Adjusted for maternal and adult son's behavior: | ||||||
| Maternal coffee consumption (5 cups per day) | −0.6* | −1.2, 0.0 | −7.5* | −15.6, 0.6 | −2.1+ | −4.3, 0.1 |
| Maternal alcohol consumption (1 drink per week) | 0.0 | −0.0, 0.1 | 0.7 | −0.3, 1.8 | 0.1 | −0.2, 0.4 |
| Maternal cigarette smoking | ||||||
| Current (yes/no) | 0.1 | −0.4, 0.6 | −3.1 | −9.7, 3.5 | −0.5 | −2.2, 1.2 |
| Past (yes/no) | −0.3 | −0.9, 0.2 | −1.1 | −8.3, 6.1 | −0.4 | −2.3, 1.4 |
| Son coffee consumption (1 cup per day) | 0.0 | −0.1, 0.1 | 0.3 | −1.0, 1.6 | 0.1 | −0.3, 0.5 |
| Son alcohol consumption (1 drink per week) | −0.0 | −0.0, 0.0 | 0.1 | −0.2, 0.4 | −0.0 | −0.1, 0.1 |
| Son cigarette smoking | ||||||
| Current (yes/no) | 0.3 | −0.3, 0.9 | −8.3* | −16.9, 0.3 | −1.4 | −3.7, 0.9 |
| Past (yes/no) | −0.0 | −0.6, 0.6 | −4.2 | −12.5, 4.0 | −1.0 | −3.4, 1.3 |
Bold values connote statistical signifance at P-value ≤ 0.10.
P-value < 0.10.
P-value ⩽ 0.05.
Concentration is cube-root transformed from units of 106 /ml.
Coefficient represents change in semen measure per unit of exposure.
CI = confidence interval (lower, upper).
Adjustment of the prenatal association for confounding (data not presented) was performed by adding each confounder (listed above in the ‘Variable construction’ section) one at a time to the final model (Table 3, model 2b).
Model estimates for maternal coffee intake reported in Table 3 are for increments of 5 cups per day, equivalent to the median level for the highest quartile minus the median level for the lowest quartile. Model estimates for the maternal coffee association presented in Table 4 are reported for three levels of intake during pregnancy, 0, 2.5 (mean) and 5 cups per day, based on the continuous measure (cups per day).
Table 4. Mean semen levels among sons estimated for increasing levels of prenatal coffee exposure. An adult follow-up Study of the Environment and Reproduction of the Child Health and Development Studies.
| Maternal coffee level during pregnancy | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 0 cups | 2.5 cups | 5 cups | Test for trend | ||||
|
|
|
|
|
||||
| Adult son's semen measures | Meana | 95% CI | Mean | 95% CI | Meana | 95% CI | P-valueb |
| Concentration (106/ml)c | |||||||
| Unadjusted | 65.9 | 52.7, 81.0 | 57.0 | 48.5, 66.4 | 49.0 | 37.5, 62.7 | 0.09 |
| Adjusted | 64.1 | 45.0, 88.1 | 51.4 | 35.7, 71.1 | 40.5 | 23.2, 64.7 | 0.06 |
| Motility (% motile) | |||||||
| Unadjusted | 50.7 | 46.7, 54.6 | 47.4 | 44.6, 50.2 | 44.1 | 39.8, 48.4 | 0.04 |
| Adjusted | 51.3 | 45.3, 57.4 | 47.6 | 41.9, 53.3 | 43.9 | 36.0, 51.7 | 0.06 |
| Morphology (% normal) | |||||||
| Unadjusted | 8.5 | 7.5, 9.5 | 7.4 | 6.7, 8.1 | 6.4 | 5.3, 7.5 | 0.01 |
| Adjusted | 8.8 | 7.2, 10.4 | 7.8 | 6.2, 9.3 | 6.7 | 4.6, 8.8 | 0.05 |
CI = confidence interval.
Estimated from linear regression. Unadjusted models include only the term for maternal coffee intake (cups per day). Adjusted models include terms for maternal coffee intake (cups per day), maternal alcohol (drinks per week), maternal smoking (yes/no), son's coffee (cups per day), son's drinking (drinks per week) and son's smoking (yes/no).
P-value for maternal coffee term (continuous).
Back transformed from cube root.
Results
A comparison of baseline demographics, health behaviors and birth characteristics for eligible subjects and study participants showed slight differences between the two groups (Table 1). There were proportionately more Hispanics and Asians among the participants, and they had proportionately more mothers who were older, multiparous, non-smoking and homemakers. The groups did not differ on mother's education, and coffee or alcohol use, nor did they differ on son's birth weight or gestational age.
Table 1.
