Abstract
Objectives
Lower birth weight (BW) re-occurs across generations, but the intermediate mechanisms remain poorly understood. One potential pathway involves cortisol, which may be elevated in women born small and in turn could lead to fetal growth restriction in offspring. To test this possibility, we evaluated whether BW predicts hypothalamic-pituitary-adrenal (HPA) function in the non-pregnant state in a cohort of young Filipino women, and whether differences in HPA function predict offspring BW.
Methods
Multiple regression relating maternal BW, adult salivary cortisol profiles and recalled offspring BW (N = 488) among participants of the Cebu Longitudinal Health and Nutrition Survey.
Results
Maternal BW related inversely to evening cortisol in adulthood (p < 0.04). Maternal BW and evening cortisol were both stronger predictors of male than of female BW (maternal BW: p < 0.0001 for males; p = 0.07 for females; bedtime cortisol: p = 0.003 for males; p = 0.3 for females). Waking and thirty minute post-waking cortisol did not predict offspring BW. Controlling for evening cortisol did not diminish the relationship between maternal and offspring BW in males or females.
Conclusions
Being born small predicted higher evening cortisol in adulthood among these young mothers. Lower maternal BW and elevated evening cortisol independently predicted giving birth to lower BW offspring, with effects greatest and only significant among males. We speculate that sex differences in sensitivity to maternal stress hormones could help explain the stronger relationships between BW and CVD risk factors reported among the males in this and other populations.
Keywords: cortisol, birth weight, intergenerational effects, sex differences, DOHaD
It is well recognized that birth weight (BW) tends to be similar in mother and offspring independent of genetic heritability (Ounstead and Ounstead 1968). Although the underlying mechanisms remain poorly understood, modifications to maternal hypothalamic-pituitary-adrenal (HPA)-axis function is one plausible contributing factor (Drake and Walker 2004). The hormone cortisol is the primary product of the HPA-axis in humans, and helps coordinate physiologic responses to various forms of environmental challenge. Although the fetus is largely shielded from maternal cortisol through the action of placental enzymes, this buffering is incomplete, thus allowing some maternal cortisol to reach the fetus (Seckl 2004). Elevated maternal cortisol is associated with reduced offspring birth size (Pike 2005). Strikingly, individuals born small often have altered HPA-axis function in adulthood (Reynolds et al. 2005; Entringer et al. 2009).
Because the gestational environment shapes adult HPA-axis function, and adult HPA-axis function influences the gestational environment experienced by the next generation, variation in HPA-axis set points established in early development could help explain the tendency for lower BW to be perpetuated across generations (Drake and Walker 2004; Kuzawa and Sweet 2009). To test this model, we evaluated the relationship between maternal and offspring BW in a large multi-generational cohort from Cebu City, Philippines, and explored the role of maternal salivary cortisol in this relationship. To evaluate the role of stable, trait-level variation in female HPA-axis function, we limited analyses to parous women whose saliva was obtained outside of pregnancy, allowing us to avoid effects of the temporary and marked regulatory changes in the HPA-axis during pregnancy (Wadhwa 2005). Because past work in this sample identified sex differences in later life outcomes related to early growth rate (Kuzawa and Adair 2003; Kuzawa et al 2010), we also evaluated whether these relationships differed by sex of offspring.
Methods
Data come from the Cebu Longitudinal Health and Nutritional Study (CLHNS), which enrolled 3327 pregnant women who gave birth to 3080 singleton, live-born offspring from 1983-1984 in Cebu City, Philippines (Adair et al 2011). The present study included 488 singleton live-born offspring born to the 308 female members of the original birth cohort who were parous by the time of questionnaire and sample collection and had all variables available. Reproductive histories recorded birth date, sex, and recalled BW and length of gestation for each offspring among study women in 2005, 2007 and 2009. Standard anthropometric techniques were used to measure standing height and weight in women. For each birth, maternal pre-pregnancy BMI was calculated using height and weight measured during the survey preceding that birth by at least nine months. We defined underweight as a BMI≤18.5; when women were younger than 18 years of age (N =33) we defined underweight status using BMI cut-offs recommended by the Childhood Obesity Working Group of the International Obesity Taskforce using the zbmicat command in Stata (Cole et al. 2000). The mothers' BW was measured by hospital nurses or birth attendants trained in the use of Salter scales, and by survey interviewers during follow-ups. The mother's gestational age at delivery was estimated from the date of last menstrual period (see Adair et al in press). This research was conducted under conditions of informed consent, and with approval of the Institutional Review Boards of the University of North Carolina (Chapel Hill), Northwestern University (Evanston, Illinois), and the Office of Population Studies Foundation (Cebu, Philippines).