Selected baseline characteristics comparing eligible and participating sons in the Study of the Environment and Reproduction, adult sons' follow-up of the CHDS
| Eligible | Provided interview | Provided interview and semen | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| n = 3471a | n = 338 | n = 196 | ||||||
|
|
|
|
||||||
| Characteristic at baseline | n | % | n | % | P-valueb | n | % | P-value b |
| Mother's race/ethnicity | ||||||||
| White | 2275 | 65.6 | 224 | 66.3 | 122 | 62.2 | ||
| African American | 850 | 24.5 | 70 | 20.7 | 45 | 23.0 | ||
| Hispanic | 89 | 2.6 | 9 | 2.7 | 8 | 4.1 | ||
| Asian | 148 | 4.3 | 28 | 8.3 | 17 | 8.7 | ||
| Multi-racial/Other | 108 | 3.1 | 7 | 2.1 | 4 | 2.0 | ||
| Unknown | 1 | – | – | 0.0082 | – | 0.0288 | ||
| Mother's age (quartiles; years) | ||||||||
| ⩽22 | 809 | 23.4 | 53 | 15.7 | 34 | 17.4 | ||
| 23–26 | 946 | 27.3 | 75 | 22.3 | 45 | 23.0 | ||
| 27–31 | 875 | 25.3 | 98 | 29.1 | 58 | 29.6 | ||
| 30 + | 835 | 24.1 | 111 | 32.9 | 59 | 30.1 | ||
| Unknown | 6 | – | 1 | – | <0.0001 | – | 0.0402 | |
| Mother's parity | ||||||||
| Primiparous | 1086 | 31.3 | 71 | 21.0 | 37 | 18.9 | ||
| Multiparous | 2385 | 68.7 | 267 | 79.0 | <0.0001 | 159 | 81.1 | 0.0002 |
| Mother's education | ||||||||
| Less than high school | 559 | 16.2 | 48 | 14.2 | 29 | 14.8 | ||
| High school/trade school | 1138 | 32.9 | 113 | 33.4 | 64 | 32.7 | ||
| College | 1764 | 51.0 | 177 | 52.4 | 103 | 52.6 | ||
| Unknown | 10 | – | – | – | 0.6442 | – | 0.8582 | |
| Mother's employment | ||||||||
| Homemaker | 1859 | 55.8 | 214 | 64.7 | 122 | 63.5 | ||
| Professional/managerial | 387 | 11.6 | 33 | 10.0 | 20 | 10.4 | ||
| Secretarial/clerical | 853 | 25.6 | 64 | 19.3 | 36 | 18.8 | ||
| Laborer (housework/factory) | 225 | 6.8 | 17 | 5.1 | 11 | 5.7 | ||
| Other | 10 | 0.3 | 3 | 0.9 | 3 | 1.6 | ||
| Unknown | 137 | – | 7 | – | 0.0075 | 4 | – | 0.0084 |
| Mother's smoking | ||||||||
| Never | 1634 | 49.1 | 169 | 51.2 | 98 | 51.0 | ||
| Current | 1131 | 34.0 | 93 | 28.2 | 47 | 24.5 | ||
| Past | 566 | 17.0 | 68 | 20.6 | 47 | 24.5 | ||
| Unknown | 140 | – | 8 | – | 0.0626 | 4 | – | 0.0044 |
| Mother's coffee consumption (quartiles) | ||||||||
| <1/2 cup per day | 699 | 23.4 | 67 | 24.8 | 44 | 27.3 | ||
| 1/2–1 cup per day | 680 | 22.8 | 49 | 18.2 | 28 | 17.4 | ||
| 2–3 cups per day | 806 | 27.0 | 69 | 25.6 | 46 | 28.6 | ||
| 4 + cups per day | 798 | 26.8 | 85 | 31.5 | 43 | 26.7 | ||
| Unknown | 488 | – | 68 | – | 0.1850 | 35 | – | 0.3767 |
| Mother's alcohol consumption (quartiles) | ||||||||
| <1 drink per weekc | 1327 | 44.5 | 131 | 48.5 | 76 | 47.2 | ||
| 1–2 drinks per week | 1072 | 35.9 | 83 | 30.7 | 53 | 32.9 | ||
| 3 + drinks per week | 585 | 19.6 | 56 | 20.7 | 32 | 19.9 | ||
| Unknown | 487 | – | 68 | – | 0.2296 | 35 | – | 0.7234 |
| Offspring birth weight (quartiles; g) | ||||||||
| ⩽2940 | 686 | 19.8 | 64 | 18.9 | 40 | 20.4 | ||
| 2941–3276 | 839 | 24.2 | 76 | 22.5 | 45 | 23.0 | ||
| 3277–3584 | 884 | 25.5 | 89 | 26.3 | 53 | 27.0 | ||
| ⩾3585 | 1062 | 30.6 | 109 | 32.3 | 0.8410 | 58 | 29.6 | 0.9417 |
| Offspring gestational age (quartiles; weeks) | ||||||||
| ⩽38 | 772 | 22.4 | 71 | 21.2 | 53 | 27.0 | ||
| 39 | 779 | 22.6 | 81 | 24.2 | 40 | 20.4 | ||
| 40 | 858 | 24.9 | 76 | 22.7 | 43 | 21.9 | ||
| ⩾41 | 1031 | 30.0 | 107 | 31.9 | 60 | 30.6 | ||
| Unknown | 31 | – | 3 | – | 0.6711 | – | 0.4168 | |
CHDS, Child Health and Development Studies.
Bold values are significant at P < 0.05.
Quartiles are based on the distribution of variables among all CHDS births. For birth weight and gestational age, quartile cutpoints were based on the distribution among liveborn CHDS births.
Eligible sample size is equivalent to total eligible (n = 3809) minus participants (n = 338).
Based on chi-square test of distribution. P-values for participants are given for the comparison of distributions between the eligible sample (n = 3471) and the participant sample (n = 338), and the comparison between the eligible sample and the sample that also provided semen (n = 196).
Owing to clumping of data, quartiles 1 and 2 are combined at this cutpoint.
In this sample of men in their mid-forties, the mean sperm concentration was 72.4 million sperms per ml of sample (standard deviation [S.D.] = 59.1), the mean percent motile was 46.8 (S.D. = 16.3) and the mean percent with normal morphology was 7.5 (S.D. = 4.2). (Additional descriptive statistics are presented in Supplementary Table 1.) Over 80% of SER subjects provided two semen samples for evaluation; the interclass correlation coefficients (ICCs) indicate good reliability between measures for the duplicate samples (ICCs = 0.70, 0.65 and 0.80 for concentration, motility and morphology, respectively). Comparison of son's age, race, education, employment status, body mass index, smoking status, history of sexually transmitted disease and frequency of sexual intercourse showed no differences between men who provided one compared with those who provided two semen samples.
Table 2 provides a description of maternal and paternal age, race/ethnicity and health behavior during their son's gestation, between 1959 and 1967, and the same information for the adult sons at their follow-up visit in 2005. Participants were largely Caucasian and African American. Mothers and fathers were younger at their enrollment in the CHDS than their sons were at the time of follow-up study participation. Mothers and fathers drank about 2.5 cups of coffee per day on average, more than the 1.5 cups per day consumed by their adult sons. Mothers drank an average of about 2 alcoholic drinks per week; however, half (47%) consumed less than 1 drink per week. The average alcohol consumption of mothers was about half that of their husbands, and both parents drank considerably less than their adult sons. Cigarette smoking was high among mothers; nearly 25% smoked during their pregnancy, whereas only 18% of adult sons identified themselves as current smokers.
Table 2. Selected characteristics of Child Health and Development Studies mothers and fathers at pregnancy enrollment (1959–1967) and of adult sons at follow-up (Study of the Environment and Reproduction, 2005).
| Mothers, 1959–1967 | Fathers,1959–1967 | Sons, 2005 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||
| Percentile | Percentile | Percentile | |||||||||||||
|
|
|
|
|||||||||||||
| n | Mean (S.D.) | 25th | 50th | 75th | n | Mean (S.D.) | 25th | 50th | 75th | n | Mean (S.D.) | 25th | 50th | 75th | |
| Age (years) | 196 | 28.5 (5.9) | 24.0 | 29.0 | 33.0 | 196 | 31.4 (7.2) | 26.0 | 30.5 | 36.0 | 196 | 43.8 (1.7) | 43.0 | 44.0 | 45.0 |
| Birth year | 196 | 1962 (1.7) | 1961 | 1962 | 1963 | ||||||||||
| Coffee consumption (cups per day) | 161 | 2.3 (2.3) | 0.3 | 2.0 | 4.0 | 139 | 2.6 (2.2) | 0.5 | 2.0 | 4.0 | 188 | 1.5 (2.4) | 0.0 | 0.8 | 2.0 |
| Alcohol consumption (drinks per week) | 161 | 2.3 (3.1) | 0.8 | 1.0 | 1.8 | 138 | 4.6 (4.6) | 1.0 | 2.5 | 8.0 | 193 | 6.1 (9.7) | 0.0 | 2.2 | 8.0 |
| Cigarettes per day (for current smokers) | 47 | 15.5 (9.7) | 10.0 | 15.0 | 20.0 | 88 | 15.9 (9.5) | 5.0 | 20.0 | 20.0 | 32 | 12.2 (9.3) | 5.0 | 10.0 | 20.0 |
| n (%) | n (%) | n (%) | |||||||||||||
|
|
|
|
|||||||||||||
| Race/ethnicity | |||||||||||||||
| Caucasian | 122 (62) | 115 (62) | 113 (58) | ||||||||||||
| African American | 45 (23) | 41 (22) | 48 (24) | ||||||||||||
| Hispanic | 8 (4) | 9 (5) | 16 (8) | ||||||||||||
| Asian | 17 (9) | 14 (8) | 18 (9) | ||||||||||||
| Other | 4 (2) | 7 (4) | 1 (1) | ||||||||||||
| Cigarette smoking | |||||||||||||||
| Current (yes/no) | 47 (24) | 92 (54) | 33 (18) | ||||||||||||
| Past (yes/no) | 47 (24) | 24 (14) | 33 (18) | ||||||||||||
| Non-smoker | 98 (51) | 53 (31) | 122 (65) | ||||||||||||
S.D. = standard deviation.