Cortisol measurement
Saliva samples were obtained at waking, 30 minutes after waking and immediately prior to bed (for protocol see Gettler et al. 2011). Cortisol was assayed in duplicate by a laboratory in Trier, Germany, using a time-resolved immunoassay with fluorometric detection (DELFIA). The intra- and inter-assay coefficients of variation (CVs) were between 4.0% and 6.7%, and 7.1% to 9.0%, respectively. Samples with CVs over 12% were rerun.
Statistical analysis
All analyses were conducted using Stata 10.1 (College Station, TX). Cortisol was log transformed and adjusted for time of saliva collection, usual wake time, exercise and illness symptoms prior to saliva collection, with residuals used in models. Multiple regression was used to determine the relationship between maternal BW, cortisol and offspring BW with maternal BMI, age, recalled offspring age at parturition, primiparity and mothers gestational age considered as covariates. In Stata, the regress command was used with the cluster option to account for non-independence of birth weights among multiparous women. While a total of 11 regressions were run, which could increase the risk of Type I error, we set alpha at 0.05 because many of the tests involved models built sequentially to test pathways and thus were not independent.
Results
Maternal BW was a significant inverse predictor of bedtime cortisol measured in young adulthood (β = -50.5± 21.5 log-nmol/g, p<0.02, R2 = 0.07), but not of other cortisol measures (both p>0.12). Maternal BW adjusted for gestational age was positively associated with recalled BW in male and female offspring, with effects stronger in males (males: β = 0.4 ± 0.09 g offspring BW/g mother's BW; p < 0.001, R2 = 0.16; females: β = 0.2 ± 0.1 g/g, p = 0.08, R2 = 0.07).
We next evaluated whether maternal HPA-axis function in young adulthood predicted offspring BW. Maternal bedtime cortisol was a significant, inverse predictor of offspring BW in males but not females (males: β = -90.6 ± 45.5 g/log-nmol/1; p = 0.05, R2 = 0.10; females: β = -46.0 ± 43.8 g/log-nmol/1, p = 0.3, R2 = 0.04)(Fig. 1a). As a result, absolute sex differences in BW were reduced in half among individuals with above-median maternal bedtime cortisol compared to individuals born to mothers with below-median bedtime cortisol (Fig. 1b). Waking cortisol was a modest inverse but non-significant predictor of offspring BW in males and females (both p > 0.1), while 30 minute post-waking cortisol was not associated with offspring BW (both p > 0.6).
Fig 1.
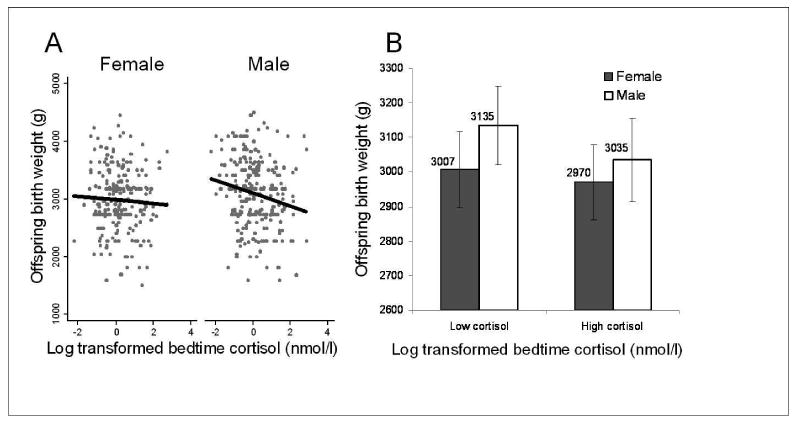
A) linear trends relating maternal evening cortisol to gestational timing-adjusted offspring birth weight plotted separately for male and female offspring (see results for slopes); B) The relationship between offspring birth weight and above- and below-median maternal bedtime cortisol, illustrating the reduction in sexual size dimorphism at higher levels of maternal cortisol.
Finally, we tested whether the higher evening cortisol among lower birth weight women might mediate the relationship between maternal BW and offspring BW. The relationship between maternal and offspring BW was essentially unchanged after adjusting for bedtime cortisol (males: maternal BW: β = 0.4 ± 0.1 g/log-nmol/1, p < 0.001, bedtime cortisol: β = -76.7 ± 47.4 g/log-nmol/1, p = 0.1, adjusted R2 = 0.18; females: maternal BW β = 0.2 ± 0.1 g/log-nmol/1, p = 0.07, bedtime cortisol: β = -35.1 ± 43.3 g/log-nmol/1, p = 0.4, adjusted R2 = 0.08).