Mothers' coffee intake was strongly correlated with their cigarette and alcohol use (Spearman correlation coefficient (rs) = 0.30, P < 0.0001 for maternal coffee v. maternal cigarette use and 0.29, P = 0.0002 for maternal coffee v. maternal alcohol use). Mother's behavior was also correlated with their husband's and the strongest associations were between like behaviors. For example, mother's coffee use was most strongly related to their husband's coffee use (rs = 0.44, P < 0.0001 for maternal v. paternal coffee use) and slightly less highly correlated with his cigarette (rs = 0.23, P < 0.0073 for maternal coffee v. paternal cigarette use) and alcohol use (rs = 0.20, P < 0.0206 for maternal coffee v. paternal alcohol use). Mother's behavior was not correlated with their son's intake of coffee, cigarettes or alcohol.
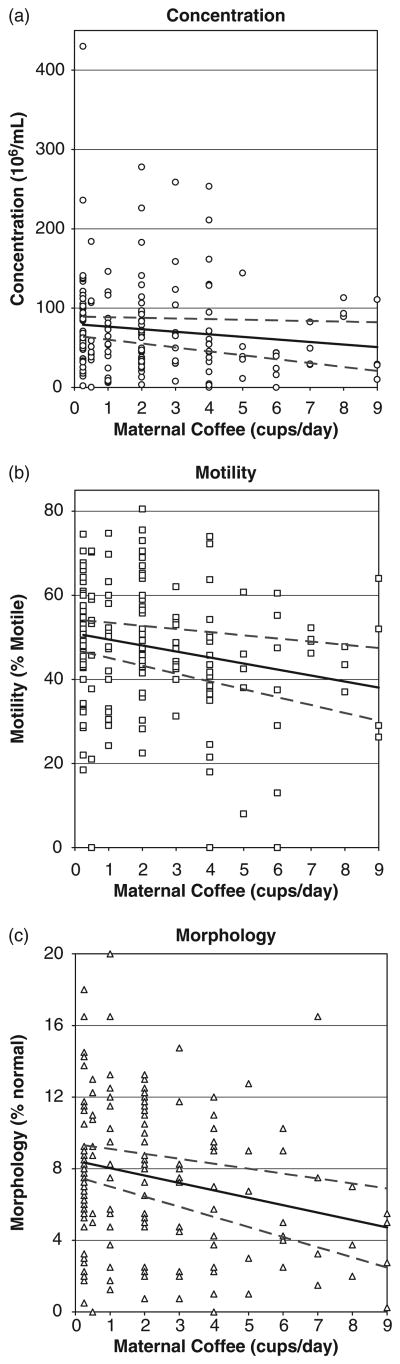
Figure 2 presents the unadjusted, data-based correlation between maternal coffee intake as a proxy for prenatal exposure and son's sperm concentration (a), motility (b) and morphology (c). Graphs show a clear decline for all semen measures with increasing maternal coffee consumption.
Fig. 2.
Distribution of son's semen measures by maternal coffee use during pregnancy, an adult follow-up study (Study of the Environment and Reproduction) of the Child Health and Development Studies.
Table 3 reports associations between prenatal and contemporary coffee, alcohol and cigarette exposure and son's semen quality estimated from linear regression models. Exposure to mother's coffee use during pregnancy was associated with a decline for all semen parameters. The prenatal coffee association was not confounded by mother's alcohol and cigarette use (Table 3, model 2a) and remained in effect after adjusting for son's contemporary use of coffee (Table 3, model 2b). Coefficients for maternal coffee consumption presented in Table 3 represent declines per 5 cups per day during pregnancy (equivalent to the median of the highest minus median of the lowest quartiles) for each semen measure. Findings for maternal alcohol and smoking and for son's contemporary coffee and alcohol use were not significant. Son's contemporary cigarette use was associated with a significant decrease in sperm motility and morphology, but not concentration. Although findings were not statistically significant for son's past cigarette use, the associations were consistent with those observed for current use. Father's behavior was not associated with son's semen measures (data not shown).
Table 4 presents maternal coffee associations with adult son's semen quality estimated from linear regression models for three levels of maternal pregnancy intake: 0 cups per day, 2.5 cups per day (mean level) and 5 cups per day (median of the highest quartile minus median of the lowest quartile). Mean sperm concentration was reduced by more than 16 million sperms for sons with high prenatal exposure (5 cups per day) compared with unexposed sons (P = 0.09). Mean percent motile sperm decreased by approximately 7 points (P = 0.04) and mean percent sperm with normal morphology decreased by 2 points (P = 0.01). The decreases in semen quality with increasing prenatal exposure, 5 cups compared with 0 cups per day, translate to proportionate reductions of approximately 25%, 13% and 25% for sperm concentration, motility and morphology, respectively. Adjustment for maternal alcohol and cigarette use and for son's contemporary coffee, alcohol and cigarette use did not affect the association between semen quality and prenatal coffee exposure.
Adjustment (individually) for mother's age, race/ethnicity and parity and for son's genital birth defects (i.e. hypospadias and cryptorchidism) and age did not explain the associations between prenatal coffee exposure and reduced semen quality (data not shown).
Adjustment for pre-examination abstinence did not explain the prenatal coffee association. However, abstinence itself was positively and significantly associated with sperm concentration [back-transformed mean sperm concentration estimated for median abstinence level (4 days) = 64; 95% CI = (52.5, 77.1), P-value = 0.02 for abstinence coefficient], but not with percent motility or morphology, consistent with other studies.24,25 The median level of pre-examination abstinence was 4 days, based on the inclusion of 12 of the 355 samples that were less than the requested 2-day abstention period. Similarly, adjustment for analysis delay (interval between ejaculation at the time of examination and the semen evaluation) did not explain the prenatal coffee association with motility. The median analysis delay was 32 min, and all but two semen evaluations were initiated within an hour of ejaculation at the time of examination.
Discussion
The most notable finding in this study is the association between maternal prenatal coffee consumption and reductions in sperm concentration, motility and normal morphology (Fig. 2 and Tables 3 and 4). Coffee associations were statistically significant for motility and morphology and consistent for all three measures, regardless of adjustment for other maternal behaviors (smoking and alcohol use) or sons' behaviors (smoking, alcohol or coffee drinking). Adjustment for maternal race/ethnicity, age and parity, which are associated with lifestyle factors that are unmeasured, did not substantially change the observed associations.
We did not calculate caffeine equivalents from coffee consumption, given a recent report that caffeine content of coffee drinks is imprecise and can introduce misclassification bias.27 Therefore, results apply to coffee consumption, and any or all of the components of coffee, including multiple caffeine metabolites and contaminants.
The only other prospective investigation of prenatal coffee exposure examined effects on semen parameters in younger men, in the age group of 18–21 years, based on a Danish pregnancy cohort with pregnancies in 1984–1987.6 Ramlau-Hansen et al. reported prenatal maternal coffee associations with reduced inhibin B and testosterone, and with a possible reduction in semen volume, but no associations with sperm concentration, motility or morphology. In this study, semen was collected from men in their 40s, and it is possible that the effects we observed apply only to older men. In addition, perhaps men in the Danish study, still in the process of completing their reproductive maturity, may not yet have observable impact from environmental stressors. It would be of great interest to study longitudinal change in semen parameters as a function of prenatal coffee exposure to learn whether reproductive senescence might occur earlier for men exposed to coffee in utero. The association between maternal prenatal coffee drinking and morphology in this study, regardless of other maternal behaviors or son's behaviors, is notable, as morphology was the strongest predictive semen parameter of infertility in the Fertile Male Study.28 However, it is unknown whether the associations we observed are large enough to impact fertility or time to pregnancy.
The inverse association between prenatal maternal coffee consumption and semen quality is consistent with animal studies. These studies have demonstrated a direct impact of the caffeine metabolite, theophylline, on the development of the seminiferous cords, embryonic precursors to the Sertoli cells.29 Studies have also shown that coffee alters hormone levels in humans, lowering estrogen30 and increasing testosterone and sex hormone-binding globulin,31 laying a foundation for another mechanism by which developing gonadal cells might be affected by prenatal coffee exposure.
In contrast to our findings for prenatal maternal coffee exposure, we found no association between son's contemporary coffee exposure and semen parameters, which is consistent with some but not all previous studies, as recently reviewed by Jensen et al.32 Few sizeable studies have reported on contemporary caffeine and semen quality. One large study of young Danish men reported that only very high levels of cola or caffeine (calculated from coffee, cola and chocolate consumption) are correlated with reduced sperm concentration and sperm count, but not with motility or morphology.32 Contemporary caffeine was also associated with higher serum testosterone in sons in the Danish pregnancy cohort.6
Our results for maternal pregnancy alcohol and maternal pregnancy smoking are largely consistent with the Danish study of younger men; there were no prenatal alcohol or smoking associations with sperm motility or morphology in either study, although in the Danish study prenatal alcohol showed a decline in sperm concentration for prenatal maternal drinking of ⩾4.5 drinks per week,9 and prenatal smoking showed a decline in sperm count and possibly sperm concentration among men exposed to >19 cigarettes per day.8
Contemporary adult cigarette use among CHDS sons was associated with a significant decrease in the percent of motile sperm, which is also consistent with the Danish cohort findings. In addition, we find evidence that adult smoking is related to a decrease in the percent of sperm with normal morphology. However, unlike the Danish study, we do not observe an association between smoking and sperm concentration (Table 3, model 1f). The smoking association for motility remains after adjustment for adult son's alcohol and coffee intake and for their prenatal exposure to maternal cigarette, alcohol and coffee use.