Discussion
We hypothesized that the gestational environment of the mother, as reflected in her own BW, would predict her adult HPA-axis function, and that this in turn would predict BW of her offspring. Consistent with this expectation, women born small did have significantly higher evening cortisol. Women who were born small had lower BW offspring, with disproportionate effects on males. Similarly, women with higher bedtime cortisol tended to give birth to smaller offspring, but this effect was stronger, and only significant, in males. Adjusting for bedtime cortisol had essentially no effect on the relationship between maternal and offspring BW, suggesting that a woman's own birth weight and HPA-axis function in adulthood are independent predictors of offspring BW.
The evidence we report for disproportionate effects of maternal cortisol on fetal growth in male offspring is a novel finding, and could shed light on prior findings of the developmental predictors of male function and health. Notably, we previously reported that BW was a stronger predictor of blood pressure and cholesterol levels measured in this cohort during adolescence (Adair et al. 2001; Kuzawa and Adair 2003), and that early postnatal weight gain disproportionately predicted adult size, muscle and life history characteristics in males (Kuzawa et al. 2010). Among species with male-biased sexual size dimorphism (SSD), such as humans, SSD tends to be reduced in the context of marginal nutrition or environmental quality (Badyaev 2002). It has been speculated that the faster growth rate of male fetuses, and the corresponding increased energy requirements, could make males more sensitive to maternal stressors (Kuzawa and Adair 2003).
In summary, being born small and having elevated evening cortisol in young adulthood independently predict giving birth to smaller newborns in this population, with both effects strongest among male offspring. Although awaiting replication in other populations and with more intensive cortisol sampling protocols, these findings do not support a strong role of basal HPA-axis function as a pathway linking BW across generations. However, the evidence reported for heightened male sensitivity to maternal stress hormones in utero could help explain our previous reports that relationships between BW and adult CVD risk factors are stronger among the males than in the females in this population. We hypothesize that these sex differences in prenatal sensitivity to maternal cortisol reflect past selection for traits related to sexual size dimorphism in humans.
Table 1. Characteristics of Mothers and Offspring.
| All (N = 308) |
Low evening cortisol (N = 154) |
High evening cortisol (N = 154) |
Pa | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Variable | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | |
| Maternal characteristics | |||||||
| Age (years) | 21.5 (0.3) | (20.8, 22.3) | 21.5 (0.31) | (20.8, 22.3) | 21.5 (0.3) | (20.8, 22.3) | 0.35 |
| Birth weight (g) | 2984 (407) | (1814, 4100) | 3010 (376) | (1956, 4082) | 2958 | (1814, 4100) | 0.26 |
| Weight (kg) | 47.2 (7.9) | (30, 85) | 48.8 (8.3) | (34, 85) | 45.6 (7.0) | (30, 64) | 0.0002 |
| Height (cm) | 150.6 (5.4) | (136.5, 167.1) | 151.2 (5.4) | (139.8, 167.1) | 149.9 (5.3) | (136.5, 162.8) | 0.039 |
| BMI (kg/m2) | 20.7 (3.0) | (14.6, 33.5) | 21.3 (3.1) | (15.7, 33.5) | 20.2 (2.8) | (14.6, 29.8) | 0.0009 |
| Underweight (%)b | 23.1% | 16.9% | 29.2% | 0.010 | |||
| Overweight (%)c | 8.1% | 10.4% | 5.8% | 0.14 | |||
| Obese (%)d | 0.01% | 1.9% | 0% | 0.15 | |||
| Cortisol | |||||||
| Waking (nmol/1) | 7.3 (3.8) | (0.4, 23.2) | 6.7 (3.2) | (0.4, 18.7) | 7.9 (4.2) | (0.9, 23.2) | 0.003 |
| 30-min post waking (nmol/1) | 8.7 (4.5) | (0.4, 34.0) | 8.0 (3.9) | (0.4, 23.6) | 9.5 (4.9) | (0.6, 34.0) | 0.012 |
| Evening (nmol/1) | 1.9 (2.2) | (0.1, 17.1) | 0.6 (0.3) | (0.1, 1.1) | 3.1 (2.5) | (1.1, 17.1) | 0.08 |
|
| |||||||
| Individual Pregnancies | (N = 488) | (N = 244) | (N = 244) | ||||
| Offspring birth weight (g) | 3032 (558) | (1500, 4500) | 3086 (552) | (1591, 4500) | 2979 (560) | (1500, 4455) | 0.03 |
| Offspring male (%) | 53% | 55% | 50% | 0.23 | |||
| Born early (<9 months gestation) | 6% | 5% | 7% | 0.24 | |||
| Primiparity (%) | 56% | 57% | 55% | 0.78 | |||
Significance level for difference between women with high and low cortisol from 2-sided t-tests for continuous variables and chi-2 for categorical variables.