Mean sperm concentration for this sample (72 million) is well above the lowest decile of 22 million sperm per ml reported as a reference for fertile men by the World Health Organization33 and comparable with the mean reported for fertile men (67 million) by the Fertile Male Study.16 Mean percent motile sperm (47%) was only slightly lower than that reported for fertile men by the Fertile Male Study (54%). However, the mean level for percent with normal morphology was considerably lower (8%) compared with the means for both fertile (14%) and infertile (11%) men reported by the Fertile Male Study.
The age of our subjects (mean of 44 years) may explain the lower percent of sperm with normal morphology in this sample. Men in the Fertile Male Study were, on average, 10 years younger than our subjects. In a semen collection pilot study among other younger CHDS sons (36–39 years old), conducted 6 years ago, normal morphology was about 12%, much closer to the Fertile Male Study.16,35 A number of studies have documented decline in both motility and morphology with age, particularly in the 30- to 50-year age group.36
Comparison of semen measures with other studies is difficult because of measurement error, sampling variability and the inherent variability of semen quality, particularly concentration, which has high normal variation.34 Semen parameters in this sample are compared with those in the Fertile Male Study because both studies relied on the UCD Andrology Laboratory to train examiners and perform the morphology evaluation.
The small sample size and modest response rate ( ˜ 35%) for the present study require cautious interpretation. However, comparison of the baseline maternal and birth characteristics of subjects who were eligible and those who participated suggest that participants were generally representative of the eligible pool (Table 1). The two groups were not different on prenatal coffee and alcohol exposure; however, participants had proportionately fewer mothers who smoked during their pregnancies. This could possibly explain the absence of a significant association between prenatal smoking and semen quality, if sons with poor semen quality exposed to maternal prenatal smoking were underrepresented. However, bias is unlikely to explain the observed association between coffee and reduced semen quality, as the distribution of maternal coffee consumption does not differ for eligible compared with participant.
Despite the small sample size, prenatal maternal coffee associations were significant for motility and morphology and achieved borderline significance for concentration (Table 3). Although these findings bear statistical significance, the magnitude of the effects of maternal prenatal coffee use on semen quality may raise ambiguity about their biological significance. What do these reductions mean for reproductive potential? Sons who were prenatally exposed to higher maternal coffee intake (5 or more cups per day) compared with those who were less exposed (less than 5 cups per day) had raw unadjusted mean sperm counts of 50 compared with 76 million, 41% v. 48% with motility and 5.6% v. 7.8% with normal morphology. Sperm measures in these highly exposed men were even lower than those for the 30% of men in this sample who never fathered a pregnancy, with mean levels of 70 million, 44% and 7.3% for concentration, motility and morphology, respectively. Furthermore, the consistency of the maternal prenatal coffee association for all three semen measures provides additional support that the findings may represent meaningful reproductive impairment.
The strengths of our study include a prospective design that minimizes recall bias, self-report of smoking and alcohol use well before those behaviors were stigmatized for pregnant women, ability to adjust simultaneously for maternal prenatal smoking, alcohol and coffee consumption, which co-vary and may have varying effects on semen parameters, ability to adjust prenatal associations for contemporary personal behavior and a standardized semen evaluation protocol originally developed for the Fertile Male Study and conducted with training by the laboratory of James Overstreet, at the UCD, where morphology was also assessed. Weaknesses are shared by many semen studies, including a low response rate (less than 50% for most studies) and consequent potential for bias, and a relatively small sample size.
We conclude that our findings support the hypothesis that prenatal, but not contemporary, coffee exposure is associated with semen quality, including concentration, motility and morphology. Negative findings for prenatal alcohol and prenatal smoking should be interpreted in light of the small sample size and lower power to detect a modest effect. Low response rates in this and most semen studies make it difficult to exclude bias as an explanation of findings. However, for prenatal coffee, there was no evidence that response varied according to maternal prenatal coffee drinking. This suggests that bias is unlikely to explain the significant associations we observed for prenatal maternal coffee use with decreased semen quality. These findings reinforce the current American Congress of Obstetricians and Gynecologists (ACOG) guidelines,37 which recommend limiting coffee intake during pregnancy.
Supplementary Material
Acknowledgments
The authors acknowledge late Jacob Yerushalmy who designed and initiated the CHDS; Barbara van den Berg, the second Director of the CHDS, who conserved the serum archive; Roberta Christianson for her role in coding and documenting the CHDS archive and for her invaluable insights about other maternal coffee effects in the CHDS based on her own previous work; Sunita Miles for managing the collection of the adult son's data; Charlene Tollner for training the study technicians to perform semen evaluations; and Cathy Treece for staining and reading the morphology slides. This research was supported by the National Institute of Environmental Health Sciences (R01 ES12231) and the National Institutes of Child Health and Human Development (N01 DK63422).
Footnotes
Supplementary material: The supplementary material referred to this article is available online at http://www.journals.cambridge.org/doh
References
- 1.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 2.Thorup J, McLachlan R, Cortes D, et al. What is new in cryptorchidism and hypospadias – a critical review on the testicular dysgenesis hypothesis. J Pediatr Surg. 2010;45:2074–2086. doi: 10.1016/j.jpedsurg.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Jorgensen N, Meyts ER, Main KM, Skakkebaek NE. Testicular dysgenesis syndrome comprises some but not all cases of hypospadias and impaired spermatogenesis. Int J Androl. 2010;33:298–303. doi: 10.1111/j.1365-2605.2009.01050.x. [DOI] [PubMed] [Google Scholar]
- 4.Mocarelli P, Gerthoux PM, Patterson DG, Jr, et al. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ Health Perspect. 2008;116:70–77. doi: 10.1289/ehp.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadeu JC, Hughes CL, Agarwal S, Foster WG. Alcohol, drugs, caffeine, tobacco, and environmental contaminant exposure: reproductive health consequences and clinical implications. Crit Rev Toxicol. 2010;40:633–652. doi: 10.3109/10408444.2010.493552. [DOI] [PubMed] [Google Scholar]
- 6.Ramlau-Hansen CH, Thulstrup AM, Bonde JP, Olsen J, Bech BH. Semen quality according to prenatal coffee and present caffeine exposure: two decades of follow-up of a pregnancy cohort. Hum Reprod. 2008;23:2799–2805. doi: 10.1093/humrep/den331. [DOI] [PubMed] [Google Scholar]
- 7.Ramlau-Hansen CH, Thulstrup AM, Olsen J, et al. Maternal smoking in pregnancy and reproductive hormones in adult sons. Int J Androl. 2008;31:565–572. doi: 10.1111/j.1365-2605.2007.00807.x. [DOI] [PubMed] [Google Scholar]
- 8.Ramlau-Hansen CH, Thulstrup AM, Storgaard L, et al. Is prenatal exposure to tobacco smoking a cause of poor semen quality? A follow-up study. Am J Epidemiol. 2007;165:1372–1379. doi: 10.1093/aje/kwm032. [DOI] [PubMed] [Google Scholar]
- 9.Ramlau-Hansen CH, Toft G, Jensen MS, et al. Maternal alcohol consumption during pregnancy and semen quality in the male offspring: two decades of follow-up. Hum Reprod. 2010;25:2340–2345. doi: 10.1093/humrep/deq140. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Lin H, Li Y, Cao J. Association between sociopsycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril. 2011;95:116–123. doi: 10.1016/j.fertnstert.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 11.Sharpe RM. Environmental/lifestyle effects on spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1697–1712. doi: 10.1098/rstb.2009.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen TK, Henriksen TB, Hjollund NH, et al. Adult and prenatal exposures to tobacco smoke as risk indicators of fertility among 430 Danish couples. Am J Epidemiol. 1998;148:992–997. doi: 10.1093/oxfordjournals.aje.a009576. [DOI] [PubMed] [Google Scholar]
- 13.Jensen TK, Henriksen TB, Hjollund NHI, et al. Caffeine intake and fecundability: a follow-up study among 430 Danish couples planning their first pregnancy. Reprod Toxicol. 1998;12:289–295. doi: 10.1016/s0890-6238(98)00002-1. [DOI] [PubMed] [Google Scholar]
- 14.van den Berg BJ, Christianson RE, Oechsli FW. The California Child Health and Development Studies of the School of Public Health, University of California at Berkeley. Paediatr Perinat Epidemiol. 1988;2:265–282. doi: 10.1111/j.1365-3016.1988.tb00218.x. [DOI] [PubMed] [Google Scholar]
- 15.Guzick DS, Carson S, Coutifaris C, et al. Efficacy of superovulation and intrauterine insemination in the treatment of infertility. National Cooperative Reproductive Medicine Network. N Engl J Med. 1999;340:177–183. doi: 10.1056/NEJM199901213400302. [DOI] [PubMed] [Google Scholar]