BMI ≤18.5
BMI ≥25 and <30
BMI ≥30
Acknowledgments
We thank Linda Adair and the researchers at the Office of Population Studies, University of San Carlos, Cebu, Philippines, for their role in study design and data collection, and the Filipino participants, who generously provided their time. Elizabeth Quinn, Jeff Huang, Katy Sharrock, Iram Azam, Divya Mallampati, Brian Dubin, Laura Rogers and Amy Desantis helped with lab work. Emma Adam advised on cortisol collection design. Funding: the National Science Foundation (BCS-0746320); fieldwork and sample collection supported by pilot funds from the Interdisciplinary Obesity Center (RR20649) and the Center for Environmental Health and Susceptibility (ES10126; project 7-2004-E). ZMT was supported by a National Science Foundation Graduate Research Fellowship during manuscript preparation.
Grants: Research supported by the National Science Foundation (BCS-0746320). Fieldwork and sample collection were also supported by pilot funds from the Interdisciplinary Obesity Center (RR20649) and the Center for Environmental Health and Susceptibility (ES10126; project 7-2004-E). ZMT is supported by a National Science Foundation Graduate Research Fellowship.
Literature Cited
- Adair LS, Kuzawa CW, Borja J. Maternal Energy Stores and Diet Composition During Pregnancy Program Adolescent Blood Pressure. Circulation. 2001;104:1034–1039. doi: 10.1161/hc3401.095037. [DOI] [PubMed] [Google Scholar]
- Adair LS, Popkin BM, Akin JS, Guilkey DK, Gultiano S, Borja J, Perez L, Kuzawa CW, McDade T, Hindin MJ. Cohort Profile: The Cebu Longitudinal Health and Nutrition Survey. Int J Epidemiol. 2011;40:619–25. doi: 10.1093/ije/dyq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev A. Growing apart: an ontogenetic perspective on the evolution of sexual size dimorphism. Trends Ecol Evol. 2002;17:369–378. [Google Scholar]
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake A, Walker B. The intergenerational effects of fetal programming: non-genomic mechanisms for the inheritance of low birth weight and cardiovascular risk. J Endocrinol. 2004;180:1–16. doi: 10.1677/joe.0.1800001. [DOI] [PubMed] [Google Scholar]
- Entringer S, Kumsta R, Hellhammer DH, Wadhwa PD, Wüst S. Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Horm Behav. 2009;55:292–298. doi: 10.1016/j.yhbeh.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Gettler LT, McDade TW, Kuzawa CW. Cortisol and testosterone in Filipino young adult men: Evidence for co-regulation of both hormones by fatherhood and relationship status. Am J Hum Biol. 2011;23:609–620. doi: 10.1002/ajhb.21187. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Adair LS. Lipid profiles in adolescent Filipinos: relation to birth weight and maternal energy status during pregnancy. Am J Clin Nutr. 2003;77:960–966. doi: 10.1093/ajcn/77.4.960. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Sweet E. Epigenetics and the embodiment of race: Developmental origins of US racial disparities in cardiovascular health. Am J Hum Biol. 2009;21:2–15. doi: 10.1002/ajhb.20822. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, McDade TW, Adair LS, Lee N. Rapid weight gain after birth predicts life history and reproductive strategy in Filipino males. PNAS. 2010;107:16800–16805. doi: 10.1073/pnas.1006008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ounsted M, Ounsted C. Maternal Regulation of Intra-Uterine Growth. Nature. 1966;212:995–997. doi: 10.1038/212995a0. [DOI] [PubMed] [Google Scholar]
- Pike IL. Maternal stress and fetal responses: Evolutionary perspectives on preterm delivery. Am J Hum Biol. 2005;17:55–65. doi: 10.1002/ajhb.20093. [DOI] [PubMed] [Google Scholar]
- Reynolds RM, Walker BR, Syddall HE, Andrew R, Wood PJ, Phillips DIW. Is there a gender difference in the associations of birthweight and adult hypothalamic- pituitary-adrenal axis activity? Eur J Endocrinol. 2005;152:249–253. doi: 10.1530/eje.1.01846. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Prenatal glucocorticoids and long-term programming. Eur J Endocrinol. 2004;151:U49–U62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]