- 16.Overstreet JW, Brazil C. Semen analysis. In: Lipshultz LI, Howard SS, editors. Infertility in the Male. 3rd. Mosby-Year Book, Inc; St Louis: 1997. pp. 487–490. [Google Scholar]
- 17.Brazil C, Swan SH, Drobnis EZ, et al. Standardized methods for semen evaluation in a multicenter research study. J Androl. 2004;25:635–644. doi: 10.1002/j.1939-4640.2004.tb02835.x. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Semen–Cervical Mucus Interaction. 4th. Cambridge University Press; Cambridge: 1999. [Google Scholar]
- 19.Brazil C, Swan SH, Tollner CR, et al. Quality control of laboratory methods for semen evaluation in a multicenter research study. J Androl. 2004;25:645–656. doi: 10.1002/j.1939-4640.2004.tb02836.x. [DOI] [PubMed] [Google Scholar]
- 20.Istvan J, Matarazzo JD. Tobacco, alcohol, and caffeine use: a review of their interrelationships. Psychol Bull. 1984;95:301–326. [PubMed] [Google Scholar]
- 21.Talcott GW, Poston WS, II, Haddock CK. Co-occurrent use of cigarettes, alcohol, and caffeine in a retired military population. Mil Med. 1998;163:133–138. [PubMed] [Google Scholar]
- 22.Zavela KJ, Barnett JE, Smedi KJ, Istvan JA, Matarazzo JD. Concurrent use of cigarettes, alcohol, and coffee. J Appl Soc Psychol. 1990;20:835–845. [Google Scholar]
- 23.Mongraw-Chaffin ML, Cohn BA, Cohen RD, Christianson RE. Maternal smoking, alcohol consumption, and caffeine consumption during pregnancy in relation to a son's risk of persistent cryptorchidism: a prospective study in the Child Health and Development Studies Cohort, 1959–1967. Am J Epidemiol. 2008;167:257–261. doi: 10.1093/aje/kwm311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mortimer D, Templeton AA, Lenton EA, Coleman RA. Influence of abstinence and ejaculation-to-analysis delay on semen analysis parameters of suspected infertile men. Arch Androl. 1982;8:251–256. doi: 10.3109/01485018208990205. [DOI] [PubMed] [Google Scholar]
- 25.De Jonge C, LaFromboise M, Bosmans E, et al. Influence of the abstinence period on human sperm quality. Fertil Steril. 2004;82:57–65. doi: 10.1016/j.fertnstert.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Shanis BS, Check JH, Bollendorf A. Interpretation and misinterpretation of semen parameters. Syst Biol Reprod Med. 1989;23:213–227. doi: 10.3109/01485018908986844. [DOI] [PubMed] [Google Scholar]
- 27.Grosso LM, Bracken MB. Caffeine metabolism, genetics, and perinatal outcomes: a review of exposure assessment considerations during pregnancy. Ann Epidemiol. 2005;15:460–466. doi: 10.1016/j.annepidem.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Guzick DS, Overstreet JW, Factor-Litvak P, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–1393. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 29.Pollard I, Locquet O, Solvar A, Magre S. Effects of caffeine and its reactive metabolites theophylline and theobromine on the differentiating testis. Reprod Fertil Dev. 2001;13:435–441. doi: 10.1071/rd01018. [DOI] [PubMed] [Google Scholar]
- 30.Petridou E, Katsouyanni K, Spanos E, et al. Pregnancy estrogens in relation to coffee and alcohol intake. Ann Epidemiol. 1992;2:241–247. doi: 10.1016/1047-2797(92)90056-v. [DOI] [PubMed] [Google Scholar]
- 31.Svartberg J, Midtby M, Bonaa KH, et al. The associations of age, lifestyle factors and chronic disease with testosterone in men: the Tromso Study. Eur J Endocrinol. 2003;149:145–152. doi: 10.1530/eje.0.1490145. [DOI] [PubMed] [Google Scholar]
- 32.Jensen TK, Swan SH, Skakkebaek NE, Rasmussen S, Jorgensen N. Caffeine intake and semen quality in a population of 2,554 young Danish men. Am J Epidemiol. 2010;171:883–891. doi: 10.1093/aje/kwq007. [DOI] [PubMed] [Google Scholar]
- 33.Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 34.Overstreet JW. Laboratory tests for human male reproductive risk assessment. Teratog Carcinog Mutagen. 1984;4:67–82. doi: 10.1002/tcm.1770040108. [DOI] [PubMed] [Google Scholar]
- 35.Cohn BA, Overstreet JW, Fogel RJ, et al. Epidemiologic studies of human semen quality: considerations for study design. Am J Epidemiol. 2002;155:664–671. doi: 10.1093/aje/155.7.664. [DOI] [PubMed] [Google Scholar]
- 36.Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril. 2001;75:237–248. doi: 10.1016/s0015-0282(00)01679-4. [DOI] [PubMed] [Google Scholar]
- 37.American Congress of Obstetricians and Gynecologists (ACOG) Nutrition During Pregnancy. Patient Education Pamphlet AP001. 2010 Aug; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.